- 1State Key Laboratory of Vascular Homeostasis and Remodeling, Department of Neurosurgery, Peking University Third Hospital, Peking University, Beijing, China
- 2Center for Precision Neurosurgery and Oncology of Peking University Health Science Center, Peking University, Beijing, China
- 3Peking University Health Science Center, Beijing, China
- 4Center for Oculocranial Pressure Instability Disorders (COPID), Henan Academy of Innovations in Medical Science (AIMS), Zhengzhou, China
- 5Peking University School of Economics, Beijing, China
Intracranial aspergillosis is uncommon but often lethal, especially in classically immunocompromised hosts. We report a 71-year-old man with poorly controlled diabetes (a non-classical risk factor) who developed bilateral frontal abscesses due to Aspergillus fumigatus. After an initial craniotomy with negative cultures and galactomannan, recurrent disease was confirmed by stereotactic biopsy with next-generation sequencing (NGS). Targeted azole therapy (voriconazole, isavuconazole) and multidisciplinary care led to marked clinical and radiographic improvement. We also pooled 343 published cases (2000–2024): overall mortality was 34.6%, and 21.8% among patients without classical immunosuppression (including some with non-classical factors such as diabetes). Improved survival in recent decades likely reflects earlier diagnosis and broader azole use, though inference is limited by case-based evidence. Early tissue diagnosis (including molecular testing), timely surgery when indicated, and CNS-penetrant azoles can yield favorable outcomes in non-classically immunosuppressed patients.
Background
Intracranial Aspergillus infection is a severe complication of invasive aspergillosis, primarily affecting immunocompromised patients (e.g., those with hematologic malignancies, transplant recipients, or on chronic corticosteroids) (1–5). Historically, reported mortality rates for intracranial aspergillosis were as high as 85% to 99%, especially in immunocompromised hosts, and when complicated by brain abscesses, the rate reportedly approaches 100% in such patients (6–14).
Aspergillus typically reaches the brain through hematogenous spread from a primary pulmonary focus or by direct extension from the paranasal sinuses (10, 15). The clinical manifestations of cerebral aspergillosis are often nonspecific, leading to delayed diagnosis (16, 17). Common symptoms include headache, altered mental status, focal neurological deficits, seizures, and visual disturbances. In patients without classical immunosuppression but with non-classical risk factors (e.g., diabetes), the course may be more indolent and may present as meningitis or granulomatous mass (18).
Intracranial aspergillosis is an exceedingly rare condition, even more so among immunocompetent patients. The existing literature primarily comprises single case reports or small retrospective studies, which provide insufficient data for robust prognostic assessments. Over the last 20–25 years, medical diagnostics and treatments have improved significantly. To contextualize our case, we compiled a pooled individual-patient analysis of reports since 2000, explicitly comparing outcomes by immune status. For terminology consistency throughout, we use “non-classically immunosuppressed” to denote patients lacking classical immunosuppressive conditions (e.g., no hematologic malignancy, transplant, HIV/AIDS, active chemotherapy, prolonged high-dose steroids, or primary immunodeficiency) but who carry risk modifiers such as diabetes.
Case presentation
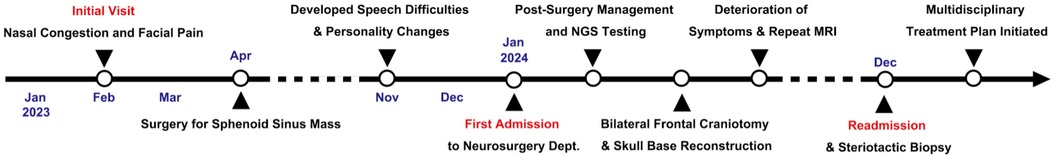
A 71-year-old man with a 17-year history of poorly controlled type 2 diabetes (HbA1c 9.5%) underwent endoscopic resection of a sphenoid sinus mass with sinusotomy for chronic sinusitis (15 months before definitive diagnosis). Histopathology showed inflammation without fungi, and symptoms initially improved. Six months later, he developed aphasia, cognitive slowing, and personality change without fever. MRI at a local hospital suggested bilateral frontal abscesses; mannitol partially improved speech.
Two months thereafter he presented to our center. MRI demonstrated bilateral frontal lesions with extensive edema and ring enhancement (Figures 1A–F); nasal endoscopy confirmed purulence (Figures 1G–I). Pre-operative CT showed anterior skull-base sclerosis and erosion consistent with osteomyelitis (Figure 3A). Laboratory tests revealed leukocytosis with neutrophilia and mildly elevated CRP; HIV screening was negative. Serum galactomannan (GM) was negative.
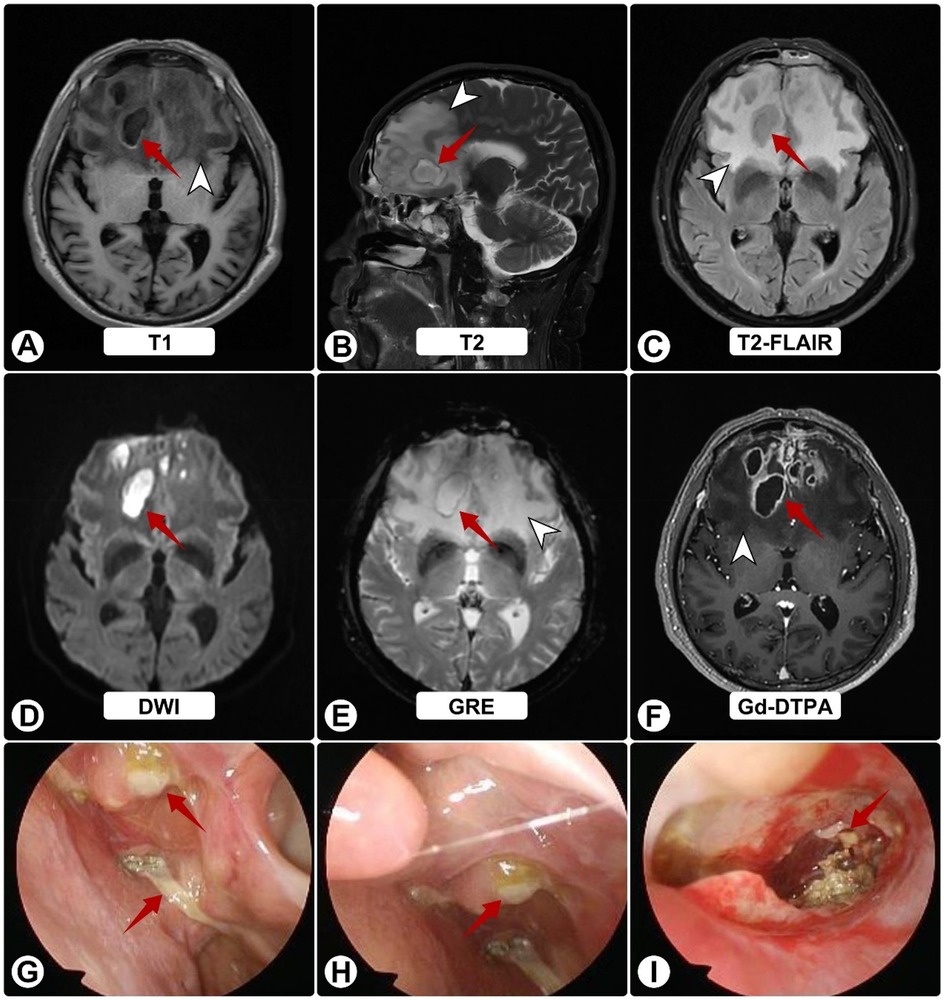
Figure 1. Brain MRI and nasal endoscopy on admission. (A–F) White arrowheads indicate surrounding edema; red arrows show abscesses. MRI shows bilateral frontal lesions appearing hyperintense on T1 (A) and T2 (B), and isointense on FLAIR (C) Post-contrast images (D–F) reveal ring enhancement of lesions with extensive vasogenic edema and midline shift. (G–I) Nasal endoscopy shows purulent sinus secretions (red arrows).
Given encapsulated abscesses with sinus and skull-base involvement, he underwent bilateral frontal craniotomy for evacuation and anterior skull-base reconstruction. Abundant pus was encountered; bacterial, fungal, and mycobacterial cultures and histopathology were negative.
Postoperatively, he received vancomycin and ceftriaxone plus fluconazole (guided by early nanopore reads suggesting possible Candida). He was discharged to complete IV antibiotics and fluconazole. Two weeks later he worsened neurologically. Follow-up MRI showed progression with persistent ring enhancement and diffusion restriction (Figures 2A–D); endoscopy again revealed purulence (Figures 2E–G).
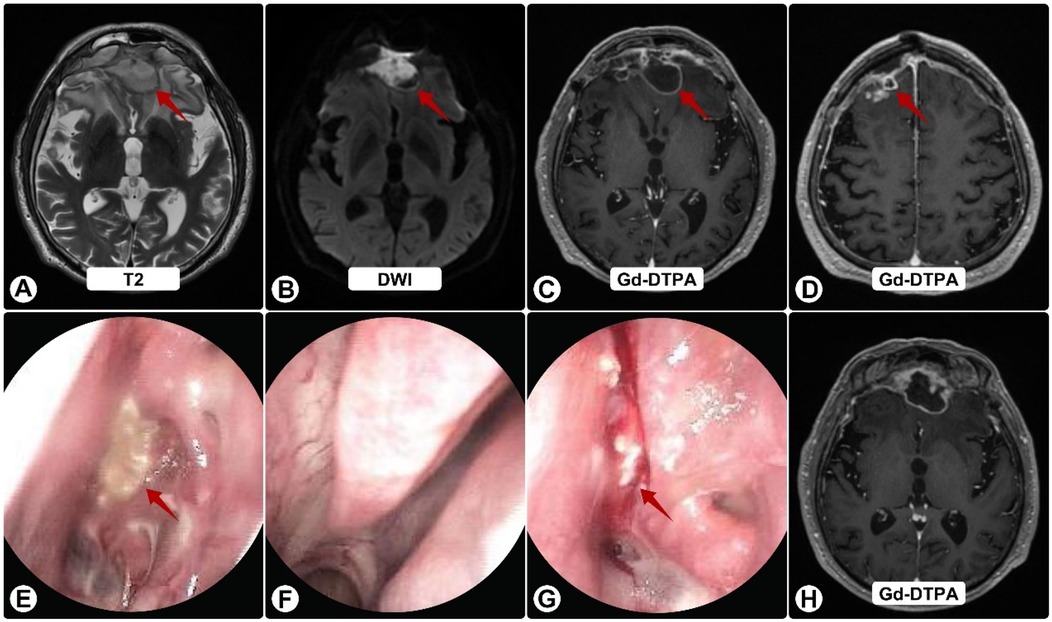
Figure 2. Follow-up brain MRI and nasal endoscopy. (A) T2-weighted image showing enlargement of frontal lesions (red arrows) with increased edema. (B) DWI shows persistent restricted diffusion. (C,D,H) Gd-DTPA–enhanced images demonstrate continued ring enhancement, with some solid components (H), (E–G) Endoscopy reveals persistent purulent secretions (red arrows) on the nasal mucosa (E), left middle meatus (F), and sphenoid sinus ostium (G), despite antibiotics.
Four months after craniotomy, stereotactic biopsy confirmed A. fumigatus by NGS; serum GM and β-D-glucan were now positive. Sinonasal biopsies demonstrated invasive hyphae. DTI tractography showed marked frontal tract loss (Figures 3B,C). OCT was normal, while visual fields showed dense bilateral defects consistent with compressive optic neuropathy (Figures 3D–F).
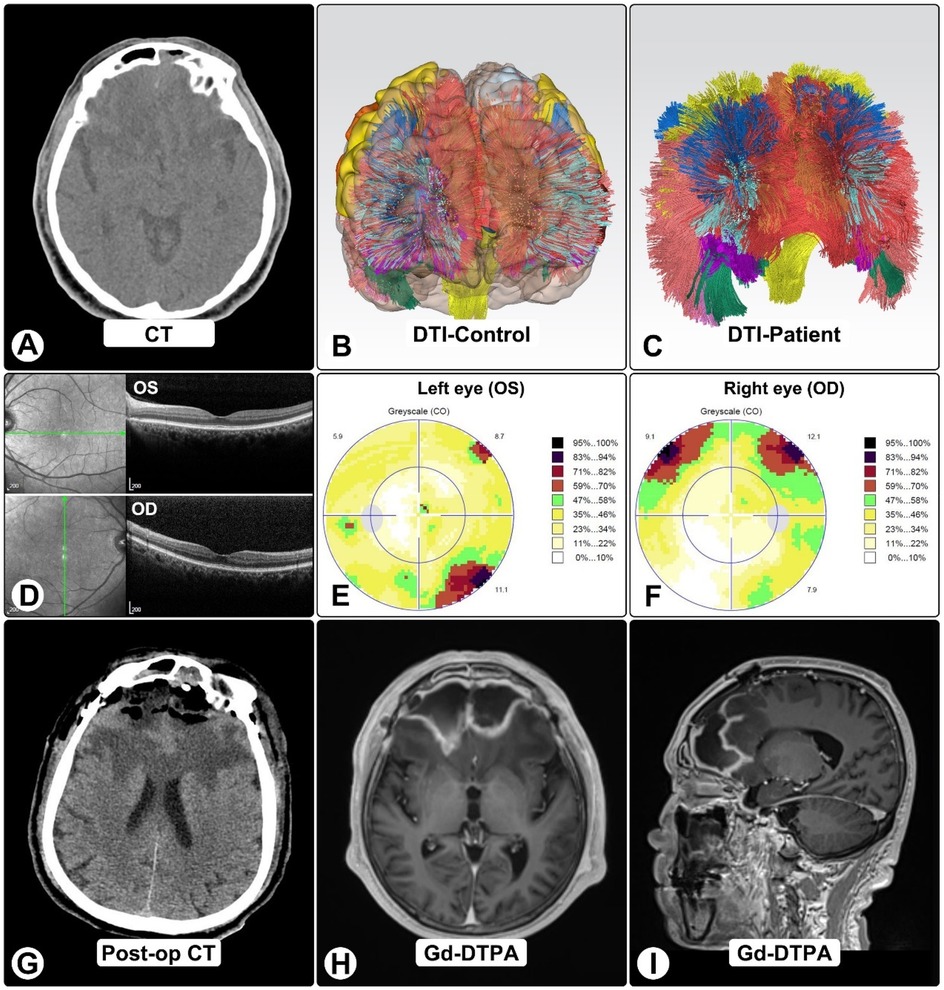
Figure 3. DTI tractography, OCT, and visual findings. (A) Pre-op CT shows bilateral frontal lesions with vasogenic edema and mass effect. (B,C) DTI tractography in normal brain (B) and patient (C), showing marked frontal tract loss, including bilateral anterior thalamic radiations, genu of corpus callosum, cingulum bundles, and forceps minor. (D) OCT imaging. (E,F) Octopus visual fields show right superior altitudinal defect (E) and left inferotemporal scotoma (F), consistent with compressive optic neuropathy. (G) Post-op CT showing expected changes after craniotomy and abscess evacuation. (H,I) One-month post-op MRI shows residual ring-enhancing lesions with reduced size and mass effect.
Multidisciplinary management with CNS-penetrant azoles (voriconazole and isavuconazole), blood-pressure control, and supportive care led to steady improvement. One-month MRI showed smaller lesions and reduced mass effect (Figures 3H,I). At six months, he was independent in daily activities with mild residual cognitive impairment and persistent visual-field loss (Figure 4).
Methods for pooled analysis
Search strategy and eligibility
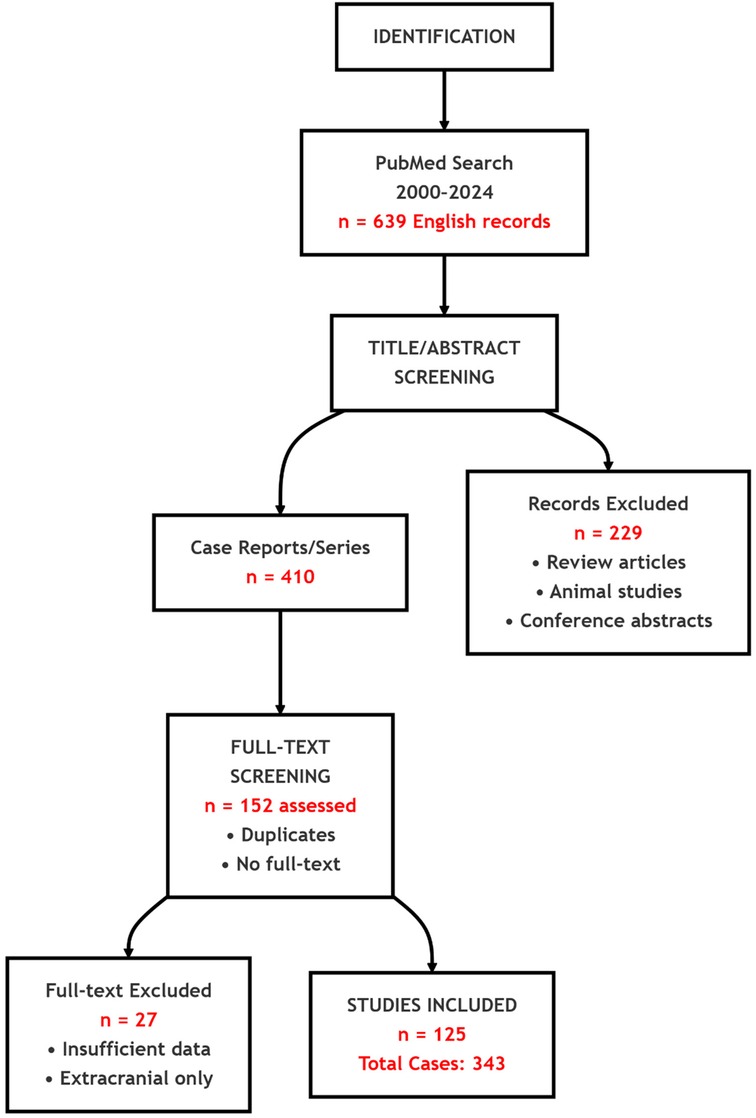
We searched PubMed for English-language reports from January 1, 2000 to May 10, 2024 using (“intracranial aspergillosis” OR “Aspergillus brain abscess” OR “central nervous system Aspergillus” OR “cerebral Aspergillus” OR “brain aspergillosis”). Inclusion required: (1) histopathological, culture, or molecular confirmation of intracranial aspergillosis; (2) radiologic evidence of an intracranial lesion (intraparenchymal or extradural); and (3) documented immune status and outcome. We excluded reviews, animal studies, conference abstracts, studies with insufficient intracranial data, and reports limited to extracranial infection. PRISMA flow: 639 records → 229 excluded at screening; after removing 258 duplicates/inaccessible texts, 152 full texts reviewed; 27 excluded; 125 studies included (343 cases) (Figure 5).
Terminology
“Classically immunocompromised” comprised hematologic malignancy, solid-organ or stem-cell transplantation, HIV/AIDS, active cytotoxic chemotherapy, prolonged high-dose corticosteroids (>20 mg/day prednisone-equivalent for >2 weeks), or primary immunodeficiency. “Non-classically immunosuppressed” included conditions such as diabetes that alter host defenses without meeting classical criteria. For clarity, we report outcomes for patients “without classical immunosuppression,” which may include non-classical factors.
Results
Among 343 patients (201 male, 107 female, 35 unspecified), there were 119 deaths, with 11 additional deaths unrelated to aspergillosis; 213 were recovered or stable at last follow-up. Overall mortality was 34.6%; mortality among patients without classical immunosuppression (including some with non-classical factors) was 21.8%. Detailed per-case data are provided in Supplementary Table S1.
Discussion and conclusion
This case underscores diagnostic pitfalls in intracranial aspergillosis, particularly when early biomarkers are negative. The GM assay has variable sensitivity: at an optical density index of 0.5, pooled sensitivity is ∼82% (≈18% false-negative rate); at 1.0, sensitivity declines to ∼72% (≈28% false-negative rate) (19–21). Thus, negative GM should not preclude biopsy or molecular testing when imaging and clinical evolution are concerning. The eventual detection of Aspergillus DNA via NGS on a biopsy specimen was pivotal, enabling targeted antifungal therapy and likely preventing a fatal outcome.
Surgery remains important for encapsulated collections, mass effect, or diagnostic uncertainty, both to decompress and to obtain tissue for definitive identification (culture, histology, PCR/NGS). In our patient, delayed confirmation by NGS ultimately enabled targeted therapy.
Therapeutically, outcomes have improved with CNS-penetrant triazoles—especially voriconazole—compared with amphotericin B or itraconazole, which historically showed poor CNS efficacy and tolerability (22–33). Isavuconazole may offer additional options, as illustrated by this case, though further CNS-specific data are needed (34, 35). Non-classical risk factors (e.g., diabetes), disruption of anatomic barriers (e.g., sinus surgery), and transient immune dysfunction can permit angioinvasion even without classical immunosuppression. Recognizing these scenarios can prompt earlier imaging, biopsy, and azole initiation (36).
In the future, standardization of molecular diagnostics, therapeutic drug monitoring for azoles, and optimized combination/sequencing strategies may further lower mortality; exploratory immunomodulatory approaches merit study but lie beyond the scope of this report.
Conclusion
Intracranial aspergillosis remains a high-stakes neurosurgical and infectious-disease emergency, yet patients without classical immunosuppression—including those with non-classical risks such as diabetes—can achieve good outcomes when clinicians maintain suspicion despite early false-negative biomarkers, pursue early tissue diagnosis with biopsy/NGS, and combine indicated surgical management with CNS-penetrant azoles (e.g., voriconazole, isavuconazole) under multidisciplinary care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent for publication (clinical details and images) was obtained from the patient. In accordance with institutional policy and journal guidance for single-patient case reports, IRB approval was not required.
Author contributions
MR: Formal analysis, Data curation, Writing – original draft, Writing – review & editing. SL: Writing – review & editing, Investigation. YD: Investigation, Writing – review & editing. JY: Conceptualization, Writing – review & editing, Funding acquisition, Resources. XC: Writing – review & editing, Resources. JY: Resources, Writing – review & editing. CY: Funding acquisition, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82371319 to CY), Beijing Nova Program (20230484356 to CY), Beijing Natural Science Foundation (7222217 to CY and QY24070 to MR), Peking University Clinical Scientist Training Program supported by “The Fundamental Research Funds for the Central Universities” (BMU2024PYJH017 to CY), Capital Health Research and Development of Special (2022-4-40918 to CY), Research Project of Peking University Third Hospital in State Key Laboratory of Vascular Homeostasis and Remodeling (2024-VHR-SY-06 to CY), Digital and Humanities Project supported by “The Fundamental Research Funds for the Central Universities” (7101503232 to J.Ye and CY), and Peking University Third Hospital Clinical Key Project (BYSYZD2021023 to CY). Funding sources had no involvement in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Acknowledgments
We thank the patient and his family who trusted us, and all the physicians and staff members who helped in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1674057/full#supplementary-material
Abbreviations
CT, computed tomography; MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; Gd-DTPA, gadolinium-diethylenetriamine pentaacetic acid; DWI, diffusion-weighted imaging; GRE, gradient echo; PLT, platelet count; CRP, C-reactive protein; PCT, procalcitonin; HIV, human immunodeficiency virus; NGS, next-generation sequencing; DTI, diffusion tensor imaging; OCT, optical coherence tomography; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; GM, galactomannan; CNS, Central Nervous System; IV, intravenous.
References
1. Cadena J, Thompson GR III, Patterson TF. Aspergillosis: epidemiology, diagnosis, and treatment. Infect Dis Clin North Am. Jun. (2021) 35(2):415–34. doi: 10.1016/j.idc.2021.03.008
2. Godoy MCB, Ferreira Dalla Pria HR, Truong MT, Shroff GS, Marom EM. Invasive fungal pneumonia in immunocompromised patients. Radiol Clin North Am. (2022) 60(3):497–506. doi: 10.1016/j.rcl.2022.01.006
3. Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, et al. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. (2020) 46(2):298–314. doi: 10.1007/s00134-019-05906-5
4. Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. (2015) 162(2):81–9. doi: 10.7326/m13-2508
5. Warris A, Lehrnbecher T, Roilides E, Castagnola E, Brüggemann RJM, Groll AH. ESCMID-ECMM guideline: diagnosis and management of invasive aspergillosis in neonates and children. Clin Microbiol Infect. (2019) 25(9):1096–113. doi: 10.1016/j.cmi.2019.05.019
6. Kumar D, Nepal P, Singh S, Ramanathan S, Khanna M, Sheoran R, et al. CNS Aspergilloma mimicking tumors: review of CNS aspergillus infection imaging characteristics in the immunocompetent population. J Neuroradiol. (2018) 45(3):169–76. doi: 10.1016/j.neurad.2017.11.001
7. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. (2001) 32(3):358–66. doi: 10.1086/318483
8. Lamoth F, Mercier T, André P, Pagani JL, Pantet O, Maduri R, et al. Isavuconazole brain penetration in cerebral aspergillosis. J Antimicrob Chemother. (2019) 74(6):1751–3. doi: 10.1093/jac/dkz050
9. Prystowsky SD, Vogelstein B, Ettinger DS, Merz WG, Kaizer H, Sulica VI, et al. Invasive aspergillosis. N Engl J Med. (1976) 295(12):655–8. doi: 10.1056/nejm197609162951206
10. Tripathi M, Mohindra S. Rhinocerebral aspergillosis. Lancet. (2018) 392(10150):e8. doi: 10.1016/s0140-6736(18)31949-4
11. Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. (2017) 45(6):737–79. doi: 10.1007/s15010-017-1042-z
12. Turgut M, Özsunar Y, Öncü S, Akyüz O, Ertuğrul MB, Tekin C, et al. Invasive fungal granuloma of the brain caused by Aspergillus fumigatus: a case report and review of the literature. Surg Neurol. (2008) 69(2):169–74; discussion 174. doi: 10.1016/j.surneu.2006.12.049
13. Epstein NE, Hollingsworth R, Black K, Farmer P. Fungal brain abscesses (aspergillosis/mucormycosis) in two immunosuppressed patients. Surg Neurol. (1991) 35(4):286–9. doi: 10.1016/0090-3019(91)90006-u
14. Elter T, Sieniawski M, Gossmann A, Wickenhauser C, Schröder U, Seifert H, et al. Voriconazole brain tissue levels in rhinocerebral aspergillosis in a successfully treated young woman. Int J Antimicrob Agents. (2006) 28(3):262–5. doi: 10.1016/j.ijantimicag.2006.04.006
15. Vanfleteren MJEGW, Dingemans A-MC, Surmont VF, Vermaelen KY, Postma AA, Oude Lashof AML, et al. Invasive aspergillosis mimicking metastatic lung cancer. Front Oncol. (2018) 8:188. doi: 10.3389/fonc.2018.00188
16. Jiao J, Zhou F, Kang H, Liu C, Yang M, Hu J. Unexpected extrapyramidal symptoms and pulmonary aspergillosis in exertional heatstroke with fulminant liver failure: a case report. J Med Case Rep. (2017) 11(1):37. doi: 10.1186/s13256-016-1184-0
17. Nadkarni T, Goel A. Aspergilloma of the brain: an overview. J Postgrad Med. (2005) 51(Suppl 1):S37–41.16519254
18. Tehrani S, Yadegarynia D, Keyvanfar A. A case-report of concurrent pulmonary and cerebral lesions in a patient with polymyositis: invasive aspergillosis or astrocytoma? Jundishapur J Microbiol. (2023) 15(11):e132821. doi: 10.5812/jjm-132821
19. Walsh TJ, Zhang SX. Emerging roles of (1→3)-β-D-glucan in cerebrospinal fluid for detection and therapeutic monitoring of invasive fungal diseases of the central nervous system. Clin Infect Dis. (2023) 78(1):11–4. doi: 10.1093/cid/ciad520
20. Hunter ES, Wilopo B, Richardson MD, Kosmidis C, Denning DW. Effect of patient immunodeficiencies on the diagnostic performance of serological assays to detect Aspergillus-specific antibodies in chronic pulmonary aspergillosis. Respir Med. Mar. (2021) 178:106290. doi: 10.1016/j.rmed.2020.106290
21. Leeflang MM, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJ, Hooft L, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. (2015) 2015(12):Cd007394. doi: 10.1002/14651858.CD007394.pub2
22. Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. (2016) 63(4):e1–60. doi: 10.1093/cid/ciw326
23. Boes B, Bashir R, Boes C, Hahn F, McConnell J, McComb R. Central nervous system aspergillosis; analysis of 26 patients. J Neuroimaging. (1994) 4(3):123–9. doi: 10.1111/jon199443123
24. Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. (1996) 23(3):608–15. doi: 10.1093/clinids/23.3.608
25. Reguera-Gomez M, Dores MR, Martinez LR. Innovative and potential treatments for fungal central nervous system infections. Curr Opin Microbiol. (2023) 76:102397. doi: 10.1016/j.mib.2023.102397
26. Lange N, Wantia N, Jörger A-K, Wagner A, Liesche F, Meyer B, et al. Fungal brain infection-no longer a death sentence. Neurosurg Rev. (2021) 44(4):2239–44. doi: 10.1007/s10143-020-01410-3
27. Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections caused by fungi. Infection. (2018) 46(4):443–59. doi: 10.1007/s15010-018-1152-2
28. Tattevin P, Bruneel F, Lellouche F, de Broucker T, Chevret S, Wolff M, et al. Successful treatment of brain aspergillosis with voriconazole. Clin Microbiol Infect. (2004) 10(10):928–31. doi: 10.1111/j.1469-0691.2004.00981.x
29. Peman J, Salavert M, Canton E, Jarque I, Roma E, Zaragoza R, et al. Voriconazole in the management of nosocomial invasive fungal infections. Ther Clin Risk Manag. (2006) 2(2):129–58. doi: 10.2147/tcrm.2006.2.2.129
30. Groll AH, Rijnders BJA, Walsh TJ, Adler-Moore J, Lewis RE, Brüggemann RJM. Clinical pharmacokinetics, pharmacodynamics, safety and efficacy of liposomal amphotericin B. Clin Infect Dis. (2019) 68(Suppl 4):S260–274. doi: 10.1093/cid/ciz076
31. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. (2002) 347(6):408–15. doi: 10.1056/NEJMoa020191
32. Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. Invasive aspergillosis disease spectrum, treatment practices, and outcomes. Medicine (Baltimore). (2000) 79(4):250–60. doi: 10.1097/00005792-200007000-00006
33. Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. (2005) 106(8):2641–5. doi: 10.1182/blood-2005-02-0733
34. Paulussen C, Hallsworth JE, Álvarez-Pérez S, Nierman WC, Hamill PG, Blain D, et al. Ecology of aspergillosis: insights into the pathogenic potency of Aspergillus fumigatus and some other Aspergillus species. Microb Biotechnol. (2017) 10(2):296–322. doi: 10.1111/1751-7915.12367
35. Benoit JM, Breznik JA, Ang JC, Bhakta H, Huynh A, Cowbrough B, et al. Immunomodulatory drugs have divergent effects on humoral and cellular immune responses to SARS-CoV-2 vaccination in people living with rheumatoid arthritis. Sci Rep. (2023) 13(1):22846. doi: 10.1038/s41598-023-50263-5
Keywords: aspergillosis, brain abscess, non-classically immunosuppressed, voriconazole, isavuconazole
Citation: Regmi M, Liu S, Dai Y, Ye J, Chen X, Yang J and Yang C (2025) Case Report: Rare invasive aspergillosis with brain abscess in a non–classically immunosuppressed patient, and pooled analysis of individual patient data (2000–2024). Front. Surg. 12:1674057. doi: 10.3389/fsurg.2025.1674057
Received: 27 July 2025; Accepted: 30 September 2025;
Published: 23 October 2025.
Edited by:
Katharina Feil, University of Tübingen, GermanyReviewed by:
Nicolas Padilla-Raygoza, Institute of Public Health of the State of Guanajuato (ISAPEG), MexicoYafei Guo, Tsinghua University, China
Copyright: © 2025 Regmi, Liu, Dai, Ye, Chen, Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenlong Yang, dmlrLnlhbmdAcGt1LmVkdS5jbg==; dmlrLnlhbmdAYmptdS5lZHUuY24=; Jun Yang, eWFuZ2pieXN5QGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Moksada Regmi
Moksada Regmi Shikun Liu1,2,3,†
Shikun Liu1,2,3,† Jun Yang
Jun Yang Chenlong Yang
Chenlong Yang