- Department of Oral and Maxillofacial Surgery, Zaozhuang Municipal Hospital, Zaozhuang, China
Objective: The study aimed to review evidence on the efficacy of submucosal (SM) administration vs. intravenous (IV) or intramuscular (IM) route of injections of dexamethasone for improving outcomes after mandibular third molar surgery.
Methods: PubMed, Embase, CENTRAL, and Google Scholar were searched for randomized controlled trials (RCTs) up to 20th May 2021. Early (2–3 days) and late (7 days) outcomes were compared between SM vs. IV or IM dexamethasone. Quality of evidence was assessed based on GRADE.
Results: Thirteen trials were included in the systematic review and 10 in the meta-analysis. Meta-analysis indicated a significant reduction in early pain with IV dexamethasone but no such difference for late pain compared to the SM group. There was no difference in early and late swelling scores between the SM and IV groups. Pooled analysis indicated no significant difference in early and late trismus between SM and IV groups. Comparing SM with IM dexamethasone, there was no significant difference in early and late pain scores. Swelling in the early and late postoperative periods was not significantly different between the two groups. There was no significant difference in early and late trismus between SM and IM groups. The quality of evidence was low for all outcomes.
Conclusion: Low-quality evidence suggests that SM infiltration of dexamethasone results in similar outcomes as compared to IV or IM administration of the drug after third molar surgeries. Further high-quality RCTs are needed to corroborate the current conclusions.
Introduction
Mandibular third molar extraction is one of the most common surgical procedures in any oral surgical practice. A large number of mandibular third molars are impacted due to lack of adequate space for eruption and require surgical intervention for their removal (1). While many of the times, impacted mandibular third molars are asymptomatic, pathologies like recurrent pericoronitis, caries, and bone loss can necessitate early surgical extraction of these teeth (2). Research also indicates that asymptomatic third molars need to be extracted when the tooth is partially impacted in the horizontal or vertical position and with incomplete roots growing close to the mandibular canal (3).
Owing to the anatomical location of the impacted third molar, any surgical procedure leads to significant soft and hard tissue trauma (4). The surgical site around the third molar is surrounded by loose connective tissue of high vascularity and dense cortical bone. Adequate bone removal is required for safe delivery of the tooth and the degree of bone removal increases with the difficulty of the extraction (5). The tissue injury inflicted on account of the surgery results in an inflammatory response leading to significant pain, swelling, and edema in the postoperative period. The surgical procedure also affects the quality of life of the individual leading to impairment of routine activities (6).
Over the years, several interventions have been reported to reduce the postoperative sequelae of third molar surgery. Laser and piezoelectric instruments have been used as an alternative to rotary instruments for bone cutting (7). A variety of mouthwashes, antibiotics, analgesics, topical gels, cryotherapy, ozone therapy, and corticosteroids have also been used to reduce pain, swelling, and trismus after third molar surgeries (8). Of these various agents, the use of corticosteroids, especially dexamethasone is a popular intervention to improve outcomes of third molar surgery (9, 10).
In conventional surgical practice, dexamethasone is routinely administered via intravenous (IV) or intramuscular (IM) route. However, for a conscious patient undergoing mandibular third molar surgery, injection at a distal site can lead to added discomfort. Administration of the drug as a submucosal (SM) injection in the buccal vestibule at the surgical site has been suggested as an alternative (11). It is postulated that local infiltration of dexamethasone at the surgical site would have a more profound anti-inflammatory effect as compared to distal site parenteral administration (12). However, whether this theory holds in the case of mandibular third molar surgeries is still unclear. Over the last decade, many systematic reviews have provided evidence that administration of dexamethasone does lead to improved outcomes following third molar surgeries (13–16). However, to the best of our knowledge, no meta-analysis has evaluated the influence of the local vs. distal route of administration of dexamethasone on postoperative outcomes. In this context, The current study was designed to systematically review the evidence and conduct a meta-analysis to compare outcomes of surgical site infiltration (SM) vs. distal site parenteral administration (IV or IM) of dexamethasone to improve outcomes of patients undergoing mandibular third molar surgeries.
Materials and Methods
This review was conducted as per the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (17) and the Cochrane Handbook for Systematic Reviews of Intervention (18). The study was not registered on any online database. We aimed to answer the following research question: Is there a difference in postoperative outcomes of pain, swelling, and trismus with SM vs. IV or IM administration of dexamethasone in patients undergoing mandibular third molar surgeries?
Literature Search
We conducted a systematic search of literature with the aid of a medical librarian. The databases of PubMed, Embase, and CENTRAL were searched electronically. Gray literature was searched using Google Scholar. We also search ClinicalTrials.gov for any ongoing trial. The search was conducted using a combination of the following keywords: “dexamethasone,” “steroid,” “corticosteroid,” “wisdom tooth,” “dental extraction,” “dental surgery,” “mandibular molar,” and “third molar” for all databases. Details are provided in Supplementary Table 1. Two reviewers (C.H. and F.L) carried out the electronic search independent of each other. We deduplicated the search results using Endnote (X9, Clarivate Analytics). The search results were assessed initially by their titles and abstracts to identify citations requiring full-text analysis. The full texts of the articles were reviewed by the two reviewers independently based on the inclusion and exclusion criteria. Any disagreements were resolved by discussion with the third reviewer (C.L.). For studies without abstract, we directly analyzed full-texts. Furthermore, we also hand-searched the bibliography of included studies for any missed references.
Eligibility Criteria
We framed the inclusion criteria of this study based on the PICOS (Population, Intervention, Comparison, Outcome, and Study design) framework. Each domain was described as follows:
Population: Patients undergoing mandibular third molar surgeries
Intervention: SM dexamethasone
Comparison: IV or IM dexamethasone. IM injections were to be given in gluteal or deltoid regions.
Outcomes: Any one of the following: Pain, swelling, or trismus.
Study design: Randomized controlled trials (RCTs) only
Exclusion criteria were: (1) Studies using any other steroid drug like methylprednisolone (2) Studies on non-surgical mandibular third molar extractions or studies on maxillary third molar extractions (3) Studies on combined maxillary and mandibular third molar extractions (4) Studies conducted on patients taking dexamethasone preoperatively (5) Studies comparing SM with intramassetric injections only (6) Non-RCTs and uncontrolled studies. (7) Studies not reporting relevant outcomes (8) Editorials, review articles, and non-English language studies.
Data Extraction and Risk of Bias Assessment
Two reviewers extracted data independently using a data extraction sheet. Data regarding the first author, publication year, study location, age and gender of the sample, sample size, type of impaction included, the dose of dexamethasone, post-operative mouthwash, antibiotics and analgesics, study outcomes, and follow-up were extracted. The aim was to compare pain, swelling, and trismus outcomes between SM vs. IV and SM vs. IM groups. Outcomes measured on postoperative day 2 or 3 were defined as early outcomes while those measured on day 7 were defined as late outcomes.
We used the recent Cochrane Collaboration's risk of bias assessment tool-2 to assess the quality of included RCTs (18). This was done by two reviewers independently. The following five domains were used for quality assessment: randomization process, deviation from intended intervention, missing outcome data, measurement of outcomes, and selection of reported result. Based on the risk of bias in individual domains, the overall bias was marked as “high risk,” “some concerns,” or “low risk.” Any disagreements related to data extraction or quality assessment were resolved by discussion. We also assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool using the GRADEpro GDT software [GRADEpro Guideline Development Tool. McMaster University, 2020 (developed by Evidence Prime, Inc.)].
Statistical Analysis
“Review Manager” [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014] was used for the meta-analysis. We normalized the pain scores on a 10-point scale. Only final pain scores were pooled for the meta-analysis. Some studies reported a change in swelling and mouth opening scores while others reported only changed scores (difference between post-operative and pre-operative values). Since pre-operative mouth opening data was available from all included studies, we converted final mouth opening scores to change in mouth opening scores based on the Cochrane methodology (18) and pooled them for a meta-analysis. However, since preoperative facial measurements were not available from all studies, we pooled final scores and change scores separately for the outcome of swelling.
Data reported on the same scale (pain and trismus) was summarized using mean difference (MD) with 95% confidence intervals (CI) while swelling data was summarized using standardized mean difference (SMD) as there were variations in the methods used by the included studies. For studies reporting data only in graphical format, Engauge Digitizer Version 12.1 was used to extract data. Median, range, and interquartile range data was converted into mean and standard deviation (SD) when required using the method of Wan et al. (19). The random-effects model was used for all the meta-analyses. Heterogeneity was assessed using the I2 statistic. I2 values of 25–50% represented low, values of 50–75% medium, and more than 75% represented substantial heterogeneity. As the total number of studies included in each meta-analysis was <10, funnel plots were not used to assess publication bias.
Results
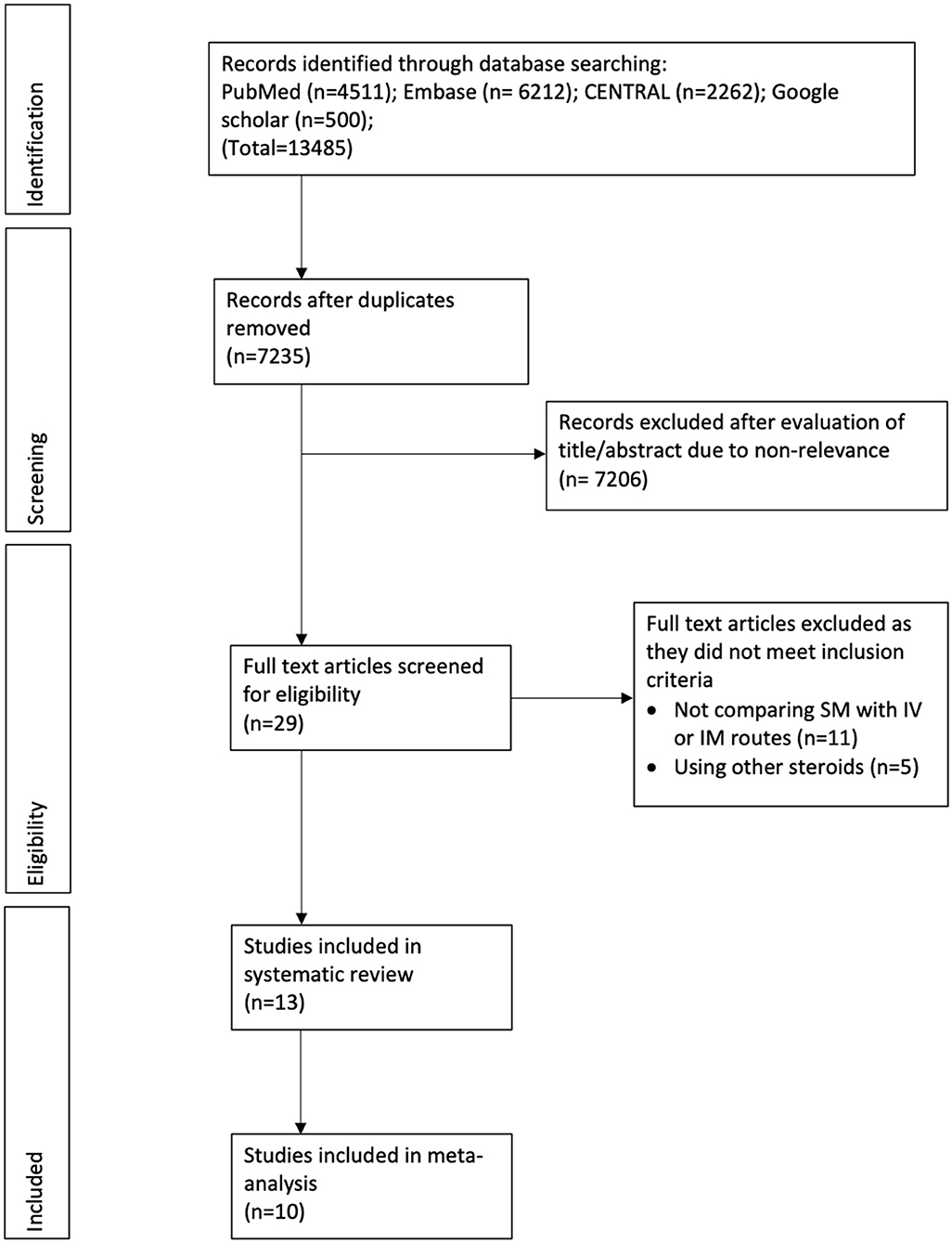
The search revealed a total of 7,235 unique records (Figure 1). A total of 29 articles were screened by their full texts of which 16 were excluded with reasons. Finally, 13 articles were included in the review (20–32).
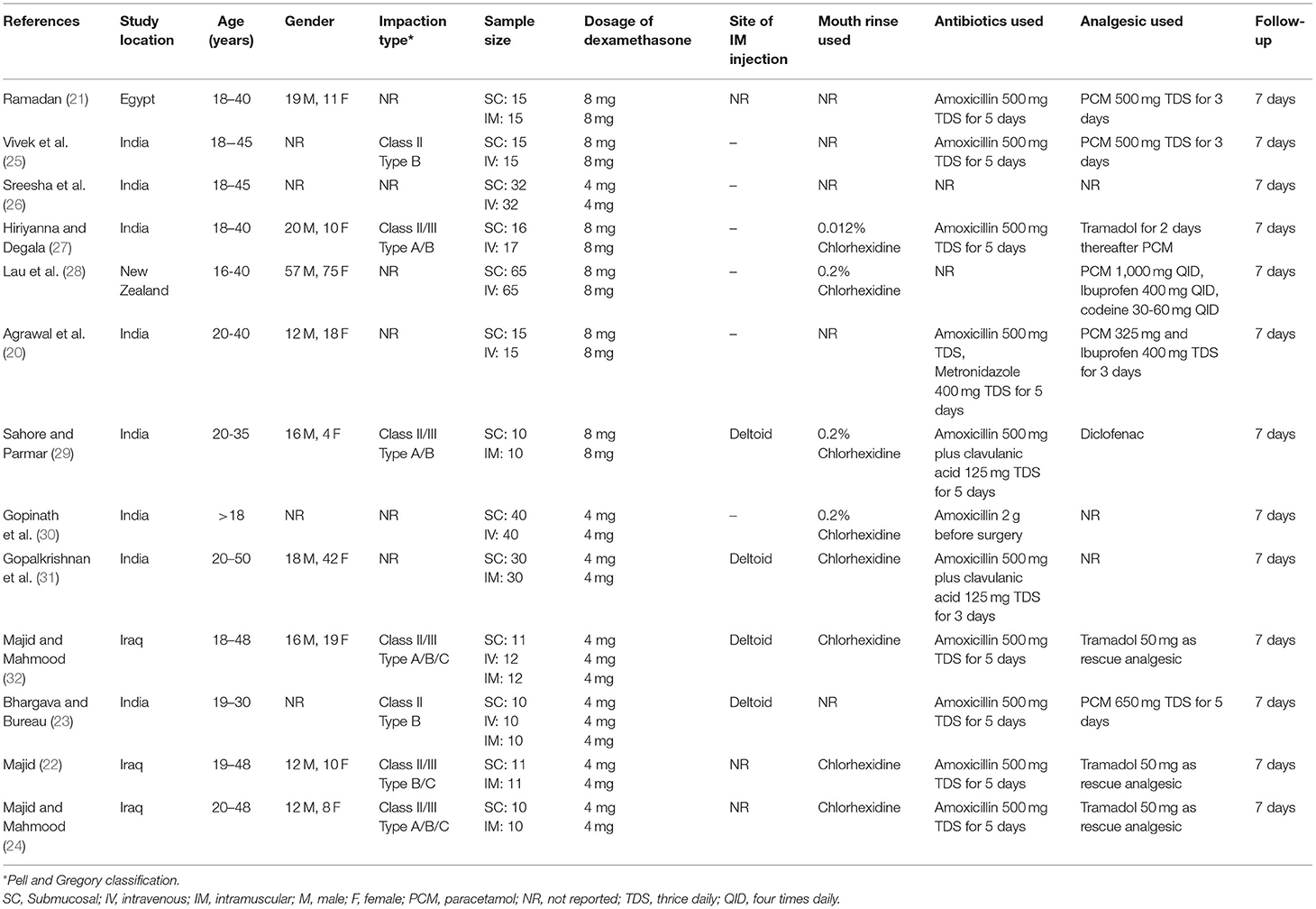
Characteristics of included studies are presented in Table 1. The majority of studies were conducted in India followed by three trials in Iraq (22, 24, 32) and one each in Egypt (21) and New Zealand (28). The sample size of the included studies varied from 10 patients per arm to up to 65 patients per arm. Seven trials (22–24, 26, 30–32) used a dose of 4 mg of dexamethasone while others used 8 mg. However, the dose of dexamethasone was the same in the intervention and comparative arms of each trial. Three of the included trials were three-arm trials comparing the SM route with both IV and IM routes (23, 25, 32). Eight studies reported the use of chlorhexidine mouthwash postoperatively (22, 24, 27–32). The follow-up duration was 7 days in all trials. Outcome data were not reported as mean and SD by three studies and these were not included in the meta-analysis (20, 30, 31).
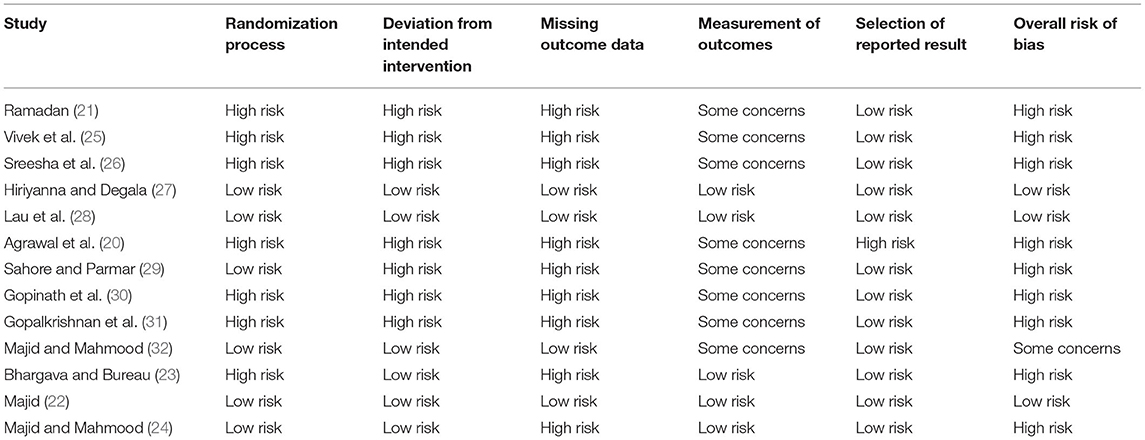
The risk of bias assessment of included studies is presented in Table 2. The risk of bias was the same for all outcomes and hence only one assessment is presented. Overall only three studies had a low risk of bias (24, 27, 28), one study (32) had some concerns while all the remaining studies had a high risk of bias.
SM vs. IV Dexamethasone
Six studies compared SM with IV dexamethasone. Meta-analysis indicated a statistically significant reduction in early pain scores in patients receiving IV dexamethasone compared to those receiving SM dexamethasone (MD: 0.58 95% CI: 0.27, 0.88 I2 = 28% p = 0.0002) (Figure 2). However, no such difference was noted between the two groups for late pain scores (MD: 0.11 95% CI: −0.14 to 0.36 I2 = 0% p = 0.40) (Figure 3).
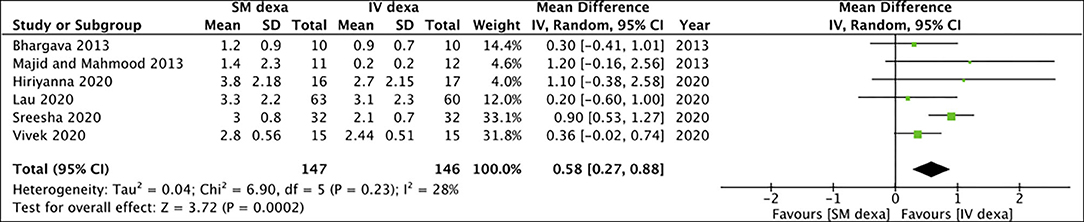
Figure 2. Meta-analysis of early pain between SM and IV dexamethasone (dexa) for third molar surgeries.
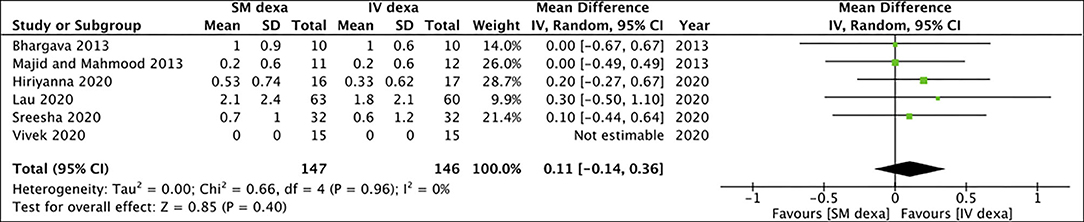
Figure 3. Meta-analysis of late pain between SM and IV dexamethasone (dexa) for third molar surgeries.
Of the six studies, three trials reported change scores for the outcome of swelling while the remaining reported final scores. Meta-analysis demonstrated no statistically significant difference in final swelling scores (SMD: −0.28 95% CI: −0.77 to 0.20 I2 = 44% p = 0.25) as well as change in swelling scores (SMD: 0.85 95% CI: −0.48 to 2.18 I2 = 89% p = 0.21) between SM and IV groups in the early postoperative period (Figure 4). Similar results were noted for late swelling outcomes for both final (SMD:−0.24 95% CI: −0.77 to 0.28 I2 = 52% p = 0.36) and change scores (SMD: −0.13 95% CI: −0.46 to 0.20 I2 = 0% p = 0.45) (Figure 5).
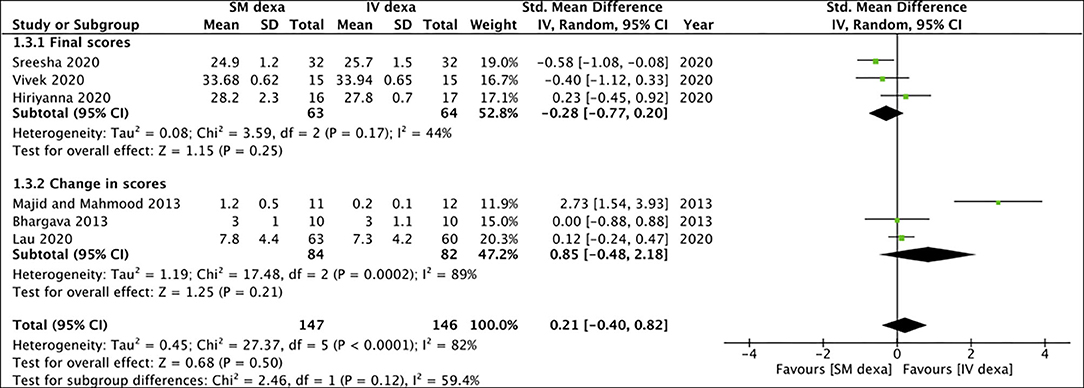
Figure 4. Meta-analysis of early swelling between SM and IV dexamethasone (dexa) for third molar surgeries. Sub-group analysis performed based on final scores or change scores.
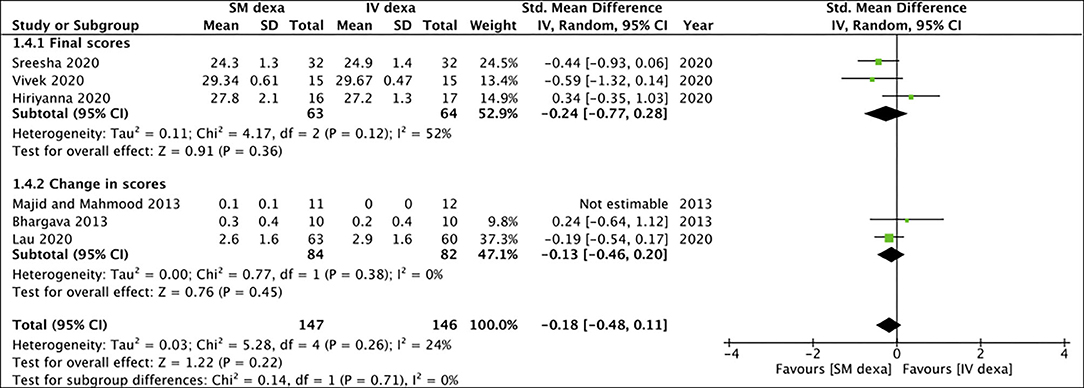
Figure 5. Meta-analysis of late swelling between SM and IV dexamethasone (dexa) for third molar surgeries. Sub-group analysis performed based on final scores or change scores.
Pooled analysis of all six studies indicated no statistically significant difference in early trismus between SM and IV dexamethasone groups (MD: −0.37 95% CI: −1.25 to 0.50 I2 = 60% p = 0.40) (Figure 6). Similar non-significant results were noted for late trismus outcomes as well (MD: 0.13 95% CI: −0.26 to 0.51 I2 = 0% p = 0.52) (Figure 7). GRADE assessment of evidence for SM vs. IV dexamethasone is presented in Supplementary Table 2. Owing to the high risk of bias in majority studies, the quality of evidence was deemed to be low for all outcomes.
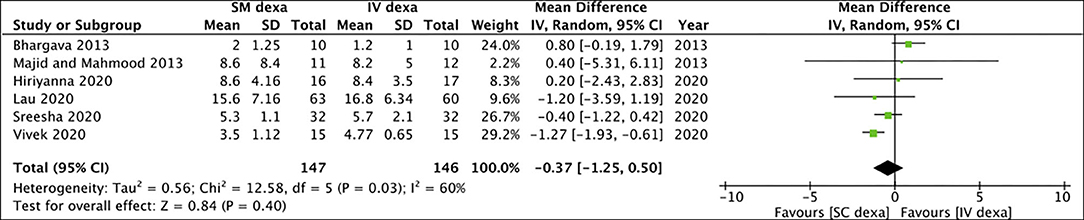
Figure 6. Meta-analysis of early trismus between SM and IV dexamethasone (dexa) for third molar surgeries.
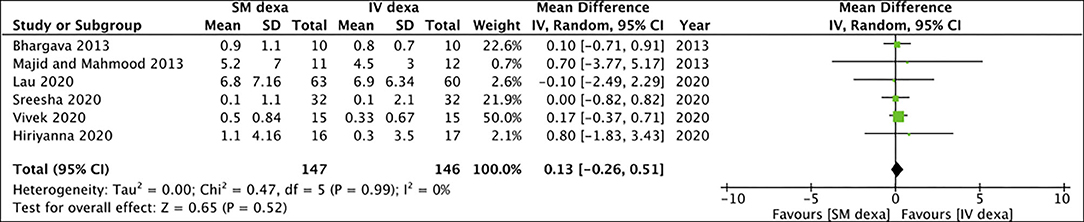
Figure 7. Meta-analysis of late trismus between SM and IM dexamethasone (dexa) for third molar surgeries.
SM vs. IM Dexamethasone
Of the seven studies included in the meta-analysis of SM vs. IM dexamethasone, five reported data on pain scores. Pooled analysis demonstrated no statistically significant difference in early (MD: −0.31 95% CI: −1.28 to 0.66 I2 = 61% p = 0.53) (Figure 8) as well as late pain scores (MD: −0.25 95% CI: −0.92 to 0.41 I2 = 86% p = 0.45) (Figure 9) between SM and IM groups.
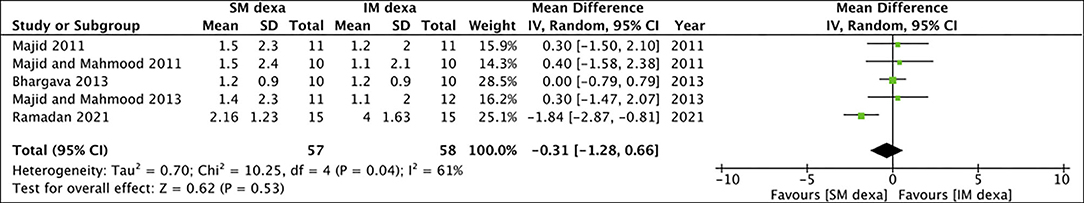
Figure 8. Meta-analysis of early pain between SM and IM dexamethasone (dexa) for third molar surgeries.
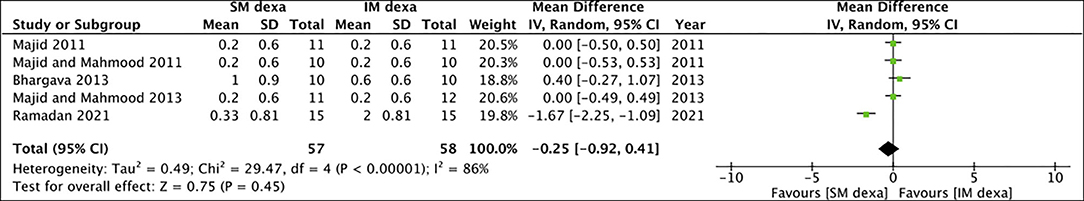
Figure 9. Meta-analysis of late pain between SM and IM dexamethasone (dexa) for third molar surgeries.
Six studies reported data on swelling of which one reported final scores while the remaining reported change scores. On meta-analysis of final swelling scores, we noted a small significant difference in early swelling between SM and IM dexamethasone groups (SMD: −0.80 95% CI: −1.55 to −0.05 p = 0.04) (Figure 10). However, non-significant results were obtained on analysis of change scores for early swelling between the two groups (SMD: 0.05 95% CI: −0.55, 0.65 I2 = 57% p = 0.87). Pooled analysis also failed to demonstrate any statistically significant difference in late swelling for studies reporting final scores (SMD: −0.54 95% CI: −1.27 to 0.19 p = 0.15) as well as for those reporting change scores (SMD: −0.06 95% CI: −0.44 to 0.32 I2 = 0% p = 0.76) (Figure 11).
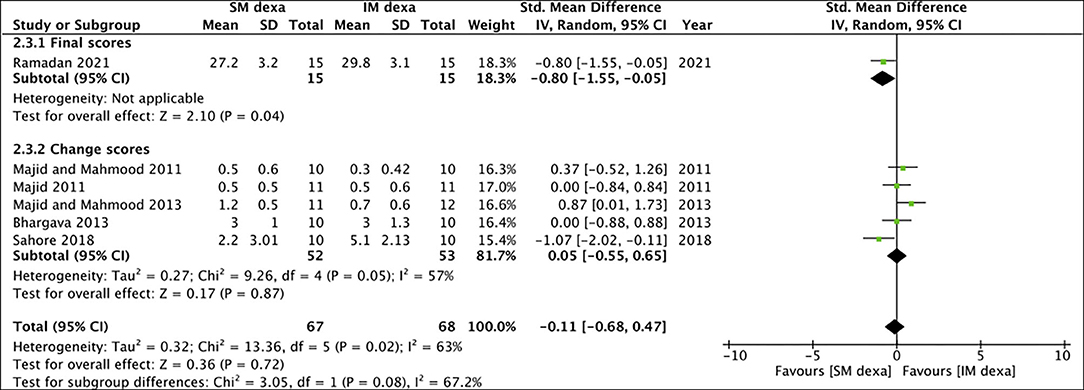
Figure 10. Meta-analysis of early swelling between SM and IM dexamethasone (dexa) for third molar surgeries. Sub-group analysis performed based on final scores or change scores.
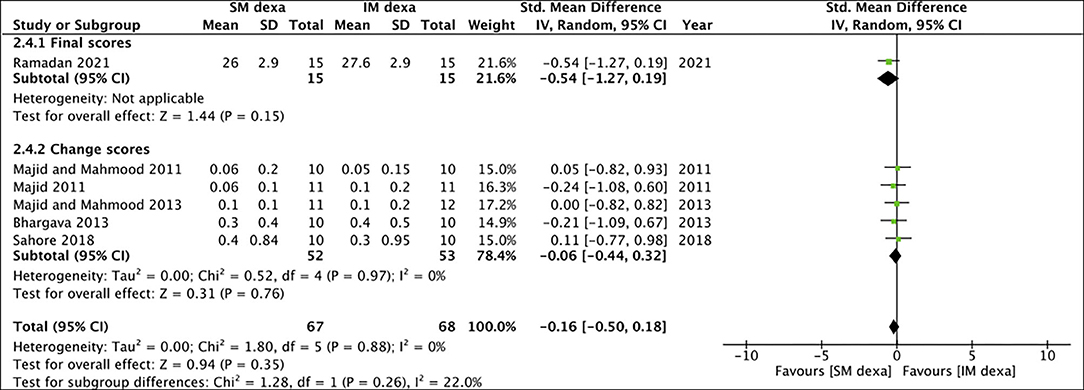
Figure 11. Meta-analysis of late swelling between SM and IM dexamethasone (dexa) for third molar surgeries. Sub-group analysis performed based on final scores or change scores.
Six studies reported data on trismus. Meta-analysis indicated no statistically significant difference in early (MD: −1.19 95% CI: −4.15 to 1.78 I2 = 47% p = 0.43) (Figure 12) and late trismus (MD: −0.03 95% CI: −0.93 to 0.88 I2 = 0% p = 0.95) (Figure 13) outcomes between SM and IM dexamethasone. GRADE assessment of evidence for SM vs. IM dexamethasone is presented in Supplementary Table 3. Owing to the high risk of bias in majority studies, the quality of evidence was deemed to be low for all outcomes.
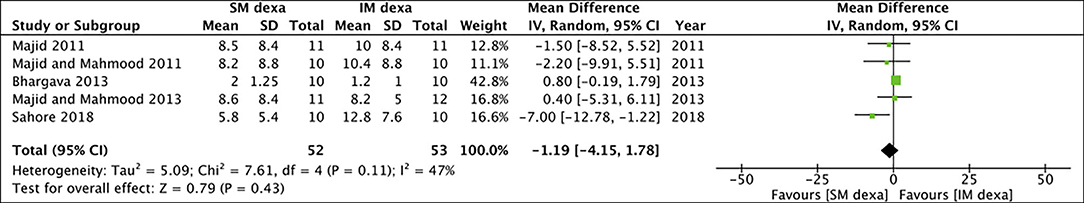
Figure 12. Meta-analysis of early trismus between SM and IM dexamethasone (dexa) for third molar surgeries.
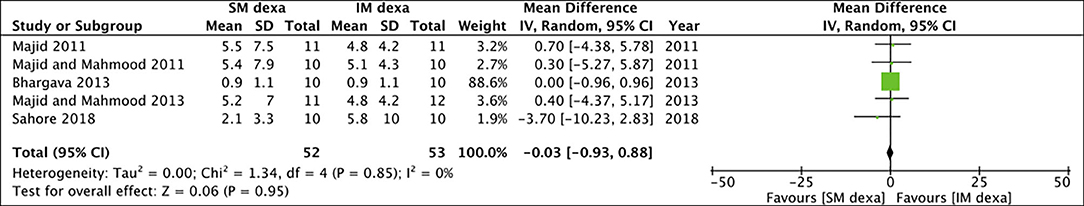
Figure 13. Meta-analysis of late trismus between SM and IM dexamethasone (dexa) for third molar surgeries.
Discussion
Our study which is the first systematic review and meta-analysis comparing outcomes of SM vs. IV or IM dexamethasone indicates that the route of administration has little impact on the outcomes of mandibular third molar surgery. We noted only a mild beneficial effect of IV dexamethasone as compared to SM dexamethasone for early pain. There were no statistically significant differences between late pain, swelling, and trismus outcomes between SM and IV or IM administration of dexamethasone.
Mandibular third molar surgeries can lead to significant postoperative morbidity due to pain, extraoral edema, and reduced mouth opening which can greatly hamper the quality of life of the patient. Consequently, administration of corticosteroids has become standard practice with third molar surgeries (9, 10). Corticosteroids act by inhibiting the enzyme phospholipase A2 which reduces the production of arachidonic acid at the inflammation site. Since arachidonic acid is an important precursor for several inflammatory mediators, inhibition of its production results in an attenuated inflammatory response with a resultant effect on adverse postoperative signs and symptoms (33). As the action of corticosteroids is primarily related to the presence of tissue injury or inflammation, infiltration of the drug directly at the surgical site should have a repository effect with direct and prolonged action on the tissues. Local infiltration should result in slow absorption of the drug with a longer duration of action as compared to distal parenteral administration of the drug with IV or IM routes (12). Indeed, Gao et al. (12) in an RCT on patients undergoing tonsillectomy have shown that tissue site infiltration of dexamethasone results in reduced pain and time to food intake as compared to patients receiving IV dexamethasone. Li et al. (34) in a recent study have demonstrated that intra-articular dexamethasone results in improved pain and swelling outcomes as compared to IV administration in patients undergoing total knee arthroplasty. A trial on patients undergoing cesarean section has also noted reduced pain scores with local infiltration of dexamethasone as compared to IV administration (35).
The results of our systematic review and meta-analysis, however, differ from the outcomes seen in other surgical specialties. On pooled analysis of SM vs. IV route, we noted a significant reduction of early pain scores with IV dexamethasone as compared to SM injections, but no difference in late pain, early and late swelling as well as early and late trismus scores between the two groups. Comparing SM vs. IM route, there was no difference in any of the outcomes of interest. Important to note is that the effect size of pain scores with IV dexamethasone in the early postoperative period was very small. Significant reduction of pain score with IV dexamethasone by 0.58 points on a 10-point pain scale may not be relevant in clinical practice. Martin et al. (36) have demonstrated that a 2.5 point reduction of pain score on the Visual Analog Scale is considered to be clinically significant for third molar surgeries.
Considering the better outcomes reported with local infiltration of dexamethasone in other surgical fields, the lack of difference in outcomes between SM and distal routes of administration is difficult to explain in the context of third molar surgeries. One possible reason could be that the high vascularity of the third molar region which receives rich blood supply from the inferior alveolar, facial and lingual arteries leads to the rapid absorption of dexamethasone after SM injections and subsequent attenuation of the local effect of the drug (37). However, to substantiate this theory there is a need for studies comparing plasma concentrations of the drug following SM and IV or IM administration. Secondly, a major component of the surgical trauma during third molar surgery results from bone removal. Research suggests that minimizing heat generation during bone cutting using piezosurgery reduces osteonecrosis and maintains viable osteocytes. The reduced trauma during bone cutting results in substantial improvements in pain, swelling, and trismus outcomes after third molar surgery (7). Therefore, theoretically, SM administration of dexamethasone may not lead to a direct action on the bone which is a major source of inflammatory mediators after surgery. Indeed, a recent study has compared outcomes of SM vs. direct intra-osseous administration of dexamethasone for third molar surgeries but found no difference in pain and swelling between the two routes (11). With just a single trial to date, there is a need for further studies comparing SM and intra-osseous administration of dexamethasone to delineate evidence on the difference between the two routes.
Other than dexamethasone, corticosteroids like methylprednisolone (MP) and triamcinolone have also been used after third molar surgeries (10). However, there is a paucity of evidence on the influence of different routes of administration of other corticosteroid drugs on mandibular third molar surgeries. In a comprehensive review, Nagori et al. (38) have demonstrated the MP administered via any route results in a reduction of early postoperative edema. However, no comparative analysis between SM and IV or IM routes could be carried out due to a lack of studies. Mukund et al. (39) in a trial comparing oral, SM, IV, and intramasseteric administration of MP reported better outcomes with the intramasseteric route after third molar surgeries. On the other hand, Selvaraj et al. (40) did not find any difference in outcomes between intramasseteric and IM injections of MP at the gluteal region.
It can be noted that two different doses of dexamethasone (4 mg or 8 mg) were used in the included studies. However, since the dosage was the same for both the intervention and comparative arms, there would have been a minimal impact of different doses on postoperative outcomes. Furthermore, studies have demonstrated that the different doses of dexamethasone (4 mg, 8 mg, or 12 mg) do not lead to statistically significant differences in outcomes after third molar surgery (41, 42). We also noted differences in the type of impactions and type of analgesics amongst the included studies. However, with every included study being an RCT, we believe the type of impaction would not have significantly altered the results. However, a split-mouth study with similar type of impactions would have indeed provided better evidence. The type of analgesic used in the included studies was the same for both intervention and control groups and therefore the influence of this confounding factor would have been minimal.
Our study has some limitations. Firstly, despite a thorough literature search, only a limited number of RCTs were available for inclusion in the review. Due to differences in comparative groups, only a small number of studies could be included in each meta-analysis. Moreover, the sample size of many studies was small and the total number of patients analyzed despite a pooled analysis was not very high. Secondly, there were only three studies with a low risk of bias (24, 27, 28). There were concerns regarding randomization, allocation concealment, and blinding of outcome measures in many studies. This may have introduced significant bias in the outcomes of the included trials. Consequently, the overall quality of evidence was deemed to be low for all our comparisons. Thirdly, the majority of the trials were from just two countries. This limits the generalizability of evidence from this meta-analysis.
Conclusion
Low-quality evidence suggests that SM infiltration of dexamethasone results in similar outcomes as compared to IV or IM administration of the drug after third molar surgeries. In clinical practice, surgeons may be more comfortable with the SM route as infiltration is simple and the drug is injected at the anesthetized surgical site. However, further high-quality RCTs are needed to corroborate the current conclusions. Future trials should be multi-centric and of large sample size to strengthen generalizability of results. The trials should also ensure adequate randomization, allocation concealment, and blinding of outcome measures to generate high-quality evidence.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CH, FL, and CL conceived and designed the study. CH and FL were involved in literature search, data collection, analyzed the data, reviewed, and edited the manuscript. FL and CL wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.714950/full#supplementary-material
References
1. de Andrade PF, Silva JNN, Sotto-Maior BS, Ribeiro CG, Devito KL, Assis NMSP. Three-dimensional analysis of impacted maxillary third molars: a cone-beam computed tomographic study of the position and depth of impaction. Imaging Sci Dent. (2017) 47:149–55. doi: 10.5624/isd.2017.47.3.149
2. Altan A, Akbulut N. Does the angulation of an impacted mandibular third molar affect the prevalence of preoperative pathoses? J Dent. (2019) 20:48–52. doi: 10.30476/DENTJODS.2019.44563
3. Ventä I. How often do asymptomatic, disease-free third molars need to be removed? J Oral Maxillofac Surg. (2012) 70:S41–7. doi: 10.1016/j.joms.2012.04.037
4. Mehrabi M, Allen JM, Roser SM. Therapeutic agents in perioperative third molar surgical procedures. Oral Maxillofac Surg Clin North Am. (2007) 19:69–84. doi: 10.1016/j.coms.2006.11.010
5. Khojastepour L, Khaghaninejad MS, Hasanshahi R, Forghani M, Ahrari F. Does the winter or pell and gregory classification system indicate the apical position of impacted mandibular third molars? J Oral Maxillofac Surg. (2019) 77:2222.e1–2222.e9. doi: 10.1016/j.joms.2019.06.004
6. Ibikunle AA, Adeyemo WL, Ladeinde AL. Oral health-related quality of life following third molar surgery with either oral administration or submucosal injection of prednisolone. Oral Maxillofac Surg. (2016) 20:343–52. doi: 10.1007/s10006-016-0571-4
7. Civak T, Ustun T, Yilmaz HN, Gursoy B. Postoperative evaluation of Er:YAG laser, piezosurgery, and rotary systems used for osteotomy in mandibular third-molar extractions. J Cranio Maxillofac Surg. (2020) 49:64–9. doi: 10.1016/j.jcms.2020.11.010
8. Cho H, Lynham AJ, Hsu E. Postoperative interventions to reduce inflammatory complications after third molar surgery: review of the current evidence. Aust Dent J. (2017) 62:412–9. doi: 10.1111/adj.12526
9. Falci SGM, Lima TC, Martins CC, Dos Santos CRR, Pinheiro MLP. Preemptive effect of dexamethasone in third-molar surgery: a Meta-analysis. Anesth Prog. (2017) 64:136–43. doi: 10.2344/anpr-64-05-08
10. Isiordia-Espinoza MA, Bologna-Molina RE, Hernández-Miramontes YA, Zapata-Morales JR, Alonso-Castro AJ, Martínez-Morales F, et al. Pharmacological control of complications following to third molar removal: evidence based on a meta-analysis. Drug Res. (2019) 69:5–11. doi: 10.1055/a-0637-8948
11. Kaewkumnert S, Phithaksinsuk K, Changpoo C, Nochit N, Muensaiyat Y, Wilaipornsawai S, et al. Comparison of intraosseous and submucosal dexamethasone injection in mandibular third molar surgery: a split-mouth randomized clinical trial. Int J Oral Maxillofac Surg. (2020) 49:529–35. doi: 10.1016/j.ijom.2019.10.006
12. Gao W, Zhang QR, Jiang L, Geng JY. Comparison of local and intravenous dexamethasone for postoperative pain and recovery after tonsillectomy. Otolaryngol Neck Surg. (2015) 152:530–5. doi: 10.1177/0194599814567856
13. Moraschini V, Hidalgo R, Porto Barboza ES. Effect of submucosal injection of dexamethasone after third molar surgery: a meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. (2016) 45:232–40. doi: 10.1016/j.ijom.2015.09.008
14. O'Hare PE, Wilson BJ, Loga MG, Ariyawardana A. Effect of submucosal dexamethasone injections in the prevention of postoperative pain, trismus, and oedema associated with mandibular third molar surgery: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. (2019) 48:1456–69. doi: 10.1016/j.ijom.2019.04.010
15. Fernandes IA, de Souza GM, Pinheiro MLP, Falci SGM. Intramuscular injection of dexamethasone for the control of pain, swelling, and trismus after third molar surgery: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. (2019) 48:659–68. doi: 10.1016/j.ijom.2018.09.014
16. Chen Q, Chen J, Hu B, Feng G, Song J. Submucosal injection of dexamethasone reduces postoperative discomfort after third-molar extraction: a systematic review and meta-analysis. J Am Dent Assoc. (2017) 148:81–91. doi: 10.1016/j.adaj.2016.09.014
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions, Second Edition. Version 6. Cochrane (2019). doi: 10.1002/9781119536604
19. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
20. Agrawal A, Chandel S, Singh N, Tiwari A, Singh A, Singh G. The efficacy of intralesional dexamethasone versus intravenous dexamethasone in surgery for impacted third molars: a randomized controlled trial. Natl J Maxillofac Surg. (2020) 11:94. doi: 10.4103/njms.NJMS_46_17
21. Ramadan N. Efficacy of various routes of dexamethasone administration on post-operative complications following impacted mandibular third molar surgeries, comparative clinical study. Egypt Dent J. (2021) 67:267–75. doi: 10.21608/edj.2020.52489.1395
22. Majid OW. Submucosal dexamethasone injection improves quality of life measures after third molar surgery: a comparative study. J Oral Maxillofac Surg. (2011) 69:2289–97. doi: 10.1016/j.joms.2011.01.037
23. Bhargava D, Sreekumar K, Deshpande A. Effects of intra-space injection of twin mix versus intraoral-submucosal, intramuscular, intravenous and per-oral administration of dexamethasone on post-operative sequelae after mandibular impacted third molar surgery: a preliminary clinical comparative study. Oral Maxillofac Surg. (2014) 18:293–6. doi: 10.1007/s10006-013-0412-7
24. Majid OW, Mahmood WK. Effect of submucosal and intramuscular dexamethasone on postoperative sequelae after third molar surgery: comparative study. Br J Oral Maxillofac Surg. (2011) 49:647–52. doi: 10.1016/j.bjoms.2010.09.021
25. Vivek GK, Vaibhav N, Shetty A, Mohammad I, Ahmed N, Umeshappa H. Efficacy of various routes of dexamethasone administration in reducing postoperative sequelae following impacted third molar surgery. Ann Maxillofac Surg. (2020) 10:61–5. doi: 10.4103/ams.ams_66_19
26. Sreesha S, Ummar M, Sooraj S, Aslam S, Roshni A, Jabir K. Postoperative pain, edema and trismus following third molar surgery – a comparitive study between submucosal and intravenous dexamethasone. J Fam Med Prim Care. (2020) 9:2454. doi: 10.4103/jfmpc.jfmpc_188_20
27. Hiriyanna NM, Degala S. Objective and subjective comparison of submucosal and intravenous routes of single-dose preoperative dexamethasone for mandibular third molar surgery—a prospective randomized observer-blind study. Oral Maxillofac Surg. (2020) 25:207–13. doi: 10.1007/s10006-020-00904-0
28. Lau AAL, De Silva RK, Thomson M, De Silva H, Tong D. Third molar surgery outcomes: a randomized clinical trial comparing submucosal and intravenous dexamethasone. J Oral Maxillofac Surg. (2021) 79:295–304. doi: 10.1016/j.joms.2020.09.020
29. Sahore M, Parmar M. Efficacy of use of single dose submucosal injection of dexamethasone in impacted mandibular third molar surgery. J Adv Med Dent Scie Res. (2018) 6:65–72.
30. Gopinath KA, Chakraborty M, Arun V. Comparative evaluation of submucosal and intravenous dexamethasone on postoperative sequelae following third molar surgery: a prospective randomized control study. Int J Oral Care Res. (2017) 5:191–5. doi: 10.5005/jp-journals-10051-0095
31. Gopalkrishnan V, Darkar H, Sahoo N. Effectiveness of submucosal v/s intramuscular dexamethasone in mandibular third molar surgeries. Int J Med Sci Clin Inv. (2015) 2:648–55.
32. Majid OW, Mahmood WK. Use of dexamethasone to minimise post-operative sequelae after third molar surgery: comparison of five different routes of administration. Oral Surg. (2013) 6:200–8. doi: 10.1111/ors.12049
33. Freire V, Bureau NJ. Injectable corticosteroids: take precautions and use caution. Semin Musculoskelet Radiol. (2016) 20:401–8. doi: 10.1055/s-0036-1594286
34. Li D, Wang Q, Zhao X, Luo Y, Kang P. Comparison of intravenous and topical dexamethasone for total knee arthroplasty: a randomized double-blinded controlled study of effects on dexamethasone administration route and enhanced recovery. J Arthroplasty. (2020) 36:1599–606. doi: 10.1016/j.arth.2020.11.019
35. Maged AM, Deeb WS, Elbaradie S, Elzayat AR, Metwally AA, Hamed M, et al. Comparison of local and intra venous dexamethasone on post operative pain and recovery after caeseream section. A randomized controlled trial. Taiwan J Obstet Gynecol. (2018) 57:346–50. doi: 10.1016/j.tjog.2018.04.004
36. Martin WJ, Ashton-James CE, Skorpil NE, Heymans MW, Forouzanfar T. What constitutes a clinically important pain reduction in patients after third molar surgery? Pain Res Manag. (2013) 18:319–22. doi: 10.1155/2013/742468
37. Kokovic V, Krsljak E, Andric M, Brkovic B, Milicic B, Jurisic M, et al. Correlation of bone vascularity in the posterior mandible and subsequent implant stability: a preliminary study. Implant Dent. (2014) 23:200–5. doi: 10.1097/ID.0000000000000057
38. Nagori SA, Jose A, Roy ID, Chattopadhyay PK, Roychoudhury A. Does methylprednisolone improve postoperative outcomes after mandibular third molar surgery? A systematic review and meta-analysis. Int J Oral Maxillofac Surg. (2019) 48:787–800. doi: 10.1016/j.ijom.2018.09.005
39. Mukund V, Singh S, Kumar S, Rath R, Tevatia S. Efficacy of various administrative techniques of methylprednisolone on oedema, trismus and pain after lower third molar surgery. Int J Dent Res. (2017) 5:186. doi: 10.14419/ijdr.v5i2.8343
40. Selvaraj L, Hanumantha Rao S, Lankupalli AS. Comparison of efficacy of methylprednisolone injection into masseter muscle versus gluteal muscle for surgical removal of impacted lower third molar. J Maxillofac Oral Surg. (2014) 13:495–8. doi: 10.1007/s12663-013-0562-z
41. Arora SS, Phull T, Kumar I, Kumar A, Kumar N, Singh H. A comparative study of the effect of two dosages of submucosal injection of dexamethasone on postoperative discomfort after third molar surgery: a prospective randomized study. Oral Maxillofac Surg. (2018) 22:225–30. doi: 10.1007/s10006-018-0699-5
Keywords: third molar surgery, steroid, pain, inflammation, intravenous, intramuscular, submucosal
Citation: Hou C, Liu F and Liu C (2021) Comparison of Submucosal With Intramuscular or Intravenous Administration of Dexamethasone for Third Molar Surgeries: A Systematic Review and Meta-Analysis. Front. Surg. 8:714950. doi: 10.3389/fsurg.2021.714950
Received: 03 June 2021; Accepted: 13 July 2021;
Published: 10 August 2021.
Edited by:
Saulo Gabriel Moreira Falci, Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), BrazilReviewed by:
Glaciele Souza, Universidade Federal dos Vales do Jequitinhonha e Mucuri, BrazilDebora Souto-Souza, Centro Universitário do Triângulo, Brazil
Copyright © 2021 Hou, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengbin Liu, bGl1Y2IxOTc2QHNvaHUuY29t
 Chengyu Hou
Chengyu Hou Chengbin Liu
Chengbin Liu