- 1Department of Orthopaedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Oncology, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of Vascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Epithelioid hemangioendothelioma (EHE) is a rare and locally aggressive tumour of vascular endothelial origin, with an estimated prevalence of less than 1 in a million. EHE can arise in any part of the body, most commonly in the liver, lungs, and skeleton, while occurrence in the blood vessels of the extremities is rare. This article reports a rare case of primary epithelioid hemangioendothelioma (EHE) of the right femoral artery. The patient was initially misdiagnosed with lower limb arterial occlusion and treated with stenting and other therapies; however, symptoms recurred, and the diagnosis of EHE was confirmed by pathological biopsy. EHE is very rare and accounts for approximately 1% of all vascular tumours. Based on pathological findings— CD31(+), CD34(−), CAMTA(+)–our patient was diagnosed with WWTR1-CAMTA1 fusion EHE.Treatment of EHE is mainly surgical. In our case, the patient underwent resection of the lesion area and the surrounding soft tissue mass, followed by a reconstruction using a left saphenous vein graft.
1 Introduction
Epithelioid hemangioendothelioma (EHE) is a rare and locally aggressive tumour of vascular endothelial origin with an estimated prevalence of less than 1 in a million (1, 2). The median age at which EHE occurs is ∼50 years old (3, 4), with a slightly higher prevalence in women than in men (5). The latest publication of the WHO Classification of Soft Tissue Tumours in 2020 states that EHE is a vascular malignant tumour, as is angiosarcoma (6). EHE can be found in any part of the body, most commonly in the liver, lungs, and bones (7). In contrast, EHE in the vessels of the extremities is rare. Fifty per cent of EHE cases are confined to small vessels and rarely occur in large veins or arteries (8). The prognosis for EHE is relatively positive, with an expected cure rate of 70%–80% if complete resection of EHE is achieved surgically and the margins are observed to be negative microscopically (R0 resection) (9). EHE is often misdiagnosed because of its low prevalence and lack of relevant tumour markers. In this case study, we share a case of EHE that was misdiagnosed as lower limb arterial occlusion, and standardised treatment enabled this patient to avoid lower limb amputation.
2 Case report
A 52-year-old woman presented to the Department of Vascular Surgery with right thigh and right lumbar buttock pain for more than 1 year, which had been aggravated for 5 months, and was diagnosed with lower limb arterial occlusion. Lower limb artery balloon dilatation was performed in September 2023, and lower limb artery balloon dilatation angioplasty + stenting was performed in May 2024, after which the pain was relieved for 2 months, and pain and discomfort later developed in the inner and outer right thigh and right lumbar and buttock regions. She was re-evaluated in the Department of Vascular Surgery and was suspected to have a stent-relatd infection. She underwent lower limb arterial exploration in the Department of Vascular Surgery on 27 November 2024. The stent implantation site was blurred with the surrounding tissues, and a specimen of the tissues around the stent was taken for biopsy. Pathology suggested epithelioid hemangioendothelioma of the right thigh. Preoperative MRI of the right thigh suggested a mass shadow around the stent of the right femoral artery with uneven enhancement, considering the possibility of malignant neoplastic lesion (Figures 1A,B). Preoperative CTA of both lower limb arteries revealed atherosclerotic changes in the lower limb arteries and changes after stent placement in the middle section of the right femoral artery (Figure 1F). Before the surgery, we consulted with vascular surgeons and oncologists: no obvious metastases were found, and complete surgical removal of the lesion was recommended. The patient underwent resection of the right femoral artery lesion area and the surrounding soft tissue mass on 23 December 2024, and a saphenous vein graft of equal length was taken from the left medial thigh. The intraoperative morphology of epithelioid hemangioendothelioma of the right femoral artery is shown in Figure 2A. Figure 2B showed the reconstruction of the right femoral artery with left saphenous vein graft after tumour resection. Postoperative CTA of both lower limb arteries was performed: the right thigh lesion was changed after resection + vascular repair, and the right femoral artery showed postoperative changes (Figure 1G). There was a limited effusion and a little pneumatosis around the upper middle segment of the right femoral artery. The right common iliac artery showed slight atherosclerosis. Postoperative pathological findings included epithelioid hemangioendothelioma of the right thigh and no tumour metastasis in the section of the right inguinal lymph node. Immunohistochemical staining showed tumour cells, ERG(+), CD31(+), CAMTA1(+), PCK(−), CD34(−), P53(20%, varying in strength), RB(+), INI-1(+), and Ki67(LI:25%). Immunohistochemical results are shown in Figure 3. A primary right femoral artery epithelioid hemangioendothelioma was identified. During surgery, we completely removed the lesion from the right femoral artery, and the surrounding lymph node biopsy was negative. We recommended regular follow-up visits for observation. Five months after surgery, the patient returned to the hospital for a follow-up visit. She was walking normally, and there were no abnormalities in the movement of her right lower limb. A video of the patient walking after surgery has been submitted in the Supplementary Material. Bilateral lower limb CTA revealed patency of the right femoral artery after great saphenous vein grafting, with no signs of recurrence. Supplementary Figure S1 shows the CTA of both lower limbs at the 5-month follow-up after surgery. Supplementary Figures S1A,B show three-dimensional images of the lower limb vessels, while Supplementary Figures S1C,D show that the right lower limb vessels are patent and no recurrence is observed.
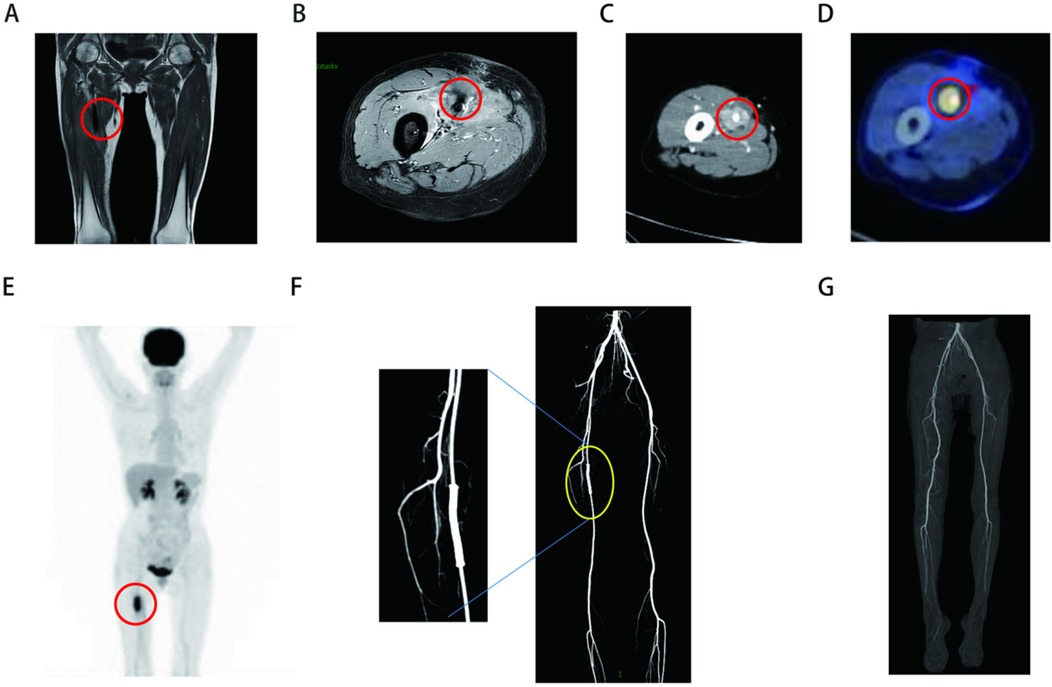
Figure 1. (A,B) Preoperative MRI of right thigh (the sequences for A and B are T1 and T1 fat compression): massive shadow around a stent in the right femoral artery with uneven enhancement. (C) Preoperative CT suggests ambiguity around the right femoral artery in the right thigh. (D,E) Preoperative PET-CT of the right femoral artery. (F) Preoperative CTA of both lower limb arteries suggests changes after stent placement in the middle right femoral artery. (G) Postoperative CTA of both lower limb arteries showed postoperative changes in the reconstruction of the right femoral artery with left saphenous vein graft after tumour resection.
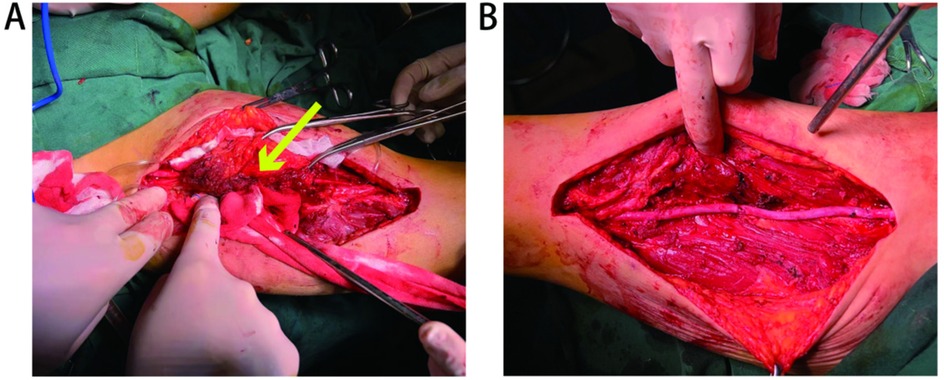
Figure 2. (A) Morphology of epithelioid hemangioendothelioma of the right femoral artery is shown by arrows. (B) Reconstruction of the right femoral artery with left saphenous vein graft after tumour resection.
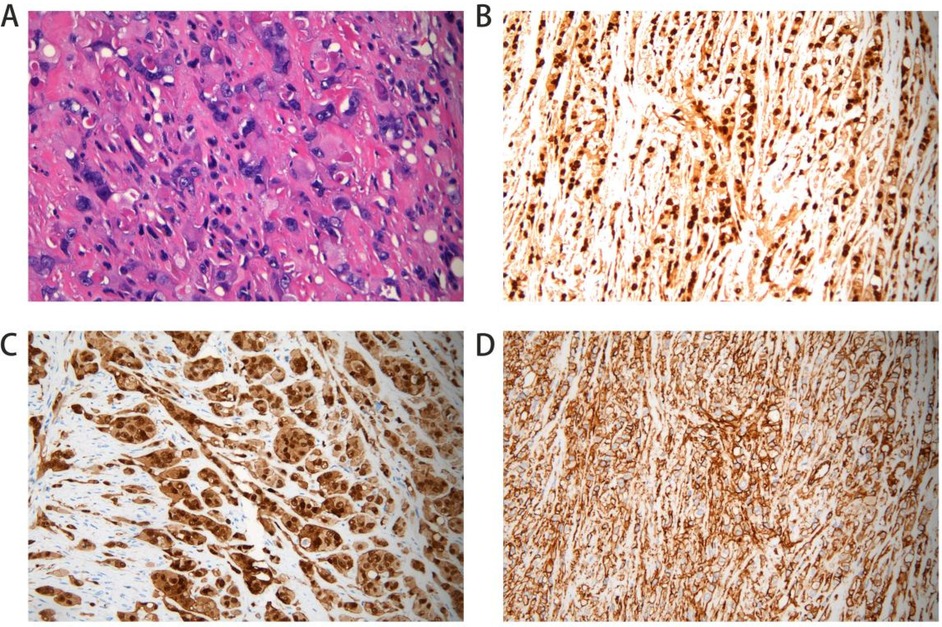
Figure 3. Postoperative pathological section. Hemotoxylin and eosin (HE) staining shows nest-like distribution of tumor cells; cells are epithelial cell-like with increased volume (A). Immunohistochemical stains of the right femoral artery tissue positive for ERG (B). Stain for CD31 was positive (C). CAMTA expression was positive (D).
3 Discussion
EHE is a subtype of vascular tumour consisting of epithelioid endothelial cells within a distinctive mucohyaline stroma (10). EHE accounts for only 1% of all vascular tumours (2) and shows a moderate female predominance in terms of sex of incidence (4). The incidence of the disease is concentrated in the middle-aged population, while its occurrence in children is extremely rare (11). Regarding the typing of EHE, the majority of EHE cases (>90%) showed WWTR1-CAMTA1 fusions, with CAMTA1 showing diffuse and strong nuclear expression. A small percentage of EHE cases (5%) showed YAP1-TFE3 fusion with diffuse nuclear TFE3 overexpression (12). The etiology of EHE has not been determined, but hepatic epithelioid hemangioendothelioma may be associated with the use of oral contraceptives (13), exposure to vinyl chloride, hepatitis, ascites, and liver injury (14).
The most common presenting symptoms of EHE in the extremities include localised pain and swelling (2). The clinical presentation of our patient was right thigh right lumbar hip pain, which is consistent with the clinical manifestations of EHE. At the same time, the patient's MRI results showed a mass shadow around the stent of the right femoral artery with uneven enhancement. The clinical presentation combined with imaging may remind us of the disease EHE. However, it is worth noting that prior to the first interventional procedure to place the stent, we did not detect any abnormalities around the right femoral artery through bilateral lower extremity CTA. This case of misdiagnosis of lower extremity arterial occlusion provides some lessons learned: overreliance on 3D vascular reconstruction from CTA while neglecting contrast-enhanced axial CT images may lead to misdiagnosis or delayed tumour diagnosis. Histological examination is the gold standard for the diagnosis of this tumour. Biopsy and immunohistochemical tests help in the diagnosis of EHE.EHE shows a typical endothelial phenotype characterised by positivity for CD31, CD34, and von Willebrand factor and occasionally for cytokeratin (15). Although EHE expresses similar molecular markers to angiosarcoma (CD31, CD34, and ERG), angiosarcoma differs greatly from EHE in terms of light microscopy morphology and often exhibits MYC amplification without CAMTA1/TFE3 expression (16). The pathological results of the two times of our patient suggested that CD31(+), CD34(−), and CAMTA(+), combined with the above EHE typing, our patient might be considered as WWTR1-CAMTA1 fusion-type EHE.
The treatment of EHE is primarily surgical, and for limited cancers, surgery is recommended as the first treatment for EHE (17). There are no standardised drug treatment protocols. However, several chemotherapeutic agents have shown good efficacy in the treatment of EHE (14). For patients not suitable for surgical treatment due to poor health, recurrence, or metastasis, VEGF inhibitors [e.g., sorafenib (18), pazopanib (19), or bevacizumab] or other chemotherapeutic agents (including thalidomide, polyethylene glycol liposomal adriamycin, and beat cyclophosphamide) may be used as adjuvant chemotherapy or neoadjuvant chemotherapy. In this case, the patient underwent resection of the right femoral artery in the area of the lesion and the surrounding soft tissue mass, and a saphenous vein graft of equal length was taken from the left medial thigh, as the tumour was relatively confined. Although 30% of this tumour metastasises (half to local lymph nodes and half to the lungs) (20), the prognosis for the disease is relatively good, with an estimated overall mortality rate of less than 20% at 5 years (21, 22). Patients with EHE who undergo complete resection of the lesion and show no evidence of metastasis preoperatively have a good postoperative prognosis. Among the seven cases reported in the literature involving primary involvement of the lower limb arteries or veins (femoral vein or artery), only one case was diagnosed with metastasis (8, 20, 23–27). The patient who experienced metastasis developed delayed pulmonary metastasis 12 years after surgery (pathological examination after resection of the affected lung revealed EHE) (27). The patient in this case underwent a follow-up examination 5 months later and showed satisfactory recovery, with normal walking ability. Bilateral lower limb CTA revealed patency of the right femoral artery after great saphenous vein grafting, with no signs of recurrence. We recommend that the patient continue to undergo bilateral lower limb CTA every 6 months, as well as enhanced CT scans of the lungs and abdomen. Some literature also suggests monitoring through circulating tumour DNA (28).
4 Conclusion
This case report describes a rare case of EHE of the right femoral artery. The patient was misdiagnosed with arterial occlusion due to lower limb pain and underwent interventional treatment, but the symptoms recurred. The diagnosis was ultimately confirmed via biopsy. The tumour was completely resected via surgery, followed by autologous saphenous vein grafting for vascular reconstruction (R0 resection). Postoperative pathology revealed CAMTA1 positivity (suggesting the WWTR1-CAMTA1 fusion subtype), with no lymph node metastasis. At the 5-month follow-up, the patient's lower limb function had recovered well, and CTA showed that the graft was patent with no signs of recurrence. This case highlights the need for vigilance in distinguishing EHE from abnormal proliferation after stenting; early radical resection combined with vascular reconstruction can effectively avoid amputation and improve prognosis. Long-term follow-up monitoring of the risk of metastasis is required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZS: Conceptualization, Investigation, Writing – original draft. JW: Investigation, Writing – review & editing. HH: Conceptualization, Writing – original draft. BW: Methodology, Supervision, Writing – original draft.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1582444/full#supplementary-material
References
1. Habeeb O, Rubin BP. The molecular diagnostics of vascular neoplasms. Surg Pathol Clin. (2019) 12(1):35–49. doi: 10.1016/j.path.2018.10.002
2. Alam SI, Nepal P, Sajid S, Al-Bozom I, Salah MM, Muneer A. Epithelioid hemangioendothelioma of the ulnar artery presenting with neuropathy. Ann Vasc Surg. (2020) 67:563.e13–e17. doi: 10.1016/j.avsg.2020.02.028
3. Liu Z, He S. Epithelioid hemangioendothelioma: incidence, mortality, prognostic factors, and survival analysis using the Surveillance, Epidemiology, and End Results database. J Oncol. (2022) 2022:2349991. doi: 10.1155/2022/2349991
4. Tsuchihashi K, Baba E. Epithelioid hemangioendothelioma—its history, clinical features, molecular biology and current therapy. Jpn J Clin Oncol. (2024) 54(7):739–47. doi: 10.1093/jjco/hyae037
5. Antonescu C. Malignant vascular tumors–an update. Mod Pathol. (2014) 27(1):S30–8. doi: 10.1038/modpathol.2013.176
6. Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: news and perspectives. Pathologica. (2021) 113(2):70–84. doi: 10.32074/1591-951X-213
7. Lau K, Massad M, Pollak C, Rubin C, Yeh J, Wang J, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. (2011) 140(5):1312–8. doi: 10.1378/chest.11-0039
8. Yven C, Gouny P, Nasr B. Epithelioid hemangioendothelioma of the lower limb, discovered by a claudication. Ann Vasc Surg. (2021) 72:665.e1–e4. doi: 10.1016/j.avsg.2020.10.017
9. Stacchiotti S, Miah AB, Frezza AM, Messiou C, Morosi C, Caraceni A, et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open. (2021) 6(3):100170. doi: 10.1016/j.esmoop.2021.100170
10. Choi JH, Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Adv Anat Pathol. (2021) 28(1):44–58. doi: 10.1097/PAP.0000000000000284
11. Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. (1986) 3(4):259–87.3303234
12. Doyle LA, Fletcher CD, Hornick JL. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol. (2016) 40(1):94–102. doi: 10.1097/PAS.0000000000000511
13. Dean PJ, Haggitt RC, O'Hara CJ. Malignant epithelioid hemangioendothelioma of the liver in young women. Relationship to oral contraceptive use. Am J Surg Pathol. (1985) 9(10):695–704. doi: 10.1097/00000478-198510000-00001
14. Kou K, Chen Y-G, Zhou J-P, Sun X-D, Sun D-W, Li S-X, et al. Hepatic epithelioid hemangioendothelioma: update on diagnosis and therapy. World J Clin Cases. (2020) 8(18):3978–87. doi: 10.12998/wjcc.v8.i18.3978
15. Weissferdt A, Moran CA. Primary vascular tumors of the lungs: a review. Ann Diagn Pathol. (2010) 14(4):296–308. doi: 10.1016/j.anndiagpath.2010.03.001
16. Cao J, Wang J, He C, Fang M. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. (2019) 9(11):2303–13.31815036
17. Niemelä M, Mäenpää H, Salven P, Summanen P, Poussa K, Laatikainen L, et al. Interferon alpha-2a therapy in 18 hemangioblastomas. Clin Cancer Res. (2001) 7(3):510–6.
18. Sangro B, Iñarrairaegui M, Fernández-Ros N. Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors. (2012) 4(2):e34. doi: 10.4081/rt.2012.e34
19. Bally O, Tassy L, Richioud B, Decouvelaere A-V, Blay J-Y, Derbel O. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res. (2015) 5:12. doi: 10.1186/s13569-014-0018-3
20. Charette S, Nehler MR, Whitehill TA, Gibbs P, Foulk D, Krupski WC. Epithelioid hemangioendothelioma of the common femoral vein: case report and review of the literature. J Vasc Surg. (2001) 33(5):1100–3. doi: 10.1067/mva.2001.111993
21. Mentzel T, Beham A, Calonje E, Katenkamp D, Fletcher CDM. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol. (1997) 21(4):363–74. doi: 10.1097/00000478-199704000-00001
22. Delin A, Johansson G, Silfverswärd C. Vascular tumours in occlusive disease of the iliac-femoral vessels. Eur J Vasc Surg. (1990) 4(5):539–42. doi: 10.1016/S0950-821X(05)80799-6
23. Wehbe R, Wang X, Newhall KA, Stoner MC, Mix DS, Pitcher GS. Epithelioid hemangioendothelioma involving the superficial femoral artery and femoral vein. J Vasc Surg Cases Innov Tech. (2025) 11(1):101645. doi: 10.1016/j.jvscit.2024.101645
24. Guo X, Yang SF, Xue GH, Chen JQ, Zhang H, Zhang L. Open surgical treatment for rare epithelioid hemangioendothelioma of the common femoral vein. J Interv Med. (2019) 2(4):178–80. doi: 10.1016/j.jimed.2019.10.010
25. Gao L, Wang Y, Jiang Y, Lai X, Wang M, Li J. Intravascular epithelioid hemangioendothelioma of the femoral vein diagnosed by contrast-enhanced ultrasonography: a care-compliant case report. Medicine. (2017) 96(49):e9107. doi: 10.1097/MD.0000000000009107
26. Minyi Y, Xintian H, Weimin L, Mier J. Intravascular epithelioid hemangioendothelioma. Int Angiol. (2011) 30(2):181–4.21460791
27. Jirasirinuphan P, Chang AL, Deepak A, Chang CK. Delayed metastatic pulmonary epithelioid hemangioendothelioma of the femoral vessels: case report and literature review. AME Case Rep. (2023) 7:1. doi: 10.21037/acr-22-57
Keywords: epithelioid hemangioendothelioma, vein graft, CAMTA1, WWTR1, surgery
Citation: Shao Z, Wang J, Wang B and Hu H (2025) Case Report: A case of epithelioid hemangioendothelioma of the right femoral artery misdiagnosed as arterial occlusion. Front. Surg. 12:1582444. doi: 10.3389/fsurg.2025.1582444
Received: 24 February 2025; Accepted: 29 July 2025;
Published: 14 August 2025.
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Huaijie Wang, Xi'an International Medical Center Hospital, ChinaSheng Dai, Weifang Traditional Chinese Hospital, China
Sweta Soni, All India Institute of Medical Sciences, India
Copyright: © 2025 Shao, Wang, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: BaiChuan Wang, d2FuZ2JhaWNodWFuLTExMkAxNjMuY29t; HongZhi Hu, MTM3MjAxMDU3NDZAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhengqi Shao
Zhengqi Shao Jian Wang
Jian Wang BaiChuan Wang
BaiChuan Wang HongZhi Hu
HongZhi Hu