- 1Department of Biomedical Informatics, College of Medicine, Konyang University, Daejeon, Republic of Korea
- 2Konyang Medical Data Research Group-KYMERA, Konyang University Hospital, Daejeon, Republic of Korea
- 3Department of Blood Management Services, Daejeon Eulji Medical Center, Eulji University, Daejeon, Republic of Korea
- 4Department of Laboratory Medicine, Daejeon Eulji Medical Center, Eulji University, Daejeon, Republic of Korea
- 5Department of Hematooncology, Daejeon Eulji Medical Center, Eulji University, Daejeon, Republic of Korea
- 6Department of Orthopaedic Surgery, Daejeon Eulji Medical Center, Eulji University, Daejeon, Republic of Korea
Background: Severe blood loss during spine surgery increases the need for blood transfusion. Transfusion carries the risks of infection, complications, and postoperative morbidity; therefore, minimizing these risks is crucial for all surgical patients.
Methods: A comprehensive literature search was conducted in PubMed, Cochrane, and EMBASE to find studies examining the effect of tranexamic acid (TXA) on spine surgeries in patients who received blood transfusion. We used the mean difference (MD) and 95% credible intervals (CrI) to analyze continuous outcomes, such as intraoperative blood loss, postoperative blood loss, hemoglobin drop, and length of hospital stay. To evaluate categorical outcomes, such as blood transfusion rate and complication rate, the odds ratios (OR) and 95% CrI were determined.
Results: A total of 38 randomized controlled trials were included, evaluating six outcomes across 10 treatment groups. Low-dose intravenous (IV) TXA combined with temperature intervention (15 mg/kg) significantly reduced intraoperative blood loss compared with placebo [MD: −112.0; 95% CrI: −211.0 to −14.9, surface under the cumulative ranking curve (SUCRA): 78.37%]. The administration of more than two doses of TXA significantly reduced intraoperative blood loss (MD: −101.0, 95% CrI: −161.0 to −44.1, SUCRA: 77.65%) and postoperative blood loss (MD: −177.0, 95% CrI: −275.0 to −92.4, SUCRA: 85.66%) compared with placebo. Both treatments significantly impacted the hemoglobin drop and blood transfusion rate.
Conclusions: Low-dose IV TXA with temperature intervention and the combined use of TXA significantly improved blood loss, hemoglobin drop, and blood transfusion rate during spine surgeries. Further studies involving larger populations are warranted and should be carefully designed to determine the potential risk of complications.
Systematic Review Registration: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024531557, identifier: CRD42024531557.
Introduction
Blood transfusions are often necessary in many surgical procedures, with cardiovascular and orthopedic surgical procedures posing the highest risks of blood transfusion (1). The number of spine fusion procedures performed in the United States increased from 54, 000 to 350, 000 between 1993 and 2007 (2). In Australia, simple lumbar fusion rates increased from 1.3 to 2.8 per 100,000 people, while complex fusion rates increased from 0.6 to 2.4 per 100,000 people from 2003 to 2013 (3). These trends are likely related to the aging population and increased average life expectancy. Elderly individuals often develop various medical comorbidities, including reduced bone mass density, osteoporosis, decreased mobility, spine degeneration and deformities, poor balance, and a higher risk of falls (4). Evidence shows that the blood transfusion rate during adult spine fusion surgery ranges from 50% to 81%. Most studies on spine surgery reported that the amount of blood loss requiring blood transfusion ranges from 650 to 2,839 ml per case (5).
Orthopedic surgeries, including spine surgery, often result in significant blood loss. Spine surgery, which involves bone resection and extensive dissection of soft tissues, is particularly associated with substantial perioperative blood loss. This can be attributed to large wound areas, prolonged operative times, and the involvement of abundant cancellous bone. Although the amount of perioperative blood loss may significantly vary among procedures depending on surgical and nonsurgical factors, it remains a major concern during spine surgery (6).
Blood transfusions increase the risk of infections, complications, coagulopathy, and postoperative morbidity (7, 8). These risks also contribute to prolonged hospital stays and negatively impact quality of life. Therefore, minimizing these risks is essential for all patients undergoing surgery. Various strategies have been employed in spine surgeries, including the use of recombinant erythropoietin and intravenous iron preparations for the preoperative correction of anemia. Additionally, hemostatic agents, autologous blood transfusions, cell savers, and tranexamic acid (TXA) have been used to reduce intraoperative and postoperative blood loss.
TXA is a synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect by blocking the lysine-binding sites on plasminogen molecules, thereby inhibiting the interaction of plasminogen and plasmin's heavy chain with lysine residues on fibrin surface (9). Reducing blood loss and blood transfusion rate is the ultimate goal of surgery. Various doses and methods of TXA administration have been explored. For instance, TXA can be administered intravenously, orally, topically, or in combination. Several studies have been conducted to determine the optimal approach (10, 11). Recently, temperature control methods for maintaining normal body temperature have also been investigated (12). However, despite these efforts, determining the most appropriate approach for administering TXA remains controversial. Therefore, no consensus or official guidelines have been established.
Although previous meta-analyses have been conducted (13), recent randomized controlled trials (RCTs) have emerged since then. Among them, providing evidence-based medicine for comprehensive decision-making, particularly incorporating new methods such as temperature intervention, remains essential. Many surgeons perform fusion surgery as their personal preference (14). Concerns also persist regarding the use of TXA due to the potential risk of myocardial infarction (MI), stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE) (6, 15).
This systematic review and network meta-analysis (NMA) evaluated RCTs to determine the optimal method of administering TXA and its effects on blood loss, blood transfusion rate, complication rate, and length of hospital stay.
Materials and methods
This study was registered in the PROSPERO database (registration number: CRD42024531557) and conducted in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (16).
Data sources and literature search
Three independent authors (SR Shim, S Han, and C Ihm) conducted a comprehensive literature search in PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane (https://www.cochranelibrary.com/search), and EMBASE (https://www.embase.com) to find articles published until the end of May 2024. No language restrictions were applied. One article was written in Chinese but the abstract was in English and we used a translation program for other necessary parts. Medical Subject Headings terms were used for searching PubMed and Cochrane, Emtree terms for EMBASE, and text keywords for identifying RCTs examining the effect of TXA on spine surgeries in patients who received blood transfusion (Supplementary Table S1).
Study selection
Studies that (1) included patients who received blood transfusion during spine surgeries; (2) investigated interventions including TXA use; (3) provided comparisons between various methods of TXA administration and a control group (normal saline); and (4) measured outcomes as mean difference (MD) in intraoperative blood loss, postoperative blood loss, hemoglobin drop, blood transfusion rate, complication rate, and length of hospital stay in RCT studies were analyzed. Following the American Association of Hip Society, American Academy of Orthopaedic Surgeons, American Association of Hip and Knee Surgeons, and American Society of Regional Anesthesia and Pain Medicine guidelines (17), TXA treatments were divided into 10 groups: high-dose IV TXA (≥20 mg/kg or >1 g; IV_high), low-dose IV TXA (<20 mg/kg or ≤1 g; IV_low), low-dose IV TXA with temperature intervention (15 mg/kg; IV_low_temp), multiple IV TXA (multiple use of TXA pre- and post-surgery; IV_multi), high-dose topical TXA (>1.5 g; Topical_high), low-dose topical TXA (≤1.5 g; Topical_low), local infiltration (bilaterally administered into the paraspinal muscles before the incision; Local_inj), combined use (more than two administrations of TXA; Combine), oral (PO) TXA, and placebo.
Meanwhile, (1) reviews, abstracts, editorials, and letters that were not original articles; (2) non-RCTs; and (3) studies with noncomparable treatments were excluded. Three authors (SR Shim, S Han, and C Ihm) independently screened the titles and abstracts to identify relevant studies, reviewed the full text of articles for data extraction, and removed duplicates using Endnote software. All authors mutually discussed and resolved disagreements and cross-checked all references.
Data extraction
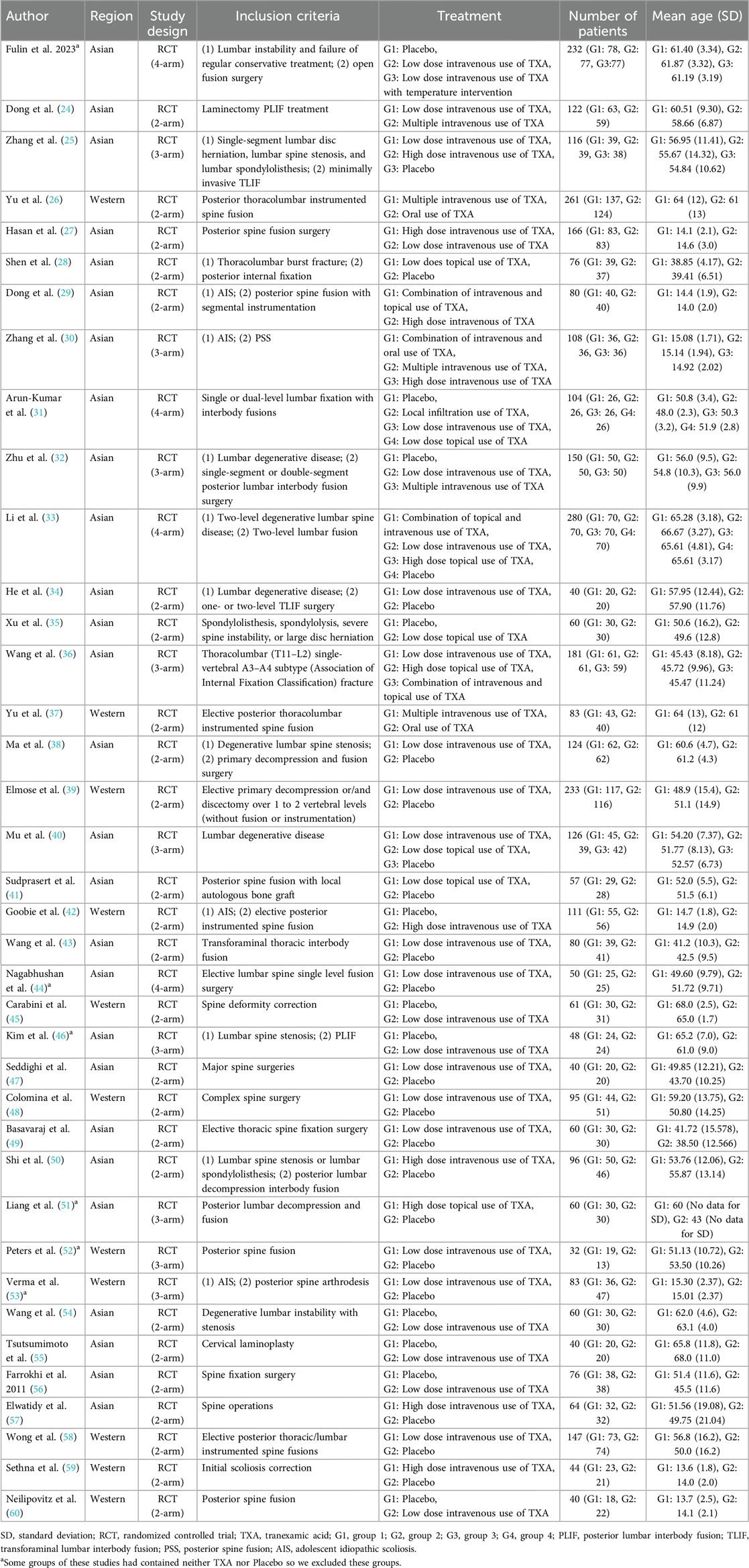
Two authors (SR Shim and S Han) used a data extraction form to categorize the primary details of the studies (first author, publication year, region, and study design), patient characteristics (number of patients, age, type of surgery, and/or disease), and technical aspects (inclusion criteria and TXA treatments; Table 1).
We used the MD to analyze continuous outcomes, such as intraoperative blood loss, postoperative blood loss, hemoglobin drop, and length of hospital stay. To evaluate categorical outcomes, such as blood transfusion rate and complication rate, the odds ratio (OR) was determined. We calculated the pooled standard deviation using the pre- and post-standard deviations when these values were not provided for MD (18). As some articles described several median outcomes, Hozo's method was used to estimate the mean and standard deviation (19).
We calculated the hemoglobin drop as the difference between the preoperative levels and last measured postoperative values. DVT and PE were included in the analysis of complication rate.
Quality assessment
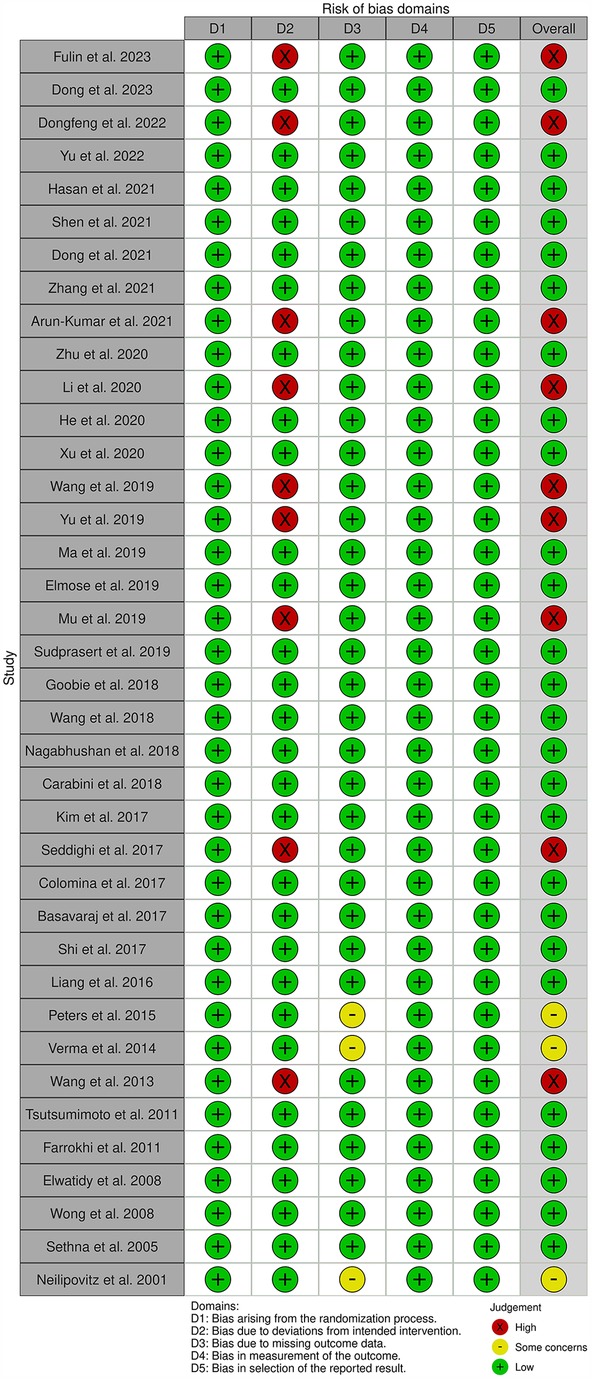
The quality of studies was evaluated using the Cochrane Collaboration Risk of Bias 2.0 (RoB 2) tool (20). The following five domains were assessed: (1) randomization process, (2) deviations from intended interventions, (3) missing data, (4) outcome measurement, and (5) selection of the reported result.
Each domain was classified as “low,” “high” or “some concerns.” The overall risk of bias was classified as “low” when all domains were rated as “low” and “some concerns” when only one domain was rated as “some concerns.” If more than two domains were rated as “some concerns” or if any domain was rated as “high,” then the overall risk of bias was “high.”
Network meta-analysis assessment of outcome findings and statistical analysis
For Bayesian NMA, specific graphical analysis was performed using the “gemtc” package in R software v.4.3.1 (R Foundation for Statistical Computing) (21). To compare the 10 TXA administration methods, a simulation was conducted by incorporating prior distributions and probabilities into the Markov Chain Monte Carlo (MCMC). Subsequently, the optimal convergence model was selected by reviewing the trace plot, normal distribution plot, and the MCMC standard error of the generated posterior distribution. Thus, the posterior probability of the effect size for each treatment was calculated. A consistency test between the direct and indirect comparisons was performed using node-splitting assessments. We used funnel plot and Egger test to assess publication bias (22).
In the Bayesian approach, the optimal probability of selecting individual treatments is determined using the generated posterior distribution. This distribution reflects the priority of each treatment, represented as the surface under the cumulative ranking curve (SUCRA); a higher SUCRA value indicates a higher rank of the intervention (21, 23). The analysis pooled the MDs and 95% credible intervals (CrI). A two-sided p-value of ≤.05 or a 95% CrI that does not contain a null value (MD = 0) was considered significant.
Results
Study selection
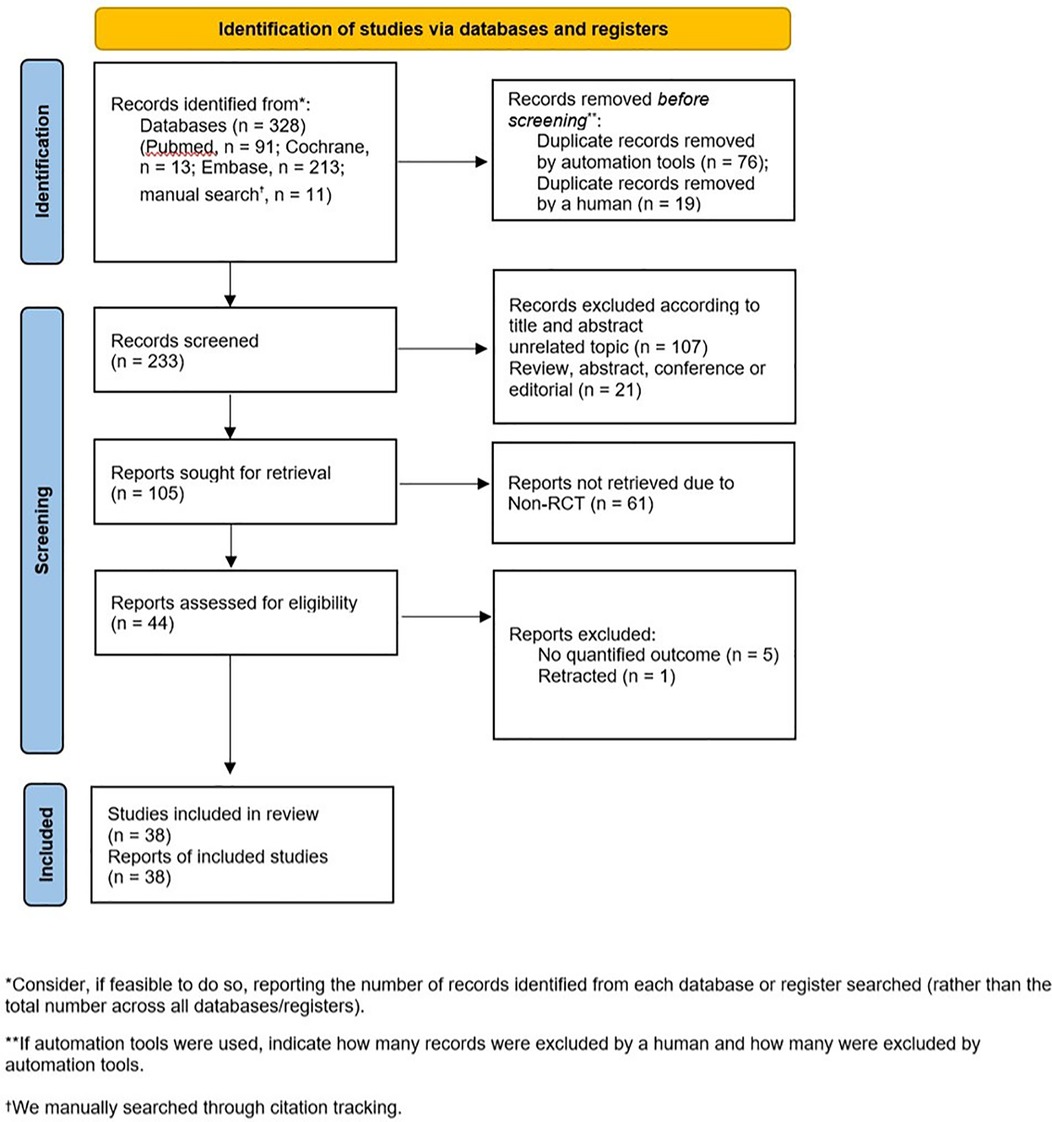
The primary search identified 317 articles from various electronic databases, including PubMed (n = 91), Cochrane (n = 13), and EMBASE (n = 213), with an additional 11 articles identified during manual search through citation tracking. We excluded 95 articles with duplicate or overlapping data, 107 articles with unrelated topics, 21 articles that were not original research, and 61 articles that were not RCTs. After a full-text review of 44 articles, six articles were further excluded: one had been retracted, while the rest had no quantified outcome. After the final selection, 38 articles were included for data extraction (Figure 1).
In total, 3,886 patients were included in 38 studies (12, 24–60), with approximately 55% being women. All studies were RCTs conducted in various regions: 27 studies were performed in Asia, while 11 studies were conducted in Western countries (Table 1). Of the included studies, 25 employed a two-arm design, while nine studies employed a three-arm design. However, four of the nine studies were classified as two-arm trials as some groups did not use TXA or a placebo. Four studies were designed as four-arm trials. However, two of these studies did not use TXA or a placebo. Hence, one was classified as a two-arm study, while the rest were classified as 3-arm studies.
Inconsistency test
The inconsistency tests for the NMA assumption were conducted using the node-splitting approach. The findings (p > 0.05) indicated consistency across direct and indirect comparisons for all outcomes.
Quality assessment
All studies were evaluated using the RoB 2 tool. The randomization process (D1) was recorded as “low” (Figure 2). However, nine studies were classified as having a “high” risk for deviations from intended interventions (D2) due to the lack of blinding. For missing data (D3), three studies were rated as “some concerns” as the number of patients between the TXA group and placebo group differed by >10%. By contrast, the outcome measurement (D4) and selection of the reported result (D5) were both rated as “low.”
Outcome findings
Through this analysis, we organized six outcomes (Figures 3, 4): intraoperative blood loss, postoperative blood loss, hemoglobin drop (Figure 3), blood transfusion rate, complication rate, and length of hospital stay (Figure 4).
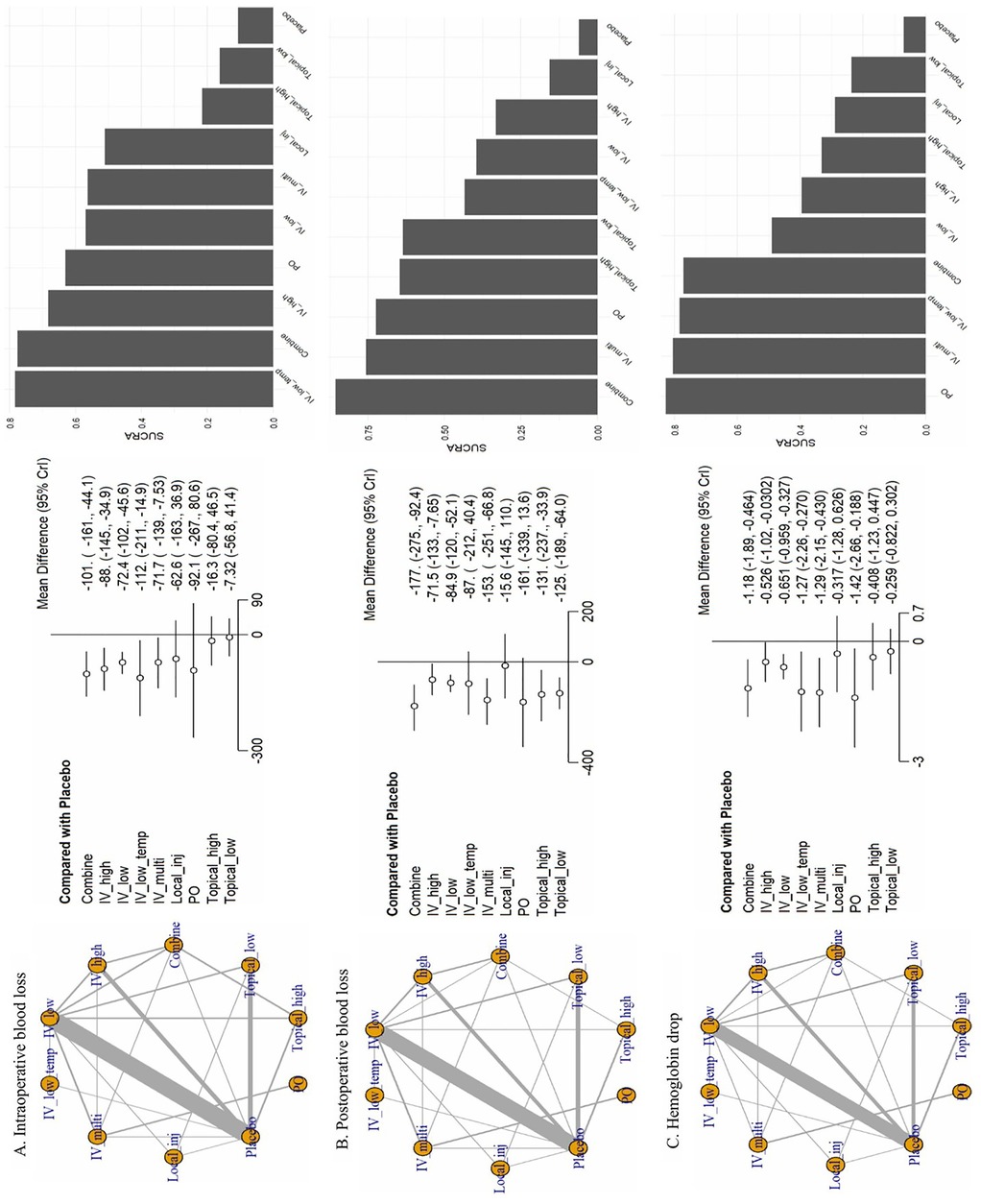
Figure 3. Network plots from the network meta-analysis. (A) Intraoperative blood loss, (B) postoperative blood loss, (C) Hemoglobin drop. CrI, credible intervals; Combine, more than two administrations of TXA; IV_high, high-dose IV TXA (≥20 mg/kg or >1 g); IV_low, low-dose IV TXA (<20 mg/kg or ≤1 g); IV_low_temp, low-dose IV TXA with temperature intervention (15 mg/kg); IV_multi, multiple use of TXA pre- and post-surgery; Local_inj, local infiltration (bilaterally administered into the paraspinal muscles before the incision; PO, oral; Topical_high, high-dose topical TXA (>1.5 g); Topical low, low-dose topical TXA (≤1.5 g).
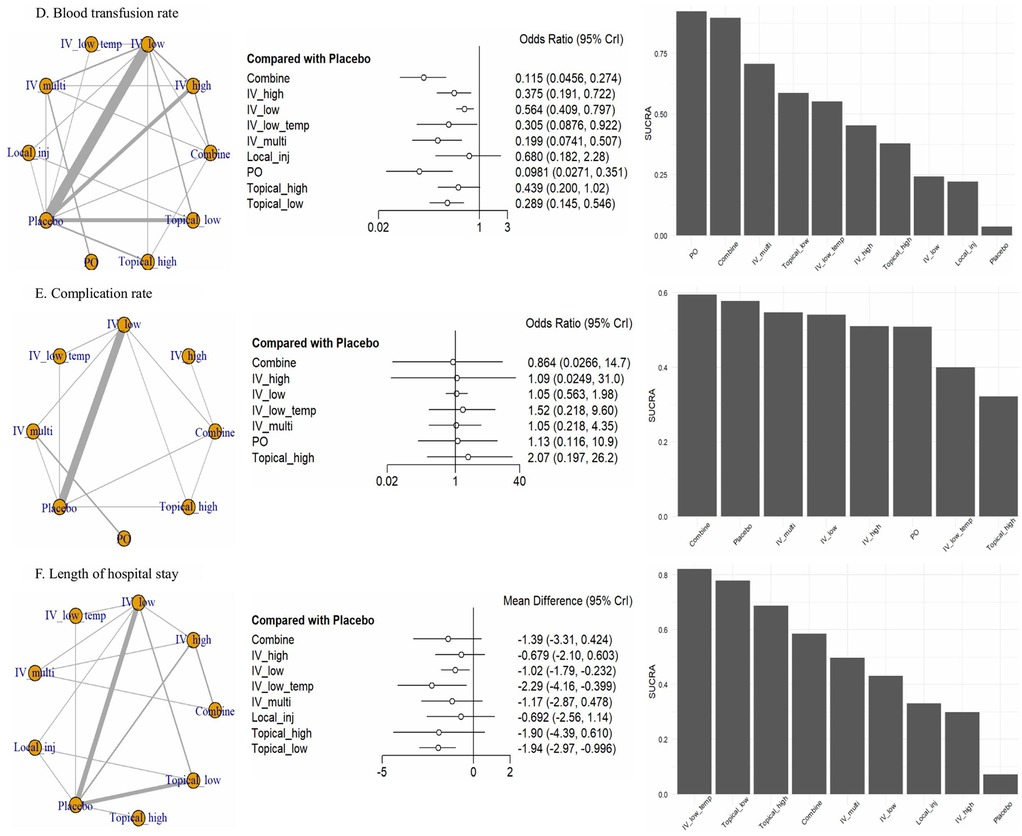
Figure 4. Network forest plot. (D) Blood transfusion rate, (E) complication rate, (F) length of hospital stay. CrI, credible intervals; Combine, more than two administrations of TXA; IV_high, high-dose IV TXA (≥20 mg/kg or >1 g); IV_low, low-dose IV TXA (<20 mg/kg or ≤1 g); IV_low_temp, low-dose IV TXA with temperature intervention (15 mg/kg); IV_multi, multiple use of TXA pre- and post-surgery; Local_inj, local infiltration (bilaterally administered into the paraspinal muscles before the incision; PO, oral; Topical_high, high-dose topical TXA (>1.5 g); Topical low, low-dose topical TXA (≤1.5 g).
For intraoperative blood loss, we included 36 studies involving 10 treatments. IV_low_temp (MD: −112.0, 95% CrI: −211.0 to −14.9), Combine (MD: −101.0, 95% CrI: −161.0 to −44.1), and IV_high (MD: −88.0, 95% CrI: −145.0 to −34.9) were significantly different compared with placebo. The following SUCRA rankings were consistent with the abovementioned findings: IV_low_temp (SUCRA: 78.37%) was the highest ranked, followed by Combine (SUCRA: 77.65%) and IV_high (SUCRA: 68.32%). On the contrary, Topical_high (SUCRA: 21.51%) and Topical_low (SUCRA: 16.17%) were the lowest ranked.
For postoperative blood loss, we analyzed 32 studies involving 10 treatments. Compared with placebo, Combine (MD: −177.0, 95% CrI: −275.0 to −92.4) and IV_multi (MD: −153.0, 95% CrI: −251.0 to −66.8) showed significant differences. SUCRA rankings were consistent, with Combine (SUCRA: 85.66%) and IV_multi (SUCRA: 75.67%) treatments achieving the highest rank, whereas Local_inj (SUCRA: 15.67%) achieved the lowest rank.
Regarding the hemoglobin drop, we analyzed 28 studies involving 10 treatments. PO (MD: −1.42, 95% CrI: −2.66 to −0.19), IV_multi (MD: −1.29, 95% CrI: −2.15 to −0.43), IV_low_temp (MD: −1.27, 95% CrI: −2.26 to −0.27), and Combine (MD: −1.18, 95% CrI: −1.89 to −0.46) showed significant differences compared with placebo. SUCRA rankings corroborated these results with the most effective being ranked in the following order: PO (SUCRA: 82.81%), IV_multi (SUCRA: 80.53%), IV_low_temp (SUCRA: 78.43%), and Combine (SUCRA: 77.13%).
For blood transfusion rate, we included 28 studies involving 10 treatments. PO (OR: 0.10, 95% CrI: 0.03–0.35), Combine (OR: 0.12, 95% CrI: 0.05–0.27), IV_multi (OR: 0.20, 95% CrI: 0.07–0.51), Topical_low (OR: 0.29, 95% CrI: 0.15–0.55), and IV_low_temp (OR: 0.31, 95% CrI: 0.09–0.92) were significantly superior to placebo. SUCRA rankings confirmed these findings, with PO (SUCRA: 92.38%), Combine (SUCRA: 89.68%), and IV_multi (SUCRA: 70.77%) ranking the highest, followed by Topical_low (SUCRA: 58.75%) and IV_low_temp (SUCRA: 55.23%), whereas Local_inj (SUCRA: 22.16%) was ranked the lowest.
In terms of complication rate, we analyzed 14 studies involving eight treatments, excluding Topical_low and Local_inj. However, the differences between the treatments were not significant compared with placebo.
In terms of the length of hospital stay, we included 19 studies involving nine treatments, excluding PO. Significant differences were only observed for IV_low_temp (MD: −2.29, 95% CrI: −4.16 to −0.40) and Topical_low (MD: −1.94, 95% CrI: −2.97 to −0.10) compared with placebo. SUCRA rankings indicated that IV_low_temp (SUCRA, 82.12%) was the highest ranked, followed by Topical_low (SUCRA: 77.86%).
Overall, the SUCRA values for ranking probabilities across outcomes indicated that TXA achieved the highest overall ranking in the NMA (Figures 3, 4).
Publication bias
Statistical methods for detecting publication bias or small-study effects are shown in Supplementary Figure S1. Funnel plots for individual outcomes (intraoperative blood loss, postoperative blood loss, hemoglobin drop, blood transfusion rate, complication rate, and length of hospital stay) showed visual symmetry. Additionally, Egger's regression analysis did not reveal any evidence of publication bias or small-study effects in this NMA (p > 0.05).
Discussion
This systematic review and NMA is the first study to evaluate the effects of low-dose IV TXA combined with temperature intervention on blood transfusion outcomes. We aimed to evaluate the effects of various TXA administration methods on blood loss, blood transfusion rate, complication rate, and length of hospital stay during spine surgery. A comprehensive NMA that closely examined 38 RCTs showed that IV_low_temp and Combine significantly improved surgical indices such as blood loss, hemoglobin drop, and blood transfusion rate. TXA has been used in various surgical procedures, demonstrating potential postoperative hemostatic benefits along with reduced blood loss and transfusion rates (61, 62). Additionally, TXA may aid in bleeding control in patients with platelet function abnormalities (63). To minimize the risk of side effects and maximize the benefits of TXA, the optimal dose and route must be carefully considered.
Various methods for administering TXA exist, with IV administration being the most commonly used in our study. IV TXA demonstrated a significant reduction in intraoperative blood loss (MD: −185.0 ml, 95% CrI: −302.1 to −67.9) (10) and estimated blood loss compared with placebo (841 vs. 1,336 ml, p = .002) (15). In our study, IV TXA also showed a reduction in most outcomes (intraoperative blood loss, postoperative blood loss, hemoglobin drop, and blood transfusion rate), positioning it as a top-priority method for TXA administration during blood transfusion. The time needed to achieve the maximum plasma levels of TXA is 30 min post-intramuscular administration, 2 h post-oral administration, and 5–15 min post-IV administration (64); therefore, IV is the recommended method.
With regard to IV doses, low-dose IV TXA has been the most commonly used (61), likely due to concerns about complications such as MI, VTE, and PE. Low-dose IV TXA reduced blood loss by 34% (p < 0.001). Meanwhile, evidence related to the effect of high-dose TXA remains lacking (11). Additionally, the low-dose IV TXA group experienced significantly reduced blood loss and required significantly fewer blood transfusions compared with the control group, with no significant differences in intraoperative and postoperative complications in patients with adolescent idiopathic scoliosis undergoing posterior spine fusion (65). In our study, low-dose IV TXA showed significant effects on five outcomes (intraoperative blood loss, postoperative blood loss, hemoglobin drop, blood transfusion rate, and length of hospital stay). It reduced intraoperative blood loss (940 ml vs. 1,280 ml, p = 0.01) (48), postoperative blood loss (29.9%, p < 0.01) (54), and the total amount of blood transfused (28%) compared with placebo (p = 0.045) (60). Additionally, high-dose IV TXA showed significant effects on four parameters (intraoperative blood loss, postoperative blood loss, hemoglobin drop, and blood transfusion rate). This treatment resulted in less intraoperative blood loss (836 ± 373 vs. 1,031 ± 484 ml, p = 0.02) (42), a 49% reduction in intraoperative blood loss (p < 0.007) and an 80% decrease in blood transfusion requirements (p < 0.008) compared with placebo (57). However, high-dose TXA showed no additional benefits (11). Consequently, low-dose TXA has more pronounced benefits with a lower risk of complications.
In addition to the TXA injection route or dose, other factors should also be considered to improve surgical outcomes. The results of our study demonstrated that the combination of low-dose IV TXA and temperature intervention (IV_low_temp) is effective in reducing intraoperative blood loss. Compared with a placebo, it showed an MD of −112.0 ml (95% CrI: −211.0 to −14.9) and ranked first in SUCRA (78.37%). This finding further underscores the significance of maintaining the core body temperature of patients. In particular, Li et al. (12) randomly assigned patients undergoing spine fusion, with one group receiving low-dose IV TXA combined with temperature control to maintain the body temperature above 36°C. Axillary temperature was monitored to assess the changes in body temperature. Warming measures included controllable electric heating blankets, fluid warmers, and operating room temperature control (24°C). This group showed superior efficacy in reducing intraoperative blood loss by −102.48 ± 141.876 ml (95% CrI: −147.152 to −57.808) (p < 0.001), decreasing hemoglobin levels by −1.25 ± 0.727 g/dl (95% CrI: −1.479 to −1.021), and length of hospital stay by −2.37 ± 0.333 d (95% CrI: −3.024 to −1.716). These findings align with our results, underscoring that temperature control enhances the hemostatic properties of TXA, effectively reducing intraoperative bleeding and subsequently improving patient outcomes. Hypothermia primarily impairs platelet function by disrupting the release of thromboxane A2, which is essential for initial platelet plug formation (66). Anesthetics inhibit the body's thermoregulation, and the low temperature in the operating rooms increases the risk of hypothermia (67). Even mild hypothermia (<1°C) significantly increased blood loss by approximately 16% and the relative risk of blood transfusion by approximately 22% (68). Maintaining perioperative normothermia effectively reduces these risks by clinically significant amounts. Therefore, this treatment has significant potential to minimize blood loss, making it one of the most effective strategies for reducing surgical risks. Further studies are needed to explain the effects of TXA on temperature management during surgery and establish standardized protocols for its use in spine surgery.
Furthermore, we defined Combine as more than two administrations of TXA that effectively and significantly reduced both intraoperative and postoperative blood loss. Compared with placebo, the MDs were −101.0 ml (95% CrI: −161.0 to −44.1) for intraoperative blood loss and −177.0 ml (95% CrI: −275.0 to −92.4) for postoperative blood loss. The significance of combined TXA administration in reducing blood loss and transfusion rates aligns with the findings of a previous study (13). The previous study only included IV and topical administration, whereas our study includes IV, topical, and PO administration, providing flexibility through various combination administration routes. Combining IV and topical TXA yields superior outcomes in reducing total blood loss and allogeneic transfusion rates (69). The combined use of TXA can stabilize the fibrinolytic system in the first 24 h after surgery, thus efficiently reducing blood loss (33). This approach indicates that maximizing the blood-clotting effect can be achieved using various TXA administration routes or doses.
Our study has some limitations. First, several individual RCTs were of low quality due to the absence of blinding. However, the overall quality assessment was deemed good as all studies employed randomization. Second, this NMA was unable to establish a closed loop owing to insufficient data, resulting in a synthesis encompassing all types of spine surgeries rather than conducting a detailed subgroup analysis of specific categories, such as cervical, lumbar, thoracic, sacral, and coccygeal surgeries. Third, this comprehensive systematic review and NMA, which integrates findings from various studies, provides an integrated perspective on TXA. However, the high heterogeneity in the study designs and patient demographics potentially influences the overall conclusions; consequently, careful consideration of methodological concerns is essential.
Evaluation of safety issues related to TXA administration, such as the risks of stroke, MI, DVT, and PE, showed that TXA did not significantly increase the incidence of these complications. Therefore, TXA can be safely used in spine surgery and effectively reduces perioperative bleeding. Our study also found that all TXA administration routes were well tolerated and safe compared with placebo.
Conclusion
Low-dose intravenous TXA with temperature intervention and the combined use of TXA significantly improved blood loss, hemoglobin drop, and blood transfusion rate during spine surgery. Further research involving a larger population and prospective design is needed to accurately quantify the effect of TXA on blood transfusion rates in the current surgical practices. Additionally, careful evaluation of TXA as a potential risk factor for complications is essential.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SS: Conceptualization, Formal analysis, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. SH: Conceptualization, Data curation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JJ: Data curation, Validation, Writing – original draft, Writing – review & editing. IH: Data curation, Validation, Writing – review & editing. YC: Validation, Writing – review & editing. CI: Conceptualization, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative Ai statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1550854/full#supplementary-material
References
1. Montroy J, Lavallée LT, Zarychanski R, Fergusson D, Houston B, Cagiannos I, et al. The top 20 surgical procedures associated with the highest risk for blood transfusion. J Br Surg. (2020) 107(13):e642–e3. doi: 10.1002/bjs.12005
2. Hughey AB, Lesniak MS, Ansari SA, Roth S. What will anesthesiologists be anesthetizing? Trends in neurosurgical procedure usage. Anesth Analg. (2010) 110(6):1686–97. doi: 10.1213/ANE.0b013e3181cbd9cc
3. Machado GC, Maher CG, Ferreira PH, Harris IA, Deyo RA, McKay D, et al. Trends, complications, and costs for hospital admission and surgery for lumbar spinal stenosis. Spine. (2017) 42(22):1737–43. doi: 10.1097/BRS.0000000000002207
4. Fehlings MG, Tetreault L, Nater A, Choma T, Harrop J, Mroz T, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. (2015) 77:S1–5. doi: 10.1227/NEU.0000000000000953
5. Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine (Phila Pa 1976). (2010) 35(9 Suppl):S47–56. doi: 10.1097/BRS.0b013e3181d833f6
6. Cheriyan T, Maier SP II, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. (2015) 15(4):752–61. doi: 10.1016/j.spinee.2015.01.013
7. Bible JE, Mirza M, Knaub MA. Blood-loss management in spine surgery. J Am Acad Orthop Surg. (2018) 26(2):35–44. doi: 10.5435/JAAOS-D-16-00184
8. Willner D, Spennati V, Stohl S, Tosti G, Aloisio S, Bilotta F. Spine surgery and blood loss: systematic review of clinical evidence. Anesth Analg. (2016) 123(5):1307–15. doi: 10.1213/ANE.0000000000001485
9. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. (1999) 57(6):1005–32. doi: 10.2165/00003495-199957060-00017
10. Rahmani R, Singleton A, Fulton Z, Pederson JM, Andreshak T. Tranexamic acid dosing strategies and blood loss reduction in multilevel spine surgery: a systematic review and network meta-analysis: tranexamic acid for multilevel spine surgery. N Am Spine Soc J. (2021) 8:100086. doi: 10.1016/j.xnsj.2021.100086
11. Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. J Br Surg. (2013) 100(10):1271–9. doi: 10.1002/bjs.9193
12. Li F, Huang X, Huang Y, Liang B, Yin D. The efficacy of temperature intervention combined with tranexamic acid in reducing blood loss and accelerating recovery during spinal fusion. Medicine (Baltimore). (2023) 102(47):e36407. doi: 10.1097/MD.0000000000036407
13. Cao Z, Li Q, Guo J, Li Y, Wu J. Optimal administration strategies of tranexamic acid to minimize blood loss during spinal surgery: results of a Bayesian network meta-analysis. Ann Med. (2022) 54(1):2053–63. doi: 10.1080/07853890.2022.2101687
14. Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis: patient selection, costs, and surgical outcomes. Spine. (1997) 22(10):1123–31. doi: 10.1097/00007632-199705150-00012
15. Choi HY, Hyun S-J, Kim K-J, Jahng T-A, Kim H-J. Effectiveness and safety of tranexamic acid in spinal deformity surgery. J Korean Neurosurg Soc. (2017) 60(1):75. doi: 10.3340/jkns.2016.0505.004
16. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162(11):777–84. doi: 10.7326/M14-2385
17. Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Bini SA, Clarke HD, et al. Tranexamic acid in total joint arthroplasty: the endorsed clinical practice guides of the American association of hip and knee surgeons, American society of regional anesthesia and pain medicine, American academy of orthopaedic surgeons, hip society, and knee society. Reg Anesth Pain Med. (2019) 44(1):7–11. doi: 10.1136/rapm-2018-000024
18. Shim SR, Kim S-J. Intervention meta-analysis: application and practice using R software. Epidemiol Health. (2019) 41:1–8. doi: 10.4178/epih.e2019008
19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:1–10. doi: 10.1186/1471-2288-5-13
20. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:1–8. doi: 10.1136/bmj.l4898
21. Shim SR, Kim S-J, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. (2019) 41:1–10. doi: 10.4178/epih.e2019013
22. Cooper H, Hedges LV, Valentine JC. The Handbook of Research Synthesis and Meta-analysis. New York: Russell Sage Foundation (2019). doi: 10.7758/9781610448864
23. Salanti G, Ades A, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
24. Dong W, Liang Y, Li D, Ma Z, Cheng M, Zhang X, et al. The effect of sequential perioperative intravenous tranexamic acid in reducing postoperative blood loss and hidden blood loss after posterior lumbar interbody fusion: a randomized controlled trial. Front Med (Lausanne). (2023) 10:1–11. doi: 10.3389/fmed.2023.1192971
25. Zhang D, Wu X, Kong Q, Wang Y, Zhang B, Feng P, et al. Prospective randomized controlled trial on the effectiveness of low-dose and high-dose intravenous tranexamic acid in reducing perioperative blood loss in single-level minimally invasive transforaminal lumbar interbody fusion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. (2022) 36(4):439–45. doi: 10.7507/1002-1892.202112015
26. Yu CC, Fidai M, Washington T, Bartol S, Graziano G. Oral is as effective as intravenous tranexamic acid at reducing blood loss in thoracolumbar spinal fusions: a prospective randomized trial. Spine (Phila Pa 1976). (2022) 47(2):91–8. doi: 10.1097/BRS.0000000000004157
27. Hasan MS, Yunus SN, Ng CC, Chan CYW, Chiu CK, Kwan MK. Tranexamic acid in pediatric scoliosis surgery: a prospective randomized trial comparing high-dose and low-dose tranexamic acid in adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine (Phila Pa 1976). (2021) 46(22):E1170–e7. doi: 10.1097/BRS.0000000000004076
28. Shen J, Yang Z, Fu M, Hao J, Jiang W. The influence of topical use of tranexamic acid in reducing blood loss on early operation for thoracolumbar burst fracture: a randomized double-blinded controlled study. Eur Spine J. (2021) 30:3074–80. doi: 10.1007/s00586-020-06626-x
29. Dong Y, Liang J, Tong B, Shen J, Zhao H, Li Q. Combined topical and intravenous administration of tranexamic acid further reduces postoperative blood loss in adolescent idiopathic scoliosis patients undergoing spinal fusion surgery: a randomized controlled trial. BMC Musculoskelet Disord. (2021) 22(1):663. doi: 10.1186/s12891-021-04562-5
30. Zhang Z, Wang LN, Yang X, Liu LM, Xiu P, Zhou ZJ, et al. The effect of multiple-dose oral versus intravenous tranexamic acid in reducing postoperative blood loss and transfusion rate after adolescent scoliosis surgery: a randomized controlled trial. Spine J. (2021) 21(2):312–20. doi: 10.1016/j.spinee.2020.10.011
31. Arun-Kumar V, Naresh-Babu J. Is there a role for preoperative local infiltration of tranexamic acid in elective spine surgery? A prospective randomized controlled trial analyzing the efficacy of intravenous, local infiltration, and topical administration of tranexamic acid. Global Spine J. (2021) 11(1):21–7. doi: 10.1177/2192568219888446
32. Zhu X, Shi Q, Li D, Wu J, Guo K, Zheng X, et al. Two doses of tranexamic acid reduce blood loss in primary posterior lumbar fusion surgery: a randomized-controlled trial. Clin Spine Surg. (2020) 33(10):E593–e7. doi: 10.1097/BSD.0000000000000999
33. Li J, Wang L, Bai T, Liu Y, Huang Y. Combined use of intravenous and topical tranexamic acid efficiently reduces blood loss in patients aged over 60 operated with a 2-level lumbar fusion. J Orthop Surg Res. (2020) 15(1):339. doi: 10.1186/s13018-020-01758-8
34. He B, Li Y, Xu S, Ou Y, Zhao J. Tranexamic acid for blood loss after transforaminal posterior lumbar interbody fusion surgery: a double-blind, placebo-controlled, randomized study. Biomed Res Int. (2020) 2020:8516504. doi: 10.1155/2020/8516504
35. Xu D, Chen X, Li Z, Ren Z, Zhuang Q, Li S. Tranexamic acid reduce hidden blood loss in posterior lumbar interbody fusion (PLIF) surgery. Medicine (Baltimore). (2020) 99(11):e19552. doi: 10.1097/MD.0000000000019552
36. Wang X, Yang R, Sun H, Zhang Y. Different effects of intravenous, topical, and combined application of tranexamic acid on patients with thoracolumbar fracture. World Neurosurg. (2019) 127:e1185–e9. doi: 10.1016/j.wneu.2019.04.095
37. Yu CC, Kadri O, Kadado A, Buraimoh M, Pawloski J, Bartol S, et al. Intravenous and oral tranexamic acid are equivalent at reducing blood loss in thoracolumbar spinal fusion: a prospective randomized trial. Spine (Phila Pa 1976). (2019) 44(11):755–61. doi: 10.1097/BRS.0000000000002954
38. Ma K, Cao C, Wang Q, Luan F, Li Q. The reduction in blood loss with an intravenous drip of tranexamic acid in decompression and fusion surgery for degenerative lumbar spinal stenosis: a randomized controlled trial. Int J Clin Exp Med. (2019) 12(5):6116–21.
39. Elmose S, Andersen MØ, Andresen EB, Carreon LY. Double-blind, randomized controlled trial of tranexamic acid in minor lumbar spine surgery: no effect on operative time, intraoperative blood loss, or complications. J Neurosurg Spine. (2019) 31(2):194–200. doi: 10.3171/2019.1.SPINE1814
40. Mu X, Wei J, Wang C, Ou Y, Yin D, Liang B, et al. Intravenous administration of tranexamic acid significantly reduces visible and hidden blood loss compared with its topical administration for double-segment posterior lumbar interbody fusion: a single-center, placebo-controlled, randomized trial. World Neurosurg. (2019) 122:e821–e7. doi: 10.1016/j.wneu.2018.10.154
41. Sudprasert W, Tanaviriyachai T, Choovongkomol K, Jongkittanakul S, Piyapromdee U. A randomized controlled trial of topical application of tranexamic acid in patients with thoracolumbar spine trauma undergoing long-segment instrumented posterior spinal fusion. Asian Spine J. (2019) 13(1):146. doi: 10.31616/asj.2018.0125
42. Goobie SM, Zurakowski D, Glotzbecker MP, McCann ME, Hedequist D, Brustowicz RM, et al. Tranexamic acid is efficacious at decreasing the rate of blood loss in adolescent scoliosis surgery: a randomized placebo-controlled trial. J Bone Joint Surg Am Vol. (2018) 100(23):2024–32. doi: 10.2106/JBJS.18.00314
43. Wang W, Duan K, Ma M, Jiang Y, Liu T, Liu J, et al. Tranexamic acid decreases visible and hidden blood loss without affecting prethrombotic state molecular markers in transforaminal thoracic interbody fusion for treatment of thoracolumbar fracture-dislocation. Spine (Phila Pa 1976). (2018) 43(13):E734–E9. doi: 10.1097/BRS.0000000000002491
44. Nagabhushan RM, Shetty AP, Dumpa SR, Subramanian B, Kanna RM, Shanmuganathan R. Effectiveness and safety of batroxobin, tranexamic acid and a combination in reduction of blood loss in lumbar spinal fusion surgery. Spine (Phila Pa 1976). (2018) 43(5):E267–e73. doi: 10.1097/BRS.0000000000002315
45. Carabini LM, Moreland NC, Vealey RJ, Bebawy JF, Koski TR, Koht A, et al. A randomized controlled trial of low-dose tranexamic acid versus placebo to reduce red blood cell transfusion during complex multilevel spine fusion surgery. World Neurosurg. (2018) 110:e572–e9. doi: 10.1016/j.wneu.2017.11.070
46. Kim K-T, Kim C-K, Kim Y-C, Juh H-S, Kim H-J, Kim H-S, et al. The effectiveness of low-dose and high-dose tranexamic acid in posterior lumbar interbody fusion: a double-blinded, placebo-controlled randomized study. Eur Spine J. (2017) 26:2851–7. doi: 10.1007/s00586-017-5230-4
47. Seddighi A, Nikouei A, Seddighi A, Zali A, Tabatabaei S, Yourdkhani F, et al. The role of tranexamic acid in prevention of hemorrhage in major spinal surgeries. Asian J Neurosurg. (2017) 12(03):501–5. doi: 10.4103/1793-5482.165791
48. Colomina MJ, Koo M, Basora M, Pizones J, Mora L, Bagó J. Intraoperative tranexamic acid use in major spine surgery in adults: a multicentre, randomized, placebo-controlled trial†. Br J Anaesth. (2017) 118(3):380–90. doi: 10.1093/bja/aew434
49. Basavaraj K, Hegde R. A randomized prospective study of efficacy of tranexamicacid on perioperative blood loss in thoracicspine fixation. Sri Lankan J Anaesthesiol. (2017) 25(1):13–8. doi: 10.4038/slja.v25i1.8182
50. Shi H, Ou Y, Jiang D, Quan Z, Zhao Z, Zhu Y. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: a randomized trial. Medicine (Baltimore). (2017) 96(1):e5718. doi: 10.1097/MD.0000000000005718
51. Liang J, Liu H, Huang X, Xiong W, Zhao H, Chua S, et al. Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: a randomized control trial. Clin Neurol Neurosurg. (2016) 147:110–4. doi: 10.1016/j.clineuro.2016.06.001
52. Peters A, Verma K, Slobodyanyuk K, Cheriyan T, Hoelscher C, Schwab F, et al. Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine (Phila Pa 1976). (2015) 40(8):E443–9. doi: 10.1097/BRS.0000000000000799
53. Verma K, Errico T, Diefenbach C, Hoelscher C, Peters A, Dryer J, et al. The relative efficacy of antifibrinolytics in adolescent idiopathic scoliosis: a prospective randomized trial. J Bone Joint Surg Am. (2014) 96(10):e80. doi: 10.2106/JBJS.L.00008
54. Wang Q, Liu J, Fan R, Chen Y, Yu H, Bi Y, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J. (2013) 22:2035–8. doi: 10.1007/s00586-013-2836-z
55. Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976). (2011) 36(23):1913–8. doi: 10.1097/BRS.0b013e3181fb3a42
56. Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. (2011) 23(4):290–6. doi: 10.1097/ANA.0b013e31822914a1
57. Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). (2008) 33(24):2577–80. doi: 10.1097/BRS.0b013e318188b9c5
58. Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. (2008) 107(5):1479–86. doi: 10.1213/ane.0b013e3181831e44
59. Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. (2005) 102(4):727–32. doi: 10.1097/00000542-200504000-00006
60. Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. (2001) 93(1):82–7. doi: 10.1097/00000539-200107000-00018
61. Mikhail C, Pennington Z, Arnold PM, Brodke DS, Chapman JR, Chutkan N, et al. Minimizing blood loss in spine surgery. Global Spine J. (2020) 10(1_suppl):71S–83. doi: 10.1177/2192568219868475
62. Lum ZC, Manoukian MA, Pacheco CS, Nedopil AJ, Giordani M, Meehan JP. Intravenous tranexamic acid versus topical aminocaproic acid: which method has the least blood loss and transfusion rates? JAAOS Glob Res Rev. (2018) 2(11):e072. doi: 10.5435/JAAOSGlobal-D-18-00072
63. Tengborn L, Blombäck M, Berntorp E. Tranexamic acid–an old drug still going strong and making a revival. Thromb Res. (2015) 135(2):231–42. doi: 10.1016/j.thromres.2014.11.012
64. Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br Vol. (2001) 83(5):702–5. doi: 10.1302/0301-620x.83b5.11745
65. Yagi M, Hasegawa J, Nagoshi N, Iizuka S, Kaneko S, Fukuda K, et al. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976). (2012) 37(21):E1336–42. doi: 10.1097/BRS.0b013e318266b6e5
66. Valeri CR, Khabbaz K, Khuri SF, Marquardt C, Ragno G, Feingold H, et al. Effect of skin temperature on platelet function in patients undergoing extracorporeal bypass. J Thorac Cardiovasc Surg. (1992) 104(1):108–16. doi: 10.1016/S0022-5223(19)34842-1
67. Sessler DI. Mild perioperative hypothermia. N Engl J Med. (1997) 336(24):1730–7. doi: 10.1056/NEJM199706123362407
68. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. J Am Soc Anesthesiol. (2008) 108(1):71–7. doi: 10.1097/01.anes.0000296719.73450.52
Keywords: systematic review, network meta-analysis, tranexamic acid, blood transfusion, spine surgeries
Citation: Shim SR, Han S, Jeong JH, Hwang I, Cha Y and Ihm C (2025) Effect of tranexamic acid in spine surgeries: a systematic review and network meta-analysis. Front. Surg. 12:1550854. doi: 10.3389/fsurg.2025.1550854
Received: 24 December 2024; Accepted: 31 March 2025;
Published: 11 April 2025.
Edited by:
Abhijit Nair, Ministry of Health, OmanReviewed by:
Habib Md Reazaul Karim, All India Institute of Medical Sciences, IndiaManamohan Rangaiah, Walsall Manor Hospital, United Kingdom
Copyright: © 2025 Shim, Han, Jeong, Hwang, Cha and Ihm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhwa Ihm, aGFuZXVsQGV1bGppLmFjLmty
†These authors have contributed equally to this work and share first authorship
 Sung Ryul Shim
Sung Ryul Shim Sangah Han
Sangah Han Ji Hun Jeong
Ji Hun Jeong Inhwan Hwang5
Inhwan Hwang5 Chunhwa Ihm
Chunhwa Ihm