- 1Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 2Clinic of Urology, University Clinical Centre of Serbia, Belgrade, Serbia
- 3Center for Radiology and Magnetic Resonance Imaging, University Clinical Centre of Serbia, Belgrade, Serbia
- 4Laboratory for Biomedical Instrumentation and Technologies, Department of Signals and Systems, University of Belgrade, School of Electrical Engineering, Belgrade, Serbia
- 5Faculty of Medicine, University Hospital Southampton, Southampton, United Kingdom
- 6Department of Urology, Fundacion Puigvert, Autonomous University of Barcelona, Barcelona, Spain
- 7National and Kapodistrian University of Athens, 2nd Department of Urology, Sismanoglio Hospital, Athens, Greece
Percutaneous nephrolithotomy (PCNL) is frequently used as the first-line treatment of large and complex stones. The key point for successful complex stone removal with minimal risk of complications is to establish the most appropriate access route. Understanding the three-dimensional (3D) relationship of kidney stones and renal collecting systems is crucial for planning and creating an optimal access route. By using a 3D volume segmentation tool a more accurate approach to the renal collecting system and stone treatment could be planned. The objective of this study was assessing the impact of 3D software in getting the desired access.
Introduction
During renal stone treatment planning, non-contrast computerized tomography (NCCT) is a highly recommended examination, used for assessing the stone size, number, and location. Non-excretory phases do not provide precise insight into detailed stone distribution inside the renal collecting system. With the use of an additional excretory phase scan, the urologist can study in-depth the relationship between the stone and the occupied pelvicalyceal system.
European Association of Urology (EAU) Urolithiasis Guidelines recommend percutaneous nephrolithotomy (PCNL) as the first-line treatment of large stones, more than 2 cm (1). Urologists usually define a renal stone as being complex based on the complicated and difficult pelvicalyceal anatomy, branching of the stone into the pelvicalyceal system, or the existence of multiple stones in various calyces. PCNL treatment of complex renal stones frequently require several access tracts and clear out the stone completely (2–4). However, the overall complication rate, as well as the transfusion rate, is higher when using multiple tracts (5–7). The key point for successful complex stone removal with minimal risk of complications is to establish the most appropriate access route (8). That means that the urologist should select and puncture that calyx(s) which will lead to complete stone extraction, while they will not increase perioperative complication rate.
Understanding the three-dimensional (3D) relationship of kidney stones and renal collecting systems is crucial for planning and creating an optimal access route. In recent years the use of advanced imaging and 3D reconstruction of CT data in the treatment of kidney stones is increasing (8). Advanced imaging systems have been shown to provide the 3D perception of target organs, surrounding anatomic structures, depth perception, and spatial orientation during endoscopic and minimally invasive surgery (9, 10).
There is a continuous search for new and improved methods that could help and refine the course of the PCNL procedure by achieving a more accurate approach to the renal collecting system and stone treatment while decreasing the risk of complications (11).
For the purpose of this study an elective semi-automated instrument was created for a more exact and quantitative evaluation of the renal collecting system and the stone by using a 3D volume segmentation tool. This could help for preoperative comprehensive PCNL access route planning but is also viable for real-time use in the operating room. The aim of this study was to assess the impact of 3D software in getting the desired access.
Methods
In this pilot case study, we enrolled 5 patients, which were selected with kidney stones, planned for PCNL procedure. CT scans were performed using a 64 multi-detector row CT scanner (General Electric) with 0.625 mm axial plane reconstruction. The scan was performed before and after contrast administration and both phases (plain and excretory) were used for the segmentation process and fusion of the 3D images into a single semi-transparent model demonstrating the stone distribution in the collecting system. While NCCT was used for stone segmentation, the excretory phase was used for collecting system segmentation. The scan delay time for excretory phase after i.v. contrast administration was 10–15 min, depending on the kidney excretion and degree of obstruction if present. The data set was routinely analyzed in Advantage Workstation Software V 4.6. The contrast medium used was Ultravist 370 mg J/mL, approximately 70–100 mL depending on the patient’s weight.
After NCCT acquisition DICOM images were used for segmentation and 3D reconstruction of the renal stones using the 3D Gastro CT Ex tool. The same software was used for segmentation and 3D reconstruction of pelvicalyceal system images obtained at the excretory phase scan. The reconstructed 3D model represented the interrelation between the pelvicalyceal system and stone with the greater anatomical details. Visualization is achieved by adjusting the scanned object transparency. Segmentations were performed to obtain stone volume (SV) and pelvicalyceal system volume (PSV). After volume rendering of the collecting system and renal stone, the stone volume (SV) was analyzed and compared with the pelvicalyceal system (PS) volume. The volumetric ratio of the stone and collecting system (SV/PS) was also analyzed.
Software Environment
3D Gastro CT Ex tool is an open-source tool developed on Python 3. This tool is the extended version of the 3D Gastro CT tool that was previously introduced (12). The previous software version supports segmentation and 3D rendering of abdominal CT scans for individual phases (native and vein phase). The new software version, whose advantages in the planning of PCNL treatment are illustrated through this paper, offers the option for 3D hybrid visualization of native and delayed phase, which will be described in detail. The following libraries and toolkits were used in the tool development process: matplotlib (13), ndimage (14), SimpleITK (15–17), and VTK (Visualization Toolkit) (18). PyQt5 binding for Qt v5 was used for designing an intuitive and user-friendly graphical interface (19). The tool offers options for reading and viewing different image formats that are supported by SimpleITK reader (e.g., Dicom, MetaImage, etc.). Export options for saving rendering results in.stl and .jpg formats are available. The source code of 3D Gastro CT Ex is available for download from the Github repository: https://github.com/milicevickatarina/3D-Gastro-CT-Extended.
Image Preprocessing
Image preprocessing of axial CT slices (512 × 512) included band shifting on a histogram, image noise re-movement, and co-registration process. First, the band [−548, 800] was shifted to [0,255] using a linear intensity transfer function. Second, image noise removal was performed by median filtering. Finally, Mattes mutual information was used as a criterion for the co-registration of native and delayed phase using the SimpleITK class Image Registration Method (sitkLinear was used as interpolator and gradient descent was used for the optimization process) (20).
Segmentation and 3D Rendering
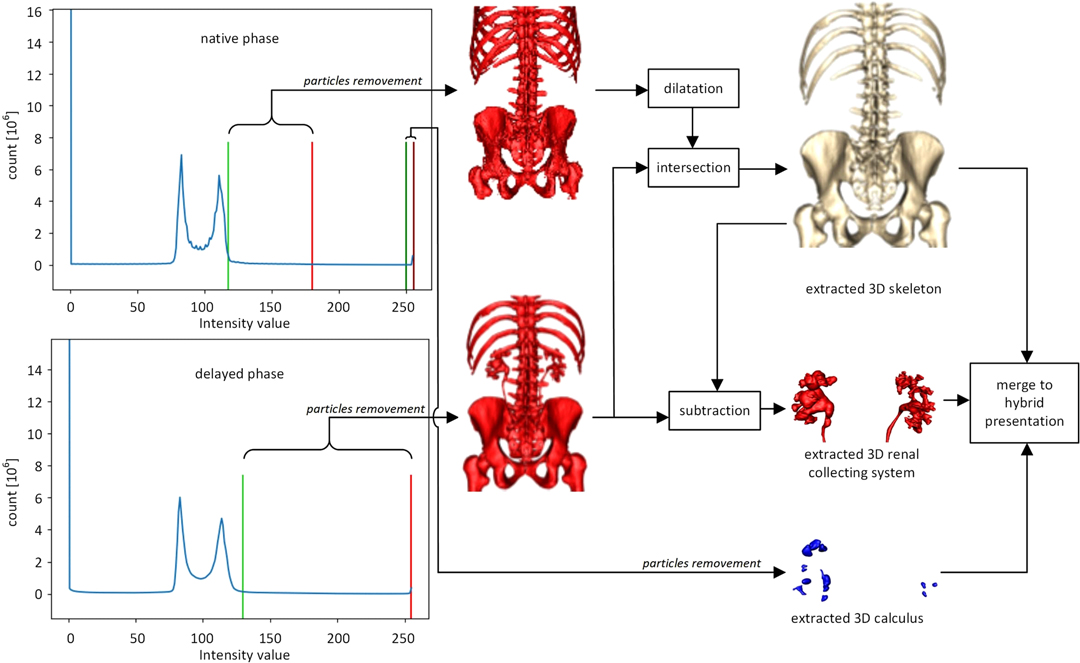
The overall segmentation process on native and delayed phases is presented in Figure 1. First, the segmentation procedure implies the calculation of histograms for the whole CT volume of both phases. Skeleton segmentation includes the following steps:
1. Volume binarization from the native phase based on the histogram thresholding method (default threshold values: lower = 117 and upper = 180, light green line and light red line on native phase histogram in Figure 1 respectively)
2. Particle removal from the volume extracted in step 1 based on opening and closing of the volume (cube structural element with the dimension 3)
3. Dilatation of the volume extracted in step 2
4. Volume binarization from the delayed phase based on the histogram thresholding method (default threshold values: lower = 130 and upper = 255, light green line and light red line on delayed phase histogram in Figure 1 respectively)
5. Particle removal from the volume extracted in step 3 based on opening and closing of the volume (cube structural element with the dimension 3)
6. The intersection of volumes obtained in step 3 and step 5.
A renal collecting system is extracted by the subtraction of volumes archived in steps 6 and 5.
Calculus segmentation includes the following steps:
1. Volume binarization from the native phase based on the histogram thresholding method (default threshold values: lower = 250 and upper = 255, dark green line and dark red line in Figure 1 respectively)
2. Particle removal from the volume extracted in the previous step is based on the opening and closing of the volume (cube structural element with the dimension 2).
3D rendering was performed by Marching Cubes algorithm (21, 22). VTK renderer was used for the implementation of rotation and zoom options (Figure 1). The processing time of DICOM files required to obtain a semi-automatic 3D image is from 45 to 90 min on a computer with the following features: processor Intel (R) Core (TM) i7-8565U CPU@1.80 GHz, 8GB RAM, graphics card NVIDIA GeForce MX250
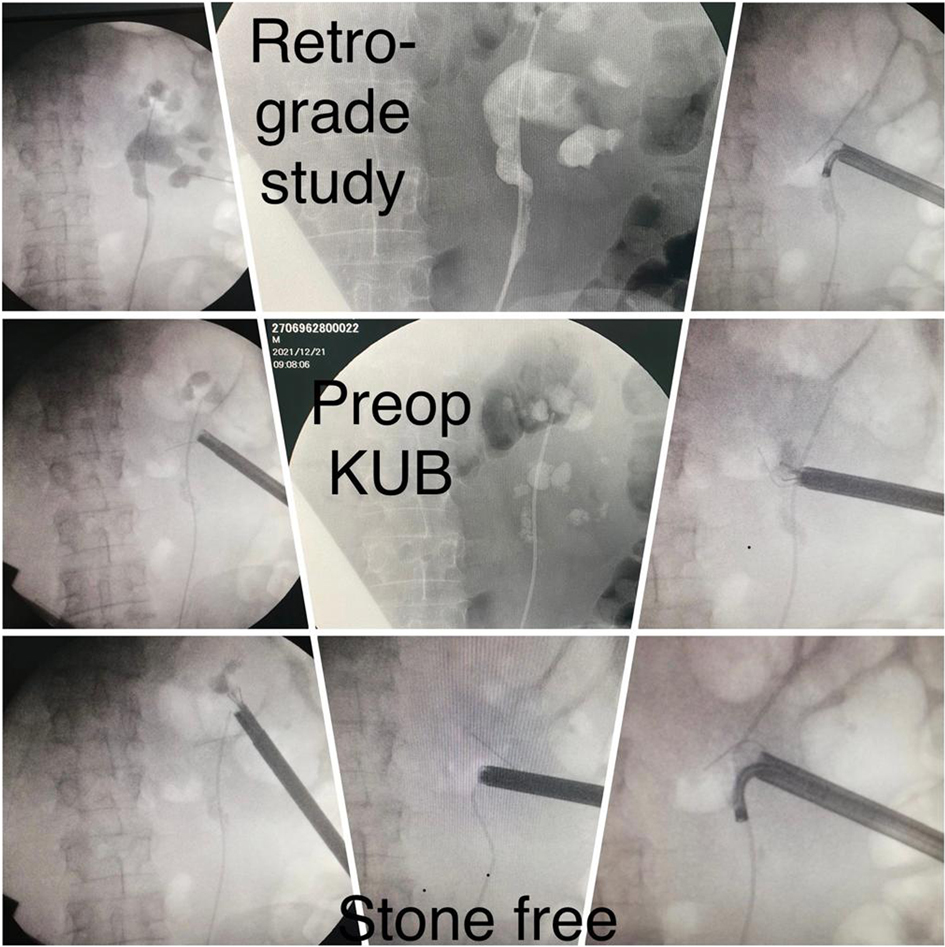
At the operating room, the patient was positioned on the table in a prone position with all the coordinates and measurements taken out from both conventional CT evaluation and 3D model translated on the skin of the lumbar area to enable the planned access route. Puncture was performed under ultrasound and pulsed fluoroscopy control after retrograde contrast injection through the ureteral catheter. Tract was dilated up to 30 Fr Amplatz sheath (standard PCNL) with the use of 26 Fr nephroscope.
Results
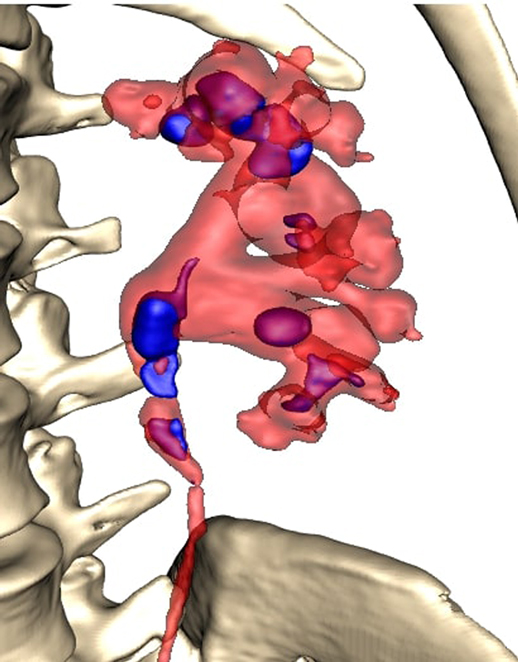
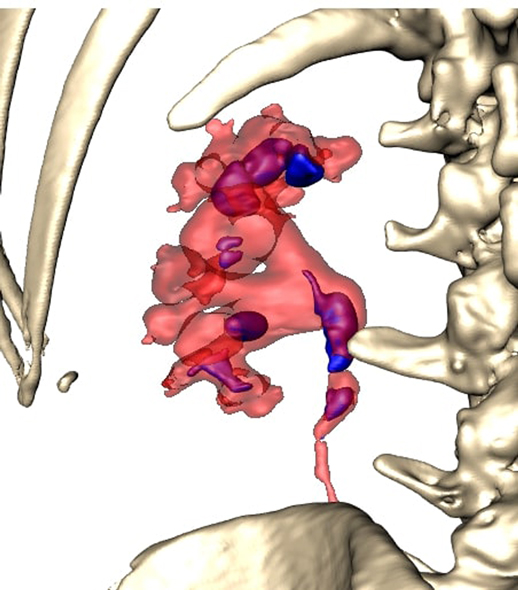
All patients were completely investigated by conventional protocols proposed by guidelines. After confirming the indication for surgery, an additional puncture strategy was made after 3D reconstruction using the 3D Gastro CT Ex tool. Images from conventional CT scanners were compared with new images and an optimal axis for percutaneous puncture was made upon case characteristics. Due to the 3D reconstructed semi-transparent images, it was possible to analyze volumetric datasets in both anterior and posterior view (Figures 2 and 3). Patients from the study group had complex renal stones (Guy stone score 3 and 4) (23). All cases were treated by using a single puncture (Figure 4). The use of flexible nephroscope was indicated by case characteristics and surgery flow. Stone-free status was achieved in all cases, confirmed by follow up imaging. No perioperative complication occurred. In our series there was no unsuccessful outcome of PCNL procedures performed regardless the use of the 3D software for puncture planning.
Discussion
The pelvicalyceal system with a complex stone represents a complex 3D structure. Thus, for accurate access route planning, the semi-transparent 3D model representing the correlation between the collecting system and stone seems to be very useful.
Rendering of the CT images with the ability to adjust the transparency of different tissue layers allowed the operator to see the stone through the renal collecting system, locate the stone and navigate the puncture needle. This semi-transparent 3D model could provide additional information during endourological procedures and therefore raise the possibility of achieving one-stage clearance of complex renal stones. The greatest advantage of using a 3D software is in creating a clear preoperative plan and selecting a target access route. Once having a clear preoperative puncture plan further steps of puncture guidance and control can be performed very precisely, usually with less fluoroscopy use. In this way the total fluoroscopy time can be reduced. As the total fluoroscopy time is a surrogate for radiation exposure, we assume that preoperative planning is a good way to also achieve the goal of reducing the use of fluoroscopy to a minimum in accordance with ALARA principles.
It has been proven that the surgeon is more comfortable with the initial puncture due to the 3D preoperative planning (24, 25). PCNL is routinely performed under 2D visualization with ultrasound and/or fluoroscopy guidance. In our technique the use of flexible nephroscope is not mandatory but flexible nephroscope should always be available during PCNL in aim to clear all stones from the pelvicalyceal system but also upper ureter.
By using a 3D model of CT images in planning the route and controlling the needle path, we would be able to use all the advantages of multidetector CT (MDCT) without raising the risk of radiation dose as it would be the case with CT fluoroscopy or Cone-beam CT guidance. 89% of success rate for percutaneous kidney access has been reported when using Cone-beam CT for guidance, but it comes with a higher radiation dose than conventional fluoroscopy guidance (26).
An accurate preoperatively defined puncture trajectory may also contribute to decreasing total fluoroscopy time to the lowest possible level. Once the operator has a clear preoperative puncture plan during fluoroscopy and ultrasound-guided PCNL procedures, fluoroscopy can be used only for access tract formation and wire position confirmation. Shortening of fluoroscopy time during PCNL is an important technical requirement to ensure safety and efficiency in PCNL while also reducing the radiation exposure of the patient and surgical team (11).
Our puncture technique involves ultrasound guidance with the assistance of pulsed fluoroscopy. Compared with our initial cases which were performed by ultrasound and continuous fluoroscopy we have already achieved significant reduction in fluoroscopy time from 155.4 s (median value) to 76.8 s (27).
If we compare the total fluoroscopy time values of PCNL procedures performed without the use of 3D software already reported in our larger series (27) with the total fluoroscopy time values of the PCNL procedures performed in this study (after creating the semi-transparent 3D model of pyelocaliceal system and stone) a significant reduction in the total fluoroscopy time is noted when 3D software was used (76.8 s vs 27.7 s).
Bearing in mind that in this study the results obtained on a small sample of five patients are presented, we cannot speak from the relevance of the statistically obtained data. This reduction can also be a result of an increased experience and skills of surgeon (OD), as his volume increased during the years.
To this study, CT images were segmented by focusing on renal stone, collecting system, and bone structures serving as a landmark for translation of measurements on the patient’s skin. Contrast is not absolutely mandatory for MDCT examination, especially in cases with damaged renal function. As the majority of patients have normal renal function the use of contrast offers a huge benefit in term of detail determination and distinction between stone and pelvicalyceal system.
Measurements of puncture angles and distances from the referent points were collected from a 3D model and translated in the operating room with accuracy. Vascular structures’ segmentation was not taken into consideration because the initial puncture of the renal collecting system was performed by an experienced urologist with real-time ultrasound guidance. The vessel segmentation process could take additional time before the procedure starting point without any substantial decrease in the rate of vascular injuries.
From the point of PCNL safety, avoidance of unnecessary puncture(s) is crucial. A greater rate of complications has been reported during calyceal stones treatment in comparison with the treatment of pelvic stones (28). When planning treatment with rigid instruments, the access route should be as straight as possible from the skin through the calyx in the axial line of the calyx through the infundibulum of the certain calyx and into the renal pelvis. Any additional intraoperative angulation of the access sheath would increase the risk of renal injury and bleeding. Every percutaneous kidney puncture is associated with a risk of bleeding. With the need for tract formation and dilatation, this risk is increased. The transfusion rate is higher in cases where multiple tracts were used during PCNL. Therefore, a decrease in access tracts is one of the goals of endourologists. The use of flexible nephroscope can decrease the need for additional puncture. In our opinion, even with flexible nephroscope the importance of optimal access axis remains of huge importance. Optimal access offers two biggest benefits: avoidance of torqueing and in that way less possibility of bleeding and better visibility during the procedure, especially in complex cases with stone distribution in different calices. Another benefit of detailed preoperative planning and selection of an optimal access route is in possibility to easily approach to all other calices that contain stones (such as in parallel calices, that sometimes can not easily be approached, even with flexible nephroscope from the punctured calyx) or might be a possible place of stone migration (usually upper calyx).
Stone burden and volume distribution in the renal collecting system is proven to be a significant determinant of the stone-free rate (SFR) (24), which was defined as no residual fragments larger than 3 mm in diameter (29, 30). In our initial experience and case series, the use of this tool helped significantly in preoperative planning and surgical strategy, allowing access to secondary calices from the main axis tract more easily.
There were no additional costs for this kind of evaluation, the software is free for use and its utilize was simple and intuitive. Collaboration with radiologists and software engineers was necessary, especially as the program offers the opportunity of rotation of the image and creating a picture in all positions used for PCNL, whether prone or supine or Valdivia modified used for retrograde intrarenal surgery (RIRS).
The limitation of the study was a shift and change in the position of the stone that can happen with the positioning of the patient. This was especially the case with smaller stones in the dilated collecting system. After 3D model calculation and taking measurements in a few cases the stone had changed its position most likely due to patient positioning. The CT scan was routinely performed while the patient was in the supine position and the PCNL procedure was performed in a prone position.
Conclusion
Having a clear and precise preoperative puncture plan is the key point to ensure efficiency in PCNL. Optimal access according to stone distribution and renal collecting system anatomy may contribute to both PCNL procedure efficacy and safety. This semi-automated segmentation tool for 3D visualization of the collecting system and stone interrelation is proven to be very useful for preoperative PCNL access planning. Currently, this might be a time-consuming process, but in the future, it should be a completely automated process with widespread use and adoption for all complex stone procedures.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article.
Author Contributions
OD is responsible for study design, data collection, data interpretation, manuscript preparation, funds collection; AF for data collection, data interpretation, manuscript preparation, literature search; KM for data interpretation, manuscript preparation, literature search; BS for manuscript preparation, data interpretation, literature search; EE for study design; AS for study design, manuscript preparation, literature search; MJ for study design, data interpretation, manuscript preparation, literature search. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. (2016) 69(3):475–82. doi: 10.1016/j.eururo.2015.07.041
2. Brehmer M, Beckman MO, Magnusson A. Three-dimensional computed tomography planning improves percutaneous stone surgery. Scand J Urol. (2014) 48(3):316–23. doi: 10.3109/21681805.2013.876552
3. Fernström I, Johansson B. Percutaneous pyelolithotomy: a new extraction technique. Scand J Urol Nephrol. (1976) 10(3):257–9. doi: 10.1080/21681805.1976.11882084
4. Raza A, Moussa S, Smith G, Tolley DA. Upper-pole puncture in percutaneous nephrolithotomy: a retrospective review of treatment safety and efficacy. BJU Int. (2008) 101(5):599–602. doi: 10.1111/j.1464-410X.2007.07388.x
5. Kukreja R, Desai M, Patel S, Bapat S, Desai M. First prize: factors affecting blood loss during percutaneous nephrolithotomy: prospective Study. J Endourol. (2004) 18(8):715–22. doi: 10.1089/end.2004.18.715
6. Turna B, Umul M, Demiryoguran S, Altay B, Nazli O. How do increasing stone surface area and stone configuration affect overall outcome of percutaneous nephrolithotomy? J Endourol. (2007) 21(1):34–43. doi: 10.1089/end.2005.0315
7. Turna B, Nazli O, Demiryoguran S, Mammadov R, Cal C. Percutaneous nephrolithotomy: variables that influence hemorrhage. Urology. (2007) 69(4):603–7. doi: 10.1016/j.urology.2006.12.021
8. Christiansen AR, Shorti RM, Smith CD, Prows WC, Bishoff JT. Intraoperative utilization of advanced imaging modalities in a complex kidney stone case: a pilot case study. World J Urol. (2018) 36(5):733–43. doi: 10.1007/s00345-018-2260-4
9. Zhang X, Chen G, Liao H. High-quality see-through surgical guidance system using enhanced 3-D autostereoscopic augmented reality. IEEE Trans Biomed Eng. (2016) 64(8):1815–25. doi: 10.1109/TBME.2016.2624632
10. Wang R, Geng Z, Zhang Z, Pei R, Meng X. Autostereoscopic augmented reality visualization for depth perception in endoscopic surgery. Displays. (2017) 48:50–60. doi: 10.1016/j.displa.2017.03.003
11. Zeng G, Zhong W, Pearle M, Choong S, Chew B, Skolarikos A, et al. European association of urology section of urolithiasis and international alliance of urolithiasis joint consensus on percutaneous nephrolithotomy. Eur Urol Focus. (2021) S2405–4569(21):00065–00071. doi: 10.1016/j.euf.2021.03.008
12. Milićević K, Durutović O, Janković M. Open-source tool for 3D segmentation and rendering of abdominal CT scans, 8th International Conference on Electrical, Electronic and Computing Engineering IcETRAN 2021, pp. 151–154, ETRAN Society, Belgrade, Academic Mind, Belgrade, Ethno Village Stanišići, Republic of Srpska. (2021).
13. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. (2007) 9(3):90–5. doi: 10.1109/MCSE.2007.55
14. Available from: https://docs.scipy.org/doc/scipy/reference/ndimage.html# (Accessed February 2022).
15. Beare R, Lowekamp BC, Yaniv Z. Image segmentation, registration and characterization in R with SimpleITK”. J Stat Softw. (2018) 86(8):6595. doi: 10.18637/jss.v086.i08
16. Yaniv Z, Lowekamp BC, Johnson HJ, Beare R. SimpleITK image-analysis notebooks: a collaborative environment for education and reproducible research. J Digit Imaging. (2018) 31(3):290–303. doi: 10.1007/s10278-017-0037-8
17. Lowekamp BC, Chen DT, Ibáñez L, Blezek D. The design of SimpleITK”. Front Neuroinform. (2013) 7(45):1–14. doi: 10.3389/fninf.2013.00045
18. Schroeder W, Martin K, Lorensen B. The Visualization Toolkit. 4th edn New York, USA: Kitware (2006).
19. Available from: https://pypi.org/project/PyQt5/ (Accessed February 2022).
20. Available from: https://simpleitk.readthedocs.io/en/master/registrationOverview.html (Accessed February 2022).
21. Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. ACM Siggraph Comput Graph. (1987) 21(4):163–9. doi: 10.1145/37402.37422
22. Available from: https://github.com/lorensen/VTKExamples (Accessed February 2022)
23. Thomas K, Smith NC, Hegarty N, Glass JM. The Guy’s stone score—grading the complexity of percutaneous nephrolithotomy procedures. Urology. (2011) 78(2):277–81. doi: 10.1016/j.urology.2010.12.026
24. Atalay HA, Canat L, Bayraktarlı R, Alkan I, Can O, Altunrende F. Evaluation of stone volume distribution in renal collecting system as a predictor of stone-free rate after percutaneous nephrolithotomy: a retrospective single-center study. Urolithiasis. (2018) 46(3):303–9. doi: 10.1007/s00240-017-0995-9
25. Keyu G, Shuaishuai L, Raj A, Shuofeng L, Shuai L, Yuan Z, et al. A 3D printing personalized percutaneous puncture guide access plate for percutaneous nephrolithotomy: a pilot study. BMC Urol. (2021) 21(1):184. doi: 10.1186/s12894-021-00945-x
26. Ritter M, Rassweiler M-C, Michel MS. The Uro Dyna-CT enables three-dimensional planned laser-guided complex punctures. Eur Urol. (2015) 68(5):880–4. doi: 10.1016/j.eururo.2015.07.005
27. Durutovic O, Dzamic Z, Milojevic B, Nikic P, Mimic A, Bumbasirevic U, et al. Pulsed versus continuous mode fluoroscopy during PCNL: safety and effectiveness comparison in a case series study. Urolithiasis. (2016) 44(6):565–70. doi: 10.1007/s00240-016-0885-6
28. Xue W, Pacik D, Boellaard W, Breda A, Botoca M, Rassweiler J, et al. Management of single large nonstaghorn renal stones in the CROES PCNL global study. J Urol. (2012) 187(4):1293–7. doi: 10.1016/j.juro.2011.11.113
29. Tanriverdi O, Boylu U, Kendirci M, Kadihasanoglu M, Horasanli K, Miroglu C. The learning curve in the training of percutaneous nephrolithotomy. Eur Urol. (2007) 52(1):206–12. doi: 10.1016/j.eururo.2007.01.001
Keywords: 3D reconstruction, 3D rendering, PCNL, puncture guidance, kidney stone
Citation: Durutović O, Filipović A, Milićević K, Somani B, Emiliani E, Skolarikos A and Janković MM (2022) 3D Imaging Segmentation and 3D Rendering Process for a Precise Puncture Strategy During PCNL – a Pilot Study. Front. Surg. 9:891596. doi: 10.3389/fsurg.2022.891596
Received: 7 March 2022; Accepted: 11 April 2022;
Published: 3 May 2022.
Edited by:
Clemens Mathias Rosenbaum, Asklepios Klinik Barmbek, GermanyReviewed by:
Noor Buchholz, U-merge Scientific Office, United KingdomAndreas Bruchbacher, Asklepios Klinik Barmbek, Germany
Copyright © 2022 Durutović, Filipović, Milićević, Somani, Emiliani, Skolarikos and Janković. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandar Filipović YWxla3NhbmRhci5maWxpcG92aWMxMUBnbWFpbC5jb20=
Speciality section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Otaš Durutović
Otaš Durutović Aleksandar Filipović
Aleksandar Filipović Katarina Milićević
Katarina Milićević Bhaskar Somani
Bhaskar Somani Esteban Emiliani6
Esteban Emiliani6 Andreas Skolarikos
Andreas Skolarikos