- 1Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Soochow University, Suzhou, China
Background: Postoperative pain after craniotomy is an important clinical concern because it might lead to brain hyperemia and elevated intracranial pressure. Considering the side effects of opioid, several studies have been conducted to investigate the effect of local anesthetics, especially the scalp block, on postoperative pain. However, the strength of evidence supporting this practice for postoperative pain after craniotomy was unclear and the best occasion of scalp block was also not identified. Therefore, we conducted a meta-analysis to evaluate the efficacy, safety, and the best occasion of scalp block for postoperative pain after craniotomy.
Methods: PubMed, Embase, and the Cochrane Library databases from database inception to October 10, 2021 were searched for all randomized controlled trials evaluating the effect of scalp block on postoperative pain after craniotomy. Data were assessed by StataMP 16 software.
Results: A total of 12 studies were included. A random-effect model was used to analyze all data. Patients under scalp block earned fewer scores than the non-scalp block group in visual analogue scale at the very early period (MD = −1.97, 95% CI = −3.07 to −0.88), early period (MD = −1.84, 95% CI = −2.95 to −0.73) and intermediate period (MD = −1.16, 95% CI = −1.84 to −0.49). Scalp block could also significantly prolong the time of the first request of rescue analgesia and reduce the use of additional analgesics without a significant difference in the incidence of complications. Subgroup analysis showed there was no significant difference in analgesia effect between pre-incision scalp block and post-incision scalp block in all periods.
Conclusion: Scalp block could lead to lower pain intensity scores, more time of the first request of rescue analgesia, and fewer analgesic drugs applied in the first 12 h after craniotomy. There was no significant difference between pre-incision and post-incision scalp block in the occurrence and severity of postoperative pain.
Introduction
Craniotomy is an effective treatment of cerebral diseases and injuries, and postoperative pain is an important clinical concern. 86% of patients probably had the pain of somatic origin, with the involvement of soft tissues and pericranial muscles (1). Elevated oxygen consumption and catecholamine release caused by postoperative pain lead to brain hyperemia and elevated intracranial pressure, which may predispose them to intracranial hematoma (2–4). Effective pain management and prevention are important to avoid these systemic changes and improve rehabilitation and long-term outcomes (5, 6). Besides, management of early postoperative pain can prevent the development of central sensitization and chronic pain states caused by surgical tissue damage (7, 8). However, pain after a craniotomy is often treated insufficiently, because of the fear that the opioid-induced sedation and miosis will mask neurological pathology. Therefore, several studies have been conducted to investigate the effect of local anesthetics, especially the scalp block, on postoperative pain (9–12).
Scalp block was a common technique in craniotomy and was widely used to reduce the hemodynamic response and incisional pain during craniotomy procedure (13–15). Analgesia could be achieved by blockade of the following nerves: greater and lesser occipital nerves, the supraorbital and supratrochlear nerves, the zygomaticotemporal nerve, the auriculotemporal nerve, and the greater auricular nerve (16–18). However, the strength of evidence supporting this practice was unclear and the best occasion of scalp block was also not identified (19–21). Therefore, we conducted a meta-analysis to evaluate the efficacy, safety, and the best occasion of scalp block for postoperative pain after craniotomy.
Methods
A meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. As our study was based on published literature data, ethical approval or patient consent was not required. Besides, only studies reporting ethical approval or patient consent were included in our meta-analysis.
Search strategy
In this meta-analysis, 2 investigators performed a systematic literature search using keywords “scalp block” and “craniotomy” in PubMed, Embase, and the Cochrane Library databases from database inception to October 10, 2021, to identify randomized clinical trials that reported scalp block vs. non-scalp block in patients scheduled for craniotomy. To avoid omissions, the reference lists of all relevant articles were manually searched.
Inclusion and exclusion criteria
Inclusion criteria were defined as follows: (1) Study type: only randomized controlled trials; (2) Population: patients aged >18 years; (3) Intervention: scalp block; (d) Studies that reported at least one of the following outcome measures: pain intensity measured by the visual analogue score, time of the first request of rescue analgesia, additional analgesia requirement in the first 24 h, and adverse events in the first 24 h.
Exclusion criteria were designed as follows: (1) Types of articles: reviews, case reports and retrospective studies; (2) Any study using methods other than placebo and systemic analgesia in the control group; (3) Any study had scalp block in all arms.
Data extraction
Two investigators extracted the following data from the included studies: (1) Number of patients; (2) occasion of scalp block; (3) Postoperative pain treatment modality (occasion and the dosage); (4) Pain intensity for all time points; (5) Time of the first request of rescue analgesia; (6) Additional analgesia requirement in the first 24 h; (7) adverse events in the first 24 h. Data was recorded on a dedicated data extraction form.
Outcome measures
The primary outcome was pain intensity measured by the visual analogue score within 48 h postoperative period. The interval of 0 to 100 pain intensity of VAS was rescaled to a standard interval of 0 to 10. In this study, pain level measurements were categorized into five time periods: (1) very early: < 2 h; (2) early: ≥2 h but <6 h; (3) intermediate: ≥6 h but <12 h; (4) late: ≥12 h but <24 h; (5) very late: ≥24 h but ≤48 h. When there were multiple intervention groups, all relevant experimental intervention groups or relevant control intervention groups were combined into one group. The average of their mean and standard deviation had been calculated at the same time.
Subgroup analysis
Subgroup analysis was conducted to evaluate efficacy in two subgroups. According to the occasion of scalp block, subgroups were classified as pre-incision scalp block and post-incision scalp block.
Risk of bias
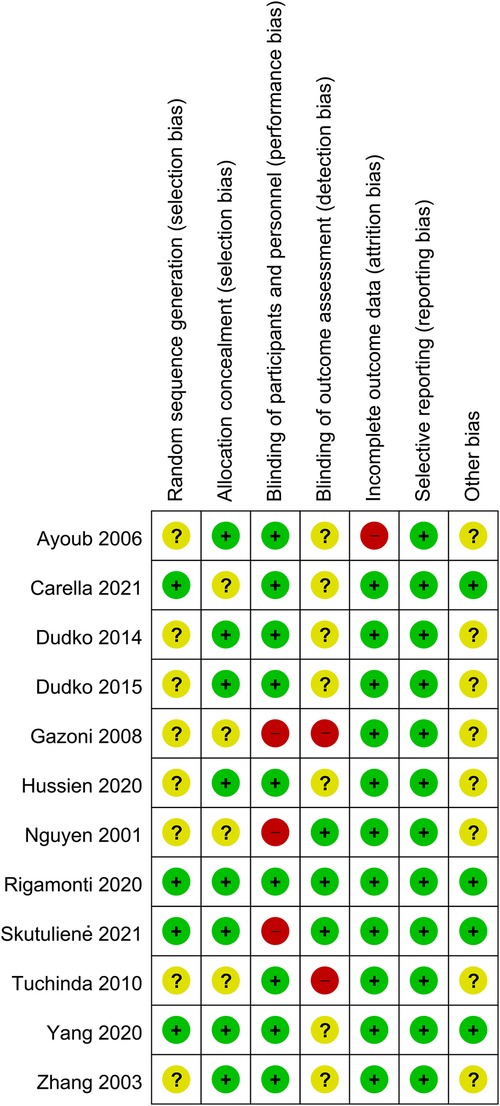
Reviews Manager 5.4 software was used to evaluate the risk of bias in each study. Two investigators used the uniform criteria of the Cochrane collaboration for assessing the risk of bias, including selection bias, performance bias, detection bias, reporting bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. The result of each aspect was divided into low risk, high risk, or unknown risk.
Statistical analysis and data synthesis
Data were assessed by StataMP 16 software. We defaulted to analyzing continuous or dichotomous outcomes as mean difference (MD) or the odds ratio (OR) using a 95% confidence interval (CI) separately. Standard mean difference (SMD) was used for additional analgesia requirements in first 24 h because the specific drug and route of administration varied among trials. Heterogeneity was classified as moderate (I2 = 25%–50%), substantial (I2 = 50%–75%) or considerable (I2 ≥ 75%) (22). Owing to the degree of heterogeneity found, a random-effect model was used (23). Subgroup analysis was performed to investigate the stability of the consolidated results. P-value <0.05 was considered as significant. To determine the source of heterogeneity and assess the influence of every single study on the final results, we excluded one study in sequence through sensitivity analysis.
Results
Study selection
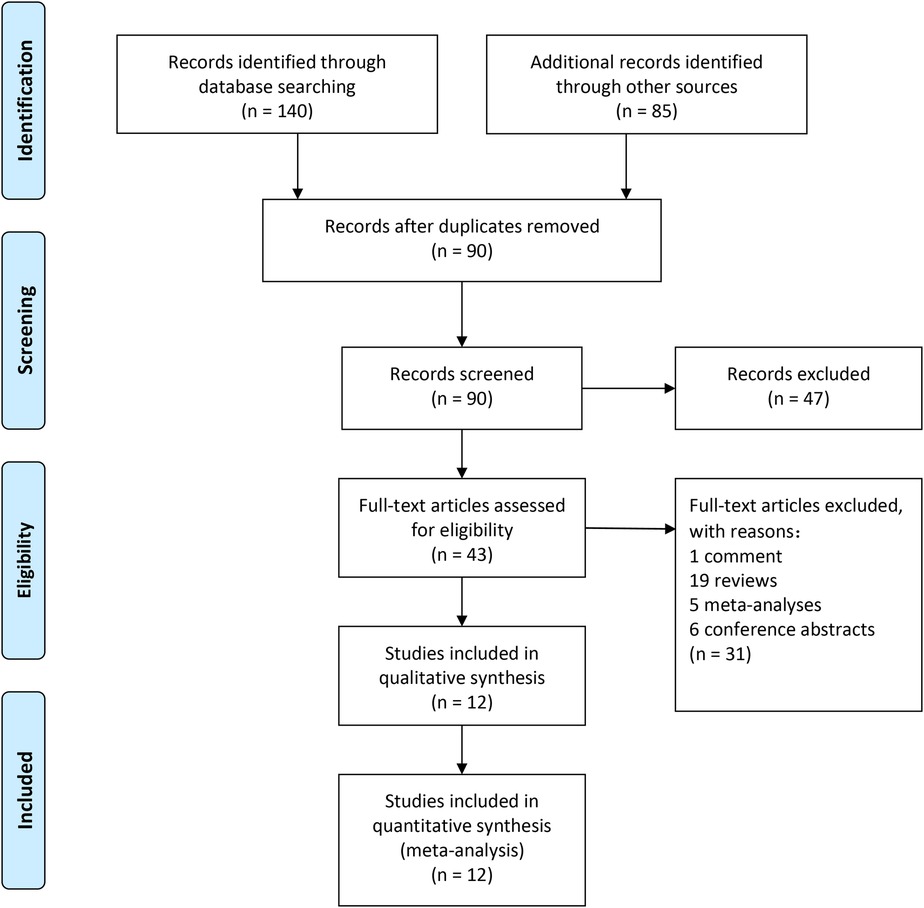
A total of 225 articles from PubMed, Embase, and Cochrane Library databases were identified. 182 records were removed because of duplication or irrelevance to the study. 31 other records were excluded because they were comments (1 record), reviews (19 records), meta-analyses (5 records), and conference abstracts (6 records). The entire process of study search, selection, and inclusion are shown in Figure 1. The overall risk of bias in studies included is presented in Figure 2.
Study characteristics
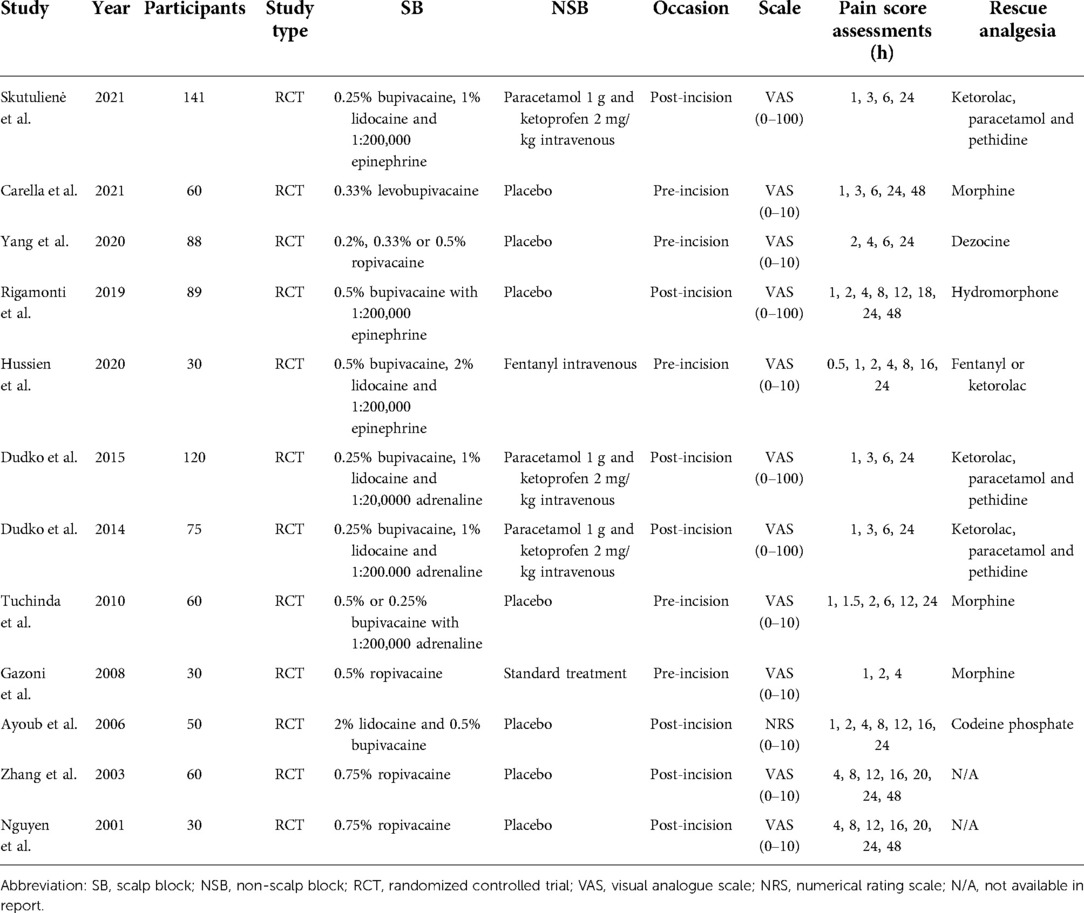
Details of the included studies are shown in Table 1. Full information on included studies could be accessed in the supplement. 8 trials (24–31) were analyzed for pain intensity outcome, 9 trials (4, 24–29, 32, 33) for additional analgesia outcome, and 5 trials (4, 25, 29, 33, 34) for adverse events outcome. Four trials had a three-arm design and two trials had a four-arm design. Data from all doses of scalp block in the same trial were combined as one experimental intervention group (28, 34). Several drugs were used for rescue analgesia, including ketorolac, paracetamol, pethidine, morphine, dezocine, hydromorphone, fentanyl, pethidine, morphine, and codeine phosphate.
Outcomes
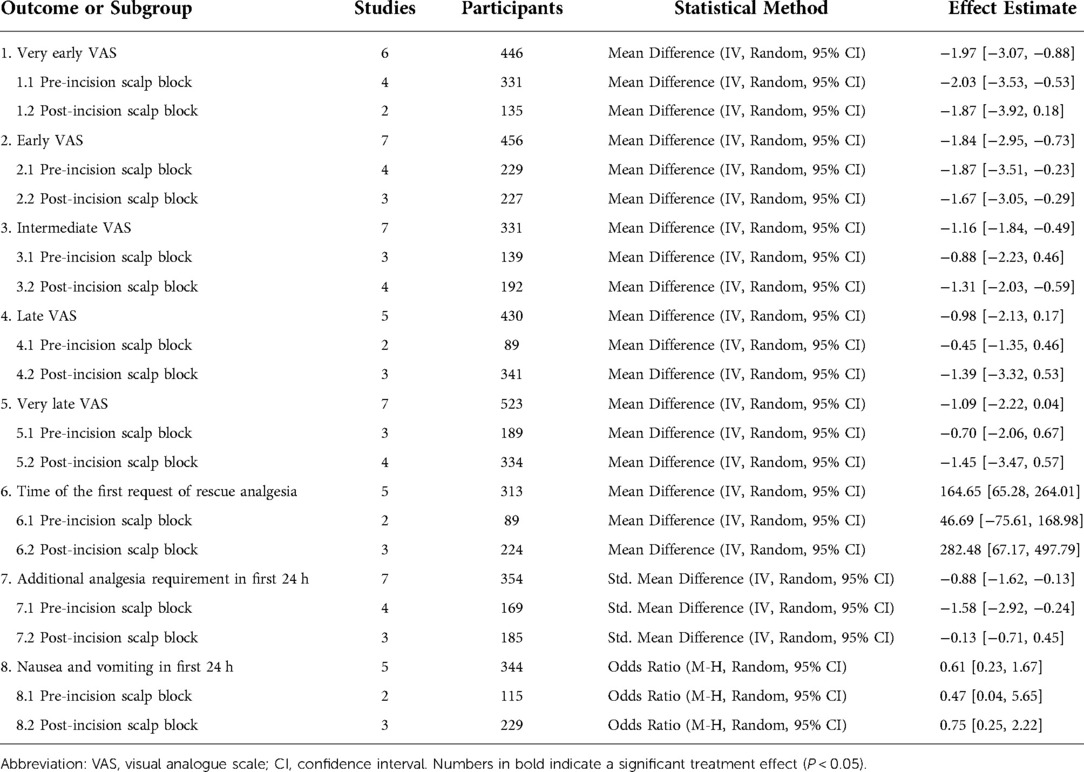
The summary of the meta-analysis results was in Table 2.
Visual analogue scale
There was lower pain intensity in patients receiving scalp block at the very early period (MD = −1.97, 95% CI = −3.07 to −0.88), early period (MD = −1.84, 95% CI = −2.95 to −0.73) and intermediate period (MD = −1.16, 95% CI = −1.84 to −0.49) than the non-scalp block group (Figure 3, Supplementary Figures S1–S2). No significant reduction in reported pain scores associated with scalp bock was found at late or very late period (Supplementary Figures S3–S4).
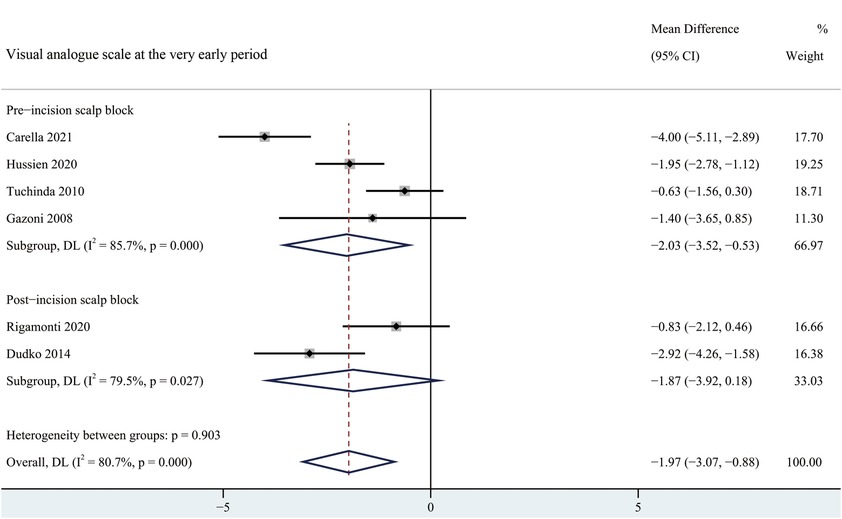
Figure 3. Forest plot summarizing meta-analysis of studies reporting very early pain score. Notes: The black solid rhombuses indicate the estimated mean difference for each randomized controlled trial and the extending lines indicate the estimated 95% CI of mean difference for each randomized controlled trial; The black hollow rhombuses indicate the estimated mean difference (95% CI) for patients in each subgroup or all patients included; Weights are from a random-effects analysis. Abbreviation: SB, scalp block; NSB, non-scalp block; CI, confidence interval.
Rescue analgesia
Six studies reported the time of the first request of rescue analgesia and there was an overall increment associated with scalp bock (MD = 164.65, 95% CI = 65.28 to 264.01). Scalp block led to a notably decrease on additional analgesia requirement in first 24 h (SMD = −0.88, 95% CI = −1.62 to −0.13).
Adverse events
Nausea and vomiting odds ratio in the first 24 h related to scalp block was 0.61 (95% CI = 0.23 to 1.67) as shown in Figure 4. None of the included trials reported any significant difference in the incidence of other complications such as local hematoma, infection, or nerve injury (24, 26–28, 30–32).
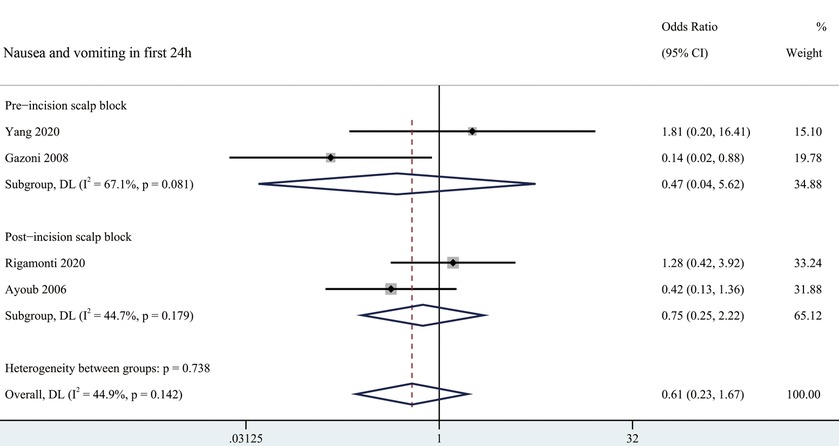
Figure 4. Forest plot summarizing meta-analysis of studies reporting nausea and vomiting in first 24 h. Notes: The black solid rhombuses indicate the estimated odds ratio for each randomized controlled trial and the extending lines indicate the estimated 95% CI of odds ratio for each randomized controlled trial; The black hollow rhombuses indicate the estimated odds ratio (95% CI) for patients in each subgroup or all patients included; Weights are from a random-effects analysis. Abbreviation: SB, Scalp Block; NSB, non-Scalp Block; CI, confidence interval.
Subgroup analysis
According to the occasion of scalp block, patients were divided into pre-incision scalp block group and post-incision scalp block group. Pre-incision scalp block was significantly effective at the very early and early period, whereas pre-incision scalp block showed a significant reduction in pain scores at early and intermediate period after surgery (Figure 3, Supplementary Figures S1–S4). There was no significant difference in the time of the first request of rescue analgesia between pre-incision scalp block and post-incision scalp block (Supplementary Figure S5). Besides, a significant reduction in the usage of analgesia requirement was found in the post-incision scalp block group (Supplementary Figure S6).
Study bias assessment
Egger's publication bias plot was used to assess the potential risk of publication bias Supplementary Figure S7) and no significant funnel plot asymmetry was observed. Results of “leave-one-out” sensitivity analysis are provided in Supplementary Figures S8–S15.
Discussion
This meta-analysis of 12 randomized controlled trials reviewed the available evidence to evaluate the efficacy and safety of scalp block for postoperative analgesia after craniotomy. Combining all studies, we found scalp block was effective in reducing short-term pain without increasing the risk of associated complications, no matter whether it was used before or after incision.
Postoperative pain after craniotomy is mainly localized in the incision and surrounding soft tissues and is less likely to be a widespread headache. The pathophysiological mechanisms of postoperative pain involve different pathways of injury perception. The effects of different analgesic techniques acted on these injury perception pathways. To block the scalp innervation and to anesthetize both the superficial layers of the scalp, local anesthetic infiltration has been accepted by many neurosurgeons, but the effect is short-lived. A study conducted by Akcil et al. (35) found anesthetic infiltration could provide effective anesthesia only in the first 10 min postoperatively, compared with systemic anesthesia. Our study showed a significant mean reduction in pain score at up to 12 h after craniotomy and the analgesic effect decreased over time. In general, the effect of local anesthetic lasts no more than 6 h. The effect of scalp block seemed to persist longer than expected, considering the duration of craniotomy. This finding might be explained by the preemptive analgesic effect. When scalp block is administered, it blocks the C fiber as well as prevents part of the inflammatory cascade, which could reduce or even eliminate the pain hypersensitivity.
Rescue analgesia is usually applied when the pain score is above a certain value, or the pain is unbearable. The longer time of first request of rescue analgesia (MD = 164.65, 95% CI = 65.28 to 264.01) and the less usage of additional analgesics (SMD = −0.88, 95% CI = −1.62 to −0.13) were found in our study. The fewer additional analgesics used postoperatively, the associated side effects like gastrointestinal bleeding caused by NSAIDs or ventilation depression caused by opioids are less likely to occur.
Concerns about postoperative pain management revolved around the side effects of sedation, miosis, nausea, and vomiting, which could probably mask some crucial clinical symptoms. Expect for a trial of Gazoni et al. (29), none of the other trials included reported any significant difference in the incidence of complications. Analysis of 5 randomized controlled trials including a total of 190 participants demonstrated that no significant variation could be found in the complications, with substantial heterogeneity. We believed that scalp block was equally safe compared to traditional analgesic methods.
We conducted a subgroup analysis to figure out the advantages and disadvantages of the different occasion of scalp block and found that there was no significant difference in analgesia effect between pre-incision scalp block and post-incision scalp block. However, we found different periods of postoperative analgesia: pre-incision scalp block was effective for analgesia at very early and early periods, but post-incision scalp block was better at the early and intermediate periods. Considering the same duration of effect of scalp block, it's important to allow effective time to cover the most critical periods. When only considering the postoperative analgesic effect, performing scalp block postoperatively was recommended. However, pre-incision scalp block might also have the advantage of blunting the hemodynamic response to noxious stimuli such as cranial stapling, skin dissection, and flap stripping, which is of great importance when patients are scheduled for awake craniotomies. In general, the occasion of scalp block should be determined upon which purpose the neurosurgeons want to achieve. We believed that pre-incision scalp block should be applied when better intraoperative analgesia was needed, otherwise post-incisional scalp block was more reliable.
Overall, Scalp block is a safe and reliable technique that can effectively reduce patients' pain in the first 12 h after craniotomy, and the results of our study supported its use in postoperative analgesia after craniotomy.
Several limitations were objectively included in this study. First, only 12 randomized controlled studies were included in the study and no more data were available to support our conclusions. Secondly, there were also methodological differences among the included trials, such as different types and doses of local anesthetics. Thirdly, we did not focus on the relevance between the duration of craniotomy and the effect of scalp block on postoperative pain after craniotomy. We hope more trials and studies related to scalp block could be conducted in the future to support our conclusion.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YC (First Author): Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft. JN (First Author): Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft. XL: Data Curation, Writing - Original Draft. JZ (Corresponding Author): Visualization, Supervision, Writing - Review /; Editing. GC (Corresponding Author): Conceptualization, Supervision, Writing- Review /; Editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.1018511/full#supplementary-material.
References
1. De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. (1996) 38:466–9; discussion 469–470. doi: 10.1097/00006123-199603000-00008
2. Haldar R, Kaushal A, Gupta D, Srivastava S, Singh PK. Pain following craniotomy: reassessment of the available options. BioMed Res Int. (2015) 2015:509164. doi: 10.1155/2015/509164
3. Hansen MS, Brennum J, Moltke FB, Dahl JB. Suboptimal pain treatment after craniotomy. Dan Med J. (2013) 60:A4569. https://ugeskriftet.dk/dmj/suboptimal-pain-treatment-after-craniotomy23461986
4. Skutulienė J, Banevičius G, Bilskienė D, Macas A. The effect of scalp block or local wound infiltration versus systemic analgesia on post-craniotomy pain relief. Acta Neurochir (Wien). (2021) 164:1375–9. doi: 10.1007/s00701-021-04886-0
5. Ayrian E, Kaye AD, Varner CL, Guerra C, Vadivelu N, Urman RD, et al. Effects of anesthetic management on early postoperative recovery, hemodynamics and pain after supratentorial craniotomy. J Clin Med Res. (2015) 7:731–41. doi: 10.14740/jocmr2256w
6. Chowdhury T, Garg R, Sheshadri V, Venkatraghavan L, Bergese SD, Cappellani RB, et al. Perioperative factors contributing the post-craniotomy pain: a synthesis of concepts. Front Med. (2017) 4:23. doi: 10.3389/fmed.2017.00023
7. Vadivelu N, Kai A, Tran D, Kodumudi G, Legler A, Ayrian E. Options for perioperative pain management in neurosurgery. J Pain Res. (2016):9:37–47. doi: 10.2147/JPR.S85782
8. Zhao L-H, Shi Z-H, Yin N-N, Zhou J-X. Use of dexmedetomidine for prophylactic analgesia and sedation in delayed extubation patients after craniotomy: a study protocol and statistical analysis plan for a randomized controlled trial. Trials. (2013) 14:251. doi: 10.1186/1745-6215-14-251
9. Stumpo V, Staartjes VE, Quddusi A, Corniola MV, Tessitore E, Schröder ML, et al. Enhanced recovery after surgery strategies for elective craniotomy: a systematic review. J Neurosurg. (2021) 135:1857–81. doi: 10.3171/2020.10.JNS203160
10. Lekprasert V, Tangjitbampenbun A, Kittiponghansa A, Boongird A, Buachai R, Ittichaikulthol W. Comparison of analgesic effect of levobupivacaine with dexmedetomidine and levobupivacaine for scalp block before supratentorial craniotomy: a randomized controlled trial. J Med Assoc Thai. (2020) 103:1028–35. doi: 10.35755/jmedassocthai.2020.10.11547
11. Chong CT. Opioid-free anaesthesia for supratentorial craniotomy for tumor resection. Reg Anesth Pain Med. (2019) 44:A140. doi: 10.1136/rapm-2019-ESRAABS2019.193
12. Darmawikarta D, Sourour M, Couban R, Kamath S, Reddy KKV, Shanthanna H. Opioid-Free analgesia for supratentorial craniotomies: a systematic review. Can J Neurol Sci. (2019) 46:415–22. doi: 10.1017/cjn.2019.57
13. Osborn I, Sebeo J. “Scalp block” during craniotomy: a classic technique revisited. J Neurosurg Anesthesiol. (2010) 22:187–94. doi: 10.1097/ANA.0b013e3181d48846
14. Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: where is the (procedure-specific) evidence? A qualitative systematic review. Eur J Anaesthesiol. (2011) 28:821–9. doi: 10.1097/EJA.0b013e32834a0255
15. Pimentel MC, Marques AI, Pires AC, André AI, Ferreira MC. Scalp nerve block: does it decrease postoperative pain after craniotomy? Eur J Pain Suppl. (2011) 5:291. doi: 10.1016/S1754-3207(11)71003-3
16. Pinosky ML, Fishman RL, Reeves ST, Harvey SC, Patel S, Palesch Y, et al. The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg. (1996) 83:1256–61. doi: 10.1097/00000539-199612000-00022
17. Kerscher C, Zimmermann M, Graf BM, Hansen E. [Scalp blocks. A useful technique for neurosurgery, dermatology, plastic surgery and pain therapy]. Anaesthesist. (2009) 58:949–58; quiz 959–960. doi: 10.1007/s00101-009-1604-2
18. Chen Y-J, Nie C, Lu H, Zhang L, Chen H-L, Wang S-Y, et al. Monitored anesthetic care combined with scalp nerve block in awake craniotomy: an effective attempt at enhanced recovery after neurosurgery. World Neurosurg. (2021) 154:e509–19. doi: 10.1016/j.wneu.2021.07.069
19. Kulikov A, Tere V, Sergi PG, Pugliese F, Lubnin A, Bilotta F. Preoperative versus postoperative scalp block combined with incision line infiltration for pain control after supratentorial craniotomy. Clin J Pain. (2021) 37:194–8. doi: 10.1097/AJP.0000000000000905
20. Guilfoyle MR, Helmy A, Duane D, Hutchinson PJA. Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth Analg. (2013) 116:1093–102. doi: 10.1213/ANE.0b013e3182863c22
21. Tsaousi GG, Logan SW, Bilotta F. Postoperative pain control following craniotomy: a systematic review of recent clinical literature. Pain Pract Off J World Inst Pain. (2017) 17:968–81. doi: 10.1111/papr.12548
22. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
23. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Carella M, Tran G, Bonhomme VL, Franssen C. Influence of levobupivacaine regional scalp block on hemodynamic stability, intra- and postoperative opioid consumption in supratentorial craniotomies: a randomized controlled trial. Anesth Analg. (2021) 132:500–11. doi: 10.1213/ANE.0000000000005230
25. Rigamonti A, Garavaglia MM, Ma K, Crescini C, Mistry N, Thorpe K, et al. Effect of bilateral scalp nerve blocks on postoperative pain and discharge times in patients undergoing supratentorial craniotomy and general anesthesia: a randomized-controlled trial. Can J Anaesth J Can Anesth. (2020) 67:452–61. doi: 10.1007/s12630-019-01558-7
26. Hussien ABM, Saleh ZT, Al Attar HAS, Nasr YM. Postoperative regional scalp block versus intravenous fentanyl for postsupratentorial craniotomy analgesia in adult patients under general anesthesia. Int J Res Pharm Sci. (2020) 11:6039–46. doi: 10.26452/ijrps.v11i4.3802
27. Dudko J, Juske M, Banevicius G. Postoperative pain management after craniotomy. Eur J Anaesthesiol. (2014) 31:241. https://www.embase.com/search/results?subaction=viewrecord/id=L71638557/from=export doi: 10.1097/00003643-201406001-00696
28. Tuchinda L, Somboonviboon W, Supbornsug K, Worathongchai S, Limutaitip S. Bupivacaine scalp nerve block: hemodynamic response during craniotomy, intraoperative and post-operative analgesia. Asian Biomed. (2010) 4:243–51. doi: 10.2478/abm-2010-0031
29. Gazoni FM, Pouratian N, Nemergut EC. Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: a pilot study. J Neurosurg. (2008) 109:44–9. doi: 10.3171/JNS/2008/109/7/0044
30. Zhang X, Ren Y. The comparison of three methods of the scalp block, wound infiltration and superficial cervical plexus block for decreasing postoperative pain after craniotomy. Chin J Misdiagnostics. (2003) 8:1148–50. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02053980/full
31. Nguyen A, Girard F, Boudreault D, Fugère F, Ruel M, Moumdjian R, et al. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg. (2001) 93:1272–6. doi: 10.1097/00000539-200111000-00048
32. Dudko J, Banevicius G, Macas A, Butenaite G. Pain relief options after craniotomy. Acta Anaesthesiol Scand. (2015) 59:59–60. doi: 10.1111/aas.12556
33. Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R. A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil-based anesthesia in neurosurgery. Anesth Analg. (2006) 103:1237–40. https://www.embase.com/search/results?subaction=viewrecord/id=L44627351/from=export doi: 10.1213/01.ane.0000244319.51957.9f
34. Yang Y, Ou M, Zhou H, Tan L, Hu Y, Li Y, et al. Effect of scalp nerve block with ropivacaine on postoperative pain in patients undergoing craniotomy: a randomized, double blinded study. Sci Rep. (2020) 10:2529. doi: 10.1038/s41598-020-59370-z
Keywords: craniotomy, meta-analysis, nerve block, postoperative pain, visual analogue scale
Citation: Chen Y, Ni J, Li X, Zhou J and Chen G (2022) Scalp block for postoperative pain after craniotomy: A meta-analysis of randomized control trials. Front. Surg. 9:1018511. doi: 10.3389/fsurg.2022.1018511
Received: 13 August 2022; Accepted: 12 September 2022;
Published: 26 September 2022.
Edited by:
Alessandro Di Rienzo, Marche Polytechnic University, ItalyReviewed by:
Riccardo Paracino, Hospital of Santa Maria della Misericordia in Perugia, ItalyBashar Alhani, University Hospitals Coventry and Warwickshire NHS Trust, United Kingdom
© 2022 Chen, Ni, Li, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jialei Zhou emhvdWppYWxlaTk5MDYyMEAxNjMuY29t Gang Chen bmp1X25ldXJvc3VyZ2VyeUAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations PRISMA, preferred reporting items for systematic reviews and meta-analyses; VAS, visual analogue scale; MD, mean difference; OR, odds ratio; CI, confidence interval; SMD, standard mean difference
 Yanting Chen1,†
Yanting Chen1,† Jialei Zhou
Jialei Zhou Gang Chen
Gang Chen