- 1M.H. Gluck Equine Research Center, Department of Veterinary Science, University of Kentucky, Lexington, KY, USA
- 2East Tennessee Clinical Research, Inc., Rockwood, TN, USA
Strongylus vulgaris is the most pathogenic nematode parasite of horses. Its extensive migration in the mesenteric blood vessels can lead to life-threatening intestinal infarctions. Recent work has shown that this parasite is still identified among managed horse populations. A serum enzyme-linked immunosorbent assay (ELISA) has been developed for the detection of migrating larvae of S. vulgaris. Previous work has documented an increase in ELISA values following larvicidal treatment with ivermectin and suggested that the target parasite antigen is primarily produced by the later larval stages. The aim of this study was to experimentally inoculate cohorts of foals with S. vulgaris, and then compare ELISA responses to early or later ivermectin treatments. Fifteen foals were held in confinement and infected orally with ~25 S. vulgaris third-stage larvae on Days 0, 7, 14, and 21. Foals were weaned on Day 43 and turned out to a pasture not previously grazed by horses. Foals remained at pasture continuously until the study was terminated on Day 196. On Day 55, foals were randomly allocated to three treatment groups of five each. Group 1 received ivermectin on Day 56, Group 2 received ivermectin on Day 112, and Group 3 foals served as untreated controls. Serum and fecal samples were collected at 28-day intervals throughout the study. Serum samples were analyzed with the S. vulgaris-specific ELISA and fecal samples were processed for fecal egg counting. The ELISA values of Group 1 foals were significantly lower than Groups 2 or 3 on Days 140–196. Both treated groups exhibited increased ELISA values following ivermectin treatment. Results indicate that the target diagnostic antigen is produced throughout the course of arterial infection with S. vulgaris, but that an early ivermectin treatment can reduce the cumulative antigen produced over the course of an infection.
Introduction
Strongylus vulgaris is considered to be the most pathogenic helminth parasite of horses due to the extensive migration of its larval stages. The predilection site for migration is the cranial mesenteric artery (CMA) and its major branches. Upon infection, exsheathed third-stage larvae penetrate the large intestinal mucosa, where they molt to the fourth larval stage (L4) within the first 5 days. The L4s then migrate proximally toward the CMA, where they arrive about 11–14 days post infection (PI). In the CMA, fourth-stage larvae (L4) molt to the immature adult stage (L5) at about 90 days PI, and migration back to the large intestine begins around Day 120 PI (1). Migrating larvae cause fibrinous endarteritis with pronounced thrombosis, formation of aneurysms, and other pathologic alterations of the vessel structures (2). Thrombo-embolism caused by L4s and immature adults has been associated with a painful and often fatal colic syndrome, characterized by ischemia and non-strangulating infarction of intestinal segments (3–5).
Historically, S. vulgaris was prevalent in virtually all grazing horses (6–8). However, this pattern changed during the 1980s, when a significant decline was attributed to the intensive anthelmintic treatment regimens typically implemented for horse populations (9, 10). Notwithstanding, subsequent surveys of managed horses across the world have documented that S. vulgaris continues to be encountered on a regular basis (11–13). One recent study found S. vulgaris DNA in fecal samples from regularly dewormed thoroughbred mares in Central Kentucky (14). Another recent study conducted in Denmark has even documented a higher occurrence of S. vulgaris on farms using a widely recommended parasite control strategy in which treatment decisions are based on pre-treatment fecal egg count levels (15). Further, a recent retrospective case-control study conducted among referred Danish equine patients documented a significant association between non-strangulating intestinal infarctions and serological evidence of S. vulgaris infection (5). Taken together, these reports emphasize the need for reliable diagnostic assays to detect S. vulgaris infections in managed horses.
Recently, a serum enzyme-linked immunosorbent assay (ELISA) was developed and validated to detect migrating S. vulgaris larvae in the bloodstream of horses (16). The assay measures host IgG(T) antibodies against a recombinant S. vulgaris SXP antigen, Strongylus vulgaris serine-X-proline (SvSXP), and returns a diagnostic sensitivity of 73.3%, a specificity of 81.0%, and a statistically significant correlation with the numbers of migrating larvae in the mesenteric arteries (16). This assay has been further evaluated and characterized in recent studies. One study performed with naturally infected foals documented the presence of maternal antibodies during the first weeks of life, and found that foals became ELISA-positive between 3 and 5 months of age (17). A second study evaluated the effect of ivermectin treatment in ELISA-positive juvenile horses and illustrated an initial increase in ELISA values following treatment, followed by a decline which was complete after 5 months (18). In the same study, an untreated control group had a significant increase of ELISA values after approximately 5 months of natural exposure to S. vulgaris infection at pasture. Taken together, these two studies illustrate that a positive ELISA result represents either current or recent infection with S. vulgaris within the preceding 5 months. Further, it appears that the SvSXP antigen may be produced primarily by later larval stages because ELISA values increase markedly about 5 months after first exposure to infection.
The purpose of this study was to test the hypothesis that SvSXP is primarily produced by the immature L5 stages present in the CMA after about 90 days PI. The hypothesis was tested by experimentally infecting cohorts of foals with S. vulgaris and then comparing SvSXP ELISA responses after early (<90 days) and later (>90 days) larvicidal treatments with ivermectin.
Materials and Methods
This was a controlled, randomized, blinded prospective clinical study conducted at a single site between July 2, 2014 and January 14, 2015. The study was reviewed and approved by East Tennessee Clinical Research’s Institutional Animal Care and Use Committee (Application No. ETCR-13-0136, approved 22MAY14).
Foals
Fifteen nursing mares with foals-at-side were acquired by East Tennessee Clinical Research in Rockwood, TN, USA. Prior to the onset of the study, mares and foals were held in confinement, and offered a commercial equine concentrate (11% protein) in quantities totaling ~0.5% of the mare’s body weight, divided into similar portions offered twice daily. Grass hay was offered twice daily in quantities totaling ~2.0% of the mare’s body weight. Water from a commercial utility was provided ad libitum.
Approximately 1–4 weeks prior to initiation of the trial, all mares and foals received a larvicidal anthelmintic regimen of fenbendazole (10 mg/kg once daily for five consecutive days; Panacur Powerpak, Merck Animal Health, Millsboro, DE, USA). Fecal egg counts were performed to confirm that the treatment worked.
The 15 foals enrolled in the study were of various light saddle breeds, including Appaloosa, Quarter Horse, Tennessee Walking Horse, and Thoroughbred, and were born between April 24 and June, 2014. Nine were females, and six were intact males. All underwent a physical examination and were found healthy prior to the onset of the study.
All foals were inoculated orally with ~25 infective third-stage larvae (L3) of S. vulgaris on Days 0, 7, 14, and 21. This infective dose has been successfully used in a previous study (19). On Day 43 (August 14, 2014), foals were separated from their dams and turned out on a pasture not previously grazed by horses, where they remained for the duration of the study. After turnout, foals were offered a commercial equine concentrate (11% protein) in quantities totaling ~0.25% of body weight once daily. Pasture forage was the primary source of fiber and energy, but supplemental grass hay was available ad libitum whenever pasture forage was inadequate for maintenance. Water was available ad libitum via automatic, frost-free hydrants.
Foals were ranked according to their arrival date. Each three consecutively ranked foals comprised a block, and foals were allocated randomly from each block to the three treatment group. Foals assigned to Groups 1 and 2 were treated orally with ivermectin (200 μg/kg; Zimecterin, Merial Ltd., Duluth, GA, USA) on Days 56 and 112, respectively. Foals assigned to Group 3 served as untreated controls. Body weights were measured with a certified scale, and fecal and serum samples were collected from each enrolled foal on Day 0 (July 2, 2014) and repeated thereafter at 28-day intervals until termination of the study on Day 196 (January 14, 2015).
Randomization of horses to study groups and preparation of individual doses of ivermectin were completed by personnel who determined no outcome measures for the duration of the trial. Administration of the anthelmintic was the responsibility of employees who remained masked to treatment assignments. Records of treatment assignments were sequestered away from masked personnel to prevent inadvertent unblinding.
Preparation and Administration of S. vulgaris Infective Larvae
Feces were collected from two mares known to have patent S. vulgaris infections. The mares were residents of the parasitology research herd of the University of Kentucky (20). Individual 20 g fecal samples were collected fresh and mixed with equal volumes of vermiculite (Infinity Fertilizers, Inc., Milan, IL, USA) and moistened with tap water until pliable. Fecal mixtures were then suspended in cheesecloth over 10 mL of tap water in humidity chambers as described by Henriksen and Korsholm (21). These chambers were incubated at ~25°C for 14 days and moistened as necessary. The fecal mixtures were then sedimented for 24 h in Baermann apparatuses consisting of wine glasses containing reservoirs in their stems.
After 24 h, the fecal mixtures were removed from the Baermann apparatus and the entire contents were centrifuged in multiple 50 mL tubes at ~200 g for 10 min. The pellets in each tube were then consolidated into a single 15 mL tube per sample and stored at 25°C until examination. For examination and enumeration of larvae present, each pellet was re-suspended and transferred to nematode counting chambers (Chalex Corp., Ketchum, ID, USA). Nematode slides were examined at 100× magnification. Approximate numbers of S. vulgaris third-stage larvae were recorded, and other observed species and stages were noted as identified according to published criteria (22). Larvae were transferred to 15 mL tubes, filled with tap water, covered with parafilm, and capped before shipment to the testing facility (East Tennessee Clinical Research).
On the days of inoculation, larval counts were conducted, and volumes of inoculum were prepared to contain ~25 infective L3s of S. vulgaris. A separate inoculum was prepared for each foal; larvae were transported to the animal facility in individual; labeled 15 mL polypropylene tubes. Larvae were administered orally to each foal using a separate syringe fitted with polyethylene tubing. After oral administration of the inoculum, the transport tube was rinsed with ~5 mL of tap water, which was aspirated into the original inoculation syringe, and administered to the recipient foal in an identical fashion.
Fecal Egg Counts
Fecal egg counts were performed in triplicate for each enrolled foal at every scheduled time point, using the mini-FLOTAC egg counting technique with a detection limit of 5 EPG (23). A saturated glucose-salt solution was used as flotation medium (specific gravity: 1.26).
SVSXP Serum ELISA
Serum samples were collected from all enrolled horses at regular, 28-day intervals throughout the study. An indirect ELISA using recombinant SvSXP protein as antigen was implemented as described previously, with duplicate measurements of each sample (16). Serum samples were diluted 1:50 and horseradish peroxidase (HRP)-conjugated goat anti-horse IgG(T) (Bethyl Laboratories, Inc., Montgomery, TX, USA) was used as a secondary antibody at a dilution of 1:40,000. The result was reported as the normalized value, percentage of a positive control (PP), in order to reduce inter-assay variability (16).
Strongylus vulgaris-Specific PCR
At termination of the study, strongyle eggs were recovered from fecal samples collected on Day 196. Strongyle eggs were analyzed for the presence of S. vulgaris DNA using a species-specific real-time PCR assay described previously (24). All positive reactions were reanalyzed to rule out false amplification. Results were reported as the cycle of threshold (Ct) for a positive PCR amplification, and no amplification in case of PCR negative tests.
Coproculture for Presence of strongylus vulgaris Larvae
The samples collected on Day 196 were also coprocultured to test for the presence of patent S. vulgaris infection. The coproculture procedure was similar to the one described previously for preparation of infective inocula. Diagnostic coprocultures utilized 10 g of feces from each sample, and the entire sediment was collected from each Baermann apparatus after 48 h of sedimentation. Larvae were identified using published morphological criteria (22).
Statistical Analyses
All analyses were carried out using SAS software (version 9.3, SAS Institute). Mixed linear models were constructed for analyzing the relationship between serum ELISA values and anthelmintic treatments (group) and time point and the interaction term between the two. The “mixed” procedure was used with repeated measures and “foal ID” as random effect. “Group,” “gender,” “age,” and “time point” were kept as class variables, while all other variables were considered continuous. The influence of all measured parameters and interactions was evaluated using traditional forward and backward elimination of variables. All variables with p-values of 0.20 or below were kept in the model. Variables were log-transformed to achieve normal distribution, where appropriate. Whenever the variables “time point,” “group,” or the interaction term “time point by group” were found significant, a “least square means” analysis was used for a Tukey’s pairwise comparison. Results were interpreted at the 0.05 significance level.
Results
The SvSXP ELISA values measured over the course of the study are presented in Figure 1. All individual ELISA values are included as supplementary material. Statistical differences between treatment groups were observed from Day 140 onward, with Group 1 having significantly lower mean ELISA values (p < 0.05) than the other two groups. Results of the Tukey’s pairwise comparison of time points within each treatment group are presented in Table 1. Whereas Group 1 had a steady increase of ELISA values over the course of the study, the two other groups exhibited increases between Days 84 and 140, after which they both reached a plateau.
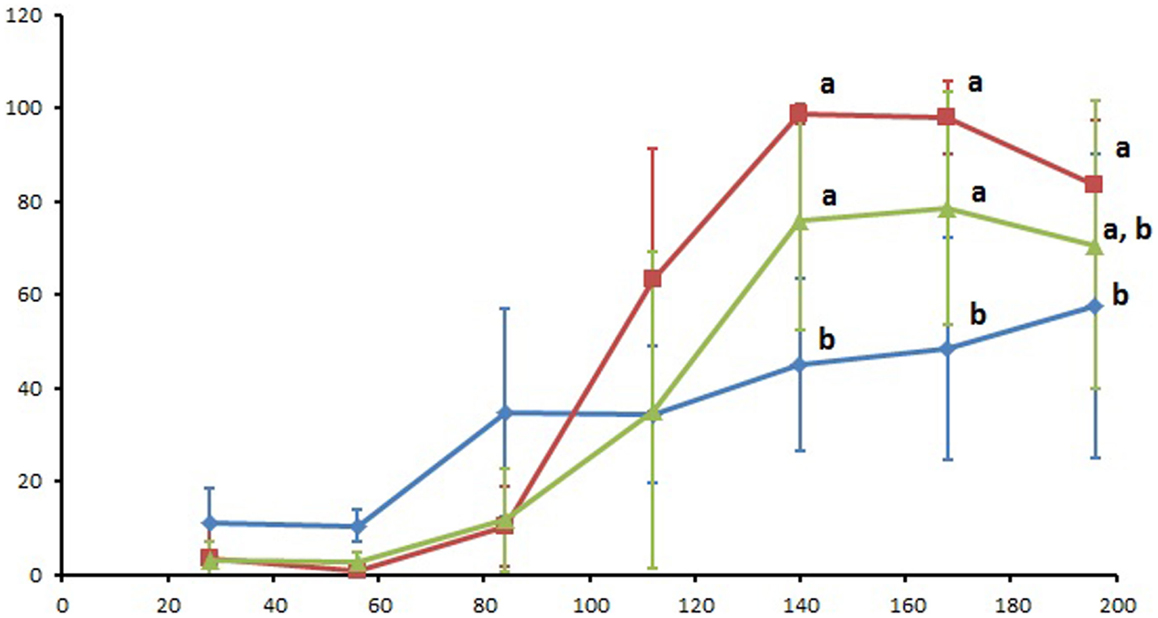
Figure 1. Serum SvSXP ELISA values presented as a percentage of a positive control over the course of the 196 study days. Blue diamonds: group 1 (treated with ivermectin on Day 56), red squares: group 2 (treated with ivermectin on Day 112), and green triangles: group 3 (no treatment control). Different letters designate statistically significant differences (p < 0.05) between groups at the given time points. Error bars represent 95% confidence intervals.
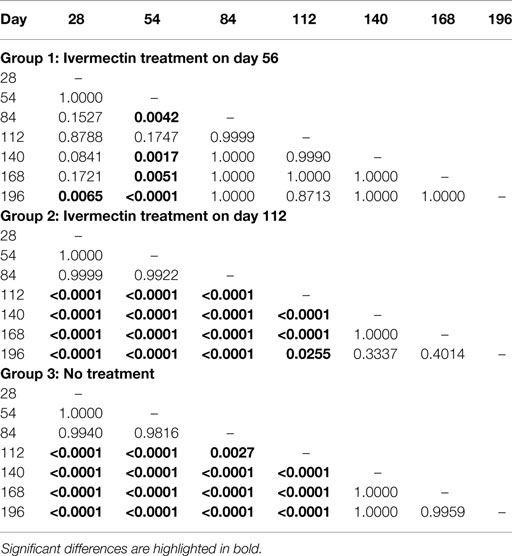
Table 1. p-Values generated in the Tukey’s pairwise comparison analysis of the SvSXP ELISA results obtained at different time points within each treatment group in the study.
Strongyle and ascarid egg count data are presented in Figure 2. The two ivermectin treatments applied in Groups 1 and 2 reduced strongyle egg counts by 73 and 100%, respectively, by the next sampling time point 28 days later. For the ascarid egg counts, no reduction was observed after the Day 56 ivermectin treatment in Group 1, whereas the Day 112 treatment in Group 2 reduced the counts by 100%.
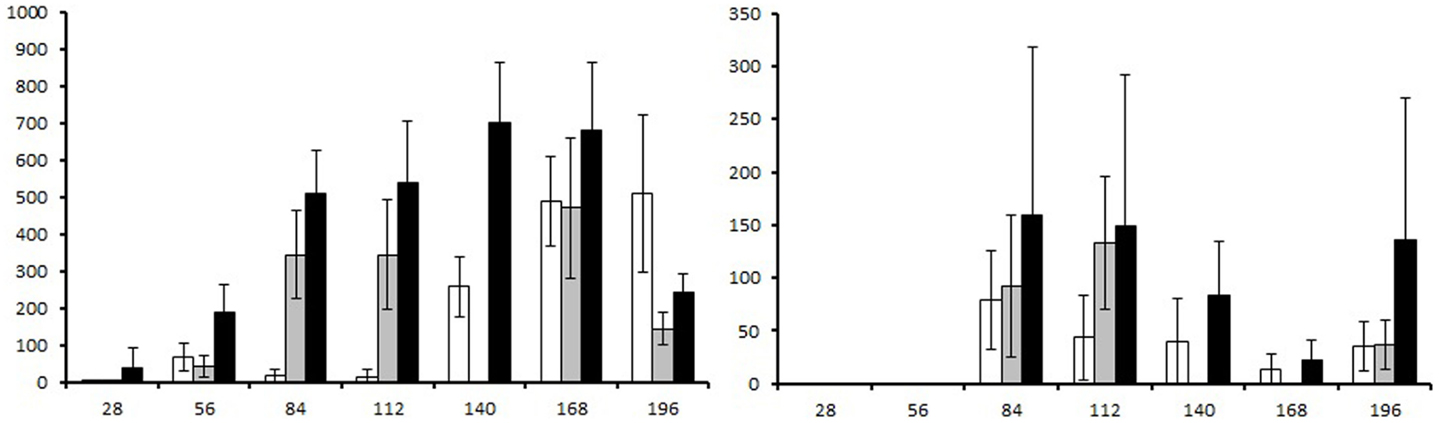
Figure 2. Fecal egg counts presented as eggs per gram (EPG) of feces generated over course of the study. Left graph: strongyle fecal egg count, right graph: ascarid fecal egg count. White columns: group 1 (ivermectin treatment on Day 56), gray columns: group 2 (ivermectin treatment on Day 112), and black columns: group 3 (no treatment control). Error bars represent 95% confidence intervals.
The coproculture and PCR results for detecting patent S. vulgaris infections at trial termination are presented in Table 2.
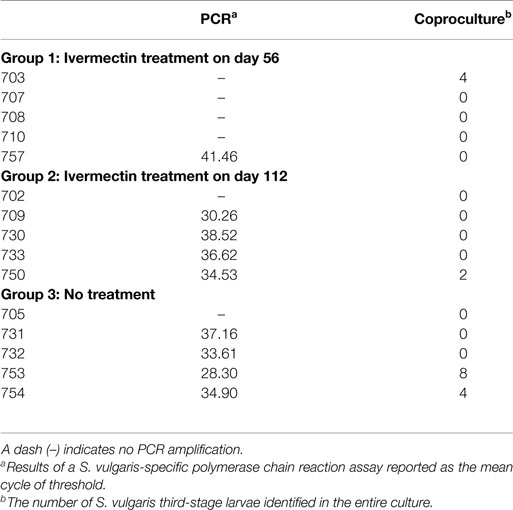
Table 2. Results of diagnostic work performed for presence patent Strongylus vulgaris infection at Day 196.
Discussion
This study generated useful new information about elaboration of S. vulgaris-specific antibodies in response to ivermectin treatment of an established infection with migrating larvae. The group treated early in the infection (Day 56) had a markedly different ELISA response than control horses or the group treated with ivermectin on Day 112. While this pattern could suggest differences in SvSXP antigen production by L4 and L5 larval stages, increased antibody levels in all groups after Day 56 (Figure 1) are evidence that antigen is undoubtedly produced by the L4 stages. It is feasible that the more pronounced ELISA responses in Group 2 and 3 foals was attributable to the continuous and cumulative production of SvSXP over the course of several months. Previous work has illustrated that this antigen is highly immunogenic (16), so continuous release is likely to stimulate a more pronounced antibody response. The abrupt increases observed after Day 84 in Groups 2 and 3 closely resemble the response observed in a cohort of foals exposed to natural infection with S. vulgaris (17). Thus, if left untreated, foals exposed to S. vulgaris infection will undergo a marked seroconversion, which can be interpreted as highly indicative of an active arterial infection.
It is worth noting that ELISA values increased following both ivermectin treatments and that this difference was statistically significant in Group 1 (Table 1). This finding is consistent with observations in a study evaluating ivermectin treatment of naturally infected juvenile horses (18), and we have hypothesized that this increased antibody production is due to dying worms which exacerbate interactions with the immune system. It has been shown that arterial stages of S. vulgaris die over an interval of 4 weeks following ivermectin treatment (25, 26). The nematocidal activity of ivermectin against migrating larvae is mediated through host immune mechanisms, and dead larvae are eventually translocated from the vessel lumen to the subendothelial tunica media of the arteries (26). Thus, it appears plausible that an increased production of antibodies to the SvSXP antigen can occur during a 4-week period following ivermectin treatment.
Table 2 suggests that ivermectin treatment was not 100% efficacious against migrating stages of S. vulgaris in the current study, and that the efficacy was perhaps even less against the later stages of infection (L5). Ivermectin is reportedly >98% efficacious against migrating larvae of S. vulgaris (25, 27, 28), so historical evidence indicates that a few larvae can survive. However, these studies all evaluated the efficacy against 56-day-old experimental infections whereas no published studies appear to have evaluated ivermectin efficacy against S. vulgaris at ~112 days post inoculation. It is worth noting that fecal samples from several horses tested PCR positive for S. vulgaris 5 months following ivermectin treatment in a recent study (18). The fecal egg count reduction observed after ivermectin administration in group 1 was less than desired, but it should be kept in mind that pre-treatment egg count levels were very low and that eggs ingested by coprophagy could constitute a plausible source of error post-treatment in this age group. Therefore, it remains possible that a portion of S. vulgaris L5 larvae can survive ivermectin treatment. However, this should be evaluated in appropriate research studies before any conclusions can be drawn. It is also worth noticing that the coproculture and the PCR were not always in perfect agreement. In a previous study, we have reported a kappa value of 0.5405 between the two tests (29). One possible explanation is that we routinely examine the entire sediment of larvae for presence of S. vulgaris, and this increases the diagnostic sensitivity to a level comparable to the PCR. Eggs of S. vulgaris are unlikely to be evenly distributed over the fecal matter, so some of the discrepancy between the two procedures could simply be due to differences between subsamples.
The survival of some larvae after ivermectin treatment would explain the steady increase of antibodies observed in Group 1 during the 5 months following treatment (Figure 1). Further, antibody levels of the other ivermectin-treated horses in Group 2 did not differ from the untreated control group (Group 3) at any of the seven time points. Again, one possible explanation is that a portion of S. vulgaris larvae survived treatment and continued their production of SvSXP. Further, we know from a recent study that ELISA values do not decrease until about 3–4 months following ivermectin treatment (18). In the present study, this means that a significant reduction was not likely to occur before the termination of the study. Further, since the prepatent period of S. vulgaris is about 6 months, the large majority of larvae should have left the vascular development site during the final months of the study. Perhaps, this exodus can explain the decline of ELISA values observed in Groups 2 and 3 during the last three time points. In other words, even the untreated control group experienced a reduction of arterial larvae during this time, so any treatment-induced effects on ELISA values in Group 2 would not be markedly different.
In summary, this study provided documentation that the SvSXP antigen is apparently produced throughout the course of arterial infection with S. vulgaris. Thus, our hypothesis that the antigen is only produced by later migrating stages can be rejected. However, an early treatment interruption will significantly reduce the concentration of serum SvSXP-specific antibodies in subsequent months. Further, this study also suggested that ivermectin efficacy against migrating S. vulgaris larvae may be <100%, but this has yet to be evaluated in appropriate studies.
Author Contributions
CRR and MKN developed the study protocol and oversaw all procedures. JCP supervised and implemented the clinical elements of the study. JS, HSG, and JLB contributed substantially to acquisition and interpretation of the data. MKN drafted the manuscript with contribution from all authors. The manuscript was critically reviewed and the final version approved by all authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by Merial Ltd. The authors are grateful to Dr. Hoyt Cheramie for his support throughout the study.
References
1. Duncan JL, Pirie HM. The life-cycle of Strongylus vulgaris in the horse. Res Vet Sci (1972) 13:374–9.
2. Duncan JL. Strongylus vulgaris infection in the horse. Vet Rec (1974) 95:34–7. doi:10.1136/vr.95.2.34
3. Enigk K. Die pathogenese der thrombotisch-embolische kolik des pferdes. Monatsh Tierheilk (1951) 3:65–74.
4. Duncan JL, Pirie HM. The pathogenesis of single experimental infections with Strongylus vulgaris in foals. Res Vet Sci (1975) 18:82–93.
5. Nielsen MK, Jacobsen S, Olsen S, Bousquet E, Pihl TH. Non-strangulating intestinal infarction associated with Strongylus vulgaris in referred Danish equine patients. Equine Vet J (2015). doi:10.1111/evj.12422
6. Bollinger O. Die kolik der pferde und das wurmaneurysma der eingeweidearterien. Akad Wiss Munchen Sitzber (1870) 1:539–44.
7. Robertson D. Intestinal parasites of Shetland Ponies in the North of Scotland. Vet Rec (1939) 51:779–81.
8. Slocombe JOD, McCraw BM. Gastrointestinal nematodes in horses in Ontario. Can Vet J (1973) 14:101–5.
9. Herd RP. The changing world of worms – the rise of the cyathostomes and the decline of Strongylus vulgaris. Comp Cont Educ Pract Vet (1990) 12:732–6.
10. Love S, Duncan JL. Could the worms have turned? Equine Vet J (1991) 23:152–4. doi:10.1111/j.2042-3306.1991.tb02745.x
11. Höglund J, Ljungström BL, Nilsson O, Lundquist H, Osterman E, Uggla A. Occurrence of Gasterophilus intestinalis and some parasitic nematodes of horses in Sweden. Acta Vet Scand (1997) 38:157–65.
12. Boxell AC, Gibson KT, Hobbs RP, Thompson RCA. Occurrence of gastrointestinal parasites in horses in metropolitan Perth, Western Australia. Aust Vet J (2004) 82:91–5. doi:10.1111/j.1751-0813.2004.tb14653.x
13. Pilo C, Altea A, Pirino S, Nicolussi P, Varcasia A, Genchi M, et al. Strongylus vulgaris (Looss, 1900) in horses in Italy: is it still a problem? Vet Parasitol (2012) 184:161–7. doi:10.1016/j.vetpar.2011.09.016
14. Lyons ET, Tolliver SC, Kuzmina T, Dzeverin II, Nielsen MK, McDowell K. Profiles of strongyle EPG values for thoroughbred mares on 15 farms in Kentucky (2012-2013). Vet Parasitol (2014) 205:646–52. doi:10.1016/j.vetpar.2014.08.001
15. Nielsen MK, Vidyashankar AN, Olsen SN, Monrad J, Thamsborg SM. Strongylus vulgaris associated with usage of selective therapy on Danish horse farms – is it reemerging? Vet Parasitol (2012) 189:260–6. doi:10.1016/j.vetpar.2012.04.039
16. Andersen UV, Howe DK, Dangoudoubiyam S, Toft N, Reinemeyer CR, Lyons ET, et al. SvSXP: a Strongylus vulgaris antigen with potential for prepatent diagnosis. Parasit Vectors (2013) 6:84. doi:10.1186/1756-3305-6-84
17. Nielsen MK, Vidyashankar AN, Gravatte HS, Bellaw J, Lyons ET, Andersen UV. Development of Strongylus vulgaris-specific serum antibodies in naturally infected foals. Vet Parasitol (2014) 200:265–70. doi:10.1016/j.vetpar.2013.12.024
18. Nielsen MK, Vidyashankar AN, Bellaw J, Gravatte HS, Cao X, Rubinson EF, et al. Serum Strongylus vulgaris-specific antibody responses to anthelmintic treatment in naturally infected horses. Parasitol Res (2015) 114:445–51. doi:10.1007/s00436-014-4201-5
19. Reinemeyer CR, Prado JC, Andersen UV, Nielsen MK, Schricker B, Kennedy T. Effects of daily pyrantel tartrate on strongylid population dynamics and performance parameters of young horses repeatedly infected with cyathostomins and Strongylus vulgaris. Vet Parasitol (2014) 204:229–37. doi:10.1016/j.vetpar.2014.05.034
20. Lyons ET, Tolliver SC, Drudge JH, Swerczek TW, Crowe MW. Common internal parasites found in the stomach, large intestine, and cranial mesenteric artery of Thoroughbreds in Kentucky at necropsy (1985-1986). Am J Vet Res (1987) 48:268–73.
21. Henriksen SA, Korsholm H. A method for culture and recovery of gastrointestinal strongyle larvae. Nord Vet Med (1983) 35:429–30.
22. Russell AF. The development of helminthiasis in Thoroughbred foals. J Comp Pathol Ther (1948) 58:107–27. doi:10.1016/S0368-1742(48)80009-3
23. Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, Sadutshang T, et al. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis (2014) 7:e2344. doi:10.1371/journal.pntd.0002344
24. Nielsen MK, Peterson DS, Monrad J, Thamsborg ST, Olsen SN, Kaplan RM. Detection and semi-quantification of Strongylus vulgaris DNA in equine faeces by real-time PCR. Int J Parasitol (2008) 38:443–53. doi:10.1016/j.ijpara.2007.07.014
25. Slocombe JOD, McCraw BM. Evaluation of ivermectin against later fourth-stage Strongylus vulgaris in ponies at two and five weeks after treatment. Can J Comp Med (1984) 48:343–8.
26. Slocombe JOD, McCraw BM, Pennock PW, Ducharme N, Baird JD. Strongylus vulgaris in the tunica media of arteries of ponies and treatment with ivermectin. Can J Vet Res (1987) 51:232–5.
27. Klei TR, Torbert BJ, Chapman MR, Turk MA. Efficacy of ivermectin in injectable and oral paste formulations against eight-week-old Strongylus vulgaris larvae in ponies. Am J Vet Res (1984) 45:183–5.
28. Slocombe JO, McCraw BM, Pennock PW, Vasey J. Effectiveness of ivermectin against later 4th-stage Strongylus vulgaris in ponies. Am J Vet Res (1982) 43:1525–9.
Keywords: Strongylus vulgaris, diagnosis, larval migration, ivermectin, ELISA
Citation: Nielsen MK, Scare J, Gravatte HS, Bellaw JL, Prado JC and Reinemeyer CR (2015) Changes in serum Strongylus vulgaris-specific antibody concentrations in response to anthelmintic treatment of experimentally infected foals. Front. Vet. Sci. 2:17. doi: 10.3389/fvets.2015.00017
Received: 07 May 2015; Accepted: 15 June 2015;
Published: 01 July 2015
Edited by:
Anja Joachim, University of Veterinary Medicine Vienna, AustriaReviewed by:
Thomas Tzelos, Moredun Research Institute, UKSmaragda Sotiraki, Veterinary Research Institute HAO-Demeter, Greece
Christina Strube, University of Veterinary Medicine Hannover, Germany
Copyright: © 2015 Nielsen, Scare, Gravatte, Bellaw, Prado and Reinemeyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Krarup Nielsen, Department of Veterinary Science, 319 M.H. Gluck Equine Research Center, University of Kentucky, Lexington, KY 40546-0099, USA,bWFydGluLm5pZWxzZW5AdWt5LmVkdQ==
 Martin Krarup Nielsen
Martin Krarup Nielsen Jessica Scare1
Jessica Scare1 Jennifer Lynn Bellaw
Jennifer Lynn Bellaw Craig Robert Reinemeyer
Craig Robert Reinemeyer