- 1Department of Radiology, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 2Department of Oncology and Southwest Cancer Center, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 3Institute of Pathology and Southwest Cancer Center, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 4Department of Radiology, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China
Background: This study aimed to investigate whether visceral adipose tissue index (VATI) is a significant risk factor for the early recurrence (ER) of HBV-related hepatocellular carcinoma (HCC) (≤5 cm) after hepatectomy.
Methods: The recruited cohort patients who were positive for hepatitis B virus, presented with surgically confirmed HCC (≤5 cm) from Army Medical University (internal training cohort: n = 192) and Chongqing Medical University (external validation group: n = 46). We measured VATI, subcutaneous adipose tissue index (SATI) via computed tomography (CT). ER was defined as recurrence within 2 years after hepatectomy. The impact of parameters on outcome after hepatectomy for HCC was analyzed.
Results: Univariate analysis showed that alpha-fetoprotein levels (p = 0.044), body mass index (BMI) (p < 0.001), SATI (p < 0.001), and VATI (p < 0.001) were significantly different between ER and non-ER groups in internal training cohort. Multivariate analysis identified VATI as an independent risk factor for ER (odds ratio = 1.07, 95% confidence interval: 1.047–1.094, p < 0.001), with a AUC of 0.802, based on the cut-off value of VATI, which was divided into high risk (≥37.45 cm2/m2) and low risk (<37.45 cm2/m2) groups. The prognosis of low risk group was significantly higher than that of high risk group (p < 0.001). The AUC value of VATI in external validation group was 0.854.
Conclusion: VATI was an independent risk factor for the ER, and higher VATI was closely related to poor outcomes after hepatectomy for HBV-related HCC (≤5 cm).
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver, accounting for approximately 80%–90% of all liver malignant tumors (1). It is also the fifth most common cancer and a leading cause of cancer-related deaths worldwide, with approximately 50% of cases occurring in China (2–4). In eastern Asia and Africa, hepatitis B virus (HBV) related-HCC is the predominant etiological factor. As suggested by clinical practice guidelines of American Association for the Study of Liver Diseases (AASLD) (5) and National Comprehensive Cancer Network (NCCN) (6), radiofrequency ablation and radical resection remain primary treatments for patients with early HCC. However, about 30% of patients are still free of disease recurrence (7).
The recurrence of HCC is classified as early recurrence (ER) (within 2 years) and non-ER (after 2 years) based on the time of recurrence after hepatectomy (2, 8). It has been reported that approximately 80% of early HCC recurrence occurs in the liver (8, 9). Furthermore, there are many risk factors for postoperative HCC recurrence including postoperative residual tumor tissuett, microvascular invasion, severe cirrhosis, portal hypertension, DNA replication, α-fetoprotein (AFP), and protein-induced by vitamin K absence-II (2, 10–13).
Lately, obesity and its associated metabolic abnormalities have been reported in previous studies as risk factors for poor prognosis in certain malignancies, including colon, endometrial, renal, and pancreatic cancers (14–16). Abdominal adipose tissue, including visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), which are significantly related to obesity and metabolic syndrome. Therefore, we hypothesized that abdominal adipose tissue is an important risk factor for ER after HCC resection. CT imaging was used to quantify abdominal adipose tissue in a rapid and precise technique.
As there have been no report regarding whether abdominal adipose tissue is a risk factor for ER after HBV-related HCC resection, the main objective of this study was to investigate the correlation between abdominal adipose tissue and ER of HBV-related HCC after hepatectomy.
Materials and methods
Patient population
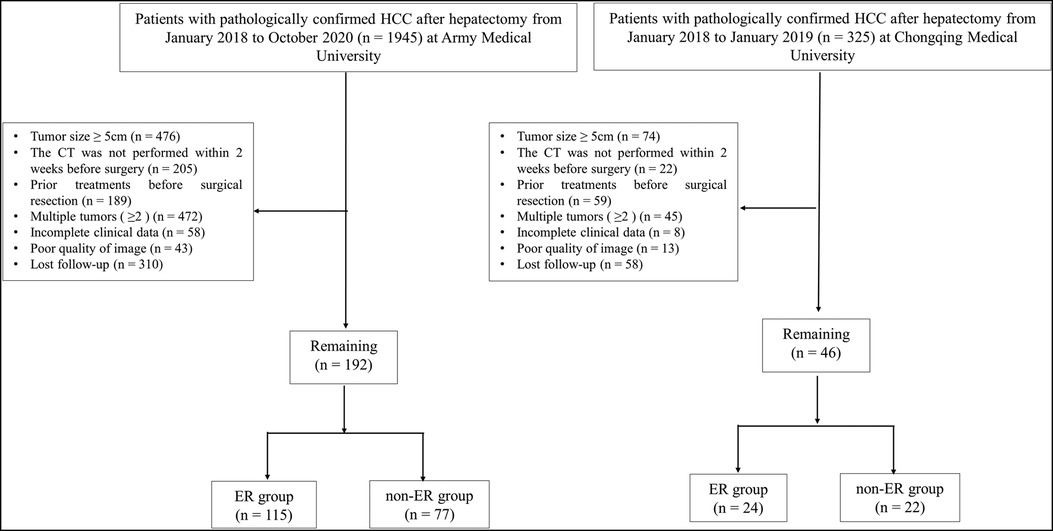
This study has been approved by the Ethics Committee of the First Affiliated Hospital (Army Medical University) (KY2021088) and the requirement for informed consent was waived. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. We collected the patients with HBV-related HCC in Army Medical University (internal training cohort) from January 2018 to October 2020, and Chongqing Medical University (external validation cohort) from January 2018 to January 2019.
The inclusion criteria were as follows: (1) pathological confirmation of HCC after surgical resection; (2) availability of complete clinical data; (3) CT performed within two weeks before surgery; (4) only a single tumor present (diameter ≤ 5 cm), excluding tumors with surrounding satellite lesions; (5) no intra or external metastasis and no visible vascular invasion; and (6) no preoperative history of other liver treatments, including radiofrequency ablation, liver resection, transcatheter arterial chemoembolization, or liver transplantation.
The exclusion criteria were as follows: (1) incomplete clinical data; (2) tumor diameter (>5 cm); (3) prior hepatic treatments before hepatectomy including radiofrequency therapy, transcatheter arterial chemoembolization, resection, or liver transplantation; (4) multiple tumors (n ≥ 2); (5) interval between the surgery date and CT examination was greater than two weeks; (6) poor image quality.
Image acquisition
The CT images of patients were collected using a dual-source CT scanner (SOMATOM Definition Flash, Siemens, Germany) and spiral CT scanner (Light Speed, GE, American). The scan range extended from the top of the diaphragm to the 4th lumbar vertebra (L4). Non-contrast scan followed by contrast-enhanced CT scans were acquired in the axial plane. Scan parameter settings were as follows: tube voltage of 120 kV, with automatic tube current, a layer thickness of 5 mm and a 1.5 mm reconstruction interval.
Image analysis
Abdominal adipose tissue were semi-automatically measured on 1.5 mm thick non-enhanced CT images using ImageJ software (https://imagej.net/software/fiji/-downloads). SAT and VAT areas were measured in adjusted-tissue Hounsfield units (HU) as follows: −190 to −30 HU. One radiologist with 10 years of experience in abdominal imaging, blinded to patients' information, evaluated cross-sectional areas of abdominal adipose tissue. A single unenhanced axial slice (layer thickness of 1.5 mm) at the L3 vertebral center was selected independently, the abdominal adipose tissue was delineated three times using ImageJ software and the average values were recorded (Figure 1). Then we used the abdominal adipose tissue divided by height (cm2/m2) to obtain the VATI and SATI as reported in the literature (17).
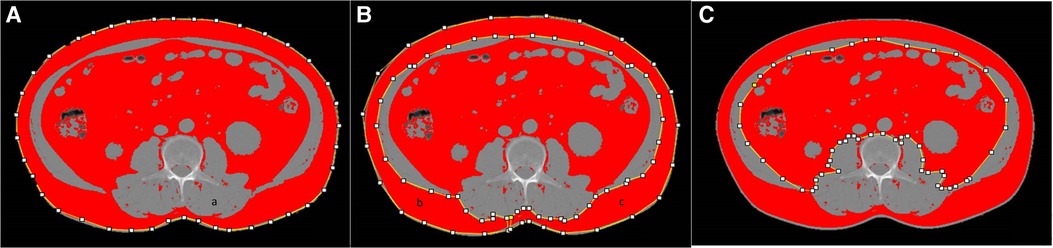
Figure 1. Example images of abdominal adipose tissue as measured by using imageJ software. total adipose (A), subcutaneous adipose (B), visceral adipose (C).
Patients with HBV-related HCC underwent routine imaging (ultrasound, CT, and/or MRI) and AFP testing at 3, 6, 12, and 24 months after hepatectomy. ER was established on typical findings on contrast-enhanced multiphase imaging [hyperenhancement in the arterial phase and washout in the portal venous or delayed phases (18)] within two years after surgery, including intrahepatic recurrence or distant metastases (19), otherwise was non-ER. The observational period for recurrence analysis was defined as the interval between surgery and recurrence or the date of the last follow-up visit before May 30, 2022.
Statistical analyses
Statistical analyses were performed using SPSS 22.0 software (Armonk, NY: IBM Corp). Variables with normal distributions are expressed as (¯x ± s), and pairwise comparisons were performed using the Student's t test. Nonnormally distributed continuous variables were analyzed using the Mann-Whitney test, while categorical variables were analyzed using the χ2-test. Univariate logistic regression was used to analyze the prognostic factors, variables with p < 0.05 were included in bivariate logistic regression analysis. Receiver operating characteristic curves were also plotted to establish VATI cut-off values associated with ER, and divided into low risk and high risk groups. RFS in the low risk and high risk was analyzed using Kaplan-Meier curves and compared using a log-rank test. For all analyses, differences with p < 0.05 were considered statistically significant.
Results
Patient characteristics
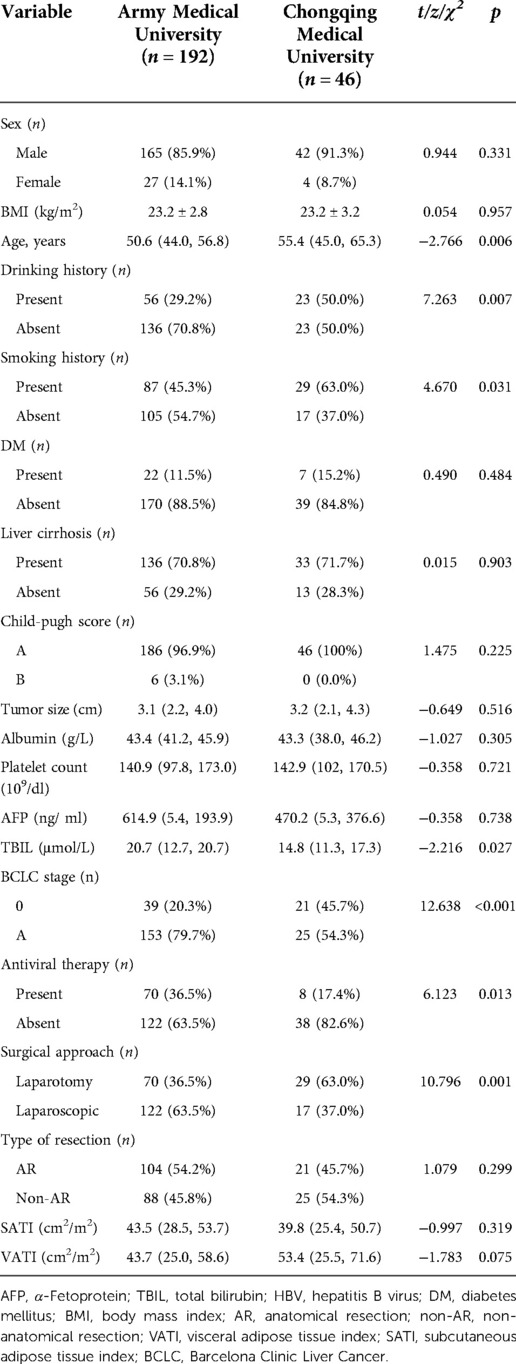
A total of 238 patients were included in the present study, including Army Medical University (internal training cohort) and Chongqing Medical University (external validation cohort) (Figure 2). The internal training cohort comprised 192 patients with a male majority population (85.9%), and the external validation cohort comprised 46 patients with a male majority population (91.3%). Body mass index (BMI), SATI and VATI were similar in the two cohorts (Table 1).
Association between parameters and early recurrence (ER) in internal training cohort
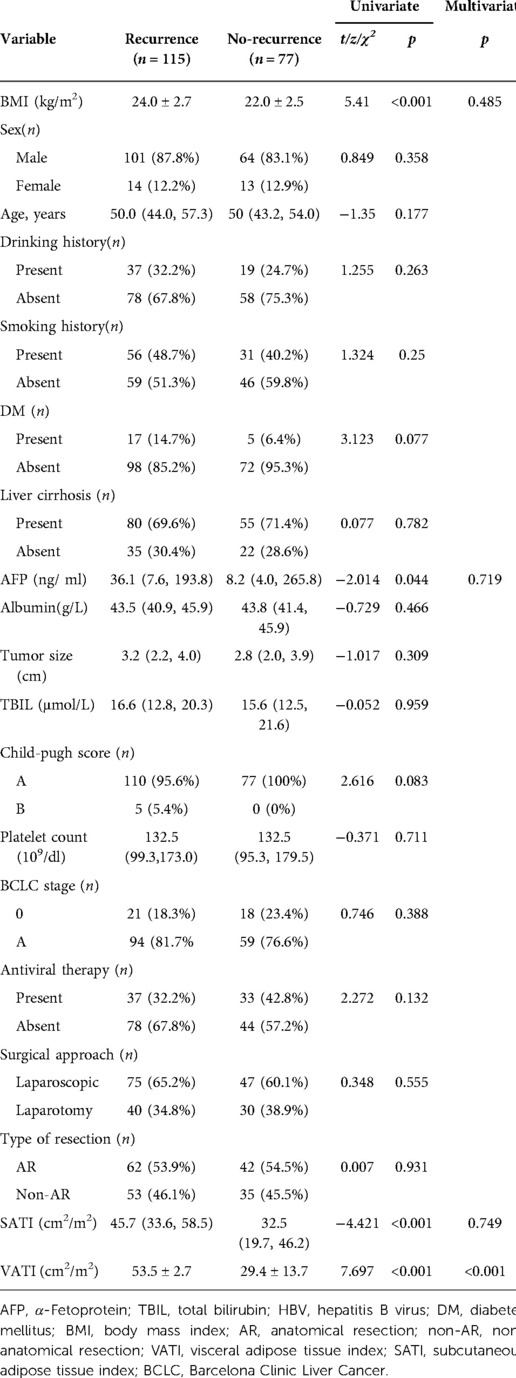
In internal training cohort, patients were divided into ER group (n = 115) and non-ER group (n = 77). Univariate analysis demonstrated clinical factors, except for AFP (p = 0.044) and (BMI) (p < 0.001), no variables showed significant differences between the ER and non-ER groups (p > 0.05).
Univariate analysis showed among VATI, SATI with ER group was higher than that of non-ER group [53.5 ± 2.7 cm2/m2 vs. 29.4 ± 13.7 cm2/m2, 45.7 cm2/m2 (33.6, 58.5) vs. 32.5 cm2/m2; p < 0.001]. Multivariate analysis showed that VATI was the only an independent risk factor (odds ratio = 1.07, 95% confidence interval: 1.047–1.094; p < 0.001) for ER (Table 2) (Figure 3).
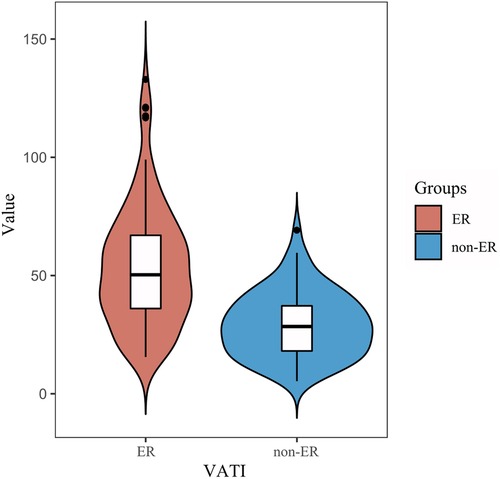
Figure 3. Comparison of the VATI between the ER and non-ER groups. An increase in visceral adipose tissue index (VATI) value was associated with an increased risk of early recurrence.
The AUC of the VATI was 0.802 (95% CI: 0.741–0.862, p < 0.001), and its sensitivity, specificity, and accuracy were 73.9%, 75.3%, and 74.6% (Figure 4), the cut-off value of VATI was 37.45 cm2/m2, then we divided into low risk (VATI < 37.45 cm2/m2) and high risk (VATI ≥ 37.45 cm2/m2) groups (Supplementary Table), and investigated the impact of VATI on PFS of HCC, the high risk group had earlier PFS compared to the low risk group (p < 0.001) (Figure 5).
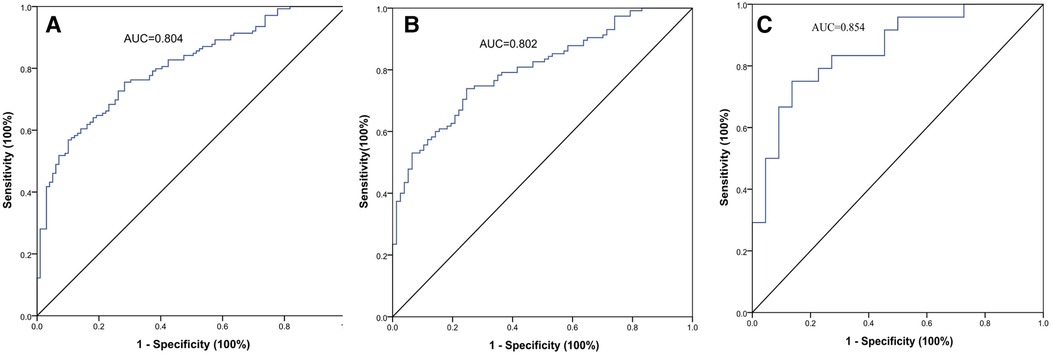
Figure 4. The ROC curves of the visceral adipose tissue index (VATI) for early recurrence (ER) of HBV-related HCC after hepatectomy. The AUC value was 0.804 in two cohort (A), 0.802 in internal training cohort (B), and 0.854 in external validation cohort (C).
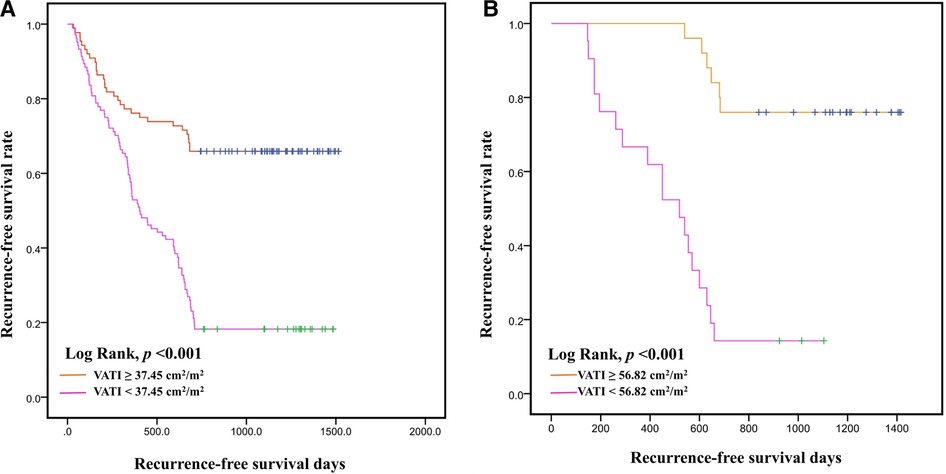
Figure 5. Kaplan–Meier curves for recurrence-free survival time. Recurrence-free survival with visceral adipose tissue index (VATI) ≥ 37.45 cm2/m2 and <37.45 m2/m2 in internal training cohort (A). Recurrence-free survival with visceral adipose tissue index (VATI) ≥ 56.82 cm2/m2 and <56.82 cm2/m2 in external validation cohort (B).
The effect of visceral adipose tissue index (VATI) on survival
In external validation cohort, patients were divided into ER group (n = 24) and non-ER group (n = 22). The AUC of the VATI in external validation cohort was 0.854 (95% CI: 0.745–0.963, p < 0.001), and its sensitivity, specificity, and accuracy were 75.0%, 86.4%, and 80.7% (Figure 4). The cut-off value of VATI was 56.82 cm2/m2, the high risk group had earlier PFS compared to the low risk group (p < 0.001) (Figure 5).
Furthermore, we analyzed the AUC of the VATI in two cohorts (n = 238), the value was 0.804, and the cut-off value of VATI was 37.45 cm2/m2, similar to internal training cohort (Figure 4).
Discussion
Obesity is a global health issue, and the association of obesity with the development of HCC has also been reported (15, 20). Studies have shown that BMI is not an ideal measure of obesity but abdominal adipose tissue distribution, including SAT and VAT (21–23). According to our previous results, the probability of positive MVI increases with increasing VAT area (24), and this is the first study to indicate that increased VAT and SAT levels are important risk factors for ER after resection of HBV-related HCC.
The results of the present study clearly showed the first evidence that obesity-related indicators, including BMI, SATI, and VATI, are associated with ER of HBV-related HCC after curative treatment. After multivariate regression analysis, the VATI was an independent risk factor, and not BMI or SATI, which are consistent with those of a previous study (22, 25, 26). An increase in VATI value was associated with an increased risk of ER, as confirmed by the external validation group (AUC = 0.854), and the high risk group had ER compared to the low risk group (p < 0.001) in both cohorts. Despite this, the VATI cut-off values of the two cohorts (37.45 vs. 56.82 cm2/m2) were discrepancy, inconsistent with Imai (47.2 cm2/m2) (25) and Montano (65 cm2/m2) (22) reports as well, nevertheless, the cut-off value for VATI was similar to the internal training cohort (37.45 cm2/m2) after analyzing all patients (n = 238) from the two cohorts. Therefore, we speculate that the cut-off value might be closely related to the sample size of the study, and racial differences might also play a key role.
The mechanism may be VAT contains more large adipocytes secreting adipokines involved in hepatocarcinogenesis compared to SAT (23). Previous studies could speculate about the inferred explanation: (1) an increase in VAT can result in a stronger inflammatory response, which is related to the inflammatory response, and the secretion of inflammatory factors directly into the liver, eventually leading to inflammation of the liver and the development of HCC as a result (22, 27–29); (2) VAT also contributes to the development of insulin resistance, wherein insulin can exert mitogenic effects by activating its receptors in precancerous and cancer cells, which results vascular damage and vascular endothelial growth factor release, and cause oncogenesis, tumor progression and poor prognosis (15, 28, 30, 31). Therefore, as pointed out by previous studies (14, 15), pharmacological or nutritional interventions, and exercise enhancement can be used to improve chronic inflammation, reduce insulin resistance, and improve the long-term survival of patients with HCC with high VAT.
In this study, univariate analysis indicated that preoperative high AFP load was a significant risk factor for ER in HBV-related HCC, consistent with the previously reported findings (1, 10). AFP has been associated with the upregulation of proteins related to cellular infiltration and distant metastasis, promoting tumor growth and progression.
Our study has several limitations. First, this study may have overlooked other variables that could affect the results due to its retrospective design. Second, all abdominal adipose tissue were manually delineated, which may have led to some errors. Therefore, we will employ deep learning artificial intelligence methods to extract abdominal CT fat with increased accuracy in future studies. Third, this study was only conducted on HBV-related HCC. The results may not be applied to other etiologies of HCC.
In conclusion, the results of this study suggest that the VATI was an independent risk factor for ER, and higher VATI was closely related to poor outcomes after hepatectomy for HBV-related HCC (≤5 cm).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Army Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
X-ML and JC conceived and designed the study; Z-QW wrote the manuscript; X-XX, H-RZ and JP collected and reviewed images; CL and JW analyzed the data; PC performed extensive editing of the manuscript; All authors contributed to the article and approved the submitted.
Funding
The authors would like to thank the X-ML, Program of National Natural Science Foundation of Chongqing (No. CSTB2022NSCQ-MSX1371).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.985168/full#supplementary-material.
References
1. Shinkawa H, Tanaka S, Takemura S, Amano R, Kimura K, Kinoshita M, et al. Nomograms predicting extra- and early intrahepatic recurrence after hepatic resection of hepatocellular carcinoma. Surgery. (2021) 169(4):922–8. doi: 10.1016/j.surg.2020.10.012
2. Xu W, Liu F, Shen X, Li R. Prognostic nomograms for patients with hepatocellular carcinoma after curative hepatectomy, with a focus on recurrence timing and post-recurrence management. J Hepatocell Carcinoma. (2020) 7:233–56. doi: 10.2147/jhc.S271498
3. Omata M, Cheng A, Kokudo N, Kudo M, Lee J, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. (2017) 11(4):317–70. doi: 10.1007/s12072-017-9799-9
4. Wang W, Guo Y, Zhong J, Wang Q, Wang X, Wei H, et al. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep. (2021) 11(1):2415. doi: 10.1038/s41598-021-82058-x
5. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD Guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). (2018) 67(1):358–80. doi: 10.1002/hep.29086
6. Benson AB 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, et al. NCCN Guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. (2017) 15(5):563–73. doi: 10.6004/jnccn.2017.0059
7. Loosen SH, Castoldi M, Jördens MS, Roy S, Vucur M, Kandler J, et al. Serum levels of circulating microRNA-107 are elevated in patients with early-stage HCC. PLoS One. (2021) 16(3):e0247917. doi: 10.1371/journal.pone.0247917
8. Amisaki M, Saito H, Tokuyasu N, Sakamoto T, Honjo S, Fujiwara Y. Prognostic value of postoperative complication for early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. (2018) 17(4):323–9. doi: 10.1016/j.hbpd.2018.03.016
9. Cha D, Jang K, Kim S, Kim Y, Kim H, Ahn S. Preoperative prediction for early recurrence can be as accurate as postoperative assessment in single hepatocellular carcinoma patients. Korean J Radiol. (2020) 21(4):402–12. doi: 10.3348/kjr.2019.0538
10. Hong Y, Cho M, Yoon K, Chu C, Yang K, Park Y, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol. (2017) 39(10):1010428317720863. doi: 10.1177/1010428317720863
11. Shinkawa H, Tanaka S, Takemura S, Ishihara T, Yamamoto K, Kubo S. Tumor size drives the prognosis after hepatic resection of solitary hepatocellular carcinoma without vascular invasion. J Gastrointest Sur. (2020) 24(5):1040–8. doi: 10.1007/s11605-019-04273-2
12. Qu C, Huang X, Liu K, Li K, Tan B, Qu L, et al. Effect of hepatitis B virus DNA replication level and anti-HBV therapy on microvascular invasion of hepatocellular carcinoma. Infect Agents Cancer. (2019) 14:2. doi: 10.1186/s13027-019-0219-8
13. Kim W, Lim T, Park P, Choi S, Kim W. Prognostic markers affecting the early recurrence of hepatocellular carcinoma with liver cirrhosis after curative resection. Int J Biol Markers. (2019) 34(2):123–31. doi: 10.1177/1724600819834306
14. Shimizu M, Tanaka T, Moriwaki H. Obesity and hepatocellular carcinoma: targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin Immunopathol. (2013) 35(2):191–202. doi: 10.1007/s00281-012-0336-6
15. Rajesh Y, Sarkar D. Molecular mechanisms regulating obesity-associated hepatocellular carcinoma. Cancers (Basel). (2020) 12(5):1290. doi: 10.3390/cancers12051290
16. González Svatetz C, Goday Arnó A. Obesity and cancer: «dangerous friendship». Med Clin (Barc). (2015) 145(1):24–30. doi: 10.1016/j.medcli.2014.05.026
17. Kimura N, Tsuchiya A, Oda C, Kimura A, Hosaka K, Tominaga K, et al. Visceral adipose tissue index and hepatocellular carcinoma are independent predictors of outcome in patients with cirrhosis having endoscopic treatment for esophageal varices. Dig Dis. (2021) 39(1):58–65. doi: 10.1159/000508867
18. Cerny M, Chernyak V, Olivié D, Billiard JS, Murphy-Lavallée J, Kielar AZ, et al. LI-RADS Version 2018 ancillary features at MRI. Radiographics. (2018) 38(7):1973–2001. doi: 10.1148/rg.2018180052
19. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9(6):682–720. doi: 10.1159/000509424
20. Siegel A, Wang S, Jacobson J, Hershman D, Lim E, Yu J, et al. Obesity and microvascular invasion in hepatocellular carcinoma. Cancer Investig. (2010) 28(10):1063–9. doi: 10.3109/07357907.2010.483500
21. Schwenzer NF, Machann J, Schraml C, Springer F, Ludescher B, Stefan N, et al. Quantitative analysis of adipose tissue in single transverse slices for estimation of volumes of relevant fat tissue compartments: a study in a large cohort of subjects at risk for type 2 diabetes by MRI with comparison to anthropometric data. Invest Radiol. (2010) 45(12):788–94. doi: 10.1097/RLI.0b013e3181f10fe1
22. Montano-Loza AJ, Mazurak VC, Ebadi M, Meza-Junco J, Sawyer MB, Baracos VE, et al. Visceral adiposity increases risk for hepatocellular carcinoma in Male patients with cirrhosis and recurrence after liver transplant. Hepatology (Baltimore, Md). (2018) 67(3):914–23. doi: 10.1002/hep.29578
23. Imai K, Takai K, Miwa T, Maeda T, Hanai T, Shiraki M, et al. Increased visceral adipose tissue and hyperinsulinemia raise the risk for recurrence of non-B non-C hepatocellular carcinoma after curative treatment. Cancers (Basel). (2021) 13(7):1542. doi: 10.3390/cancers13071542
24. Wu ZQ, Lu H, Xie Q, Cheng J, Ma KS, Hu XF, et al. Preoperative assessment of abdominal adipose tissue to predict microvascular invasion in small hepatocellular carcinoma. J Clin Transl Hepatol. (2022) 10(2):184–9. doi: 10.14218/JCTH.2021.00126
25. Imai K, Takai K, Maeda T, Watanabe S, Hanai T, Suetsugu A, et al. Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget. (2018) 9(18):14058–67. doi: 10.18632/oncotarget.24500
26. Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. (2009) 58(6):839–44. doi: 10.1136/gut.2008.164053
27. Healy L, Howard J, Ryan A, Beddy P, Mehigan B, Stephens R, et al. Metabolic syndrome and leptin are associated with adverse pathological features in Male colorectal cancer patients. Colorectal Dis. (2012) 14(2):157–65. doi: 10.1111/j.1463-1318.2011.02562.x
28. Yamamoto A, Kikuchi Y, Kusakabe T, Takano H, Sakurai K, Furui S, et al. Imaging spectrum of abnormal subcutaneous and visceral fat distribution. Insights Imaging. (2020) 11(1):24. doi: 10.1186/s13244-019-0833-4
29. Wu T, Chang S, Li C, Hsu Y, Chen T, Lee W, et al. Severe hepatitis promotes hepatocellular carcinoma recurrence via NF-κB pathway-mediated epithelial-mesenchymal transition after resection. Clin Cancer Res. (2016) 22(7):1800–12. doi: 10.1158/1078-0432.Ccr-15-0780
30. Imai K, Takai K, Hanai T, Suetsugu A, Shiraki M, Shimizu M. Homeostatic model assessment of insulin resistance for predicting the recurrence of hepatocellular carcinoma after curative treatment. Int J Mol Sci. (2019) 20(3):605. doi: 10.3390/ijms20030605
Keywords: visceral adipose tissue, HBV, HCC, early recurrence, abdominal adipose tissue, computed tomography
Citation: Wu Z, Cheng J, Xiao X, Zhang H, Wang J, Peng J, Liu C, Cai P and Li X (2023) Preoperative prediction of early recurrence of HBV-related hepatocellular carcinoma (≤5 cm) by visceral adipose tissue index. Front. Surg. 9:985168. doi: 10.3389/fsurg.2022.985168
Received: 15 July 2022; Accepted: 4 November 2022;
Published: 6 January 2023.
Edited by:
Gabriel Sandblom, Karolinska Institutet (KI), SwedenReviewed by:
Imelda Loho, Dharmais Hospital National Cancer Center, IndonesiaUlysses Torres, Fleury S.A. (Brazil), Brazil
© 2023 Wu, Cheng, Xiao, Zhang, Wang, Peng, Liu, Cai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Cai NTMwMzA1OTQyQHFxLmNvbQ== Xiao-ming Li MzU5MjYxMDY5QHFxLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Zong-qian Wu1,†
Zong-qian Wu1,† Jian Wang
Jian Wang Chen Liu
Chen Liu Ping Cai
Ping Cai Xiao-ming Li
Xiao-ming Li