- China Institute of Veterinary Drug Control, Beijing, China
Avian tuberculosis is a chronic, contagious zoonotic disease affecting birds, mammals, and humans. The disease is most often caused by Mycobacterium avium spp. avium (MAA). Strain resources are important for research on avian tuberculosis and vaccine development. However, there has been little reported about the newly identified MAA strain in recent years in China. In this study, a new strain was isolated from a fowl with symptoms of avian tuberculosis by bacterial culture. The isolated strain was identified to be MAA by culture, staining, and biochemical and genetic analysis, except for different colony morphology. The isolated strain was Ziehl-Zeelsen staining positive, resistant to p-nitrobenzoic acid, and negative for niacin production, Tween-80 hydrolysis, heat stable catalase and nitrate production. The strain had the DnaJ gene, IS1245, and IS901, as well. Serum agglutination indicated that the MAA strain was of serotype 1. The MAA strain showed strong virulence via mortality in rabbits and chickens. The prepared tuberculin of the MAA strain had similar potency compared to the MAA reference strain and standard tuberculin via a tuberculin skin test. Our studies suggested that this MAA strain tends to be a novel subtype, which might enrich the strain resource of avian tuberculosis.
Introduction
Avian tuberculosis is a serious, chronic infectious zoonotic disease in poultry, pet, or captive birds, animals, and humans that is caused by Mycobacterium avium spp. avium (MAA), a subspecies of the M. avium complex (MAC). Avian tuberculosis was classified as a List B disease by the World Organization for Animal Health and as a third-class animal disease in China. Clinical manifestations in birds include emaciation, depression, and diarrhea, and the prominent feature is the formation of caseous tubercular nodules and granulomas with calcification in the liver, spleen, intestine, and bone marrow (1–3). Humans exposed to infected birds may acquire a zoonotic infection, particularly in immunocompromised people such as those with HIV (3, 4). Once fowls in poultry farms are infected, avian tuberculosis can persist for a long time and is difficult to eradicate, due to the chronic carrier state and shedding of bacteria by infected fowls (5, 6). This disease can have a serious economic impact associated with mortality, morbidity, and a reduction of egg production (1, 6). The best way is to quarantine and slaughter the infected ones (6, 7), as well as antibiotic treatment with the infected individuals (8, 9).
The MAC is a group of closely related non-tuberculous mycobacterial species and subspecies which includes both veterinary and opportunistic human pathogens. In addition to birds, MAC may also infect different animal species, such as swine, cattle, deer, sheep, goat, horses, cats, dogs, and exotic species. The species within MAC are divided into the following subspecies according to taxonomical classification: MAA, the etiological agent of avian tuberculosis; M. avium spp. paratuberculosis, the etiological agent of Johne’s disease; M. avium spp. silvaticum, previously called “wood pigeon Mycobacterium”; M. avium spp. hominissuis, frequently isolated from pig and human and M. intracellulare, a closely related pathogen of birds with a lower prevalence (2, 6, 10). Although the subspecies of MAC differ greatly in their host range and growth potential, it has been reported that tuberculous lesions caused by MAA, M. avium spp. paratuberculosis, M. avium spp. hominissuis, and M. avium spp. silvaticum are indistinguishable (11–15). In addition, coinfection with different members of M. tuberculosis complex and M. avium subspecies or with other mycobacterial species combinations is not rare in animal and human hosts (16, 17). Accurate identification and discrimination of mycobacterial species and subspecies is essential to determine their significance, pathogenicity, diagnosis, epidemiology, and most beneficial control program (18–20).
The identification and preservation of microbial strains is an important strategic resource for disease epidemiology research and disease prevention. Since the first report of avian tuberculosis in 1978 in China, there have been additional reports about the disease (7). As known, avian tuberculosis is sporadic and has a low incidence. Recent years, there were reports about incidence of avian disease in the poultry farm, but not large scale occurrences of disease (7, 21, 22). Isolation and identification of MAA strains from pathological materials could provide abundant resources for avian tuberculosis research. Although more attention has recently been paid to avian tuberculosis research, fewer MAA strains have been isolated and identified in recent years because of the high biological safety risks and low economic value of MAA strains in China. There were almost no reports regarding the isolation of MAA strains from diseased species, and no systematic studies were carried out on the characteristics of MAA strains.
In this work, we first isolated an MAA strain from the typical tubercular nodules of a fowl with possible avian tuberculosis symptoms. We then systematically identified the isolated strain via a series of assays and further investigated the virulence and potency of the identified strain.
Materials and Methods
Ethics Statement
The present study was approved by the Laboratory Animal Ethics Committee of China Institute of Veterinary Drug Control and was also approved by the Ministry of Agriculture and the Bureau of Animal Husbandry. The experiments were performed in compliance with the “Regulations of the People’s Republic of China on the Administration of Experimental Animals” and the “Guidelines for the ethical review of experimental animal welfare in Beijing.”
Mycobacterium avium spp. avium strains were cultured in an air-conditioned, air filtered, biosafety level III facility. The experimental use of serum samples, including sample collection, handling, testing, and personal protection, complied with the General Requirements for Laboratory Biological Safety of China, GB19489 (2008).
MAA Strains
Mycobacterium avium spp. avium strains CVCC275, CVCC276, and CVCC277 were the virulent reference strains for serotype 1, serotype 2, and serotype 3, respectively. MAA strain CVCC68201 was the virulent strain for avian tuberculin purified protein derivative (PPD) production and inspection (23).
Tissue Origin and Examination
One fowl showing the clinical signs (swollen joints, lameness, emaciation, tubercle formation under the skin, and granulomas in the conjunctival sac) of avian tuberculosis and poor health was transferred to the avian diseases section, Shandong Agricultural University, College of Animal Science and Veterinary Medicine. The fowl was euthanized and underwent a necropsy examination, and tissue containing typical tubercles was collected in 50 mL screw cap containers, packed in dry ice chambers, and then sent to the China Institute of Veterinary Drug Control.
After thawing the tissue samples, portions were formalin fixed, embedded in paraffin blocks, and stained by the Ziehl-Neelsen (Z-N) technique to detect Mycobacteria (24). An additional tissue sample was fixed in neutral-buffered formalin and embedded in paraffin wax. Thereafter, the sample was sectioned, stained and transported to perform histopathological examination.
Mycobacterial Isolation
Approximately 1 g of tissues were pooled into a test tube containing 1 mL of sterile PBS and homogenized for 1 min. Two hundred microliters of the mixture was inoculated onto two slopes of Petragnani medium (25). The inoculated slopes were incubated at 37°C for 4 weeks. Colony morphology was visualized with the naked eye.
Grown Colony Staining
The grown colony on the slope was checked by Gram staining and Z-N acid-fast staining (24, 26). The studies were carried out using a Gram-staining kit and Z-N staining kit (Land Bridge Technology Co., Ltd., Beijing, China) according to the manufacturer’s instructions. The colony, confirmed to contain Gram-positive bacteria and acid-fast bacteria (AFB), was streaked out for further study.
Biochemical Identification of the AFB Isolates
Biochemical identification of the AFB isolates was based on multiple tests, which included the p-nitrobenzoic acid (PNB) growth assay, niacin production, Tween-80 hydrolysis, heat stable catalase activity, and nitrate reductase activity (27, 28).
PNB Growth Assay
The AFB isolates were screened by a growth test on a slope of Petragnani medium containing 500 µg/mL PNB (29). The inoculated slope was incubated at 37°C for 4 weeks, and then growth conditions were visualized.
Niacin Production Assay
The AFB isolates were washed off from the slope of Petragnani medium. Next, 0.9 mL of bacterial suspension was added to 0.2 mL of 3% benzidine ethanol, followed by the addition of 0.2 mL of 10% hydrogen bromide. The color reaction and precipitation reaction were observed.
Tween-80 Hydrolysis Assay
The Neutral Red/Tween-80 solution was prepared by adding 0.5 mL of 0.8% Neutral Red to 100 mL of 0.5% Tween-80/PBS solution (pH 7.0), followed by sterilization at 116°C for 20 min. The AFB isolates were washed off from the slope and resuspended to a final concentration of 10 mg/mL with PBS. Then, 0.5 mL of bacterial suspension was added to 2 mL Neutral Red/Tween-80 solution and incubated at 37°C for 10 days. The color change of the Neutral Red/Tween-80 solution was observed daily.
Heat Stable Catalase Assay
First, 0.5 mL of the bacterial suspension prepared above (10 mg/mL) was added to a test tube and incubated at 68°C for 20 min. Then, 0.5 mL of 30% H2O2 in 10% Tween-80 (pH 7.0) was added to the bacterial culture. The evolution of O2 gas caused frothing indicative of catalase activity, whereas the absence of O2 gas bubbles demonstrated a loss of enzymatic activity.
Nitrate Reductase Assay
Nitrate reduction was performed by the classical procedure with liquid reagent. First, 10 mM NaNO3/PBS solution (pH 7) was prepared and sterilized at 116°C for 20 min. Next, 0.5 mL of the bacterial suspension prepared above (10 mg/mL) was added to 2 mL of NaNO3/PBS solution. The mixture was incubated at 37°C for 2 h, followed by the addition of 100 µL of 18% HCl and 100 µL of 0.2% sulfanilamide solution and later by 100 µL of 0.1% naphthylethylenediamine solution. The system was stored at 2–8°C for 2 weeks, and the color reaction was visualized.
Genetic Identification of the AFB Isolates
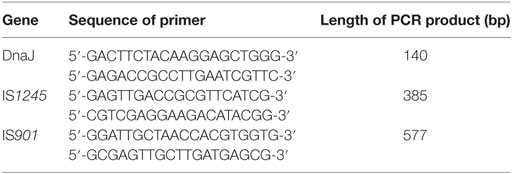
The AFB isolates were examined by PCR for the detection of DnaJ, IS1245, and IS901 gene fragments (30). The colony on Petragnani medium was washed off from the slope and washed with PBS. After heat inactivation at 60°C for 2 h, genomic DNA was extracted using a genomic DNA purification kit (Promega, Madison, WI, USA). Primers were designed for amplification of DnaJ, IS1245, and IS901 fragments (Table 1) (30). Multiplex PCR was programmed as 1 cycle at 96°C for 2 min, 35 cycles at 96°C for 10 s, 58°C for 10 s, 72°C for 1 min, and 1 cycle at 72°C for 2 min. The PCR products were visualized by DNA gel electrophoresis.
Serological Identification of the AFB Isolates
The serotype of the AFB isolates was identified by the serum agglutination method as described below (31). The antigens of serotype 1, 2, and 3 were prepared from MAA reference strains CVCC275, CVCC276, and CVCC277, respectively. The AFB isolates, as well as the three strains, were cultured on Lowenstein-Jensen solid medium at 37°C for 18 days. The bacteria were washed off using 200 mL of sterile PBS containing sterile glass beads and inactivated at 37°C for 7 days with shaking up to four times a day. The mixture was centrifuged to remove the supernatant. After washing three times, the bacterial pellet was suspended in 0.4% sodium citrate/PBS to a concentration of approximately 1010 CFU/mL. The suspended bacteria were used as antigen for the serum agglutination assay.
The AFB isolates were cultured on Petragnani medium at 37°C for 4 weeks. The bacterial culture was washed off using sterile PBS containing sterile glass beads and centrifuged at 10,000 rpm for 5 min to remove the supernatant. The bacterial pellet was weighed and resuspended in sterile PBS to a concentration of 0.1 mg/mL. Three 8-week-old SPF chickens were inoculated intravenously with 0.2 mL of the above bacterial suspension, with the same amount of PBS as a control. After 60 days, sera were collected from the wing vein for the serum agglutination assay.
For the serum agglutination assay, one drop of serum from the wing vein and one drop of bacterial suspension were mixed on a slide. After 1 min, the results were graded as negative (−), weakly positive (+), positive (++), and strongly positive (+++), according to agglutination degree as no agglutination (−), less than 25% agglutination (+), approximately 50% agglutination (++), and more than 75% agglutination (+++), respectively.
Virulence
The AFB isolates were cultured on Petragnani medium at 37°C for 4 weeks. The bacterial culture was washed off using sterile PBS and centrifuged at 10,000 rpm for 5 min to remove the supernatant. The bacterial pellet was weighed. The virulence of the AFB isolates in rabbits and chickens was studied according to the “The People’s Republic of China veterinary biological product regulation” (25).
For virulence in rabbits, the above bacterial pellet was resuspended in sterile PBS to a final concentration of 0.5, 1, 2, or 4 mg/mL. Six healthy rabbits at 2 kg of body weight (Albino, Vital River) were inoculated intravenously in the ear with 1 mL of the bacterial suspension, while the same amount of PBS was used as a control. The inoculated rabbits were observed for 30 days, and survival was recorded.
For virulence in chickens, the above bacterial pellet was resuspended in sterile PBS to a final concentration of 0.5, 1.25, 2.5, or 5 mg/mL. Five 8-week-old SPF chickens (Vital River) were inoculated intravenously with 0.2 mL of the bacterial suspension, while the same amount of PBS was used as a control. The inoculated rabbits were observed for 60 days, and survival was recorded.
Potency and Specificity
Preparation of PPD of Avian Tuberculin
Avian tuberculin was prepared according to the rules for tuberculin production and inspection (25), as described briefly below. The AFB isolates were streaked out from the Petragnani slope medium to 200 mL of Sauton liquid medium and grown at 37°C for 40–45 days to prepare the seed strain. The seed strain was inoculated into 1,000 mL of Sauton liquid medium and grown at 37°C for 90 days. The culture was inactivated at 121°C for 30 min and filtered through a hollow fiber membrane to remove the bacteria. The filtrate was mixed with 40% (w/v) TCA solution to a final concentration of 4%. The mixture was incubated overnight at 2–8°C. The pellets were collected and washed three times with 1% TCA solution. The pellets were centrifuged at 4°C, 5,000 rpm for 30 min, and the pellet was dried and weighed. The pellet was dissolved in 1 M NaOH, adjusted to pH 7.4 using 1 M HCl, and filtered through sterilizing Chua’s filter. The final product was the avian tuberculin PPD for further testing, referred to as PPD-AFB. The avian tuberculin PPD from CVCC68201 (PPD-CVCC68201) was prepared using the same procedure.
Preparation of Allergen and Sensitization of Guinea Pigs
Mycobacterium avium spp. avium strain CVCC68201 was cultured at 37°C for 20 days. The bacteria were scraped from the slope, weighed, dissolved in sterile PBS, and inactivated at 121°C for 30 min. The bacterial suspension was mixed with Fruend’s incomplete adjuvant to prepare an emulsion at a concentration of 40 mg/mL bacteria. The emulsion was subpackaged and inactivated at 80°C water bath for 2 h, and the emulsion was used as the allergen. Sixteen guinea pigs at 400 g of body weight (Hartly, Vital River) were sensitized with 0.5 mL of allergen by deep intramuscular injection in the inner thigh and maintained for 35 days under specific pathogen-free conditions. After the sensitization period, guinea pigs were shaved in a 3 cm2 area on the hip, at the opposite side of allergen injection. The standard avian tuberculin PPD (PPD-S, IVDC) was diluted 100-fold, and 0.1 mL diluted standard avian tuberculin PPD was injected into the shaved area of the guinea pigs. The diameter of reactions on the skin was measured after 24 h, and the guinea pig was positive for sensitization if the area of skin thickness was over 1 cm2.
Potency Calibration and Specificity Test of Avian Tuberculin PPD
The potency calibration of PPD was carried out according to the relevant rules (24–26, 32). For the potency calibration, the PPD-AFB was diluted to two concentrations of 0.1 and 0.25 dose/mL. Additionally, the PPD-S and PPD-CVCC68201 were diluted to two concentrations of 100 and 250 IU/mL. The shaved area of the sensitized guinea pigs was divided into two equal blocks. Each block contained three randomized injection sites resulting in six total injections, with a replicated injection of each PPD concentration in each guinea pig. Guinea pigs were injected intradermally with a volume of 0.1 mL of designated PPDs. A total of 12 guinea pigs (6 guinea pigs for each PPD concentration) were used for PPD inoculation. An additional 4 guinea pigs were used as negative controls. No adverse effect resulted from the procedures used, and the animals were monitored daily by the animal care staff. After 24 h, the skin thickness was measured using Vernier calipers.
The specificity of PPD testing was performed as described below (25, 33). Six guinea pigs at 400 g of body weight (Hartly, Vital River) were shaved in an area (3 cm × 9 cm) on both sides of the chest. PPD-AFB, PPD-S, and PPD-CVCC68201 were diluted to 2 × 104 IU/mL. Similar to the potency test, the guinea pigs were injected intradermally with one volume of 0.1 mL of designed PPDs with replicated injections of each PPD. After 24 h, the skin inflammatory reaction was observed.
Statistical Analysis
The statistical package SPSS and one-way ANOVA method were used for data analyses. The results were expressed as the mean ± SD. The p-values were considered significant at (*p<0.05).
Results
Identification of the Pathological Tissue
The pathological tubercle contained a yellow-white cheese-like substance with a coated envelope after cutting. After smearing and Z-N staining, a large number of mycobacteria were observed (data not shown). With further histopathological examination of the tubercle, caseous necrosis appeared in the center of the tubercle with a large number of lymphoid cells, epithelioid cells, and Langerhans multinucleated giant cells (Figure 1A). This was a typical avian tuberculosis symptom.
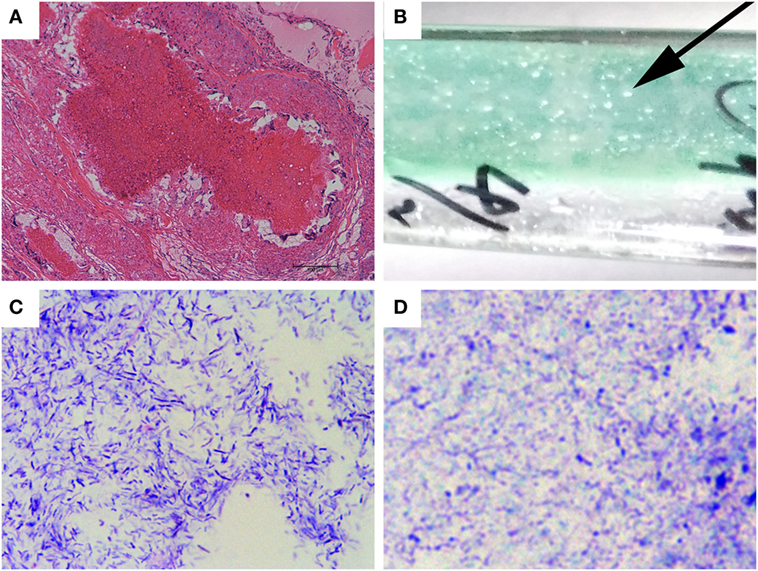
Figure 1. Tubercle staining and mycobacterial isolation. (A) Histopathological staining of the pathological tubercle from the diseased fowl. Bar, 100 µm. (B) Growth of isolated mycobacteria colonies from the tissue homogenate on a slope of Petragnani medium. The arrow indicates a typical clone. (C,D) Photographies of the mycobacterial colonies after Gram staining (C) and Z-N acid-fast staining [(D), 1,000×].
On the slopes of the Petragnani medium inoculated with homogenized tissue, transparent, round, thick, drip-like colonies were observed (Figure 1B, arrow) after incubation at 37°C for 4 weeks. The colonies did not have a yellowish-white, cream like appearance (24, 25, 34). While streaking, the colonies had a threadlike filament (data not shown). The colonies were further tested by Gram staining and Z-N staining. As shown in Figure 1C, the colonies were Gram-positive, bluish-purple staining, and contained straight or curved rod-shaped bacteria. Additionally, the grown colonies were Z-N staining positive with red staining and a length of 1–3 µm (Figure 1D). The above results indicated that the colonies were AFB, i.e., mycobacteria.
Biochemical Characterization of AFB Isolates
Five different biochemical tests, including resistance to PNB, niacin production, Tween-80 hydrolysis, heat stable catalase and nitrate production, were applied to the AFB isolates (Table 2). The AFB isolates showed positive results for resistance to PNB. For the niacin production test, a clear pellet, not a peach red pellet was observed, indicating a negative result. The test for Tween-80 hydrolysis was negative, indicated by no color change of from neutral red. The heat stable catalase activity was negative, as no bubble was generated in the reaction solution. The reaction system was very light pink, suggesting the absence of nitrate reductase in the AFB isolates. From the above biochemical tests, the AFB isolates most closely resembleMAA in their enzymatic activities.
Genetic Characterization of AFB Isolates
Using multiplex PCR, the AFB isolates and the CVCC6801 strain produced three bands (140, 385, and 577 bp) corresponding to the DnaJ gene, IS1245, and IS901 (Figure 2). As the isolates were IS901+, IS1245+, and dnaJ+ (30, 35), the isolates were of the MAA strain.
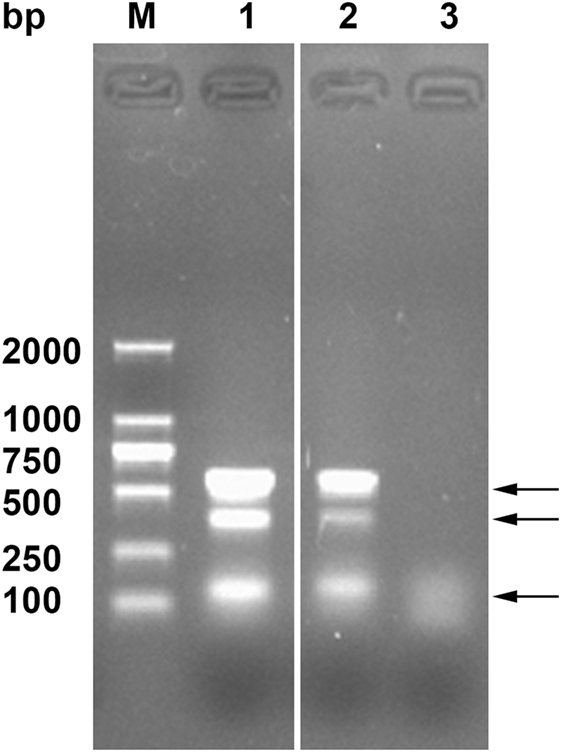
Figure 2. Genetic identification of the acid-fast bacteria (AFB) isolates by Multiplex PCR. Lane M, DNA ladder, Lane 1, AFB isolates, Lane 2, CVCC68201, and Lane 3, PBS. The arrows indicate the three typical amplified bands of DnaJ gene, IS1245, and IS901.
Serological Characterization of AFB Isolates
The MAA strain has three serotypes: serotype 1, serotype 2, and serotype 3 (2). Using the serum agglutination method, the antigen prepared from AFB isolates showed a strong agglutination reaction to the serum from chickens infected with the CVCC275 strain but no agglutination reaction to the serum from chickens infected with the CVCC276 and CVCC277 strains (Table 3). The MAA strains CVCC275, CVCC276, and CVCC277 were the reference strains for serotype 1, serotype 2, and serotype 3, respectively. The results suggested that the AFB isolates were an MAA strain of serotype 1.
Virulence of the AFB Isolates
To evaluate the virulence of the AFB isolates, mortality in rabbits and chickens was recorded during the observation period post-injection of the prepared bacteria. As shown in Figure 3A, the six uninfected rabbits remained healthy and alive until the 30th day of the observation period. Among the six rabbits injected with 0.5 mg of bacteria, three of the rabbits died, and three remained alive until the end of the observation period. For the remaining three groups of rabbits inoculated with 1, 2, and 4 mg of bacteria, all rabbits died during the observation period. Moreover, the rabbits died at earlier dates when inoculated with higher doses of bacteria.
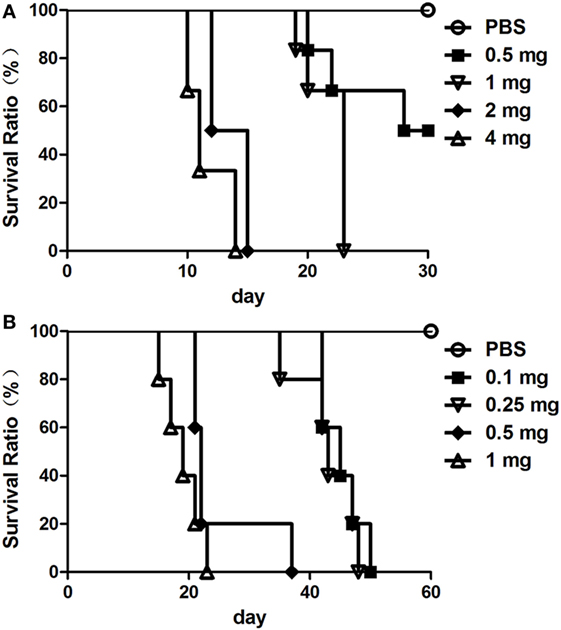
Figure 3. The virulence of the acid-fast bacteria (AFB) isolates. Five groups of healthy rabbit (A) and chicken (B) was inoculated with different amounts of Mycobacterium avium spp. avium bacteria. The virulence of the AFB isolates is represented by the survival ratio of rabbits (A) and chickens (B).
As shown in Figure 3B, the five uninfected chickens remained healthy and alive until the 60th day of the observation period. All of the chickens in the groups injected with different doses of bacteria, from 0.1 to 1.0 mg, died during the 60 days observation period. Additionally, the chickens died earlier when given a higher inoculation dose of bacteria.
According to the definition of MAA virulence (25), the results indicated that the AFB isolates were MAA strain with strong virulence.
Potency and Specificity of the PPD Prepared from AFB Isolates
The potency of the AFB isolates was evaluated by calibrating the prepared avian tuberculin PPD from the AFB isolates. As shown in Figure 4, PPD-AFB, PPD-S and PPD-CVCC68201 showed no significant difference when analyzed for indurations (skin thickness) in sensitized guinea pigs. Thus, the PPD prepared from the AFB isolates had a similar potency to the standard PPD and the PPD from the reference MAA strain, and the AFB isolates had a similar potency to the reference MAA strain.
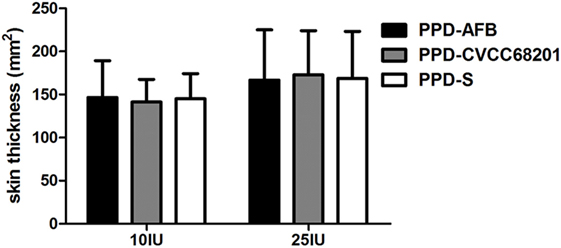
Figure 4. The potency of the acid-fast bacteria (AFB) isolates. The purified protein derivative (PPD) was prepared from the AFB isolates and was calibrated by traditional PPD skin test. The potency is represented by the area of skin thickness after PPD injection on sensitized guinea pigs.
The specificity of the AFB isolates was analyzed by the skin test in unsensitized guinea pigs with PPD-AFB, PPD-CVCC68201, and PPD-S. Similar to PPD-CVCC68201 and PPD-S, PPD-AFB could not induce a skin inflammatory reaction (Table 4). Thus, the PPD prepared from the AFB isolates was specific for PPD prepared from the MAA strains.

Table 4. Specificity of the p-nitrobenzoic acid (PPD) prepared from the acid-fast bacteria isolates.
Discussion
In this work, we isolated an MAA strain from caseous tubercular nodules of a fowl with avian tuberculosis. The isolated strain was a typical MAA strain of serotype 1, genotype IS901+ and IS1245+ according to biochemical, serological and genetic identification. The identified MAA strain was a virulent strain and showed similar PPD potency as the reference MAA strain. Interestingly, our study revealed that this isolated MAA strain showed a novel colony appearance not previously reported.
Diagnosis of avian tuberculosis is frequently based on clinical symptoms, postmortem gross lesions and Z-N staining of the smear. However, Z-N staining for demonstrating acid-fast bacilli is limited because of its low sensitivity and detection rate (36). Serological diagnosis method such as hemagglutination, complement fixation, ELISA, and Western-blot analysis might be most useful in late-stage infections based on the process of mycobacteria evading the host immune system. Also the request of specific antigen might limit the board application of these methods (37). The PCR approaches, including PCR, multiplex PCR, PCR restriction fragment length polymorphism (RFLP), and mycobacterial interspersed repetitive units and variable number tandem repeats (MIRU-VNTR) typing are also capable of specifically detecting DNA fragments, thus acting as a diagnostic procedure (30, 35, 38, 39). However, PCR products can have false positive results because of contamination (40). The PPD skin test is the only method recommended by OIE, but the result reading is affected by the dose and injection manner (24). Comparing everything, the gold standard technique for avian tuberculosis is culturing from diseased tissue for isolation, genotypic and phenotypic characterization of the suspect pathogen (2). This method is reliable, but it requires more time and high quality culture medium.
For the conventional culturing technique of the MAA strain, it is important to use the proper selective medium (41). It was reported that M. avium grows best on media such as Lowenstein-Jensen medium, Herrold’s medium and Middlebrook medium (6), which were also recommended by the World Organization for Animal Health as the culturing medium of MAA (24). In this study, we used Petragnani medium to isolate the MAA strain from the tubercle (25, 26). The Petragnani medium was reported to have similar colony yielding ratio as Lowenstein-Jensen medium for Mycobacterium tuberculosis (42). As known, culturing of mycobacteria is time-consuming to complete, as it takes at least 4–8 weeks for visible colonies to appear (5, 37). For MAA strain, the culture should be incubated for at least 12 weeks using Lowenstein-Jensen medium, Herrold’s medium and Middlebrook medium (43). It took only 4 weeks to observe transparent, round, thick, drip-like colonies on Petragnani medium (Figure 1B). Thus, culturing Petragnani medium for isolation of MAA strain was time-saving and offered greater efficiency for avian diagnosis.
The traditional method for species and subspecies typing of Mycobacterium was according to growth on distinct medium and morphology of the grown colony. The MAA strain usually had yellowish-white, round, and cream-like colonies (25) (Insights into the mycobacterium infection). In our study, the isolated MAA strain had a novel colony appearance, as round, thick, drip-like colonies (Figure 1B). MAA forms colonies of different morphologies, including smooth transparent, smooth opaque, and rough, which might influence pathogenicity, virulence, drug susceptibility and macrophage survival (44–46). Here, this isolated MAA strain had similar virulence and PPD potency compared to the MAA reference strain CVCC68201 (Figures 3 and 4). This finding might indicate that this isolated MAA strain might be of a novel subtype and have differences in some unknown phenotype. However, the traditional method was limited in use because of the high biosafety requirement, and it was more time-consuming and required more complicated techniques. Genetic approaches would applied for further subtyping of this newly isolated MAA strain, as RFLP and DNA sequencing of distinct gene cluster for subtyping of diverse MAA isolates (47, 48), short sequence repeats sequencing and MIRU-VNTR typing for subtyping of M. avium spp. paratuberculosis (49–51). For example, IS901 RFLP typing was used to discriminate MAA field isolates (52, 53). As reported, a cluster of genes were proved to be related to colony morphology, such as MAP1156 (diacyglycerol O-acyltransferase), MAP 1152 (PPE protein), and Lsr2 etc. in M. avium spp. paratuberculosis (54), MAV_4334 (nitroreductase family protein), MAV_5106 (phosphoenolpyruvate carboxykinase), MAV_1778 (GTP-binding protein LepA), etc. in M. avium spp. hominissuis (55). In addition, glycopeptidolipids and its biosynthesis were reported to be related to the surface properties and colony morphology of mycobacteria (56–58). DNA sequencing of these gene clusters might give a clue for further subtyping of this isolated MAA strain with a round, thick, drip like colony appearance (Figure 1B).
Virulence is a reflection of pathogenicity of the bacterial pathogen. The virulence of MAA is affected by various factors and could change with different hosts and environments (59). The extent of intracellular replication in cell cultures, guinea pigs and mice is widely used as a measure for mycobacterial virulence (60, 61). Additionally, the mortality of animals inoculated with mycobacteria is also used as an indicator of virulence (25, 62). The definition of the MAA virulent strain is that inoculation of 0.1–1.0 mg bacteria would lead to the death of chickens or that 1 mg of bacteria would lead to the death of rabbits (23, 25). The isolated MAA strain here was proven to have strong virulence (Figure 3). Compared to intracellular bacterial replication or macrophage infection (59, 63), it was more intuitive to use mortality as an indicator of virulence, especially for a bacterial pathogen of strong virulence.
In conclusion, in this study, we isolated and identified a strain of M. avium spp. avium. The virulence and the derivative PPD of the strain were also characterized. Our work provided a novel reference strain for the intensive study of avian tuberculosis. This work not only enriched strain resources for M. avium spp. avium but also provided a realistic candidate strain for further development of M. avium spp. avium vaccines.
Ethics Statement
The present study was approved by the Laboratory Animal Ethics Committee of China Institute of Veterinary Drug Control and was also approved by the Ministry of Agriculture and the Bureau of Animal Husbandry. The experiments were performed in compliance with the “Regulations of the People’s Republic of China on the Administration of Experimental Animals” and the “Guidelines for the ethical review of experimental animal welfare in Beijing.” MAA strains were cultured in an air-conditioned, air filtered, biosafety level III facility. The experimental use of serum samples, including sample collection, handling, testing, and personal protection, complied with the General Requirements for Laboratory Biological Safety of China, GB19489 (2008).
Author Contributions
LZ conceived and designed the experiments and wrote the whole manuscript. YP, JY, TW, ZB, and HZ provided assistance of the experiments. YQ revised the manuscript. JD supervised the experiments and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Key R&D Program of China (2016YFD0500902).
References
1. Marco I, Domingo M, Lavin S. Mycobacterium infection in a captive-reared capercaillie (Tetrao urogallus). Avian Dis (2000) 44:227–30. doi:10.2307/1592531
3. Slany M, Ulmann V, Slana I. Avian mycobacteriosis: still existing threat to humans. Biomed Res Int (2016) 2016:4387461. doi:10.1155/2016/4387461
4. Martin G, Schimmel D. [Mycobacterium avium infections in poultry – a risk for human health or not?]. Dtsch Tierarztl Wochenschr (2000) 107:53–8.
6. Dhama K, Mahendran M, Tiwari R, Dayal Singh S, Kumar D, Singh S, et al. Tuberculosis in birds: insights into the Mycobacterium avium infections. Vet Med Int (2011) 2011:712369. doi:10.4061/2011/712369
8. Buur J, Saggese MD. Taking a rational approach in the treatment of avian mycobacteriosis. Vet Clin North Am Exot Anim Pract (2012) 15:57–70,vi. doi:10.1016/j.cvex.2011.12.001
9. Saggese MD, Tizard I, Gray P, Phalen DN. Evaluation of multidrug therapy with azithromycin, rifampin, and ethambutol for the treatment of Mycobacterium avium subsp avium in ring-neck Doves (Streptopelia risoria): an uncontrolled clinical study. J Avian Med Surg (2014) 28:280–9. doi:10.1647/2012-067R1
10. Rindi L, Garzelli C. Genetic diversity and phylogeny of Mycobacterium avium. Infect Genet Evol (2014) 21:375–83. doi:10.1016/j.meegid.2013.12.007
11. Mackintosh CG, De Lisle GW, Collins DM, Griffin JF. Mycobacterial diseases of deer. N Z Vet J (2004) 52:163–74. doi:10.1080/00480169.2004.36424
12. Turenne CY, Collins DM, Alexander DC, Behr MA. Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium are independently evolved pathogenic clones of a much broader group of M. avium organisms. J Bacteriol (2008) 190:2479–87. doi:10.1128/JB.01691-07
13. Agdestein A, Johansen TB, Kolbjornsen O, Jorgensen A, Djonne B, Olsen I. A comparative study of Mycobacterium avium subsp. avium and Mycobacterium avium subsp. hominissuis in experimentally infected pigs. BMC Vet Res (2012) 8:11. doi:10.1186/1746-6148-8-11
14. Ronai Z, Csivincsik A, Dan A, Gyuranecz M. Molecular analysis and MIRU-VNTR typing of Mycobacterium avium subsp. avium, ‘hominissuis’ and silvaticum strains of veterinary origin. Infect Genet Evol (2016) 40:192–9. doi:10.1016/j.meegid.2016.03.004
15. Arrazuria R, Juste RA, Elguezabal N. Mycobacterial infections in rabbits: from the wild to the laboratory. Transbound Emerg Dis (2017) 64:1045–58. doi:10.1111/tbed.12474
16. Sharma K, Sharma A, Modi M, Singh G, Kaur H, Varma S, et al. PCR detection of co-infection with Mycobacterium tuberculosis and Mycobacterium avium in AIDS patients with meningitis. J Med Microbiol (2012) 61:1789–91. doi:10.1099/jmm.0.045898-0
17. Arrazuria R, Sevilla IA, Molina E, Perez V, Garrido JM, Juste RA, et al. Detection of Mycobacterium avium subspecies in the gut associated lymphoid tissue of slaughtered rabbits. BMC Vet Res (2015) 11:130. doi:10.1186/s12917-015-0445-2
18. Chiers K, Deschaght P, De Baere T, Dabrowski S, Kotlowski R, De Clercq D, et al. Isolation and identification of Mycobacterium avium subspecies silvaticum from a horse. Comp Immunol Microbiol Infect Dis (2012) 35:303–7. doi:10.1016/j.cimid.2012.01.011
19. Ahlstrom C, Barkema HW, Stevenson K, Zadoks RN, Biek R, Kao R, et al. Limitations of variable number of tandem repeat typing identified through whole genome sequencing of Mycobacterium avium subsp. paratuberculosis on a national and herd level. BMC Genomics (2015) 16:161. doi:10.1186/s12864-015-1387-6
20. Sevilla IA, Molina E, Elguezabal N, Perez V, Garrido JM, Juste RA. Detection of mycobacteria, Mycobacterium avium subspecies, and Mycobacterium tuberculosis complex by a novel tetraplex real-time PCR assay. J Clin Microbiol (2015) 53:930–40. doi:10.1128/JCM.03168-14
21. Xu Q, Zhou S, Li Z, Bi D. Diagnosis of avian tuberculosis. Chin J Vet Sci (2001) 31:30–1. doi:10.3969/j.issn.1673-4696.2001.03.016
22. Zhang Y, Cai J, Shen Y. Diagnosis and therapy of avian tuberculosis. Poul Husbandry Dis Control (2002) 11:28–9.
23. China Institute of Veterinary Drug Control, China Veterinary Culture Collection Center. CVCC Catalogue of Culture. Beijing, China: China Agricultural Science and Technology Publishing Company (2008).
24. Office International Des Epizooties. OE Terrestrial Manual. Paris, France: Office International Des Epizooties (2014).
25. Ministry of Agriculture of the People’s Republic of China. The People’s Republic of China Veterinary Biological Product Regulation. Beijing, China: Chemical Industry Press (2000). p. 164–6.
26. General Administration of Quality Supervision, Inspection and Quarantine, People’s Republic of China. Industry standard for import and export inspection and quarantine. In: Certification and Accreditation Administration of the Peoples Republic of China, editor. Quarantine Protocol for Animal Tuberculosis. Beijing: Standards Press of China (2011). p. 4–5.
27. Levy-Frebault VV, Portaels F. Proposed minimal standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int J Syst Bacteriol (1992) 42:315–23. doi:10.1099/00207713-42-2-315
28. Yang Z, Fang H. Human and Animal Pathogenic Bacteria. Shijiazhuang, Hebei: Hebei Science & Technology Press (2003).
29. Sharma B, Pal N, Malhotra B, Vyas L. Evaluation of a rapid differentiation test for Mycobacterium tuberculosis from other mycobacteria by selective inhibition with P-nitrobenzoic acid using MGIT 960. J Lab Physicians (2010) 2:89–92. doi:10.4103/0974-2727.72157
30. Moravkova M, Hlozek P, Beran V, Pavlik I, Preziuso S, Cuteri V, et al. Strategy for the detection and differentiation of Mycobacterium avium species in isolates and heavily infected tissues. Res Vet Sci (2008) 85:257–64. doi:10.1016/j.rvsc.2007.10.006
31. Chen X, Mao K, Luo Y. Diagnosis of avian tuberculosis with the avian Tubercula plain agglutination test. Chin J Vet Drug (1999) 33:3–5.
32. General Administration of Quality Supervision, Inspection and Quarantine, People’s Republic of China. National standards of the People’s Republic of China. In: National Technical Standardization Committee of Animal Inspection and Quarantine, editor. Diagnostic Techniques for Tuberculosis of Animal. Beijing: General Administration of Quality Supervision, Inspection and Quarantine (2002). p. 181–82.
33. Haifeng X. Testing method for the specificity of purified protein derivative. Chin J Vet Drug (1990) 24:15–8.
34. Mahon CR, Lehman DC, Manuselis G. Textbook of Diagnostic Microbiology. Maryland Heights, MO: Saunders (2011).
35. Bartos M, Hlozek P, Svastova P, Dvorska L, Bull T, Matlova L, et al. Identification of members of Mycobacterium avium species by Accu-Probes, serotyping, and single IS900, IS901, IS1245 and IS901-flanking region PCR with internal standards. J Microbiol Methods (2006) 64:333–45. doi:10.1016/j.mimet.2005.05.009
36. Garg SK, Tiwari RP, Tiwari D, Singh R, Malhotra D, Ramnani VK, et al. Diagnosis of tuberculosis: available technologies, limitations, and possibilities. J Clin Lab Anal (2003) 17:155–63. doi:10.1002/jcla.10086
37. Dahlhausen B, Tovar DS, Saggese MD. Diagnosis of mycobacterial infections in the exotic pet patient with emphasis on birds. Vet Clin North Am Exot Anim Pract (2012) 15:71–83,vi. doi:10.1016/j.cvex.2011.11.003
38. Saito H, Tomioka H, Sato K, Tasaka H, Dawson DJ. Identification of various serovar strains of Mycobacterium avium complex by using DNA probes specific for Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol (1990) 28:1694–7.
39. Inagaki T, Nishimori K, Yagi T, Ichikawa K, Moriyama M, Nakagawa T, et al. Comparison of a variable-number tandem-repeat (VNTR) method for typing Mycobacterium avium with mycobacterial interspersed repetitive-unit-VNTR and IS1245 restriction fragment length polymorphism typing. J Clin Microbiol (2009) 47:2156–64. doi:10.1128/JCM.02373-08
40. Ritelli M, Amadori M, Tagliabue S, Pacciarini ML. Use of a macrophage cell line for rapid detection of Mycobacterium bovis in diagnostic samples. Vet Microbiol (2003) 94:105–20. doi:10.1016/S0378-1135(03)00080-4
41. Tell LA, Woods L, Cromie RL. Mycobacteriosis in birds. Rev Sci Tech (2001) 20:180–203. doi:10.20506/rst.20.1.1273
42. Martin RS, Sumarah RK, Robart EM. Comparison of four culture media for the isolation of Mycobacterium tuberculosis: a 2-year study. J Clin Microbiol (1975) 2:438–40.
43. Mayahi M, Mosavari N, Esmaeilzadeh S, Parvandar-Asadollahi K. Comparison of four different culture media for growth of Mycobacterium avium subsp. avium isolated from naturally infected lofts of domestic pigeons. Iran J Microbiol (2013) 5:379–82.
44. Belisle JT, Brennan PJ. Molecular basis of colony morphology in Mycobacterium avium. Res Microbiol (1994) 145:237–42. doi:10.1016/0923-2508(94)90024-8
45. Reddy VM, Luna-Herrera J, Gangadharam PR. Pathobiological significance of colony morphology in Mycobacterium avium complex. Microb Pathog (1996) 21:97–109. doi:10.1006/mpat.1996.0046
46. Cangelosi GA, Palermo CO, Bermudez LE. Phenotypic consequences of red-white colony type variation in Mycobacterium avium. Microbiology (2001) 147:527–33. doi:10.1099/00221287-147-3-527
47. Krzywinska E, Schorey JS. Characterization of genetic differences between Mycobacterium avium subsp. avium strains of diverse virulence with a focus on the glycopeptidolipid biosynthesis cluster. Vet Microbiol (2003) 91:249–64. doi:10.1016/S0378-1135(02)00292-4
48. Parvandar-Asadollahi K, Mosavari N, Mayahi M. Genotyping of Mycobacterium avium subsp. avium isolates from naturally infected lofts of domestic pigeons in Ahvaz by IS901 RFLP. Iran J Microbiol (2015) 7:260–4.
49. Castellanos E, Romero B, Rodriguez S, De Juan L, Bezos J, Mateos A, et al. Molecular characterization of Mycobacterium avium subspecies paratuberculosis types II and III isolates by a combination of MIRU-VNTR loci. Vet Microbiol (2010) 144:118–26. doi:10.1016/j.vetmic.2009.12.028
50. Ricchi M, Barbieri G, Cammi G, Garbarino CA, Arrigoni N. High-resolution melting for analysis of short sequence repeats in Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett (2011) 323:151–4. doi:10.1111/j.1574-6968.2011.02371.x
51. Ricchi M, Barbieri G, Taddei R, Belletti GL, Carra E, Cammi G, et al. Effectiveness of combination of mini-and microsatellite loci to sub-type Mycobacterium avium subsp. paratuberculosis Italian type C isolates. BMC Vet Res (2011) 7:54. doi:10.1186/1746-6148-7-54
52. Dvorska L, Bull TJ, Bartos M, Matlova L, Svastova P, Weston RT, et al. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. J Microbiol Methods (2003) 55:11–27. doi:10.1016/S0167-7012(03)00092-7
53. Moravkova M, Lamka J, Slany M, Pavlik I. Genetic IS901 RFLP diversity among Mycobacterium avium subsp. avium isolates from four pheasant flocks. J Vet Sci (2013) 14:99–102. doi:10.4142/jvs.2013.14.1.99
54. Rathnaiah G, Lamont EA, Harris NB, Fenton RJ, Zinniel DK, Liu X, et al. Generation and screening of a comprehensive Mycobacterium avium subsp. paratuberculosis transposon mutant bank. Front Cell Infect Microbiol (2014) 4:144. doi:10.3389/fcimb.2014.00144
55. Khattak FA, Kumar A, Kamal E, Kunisch R, Lewin A. Illegitimate recombination: an efficient method for random mutagenesis in Mycobacterium avium subsp. hominissuis. BMC Microbiol (2012) 12:204. doi:10.1186/1471-2180-12-204
56. Deshayes C, Laval F, Montrozier H, Daffe M, Etienne G, Reyrat JM. A glycosyltransferase involved in biosynthesis of triglycosylated glycopeptidolipids in Mycobacterium smegmatis: impact on surface properties. J Bacteriol (2005) 187:7283–91. doi:10.1128/JB.187.21.7283-7291.2005
57. Johansen TB, Agdestein A, Olsen I, Nilsen SF, Holstad G, Djonne B. Biofilm formation by Mycobacterium avium isolates originating from humans, swine and birds. BMC Microbiol (2009) 9:159. doi:10.1186/1471-2180-9-159
58. Mukherjee R, Chatterji D. Glycopeptidolipids: immuno-modulators in greasy mycobacterial cell envelope. IUBMB Life (2012) 64:215–25. doi:10.1002/iub.602
59. Viale MN, Park KT, Imperiale B, Gioffre AK, Colombatti Olivieri MA, Moyano RD, et al. Characterization of a Mycobacterium avium subsp. avium operon associated with virulence and drug detoxification. Biomed Res Int (2014) 2014:809585. doi:10.1155/2014/809585
60. Tateishi Y, Hirayama Y, Ozeki Y, Nishiuchi Y, Yoshimura M, Kang J, et al. Virulence of Mycobacterium avium complex strains isolated from immunocompetent patients. Microb Pathog (2009) 46:6–12. doi:10.1016/j.micpath.2008.10.007
61. Li YJ, Danelishvili L, Wagner D, Petrofsky M, Bermudez LE. Identification of virulence determinants of Mycobacterium avium that impact on the ability to resist host killing mechanisms. J Med Microbiol (2010) 59:8–16. doi:10.1099/jmm.0.012864-0
62. Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME, et al. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops x M. saxatilis and zebrafish Danio rerio. Dis Aquat Organ (2008) 79:107–18. doi:10.3354/dao01891
Keywords: avian tuberculosis, Mycobacterium avium spp. avium, isolation, identification, characterization
Citation: Zhu L, Peng Y, Ye J, Wang T, Bian Z, Qin Y, Zhang H and Ding J (2018) Isolation, Identification, and Characterization of a New Highly Pathogenic Field Isolate of Mycobacterium avium spp. avium. Front. Vet. Sci. 4:243. doi: 10.3389/fvets.2017.00243
Received: 13 September 2017; Accepted: 20 December 2017;
Published: 15 January 2018
Edited by:
Joy Scaria, South Dakota State University, United StatesReviewed by:
Kuldeep Dhama, Indian Veterinary Research Institute (IVRI), IndiaShankar Thangamani, Midwestern University, United States
Copyright: © 2018 Zhu, Peng, Ye, Wang, Bian, Qin, Zhang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangquan Zhu, MTM2NzM5MTg5NEBxcS5jb20=;
Jiabo Ding, ZGluZ2ppYWJvQDEyNi5jb20=
 Liangquan Zhu
Liangquan Zhu Yong Peng
Yong Peng Tuanjie Wang
Tuanjie Wang Jiabo Ding
Jiabo Ding