- 1Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Shanghai Key Laboratory of Lung Inflammation and Injury, Shanghai, China
- 3Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
Background: Airflow obstruction is a critical element of chronic airway diseases. This study aimed to evaluate the impact of preoperative airflow obstruction on the prognosis of patients following surgery for esophageal carcinoma.
Methods: A total of 821 esophageal cancer patients were included and classified into two groups based on whether or not they had preoperative airflow obstruction. Airflow obstruction was defined as a forced expiration volume in the first second (FEV1)/forced vital capacity (FVC) ratio below the lower limit of normal (LLN). A retrospective analysis of the impact of airflow obstruction on the survival of patients with esophageal carcinoma undergoing esophagectomy was performed.
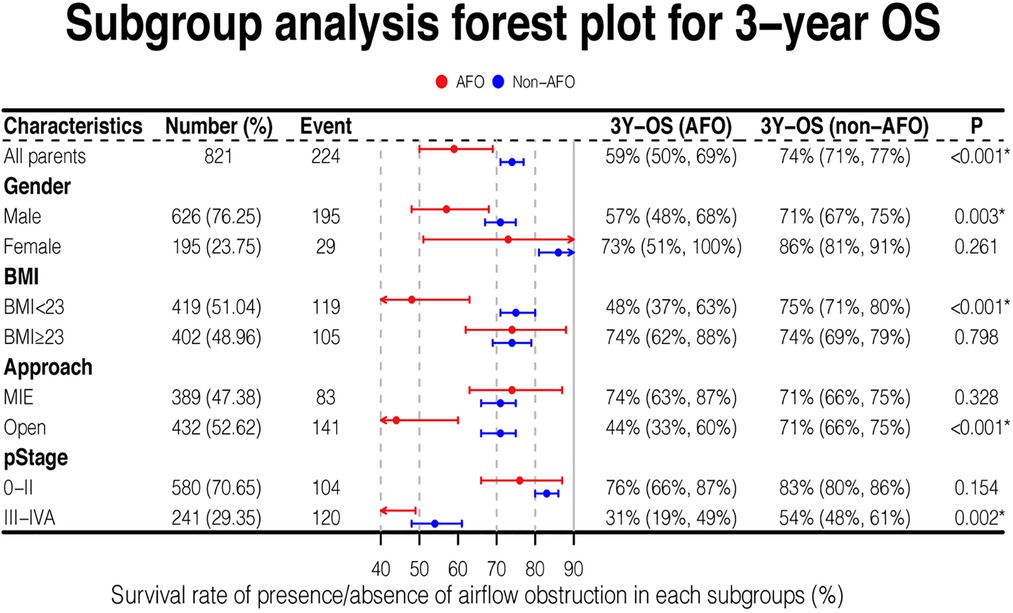
Results: Patients with airflow obstruction (102/821, 12.4%) had lower three-year overall (42/102, 58.8%) and progression-free survival rate (47/102, 53.9%) than those without airflow obstruction (P < 0.001). Multivariate analyses showed that airflow obstruction was an independent risk factor for overall survival (Hazard Ratio = 1.66; 95% CI: 1.17–2.35, P = 0.004) and disease progression (Hazard Ratio = 1.51; 95% CI: 1.1–2.08; P = 0.01). A subgroup analysis revealed that the above results were more significant in male patients, BMI < 23 kg/m2 patients or late-stage cancer (stage III-IVA) (P = 0.001) patients and those undergoing open esophagectomy (P < 0.001).
Conclusion: Preoperative airflow obstruction defined by FEV1/FVC ratio below LLN was an independent risk factor for mortality in esophageal cancer patients after trans-thoracic esophagectomy. Comprehensive management of airflow obstruction and more personalized surgical decision-making are necessary to improve survival outcomes in esophageal cancer patients.
Introduction
Esophageal cancer (EC) is a highly aggressive malignancy with an inferior prognosis of 5-year survival rate of about 20% over the past decade worldwide (1). The incidence and healthcare burden of esophageal cancer in Eastern Asia were higher than in the rest of the world over the past decades (2). Though esophagectomy is an essential treatment for esophageal cancer, it is associated with a high incidence of postoperative complications (3, 4), and overall outcomes are still poor for late-stage esophageal cancer, especially in squamous cell cancer (5, 6).
Lung function is a criterion for eligibility for radical esophagectomy (7). Esophageal cancer patients undergoing esophagectomy should have good or at least not poor lung function, as many patients with severe chronic pulmonary disease are unsuitable for thoracic surgery. It is widely accepted that smoking is one of the relevant risk factors for esophageal cancer and chronic obstructive airway disease (8). Previous research demonstrated a high degree of overlap (7.1%–25%) of operable esophageal cancer patients with chronic obstructive pulmonary diseases (COPD) or asthma (9–11). Furthermore, chronic airway obstruction is directly related to the morbidity of esophagectomy, particularly concerning pulmonary complications and anastomotic leaks (10, 11). However, studies on the outcomes of patients with esophageal cancer and COPD or asthma were limited to postoperative morbidity rather than survival status. Though preoperative low vital capacity decreased the survival rate after radical esophagectomy for cancer (12), the impact of preoperative airway obstruction on long-term survival is unclear. Thus, an accurate assessment of the risk of airway obstruction in esophageal cancer patients is essential. We conducted this study to investigate the impact of preoperative airway obstruction on survival outcomes in patients with esophageal cancer after trans-thoracic esophagectomy. These findings shed light on patients’ long-term airway management after esophageal cancer surgery.
Materials and methods
Population
This is a single-center, retrospective cohort study. From June 2012 to December 2015, 1,016 Chinese patients with esophageal cancer admitted to Zhongshan Hospitals, Fudan University (Shanghai, China), were evaluated and enrolled in the present study. All patients underwent radical trans-thoracic esophagectomy (Ivor-Lewis or McKeown procedure) with gastroesophageal reconstruction. Forty-four patients lost to follow-up, 149 patients without retrieved preoperative spirometry records, and two patients with distant metastasis at the time of diagnosis (M1) were excluded from the sample (Figure 1). This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University.
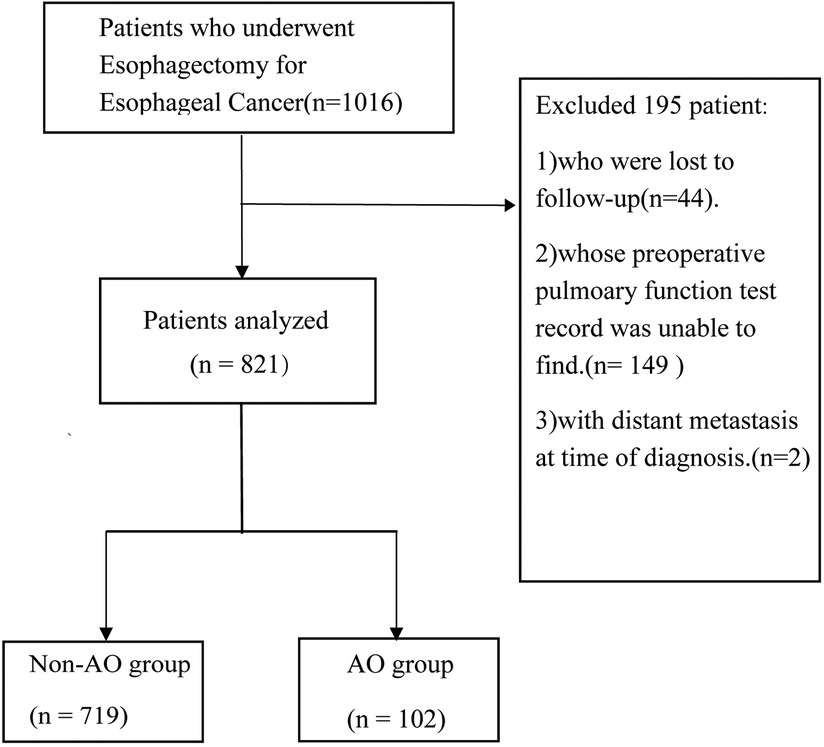
Figure 1. Study profile. Schematic diagram showing the study profile. The AFO group included patients with an FEV1/FVC ratio below the LLN. AFO: airflow obstruction.
Data collection for baseline patients' characteristics
Esophageal carcinoma and the stage were pathologically determined.
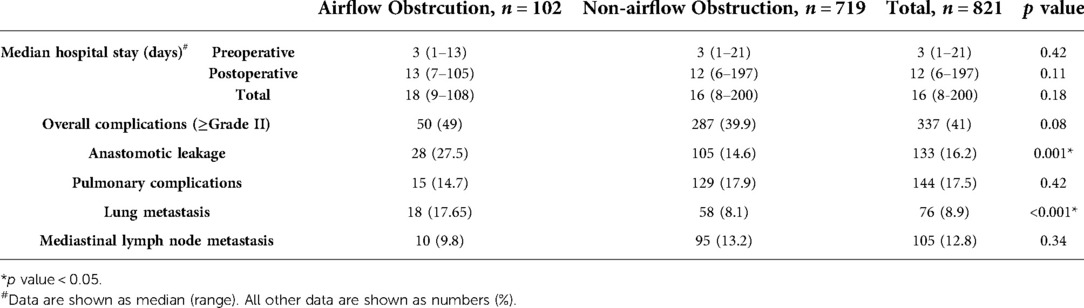
Information about patient characteristics and short-term postoperative complications before hospital discharge was obtained from the patient's medical records. Demographic characteristics, clinicopathological features, pulmonary function, and details of postoperative complications were collected and summarized in Tables 1, 2.

Table 1. Baseline demographics, clinicopathological and spirometric characteristics of Non-AFO and AFO patients.
Evaluation of preoperative pulmonary function variables by spirometry
Spirometry was performed in Zhongshan hospital according to the ATS standards (13). Airflow obstruction was defined as a forced expiration volume in the first second (FEV1)/forced vital capacity (FVC) ratio was below the lower fifth percentile of a large healthy Chinese reference group (lower limit of normal, LLN) (14–16). The lower limit of normal (LLN) of FEV1/FVC was calculated with the formula in Supplementary Table S1. A website was developed by our team for convenient calculation and diagnosis of airflow obstruction (https://drpulmonary.shinyapps.io/AOdiagnosistool/). Given that FEV1/FVC decreases with increased age and most of the study population were over 50 years old, LLN definition of airflow obstruction was used to minimize false positives.
Postoperative complications
Postoperative complications, including pulmonary complications (e.g., pneumonia, acute respiratory distress syndrome, and aspiration), anastomotic leakage, surgical site infection, cardiac complications, chyle leakage, thromboembolic events, recurrent laryngeal nerve paresis, and other complications were summarized. The severity of postoperative complications was classified according to the Clavien-Dindo classification as instructed by the International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy (17). Overall complications were defined as grade II and higher according to the Clavien-Dindo classification.
Follow-Up and definition of recurrence
In principle, patients were reviewed through in-clinic follow-ups every three months in the first year and every six months after that for at least 3 years. Computed tomography of the neck, chest, and abdomen was examined every six months. Disease progression was defined as local recurrence of primary esophageal cancer, distant metastasis, or death due to any cause.
Statistical analysis
All collected data were manually checked for completeness and consistency, and the continuous variables were tested for normality using the Shapiro–Wilk test. Normally distributed variables were compared using the t-test, and non-normally distributed ones were compared using the Mann–Whitney U test between airflow obstruction and non-airflow obstruction groups. Comparisons between the proportions were made using the χ2 test or Fisher's exact test. Survival was calculated using Kaplan–Meier survival curves and compared using the log-rank test. P < 0.05 was considered significant. Median follow-up time was calculated using the reverse Kaplan–Meier method (18). The Cox proportional hazards model was used for the univariate and multivariate analyses to identify independent risk factors associated with survival. Risk-adjusted, restricted cubic splines with 4 knots were used to model the possible non-linearity of the association between BMI and the risk of all-cause death (19, 20). The R Code for restricted cubic splines analysis is available on the GitHub repository: https://github.com/longerham/RCS#rcs. Data analysis was performed using R Foundation Statistical software (R 3.2.2) with ggplot2, forest plot, and survival packages (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Distribution of characteristics in the study population
Among included 821 patients with esophageal cancer, 102 patients were with airflow obstruction (FEV1/FVC < LLN, AFO group), and the remaining 719 patients were classified as non-airflow obstruction (FEV1/FVC ≥ LLN, non-AFO group) patients. Table 1 showed that non-airflow obstruction patients were younger than airflow obstruction patients (mean 60.9 vs. 62.5 years; P < 0.001). Airflow obstruction was associated with male (P < 0.001), lower BMI (mean 22.2 vs. 22.9 kg/m2; P = 0.019), smoking history (P = 0.003), and squamous cell carcinoma (P = 0.018) No significant differences in tumor grades (G), pathological T factor (pT); pathological N factor (pN), perineural invasion (PNI), lymph-vascular space invasion (LVSI), tumor length or tumor locations between two groups were discovered. Table 1 also demonstrated the differences in spirometric variables and operative procedures between AFO and non-AFO groups. FEV1/FVC, FEV1, %VC predicted, and DLCO variables in AFO group were significantly lower than those in non-AFO group.
Short-term outcomes in AFO and non-AFO groups
Length of hospital stay and incidence of overall complications, pulmonary complications, and anastomotic leaks were given in Table 2. Airflow obstruction patients showed significantly higher rate of anastomotic leakage than non-airflow obstruction patients (27.5% vs. 14.6%; P < 0.001). However, there were no significant differences between the groups in the length of hospital stay and rates of pulmonary complications.
Impact of airflow obstruction on survival of esophageal cancer patients
The median follow-up time was 54 months for all patients, while the median follow-up time was 53.6 months (95% CI: 51.9–56.1) in non-AFO group and 55.9 months (95% CI: 52.2–59.1) in AFO group (P = 0.61). The 3-year overall survival (OS) rates were 75.5% and 58.82%, and 3-year progression-free survival (PFS) rates were 67.5% and 53.92% in non-airflow obstruction and airflow obstruction groups, respectively. The airflow obstruction patients' OS and PFS rates were significantly worse than those of non-obstruction patients (P < 0.001 and P = 0.002, respectively, Figure 2). Table 3 presents a multivariate Cox regression analysis performed on factors showing significance in the univariate analysis (age, gender, smoking status, surgical approach, pT, pN, G, PNI, LVSI, and anastomotic leakages). Airflow obstruction turned out to be an independent risk factor for OS (Hazard Ratio = 1.66; 95%CI: 1.17–2.35, P = 0.004) and PFS (Hazard Ratio = 1.51; 95% CI: 1.1–2.08; P = 0.01) in esophageal cancer patients.
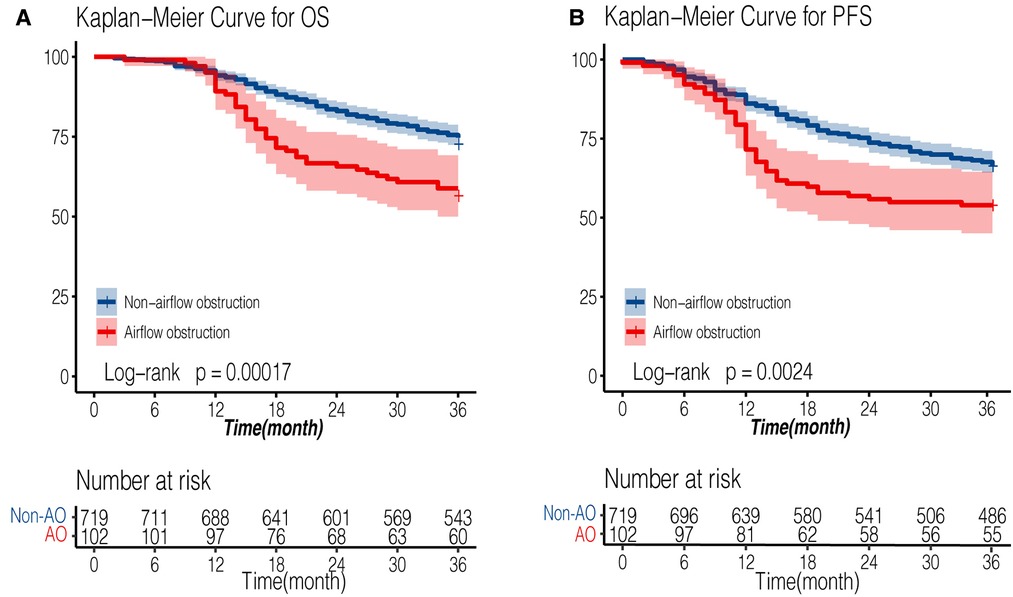
Figure 2. Kaplan-Meier survival curve of AFO and non-AFO groups in patients undergoing esophagectomy for esophageal cancers. (A): overall survival curve; (B): progression-free survival curve). AFO: airflow obstruction.
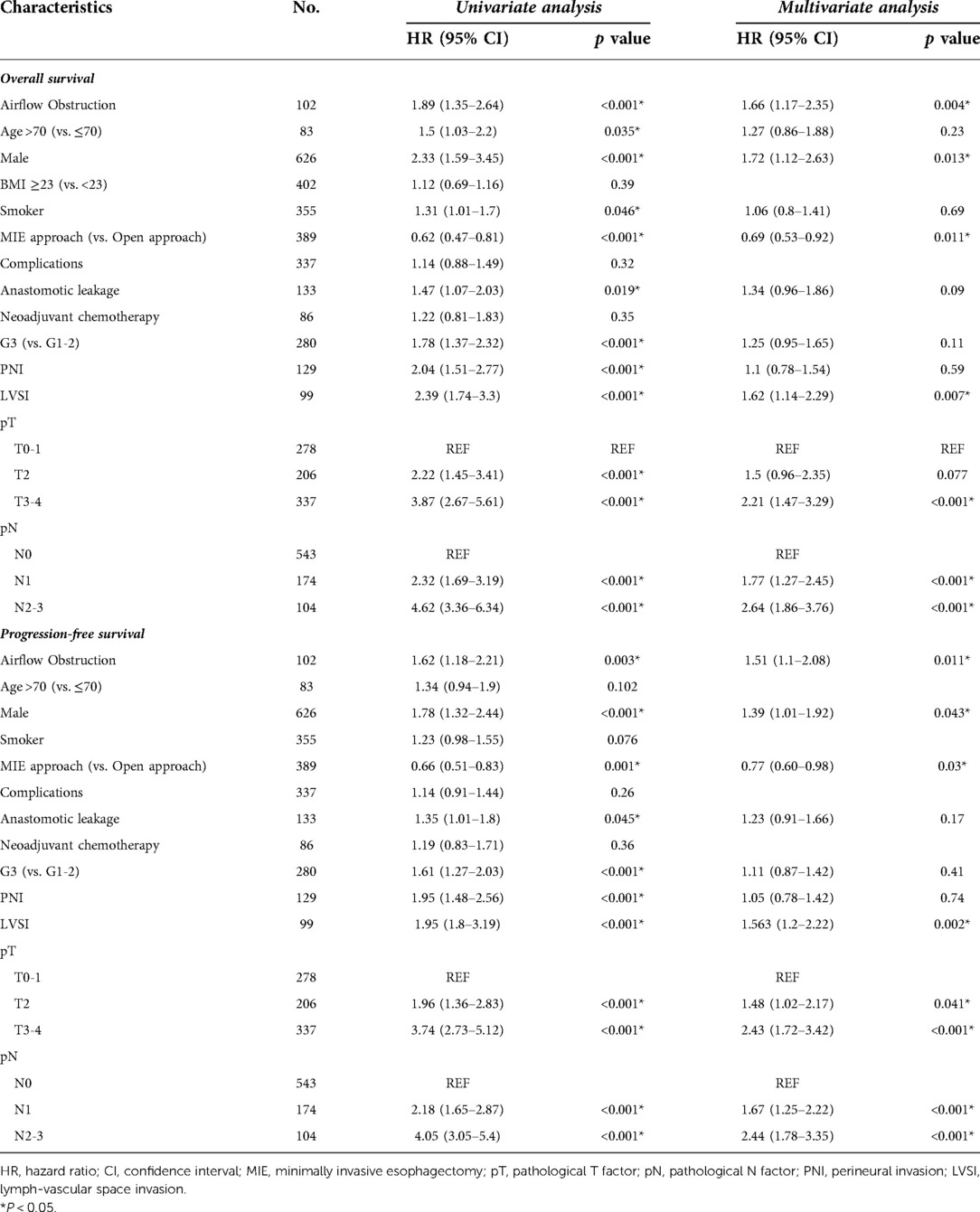
Table 3. Cox proportional hazards regression models for predictors of overall survival (OS) and progression-free survival (PFS).
Subgroup survival analysis
Overall survival stratified by several covariates was analyzed. When patients were male (P = 0.003), with BMI < 23 kg/m2 (P < 0.001), with late-stage cancer (stage III-IVA) (P = 0.002), or undergoing open esophagectomy (P < 0.001), the overall survival was significantly shorter in AFO group compared with non-AFO group. Other covariates showed no differences in survival between the two groups (Figure 3).
Notably, the 3-year survival rate of airflow obstruction with open surgical procedure or stage III-IVA was 44% and 31%, respectively, which were much lower than those in any other subgroups analyzed.
Impact of airflow obstruction with BMI < 23 kg/m2 on survival of esophageal cancer patients
Among all baseline variables, BMI was significantly lower in the obstruction group than in non-airflow obstruction group (22.2 vs. 22.9, P = 0.019). We evaluated the comprehensive impact of airflow obstruction and BMI on survival. A BMI of 23 kg/m2 is used to distinguish whether a patient is overweight. Patients with both BMI < 23 kg/m2 and airflow obstruction showed inferior outcomes (3-year OS: 48%, Figure 3), which was significantly worse than that of patients in the other three groups (all P < 0.05, Figure 4). However, the BMI value was not related to the overall survival of the entire study population (Table 3).
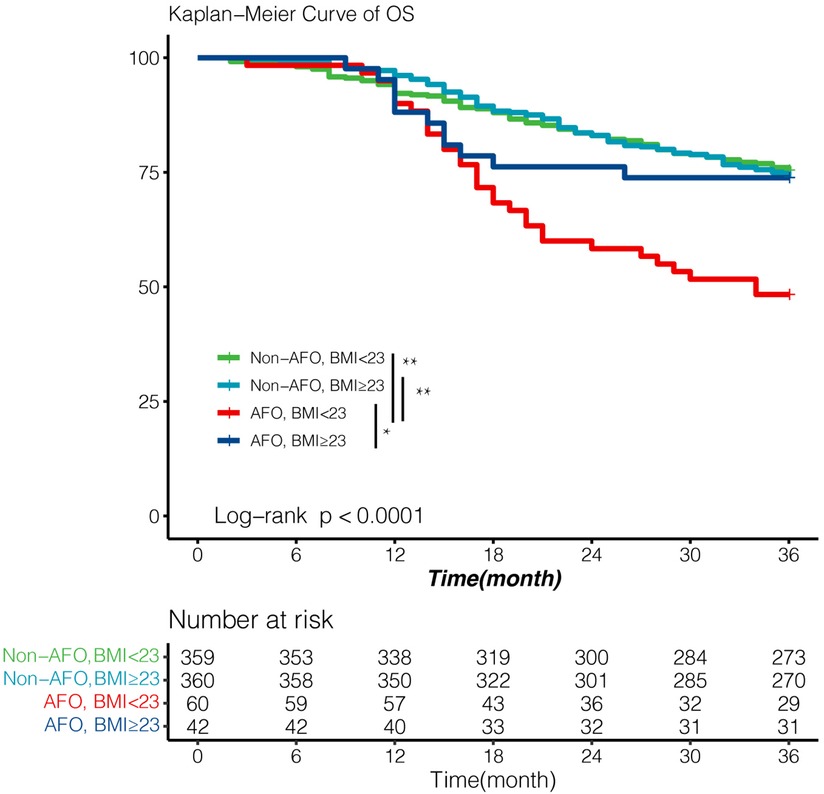
Figure 4. Survival according to airflow obstruction and BMI with cut-off value 23 kg/m2. Patients with both airflow obstruction and BMI < 23 showed significantly poor OS (3-year OS; 48%). Survival outcomes of other three patients were essentially equivalent (3-year OS; 75%, 74.0%, 74% respectively). Survival was analyzed by Kaplan–Meier method using the log-rank test. AFO: airflow obstruction. BMI:body mass index. *p value < 0.05, **p value < 0.01.
To further validate this finding, we performed a univariate Cox regression analysis in AFO group (Supplementary Table S2). Variables with P < 0.05 in the univariate Cox regression analysis were included in the multivariate Cox proportional splines model to reflect the non-linear relation between all-cause mortality and BMI as a continuous variable. Hazard ratios of mortality decreased more as BMI increased (Supplementary Figure S1B) in airflow obstruction patients, compared with that in the whole population (Supplementary Figure S1A).
Airflow obstruction promotes lung metastasis in esophageal cancer patients
It was noteworthy that lung metastasis was associated with airflow obstruction (Table 2, P = 0.01). The evaluation of risk factors for lung metastasis in esophageal cancer patients is shown in Table 4. In multivariate Cox regression analysis, airflow obstruction was associated with a significantly increased probability (Hazard Ratio = 2.22; 95% CI: 1.31–3.78; P = 0.003) of lung metastasis from the primary tumor. The risk for lung metastasis also significantly increased when the pathological N factor was larger than 0 (Hazard Ration = 1.73; 95% CI: 1.07–2.78; P = 0.024).
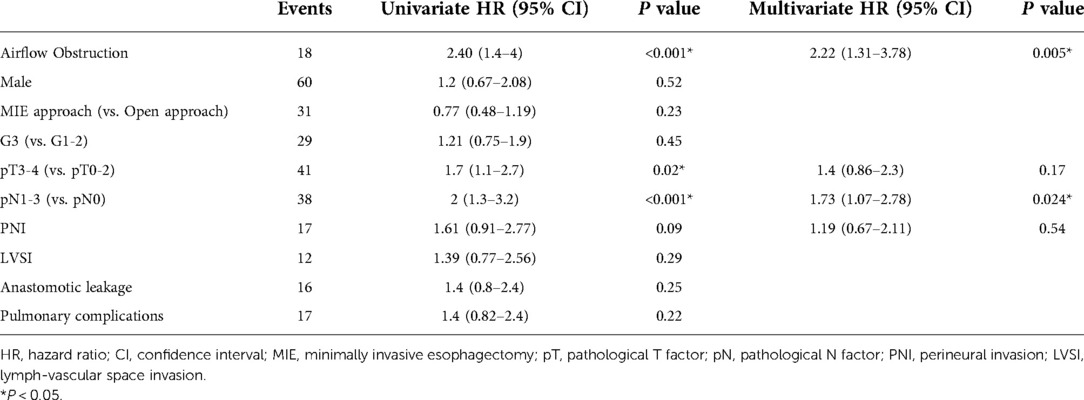
Table 4. Univariate and multivariate Cox proportional hazards regression analysis for the evaluation of risk factors for lung metastasis within 3 years after esophagectomy.
Discussion
This is a single-center-based retrospective cohort study of patients with esophageal cancer. And it is the first study on the prognosis impact of preoperative airflow obstruction defined as FEV1/FVC < LLN for esophageal cancer. Our findings suggest that (i) airflow obstruction was observed in 12.4% of patients receiving esophageal cancer surgery, (ii) preoperative airflow obstruction was an independent prognostic factor for 3-year OS and PFS following trans-thoracic esophagectomy. (iii) preoperative airflow obstruction was an independent risk factor for pulmonary metastasis in esophageal cancer.
The impact of airway obstruction on patients' survival outcomes should not be surprising. Trans-thoracic esophagectomy affects the activity of the chest wall and the lung. Meanwhile, the stomach moves upward and squeezes into the lungs after esophagogastrostomy, resulting in limited pulmonary dilatation and accelerated lung function decline. Patients with chronic airflow obstruction diseases (COPD and asthma, for instance) may be more susceptible to anastomotic leakages and infections, which detrimentally affect survival by delaying recovery or leading to death (21, 22).
Subgroup analysis shed light on the most sensitive population to airflow obstruction. Minimally invasive esophagectomy (MIE) could reduce the response of the organism, accelerate recovery and maintain postoperative pulmonary function (23, 24). Patients with airflow obstruction may particularly benefit from MIE. Moreover, airflow obstruction worsened survival of stage III-IVA esophageal cancer; but showed no difference in patients with stage 0-II cancer. This is probably because late-stage cancer patients have deteriorating disease manifestations and declining quality of life (25, 26). The presence of airflow obstruction worsens the cognitive and overall status at certain levels (27, 28), playing an adjunctive role in the lethal effects of EC. But in the early stages, the follow-up was relatively short, and most of them did not experience the outcome event. The sex difference might be because insufficient female patients led to investigation bias.
A previous study demonstrated that patients with lower BMI had a faster FEV1/FVC decline and more symptoms than patients with higher BMI (29). In line with these prior results (30, 31), patients with airflow obstruction in our study had lower BMI. It is noteworthy that patients with airflow obstruction but BMI ≥23 kg/m2 exhibited as good survival outcomes as the non-airflow obstruction group, which suggested higher BMI could be protective in esophageal cancer patients complicated with airflow obstruction. Therefore, we assume that BMI or overall nutrition status could partly explain our findings on survival outcomes.
Another interesting phenomenon was that airway obstruction facilitated the lung spread of esophageal cancer. This finding echoes the impact of smoking (32) since smoking is highly correlated to airflow obstruction. The “seed-and-soil hypothesis” partially explains this finding (33, 34). Airway obstruction usually coexists with the remodeling of the airway epithelium and alterations of the distribution of inflammatory cells, providing an ideal micro-environment (soil) for tumor cells (seed) colonization and growth (35). Therefore, our findings shed new light on the mechanism of lung metastasis of esophageal cancer.
Unfortunately, in our study, only 25 (25/102, 24.5%) were diagnosed with chronic obstructive airway diseases before the esophagectomy. Almost all patients (95/102, 93.1%) were without sustained lung-directed therapy. Although undiagnosed airflow obstruction subjects appeared healthier than those with a diagnosis, their prognosis was worse than subjects without airflow obstruction15. Our work suggests that preoperative airflow obstruction and potential obstructive airway diseases should be given more attention. Perioperative and long-term airway intervention deserves further investigation to improve survival outcomes.
There are some limitations to our study. First, the median follow-up duration was 54 months in the whole study population, while more extended follow-up periods may provide detailed information on EC prognosis, especially in stage 0-II patients. Secondly, the sample size of patients receiving neoadjuvant therapy was not enough. The interaction between airflow obstruction and neoadjuvant treatment remains to be demonstrated. Finally, 93.5% of patients in our cohort were with esophageal squamous cell carcinoma, whose BMI was generally lower than average (36). It remains unclear whether our conclusions apply to western countries, where adenocarcinoma is the primary pathological type.
Conclusion
Airflow obstruction is a common comorbidity in patients with esophageal cancer. Patients with airflow obstruction had more postoperative complications and shorter 3-year OS and PFS after trans-thoracic surgery for esophageal cancer. BMI or overall nutrition status could partly explain these effects. More attention is needed to manage airflow obstruction in esophageal cancer patients comprehensively. We should incorporate the patient's respiratory condition into the surgical decision-making process to reach a better prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Zhongshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KL: data collection, data analysis, and manuscript writing; XW: data collection, data management. TW: manuscript editing and data management. ZG & YS: data collection; DY & HW: project development, data management, and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by the State's Key Project of Research and Development Plan in China (Project number 2017YFC1310602, 2017YFC1310600); National Natural Science Foundation of China (Project number 81970069, 82170091); Smart Healthcare Project of Zhongshan Hospital, Fudan University (2020ZHZS18); Shanghai Municipal Key Clinical Specialty (shslczdzk02201); Science and Technology Commmission of Shanghai Municipality (20DZ2261200).
Acknowledgment
We greatly appreciate the assistance of the staff of the Department of Thoracic Surgery, Zhongshan Hospital, Fudan University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.966340/full#supplementary-material.
Abbreviations
EC, esophageal cancer; AFO, airflow obstruction; LLN, lower limit of normal; FEV1, forced expiration volume in the first second; FVC, forced vital capacity; OS, overall survival; PFS, progression-free survival; G, grade of tumor differentiation; pT, pathological T factor; pN, pathological N factor; BMI, body mass index; MIE, minimally invasive esophagectomy.
References
1. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. (2017) 390:2383–96. doi: 10.1016/S0140-6736(17)31462-9
2. Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, et al. Global burden, risk factors, and trends of esophageal cancer: an analysis of cancer registries from 48 countries. Cancers (Basel). (2021) 13:141. doi: 10.3390/cancers13010141
3. Klevebro F, Elliott JA, Slaman A, Vermeulen BD, Kamiya S, Rosman C, et al. Cardiorespiratory comorbidity and postoperative complications following esophagectomy: a European multicenter cohort study. Ann Surg Oncol. (2019) 26:2864–73. doi: 10.1245/s10434-019-07478-6
4. Weksler B, Sullivan JL. Survival after esophagectomy: a propensity-matched study of different surgical approaches. Ann Thorac Surg. (2017) 104:1138–46. doi: 10.1016/j.athoracsur.2017.04.065
5. Rice TW, Patil DT, Blackstone EH. 8th Edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. (2017) 6:119–30. doi: 10.21037/acs.2017.03.14
6. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of locally advanced esophageal carcinoma: aSCO guideline. J Clin Oncol. (2020) 38:2677–94. doi: 10.1200/JCO.20.00866
7. Yoshida N, Harada K, Iwatsuki M, Baba Y, Baba H. Precautions for avoiding pulmonary morbidity after esophagectomy. Ann Gastroenterol Surg. (2020) 4:480–4. doi: 10.1002/ags3.12354
8. Ho CH, Chen YC, Wang JJ, Liao KM. Incidence and relative risk for developing cancer among patients with COPD: a nationwide cohort study in Taiwan. BMJ Open. (2017) 7:e013195. doi: 10.1136/bmjopen-2016-013195
9. Molena D, Mungo B, Stem M, Lidor AO. Incidence and risk factors for respiratory complications in patients undergoing esophagectomy for malignancy:a NSQIP analysis. Semin Thorac Cardiovasc Surg. (2014) 26:287–94. doi: 10.1053/j.semtcvs.2014.12.002
10. Wei R, Dong W, Shen H, Ni Y, Zhang T, Wang Y, et al. Predictive effects of lung function test on postoperative pneumonia in squamous esophageal cancer. Sci Rep. (2016) 6:23636. doi: 10.1038/srep23636
11. Ohi M, Toiyama Y, Omura Y, Ichikawa T, Yasuda H, Okugawa Y, et al. Risk factors and measures of pulmonary complications after thoracoscopic esophagectomy for esophageal cancer. Surg Today. (2019) 49:176–86. doi: 10.1007/s00595-018-1721-0
12. Sugawara K, Mori K, Okumura Y, Yagi K, Aikou S, Uemura Y, et al. Preoperative low vital capacity influences survival after esophagectomy for patients with esophageal carcinoma. World J Surg. (2020) 44:2305–13. doi: 10.1007/s00268-020-05450-0
13. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. (2005) 26:948–68. doi: 10.1183/09031936.05.00035205
14. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2021) 203:24–36. doi: 10.1164/rccm.202009-3533SO
15. Quanjer PH, Enright PL, Miller MR, Stocks J, Ruppel G, Swanney MP, et al. The need to change the method for defining mild airway obstruction. Eur Respir J. (2011) 37:720–2. doi: 10.1183/09031936.00135110
16. Jian W, Gao Y, Hao C, Wang N, Ai T, Liu C, et al. Reference values for spirometry in Chinese aged 4-80 years. J Thorac Dis. (2017) 9:4538–49. doi: 10.21037/jtd.2017.10.110
17. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, et al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg. (2015) 262:286–94. doi: 10.1097/SLA.0000000000001098
18. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. (1996) 17:343–6. doi: 10.1016/0197-2456(96)00075-X
19. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. doi: 10.1002/sim.4780080504
20. Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in cox models for exposure-response relationships. Stat Med. (2007) 26:3735–52. doi: 10.1002/sim.2848
21. Andreou A, Biebl M, Dadras M, Struecker B, Sauer IM, Thuss-Patience PC, et al. Anastomotic leak predicts diminished long-term survival after resection for gastric and esophageal cancer. Surgery. (2016) 160:191–203. doi: 10.1016/j.surg.2016.02.020
22. Saunders JH, Yanni F, Dorrington MS, Bowman CR, Vohra RS, Parsons SL, et al. Impact of postoperative complications on disease recurrence and long-term survival following oesophagogastric cancer resection. Br J Surg. (2020) 107:103–12. doi: 10.1002/bjs.11318
23. Otani T, Ichikawa H, Hanyu T, Ishikawa T, Kano Y, Kanda T, et al. Long-Term trends in respiratory function after esophagectomy for esophageal cancer. J Surg Res. (2020) 245:168–78. doi: 10.1016/j.jss.2019.07.040
24. Briez N, Piessen G, Torres F, Lebuffe G, Triboulet JP, Mariette C. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg. (2012) 99:1547–53. doi: 10.1002/bjs.8931
25. Bracken-Clarke D, Farooq AR, Horgan AM. Management of locally advanced and metastatic esophageal cancer in the older population. Curr Oncol Rep. (2018) 20:99. doi: 10.1007/s11912-018-0745-3
26. Lagergren P, Avery KN, Hughes R, Barham CP, Alderson D, Falk SJ, et al. Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer (2007) 110:686–93. doi: 10.1002/cncr.22833
27. Odeyemi YE, Meda E, Ogundipe F, Russ E, Mehari A, Obisesan T, et al. Airflow obstruction, cognitive function and mortality in a US national cohort: nHANES-III. Clin Respir J. (2018) 12:1141–9. doi: 10.1111/crj.12643
28. Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the United States. Thorax. (2011) 66:49–54. doi: 10.1136/thx.2010.147041
29. Luoto J, Pihlsgård M, Wollmer P, Elmståhl S. Relative and absolute lung function change in a general population aged 60–102 years. Eur Respir J. (2019) 53:1701812. doi: 10.1183/13993003.01812-2017
30. Burney P, Patel J, Minelli C, Gnatiuc L, Amaral AFS, Kocabaş A, et al. Prevalence and population attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med. (2020) 203:1353–65. doi: 10.1164/rccm.202005-1990OC
31. Graff S, Bricmont N, Moermans C, Henket M, Paulus V, Guissard F, et al. Clinical and biological factors associated with irreversible airway obstruction in adult asthma. Respir Med. (2020) 175:106202. doi: 10.1016/j.rmed.2020.106202
32. Abrams JA, Lee PC, Port JL, Altorki NK, Neugut AI. Cigarette smoking and risk of lung metastasis from esophageal cancer. Cancer Epidemiol Biomarkers Prev. (2008) 17:2707–13. doi: 10.1158/1055-9965.EPI-08-0232
33. Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. (1989) 8:98–101. doi: 10.1016/s0140-6736(00)49915-0
34. Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. (2003) 3:453–8. doi: 10.1038/nrc1098
35. Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. (2013) 13:233–45. doi: 10.1038/nrc3477
Keywords: esophageal cancer, survival, airflow obstruction, lung function, decision-making
Citation: Lang K, Wang X, Wei T, Gu Z, Song Y, Yang D and Wang H (2023) Concomitant preoperative airflow obstruction confers worse prognosis after trans-thoracic surgery for esophageal cancer. Front. Surg. 9:966340. doi: 10.3389/fsurg.2022.966340
Received: 10 June 2022; Accepted: 28 October 2022;
Published: 16 January 2023.
Edited by:
Fernando A. M. Herbella, Federal University of São Paulo, BrazilReviewed by:
Mohamed Rahouma, Weill Cornell Medical Center, NewYork-Presbyterian, United StatesMehmet Ali Bedirhan, Yedikule Teaching Hospital, Turkey
© 2023 Lang, Wang, Wei, Gu, Song, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang d2FuZy5oYW9AenMtaG9zcGl0YWwuc2guY24= Dong Yang eWFuZy5kb25nQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Ke Lang
Ke Lang Xiaocen Wang1,†
Xiaocen Wang1,† Dong Yang
Dong Yang Hao Wang
Hao Wang