- Department of General Surgery, People’s Hospital of Rongchang District, Chongqing, China
The effect of probiotics on postoperative infectious complications and nutritional status in patients with gastric cancer is still controversial, and a comprehensive search and analysis of the current relevant evidence is necessary. Our study aimed to define the effects of probiotics on surgical outcomes in gastric cancer patients undergoing surgery. Up to June 20, 2023, Embase, PubMed, Web of Science, and Cochrane databases were searched for randomized controlled trials of probiotics in gastric cancer patients undergoing surgery. Relative risk (RR) or mean difference (MD) was used to calculate the effect sizes using RevMan 5.3. A total of nine studies reporting on 861 participants were included. Perioperative supplementation with probiotics did not improve weight loss (MD 0.73 kg; 95% CI: −0.56, 2.02) or serum prealbumin levels (MD 9.48 mg/L 95% CI: −3.43, 22.40), but did reduce the incidence of postoperative infectious complications (RR 0.46, 95% CI 0.28, 0.77), shorten the time to first exhaust (MD −11.27 h; 95% CI: −16.83, −5.70), the time to first defecation (MD −15.71 h; 95% CI: −25.62, −5.79), and the length of hospital stay (MD −0.94 days; 95% CI: −1.33, −0.55), and increase serum albumin levels (MD 0.73 g/L; 95% CI: 0.01, 1.46) in gastric cancer patients undergoing surgery. Probiotics are effective in preventing postoperative infectious complications, promoting postoperative recovery, and improving nutritional status in gastric cancer patients undergoing surgery. Our study highlights the importance of probiotics for healthcare systems and offers a potential strategy to improve the prognosis and reduce the medical burden of gastric cancer patients undergoing surgery.
1. Introduction
Gastric cancer is one of the most common malignancies worldwide and the fourth most common cause of cancer-related death, with more than 1,000,000 new cases diagnosed each year (1). In the United States and Europe, the five-year survival rate for advanced gastric cancer is less than 30 percent (2). Surgical resection is considered to be the most effective treatment for gastric cancer. In recent years, although many adjuvant therapies have been proposed, the postoperative morbidity and mortality of gastric cancer are still significant (3). Postoperative morbidity of gastric cancer has been reported to range from 1.88% to 59.8% (4), and a recent large national study in Germany showed that the in-hospital mortality after elective gastric cancer surgery was as high as 6.2% (5). Postoperative complications of gastric cancer surgery can lead to longer hospitalization days and increased hospitalization costs, among which intestinal obstruction is a common postoperative complication of gastric cancer (3). In addition, patients with gastric cancer are often accompanied by severe malnutrition (6), which can also lead to increased postoperative morbidity and mortality, and even affect the overall survival of patients (2). How to promote postoperative recovery of intestinal function, improve the nutritional status of patients, and reduce postoperative complications in patients with gastric cancer has become a focus of extensive attention by researchers.
Gastric surgery typically destroys the gastrointestinal mucosal barrier, resulting in increased permeability of the gastrointestinal mucosa and disorders of intestinal flora, and damage to the normal immune system (7). Gut microflora is a potential target for improving perioperative outcomes of gastric cancer. Probiotics are a group of living microbes that are beneficial when consumed in moderate amounts (8). Probiotics have been widely used to treat a variety of gastrointestinal diseases due to their extensive beneficial activities such as anti-inflammatory, anti-tumor, and immunomodulatory effects (9–13). Probiotics have shown great potential in preventing complications from intestinal surgery (14). The meta-analysis by Chowdhury et al. showed that probiotics can effectively reduce the incidence of complications such as postoperative infection in abdominal surgery (15). Probiotics could also promote recovery of intestinal function in patients undergoing colorectal surgery (16). However, relatively fewer studies explored the effect of probiotics on gastric cancer surgery, and there are inconsistent results among the studies. In addition, there is a lack of meta-analyses to summarize the current inconsistent evidence.
We hypothesized that probiotic supplementation would reduce postoperative infectious complications, promote postoperative recovery, and improve nutritional status. Here, we conducted a comprehensive search of relevant clinical studies and conducted a meta-analysis to explore the impact of probiotics supplementation on gastric cancer surgery.
2. Methods
2.1 Search strategy
This meta-analysis was successfully registered on PROSPERO (registration no. CRD42023440075). A systematic search for the current meta-analysis was conducted on material published from inception, to June 20, 2023, in the Cochrane Library, EMBASE, PubMed, and the Web of Science databases, using the following search string: (Gastric Neoplasm OR Stomach Neoplasm OR Gastric Cancer OR Stomach Cancer OR Gastric Tumor OR Stomach Tumor OR Gastric Carcinoma OR Stomach Carcinoma) AND (probiotics OR probiotic OR Bifidobacterium OR Lactobacillus OR Enterococcus OR Limosilactobacillus) (Table 1). No language restrictions were imposed. The list of references for the relevant reviews and included studies were also searched.
2.2. Study selection
Inclusions contain: (1) randomized controlled trials (RCTs) (2) intervention with probiotics (any species, dose, and strain), (3) comparing with standard diet or placebo, (4) patients with gastric cancer who underwent operation (5) the outcomes included any of the following: the time to first exhaust, weight, serum prealbumin level, serum albumin level, the time to first defecation, length of hospital stay, and the incidence of postoperative complications. Primary outcome indicator was the incidence of postoperative infectious complications, and the secondary outcome were the time to first exhaust, weight, serum prealbumin level, serum albumin level, the time to first defecation, and length of hospital stay.
Exclusions contain: review, non-randomized studies, conference abstracts, case reports, letters to the editor, technical reports, and non-human studies.
2.3. Data extraction
Data, including study type, type of surgery, country, sample size, age, diagnosis, sex, the first author, year of publication, intervention type, control groups, number of treatment days, and outcomes were extracted from each study. If relevant data could not be extracted from the literature, we tried to contact the corresponding author of the study to obtain relevant information. When an outcome data was recorded at different timepoints in one study, the most recent measured data after the intervention was completed was selected as the outcome data.
2.4. Quality assessment
Methodological quality of all eligible trials was assessed using the Cochrane Collaboration's risk-of-bias tool (17): (1) the randomizing process, (2) allocation concealment, (3) participant and operator blinding, (4) blinding of outcome assessment, (5) incomplete data, (6) selective reporting, and (7) other biases. Two authors (Ye and Zhang) independently conducted literature retrieval, study selection, data extraction, and quality assessment of the methodology included in the study. When there were inconsistencies between the two authors, they were discussed and resolved by a third author (Ye, Dong and Zhang).
2.5. Statistical analysis
Relative risk (RR) with 95% confidence intervals (CI) were calculated for qualitative variables and mean difference (MD) for quantitative outcomes (17). Heterogeneity between studies was assessed using the I2 statistic (18). The random effects model was selected when I2 was >50%. Otherwise, the fixed-effect model was selected. To explore the robustness of the results, one-study exclusion test was used to examine the impact of each study on the pooled effect size. Analysis was conducted using Review 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2014; Copenhagen, Denmark). P < 0.05 was considered significant.
3. Results
3.1. Selected studies
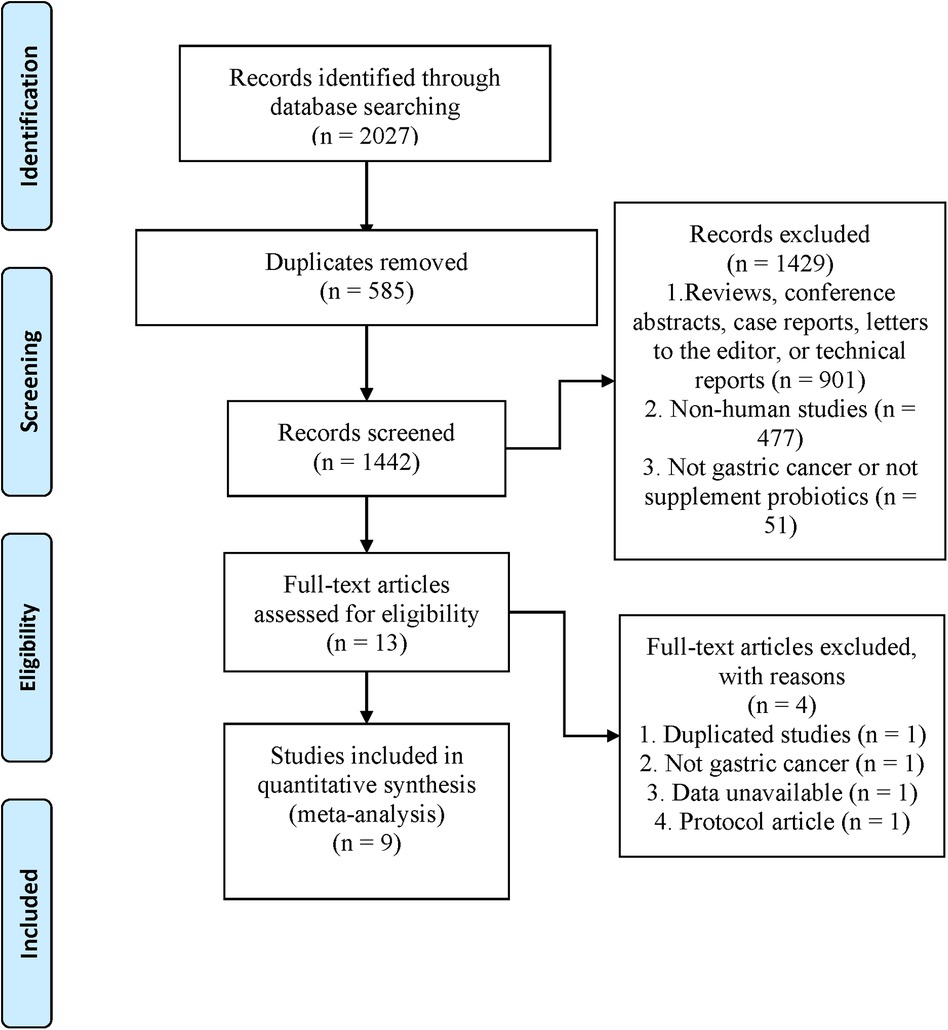
Our search strategy identified 2,027 records. 1,442 were retained after 585 duplicates were excluded. 1,429 articles were excluded by reading titles and abstracts (The details are summarized in Figure 1), and the remaining 13 articles were evaluated for full text. Finally, nine trials (19–27) were eligible and included for meta-analysis (Figure 1).
3.2. Study characteristics
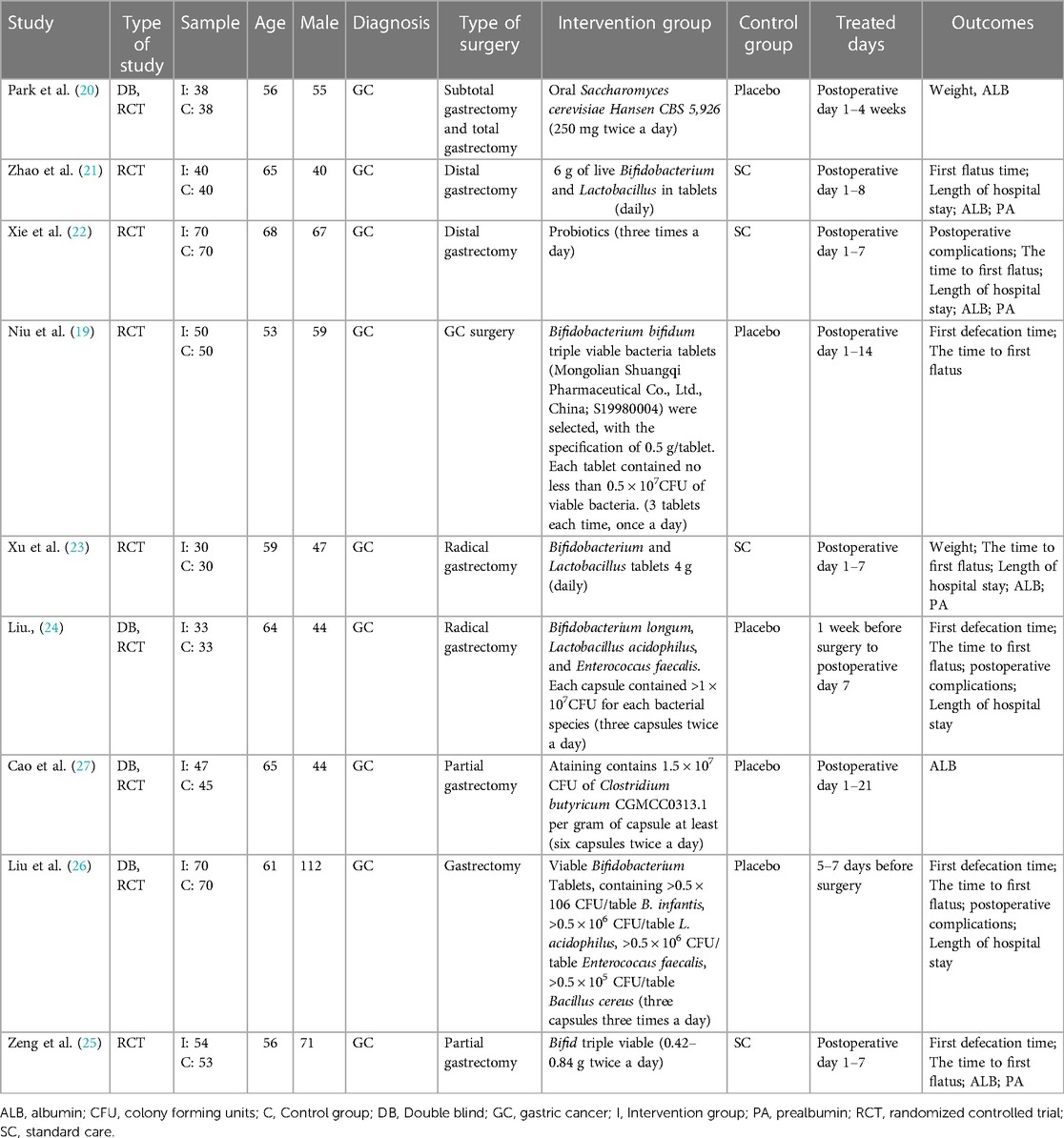
Nine studies (19–27) with a total of 861 participants were published between 2006 and 2023. Eight of the studies (19, 21–27) were conducted in China and the remaining one in South Korea (20). The number of participants ranged from 60 to 140. The probiotics used in six studies (19, 21, 23–26) included Bifidobacterium, one (27) used Clostridium butyricum CGMCC0313.1, one used Saccharomyces cerevisiae Hansen CBS 5926 (20), and one (23) did not explicitly describe the probiotic species (Table 2). The duration of intervention ranged from 7 to 28 days.
3.3. Quality assessment
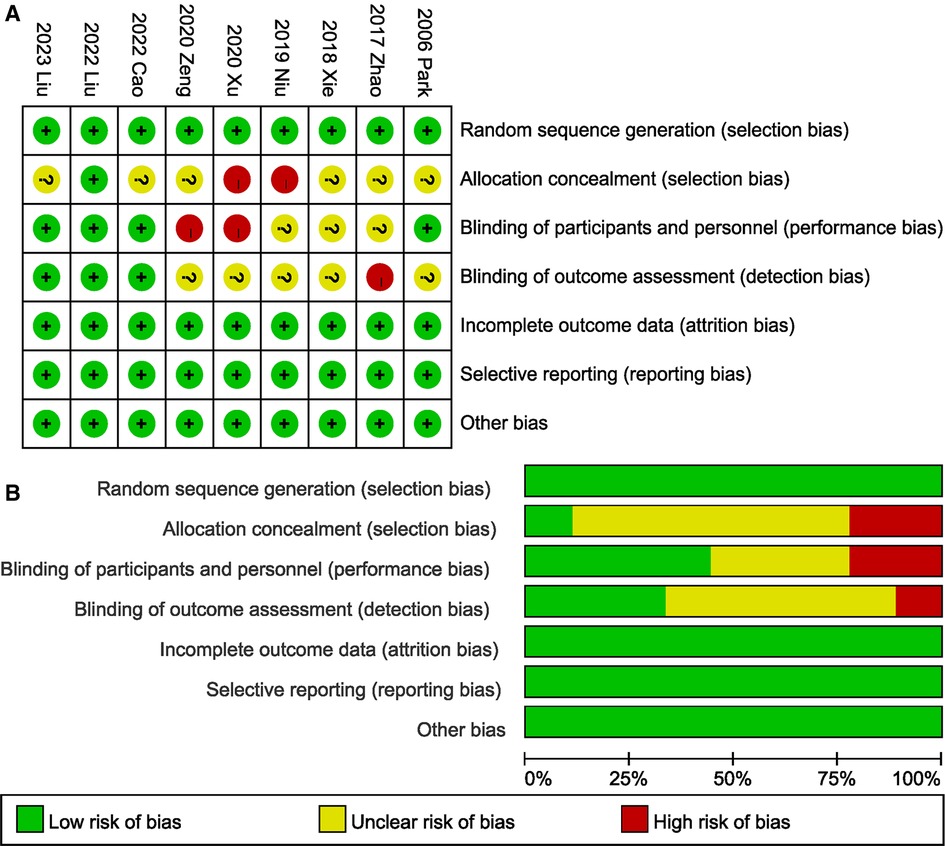
All of the trials (19–24, 26, 27) described their specific methods of randomization (Figure 2). Four tudies (20, 24, 26, 27) adopted a double-blind design, and blinded method for evaluating results were evaluated as a low bias risk in three (24, 26, 27) of the studies. The randomization scheme was appropriately hidden in one study (24). Selective reporting, incomplete outcome data, and other bias sources in all studies were evaluated as a low bias risk.
3.4. Meta-analysis
3.4.1. Effects of probiotics on the incidence of postoperative infectious complications
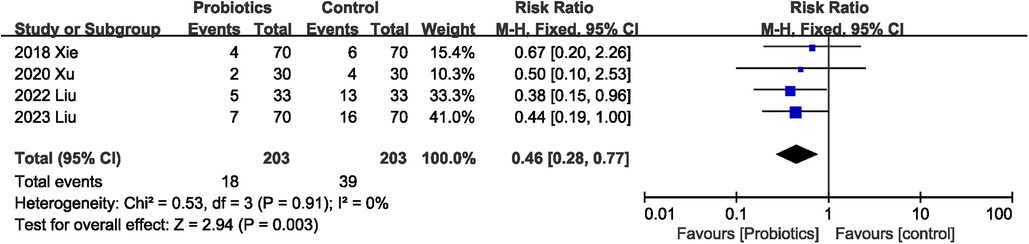
A total of 406 participants in four studies (22–24, 26) mentioned postoperative infectious complications. The pooled effect was calculated with random-effect model. Probiotics supplementation was associated with lower risk of postoperative infectious complications (RR 0.46, 95% CI 0.28, 0.77; Heterogeneity: I2 = 0%, P = 0.91) (Figure 3).
3.4.2. Effects of probiotics on the time to first exhaust
A total of 693 participants in seven studies (19, 21–26) reported this outcome, all of which observed that probiotics consumption was associated with a reduction in the time to first exhaust. The overall effect indicated that probiotics could shorten the first exhaust time (MD −11.27 h; 95% CI: −16.83, −5.70), with significant heterogeneity (I2 = 91%, P < 0.00001) between studies (Figure 4).
3.4.3. Effects of probiotics on the time to first defecation
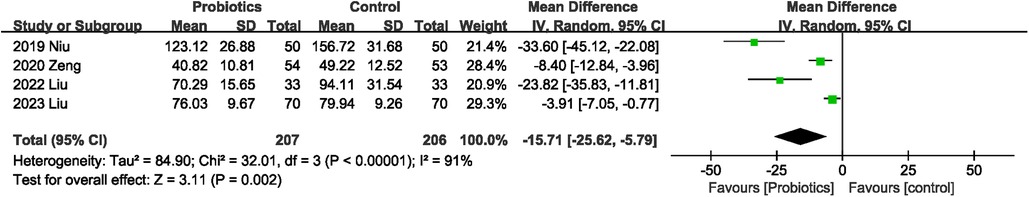
Four studies (19, 24–26) reported data for time to first defecation, and all showed that probiotics reduced time to first defecation. Pooled results indicated that probiotics consumption reduced the time to first defecation, with significant heterogeneity (MD −15.71 h; 95% CI: −25.62, −5.79; Heterogeneity: I2 = 91%, P < 0.00001) between studies (Figure 5).
3.4.4. Effects of probiotics on length of hospital stay
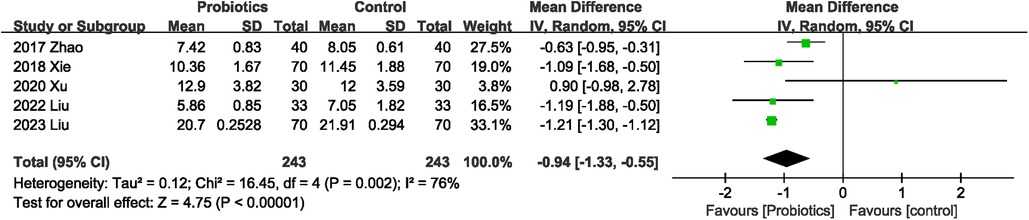
Length of hospital stay was mentioned in five (21–24, 26) of the nine RCTs. Four studies (21, 22, 24, 26) showed that probiotics were associated with shorter length of hospital stay, while one (23) study observed no significant difference in length of hospital stay between the probiotics and control groups. Probiotics were associated with a significant reduction in length of hospital stay (MD −0.94 days; 95% CI: −1.33, −0.55), with significant heterogeneity between studies (I2 = 76%, P = 0.002; Figure 6).
3.4.5. Effects of probiotics on weight loss
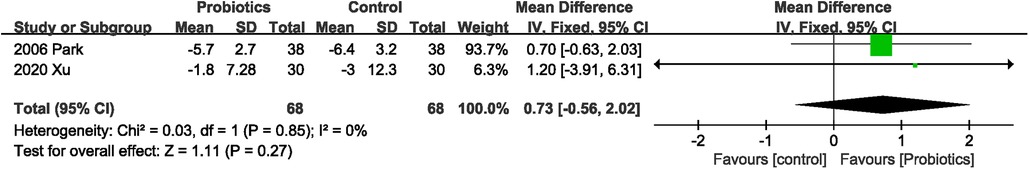
The combined effect size of the two studies (20, 23) showed that probiotics did not improve weight loss (MD 0.73 kg; 95% CI: −0.56, 2.02) in patients with gastric cancer compared with controls. No significant heterogeneity (I2 = 0%, P = 0.85) was observed between studies (Figure 7).
3.4.6. Effects of probiotics on serum albumin levels
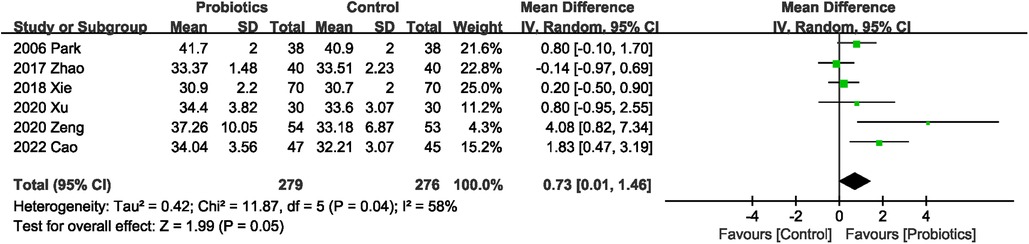
Six studies (20–23, 25, 27) described serum albumin levels, and probiotics supplementation significantly improved albumin levels, with high heterogeneity (MD 0.73 g/L; 95% CI: 0.01, 1.46; Heterogeneity: I2 = 58%, P = 0.04) between studies (Figure 8).
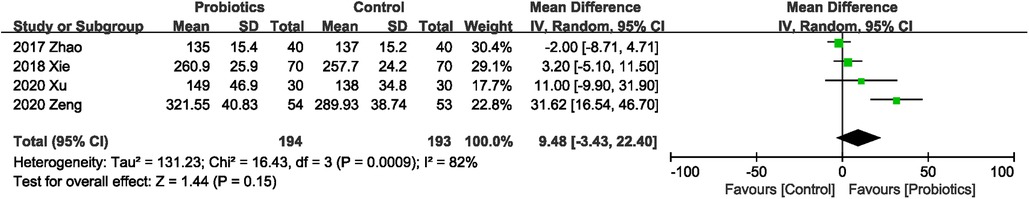
3.4.7. Effects of probiotics on serum prealbumin levels
A total of 387 participants in four studies (21–23, 25) reported serum prealbumin levels, and probiotics did not increase serum prealbumin levels compared with controls, with significant heterogeneity between studies (MD 9.48 mg/L; 95% CI: −3.43, 22.40; Heterogeneity: I2 = 82%, P = 0.0009) (Figure 9).
3.5. Sensitivity analysis
The results of the sensitivity analysis showed that the total effect size of the time to first exhaust, the incidence of postoperative infectious complications, length of hospital stay, and serum prealbumin levels were not affected by the elimination of any one study. The total effect size for the time to first defecation changed when the study by Zeng et al. (25) (MD −19.86 h; 95% CI: −40.08, 0.36; Heterogeneity: I2 = 94%, P < 0.00001) was excluded. Sensitivity analysis indicated that Park et al.’ study (21) (MD 0.79; 95% CI: −0.14, 1.72), Cao et al.’ study (27) (MD 0.49; 95% CI: −0.20, 1.18), Zeng et al.’ study (25) (MD 0.54; 95% CI: −0.06, 1.14) and Xu et al.’ study (23) (MD 0.76; 95% CI: −0.07, 1.58) prominently affected the total effect size of serum albumin levels.
4. Discussion
To our knowledge, this is the first meta-analysis to evaluate the effects of probiotics on postoperative infectious complications and nutritional status in gastric cancer patients undergoing surgery. The results of this meta-analysis showed that probiotics supplementation did not improve weight loss and serum prealbumin level, but could shorten the first exhaust time, the time to first defecation, and the length of hospital stay, improve serum albumin level, and reduce the incidence of postoperative infectious complications. The results of this study have important clinical significance. Gastric cancer surgery is associated with high morbidity and mortality, and postoperative complications and poor nutritional status not only prolong the length of hospital stay and bring heavy economic burden to patients, but also are associated with poor long-term prognosis (4). The results of this study confirm the effect of probiotics on the prevention of postoperative infectious complications and the improvement of nutritional status in patients with gastric cancer. Probiotics may be a potential strategy to improve the prognosis and reduce the medical burden of gastric cancer patients undergoing surgery.
In recent years, the application of probiotics in reducing perioperative complications of abdominal surgery has become a hot spot in clinical research. Even for some highly invasive procedures, such as pancreaticoduodenectomy, probiotics are still effective (28). This is consistent with the conclusion of this study. Postoperative ileus is one of the most common complications in patients with gastric cancer, with abdominal pain, abdominal distension, weak defecation, nausea and vomiting as the main clinical manifestations, and is one of the main reasons for prolonged hospital stay (29, 30). Recovery of intestinal obstruction is fastest in the small intestine (8–12 h), followed by the stomach (1–2 days), and finally the colon (3–5 days) (30). Several randomized controlled clinical studies have shown that probiotics can promote recovery of intestinal function after abdominal surgery such as colon cancer surgery and pancreaticoduodenal surgery (16, 28, 31), similar to our results. In addition, the effect of probiotics on shortening hospital stay in patients with gastric cancer may also be related to the rapid recovery of intestinal function by probiotics.
The mechanism by which probiotics confer a benefit to gastric cancer surgery is not yet clear, which may be related to the following aspects: First, surgery leads to intestinal microflora imbalance and intestinal microflora translocation, while probiotics can protect the intestinal mucosal barrier and reduce intestinal microflora imbalance and translocation (32, 33); Second, probiotics can enhance the body’s immune function (32); Finally, probiotics can also improve intestinal motility by regulating the fermentation products of intestinal flora (31).
Malnutrition is a common and painful problem for people with gastric cancer, which can be exacerbated by gastric cancer surgery and stress (34, 35). Malnutrition is one of the damaging factors of immune function and is closely related to the increase of hospital stay and mortality (35, 36). Weight loss alone or in combination with laboratory parameters such as albumin and prealbumin is a major indicator of malnutrition (35). Therefore, the effects of probiotics on weight loss, serum albumin and serum prealbumin in gastric cancer patients undergoing surgery were analyzed in this study. Albumin is an important predictor of short-term complications after gastric cancer surgery (37). Our meta-analysis showed that probiotics were associated with improved serum albumin levels. Similarly, Zheng et al. (38) found that supplementation with Bifidobacterium tetravaccine tablets seven days after partial gastrectomy significantly improved nutritional parameters, including albumin and total protein. The combined results showed that probiotics did not reduce weight loss, but all the included studies (20, 23) showed a trend of improvement in weight loss, which may be related to the small number of studies included. More trials are needed in the future to explore the effect of probiotics on weight loss in gastric cancer patients undergoing surgery.
Several strength points warrant mention: on the one hand, only RCTs were included, which improves the reliability of the conclusions of our study. On the other hand, we conducted extensive literature search (besides database retrieval, hand search was used).
There are several limitations in our meta-analysis. First, only nine trials were included and included some trials with a small sample size. Secondly, there is a large heterogeneity in some outcome indicators in this study, which may be related to the differences in the species, dose and length of treatment of probiotics used between studies. However, due to the limited number of studies included, we were unable to perform subgroup analysis and meta-regression. In addition, some outcome measures, such as the time to first defecation and serum albumin levels, are not robust enough, and more studies are needed to further explore the effects of probiotics on these indicators. Finally, only four of the studies included had a double-blind design.
In conclusion, our study shows that probiotics supplementation can effectively prevent postoperative infectious complications, promote postoperative recovery, and improve nutritional status in patients undergoing gastric cancer surgery. But given some limitations, these results need to be interpreted with caution. Large, high-quality prospective studies are needed to verify the benefits of probiotics in gastric cancer patients undergoing surgery.
Author contributions
WY: Data curation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. BD: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. (2020) 38:28–42. doi: 10.1016/j.clnesp.2020.05.007
3. Liu D, Jing X, Cao S, Liu X, Tan X, Jiang H, et al. Impact of drinking Chinese green tea on postoperative short outcomes for gastric cancer: a randomized controlled trial. Eur J Clin Nutr. (2021) 75:1568–77. doi: 10.1038/s41430-021-00868-8
4. Li J, Zhang Y, Hu DM, Gong TP, Xu R, Gao J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: a systematic review and meta-analysis of 64 follow-up studies. Asian Journal of Surgery. (2020) 43(7):719–29. doi: 10.1016/j.asjsur.2019.10.007
5. Diers J, Baum P, Wagner JC, Matthes H, Pietryga S, Baumann N, et al. Hospital volume following major surgery for gastric cancer determines in-hospital mortality rate and failure to rescue: a nation-wide study based on German billing data (2009-2017). Gastric Cancer. (2021) 24(4):959–69. doi: 10.1007/s10120-021-01167-8
6. Xin F, Mzee SAS, Botwe G, He H, Zhiyu S, Gong C, et al. Short-term evaluation of immune levels and nutritional values of EN versus PN in gastric cancer: a systematic review and a meta-analysis. World J Surg Oncol. (2019) 17(1):114. doi: 10.1186/s12957-019-1658-9
7. Yang Z, Wu Q, Liu Y, Fan D. Effect of perioperative probiotics and synbiotics on postoperative infections after gastrointestinal surgery: a systematic review with meta-analysis. JPEN J Parenter Enteral Nutr. (2017) 41(6):1051–62. doi: 10.1177/0148607116629670
8. Tang G, Zhang L. Update on strategies of probiotics for the prevention and treatment of colorectal cancer. Nutr Cancer. (2020) 74:1–12. doi: 10.1080/01635581.2020.1865420
9. Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. (2017) 9(6):555. doi: 10.3390/nu9060555
10. Jahanshahi M, Maleki Dana P, Badehnoosh B, Asemi Z, Hallajzadeh J, Mansournia MA, et al. Anti-tumor activities of probiotics in cervical cancer. J Ovarian Res. (2020) 13(1):68. doi: 10.1186/s13048-020-00668-x
11. Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, et al. Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol. (2019) 234(10):17127–43. doi: 10.1002/jcp.28473
12. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. (2019) 25(5):716–29. doi: 10.1038/s41591-019-0439-x
13. Nada HG, Sudha T, Darwish NH, Mousa SA. Lactobacillus acidophilus and Bifidobacterium longum exhibit antiproliferation, anti-angiogenesis of gastric and bladder cancer: impact of COX2 inhibition. PharmaNutrition. (2020) 14;100219.
14. Darbandi A, Mirshekar M, Shariati A, Moghadam MT, Lohrasbi V, Asadolahi P, et al. The effects of probiotics on reducing the colorectal cancer surgery complications: a periodic review during 2007-2017. Clin Nutr. (2020) 39(8):2358–67. doi: 10.1016/j.clnu.2019.11.008
15. Chowdhury AH, Adiamah A, Kushairi A, Varadhan KK, Krznaric Z, Kulkarni AD, et al. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. (2020) 271(6):1036–47. doi: 10.1097/sla.0000000000003581
16. Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg. (2016) 40(8):1985–92. doi: 10.1007/s00268-016-3499-9
17. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated march 2011). The cochrane collaboration. Cochrane: Cochrane Collaboration (2011). Available from: www.cochrane-handbook.org
18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
19. Niu W, Du X, Zhang Q, Kang C. Effects of bifidobacteria use after gastric cancer surgery on intestinal flora and serum corticotropin-releasing factor. Curr Top Nutraceutical Res. (2019) 17(4):422–5.
20. Park DJ, Lee HJ, Lee KU, Yang HK. Effect of Oral Saccharomyces Cerevisiae Hansen CBS 5926 Therapy on Gastrointestinal Symptoms and Nutrition in Gastrectomized Patients (2006).
21. Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Rao Z, et al. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: a prospective randomized and controlled trial. Medicine (Baltimore). (2017) 96(43):e8418. doi: 10.1097/md.0000000000008418
22. Xie H, Lu Q, Wang H, Zhu X, Guan Z. Effects of probiotics combined with enteral nutrition on immune function and inflammatory response in postoperative patients with gastric cancer. J Buon. (2018) 23(3):678–83.30003737
23. Xu R, Xiao S, Ding Z, Zhao P. Does early postoperative enteral ecoimmunonutrition enhance intestinal function in gastric cancer? Asia Pac J Clin Nutr. (2020) 29(3):469–75. doi: 10.6133/apjcn.202009_29(3).0004
24. Liu G, Cao S, Liu X, Li Z, Tian Y, Zhang X, et al. Effect of perioperative probiotic supplements on postoperative short-term outcomes in gastric cancer patients receiving neoadjuvant chemotherapy: a double-blind, randomized controlled trial. Nutrition. (2022) 96:111574. doi: 10.1016/j.nut.2021.111574
25. Zeng X, Yang SW, Yang HQ, Chen Y, Pan QJ. Effect of bifid triple viable combined with enteral nutrition support on gastrointestinal function and nutritional indexes in patients with gastric cancer after operation. World Chinese Journal of Digestology. (2020) 28(11):410–6. doi: 10.11569/wcjd.v28.i11.410
26. Liu W, Zheng C, Li Q, Xu T, Cao W, Shi M, et al. Preoperative oral probiotics relieve insulin resistance and gut dysbacteriosis in patients with gastric cancer after gastrectomy. J Funct Foods. (2023) 101:10. doi: 10.1016/j.jff.2023.105426
27. Cao W, Zheng C, Xu X, Jin R, Huang F, Shi M, et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front Immunol. (2022) 13:1076245. doi: 10.3389/fimmu.2022.1076245
28. Folwarski M, Dobosz M, Małgorzewicz S, Skonieczna-Żydecka K, Kaźmierczak-Siedlecka K. Effects of Lactobacillus rhamnosus GG on early postoperative outcome after pylorus-preserving pancreatoduodenectomy: a randomized trial. Eur Rev Med Pharmacol Sci. (2021) 25(1):397–405. doi: 10.26355/eurrev_202101_24407
29. Liang WQ, Zhang KC, Cui JX, Xi HQ, Cai AZ, Li JY, et al. Nomogram to predict prolonged postoperative ileus after gastrectomy in gastric cancer. World J Gastroenterol. (2019) 25(38):5838–49. doi: 10.3748/wjg.v25.i38.5838
30. Liu Q, Jiang H, Xu D, Jin J. Effect of gum chewing on ameliorating ileus following colorectal surgery: a meta-analysis of 18 randomized controlled trials. Int J Surg. (2017) 47:107–15. doi: 10.1016/j.ijsu.2017.07.107
31. Bajramagic S, Hodzic E, Mulabdic A, Holjan S, Smajlovic SV, Rovcanin A. Usage of probiotics and its clinical significance at surgically treated patients sufferig from colorectal carcinoma. Med Arch. (2019) 73(5):316–20. doi: 10.5455/medarh.2019.73.316-320
32. Arumugam S, Lau CS, Chamberlain RS. Probiotics and synbiotics decrease postoperative sepsis in elective gastrointestinal surgical patients: a meta-analysis. J Gastrointest Surg. (2016) 20(6):1123–31. doi: 10.1007/s11605-016-3142-y
33. Liu PC, Yan YK, Ma YJ, Wang XW, Geng J, Wang MC, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. (2017) 2017:6029075. doi: 10.1155/2017/6029075
34. Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, et al. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). (2015) 94(31):e1311. doi: 10.1097/md.0000000000001311
35. Nikniaz Z, Somi MH, Nagashi S, Nikniaz L. Impact of early enteral nutrition on nutritional and immunological outcomes of gastric cancer patients undergoing gastrostomy: a systematic review and meta-analysis. Nutr Cancer. (2017) 69(5):693–701. doi: 10.1080/01635581.2017.1324996
36. Cheng Y, Zhang J, Zhang L, Wu J, Zhan Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: a systematic review and meta-analysis. BMC Gastroenterol. (2018) 18(1):11. doi: 10.1186/s12876-018-0741-y
37. Liu ZJ, Ge XL, Ai SC, Wang HK, Sun F, Chen L, et al. Postoperative decrease of serum albumin predicts short-term complications in patients undergoing gastric cancer resection. World J Gastroenterol. (2017) 23(27):4978–85. doi: 10.3748/wjg.v23.i27.4978
Keywords: probiotics, gastric cancer, infectious complications, clinical outcomes, meta-analysis
Citation: Ye W, Dong B, Li G and Zhang Y (2023) The effect of probiotics on surgical outcomes in patients with gastric cancer: a meta-analysis of randomized controlled trials. Front. Surg. 10:1254597. doi: 10.3389/fsurg.2023.1254597
Received: 7 July 2023; Accepted: 3 October 2023;
Published: 13 October 2023.
Edited by:
J. Antonio Quiros, Icahn School of Medicine at Mount Sinai, United States© 2023 Ye, Dong, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqiang Zhang cWd4bWxAMTI2LmNvbQ==
 Wei Ye
Wei Ye Bo Dong
Bo Dong Yuqiang Zhang
Yuqiang Zhang