- Department of Neurosurgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: As the pathogenesis of plurihormonal pituitary adenoma (PPA) is unclear and the diagnostic criteria are inconsistent, clinicians still find it challenging to diagnose. To analyze the relationship between clinical and pathological characteristics in PPA.
Methods: The clinical data of patients with 70 PPAs admitted during 2008–2010 and 2019–2020 were collected and analyzed. In particular, hormone examination using cell culture supernatant was performed to confirm PPA cases from 2019 to 2020.
Results: PPA accounted for 13% of all pituitary cases recorded in the same period. There were 30 men and 40 women. Fifty-three percent of patients had one endocrine manifestation, and 1% presented with two endocrine symptoms. However, none of the patients had three endocrine manifestations. The level of one and two types of hormones was elevated in 52 (74.3%) and 5 (7.1%) patients, respectively and that of three types of hormones was increased only in one patient. Immunohistochemical staining for PRL + TSH or FSH/LH was most commonly performed (n = 17), followed by that for PRL + GH + ACTH and PRL + GH + TSH or FSH/LH (n = 14) and PRL + ACTH (n = 10). The primary culture results in vitro were consistent with the pathological findings in five (41.7%) patients. Moreover, 4 of 12 patients diagnosed with PPA during 2019–2020 tested positive for SOX2.
Conclusion: The pathogenesis of PPA remains elusive due to the lack of specific clinical symptoms and endocrine changes. Examination of hormones on tumor culture supernatant is helpful for its diagnosis.
Introduction
Plurihormonal pituitary adenoma (PPA) is a type of pituitary adenoma that expresses two or more types of pituitary adenoma hormones in addition to growth hormone/prolactin or follicle-stimulating hormone β subunit/luteinizing hormone β-subunit (1). PPA expresses multiple hormones. However, clinically, it is commonly characterized by the expression of one type of hormone, or patients are asymptomatic. Due to the use of poor diagnostic techniques, PPA is often misdiagnosed in the past. With the development of technologies such as electron microscopy, immunoelectron microscopy, and immunohistochemistry, as well as a better understanding of this tumor, the proportion of patients diagnosed with PPA has been increasing.
As the pathogenesis of PPA is unclear and the diagnostic criteria are inconsistent, clinicians still find it challenging to diagnose. At present, most PPAs are diagnosed based on the following aspects: (1) clinical symptoms and endocrine activity, (2) imaging and intraoperative findings, (3) histology, (4) immunohistochemistry, and (5) ultrastructure (2). However, due to limitations in testing personnel and economic conditions, the wide use of the diagnostic standard, particularly ultrastructure testing, is challenging to implement in clinical practice. Therefore, there are still challenges such as perfecting the diagnostic criteria for PPA and simplifying the diagnostic process, and these should be addressed urgently. The current study aimed to analyze the relationship among clinical manifestations, serological hormone levels, and immunohistochemical results in patients with PPA. Moreover, the clinicopathological characteristics of PPA were analyzed, and the diagnostic criteria for PPA were improved.
Patients and Methods
The clinical data of patients with PPA admitted to our department from 2008 to 2010 were retrospectively analyzed. Moreover, the clinical, imaging, and laboratory examination data were collected. The inclusion criteria were as follows: (1) patients who underwent complete pituitary magnetic resonance imaging and serum endocrinology examination before surgery and (2) those who had pathological and immunohistochemical examination of tumor tissue, sellar dura, and sphenoid sinus mucosa samples collected during surgery and who have a detailed pathological report. In addition, the imaging and laboratory examination data of patients diagnosed with pituitary adenoma from 2019 to 2020 were prospectively analyzed. Pit-1, SF-1, or T-pit were immunohistochemical stained since 2020 and only three patients had the result of transcription factors. The primary culture of tumor tissues was performed to detect the secretion of tumor cells.
Primary Culture and Hormone Detection
On the first day, the fresh pituitary tumor tissue samples collected during surgery were soaked in 2 mL DMEM medium containing 10% fetal bovine serum, 100 mg/L streptomycin, 105 U/L penicillin, 10 mmol/L HEPES, and essential amino acids. Then, they were quickly transported to the laboratory and were placed in a biological safety cabinet. Ophthalmological scissors was used to cut the tumor specimens into small tissue sections measuring <1 mm3 in a 35-mm diameter petri dish. Then, they were rinsed with PBS and centrifuged three times at 1,000 r/min each for 5 min. The supernatant was discarded. Then, 2 mL DMEM medium was used. The tumor tissue was cultured in an incubator at a constant temperature of 37°C for 24 h. Next, the supernatant was discarded and replaced with 2 mL DMEM medium. The tumor was cultivated in an incubator at a constant temperature incubator of 37°C. Then, the supernatant was collected after culturing for another 24 h. The supernatant culture solution was placed into 15-mL centrifuge tubes, and the presence of TSH and ACTH in the culture medium was evaluated with the Roche electrochemiluminescence method. Next, the Beckman DXI chemiluminescence method was utilized to detect GH, PRL, FSH, and LH in the tumor culture medium.
Statistical Analysis
All statistical analyses were conducted with the open-source statistical package R version 4.0.4. Continuous variables were presented as mean ± standard deviation.
Results
Clinical Data
In total, 535 patients with pituitary adenoma were treated. Among them, 70 had PPA (58 diagnosed in 2008–2010 and 12 in 2019–2020). The clinical data of 70 patients (30 men and 40 women) were collected. The follow-up period ranged from 3 to 156 months, and patients were aged 13–70 (average: 41) years.
Immunohistochemistry Result and Its Relationship With Tumor Size and Aggressiveness
Histochemical staining for PRL + GH + ACTH was most commonly performed, and this method was used to diagnose 14 patients (n = 3, microadenoma; n = 5, large adenoma; n = 6, unknown; and n = 2, intraoperative and postoperative diagnoses of invasive pituitary tumor). In total, 14 patients tested positive for PRL + GH + TSH or FSH or LH or FSH/LH. Among them, one presented with microadenoma and 12 with a large adenoma. However, the type of tumor was unknown in one patient, and eight patients presented with invasive pituitary tumor after surgery. Moreover, 17 patients tested positive for PRL + TSH or FSH or LH or FSH/LH. Among them, two presented with microadenomas and eight with a large adenomas. Then, the type of tumor was unknown in seven cases, and seven patients were diagnosed with invasive pituitary tumors after surgery. Table 1 shows the immunohistochemical results and tumor size and invasiveness.
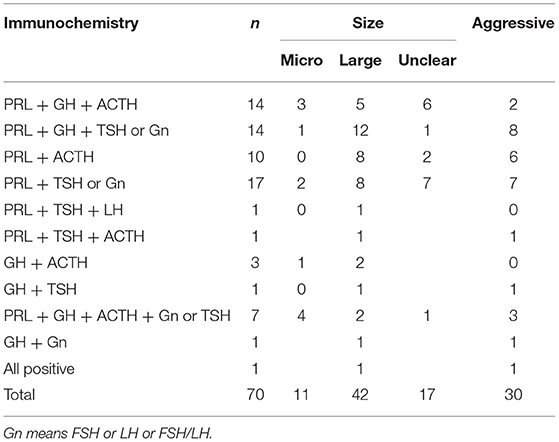
Table 1. The combination of immunohistochemistry and its relationship with tumor size and aggressiveness.
Relationship Among Immunohistochemistry Results, Clinical Hormone Levels, and Endocrine Symptoms
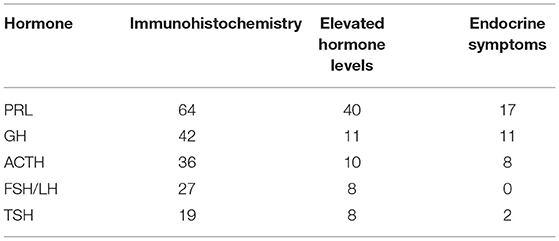
In total, 40, 11, 10, 8, and 4 patients had high serum PRL, GH, ACTH, TSH, and GH and PRL levels, respectively. Moreover, one had elevated GH and ACTH levels, and only one patient presented with high PRL, TSH, and ACTH levels (Table 2). The positivity rate of PRL immunohistochemistry was the highest, accounting for 91% of all cases. Only 57 had high serum hormone levels, and only 24% presented with endocrine symptoms. The positivity rate of GH immunohistochemistry was 60%. Only 16% of patients had high serum hormone levels. Moreover, 16% had endocrine symptoms. The positivity rate of ACTH immunohistochemistry was 51%. Only 14 had high serum hormone levels, and 11% had endocrine symptoms. Table 3 shows the relationship among immunohistochemistry positivity rates, serum hormones levels, and endocrine symptoms.
Endocrine Test Using Primary Culture Medium and Immunohistochemistry of Tumor Tissues
In 5 of 12 patients, the endocrine evaluation findings were consistent with the pathological examination results. However, these two examinations obtained conflicting data. That is, the tumor was a silent type of PPA. Table 4 shows the endocrine examination findings in 12 patients.
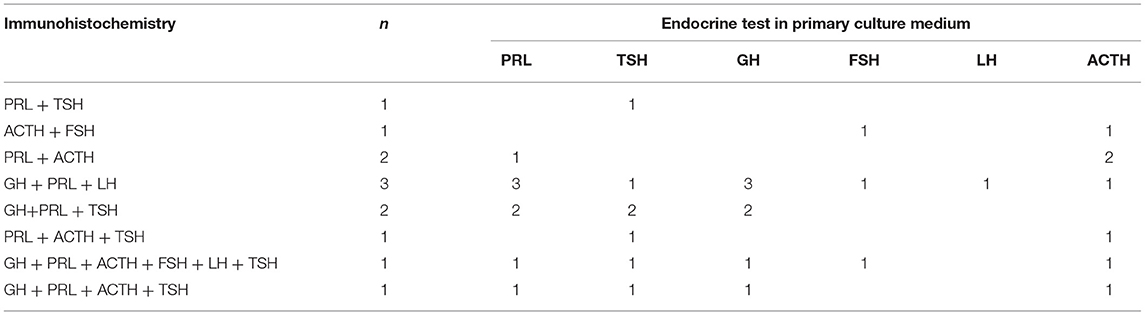
Table 4. Comparison of endocrine test in primary culture medium and immunohistochemical test of tumor tissue.
SOX2 Positivity Rate in Multihormonal Adenoma
Of 12 patients with PPAs, two tested positive for SOX2. Then, one was partially positive, one was 1% positive, and four patients were positive. The positivity rate was 33%.
Discussion
The diagnosis of PPAs based on clinical symptoms, serum hormone levels, and HE staining results remains debatable (3). Some scholars recommend that all pituitary hormone components in tumor cells should be identified using immunohistochemical methods (3, 4). However, the hormone components detected by immunohistochemistry don't cause elevation of the corresponding serum hormones and produce corresponding endocrine symptoms. For example, in this study (as shown in Table 3), 36 patients tested positive for ACTH and 19 for TSH. However, only 10 patients had high ACTH levels, and 8 had elevated serum TSH levels. Further, only eight and two patients presented with Cushing's syndrome and hyperthyroidism, respectively. The possible reason is that the hormone secreted by the tumor is not biologically active, or it has lost activity after entering the blood circulation (3). In addition, three patients underwent multiple surgeries due to relapse, and the immunohistochemistry results were inconsistent. Thus, the efficacy of immunohistochemistry alone in diagnosing PPA is still debated.
The latest classification of pituitary tumors is based on transcription factors and differentiation drivers in the differentiation pathway of pituitary cells. However, they cannot completely reflect the different endocrine disorders in PPA. Currently, the diagnosis of PPAs is still controversial, and the use of serum hormone levels, clinical manifestations, and pathological results alone is not sufficient. Moreover, few types of multihormonal adenomas are difficult to diagnose (3, 5). Hormonal examination using a tumor cell supernatant cultured in vitro is a good diagnostic method. Russel et al. succeeded in culturing pituitary adenoma cells in vitro for the first time in 1959, and several studies have assessed the primary culture of pituitary adenoma cells. Adams et al. (6) attempted to culture pituitary tumor tissues in vitro to detect hormone secretion. Results showed that this method was convenient and quick, and whole tumor tissues could be used, which has a unique role in diagnosing pituitary adenoma. Some subsequent studies (7–9) reported that non-functional adenomas can secrete LH, FSH, and PRL in cell culture in vitro. The in vitro culture experiment of functional pituitary adenoma (10–13) has shown that functional pituitary adenoma has a good secretory function in vitro. Moreover, previous studies (14, 15) have revealed that the hormones secreted by pituitary adenomas in vitro were often different from those observed in immunohistochemistry and serology. Therefore, primary tumor culture in vitro is important in assessing the etiology, pathogenesis, diagnosis, and treatment of tumors.
In this study, the tissue samples of 12 patients with PPA were cultured in vitro. In most cases, the secretion of multiple hormones could be evaluated. Results showed that only four patients had a single hormone secretion, and the corresponding clinical manifestations were observed. In one case, all pituitary hormones were detected, and some normal pituitary tissues were mixed in the tumor tissue. In 5 of 11 patients, the immunohistochemistry results of six hormones were consistent with in vitro culture findings. However, in six patients, the immunohistochemistry results of six hormones were in contrast with in vitro culture results. This may be caused by the following: (1) The hormone cell level is low (16). (2) Tumor cells synthesize hormones. However, they are not secreted outside of the cell, or they degrade immediately after secretion (17). (3) Hormones only have immunological activity, not biological (16, 18). Moreover, in three patients, the endocrinologic examination results of the primary tumor culture were in accordance with the serological result. This could be attributed to the following: (1) The cell count is low and is not enough to identify significant serum changes and clinical symptoms (16). (2) After the tumor compresses the normal pituitary gland, pituitary gland atrophy and hormone levels decrease. Meanwhile, the tumor cells produce low hormone levels. The two can cancel each other out, resulting in a relatively normal serum hormone level and absence of clinical manifestations indicating excessive hormone secretion. (3) Patients may have abnormal receptors for this hormone (19). (4) The secreted hormones have been inactivated in the circulation. Tumor culture conditions in vitro are relatively stable, which can give full play to the function of the whole tissue. In addition, the hormone secretion time is long, easy to accumulate, and the concentration is high. It has certain advantages in diagnosing the endocrine function of tumors. Hence, it has certain advantages and can be used along with other diagnostic methods. However, more specific and effective culture conditions must be explored.
Regarding the pathological assessment of PPA, some scholars (18) believe that it is transformed from certain cells during the normal early development of the pituitary gland. In addition, previous studies (20, 21) have shown that it is transformed from stem cells. In this study, the SOX2 stem cell markers were stained in 12 patients diagnosed with PPA from 2019 to 2020. Only four patients tested positive, and the positivity rate is of 33%. The pathogenesis of PPA could not be completely explained by the origin theory of stem cells. Therefore, the pathogenesis and tumor origin of PPA must be further assessed.
Conclusion
PPA is commonly characterized by a large adenoma with an aggressive biological behavior. The immunohistochemistry of tumor tissues in patients with PPA shows positivity to multiple hormones. However, it does manifest as increased corresponding hormones in serum and clinical symptoms, but manifested as endocrine symptoms of only one increased serum hormone or non-functioning state. PPA is often diagnosed based on clinical manifestations, serum hormone levels, tumor immunohistochemical staining and hormone evaluation (using a supernatant for tumor cells cultured in vitro).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.826720/full#supplementary-material
References
1. Liu X, Jiao Y, Wang R. Interpretation and enlightenment of the 2017 edition of the World Health Organization's classification of pituitary tumors. Chin Med J. (2018) 98:641–2. doi: 10.3760/cma.j.issn.0376-2491.2018.09.001
2. Teng L, Lu D. Comparison of classification of pituitary tumors between WHO (2004 edition) and WHO (2000 edition). Chin J Diag Pathol. (2007) 14:7–9. doi: 10.3969/j.issn.1007-8096.2007.03.001
3. Wei L, Yue Z, Wang S. Immunopathological study of plurihormonal pituitary adenomas. Chin J Neurosurg. (2008) 13:208. doi: 10.l13798/j.issn.1009-153x.2008.04.025
4. Ho DM, Hsu CY, Ting LT, Chiang H. Plurihormonal pituitary adenomas: immunostaining of all pituitary hormones is mandatory for correct classification. Histopathology. (2001) 39:310–9. doi: 10.1046/j.1365-2559.2001.01204.x
5. Zhou F, Zhang M, Lei T. Analysis of clinical and pathological characteristics of GH-secreling pituitary adenomas and mixed pituitary adenomas in 120 patients. Chin J Clin Neurosurg. (2010) 15:92. doi: 10.3969/j.issn.1009-153X.2010.02.009
6. Adams EF, Mashiter K. Role of cell and explant culture in the diagnosis and characterization of human pituitary tumours. Neurosurg Rev. (1985) 8:135–40. doi: 10.1007/BF01815438
7. Surmont DW, Winslow CL, Loizou M, White MC, Adams EF, Mashiter K. Gonadotrophin and alpha subunit secretion by human 'functionless' pituitary adenomas in cell culture: long term effects of luteinizing hormone releasing hormone and thyrotrophin releasing hormone. Clin Endocrinol. (1983) 19:325–36. doi: 10.1111/j.1365-2265.1983.tb00006.x
8. Croue A, Beldent V, Rousselet MC, Guy G, Rohmer V, Bigorgne JC, et al. Contribution of immunohistochemistry, electron microscopy, and cell culture to the characterization of non-functioning pituitary adenomas: a study of 40 cases. Hum Pathol. (1992) 23:1332–9. doi: 10.1016/0046-8177(92)90051-4
9. Mashiter K, Adams E, Van Noorden S. Secretion of LH, FSH, and PRL shown by cell culture and immunocytochemistry of human functionless pituitary adenomas. Clin Endocrinol. (1981) 15:103–12. doi: 10.1111/j.1365-2265.1981.tb00643.x
10. Zhang Z, Lei Tand Zhu Y. Study on cell culture and hormone secretion in pituitary prolactin-secreting adenomas in vitro. J Huazhong Univ Sci. Technol Med Sci. (2001) 30:386–8. doi: 10.3870/j.issn.1672-0741.2001.04.034
11. Gao P, Wang H, Shi Y, Wang M, Jia C, Chen Y, et al. Pituitary prolactin—secreting adenom a cells in vitro experimental study. Guide China Med. (2012) 10:33–4. doi: 10.15912/j.cnki.gocm.2012.04.216
12. Li J, Shi J, Wang H, Yin H. Characteristics of cell culture and hormone secretion by human functioning pituitary adenomas. J Med Postgrad. (2002) 15:306–8. doi: 10.3969/j.issn.1008-8199.2002.04.009
13. White MC, Newland P, Daniels M, Turner SJ, Mathias D, Teasdale G, et al. Growth hormone secreting pituitary adenomas are heterogeneous in cell culture and commonly secrete glycoprotein hormone alpha-subunit. Clin Endocrinol. (1986) 25:173–9. doi: 10.1111/j.1365-2265.1986.tb01679.x
14. Bao W, Yang D, Zhang F, Li S, Mao R, Lu H, et al. Hormone determination of pituitary adenoma cells cultured in vitro. Chin Neurosurg J. (1995) 11:86–8.
15. Tong J, Tao X. Study on the culture and hormone secretion of pituitary adenoma cells in vitro. Zhejiang Med J. (1990) 12:4–7.
16. Scheithauer BW, Horvath E, Kovacs K, Laws ER, Randall RV, Ryan N. Plurihormonal pituitary adenomas. Semin Diagn Pathol. (1986) 3:69–82.
17. Zhou Q, Ma H. The immunohistochemical, transmission and immunoelectron microscopic observation of ACTH-secreting pituitary adenomas and plurihormonal pituitary adenomas containing ACTH. Jiangsu Med J. (1995) 21:597–8. doi: 10.19460/j.cnki.0253-3685.1995.09.013
18. McComb DJ, Bayley TA, Horvath E, Kovacs K, Kourides IA. Monomorphous plurihormonal adenoma of the human pituitary. A histologic, immunocytologic, and ultrastructural study. Cancer. (1984) 53:1538–44. doi: 10.1002/1097-0142(19840401)53:73.0.CO;2-I
19. Felix I, Asa SL, Kovacs K, Horvath E. and Smyth HS. Recurrent plurihormonal bimorphous pituitary adenoma producing growth hormone, thyrotropin, and prolactin. Arch Pathol Lab Med. (1994) 118:66–70. doi: 10.1097/00000478-199401000-00012
20. Kuzuya N, Inoue K, Ishibashi M, Murayama Y, Koide Y, Ito K, et al. Endocrine and immunohistochemical studies on thyrotropin (TSH)-secreting pituitary adenomas: responses of TSH, alpha-subunit, and growth hormone to hypothalamic releasing hormones and their distribution in adenoma cells. J Clin Endocrinol Metab. (1990) 71:1103–11. doi: 10.1159/000181836
21. Matsuno A, Sasaki T, Mochizuki T, Fujimaki T, Sanno N, Osamura Y, et al. A case of pituitary somatotroph adenoma with concomitant secretion of growth hormone, prolactin, and adrenocorticotropic hormone–an adenoma derived from primordial stem cell, studied by immunohistochemistry, in situ hybridization, and cell culture. Acta Neurochir. (1996) 138:1002–7. doi: 10.1007/BF01411291
Keywords: plurihormonal pituitary adenoma, clinicopathological characteristics diagnosis, personalized therapy, cell culture, SOX2
Citation: Shi R, Wan X, Yan Z, Tan Z, Liu X and Lei T (2022) Clinicopathological Characteristics of Plurihormonal Pituitary Adenoma. Front. Surg. 9:826720. doi: 10.3389/fsurg.2022.826720
Received: 01 December 2021; Accepted: 02 February 2022;
Published: 25 February 2022.
Edited by:
Vadim Byvaltsev, Irkutsk State Medical University, RussiaReviewed by:
Dmitrii Guliaev, Almazov National Medical Research Centre, RussiaRyuhei Kitai, University of Fukui, Japan
Copyright © 2022 Shi, Wan, Yan, Tan, Liu and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Lei, dGxlaUB0amgudGptdS5lZHUuY24=
†These authors have contributed equally to this work
 Ruoyu Shi†
Ruoyu Shi† Zhoubin Tan
Zhoubin Tan Xiaojin Liu
Xiaojin Liu Ting Lei
Ting Lei