- 1Department of Gynecologic Oncology, the First Hospital of Jilin University, Changchun, China
- 2Department of Cardiac Surgery, the First Hospital of Jilin University, Changchun, China
Aim: To establish prediction models for 2-year overall survival of ovarian cancer patients with metastasis.
Methods: In total, 4,929 participants from Surveillance, Epidemiology, and End Results (SEER) database were randomly divided into the training set (n = 3,451) and the testing set (n = 1,478). Univariate and multivariable regression were conducted in the training set to identify predictors for 2-year overall survival of metastatic ovarian cancer patients. The C-index was calculated for assessing the performance of the models. The nomogram for the model was plotted. The prediction value of the model was validated in the testing set. Subgroup analysis were performed concerning surgery and chemotherapy status of patients and the metastatic site of ovarian cancer in the testing set. The calibration curves were plotted and the decision curve analysis (DCA) were conducted.
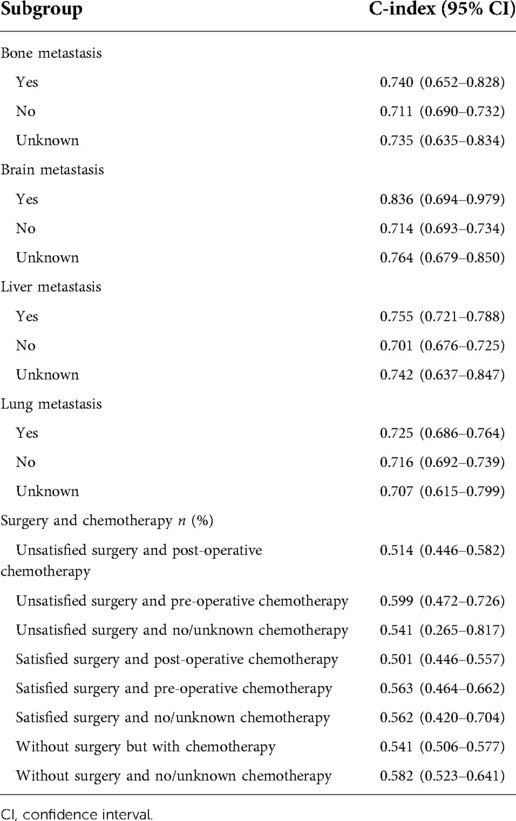
Results: At the end of follow-up, 2,587 patients were survived and 2,342 patients were dead within 2 years. The 2-year survival rate was 52.5%. The prediction models were constructed based on predictors including age, radiation, surgery and chemotherapy, CA125, and bone, liver, and lung metastasis. The prediction model for 2-year overall survival of ovarian cancer patients with metastasis showed good predictive ability with the C-index of the model of 0.719 (95% CI: 0.706–0.731) in the training set and 0.718 (95% CI: 0.698–0.737) in the testing set. In terms of patients with bone metastasis, the C-index was 0.740 (95% CI: 0.652–0.828) for predicting the 2-year overall survival of ovarian cancer patients. The C-index was 0.836 (95% CI: 0.694–0.979) in patients with brain metastasis, 0.755 (95% CI: 0.721–0.788) in patients with liver metastasis and 0.725 (95% CI: 0.686–0.764) in those with lung metastasis for predicting the 2-year overall survival of ovarian cancer patients.
Conclusion: The models showed good predictive performance for 2-year overall survival of metastatic ovarian cancer patients.
Introduction
Ovarian cancer is a prevalent and deadly malignant cancer in females that is often diagnosed at an advanced stage with extensive metastasis (1). Based on the data of Globocan, there were 295,414 newly diagnosed ovarian cancer patients and 184,799 deaths registered in 2018 (2). A previous study has reported that in the United States, there was about 21,750 newly diagnosed ovarian cancer patients and 13,940 deaths caused by ovarian cancer in 2020 (1). There was 55,342 newly diagnosed ovarian cancer patients in China in 2020, and the incidence was about 2.6% (3). Another study found that about 490,142 deaths due to ovarian was reported among 19,296,319,576 person-years at risk for women in China from 1990 to 2019 (4). Ovarian cancer is often silently spilled and patients were mainly diagnosed as an advanced disease, which becomes one of the most lethal gynecological malignancy (5). Approximately 70% of ovarian cancer patients were diagnosed with synchronous distant metastases due to the limited early, specific symptoms and effective screening strategies (6). Liver was identified to be the most common distant metastatic organ of ovarian cancer in stage IV which accounted for 37%–57%, followed by distant lymph nodes, lung, bone and brain (7, 8). Metastases is a major cause of mortality in ovarian cancer patients, and the 5-year survival rate was only 29% among women diagnosed with distant-metastatic ovarian cancer (9). Despite the continuous improvement of chemotherapy drugs and advancement of surgical techniques, the 5-year survival rate of ovarian cancer patients is still not more than 50% (10). The 2-year recurrence free survival of ovarian cancer patients was about 48%, less than 50% (11). To identify factors associated with the mortality of patients with metastatic ovarian cancer is essential for improving the prognosis of these patients.
The diagnosis at stage IV at the beginning of treatment sorrowfully decreases the confidence of doctors, patients and their families (12). This may affect the selection of treatments in stage IV ovarian cancer patients, especially in less developed regions. Many patients give up treatment in despair, some patients only choose short-term chemotherapy, and some patients choose standard surgery and chemotherapy, and the prognoses were not the same (13–15). The poor prognosis of metastatic ovarian cancer patients may due to the differences of patients’ metastatic sites and conditions (7). On the other hand, it depends on the decision-making of patients and their families for choosing what kind of treatments. At this point, it is particularly important for patients to make decisions about which treatment can achieve the maximum benefit (16). So the prediction model for the prognosis of metastatic ovarian cancer patients is necessary for both the clinicians and patients choosing the best treatment despite their stages.
In previous studies, various prediction models were established to evaluate the survival of ovarian cancer patients. Zhao et al. constructed a prediction model and plotted a nomogram for predicting the cancer-specific survival of stage II–IV epithelial ovarian cancer patients receiving surgery and chemotherapy (17). Inci et al. conducted a prospective study and established a prediction model for the surgical outcome in ovarian cancer based on frailty index (18). Wang et al. built a prediction model for the survival outcome among epithelial ovarian cancer patients with site-distant metastases (19). Up to date, the prediction model for the survival of all ovarian cancer population such as non-epithelial ovarian cancer patients with metastasis was still lacking (20).
In this study, based on the data from Surveillance, Epidemiology, and End Results (SEER) database, we planned to establish prediction models to predict the 2-year overall survival of metastatic ovarian cancer patients for identifying those who at high possibility to survive within 2 years. Subgroup analysis was conducted concerning the surgery status of patients and the metastatic site of ovarian cancer.
Methods
Study design and population
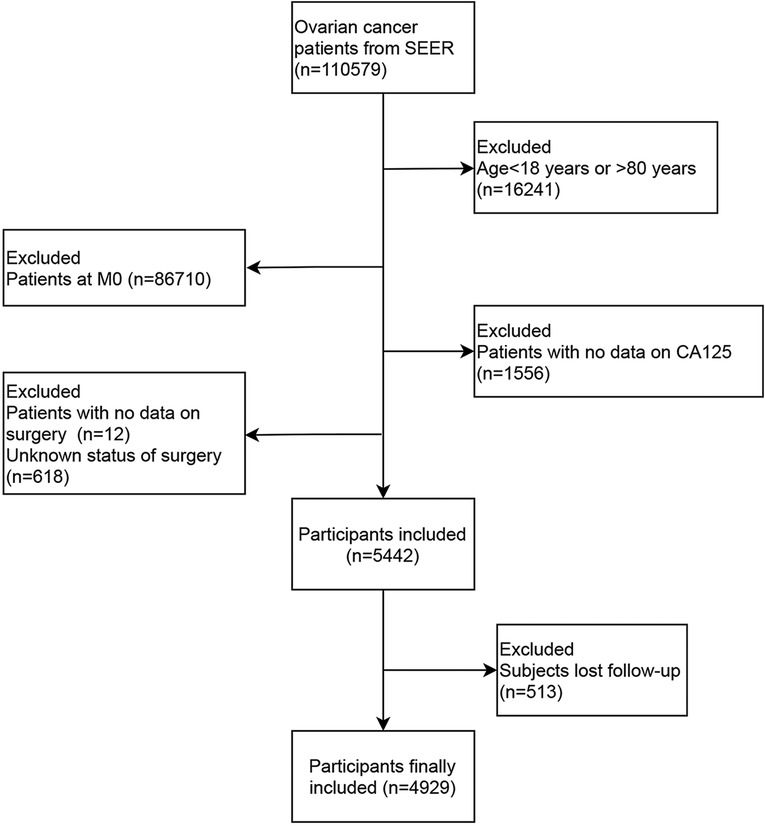
The current study was a cohort study collecting the data of 110,579 ovarian cancer patients from SEER database between 2010 and 2015. SEER database (seer.cancer.gov) is a free access database collecting demographics, tumor characteristics, nodal staging, surgery information, vital status, and follow-up information of patients from 18 cancer registries, which covers about 27.8% of the U.S. population (21). Ovarian cancer in patients was defined by the Site recode ICD-0-3/WHO 2008 (International Classification of Diseases for Oncology, 3rd edition). Patients who were diagnosed with ovarian cancer at the age <18 years old or >80 years old were excluded. Patients without metastasis (M0 stage) were not analyzed. Those who lost the data on surgery, cancer antigen 125 (CA125) and follow-up were excluded. Subjects who were unclear about whether they received surgery were also excluded. Finally, 4,929 subjects were involved and followed up in our study. At the end of the follow-up, 2,587 patients were survived and 2,342 patients were dead.
Potential prognostic predictors
The potential predictors for the 2-year survival of metastatic ovarian cancer patients were collected including age at diagnosis (years), race (American, Asian, Black, White or unknown), marital status (married, separated, divorced, single, widowed, unknown or unmarried), tumor size (0–1, 1–5 or 5–10 cm), Grade (I, II, III, IV or unknown), AJCC T stage (T0, T1, T2, T3 or unknown), AJCC N stage (N0, N1 or unknown), laterality (one side or paired site), receiving radiation treatment (none/unknown, refused or yes), receiving chemotherapy treatment (no/unknown or yes), surgery (satisfied, unsatisfied or without surgery), receiving surgery and chemotherapy (unsatisfied surgery and post-operative chemotherapy, unsatisfied surgery and pre-operative chemotherapy, unsatisfied surgery and no/unknown chemotherapy, satisfied surgery and post-operative chemotherapy, satisfied surgery and pre-operative chemotherapy, satisfied surgery and no/unknown chemotherapy, without surgery but with chemotherapy or without surgery and no/unknown chemotherapy), CA125 positive (>35 U/ml) or not, bone, brain, liver, and lung metastasis status (no, yes or unknown).
Outcome variables
The outcome in this study was the overall survival of patients with metastatic ovarian cancer within 2 years. The follow-up time was 2 years and ended in 2015.
Statistical analysis
The measurement data of normal distribution were shown as mean ± standard deviation (mean ± SD), and the independent sample t test was used for comparison between groups. The non-normal data were exhibited as the median quartile [M (Q1, Q3)], and differences between groups were compared by the Mann-Whitney U rank sum test. Enumeration data were described as n (%). Comparison between groups was conducted by chi-square test or Fisher's exact probability method. Statistical analyses were subjected to two-sided test, and the test level was set as α = 0.05. All data were randomly sampled. After setting the random seed, 70% of the randomly sampled data were grouped into the training set (n = 3,451) and the remaining 30% were used as the testing set (n = 1,478). The Kaplan-Meier method was employed for estimating 2-year survival of metastatic ovarian cancer patients in the training set. Then the equilibrium between the training set and testing set was assessed. This study was a cohort study and involved follow-up, Cox proportional hazards model was used for analysis. Univariate and multivariable Cox proportional hazards analyses were conducted in the training set to identify predictors for 2-year overall survival of metastatic ovarian cancer patients. The C-index was calculated for assessing the performance of the models. The nomogram of the model for quickly evaluating the overall survival rate of patients was plotted. The area under the curve (AUC) values of the models for predicting the survival of metastatic ovarian cancer patients at different time were measured. The calibration curves and the decision curve analysis (DCA) were plotted in the training set to evaluate the clinical applicability of the nomogram. Subgroup analysis were performed concerning surgery status of patients and the metastatic site of ovarian cancer in the testing set. SAS 9.4 was applied for data analysis and R 4.0.3 was utilized to establish the prediction model. P < 0.05 was regarded as statistical difference.
Results
The baseline characteristics of subjects
In the present study, the data of 110,579 ovarian cancer patients from SEER database between 2010 and 2015 were collected. After excluding patients who were diagnosed with ovarian cancer at the age <18 years old or >80 years old (n = 16,241), patients at M0 stage (n = 86,710), patients who lost the data on surgery (n = 12), CA125 (n = 1,556) or who were unclear about whether they received surgery or not (n = 681), 5,442 patients were included. Among them, 513 patients lost follow-up, and 4,929 patients were finally analyzed. The screen process was presented in Figure 1.
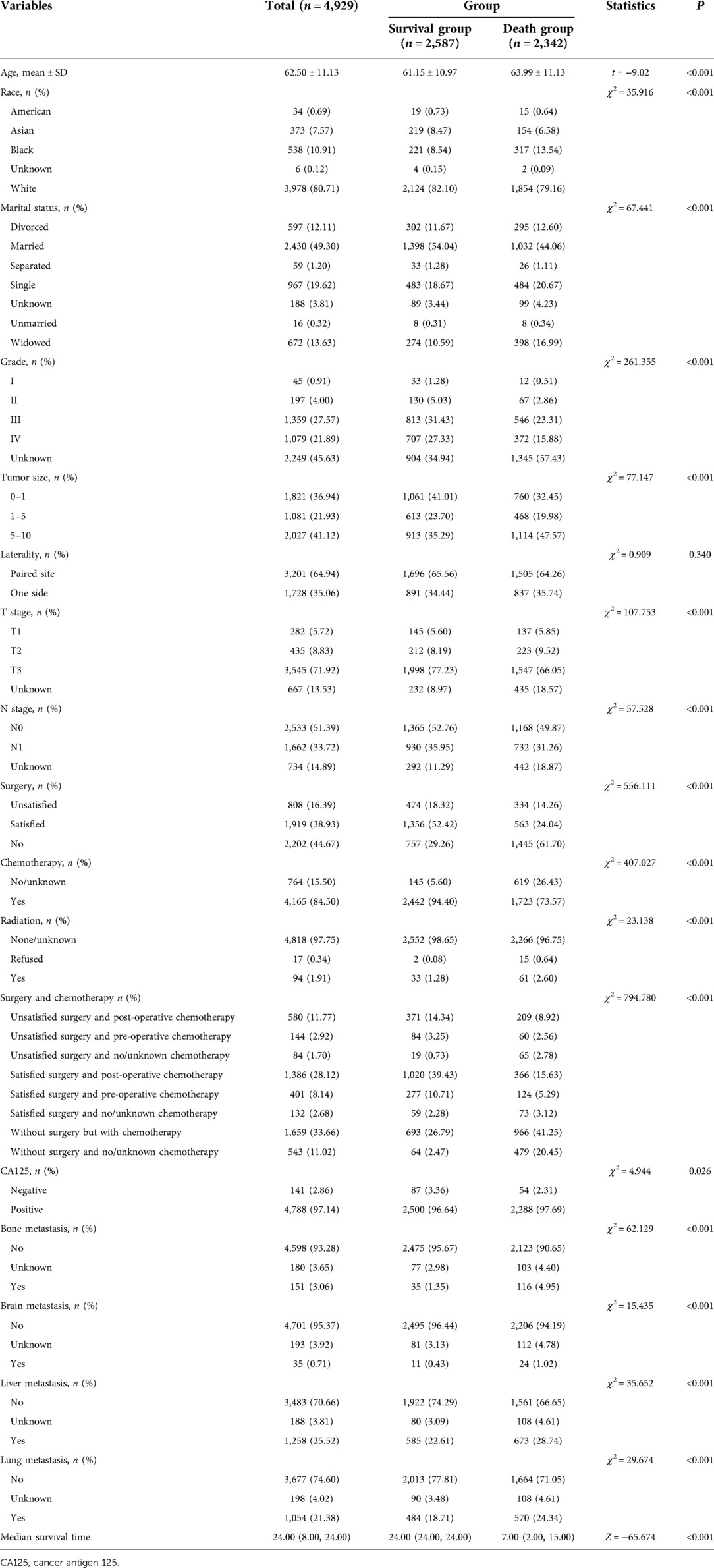
Among all participants, the average age was 62.50 years. 1,919 patients had satisfied results of the surgery, accounting for 38.93%, and 808 patients had unsatisfied results of surgery, accounting for 16.39%. 4,788 patients had positive CA125, which accounted for 97.14%. 151 patients suffered from bone metastasis, accounting for 3.06%, 35 patients had brain metastasis, accounting for 0.71%, 1,258 patients had liver metastasis, accounting for 25.52% and 1,054 patients had lung metastasis, accounting for 21.38%. The median follow-up time was 24 months, and 2,587 patients were survived and 2,342 patients were dead within 2 years. The 2-year survival rate was 52.5% (Table 1).
Equilibrium analysis of the data in the training set and the testing set
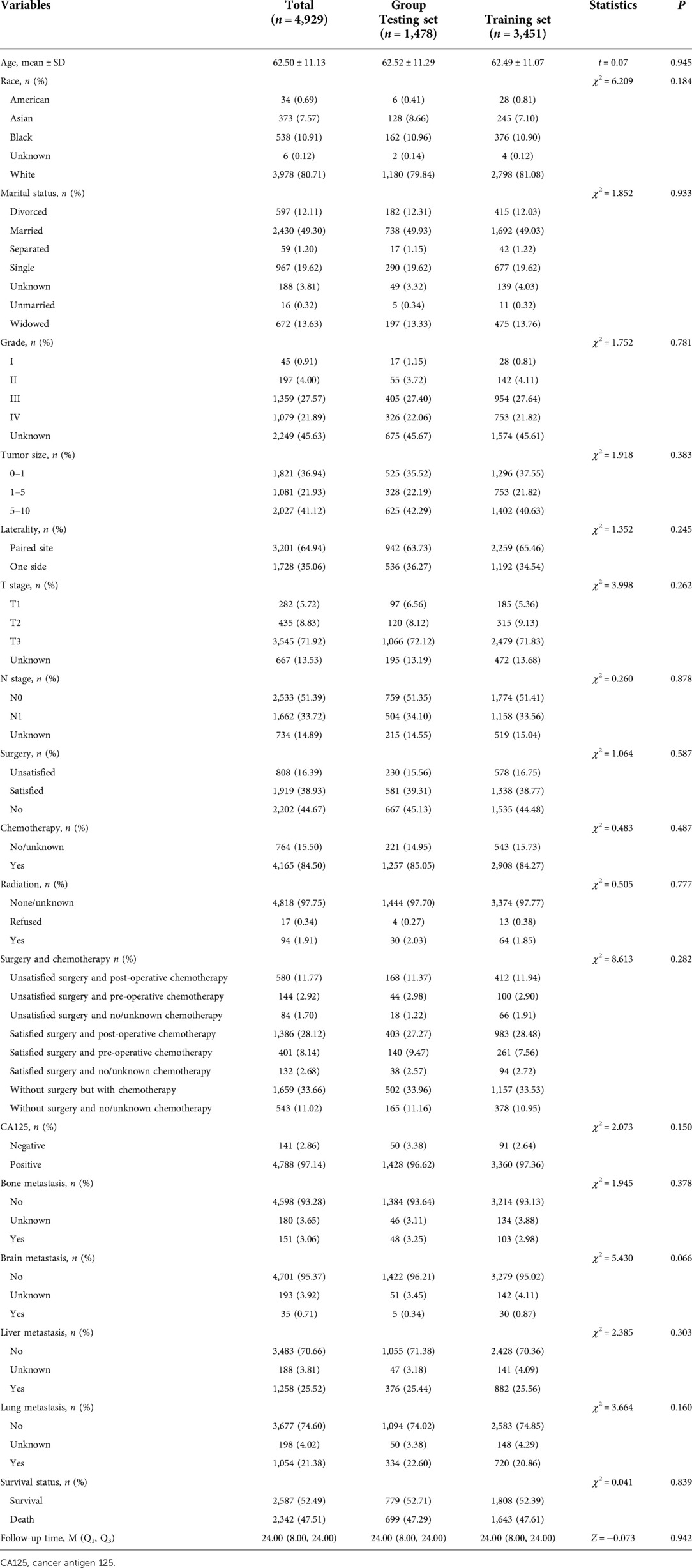
As shown in Table 2, after the random division of the training set and the testing set, the results of equilibrium analysis revealed that there was no statistical difference in the demographic and clinical characteristics of patients with metastatic ovarian cancer between the training set and the testing set (all P > 0.05). The survival curve of patients in the training set was shown in Figure 2.
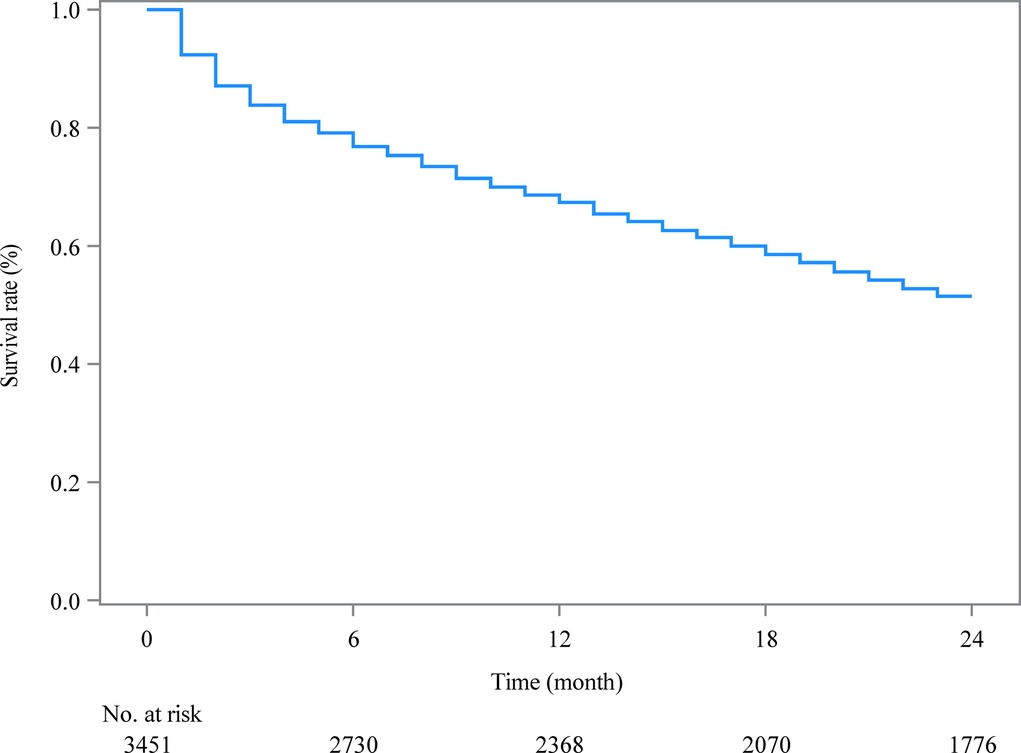
Figure 2. The Kaplan-Meier curve estimating 2-year survival of metastatic ovarian cancer patients in the training set.
Predictors for 2-year overall survival of metastatic ovarian cancer patients
According to the data in Table 3, age, race, marital status, grade, tumor size, T stage, N stage, surgery, chemotherapy, radiation therapy, receiving radiation treatment, surgery and chemotherapy treatments, CA125, bone metastasis, brain metastasis, liver metastasis, and lung metastasis were potential predictors for 2-year overall survival of patients with metastatic ovarian cancer.
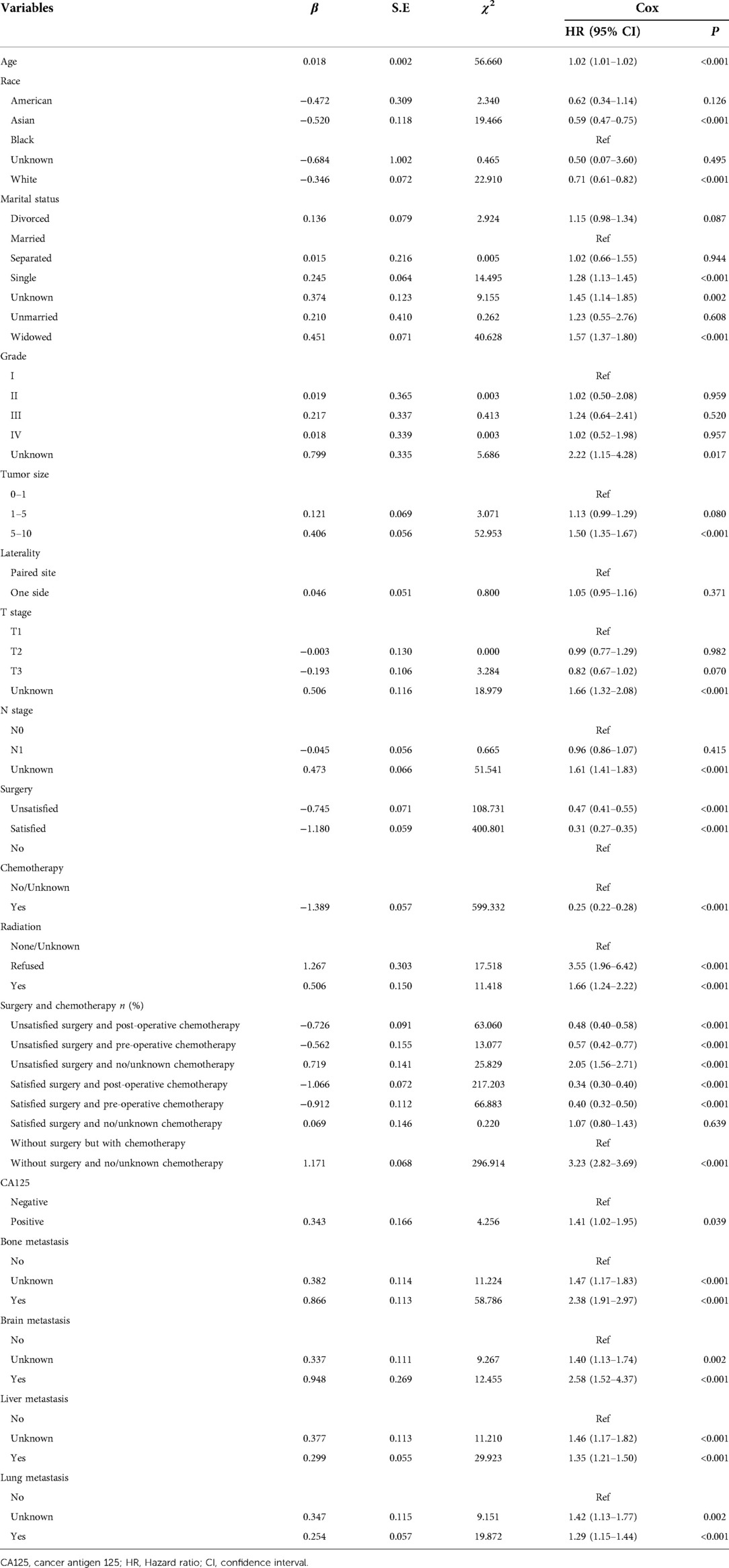
Table 3. Univariate analysis for screening the predictors for 2-year overall survival of metastatic ovarian cancer patients.
The results of multivariate cox regression delineated that older ager was associated with higher risk of 2-year overall mortality (HR = 1.01, 95% CI: 1.01–1.01) among metastatic ovarian cancer patients. Patients with unsatisfied surgery and post-operative chemotherapy were linked with decreased risk of 2-year overall mortality (HR = 0.51, 95% CI: 0.43–0.61). Patients received unsatisfied surgery and pre-operative chemotherapy were correlated with decreased risk of 2-year overall mortality (HR = 0.58, 95% CI: 0.43–0.79). The 2.30-fold risk of 2-year overall mortality (HR = 2.30, 95% CI: 1.74–3.04) was observed in patients received unsatisfied surgery and no/unknown chemotherapy. Patients with satisfied surgery and post-operative chemotherapy (HR = 0.37, 95% CI: 0.32–0.42) as well as satisfied surgery and pre-operative chemotherapy (HR = 0.40, 95% CI: 0.32–0.49) were linked with decreased risk of 2-year overall mortality. For those without surgery and no/unknown chemotherapy, the risk of 2-year overall mortality (HR = 3.28, 95% CI: 2.87–3.76) was elevated. The risk of 2-year overall mortality (HR = 1.87, 95% CI: 1.35–2.60) was found in patients with positive CA125. Those with bone (HR = 1.56, 95% CI: 1.25–1.96), liver (HR = 1.34, 95% CI: 1.20–1.49) or lung metastasis (HR = 1.22, 95% CI: 1.09–1.37) were associated with elevated risk of overall mortality (Table 4).

Table 4. Multivariable analysis for screening the predictors for 2-year overall survival of metastatic ovarian cancer patients.
Construction and validation of the prediction model for 2-year overall survival of metastatic ovarian cancer patients
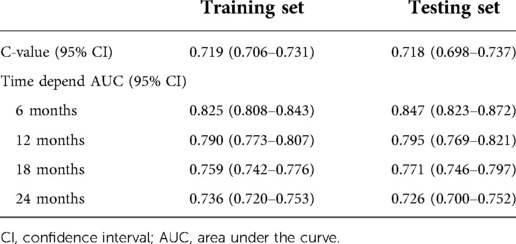
The prediction model was established based on the predictors including age, radiation therapy, surgery and chemotherapy, CA125, bone metastasis, brain metastasis, liver metastasis, and lung metastasis. The C-index of the model for predicting the 2-year overall survival of metastatic ovarian cancer patients was 0.719 (95% CI: 0.706–0.731) in the training set and 0.718 (95% CI: 0.698–0.737) in the testing set (Table 5). The AUCs of the model for predicting the overall survival of metastatic ovarian cancer patients was 0.825 (95% CI: 0.808–0.843) at 6-month, 0.790 (95% CI: 0.773–0.807) at 1-year, 0.759 (95% CI: 0.742–0.776) at 18-month and 0.736 (95% CI: 0.720–0.753) at 2-year in the training set (Figure 3). In the testing set, the AUCs was 0.847 (95% CI: 0.823–0.872) for predicting 6-month overall survival, 0.795 (95% CI: 0.769–0.821) for predicting 1-year overall survival, 0.771 (95% CI: 0.746–0.797) for predicting 18-month survival and 0.726 (95% CI: 0.700–0.752) for predicting 2-year overall survival (Figure 4). The calibration curves of the prediction model for predicting the 6-month mortality (Figure 5A), 1-year mortality (Figure 5B), 18-month mortality (Figure 5C) and 2-year mortality (Figure 5D) of metastatic ovarian cancer patients were shown, which depicted that the prediction values of the model in the training set deviated slightly from the perfected model, but was close to matching, indicating the prediction model had good agreement between the predictive probability and the actual probability. The nomogram for the model was presented in Figure 6. The DCA curve revealed that the use of the nomogram to predict the 2-year overall survival of ovarian cancer patients increased the net benefit than use no model, suggesting that the model might help the clinicians quickly identify those at high risk of 2-year mortality (Supplementary Figure S1).
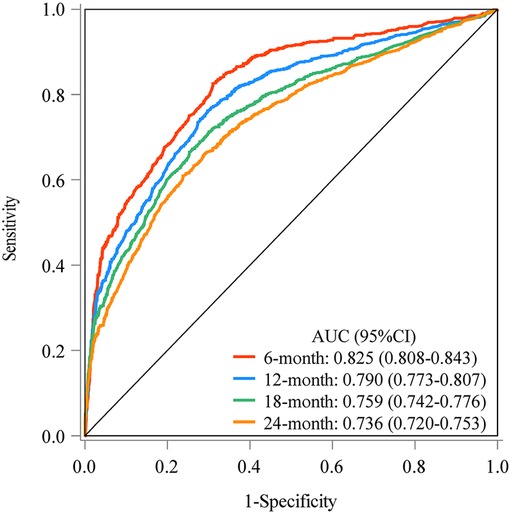
Figure 3. The AUCs of the model for predicting the overall survival of metastatic ovarian cancer patients at different time points in the training set.
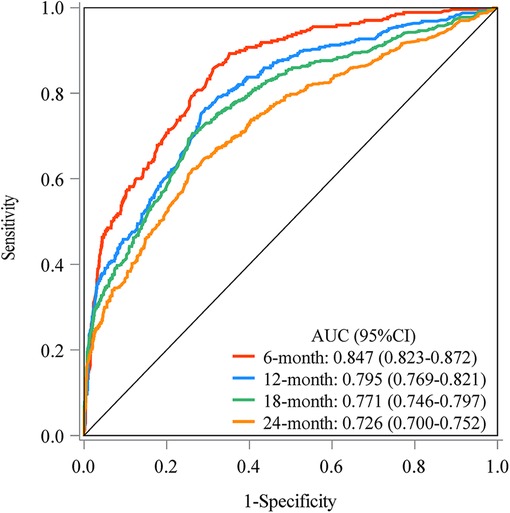
Figure 4. The AUCs of the model for predicting the overall survival of metastatic ovarian cancer patients at different time points in the testing set.
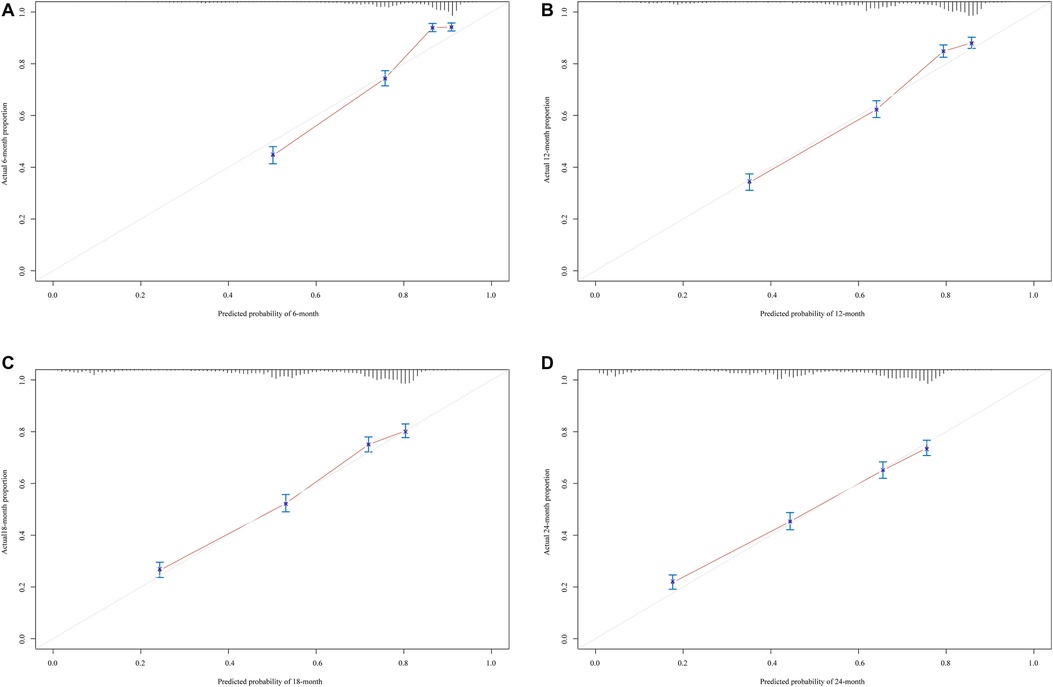
Figure 5. The calibration curves of the prediction model for predicting the 6-month mortality (A), 1-year mortality (B) and 18-month mortality (C) and 2-year mortality (D) of metastatic ovarian cancer patients.
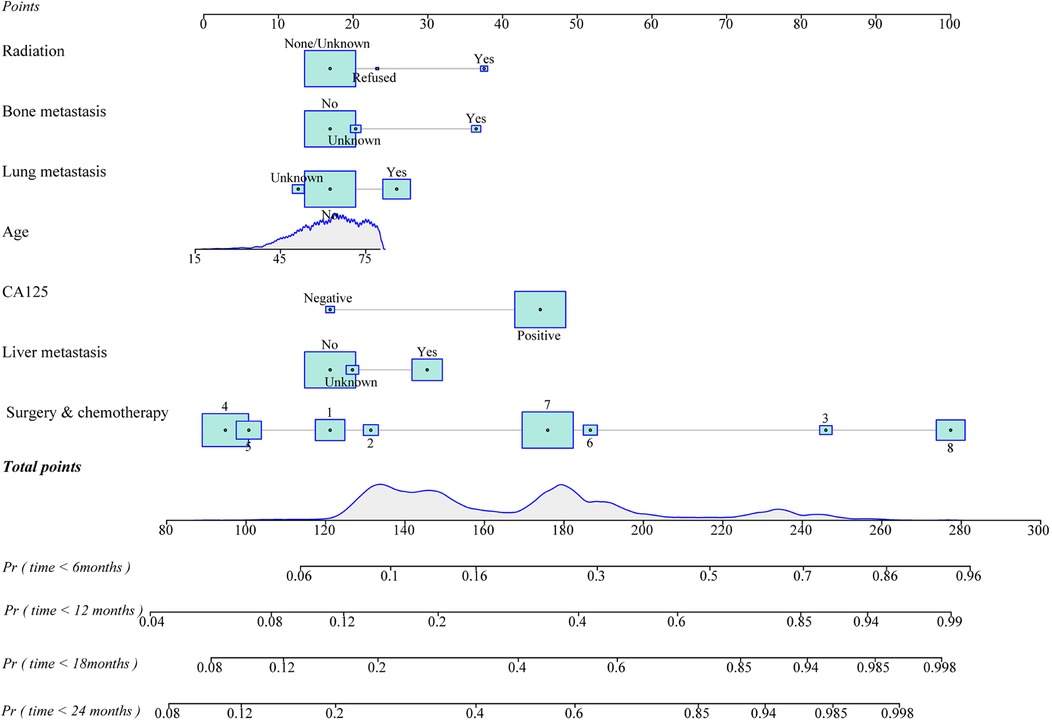
Figure 6. The nomogram of the prediction model for overall survival of metastatic ovarian cancer patients at different time points (1. unsatisfied surgery and post-operative chemotherapy, 2. unsatisfied surgery and pre-operative chemotherapy, 3. unsatisfied surgery and no/unknown chemotherapy, 4. satisfied surgery and post-operative chemotherapy, 5. satisfied surgery and pre-operative chemotherapy, 6. satisfied surgery and no/unknown chemotherapy, 7. without surgery but with chemotherapy or without surgery and 8. no/unknown chemotherapy).
Subgroup analysis of the predictive value of the model for the 2-year survival of metastatic ovarian cancer patients
In terms of ovarian cancer patients with bone metastasis, the C-index of the model for predicting the 2-year survival was 0.740 (95% CI: 0.652–0.828). The C-index of the model for predicting the 2-year survival was 0.836 (95% CI: 0.694–0.979) in ovarian cancer patients with brain metastasis. With regard to the status of liver metastasis, the C-index of the model for predicting the 2-year survival was 0.755 (95% CI: 0.721–0.788) for those with liver metastasis. The C-index of the model for the prediction of 2-year survival of metastatic ovarian cancer patients with lung metastasis was 0.725 (95% CI: 0.686–0.764) (Table 6). For patients with satisfied surgery and post-operative chemotherapy, the C-index of the model for predicting the 2-year overall survival of metastatic ovarian cancer patients was 0.501 (95% CI: 0.446–0.557). The C-index was 0.563 (95% CI: 0.464–0.662) for those with satisfied surgery and pre-operative chemotherapy.
Discussion
In this study, the data of 4,929 ovarian cancer patients with metastasis were extracted from SEER database to establish a prediction model for the 2-year survival of those patients based on the screened predictors. The nomogram delineated good predictive value for the 2-year overall survival, with the AUC of 0.726 for 2-year survival in the testing set. Subgroup analysis revealed that the model had good predictive value for patients with bone, brain, liver or lung metastasis. The findings of our study might help identify metastatic ovarian cancer patients with high possibility of survival and provide confidence for the clinicians and patients to receiving proper treatment. For those who predicted with risk of death, timely interventions should be applied for improving their prognosis.
The present study constructed prediction models for 2-year mortality of metastatic ovarian cancer patients based on the predictors including age, radiation therapy, surgery and chemotherapy, CA125, bone metastasis, brain metastasis, liver metastasis, and lung metastasis. Currently, several models were established for predicting the cancer-specific survival of stage II–IV epithelial ovarian cancer patients receiving surgery and chemotherapy (17) or for the surgical outcome in ovarian cancer (18) as well as for the survival outcome in epithelial ovarian cancer patients with site-distant metastases (19). There was still no prediction model for the survival of all ovarian cancer population with different types of metastasis. This study constructed a prediction model for 2-year overall survival of ovarian cancer patients with different kinds of metastasis. In our prediction model, the C-index was 0.722 in the training set and 0.721 in the testing set for predicting the 2-year overall survival of metastatic ovarian cancer patients, which suggested that the model had good predictive ability. Additionally, the AUCs and calibration curves of the model at different time point also indicated that the predictive value of the model for 6-month, 1-year, 18-month and 2-year mortality of metastatic ovarian cancer patients were good. The nomogram of the prediction model was plotted, which could quickly and easily calculate the total score of each patient and obtain the probability of 6-month, 1-year, 18-month and 2-year mortality in the patient. The DCA curves indicated the nomograms were clinically useful. Our model might provide an effective and convenient tool for identifying metastatic ovarian cancer patients who were at high risk of mortality within 2 years and timely interventions should be applied for these patients to improve their prognosis. The model could also help find those with high possibility to survive in 2 years. This might greatly improve the confidence of clinicians and adherence of patients for the treatments. Also, subgroup analysis demonstrated that the model had good predictive performance in patients with bone, brain, liver or lung metastasis, these indicated that the model could be applied in evaluating the risk of mortality in specific metastatic ovarian cancer patients.
CA125 has been widely proposed to be a valuable biomarker in cancer cell growth and survival pathways, tumorigenesis, metastasis and prognosis (22). Previous studies reported that CA125 level is increased in 80% of the patients with epithelial ovarian cancer and 90% of women with epithelial ovarian cancer at advanced stage (23). CA125 has been validated as an important independent indicator correlated with the risk of death of ovarian cancer patients (17). Preoperative serum CA125 level was validated to be a predictor for the extent of cytoreduction in patients with advanced stage epithelial ovarian cancer (24). Rong et al. indicated that early clearance of serum CA125 could predict the platinum response and prognosis in patients with ovarian cancer (25). The findings of these studies gave evidence to the results of our study, which showed that CA125 was an essential predictor for the mortality of ovarian cancer patients with metastasis. Compared with patients have lymphatic metastasis, those with organ metastases showed a lower 5-year overall survival and worse prognosis (26). In the current study, ovarian cancer patients with lung, liver, bone and brain metastases were correlated with the survival of these patients. For patients with lung, liver, bone and brain metastases, attention should be paid at the initial diagnosis and fully evaluation of the condition of patients should be conducted to choose the better plan for the treatment of these patients (27).
For ovarian cancer patients at stage IV, due to spread of disease beyond the ovary, the treatment is more challenging (28). Surgery was regarded as a vital part in the treatment of ovarian cancer patients (29, 30). Complete cytoreductive surgery was reported to be an important modifiable prognostic factor influencing the overall survival time of patients with ovarian cancer (31). As a practice recommended by the National Comprehensive Cancer Center (NCCN), complete cytoreduction to no gross residual disease is a vital factor affecting the progression-free and overall survival of ovarian cancer patients at stage IV (32, 33). Another study also indicated that complete surgical cytoreduction had the best survival benefit in advanced epithelial ovarian cancer patients (34). Balkhy et al. indicated that surgery was the cornerstone of treatment for most stages of non-epithelial ovarian cancers, which might correlate with the prognosis of those patients (35). A retrospective review showed that ovarian cancer patients receiving intraperitoneal chemotherapy were associated with improved 10-year survival of these patients (36). There was evidence indicated that in primary debulking surgery combined with first-line chemotherapy might be the standard treatment for advanced epithelial ovarian cancer patients (37). A multicenter, randomized, phase 3 trial found that secondary cytoreduction followed by chemotherapy might increase the progression-free survival compared with chemotherapy alone in patients with platinum-sensitive relapsed ovarian cancer (38). Other studies were also trying to identify the optimal timing for cytoreduction and hyperthermic intraperitoneal chemotherapy in advanced ovarian cancer patients (39, 40). In this study, regardless of the outcomes of surgery and time of chemotherapy, those received surgery treatment might be associated with lower risk of overall mortality compared with patients who did not undergo surgery treatment. The elevated risk of mortality was observed in patients without both surgery and chemotherapy. These suggested that surgery and chemotherapy should be operated in ovarian cancer patients based on the feasibility conditions of patients to improve the outcome of these patients. In addition, chemotherapy might be associated with some side-effects such as fatigue in patients, and intervention strategies should be proposed to improve the management of these patients during their treatment and in the long term (41). In the past, ovarian cancer was found to be a radiosensitive tumor, and radiation was one of the main therapies for patients with epithelial ovarian cancer, which was applied to manage patients with low residual volumes of disease (42). Nowadays, radiotherapy is replaced by some other therapies in the treatment of ovarian cancer as ovarian cancer is easily to metastasize to other organs, especially in the pelvic and abdominal cavity. Too many organs need irradiation, which may cause more complications and result in poor outcomes in these patients (43). Hreshchyshyn et al. identified that for women with epithelial ovarian cancer at stage I receiving surgery treatment, those who subsequently received radiotherapy had highest recurrence rate (44). Clinicians should evaluate the health status of ovarian cancer patients and whether radiotherapy can be applied for patients should be carefully assessed.
Several limitations existed in the current study. Firstly, all the data were extracted from SEER database, and the participants were mainly White people, whether the prediction model was suitable for Chinese people still needs validation in more studies. Secondly, the variables associated with the risk of mortality in patients with metastatic ovarian cancer were not comprehensive, variables such as the basic demographic characteristic including body mass index and laboratory characteristic including the status of BRCA were not included. BRCA status has been widely tested in ovarian cancer patients in recent years and BRCA positive patients are more sensitive to chemotherapy drugs than negative patients (45, 46), which might be associated with the prognosis of patients. Thirdly, the detailed surgery information such as whether adequate lymphadenectomy was performed could not be extracted from SEER (47). Fourthly, external validation of the results of our study were not performed. Studies with more reliable variables especially surgery information, the status of BRCA and external validation of prediction models were required to validate the findings of this study.
Conclusion
Our study assessed establish prediction models for 2-year overall survival of ovarian cancer patients with metastasis based on the data of 4,929 metastatic ovarian cancer patients from SEER database. The models showed good predictive performance for 2-year overall survival of ovarian cancer patients with metastasis. In addition, the model presented good predictive value for patients with bone, brain, liver and lung metastasis. The results in this study might help identify metastatic ovarian cancer patients with high possibility of survival and provide a reference for the clinicians and patients to choose a better treatment to improve the prognosis of patients with metastatic ovarian cancer.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: SEER database, https://seer.cancer.gov/.
Ethics statement
Ethical approval was not provided for this study on human participants because data used in this study was publicly avaliable. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YW and YY designed the study. YW wrote the manuscript. XS, HD and ML collected, analyzed and interpreted the data. YY critically reviewed, edited and approved the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.974536/full#supplementary-material.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. COPD. Copd. Available at: https://www.who.int/en/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (Accessed August 6, 2021).
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Wang Z, Guo E, Yang B, Xiao R, Lu F, You L, et al. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: cervical, ovarian and uterine cancer. Gynecol Oncol. (2021) 163(2):358–63. doi: 10.1016/j.ygyno.2021.08.029
5. Zhang Y, Luo G, Li M, Guo P, Xiao Y, Ji H, et al. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer. (2019) 19(1):984. doi: 10.1186/s12885-019-6139-6
6. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. (2019) 35(2):151–6. doi: 10.1016/j.soncn.2019.02.001
7. Deng K, Yang C, Tan Q, Song W, Lu M, Zhao W, et al. Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol. (2018) 150(3):460–5. doi: 10.1016/j.ygyno.2018.06.022
8. Gardner AB, Charo LM, Mann AK, Kapp DS, Eskander RN, Chan JK. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: most common locations and outcomes. Clin Exp Metastasis. (2020) 37(1):107–13. doi: 10.1007/s10585-019-10007-0
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin. (2018) 68(1):7–30. doi: 10.3322/caac.21442
10. Cai Z, Liu Q. Understanding the global cancer statistics 2018: implications for cancer control. Sci China Life Sci. (2021) 64(6):1017–20. doi: 10.1007/s11427-019-9816-1
11. Benoit L, Koual M, Le Frère-Belda MA, Zerbib J, Fournier L, Nguyen-Xuan HT, et al. Risks and benefits of systematic lymphadenectomy during interval debulking surgery for advanced high grade serous ovarian cancer. Eur J Surg Oncol. (2022) 48(1):275–82. doi: 10.1016/j.ejso.2021.10.027
12. Chen JP, Huang QD, Wan T, Tu H, Gu HF, Cao JY, et al. Combined score of pretreatment platelet count and CA125 level (PLT-CA125) stratified prognosis in patients with FIGO stage IV epithelial ovarian cancer. J Ovarian Res. (2019) 12(1):72. doi: 10.1186/s13048-019-0544-y
13. Kumari A, Thakur M, Saha SC, Suri V, Prasad GRV, Patel FD, et al. To compare the optimal cytoreduction rate in advanced epithelial ovarian cancer stage III/IV after 3 versus 6 cycles of neoadjuvant chemotherapy. J Obstet Gynaecol. (2021) 41(4):616–20. doi: 10.1080/01443615.2020.1787967
14. Weeks KS, Lynch CF, West M, Carnahan R, O'Rorke M, Oleson J, et al. Gynecologic oncologist impact on adjuvant chemotherapy care for stage II-IV ovarian cancer patients. Gynecol Oncol. (2022) 164(1):3–11. doi: 10.1016/j.ygyno.2021.11.001
15. Sørensen SM, Høgdall C, Mosgaard BJ, Dalgaard MIR, Jensen MP, Fuglsang K, et al. Residual tumor and primary debulking surgery vs interval debulking surgery in stage IV epithelial ovarian cancer. Acta Obstet Gynecol Scand. (2022) 101(3):334–43. doi: 10.1111/aogs.14319
16. El Helali A, Kwok GST, Tse KY. Adjuvant and post-surgical treatment in non-epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. (2022) 78:74–85. doi: 10.1016/j.bpobgyn.2021.06.001
17. Zhao L, Yu P, Zhang L. A nomogram to predict the cancer-specific survival of stage II-IV epithelial ovarian cancer after bulking surgery and chemotherapy. Cancer Med. (2021) 10(13):4344–55. doi: 10.1002/cam4.3980
18. Inci MG, Anders L, Woopen H, Richter R, Guzel D, Armbrust R, et al. Frailty index for prediction of surgical outcome in ovarian cancer: results of a prospective study. Gynecol Oncol. (2021) 161(2):396–401. doi: 10.1016/j.ygyno.2021.02.012
19. Wang B, Wang S, Ren W. Development and validation of a nomogram to predict survival outcome among epithelial ovarian cancer patients with site-distant metastases: a population-based study. BMC Cancer. (2021) 21(1):609. doi: 10.1186/s12885-021-07977-4
20. Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29(Suppl 4):iv1–iv18. doi: 10.1093/annonc/mdy001
21. Yang C, Lei C, Zhang Y, Zhang J, Ji F, Pan W, et al. Comparison of overall survival between invasive lobular breast carcinoma and invasive ductal breast carcinoma: a propensity score matching study based on SEER database. Front Oncol. (2020) 10:590643. doi: 10.3389/fonc.2020.590643
22. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. (2021) 1875(2):188503. doi: 10.1016/j.bbcan.2021.188503
23. Asali A, Haj-Yehia N, Zehavi T, Perry T, Beiner M, Fishman A, et al. High grade, advanced, serous ovarian cancer with low serum CA125 levels. J Obstet Gynaecol. (2021) 41(7):1107–11. doi: 10.1080/01443615.2020.1835844
24. Merlo S, Besic N, Drmota E, Kovacevic N. Preoperative serum CA-125 level as a predictor for the extent of cytoreduction in patients with advanced stage epithelial ovarian cancer. Radiol Oncol. (2021) 55(3):341–6. doi: 10.2478/raon-2021-0013
25. Rong Y, Li L. Early clearance of serum HE4 and CA125 in predicting platinum sensitivity and prognosis in epithelial ovarian cancer. J Ovarian Res. (2021) 14(1):2. doi: 10.1186/s13048-020-00759-9
26. Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. (2014) 101(1):E1–E12. doi: 10.1684/bdc.2013.1882
27. Canaz E, Grabowski JP, Richter R, Braicu EI, Chekerov R, Sehouli J. Survival and prognostic factors in patients with recurrent low-grade epithelial ovarian cancer: an analysis of five prospective phase II/III trials of NOGGO metadata base. Gynecol Oncol. (2019) 154(3):539–46. doi: 10.1016/j.ygyno.2019.06.014
28. Wong DH, Mardock AL, Manrriquez EN, Lai TS, Sanaiha Y, Sinno AK, et al. Trends in extent of surgical cytoreduction for patients with ovarian cancer. PloS One. (2021) 16(12):e0260255. doi: 10.1371/journal.pone.0260255
29. Ferron G, Narducci F, Pouget N, Touboul C. Surgery for advanced stage ovarian cancer: article drafted from the French guidelines in oncology entitled “initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa. Gynecol Obstet Fertil Senol. (2019) 47(2):197–213. doi: 10.1016/j.gofs.2019.01.003
30. Cheung A, Shah S, Parker J, Soor P, Limbu A, Sheriff M, et al. Non-epithelial ovarian cancers: how much do we really know? Int J Environ Res Public Health. (2022) 19(3):1106. doi: 10.3390/ijerph19031106
31. Vincent L, Jankowski C, Ouldamer L, Ballester M, Bendifallah S, Bolze PA, et al. Prognostic factors of overall survival for patients with FIGO stage IIIc or IVa ovarian cancer treated with neo-adjuvant chemotherapy followed by interval debulking surgery: a multicenter cohort analysis from the FRANCOGYN study group. Eur J Surg Oncol. (2020) 46(9):1689–96. doi: 10.1016/j.ejso.2020.04.029
32. Wallace S, Kumar A, Mc Gree M, Weaver A, Mariani A, Langstraat C, et al. Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol. (2017) 145(1):21–6. doi: 10.1016/j.ygyno.2017.01.029
33. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 1.2019. J Natl Compr Cancer Network. (2019) 17(8):896–909. doi: 10.6004/jnccn.2019.0039
34. Yao SE, Tripcony L, Sanday K, Robertson J, Perrin L, Chetty N, et al. Survival outcomes after delayed cytoreduction surgery following neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Gynecol Cancer. (2020) 30(12):1935–42. doi: 10.1136/ijgc-2020-001658
35. Balkhy AL, Saleh ER, Jabali HI, Al-Jifree HM, Alwazzan AB. Demographic features, clinical characteristics, and prognostic factors of non-epithelial ovarian tumors at Princess Noorah Oncology Center, National Guard Hospital, Jeddah, Saudi Arabia. Saudi Med J. (2022) 43(2):208–12. doi: 10.15537/smj.2022.43.2.20210433
36. Adambekov S, Lopa S, Edwards RP, Lemon L, Wang S, Taylor SE, et al. Survival and recurrence after intraperitoneal chemotherapy use: retrospective review of ovarian cancer hospital registry data. Cancer Med. (2020) 9(20):7388–97. doi: 10.1002/cam4.3340
37. Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. (2019) 30(5):672–705. doi: 10.1093/annonc/mdz062
38. Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22(4):439–49. doi: 10.1016/S1470-2045(21)00006-1
39. Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. (2015) 22(5):1570–5. doi: 10.1245/s10434-014-4157-9
40. Kireeva GS, Gafton GI, Guseynov KD, Senchik KY, Belyaeva OA, Bespalov VG, et al. HIPEC in patients with primary advanced ovarian cancer: Is there a role? A systematic review of short- and long-term outcomes. Surg Oncol. (2018) 27(2):251–8. doi: 10.1016/j.suronc.2018.05.006
41. Gernier F, Ahmed-Lecheheb D, Pautier P, Floquet A, Nadeau C, Frank S, et al. Chronic fatigue, quality of life and long-term side-effects of chemotherapy in patients treated for non-epithelial ovarian cancer: national case-control protocol study of the GINECO-vivrovaire rare tumors INCa French network for rare malignant ovarian tumors. BMC Cancer. (2021) 21(1):1147. doi: 10.1186/s12885-021-08864-8
42. Durno K, Powell ME. The role of radiotherapy in ovarian cancer. Int J Gynecol Cancer. (2022) 32(3):366–71. doi: 10.1136/ijgc-2021-002462
43. Iorio GC, Martini S, Arcadipane F, Ricardi U, Franco P. The role of radiotherapy in epithelial ovarian cancer: a literature overview. Med Oncol. (2019) 36(7):64. doi: 10.1007/s12032-019-1287-8
44. Hreshchyshyn MM, Park RC, Blessing JA, Norris HJ, Levy D, Lagasse LD, et al. The role of adjuvant therapy in stage I ovarian cancer. Am J Obstet Gynecol. (1980) 138(2):139–45. doi: 10.1016/0002-9378(80)90024-1
45. Amin N, Chaabouni N, George A. Genetic testing for epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. (2020) 65:125–38. doi: 10.1016/j.bpobgyn.2020.01.005
46. Xu K, Yang S, Zhao Y. Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget. (2017) 8(1):285–302. doi: 10.18632/oncotarget.12306
Keywords: ovarian cancer, metastasis, prediction, two-year mortality, SEER
Citation: Wang Y, Shan X, Dong H, Li M and Yue Y (2022) Prediction for 2-year mortality of metastatic ovarian cancer patients based on surveillance, epidemiology, and end results database. Front. Surg. 9:974536. doi: 10.3389/fsurg.2022.974536
Received: 21 June 2022; Accepted: 24 August 2022;
Published: 15 September 2022.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Ruotao Xiao, Peking University Third Hospital, ChinaMohamed M. Abdel-Daim, Suez Canal University, Egypt
© 2022 Wang, Shan, Dong, Li and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Yue eXVleUBqbHUuZWR1LmNu
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yongxin Wang
Yongxin Wang Xue Shan2
Xue Shan2 He Dong
He Dong Ying Yue
Ying Yue