- 1Department of Gynecology-Andrology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium
- 2Department of Obstetrics and Gynecology, Clinique Saint-Jean, Brussels, Belgium
- 3Tumor Infiltrating Lymphocytes Group - De Duve Institute, Université Catholique de Louvain, Brussels, Belgium
- 4Department of Obstetrics and Gynecology, Tygerberg Hospital, University of Stellenbosch, Cape Town, South Africa
- 5Institut de Recherche Expérimentale et Clinique, Université catholique de Louvain, Brussels, Belgium
Objective: To describe the available knowledge on vulvo-perineal endometriosis including its diagnosis, clinical management and recurrence rate.
Methods: We followed the PRISMA guidelines for Systematic Reviews and our study was prospectively registered with PROSPERO (CRD42020202441). The terms “Endometriosis” and “Perineum” or “Vulva” were used as keywords. Cochrane Library, Medline/Pubmed, Embase and Clinicaltrials.gov were searched. Papers in English, Spanish, Portuguese, French or Italian from inception to July 30, 2020 were considered. Reference lists of included articles and other literature source such as Google Scholar were also manually scrutinized in order to identify other relevant studies. Two independent reviewers screened potentially eligible studies according to inclusion criteria.
Results: Out of 539 reports, 90 studies were eligible including a total of 283 patients. Their mean age was 32.7 ± 7.6 years. Two hundred sixty-three (95.3%) presenting with vulvo-perineal endometriosis have undergone either episiotomy, perineal trauma or vaginal injury or surgery. Only 13 patients (4.7%) developed vulvo-vaginal endometriosis spontaneously i.e., without any apparent condition favoring it. The reasons that motivated the patients to take medical advice were vulvo-perineal cyclical pain increasing during menstruations (98.2% of the patients, n = 278). Out of the 281 patients for whom a clinical examination was described, 274 patients (97.5%) showed a vulvo-perineal nodule, mass or swelling while six presented with bluish cutaneous lesions (2.1%) and 1 with bilateral polyps of the labia minora (0.4%). All but one patients underwent surgical excision of their lesions but only 88 patients (28.1%) received additional hormonal therapy. The recurrence rate was 10.2% (29 patients) considering a median follow-up period of 10 months (based on 61 studies).
Conclusion: In conclusion, vulvo-perineal endometriosis is a rare entity with approximately 300 cases reported in the literature since 1923. With the available knowledge shown in this systematic review, we encourage all practitioners to think about perineal endometriosis in case of perineal cyclical pain with or without previous perineal damage. Diagnosis should be done with clinical exam, perineal ultrasound and pelvic MRI when available. In case of anal sphincter involvement, perianal ultrasound should be performed. Surgical excision of the lesion should be realized in order to remove the lesion and to confirm the diagnosis histologically. Hormonal treatment could be proposed to attempt to decrease the size of a large lesion before surgery or to avoid recurrence of the lesion. As evidence-based approach to the diagnosis, treatment and recurrence rate of affected patients remains a challenge given its low prevalence, the variations in management found in the articles included and the limited quality of available studies, we suggest that a prospective database on vulvo-perineal endometriosis should be generated to increase knowledge but also awareness among healthcare professionals and optimize patients' care.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42020202441.
Introduction
Endometriosis is a complex benign disease characterized by an estrogen-dependent chronic inflammatory process and is defined as the presence of endometrial glands and stroma-like tissue outside the uterine cavity, most often located in the pelvis (1), although extrapelvic sites have been described, including the urinary and gastro-intestinal tracts, the nervous system, the thoracic cavity and diaphragm as well as some (sub-)cutaneous sites (2–5). It occurs in ~10% of women of reproductive-age and in 35–50% of women with infertility and chronic pelvic pain (6–8).
While extrapelvic endometriosis is a relatively uncommon condition, accounting for ~12% of all cases of endometriosis (9), perineal endometriosis is an even rarer entity. Cutaneous and subcutaneous endometriotic lesions have been observed in surgical scars following laparoscopy, laparotomy, vulvo-vaginal surgery, episiotomy and obstetrical lacerations whether surgically repaired or not (3). Perineal endometriosis may involve the skin and/or subcutaneous tissue of the vulva and perineum but also the perianal sphincteric muscular tissue (10). Following the first case of perineal endometriosis reported in 1923 (11), most of what is known of this commonly misdiagnosed entity comes from case reports published over the past 60–70 years.
Our study aims to identify all reported cases of vulvo-perineal endometriosis published in the literature in order to describe diagnostic processes, treatments and recurrence rates of this uncommon type of endometriosis and help practitioners to achieve prompt diagnosis and optimize patients' outcomes.
Methods
This study followed the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (12) and was prospectively registered within the International Prospective Register of Systematic Review (PROSPERO) on the 4th of August 2020 (number CRD42020202441).
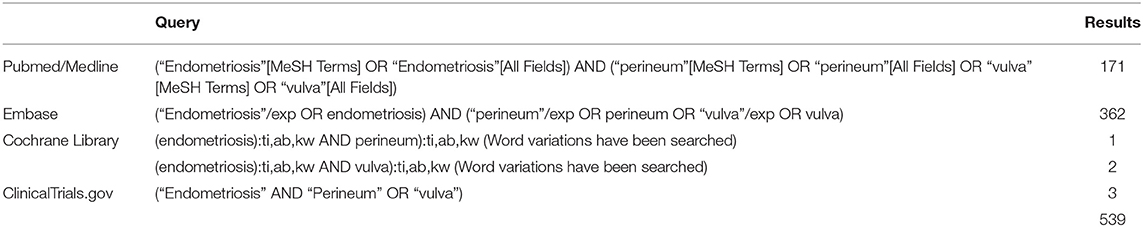
The following search engines and electronic databases were searched on July 30, 2020 by one author (CM): Cochrane Library, Medline/Pubmed, Embase and Clinicaltrials.gov. The terms “Endometriosis” and “Perineum” or “Vulva” were used as keywords to recover all possible publications. Table 1 shows the queries and record numbers for the different databases used. No restrictions regarding language, type or date of publication were initially applied. Reference lists of included articles and other literature sources such as Google Scholar were also manually scrutinized in order to identify additional relevant studies. Articles in English, Spanish, Portuguese, French or Italian were considered. Review articles and studies describing exclusively lesions involving the abdominal wall or the inguinal area, or malignant transformation of endometriosis were excluded. Two reviewers (CM, ZCA) independently screened titles and abstracts of the search output to identify potentially eligible studies and cross-examined their results.
Results
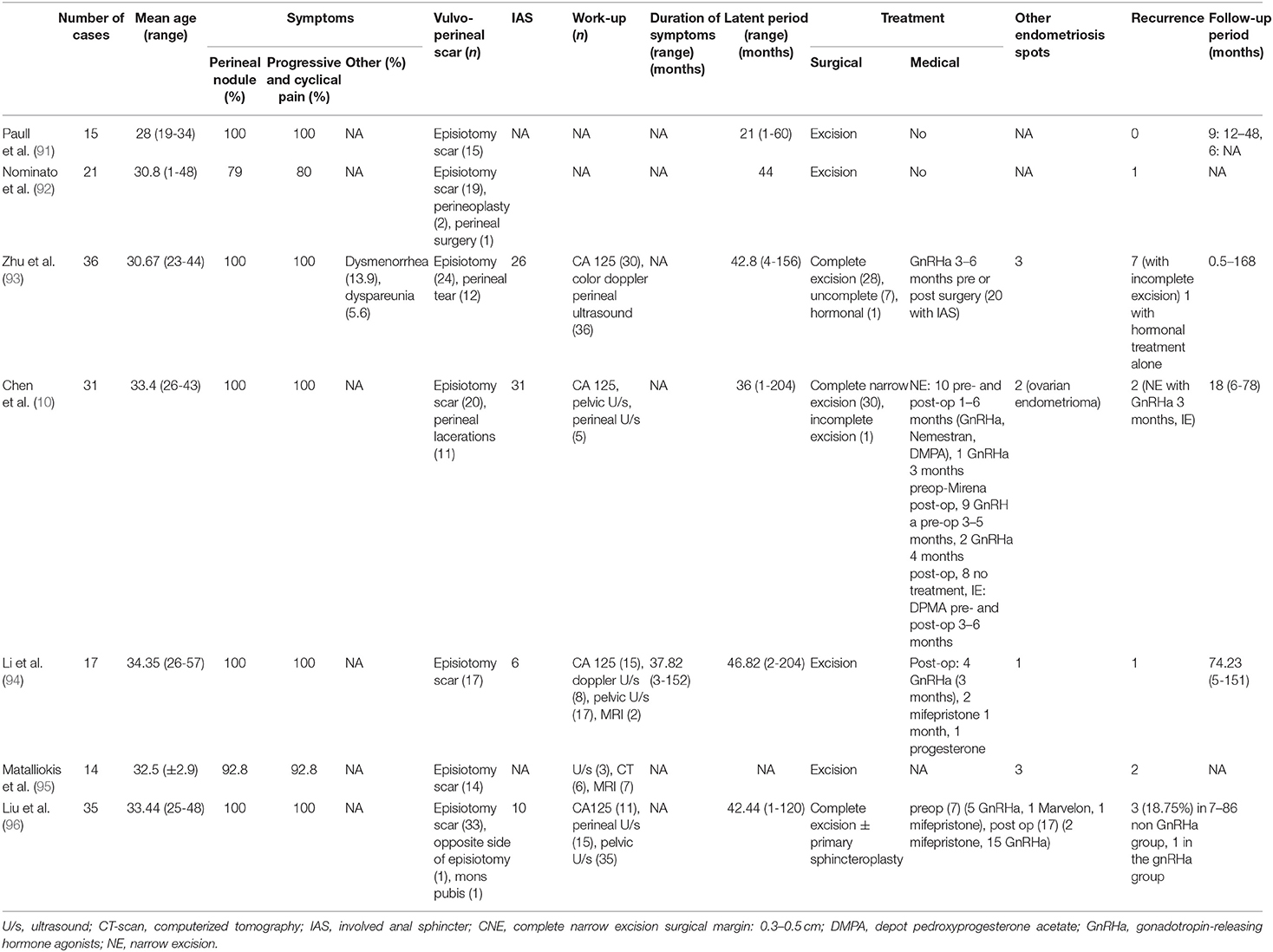
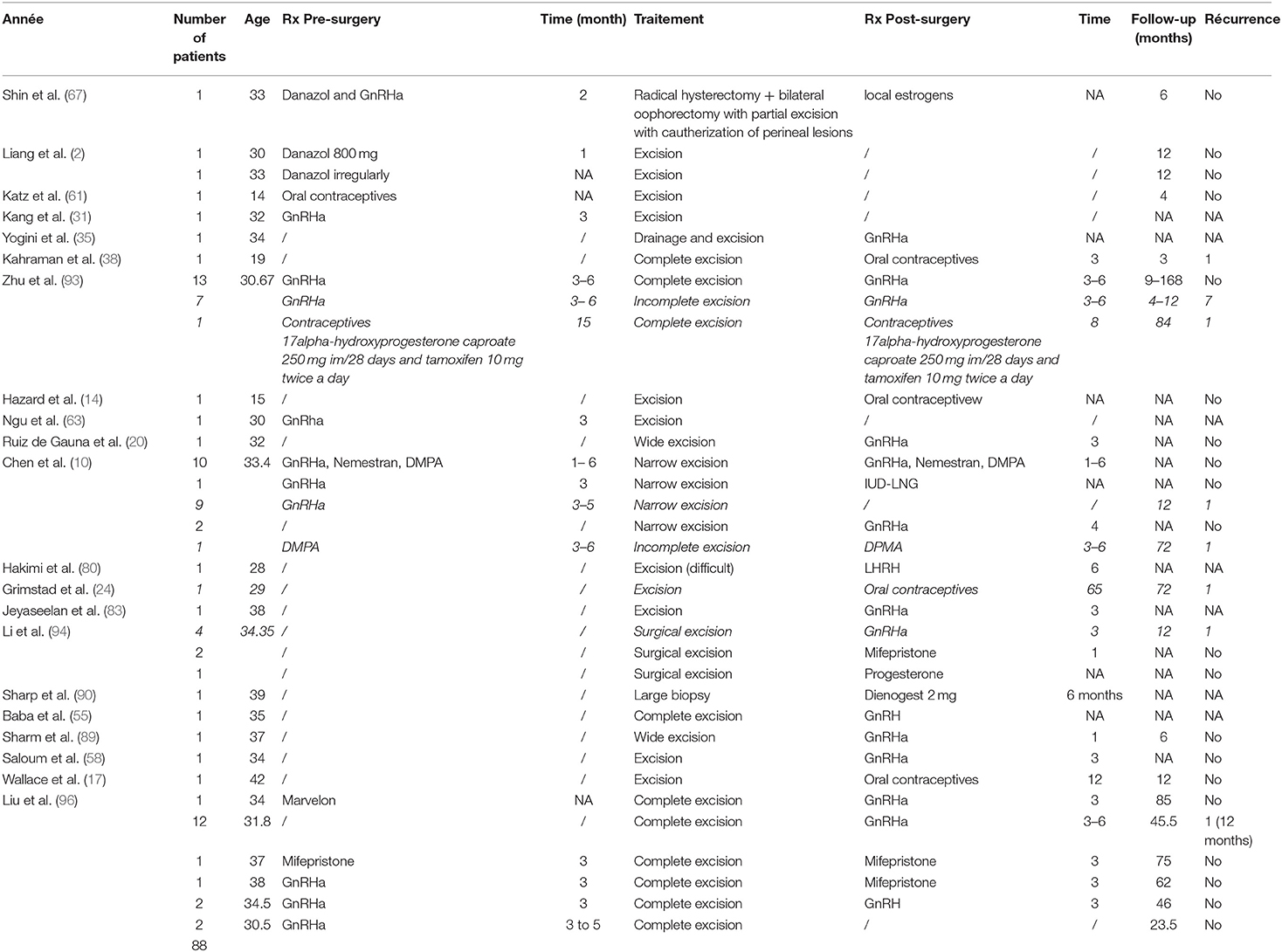
Figure 1 shows the flow chart of the literature search. Our search strategy yielded a total of 457 potentially eligible studies. Ninety articles published between 1956 and 2020 were eventually included in our review. Eighty-three articles were case reports or case series including one to eight patients (2, 9, 11, 13–90) and seven studies described retrospective cohorts of 14–36 patients (10, 91–96). The main results of the eight retrospectives studies can be found in Table 2.
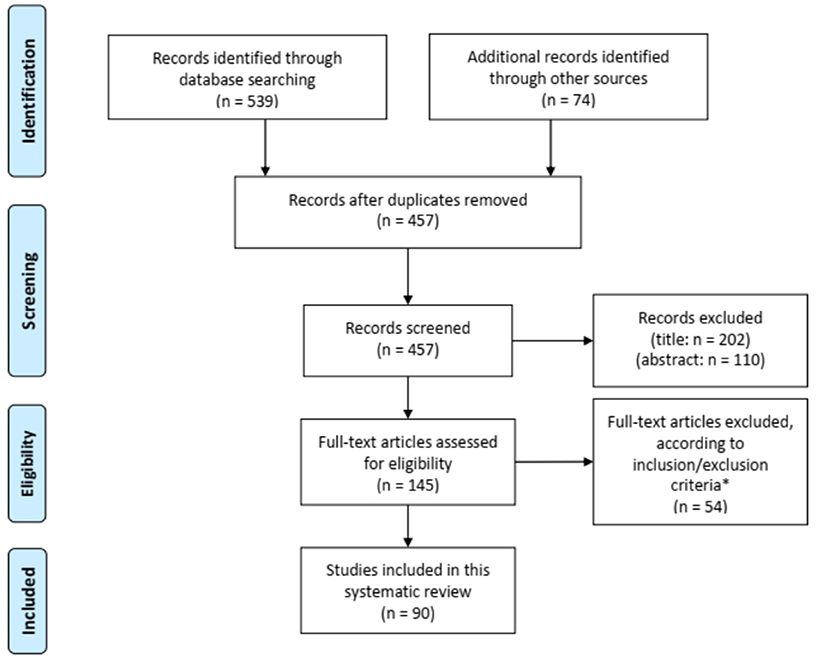
Figure 1. Flow diagram for study selection. *Seven articles could not be retrieved despite contacting the corresponding author.
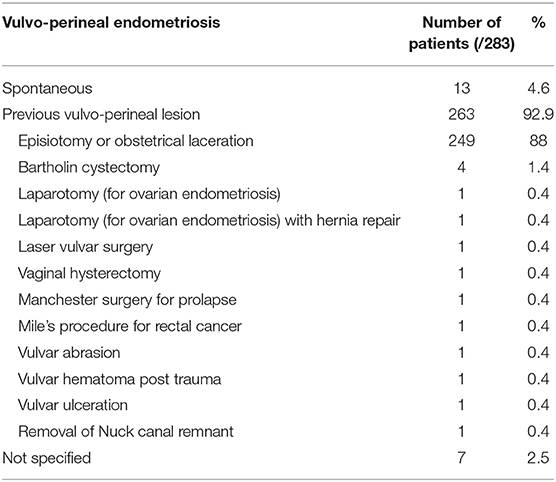
Two hundred and eighty-three patients, with a mean age of 32.7 ± 7.6 years and an age range from 14 to 69 years at diagnosis, were included. While 263 patients (95.3%) had undergone previous episiotomy, obstetrical lacerations, whether repaired or not, perineal trauma or vaginal surgery or injury, only 13 patients (4.6%) developed spontaneous vulvo-vaginal endometriosis i.e., in the absence of the most frequently associated conditions (Table 3). Among the latter, lesions developed in the Bartholin gland in 6 cases (21, 44, 48, 62, 70, 80). Previous history wasn't described for seven patients (40, 45, 47, 74).
The median latent period i.e., the time between perineal trauma or surgery and occurrence of symptoms was 2.5 years, ranging from 1 month to 14 years.
Incidence rates were reported in two studies, ranging between 0.01 and 0.06% after vaginal deliveries (84, 92). The incidence of anal sphincter involvement was reported only by Chen et al. who described a 0.37% incidence of perineal endometriosis among women treated surgically for endometriosis regardless of its location with 0.18% of patients presenting with anal sphincter involvement (10).
Main complaints were vulvar and/or perineal cyclical pain increasing during menstruations for 278 patients (98.2%). Five patients (1.8%) presented exclusively with other symptoms, e.g., painful bilateral polyps of the labia minora, cyclical bleeding, anal pruritus or infertility. Concurrent pelvic endometriosis occurred in 19 patients (6.1%). The median duration of symptoms before medical care was 12 months, ranging from 2 weeks to 20 years.
Out of the 281 patients for whom a clinical examination was described, 274 patients (97.5%) showed a vulvo-perineal nodule, mass or swelling, including one with vulvar ulceration, while 6 presented with bluish cutaneous lesions (2.1%) and one with bilateral polyps of the labia minora (0.4%).
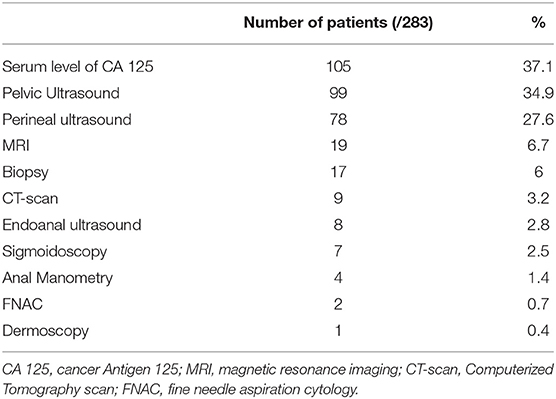
The different workups conducted in the 283 patients are described in Table 4. The most common workup assessment was the serum level of cancer antigen 125 (CA125), measured in 105 patients (37.1%), followed by pelvic ultrasound (34.9%) and perineal ultrasound (27.%). Other workup examinations were performed in <10% of the cases. Slightly elevated serum levels of CA125 were found in 6.5–46.7% of patients with perineal endometriosis (10, 93, 94, 96). Pelvic ultrasound was mostly performed to exclude pelvic endometriosis. Perineal and endoanal ultrasound (50, 93, 94, 96), as well as magnetic resonance imaging (MRI) (94, 95) helped to describe precisely the size of the lesions and to diagnose and assess the extent of the anal sphincter involvement. Well-defined hypoechoic solid or cystic masses with hyperechoic spots or strands representing fibrosis within the scar tissue have been described (79, 97). Increased vascularity and spiculated borders with a single vessel entering the nodule from the periphery has also been observed (98). Fine needle aspiration cytology (FNAC) or biopsy of the lesions was reported in 19 patients (6.1%) and confirmed the diagnosis before surgical excision in 16 patients (84.2%) while it didn't confirm the presence of endometriosis in two patients [granulation tissue and old hemorrhage (22, 63)]. One biopsy result was not described (36).
All but one patients (282/283) underwent surgical excision of the perineal mass. In the patient where the perineal lesion was left, a total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed to induce menopause and decrease pain-related symptoms (69). The detailed technique of the different surgical procedures was usually not described, except in two studies mentioning the following specifications i.e., narrow excision with deep surgical margins of 0.3–0.5 cm (10) or complete excision with a surgical margin of 0.5–1 cm (94). “Excision” or “surgical excision” without further details was mentioned for 148 patients (52.5%). Amongst the remainder, 73 had a “complete excision” (25.6%), 32 benefited from a “complete narrow excision” (11.3%) and 10 patients from a “wide excision” (3.5%). Eight surgeries were described as “incomplete” (2.8%). Histological findings confirmed endometriosis in all cases. Eighty-eight patients (28.1%) received hormonal therapy, either pre-or post-operatively for 3–6 months (Table 5).
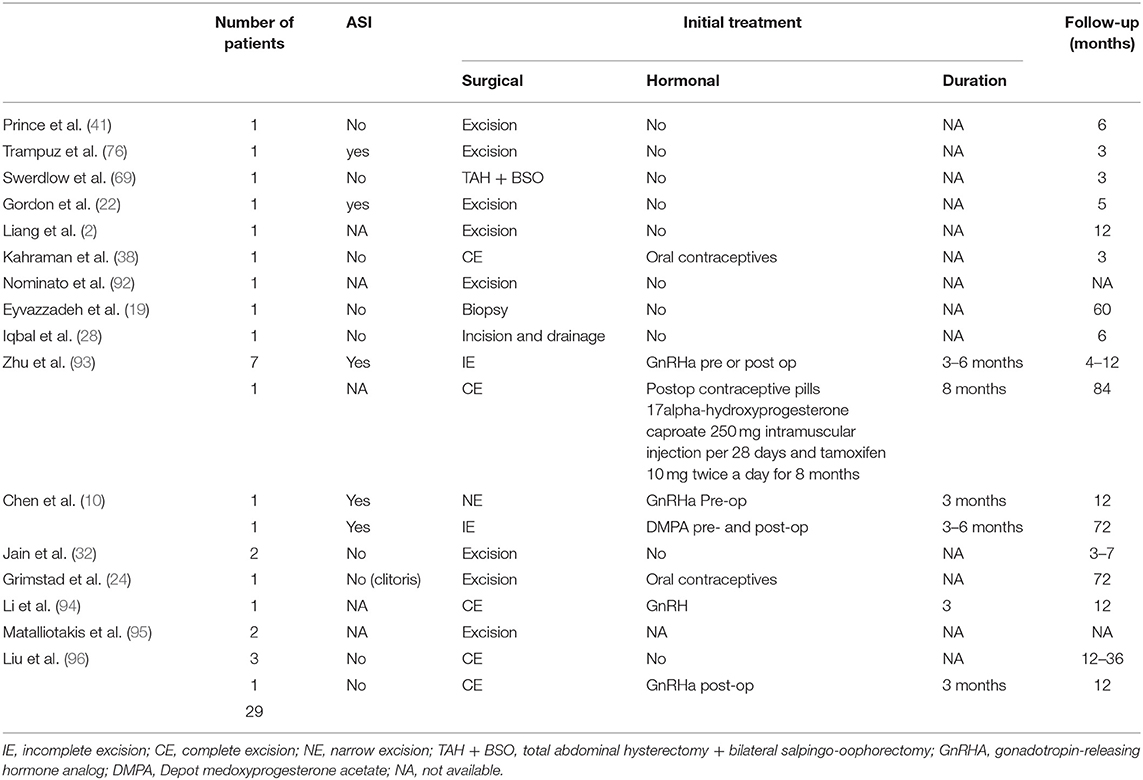
A follow-up period was mentioned in 61 studies, with a median value of 10 months (range from 1 to 108 months). Recurrent lesions have been reported in 29 patients (10.2%) and are presented in Table 6. Out of these patients with recurrence, 13 benefited from hormonal treatment pre-or post-operatively (44.8%) (nine GnRHa, three oral contraceptives, and one DMPA) while 16 didn't receive any additional treatment.
Discussion
While vulvo-perineal endometriosis presents mostly after perineal trauma, its exact etiology remains unclear, even if major progress has been achieved in the field over the last decades. Etiopathogenesis of endometriosis has generally been related to endometrial implantation, coelomic metaplasia, lymphatic dissemination and hematogenous spread (1) and the origin of extrapelvic endometriosis is not well-deciphered. As pelvic endometriosis can be considered as three separate entities (peritoneal, ovarian and recto-vaginal (deep) lesions) with different pathogeneses (99, 100), vulvo-perineal endometriosis could potentially also be separated between cystic and nodular lesions with distinct etiologies and treatment. Direct mechanical implantation seems to be the most plausible hypothesis for explaining scar endometriosis after obstetrical and gynecological procedures. According to this theory, mechanical dissemination during normal vaginal delivery, for example, allows transplantation of viable decidual endometrial cells into the episiotomy wound or perineal tear (2, 34, 53, 93, 101). We must note that scar endometriosis may as well-rarely be seen after a number of general surgical procedures like appendicectomy, inguinal hernial repair, laparoscopic cholecystectomy or even laparoscopic gastric by-pass (9). Nevertheless, perineal endometriosis has also been described in patients without any previous vulvo-vaginal trauma. In that respect, we found 13 cases of primary perineal endometriosis without vaginal birth, previous perineal injury in the literature. Different explanations of the pathogenesis of spontaneously developing perineal endometriotic lesions have been put forward with the most likely being lymphovascular dissemination (39, 53). However, the presence of endometriosis in the labia majora could also be explained by the direct spread of pelvic endometriosis along the round ligaments or Nuck canal's remnants while a solitary focus in the Bartholin's gland could theoretically be attributed to coelomic metaplasia (57). Eventually, other factors, such as immunological, genetic and familial factors, could be involved in the pathogenesis of this disease (94, 102).
Few studies have reported the incidence or prevalence of vulvo-perineal endometriosis. After vaginal delivery, the highest incidence rate of perineal endometriosis was reported by Nominato et al. representing 0.06% of patients, compared to 0.2% abdominal scar endometriosis after cesarean section (92), while perineal endometriosis only represents a proportion of 0.37% of women treated surgically for endometriosis (10).
Besides the rarity of vulvo-perineal endometriosis, its variability in clinical presentation makes this condition hardly recognized by some healthcare professionals leading to a delayed diagnosis. Misdiagnoses have also been reported such as herpes outbreak or perianal sepsis in the presence of vulvar pain with ulcerations or perianal swelling, respectively (14, 28). For instance, we showed a mean duration of symptoms of 12 months, ranging from 2 weeks to 20 years in our review meaning that half of them had to endure their pain for more than a year before being correctly diagnosed (94).
Early diagnosis and treatment are however recommended in order to prevent adverse complications such as long-term psychological distress, progressive involvement of the surrounding and adjacent tissues such as anal sphincter or rectum and potential malignant degeneration.
A detailed medical and surgical history associated with a thorough clinical exam are of great importance for accurate diagnosis. Good clinical vulvar and vaginal examination, including speculum and bimanual examination, are primordial to fully describe the perineal lesions. Rectal examination should be performed routinely in case of perineal lesions especially if suspicion of anal sphincter involvement (ASI). Clinically, endometriosis of the perineum and vulva presents as ill-defined papule or nodule, generally hard, usually located near a surgical scar, potentially skin-colored, dark-red, brown, or blue-black cystic (10, 103). It is mainly accompanied by cyclic pain and swelling, or periodic leakage of dark colored fluid during menses attributable to the fact that endometrial implants behave like normal endometrium. Some cases show neither discoloration of the perineal skin, nor local swelling nor intermittent leakage (32). Other symptoms can include dyspareunia. Interestingly, Zhu et al. described three criteria i.e., history of past perineal tear of episiotomy during vaginal delivery, presence of a tender nodule or mass at the perineal lesion on clinical exam and history of progressive and cyclic perineal pain which when all met provide a 100% positive predictive value (93). Concomitant pelvic endometriosis was found in 6.1% of patients in our systematic review. These results concord with the literature suggesting that scar endometriosis is not a risk factor for pelvic endometriosis (104, 105).
The differential diagnosis in such patients includes, but is not limited to, anal fistula, abscesses, suture granulomas, Bartholin cysts or bartholinitis, hernias, hematoma, sebaceous cyst, lipoma, herpes, neoplastic tissue or metastatic carcinoma, traumatic neuroma, desmoid tumor and anal melanoma (9, 28, 42, 45, 46). A perineal mass discovered in menopaused women should be considered as malignant until proven otherwise. Malignant degeneration occurs infrequently for cutaneous endometriosis representing 0.3–1% of endometriosis located in surgical scars (106). It is difficult to distinguish benign from malignant perineal endometriosis based on symptoms and clinical examination and a biopsy or surgical excision will always be necessary to confirm the diagnosis of malignant transformation (13). Histological observations are dominated by endometrioid carcinoma and sarcoma (107), but can also present as dermatosarcoma, clear cell carcinoma and serous papillary cystadenocarcinoma (87, 106, 108–116). As malignant transformation appears uncertain, unpredictable and may be very delayed, long-term follow-up is recommended.
The work-up appeared to be very variable depending on the medical team dealing with the patients. It was therefore difficult to evaluate the sensibility and specificity of each exam for perineal endometriosis as most of them have been realized in only a tiny proportion of the patients in this study. Levels of serum CA125 did not seem to be effective in diagnosing perineal endometriosis since it was usually normal or slightly increased. With regard to perineal ultrasonography, variable sonographic features were seen, as it is the case for abdominal wall endometriosis, which could make the diagnostic process more challenging but it remains useful to describe precisely the size of the lesions and to assess the extent of the ASI. Preoperative endoanal ultrasonography has also been described as a reliable technique for visualizing perianal endometriosis and diagnosing ASI, enabling the surgeon to determine the extent of an operative procedure and the possible need for a sphincteroplasty (46, 86). Ultrasonographic features of the lesion are usually similar to those observed with perineal ultrasonography with the advantage that it better reveals the involvement of the anal sphincter (10). Even if only 6.1% of the patients in this review benefited from this exam, magnetic resonance imaging (MRI) could become the modality of choice for perineal imaging (117, 118) as pelvic MRI has greater sensitivity (90–92%) and specificity (91–98%) for the diagnosis of endometriomas when compared to other non-invasive methods (119, 120). Vulvo-perineal localizations are easily identified on T1-weighted fat-suppressed images as hyper-intense spots within the perineum. Multilobular mass with inner hemorrhage, localized or diffuse vulvovaginal wall thickening, hemorrhagic or spiculated masses and distortion can also be observed (94, 117). MRI also helps in assessing the extent of the anal sphincter involvement (35). Depending on the availability in each center, we suggest that pelvic and perineal ultrasound as well as MRI should be performed in all patients and associated with endoanal ultrasound when there is a suspicion of ASI.
Histology is the hallmark of diagnosis which shows endometrial glands, stroma, and hemosiderin pigment. Generally, diagnosis is easy with a microscopic examination of a standard hematoxylin and eosin (H&E)-stained slide. Immunostaining for CD10 (neprilysin, a cell-surface metalloendopeptidase expressed in normal and ectopic endometrial stroma) increases the sensitivity compared to H&E staining (17, 19). Evidencing the estrogen receptor (ER) and progesteron receptor (PR) may help to identify endometrial glands (121). Furthermore, it is important to keep in mind that cutaneous endometriotic lesions show a broad spectrum of metaplastic changes and that all types of müllerian differentiation can be discovered (122) which make the diagnosis on FNAC or biopsy challenging for the unexperimented histologists.
Treatment of vulvo-perineal endometriosis includes usually surgical excision with or without hormonal suppression (GnRHa, oral contraceptives, progestins) (2, 10, 15, 123). It seems that complete excision should be the treatment of choice as it decreases the risk of recurrence (10, 93) and could reduce consequently the risk of malignant degeneration (109). Care must be applied to avoid rupturing the mass during surgery with its consecutive risk of re-implantation or leaving endometriotic remnants. To this end, excision of surrounding fibrous tissue has been suggested (94) although recommendations with respect to the surgical technique, e.g., surgical margins needed to decrease the risk of recurrence, are not available so far. Precise description of the surgical procedure including technique and margins is most often missing and awaited in future studies. When the anal sphincter is involved, complete narrow excision or wide excision of the endometrial tissue with a good healthy margin have been proposed with primary sphincteroplasty using the apposition or overlapping technique (10). Although symptomatic relief could be achieved with hormonal intervention, complete surgical excision still remains the best treatment for perineal endometriosis and often leads to permanent cure (71). As expected, large and deep lesions to the muscle or the fascia might be more difficult to excise completely. In large lesions, complete excision of the lesion may entail a synthetic mesh placement, tissue transfer for closure after resection (124) or combined surgery with gynecologic and plastic surgeons (125). It is however important to keep in mind that endometriosis remains a benign condition allowing conservative surgery and that decaying surgery is not recommended even in very large lesions. The type of resection should be based on the patient's age and desire for future pregnancy and the decision should be made only after possible outcomes of the different approaches have been discussed with the patient (86). Some authors have suggested that wide excision with primary sphincteroplasty could be optimal in younger patients, obviating the need for additional therapy, while narrow or incomplete excision with subsequent hormonal therapy could be advantageous in older patients closer to menopause to lessen the risk of incontinence due to sphincter resection (93). 28.1% of patients in this review received hormonal treatment pre-or post-operatively. As described in pelvic endometriosis, hormonal treatment could stabilize the size of cystic lesions and reduce pain as endometriosis is an estrogen-dependent process (126–129). Some authors suggested that massive lesions with anal sphincter involvement should be treated by hormonal therapy before surgery to reduce the size of the perineal mass (63). It should be noted that perineal endometriosis persists with medical treatment alone as it was always found on histology when hormonal treatment was followed by surgical excision. Various authors have reported the administration of a gonadotropin-releasing hormone analog to prevent recurrence (10, 31, 96). When complete wide excision, as reported by Zhu et al. (14), was performed, the recurrence rate was lower (3.3%) than the overall rate of 9.3% found in our review compiling all kinds of excisions, suggesting that recurrence was presumably due to incomplete removal of the lesions rather than to the absence of hormonal treatment (14). In addition, preoperative hormone therapy did not improve outcomes compared to surgery alone in patients with ASI (10). Results on hormonal therapy for abdominal wall or abdominal scar endometriosis are similar to those presented in this review showing a possible temporary relief of symptoms or potential slight reduction of the lesions' size easing the surgical resection but the bulk of evidence shows a low degree of efficacy. Currently, available data do not comment on best practices for the perioperative management of cutaneous endometriosis (104, 105, 130–134).
The limitations of this systematic review include the level of evidence due to the nature of the studies i.e., retrospective and case reports and the important variations in clinical management of vulvo-perineal endometriosis described in the available studies such as methods of diagnosis, surgery procedure's details and hormonal therapy. At present, there are no comparative studies to provide accurate and evidence-based guidelines regarding optimal diagnostic methods, treatment options and outcomes for endometriosis involving the perineum.
Although evidence-based guidelines cannot be retrieved from this systematic review due to the reasons mentioned above, Table 7 summarizes all the suggested recommendations based on our results and on the available literature. To improve our knowledge on this rare condition, we suggest developing a international database on vulvo-perineal endometriosis. Any future study regarding this type of endometriosis should include the data described in Table 8.
In conclusion, vulvo-perineal endometriosis is a rare entity with ~300 cases reported in the literature since 1923. With the available knowledge shown in this systematic review, we encourage all practitioners to think about perineal endometriosis in case of perineal cyclical pain with or without previous perineal damage. Diagnosis should be done with clinical exam, perineal ultrasound and pelvic MRI when available. In case of anal sphincter involvement, perianal ultrasound should be performed. Surgical excision of the lesion should be realized in order to remove the lesion and to confirm the diagnosis histologically. Hormonal treatment could be proposed to attempt to decrease the size of a large lesion before surgery or to avoid recurrence of the lesion. As evidence-based approach to the diagnosis, treatment and recurrence rate of affected patients remains a challenge given its low prevalence, the variations in management found in the articles included and the limited quality of available studies, we suggest that a prospective database on vulvo-perineal endometriosis should be generated to increase knowledge but also awareness among healthcare professionals and optimize patients' care.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CM: search strategy, screening of studies, data extraction, manuscript writing, and final revision. ZC: screening of studies. J-LS, ML, and PJ: final revision. VT: screening of studies and language revision. CW: search strategy, manuscript writing, and final revision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. (2014) 10:261–75. doi: 10.1038/nrendo.2013.255
2. Liang CC, Tsai CC, Chen TC, Soong YK. Management of perineal endometriosis. Int J Gynecol Obstet. (1996) 53:261–5. doi: 10.1016/0020-7292(95)02592-8
3. Davis AC, Goldberg JM. Extrapelvic endometriosis. Semin Reprod Med. (2017) 35:98–101. doi: 10.1055/s-0036-1597122
4. Andres MP, Arcoverde FV, Souza CC, Fernandes LF, Simoes Abrao MS, Kho RM. Extrapelvic endometriosis: a systematic review. J Minim Invasive Gynecol. (2020) 27:373–89. doi: 10.1016/j.jmig.2019.10.004
5. Jubanyik KJ, Comite F. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. (1997) 24:411–40. doi: 10.1016/S0889-8545(05)70311-9
7. Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocr Rev. (2019) 40:1048–79. doi: 10.1210/er.2018-00242
8. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. (2010) 362:2389–98. doi: 10.1056/NEJMcp1000274
9. Cinardi N, Franco S, Centonze D, Giannone G. Perineal scar endometriosis ten years after Miles' procedure for rectal cancer: case report and review of the literature. Int J Surg Case Rep. (2011) 2:150–3. doi: 10.1016/j.ijscr.2011.04.001
10. Chen N, Zhu L, Lang J, Liu Z, Sun D, Leng J, et al. The clinical features and management of perineal endometriosis with anal sphincter involvement: a clinical analysis of 31 cases. Hum Reprod. (2012) 27:1624–7. doi: 10.1093/humrep/des067
11. Dougherty LS, Hull T. Perineal endometriosis with anal sphincter involvement: report of a case. Dis Colon Rectum. (2000) 43:1157–60. doi: 10.1007/BF02236565
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Catherwood AE, Cohen ES. Endometriosis with decidual reaction in episiotomy scar. Am J Obstet Gynecol. (1951) 62:1364–6. doi: 10.1016/0002-9378(51)90068-3
14. Hazard DB, Harkins GJ. A case of cutaneous endometriosis following vulvar injury. J Endometr. (2011) 3:171–3.
15. Cheng DL, Heller DS, Oh C. Endometriosis of the perineum; report of two new cases and a review of literature. Eur J Obstet Gynecol Reprod Biol. (1991) 42:81–4. doi: 10.1016/0028-2243(91)90165-H
16. Demir M, Yildiz A, Ocal I, Yetimalar MH, Kilic D, Yavasi O. Endometriosis in episiotomy scar: a case report. J Cases Obstet Gynecol. (2014) 1:8–10.
17. Wallace E, Marin S, Elkattah R. Vulvar endometriosis in the setting of a traumatic neuroma. J Endometr Pelvic Pain Disord. (2019) 11:49–51. doi: 10.1177/2284026519828909
18. Kirk EW. Endometrioma in the posterior half of the labium majus. J Obstet Gynaecol Br Emp. (1950) 57:237–9. doi: 10.1111/j.1471-0528.1950.tb05233.x
19. Eyvazzadeh AD, Smith YR, Lieberman R, Quint EH. A rare case of vulvar endometriosis in an adolescent girl. Fertil Steril. (2009) 91:929.e9–11. doi: 10.1016/j.fertnstert.2008.10.026
20. Ruiz de Gauna B, Rodriguez D, Cabré S, Callejo J. A case of endometriosis in episiotomy scar with anal sphincter involvement. Int J Clin Med. (2011) 2:624–6. doi: 10.4236/ijcm.2011.25104
21. Gocmen A, Inaloz HS, Sari I, Inaloz SS. Endometriosis in the Bartholin gland. Eur J Obstet Gynecol Reprod Biol. (2004) 114:110–1. doi: 10.1016/j.ejogrb.2003.07.004
22. Gordon PH, Schottler JL, Balcos EG, Goldberg SM. Perianal endometrioma: report of five cases. Dis Colon Rectum. (1976) 19:260–5. doi: 10.1007/BF02590916
23. Gorkem U, Efeturk T, Guney G, Gungor T. A case of vulvar endometrioma mimicking a bartholin cyst. J Cases Obstet Gynecol. (2016) 3:57–60. doi: 10.1093/jscr/rjv169
24. Grimstad FW, Carey E. Periclitoral endometriosis: the dilemma of a chronic disease invading a rare location. J Minim Invasive Gynecol. (2015) 22:684–6. doi: 10.1016/j.jmig.2015.02.002
25. Healy JJ, Hills FH. Bilateral endometriosis of the vulva. Am J Obstet Gynecol. (1956) 72:1361–3. doi: 10.1016/0002-9378(56)90801-8
26. Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. (2016) 20:e48–9. doi: 10.1097/LGT.0000000000000203
27. Gözükara I, Karapinar OS, HakverdiI AU. Endometriosis of episiotomy scar. J Turkish Ger Gynecol Assoc. (2016) 17:S166.
28. Iqbal M, Thumbe V, Dhange R, Chan SY, Bhalerao S. Perianal endometriosis mimicking recurrent perianal abscess. Case Rep Gastroenterol. (2009) 3:414–7. doi: 10.1159/000250787
29. Cheleden J. Endometriosis of the perineum. Report of two cases. South Med J. (1968) 61:1313–4. doi: 10.1097/00007611-196812000-00011
30. Kouach J, El Hassani M, Moussaoui DR, Mohamed D. Vulvar endometriosis. J Obstet Gynaecol Canada. (2008) 30:1095. doi: 10.1016/S1701-2163(16)33014-6
31. Kang SK, Lee MW, Choi JH, Sung KJ, Moon KC, Koh JK. Cutaneous endometriosis: a combination of medical and surgical treatment. J Dermatolog Treat. (2002) 13:189–92. doi: 10.1080/09546630212345677
32. Jain D. Perineal scar endometriosis: a comparison of two cases. BMJ Case Rep. (2013) 2013:bcr2013010051. doi: 10.1136/bcr-2013-010051
33. AbdullGaffar B, Keloth TR, Raman LG, Mahmood S, Almulla A, AlMarzouqi M, et al. Unusual benign polypoid and papular neoplasms and tumor-like lesions of the vulva. Ann Diagn Pathol. (2014) 18:63–70. doi: 10.1016/j.anndiagpath.2013.11.005
34. Luterek K, Barcz E, Bablok L, Wierzbicki Z. Giant recurrent perineal endometriosis in an episiotomy scar - a case report. Ginekol Pol. (2013) 84:726–9. doi: 10.17772/gp/1631
35. Yogini KD, Balasubramaniam D, Karunanithi S, Parthasarathi R. Perianal endometriosis: a rare presentation of extrapelvic endometriosis. J SAFOG. (2019) 11:138–9. doi: 10.5005/jp-journals-10006-1659
36. Natale KE, Royer MC, Rush WL, Luption GP. Cutaneous deciduosis: a report of two cases of an unusual pseudomalignancy. J Cutan Pathol. (2012) 39:777–80. doi: 10.1111/j.1600-0560.2012.01907.x
37. Kanellos I, Kelpis T, Zaraboukas T, Betsis D. Perineal endometriosis in episiotomy scar with anal sphincter involvement. Tech Coloproctol. (2001) 5:107–8. doi: 10.1007/s101510170009
38. Kahraman K, Sonmezer M, Gungor M. Recurrent vulvar-perineal endometriosis. Artemis. (2003) 4:77−9.
39. Zhu L, Lang J, Wong F, Guo L. Perineal endometriosis without perineal trauma: a case report. Chin Med J (Engl). (2003) 116:639–40.
40. Ferreira LA. Endometriosis as a differential diagnosis on vulvar lump: a case report. Int J Gynecol Obstet. (2018) 143:742.
41. Prince LN, Abrams J. Endometriosis of the perineum; review of the literature and case report. Am J Obstet Gynecol. (1957) 73:890–3. doi: 10.1016/0002-9378(57)90402-7
42. Laadioui M, Alaoui F, Jayi S, Bouguern H, Chaara H, Melhouf MA. Deep perineal endometriosis on episiotomy scar: about a rare case. Pan Afr Med J. (2013) 16:112. doi: 10.11604/pamj.2013.16.112.3415
44. Aydin Y, Atis A, Polat N. Bilateral endometrioma of Bartholin glands accompanying ovarian endometrioma. J Obstet Gynaecol. (2011) 31:187–9. doi: 10.3109/01443615.2010.541303
45. Corazza M, Schettini N, Pedriali M, Toni G, Borhi A. Vulvar endometriosis. A clinical, histological and dermoscopic riddle. J Eur Acad Dermatology Venereol. (2020) 34:e321–2. doi: 10.1111/jdv.16257
46. Watanabe M, Kamiyama G, Yamazaki K, Hiratsuka K, Takata M, Tsunoda A, et al. Anal endosonography in the diagnosis and management of perianal endometriosis: report of a case. Surg Today. (2003) 33:630–2. doi: 10.1007/s00595-003-2545-z
47. Marcos MF, Marchitelli C, Sluga C, Secco G, Gorgoza S, Martinez MM. Vulvar endometriosis: A series of 4 cases. J Low Genit Tract Dis. (2017) 21:S5–6. doi: 10.1097/LGT.0000000000000269
48. Matseoane S, Harris T, Moscowitz E. Isolated endometriosis in a Bartholin gland. N Y State J Med. (1987) 87:575–6.
49. Mazzeo C, Gammeri E, Foti A, Rossitto M, Cucinotta E. Vulvar endometriosis and Nuck canal. Ann Ital Chir. (2014) 85:1–4.
50. Mişina A, Zaharia S, Harea P, Fuior-Bulhac L, Petrovici V, şor E, et al. Endometriosis of the vulva and perineum. Arta Medica. (2020) 1:4–8.
51. Nussbaum W, Motyloff L Endometriosis of the vulva during pregnancy. Am J Obstet Gynecol. (1957) 73:215–6. doi: 10.1016/S0002-9378(16)37286-6
52. Mahmud N, Kusuda N, Ichinose S, Gyotoku Y, Nakajima H, Ishimaru T, et al. Needle aspiration biopsy of vulvar endometriosis: a case report. Acta Cytol. (1992) 36:514–6.
53. Nasu K, Okamoto M, Nishida M, Narahara H. Endometriosis of the perineum. J Obstet Gynaecol Res. (2013) 39:1095–7. doi: 10.1111/jog.12003
54. Odobasic A, Pasic A, Iljazovic-Latifagic E, Arnautalic L, Odobasic Ad, Idrizovic E, et al. Perineal endometriosis: a case report and review of the literature. Tech Coloproctol. (2010) 14(Suppl. 1):S25–7. doi: 10.1007/s10151-010-0642-8
55. Baba AA, Dar AM, Parray AA, Khan MA, Laway MA, Chowdri NA. Perineal scar endometriosis. Indian J Colo-Rectal Surg. (2018) 1:30. doi: 10.4103/IJCS.IJCS_2_18
56. Brug P, Gueye N-A, Bachmann G. Vulvar endometriosis presenting with dyspareunia: a case report. J Reprod Med. (2012) 57:175–7.
57. Singh P, Bhutia K, Gudi MA, Chuan HH. Endometriotic foci in bartholin's cyst. J Gynecol Surg. (2017) 33:111–3. doi: 10.1089/gyn.2016.0061
58. Saloum NM, Qureshi S, Ibrahim SA, Alrashid A. A rare case report of endometriosis in an episiotomy scar without anal sphincter involvement. EC Gynaecol. (2018) 12:466–70.
59. Kistner RW, Younge PA. Endometriosis occurring in a vaginoperineal fistula. Am J Obstet Gynecol. (1952) 63:455–7. doi: 10.1016/S0002-9378(15)32847-7
60. Ramsey WH. Endometrioma involving the perianal tissues: report of a case. Dis Colon Rectum. (1971) 14:366–7. doi: 10.1007/BF02553424
61. Katz Z, Goldchmit R, Blickstein I. Post-traumatic vulvar endometriosis. Eur J Pediatr Surg. (1996) 6:241–2. doi: 10.1055/s-2008-1066519
62. Robotti G, Canepari E, Torresi M. Premenstrual inguinal swelling and pain caused by endometriosis in the Bartholin gland: a case report. J Ultrasound. (2015) 18:71–2. doi: 10.1007/s40477-014-0076-7
63. Ngu SF, Cheung VY. Vulvar endometriosis. J Obstet Gynaecol Canada. (2011) 33:891. doi: 10.1016/S1701-2163(16)35007-1
64. Sayfan J, Benosh L, Segal M, Orda R. Endometriosis in episiotomy scar with anal sphincter involvement - Report of a case. Dis Colon Rectum. (1991) 34:713–6. doi: 10.1007/BF02050357
65. Shanmuga Jayanthan S, Shashikala G, Arathi N, S. Perineal scar endometriosis. Indian J Radiol Imaging. (2019) 29:457–61. doi: 10.4103/ijri.IJRI_366_19
66. Barisic GI, Krivokapic Z V, Jovanovic DR. Perineal endometriosis in episiotomy scar with anal sphincter involvement: report of two cases and review of the literature. Int Urogynecol J. (2006) 17:646–9. doi: 10.1007/s00192-005-0022-5
67. Shin HC, Kim TH, Kim SW, Kim DS, Park CH, Huh CK. Perineal endometriosis. Ann Dermatol. (1994) 6:196–9. doi: 10.5021/ad.1994.6.2.196
68. Sully L. Endometrioma of the perineum associated with episiotomy scars. Scott Med J. (1977) 22:307–9. doi: 10.1177/003693307702200422
69. Swerdlow DB. Endometrioma masquerading as an anorectal abscess: report of a case. Dis Colon Rectum. (1975) 18:620–2. doi: 10.1007/BF02587146
70. Heijink T, Bogers H, Steensma A. Endometriosis of the Bartholin gland: a case report and review of the literature. J Med Case Rep. (2020) 14:85. doi: 10.1186/s13256-020-02424-7
71. Tam T, Huang S. Perineal endometriosis in an episiotomy scar: case report and review of literature. J Endometr. (2012) 4:93–6. doi: 10.5301/JE.2012.9410
72. Tornqvist B. Endometriosis in vaginal, vulvar and perineal scars. Acta Obstet Gynecol Scand. (1949) 28:485–9. doi: 10.3109/00016344909155706
73. Torres-Cepeda D, Reyna-Villasmil E, Santos-Bolívar J. Endometriosis vulvar. Reporte de Caso (Vulvar Endometriosis. Case Report). Av en Biomed. (2014) 3:38–41.
74. Ümit C, Kokanali MK, Erkilinç S, Doganay M. Spontanous vulvar endometriosis: report of a case. Gynecol Obs Reprod Med. (2014) 20:119–21.
75. Dadhwal V, Sharma A, Khoiwal K, Nakra T. Episiotomy scar endometriosis. Med J Armed Forces India. (2018) 74:297–9. doi: 10.1016/j.mjafi.2017.06.004
76. Trampuz V. Endometriosis of the perineum. A report of 5 new cases. Am J Obstet Gynecol. (1962) 84:1522–5. doi: 10.1016/S0002-9378(16)35801-X
77. Buda A, Ferrari L, Marra C, Passoni P, Perego P, Milani R. Vulvar endometriosis in surgical scar after excision of the Bartholin gland: report of a case. Arch Gynecol Obstet. (2008) 277:255–6. doi: 10.1007/s00404-007-0458-6
78. Turan V, Ergenoglu M, Yeniel O, Emiroglu G, Ulukus M, Zekioglu O. Vulvar endometrioma: a case report. J Nepal Med Assoc. (2011) 51:87–9. doi: 10.31729/jnma.250
79. Gunes M, Kayikcioglu F, Ozturkoglu E, Haberal A. Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures. J Obstet Gynaecol Res. (2005) 31:471–5. doi: 10.1111/j.1447-0756.2005.00322.x
80. Hakimi I. Endometriosis in the Bartholin gland: a case report. J Gynecol Obstet. (2014) 2:75. doi: 10.11648/j.jgo.20140205.12
81. Hamdi A, Gharsa A, Jaziri D, Sfar E, Chelli D. Perineal endometriosis without perineal trauma: a case report. Chin Med J. (2015) 6:1–3. doi: 10.4172/2155-9554.10000293
82. Canlorbe G, Laas E, Cortez A, Daraï E. Spontaneous hymeneal endometriosis: a rare cause of dyspareunia. BMJ Case Rep. (2014) 2014:bcr2013202299. doi: 10.1136/bcr-2013-202299
83. Jeyaseelan S, Kwatra N. A rare case of episiotomy scar endometriosis. J Obstet Gynecol India. (2016) 66(Suppl. 2):654–5. doi: 10.1007/s13224-015-0800-z
84. Leite GKC, De Carvalho LFP, Korkes H, Guazzelli TF, Kenj G, Viana ADT. Scar endometrioma following obstetric surgical incisions: retrospective study on 33 cases and review of the literature. São Paulo Med J. (2009) 127:270–7. doi: 10.1590/S1516-31802009000500005
85. Rajesh Kamble V, Gawande MS. Preoperative magnetic resonance evaluation of perineal endometriosis in episiotomy scar with anal sphincter involvement. Panacea J Med Sci. (2016) 6:170.
86. Toyonaga T, Matsushima M, Tanaka Y, Nozawa M, Sogawa N, Kanyama H, et al. Endoanal ultrasonography in the diagnosis and operative management of perianal endometriosis: report of two cases. Tech Coloproctol. (2006) 10:357–60. doi: 10.1007/s10151-006-0309-7
87. Xu S, Wang W, Sun LP. Comparison of clear cell carcinoma and benign endometriosis in episiotomy scar - two cases report and literature review. BMC Womens Health. (2020) 20:11. doi: 10.1186/s12905-020-0880-5
88. Cai S-Q, Zheng M, Man X-Y. Perineal endometriosis: a case report. Int J Clin Exp Med. (2014) 7:2939–40.
89. Sharma N, Khan DA, Jethani R, Baruah S, Dey B. Perineal endometriosis in an episiotomy scar: a case report and review of literature. Gynecol Obs Case Rep. (2018) 4:66. doi: 10.21767/2471-8165.1000066
90. Sharp C, Kulkarni M, Rosamilia A, Tsaltas J. Vulval endometriosis following vaginal hysterectomy. J Minim Invasive Gynecol. (2020) 27:1453–4. doi: 10.1016/j.jmig.2020.02.006
91. Paull T, Tedeschi LG. Perineal endometriosis at the site of episiotomy scar. Obstet Gynecol. (1972) 40:28–34. doi: 10.1097/00006250-197207000-00006
92. Nominato NS, Victor Spyer Prates LF, Lauar I, Morais J, Maia L, Geber S. Endometriose de cicatriz cirúrgica: estudo retrospectivo de 72 casos Scar endometriosis: a retrospective study of 72 patients. Rev Bras Ginecol Obstet. (2007) 29:403–7. doi: 10.1590/S0100-72032007000800007
93. Zhu L, Lang J, Wang H, Liu Z, Sun D, Leng J, et al. Presentation and management of perineal endometriosis. Int J Gynecol Obstet. (2009) 5:230–2. doi: 10.1016/j.ijgo.2009.01.022
94. Li J, Shi Y, Zhou C, Lin J. Diagnosis and treatment of perineal endometriosis: review of 17 cases. Arch Gynecol Obstet. (2015) 292:1295–9. doi: 10.1007/s00404-015-3756-4
95. Matalliotakis M, Matalliotaki C, Zervou MI, Krithinakis K, Goulielmos GN, Kalogiannidis I. Abdominal and perineal scar endometriosis: retrospective study on 40 cases. Eur J Obstet Gynecol Reprod Biol. (2020) 252:225–7. doi: 10.1016/j.ejogrb.2020.06.054
96. Liu Y, Pi R, Luo H, Wang W, Zhao X, Qi X. Characteristics and long-term outcomes of perineal endometriosis: a retrospective study. Medicine. (2020) 99:e20638. doi: 10.1097/MD.0000000000020638
97. Park SB, Kim JK, Cho KS. Sonography of endometriosis in infrequent sites. J Clin Ultrasound. (2008) 36:91–7. doi: 10.1002/jcu.20431
98. Guerriero S, Conway F, Pascual MA, Graupera B, Ajossa S, Neri M, et al. Ultrasonography and atypical sites of endometriosis. Diagnostics. (2020) 10:345. doi: 10.3390/diagnostics10060345
99. Donnez J, Nisolle M, Casanas-Roux F, Brion P, Da Costa Ferreira N. Stereometric evaluation of peritoneal endometriosis and endometriotic nodules of the rectovaginal septum. Hum Reprod. (1996) 11:224–8. doi: 10.1093/oxfordjournals.humrep.a019024
100. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. (1997) 68:585–96. doi: 10.1016/S0015-0282(97)00191-X
101. Steck WD, Helwig EB. Cutaneous endometriosis. JAMA. (1965) 191:167–70. doi: 10.1001/jama.1965.03080030011002
102. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. (2019) 111:327–40. doi: 10.1016/j.fertnstert.2018.10.013
103. Haley JC, Mirowski GW, Hood AF. Benign vulvar tumors. Semin Cutan Med Surg. (1998) 17:196–204. doi: 10.1016/S1085-5629(98)80014-X
104. Tatli F, Gozeneli O, Uyanikoglu H, Uzunkoy A, Yalcin HC, Ozgonul A, et al. The clinical characteristics and surgical approach of scar endometriosis: a case series of 14 women. Bosn J Basic Med Sci. (2018) 18:275–8. doi: 10.17305/bjbms.2018.2659
105. Horton JD, Dezee KJ, Ahnfeldt EP, Wagner M. Abdominal wall endometriosis: a surgeon's perspective and review of 445 cases. Am J Surg. (2008) 196:207–12. doi: 10.1016/j.amjsurg.2007.07.035
106. Han L, Zheng A, Wang H. Clear cell carcinoma arising in previous episiotomy scar: a case report and review of the literature. J Ovarian Res. (2016) 9:1. doi: 10.1186/s13048-016-0211-5
107. Heaps JM, Nieberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol. (1990) 75:1023–8.
108. Kwon YS, Nam JH, Choi G. Clear cell adenocarcinoma arising in endometriosis of a previous episiotomy site. Obstet Gynecol. (2008) 112 (2 Pt 2):475–7. doi: 10.1097/AOG.0b013e318179475b
109. Chene G, Darcha C, Dechelotte P, Mage G, Canis M. Malignant degeneration of perineal endometriosis in episiotomy scar, case report and review of the literature. Int J Gynecol Cancer. (2007) 17:709–14. doi: 10.1111/j.1525-1438.2007.00822.x
110. Hitti IF, Glasberg SS, Lubicz S. Clear cell carcinoma arising in extraovarian endometriosis: report of three cases and review of the literature. Gynecol Oncol. (1990) 39:314–20. doi: 10.1016/0090-8258(90)90259-N
111. Kholová I, Ryska A, Dedic K. Composite tumor consisting of dermatofibrosarcoma protuberans and giant cell fibroblastoma associated with intratumoral endometriosis. Pathol Res Pract. (2001) 197:263–7. doi: 10.1078/0344-0338-00045
112. Kojima N, Yoshida H, Uehara T, Ushigusa T, Asami Y, Shiraishi K, et al. Primary clear cell adenocarcinoma of the vulva: a case study with mutation analysis and literature review. Int J Surg Pathol. (2019) 27:792–7. doi: 10.1177/1066896919848823
113. Mesko JD, Gates H, McDonald TW, Youmans R, Lewis J. Clear cell ('Mesonephroid') adenocarcinoma of the vulva arising in endometriosis: a case report. Gynecol Oncol. (1988) 29:385–91. doi: 10.1016/0090-8258(88)90241-7
114. Bolis GB, Maccio T. Clear cell adenocarcinoma of the vulva arising in endometriosis. A case report. Eur J Gynaecol Oncol. (2000) 21:416–7.
115. Todd RW, Kehoe S, Gearty J. A case of clear cell carcinoma arising in extragonadal endometriosis. Int J Gynecol Cancer. (2000) 10:170–2. doi: 10.1046/j.1525-1438.2000.00024.x
116. Buppasiri P, Kleebkaow P, Tharanon C, Aue-Aungkul A, Kietpeerakool C. Clear cell carcinoma arising in vulvar endometriosis. Case Rep Pathology. (2018) 2018:4263104. doi: 10.1155/2018/4263104
117. Ssi-Yan-Kai G, Thubert T, Rivain AL, Prevot S, Deffieux X, De Laveaucoupet J. Female perineal diseases: spectrum of imaging findings. Abdom Imaging. (2015) 40:2690–709. doi: 10.1007/s00261-015-0427-7
118. Hosseinzadeh K, Heller MT, Houshmand G. Imaging of the female perineum in adults. Genitourinary Imaging. (2012) 32:E129–68. doi: 10.1148/rg.324115134
119. Bazot M, Darai E, Hourani R, Thomassin I, Cortez A, Uzan S, et al. Deep pelvic endometriosis: MR imaging for diagnosis and prediction of extension of disease. Radiology. (2004) 232:379–89. doi: 10.1148/radiol.2322030762
120. Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. (2006) 16:285–98. doi: 10.1007/s00330-005-2882-y
121. Cazacu E. Histological and immunohistochemical study of extragenital endometriosis. Virchows Arch. (2014) 465:S356.
122. Kazakov D V, Ondic O, Zamecnik M, Shelekhova K V, Mukensnabl P, Hes O, et al. Morphological variations of scar-related and spontaneous endometriosis of the skin and superficial soft tissue: a study of 71 cases with emphasis on atypical features and types of müllerian differentiations. J Am Acad Dermatol. (2007) 57:134–46. doi: 10.1016/j.jaad.2006.11.036
123. Zhu L, Wong F, Lang JH. Perineal endometriosis after vaginal delivery - Clinical experience with 10 patients. Aust New Zeal J Obstet Gynaecol. (2002) 42:565–7. doi: 10.1111/j.0004-8666.2002.548_12.x
124. Uzunçakmak C, Güldaş A, Özçam H, Dinç K. Scar endometriosis: a case report of this uncommon entity and review of the literature. Case Rep Obstet Gynecol. (2013) 2013:1–4. doi: 10.1155/2013/386783
125. Galdino JDL, Costa RSC, De Oliveira JG. Reconstruction of abdominal wall and vulvar defects after endometriosis resection. Rev Bras Cir Plást. (2019) 34:355–61. doi: 10.5935/2177-1235.2019RBCP0208
126. Sauvan M, Chabbert-Buffet N, Canis M, Collinet P, Fritel X, Geoffron S, et al. Medical treatment for the management of painful endometriosis without infertility: CNGOF-HAS endometriosis guidelines. Gynecol Obstet Fertil Senol. (2018) 46:267–72. doi: 10.1016/j.gofs.2018.02.028
127. Geoffron S, Legendre G, Daraï E, Chabbert-Buffet N. Traitement médical de l'endométriose : prise en charge de la douleur et de l'évolution des lésions par traitement hormonal et perspectives thérapeutiques. Press Medicale. (2017) 46 (12P1):1199–211. doi: 10.1016/j.lpm.2017.10.005
128. Bedaiwy MA, Allaire C, Alfaraj S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil Steril. (2017) 107:537–48. doi: 10.1016/j.fertnstert.2016.12.024
129. Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Exp Opin Pharmacother. (2018) 19:1109–25. doi: 10.1080/14656566.2018.1494154
130. Kulkarni N, Patil A, Patel R. Study of preoperative GnRh agonist in cutaneous scar endometriosis. Int J Reprod Contracept Obstet Gynecol. (2016) 5:3191–4. doi: 10.18203/2320-1770.ijrcog20163010
131. Mistrangelo M, Gilbo N, Cassoni P, Micalef S, Faletti R, Miglietta C, et al. Surgical scar endometriosis. Surg Today. (2014) 44:767–72. doi: 10.1007/s00595-012-0459-3
132. Purvis RS, Tyring SK. Cutaneous and subcutaneous endometriosis. J Dermatol Surg Oncol. (1994) 20:693–5. doi: 10.1111/j.1524-4725.1994.tb00456.x
133. Raffi L, Suresh R, McCalmont TH, Twigg AR. Cutaneous endometriosis. Int J Womens Dermatol. (2019) 5:384–6. doi: 10.1016/j.ijwd.2019.06.025
Keywords: endometriosis, perineum, vulva, episiotomy, perineal pain, cyclical pain, perineal nodule, extrapelvic endometriosis
Citation: Maillard C, Cherif Alami Z, Squifflet J-L, Luyckx M, Jadoul P, Thomas V and Wyns C (2021) Diagnosis and Treatment of Vulvo-Perineal Endometriosis: A Systematic Review. Front. Surg. 8:637180. doi: 10.3389/fsurg.2021.637180
Received: 02 December 2020; Accepted: 06 April 2021;
Published: 11 May 2021.
Edited by:
Salim Alfred Bassil, Al-Arz Hospital, LebanonReviewed by:
Mohd Faizal Ahmad, National University of Malaysia, MalaysiaSvend Lindenberg, Copenhagen Fertility Center, Denmark
Copyright © 2021 Maillard, Cherif Alami, Squifflet, Luyckx, Jadoul, Thomas and Wyns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte Maillard, Y2hhcmxvdHRlLm1haWxsYXJkQHVjbG91dmFpbi5iZQ==
 Charlotte Maillard
Charlotte Maillard Zineb Cherif Alami2
Zineb Cherif Alami2 Jean-Luc Squifflet
Jean-Luc Squifflet Mathieu Luyckx
Mathieu Luyckx Pascale Jadoul
Pascale Jadoul Christine Wyns
Christine Wyns