- 1Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, United States
- 2Comparative Medicine Institute, North Carolina State University, Raleigh, NC, United States
Equine asthma syndrome (EAS) is a common problem that affects horses of any age. Severe EAS is reported to affect 10–20% of adult horses in the northern hemisphere, while mild/moderate EAS is reported to affect 60–100% of adult horses, depending on the population and geographic region. For both severe and mild/moderate EAS, the presence of lower airway inflammation is attributed to airborne “triggers” such as dust, mold, and bacterial components that horses encounter in hay and stable-environments; and treatment recommendations for horses with EAS often include full-time pasture turnout. The caveat to this recommendation is horses with summer-pasture associated EAS (SP-EAS), who experience allergic lower airway inflammation when exposed to summer pasture. The prevalence of EAS in horses on pasture that do not have SP-EAS has not been reported. The purpose of this study was to use bronchoalveolar lavage (BAL) cytology to determine the prevalence of EAS in a herd of pastured, adult research horses with no history of respiratory disease. The horses were members of a teaching animal herd housed on pasture in the southeastern United States and fed round-bale Bermuda-grass hay. BAL fluid (BALF) cytology was analyzed in both summer (May–August 2017) and winter (November 2017–February 2018). Similar to previous reports, the prevalence of severe EAS in our study population was 10% in summer and 4.3% in winter. The prevalence of mild/moderate EAS was 60% in summer and 87% in winter. The high prevalence of mild/moderate EAS in this population was unexpected, given the 24-h, year-round pasture environment and the lack of history of respiratory disease. Additionally, 61.1% of horses with both summer and winter data had a different BALF cytology profile between the two seasons. To the authors' knowledge, this is the first study to use BAL cytology to diagnose, and monitor changes in, EAS phenotype in pastured adult horses. These results help to inform discussions regarding prevalence of EAS in pastured, adult horses in the southeastern region of North America.
Introduction
Equine asthma syndrome (EAS) is a non-infectious, inflammatory respiratory disease that includes a mild/moderate form, historically referred to as inflammatory airway disease (IAD), and a severe form, historically referred to as recurrent airway obstruction (RAO), heaves or summer pasture associated recurrent airway obstruction (SPRAO) (1). Both mild/moderate and severe EAS are characterized by lower airway inflammation and excessive mucus in the airways. While severe EAS is often diagnosed by history and clinical signs alone, bronchoalveolar lavage fluid (BALF) cytology, or other advanced diagnostic such as pulmonary function testing, is needed to diagnose mild/moderate EAS. BALF cytology is also used to monitor lower airway inflammation in response to environmental management and medication in horses with severe EAS.
BALF cytology from horses with severe asthma is characterized by moderate to severe neutrophilia (≥20–25%) as well as decreased % lymphocytes and % macrophages. BALF cytology from horses with mild/moderate EAS usually reveals mild to moderate increase in % neutrophils, % eosinophils, and/or % mast cells (1); however, different phenotypes have been recognized and associated with varying clinical signs and proinflammatory cytokine expression patterns. Specifically, an increase in % mast cells has been associated with airway hyperreactivity and altered pulmonary function (2, 3) as well as increase in IL-4 and IL-5 (4) while an increase in % neutrophils has been associated with cough (2) and increase in IL-17 and IL-23 (4, 5).
The pathophysiology of EAS is complex and significant research efforts have focused on trying to understand the contribution of multiple factors including genetics (6, 7), environment (8–11) and viral infection (12, 13). Stabling of horses has been reported as a risk factor (14), as non-infectious aerosolized particles are thought to play a key role in the development of EAS. However, the prevalence of mild/moderate and severe lower airway inflammation in horses that are not stabled has not been reported. In this study, we used BALF cytology to determine the prevalence of EAS in a university owned equine teaching herd in summer and winter. The horses were housed on pasture year-round at a veterinary teaching hospital and had no history of respiratory disease. Our hypothesis was that airway inflammation would be uncommon in pastured horses with no history of respiratory disease, and that BALF cytology would be consistent in individual horses regardless of season.
Materials and Methods
The experimental design of this investigation was an observational, cross-sectional study. All samples were collected between May and August 2017 (summer samples) and between November and February 2018 (winter samples).
Horses
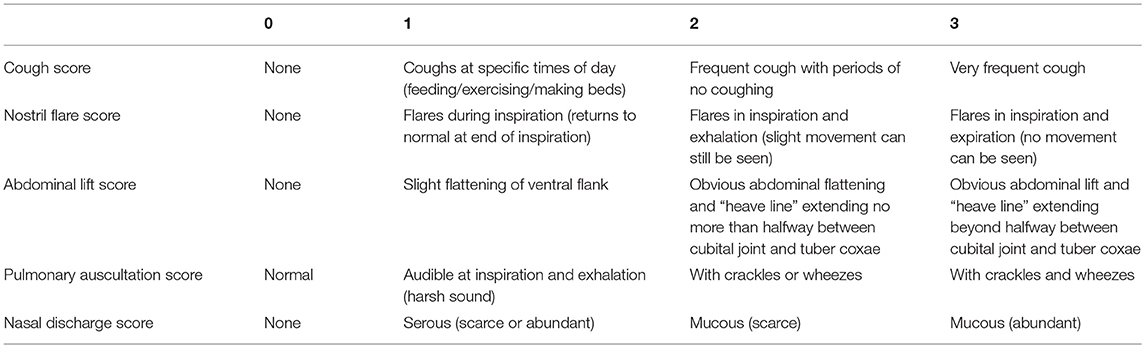
The North Carolina State University Institutional Animal Care and Use Committee (IACUC #16-074-O) approved all procedures performed for the purposes of this study. Twenty horses were included in the summer and 25 horses were included in the winter. Horses included in this study were university owned teaching animals with no history of respiratory disease. Horses ranged in age from 6 to 24 years and were of mixed breed and gender. The horses were kept on pasture in North Carolina (southeastern United States) and received no medications for the duration of this study. Each horse received a physical exam, clinical score (Table 1) and BAL. The clinical score rubric was similar to those previously described, with slight modifications (15, 16).
BALF Collection and Sample Processing
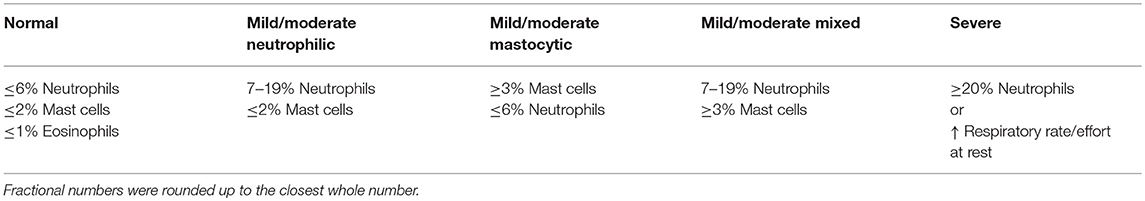
Horses were sedated with detomidine (0.005–0.01 mg/kg IV) and butorphanol (0.02–0.04 mg/kg IV). A cuffed catheter was passed nasotracheally until wedged in a secondary or tertiary bronchus where 300 total mL of warmed sterile saline solution was infused and re-aspirated, 150 mL at a time. The aspirated fluid was pooled and submitted for cytological analysis by blinded clinical pathologists. Direct, concentrated and cytospin preparation slides were examined, and a 300 cell differential count was performed as part of routine cytological analysis. BALF cytology was used to assign horses to one of three groups: normal, mild/moderate inflammation or severe inflammation. Criteria for BALF cytology classification was similar to previously published guidelines (1) (Table 2), with slight modification to the cutoff used for normal and severe % neutrophils. Subtypes of mild to moderate inflammation were further categorized as mild to moderate neutrophilic inflammation (7–19% neutrophils but ≤2% mast cells), mild to moderate mastocytic inflammation (≥3% mast cells but ≤6% neutrophils) and mild to moderate mixed inflammation (7–19% neutrophils and ≥3% mast cells) (2, 17, 18).
Statistical Analysis
Clinical characteristics and BALF cytology results were analyzed by One-way ANOVA with Tukey's post-hoc test, except for sex, which was compared by Chi-square test. BALF total and differential cell counts were compared between summer and winter by two-tail student t-test, and prevalence of each disease category was compared by Fisher's exact test (due to small sample size), except for mild/moderate neutrophilic inflammation, which was compared by Chi-square test. P < 0.05 were considered significant. All analyses were performed using GraphPad Prism (Version 7.0 and 8.0, GraphPad Software, La Jolla, CA).
Results
Summer Samples
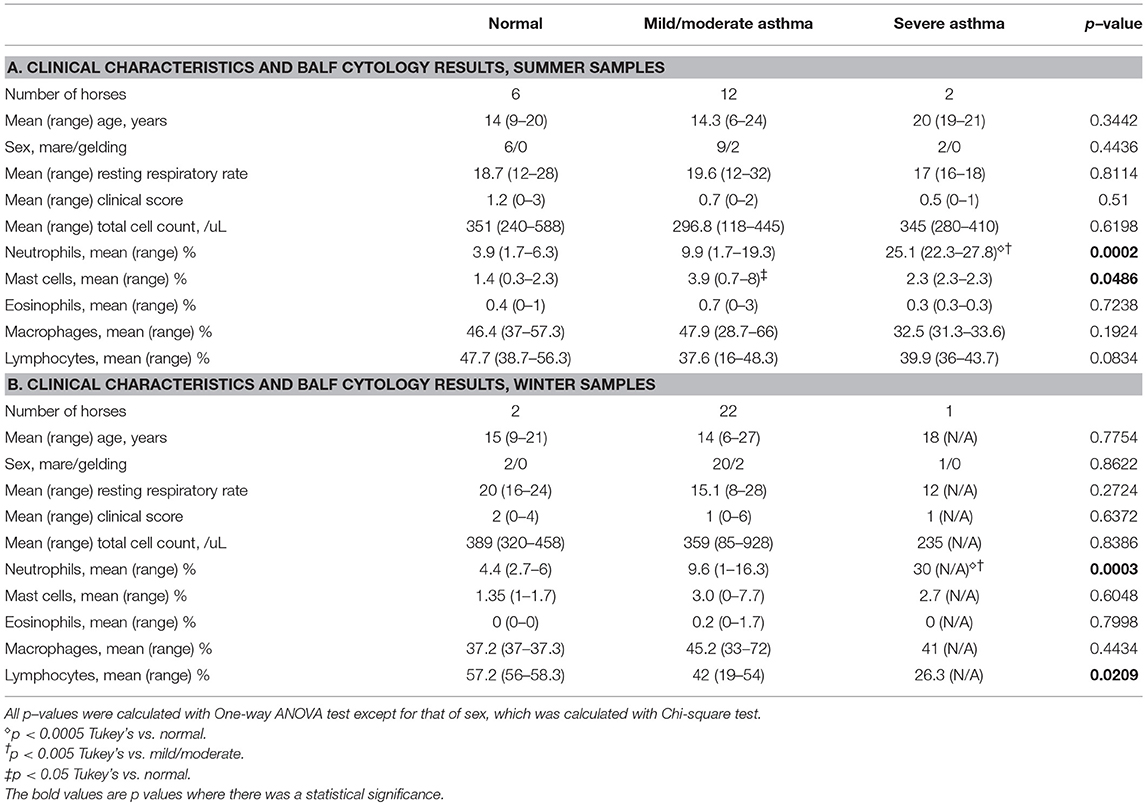
Twenty horses were sampled during the summer period. The study population and clinical data are summarized in Table 3A. Of the 20 horses sampled, 6 (30%) had normal BALF cytology, 12 (60%) had mild/moderate inflammation and 2 (10%) had severe inflammation. There was no significant difference in the mean age among groups. Although more mares were sampled compared to geldings, the sex distribution was not significantly different among groups. There was no significant difference in mean clinical scores between groups. The mean ± SD BALF % neutrophils was significantly different in the severe group vs. normal and mild/moderate groups (p < 0.0005). Interindividual variability of % neutrophils in each disease category is illustrated in Figure 1. The mean ± SD % mast cells of the mild/moderate group was significantly different compared to mean % mast cells for the normal and severe groups (p < 0.05).
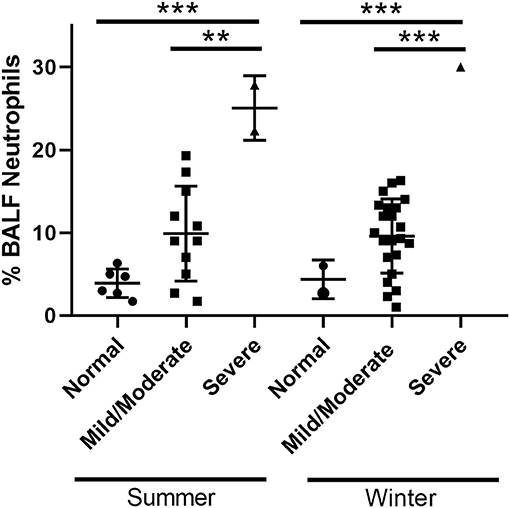
Figure 1. Interindividual variability. BALF neutrophil percentages of horses in each disease category. **p < 0.005 and ***p < 0.0005.
Winter Samples
Twenty-three horses were sampled during the winter period. The study population and clinical data are summarized in Table 3B. Of the 23 horses sampled, 2 (8.7%) had normal BALF cytology, 20 (87%) had mild/moderate inflammation and 1 (4.3%) had severe inflammation. There was no significant difference in the mean age among groups. Similar to the summer samples, more mares were sampled compared to geldings, but the sex distribution was not significantly different among groups. No significant difference was found in mean clinical scores between groups. The mean ± SD % neutrophils was significantly different in the severe group vs. normal and mild/moderate groups (p < 0.0005). Interindividual variability of % neutrophils in each disease category are illustrated in Figure 1. The mean ± SD % lymphocytes of the severe group was significantly different compared to that for the normal group (p < 0.05). This decrease in % lymphocytes has been reported previously in horses with severe EAS (1), and is likely due to the increased % neutrophils in the severe group.
Seasonal Comparison
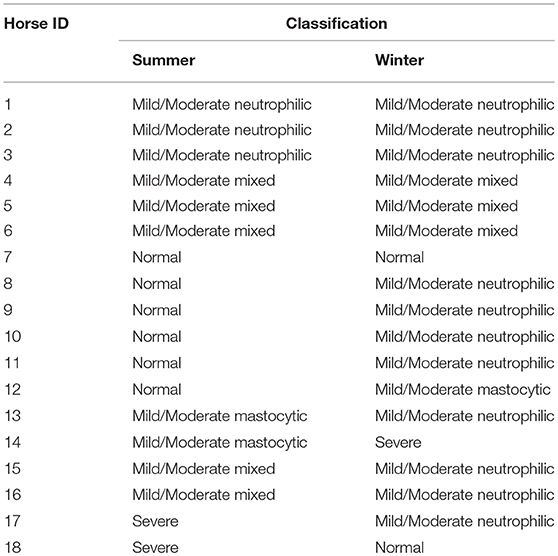
Eighteen horses had data from both summer and winter. Overall, there was a significant difference in the prevalence of mild/moderate neutrophilic inflammation in summer vs. winter samples (p = 0.0295). Additionally, based on BALF cytology, 11 (61.1%) horses had a different disease classification or subtype classification between the two seasons, while the BALF cytology profiles remained consistent in 7 (38.9%) horses. The disease classifications are summarized in Table 4.
Discussion
Severe EAS is reported to affect 10–20% of adult horses in the northern hemisphere and other temperate climates (19, 20). In our study population, 2 of 20 (10%) horses in the summer, and 1 of 23 (4.3%) in the winter, had BALF cytology consistent with severe EAS. One horse had severe EAS in the summer but normal BALF cytology in the winter, suggesting a diagnosis of summer-pasture associated EAS (SP-EAS, also referred to as summer pasture associated recurrent airway obstruction or SPRAO), which had not been previously diagnosed. While the prevalence of severe EAS in our teaching horse population was similar to previous reports, we were surprised by the high prevalence of mild/moderate EAS, given the lack of history of respiratory disease in these horses, and their 24-h pasture environment. Potential explanations for the high prevalence of mild/moderate lower airway inflammation in this herd include: continuous access to round bale hay and exposure to air pollution due to pasture location in an urban setting and close proximity of pasture to a heavily traveled 4-lane road. Hay feeding causes increased particulate in the breathing zone, which can lead to airway inflammation (21, 22). Additionally, the method of hay processing also affects the airway health of horses. Horses fed round bale hay are known to have an elevated risk of airway inflammation compared to those fed square bale hay (23, 24). This finding is due to higher numbers of thermophilic actinomycetes found in round bale hay (25) and increased dust exposure for horses that keep their faces immersed in the round bale while eating. Air pollution is another cause of increased particulate concentration. In people, there is a strong association between air pollutant levels and increased frequency and severity of asthma attacks (26–28). Similar to humans, traffic pollution has also been associated with airway inflammation in horses (10).
Compared with severe EAS, fewer studies have investigated the prevalence and/or environmental factors associated with mild/moderate EAS. In one study comparing airway inflammation and mucus in two age groups of sport horses housed in a conventional stable environment, 100% of the horses had increased BALF % neutrophils and/or % mast cells, despite the lack of history or clinical signs of respiratory disease (29). In an observational study in a population of racing thoroughbreds, evidence of mild EAS was found in 80% (78/98) of BALF samples from 52 out of 64 horses (15). In these studies, as well as others, investigators conclude that stable dust likely contributes to the high prevalence of mild/moderate EAS in performance horses. Indeed, mild/moderate EAS is often only investigated in sport horses with performance “issues”. Therefore, the prevalence of mild/moderate EAS in non-exercised horse, and the clinical relevance of mild/moderate EAS in horses with no clinical signs or history of poor performance, is largely unknown. Results from our study suggest that non-infectious lower airway inflammation may be a common finding, even in horses not exposed to stabling environments.
In our study population, the neutrophilic subtype of mild/moderate EAS was more prevalent in winter (Figure 2). Increased BALF % neutrophils in winter has been reported in other population of horses as well. In a previous report, Riihimaki et al. evaluated differences in air quality and markers of airway inflammation by season and found a trend toward elevation of BALF % neutrophils during winter. Riihimaki et al. also report increased expression of IL-6 mRNA in BAL cells and inhalable dust and 1,3-β-glucan in the breathing zone during winter (11). Similarly, Hansen et al. report significantly higher BALF % neutrophils and mucus score in November compared to May (30). In these studies investigators suggest that client-owned horses are stabled more in winter, leading to an increase in exposure to airborne irritants and a concomitant airway neutrophilia. In our population, however, increased stabling was not a potential cause of increased lower airway neutrophils, as the horses in our study group were kept on pasture year-round.
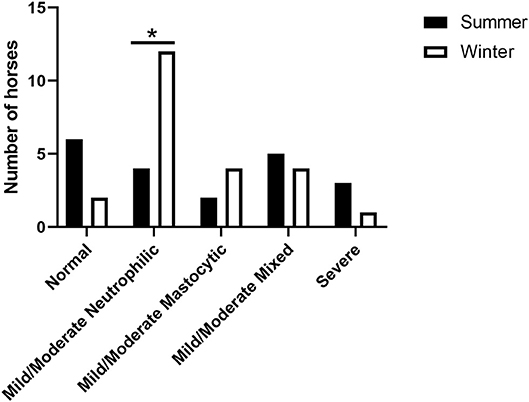
Figure 2. Seasonal distribution of categorized inflammatory subtype. The prevalence of mild/moderate neutrophilic inflammation was significantly higher (*p < 0.05) in winter compared to summer.
One possible explanation for the seasonal difference in BALF cytology profile is change in temperature and humidity. Cold and dry air is known to exacerbate asthma. Asthma-like respiratory symptoms and chronic airway inflammation are reported by athletes who perform repeated exercise while breathing cold air (termed “ski asthma”) (31, 32). Airway dehydration is reported to trigger bronchoconstriction, and altered airway hydration is associated with airway neutrophilia and increased levels of IL-8 (33). In dogs, persistent airway neutrophilic and eosinophilic inflammation, obstruction and hyperreactivity have been demonstrated following challenge with cold and dry air (34). Similarly, in two studies in healthy adult exercising horses, Davis et al. report that horses that breathe cold air during exercise experience significant upregulation of TH2 cytokines IL-4, IL-5, and IL-10 (35) and proinflammatory cytokines IL-1, IL-6 and IL-8 in BALF cells, as well as a significant increase in the BALF % neutrophils (36). Another possible explanation for the seasonal difference we observed in airway neutrophilia is change in hay consumption. Horses spend increased amount of time eating from round bale hay during cold months due to dormant pasture and increased caloric demand, both of which result in increased exposure to dust and molds. Taken together, seasonal changes in ambient air temperature and humidity, as well as increased consumption of round bale hay, may be contributing to the increased neutrophilic inflammation we observed in our population in the winter.
In addition to investigating the prevalence of EAS in our teaching herd, we also sought to determine whether BAL cytology from individual horses was consistent over time. Eleven out of 18 horses (61.1%) in our study group with both summer and winter BALF cytology data had differing cytology profiles in summer vs. winter. This finding was the opposite of what we hypothesized and somewhat surprising, given the consistent environment of our study. Previous reports on the consistency of BALF cell differential counts in individual horses are conflicting. BAL cytology results reported by Thomas et al. show diagnostically relevant differences in BALF % neutrophils in ponies in clinical remission from heaves, as well as healthy ponies, when samples were collected 2–15 weeks apart (37); while findings from Jean et al. show consistent BAL cytology from horses sampled monthly for a period of 3 months (38). Differences in housing could explain the apparent discrepancies in these reports, as ponies in the Thomas et al. study were housed on pasture and horses in the Jean et al. study were stabled. Another important factor to consider when assessing the prevalence of lower airway inflammation in horses is variation during BAL procedure and sample processing that contribute to cytologic findings. Multiple variables including volume of fluid instilled (38, 39), number of aliquots collected (38, 40), sampling site (38, 41), slide preparation (42), and cell counting methods (43) are reported to affect differential cell counts, making characterization of mild to moderate lower airway inflammation challenging. The resulting variability in cytologic findings makes it difficult to establish definitive cutoff values for the classification of EAS. Nevertheless, results reported here, combined with findings reported by Thomas et al. could indicate that the BALF cytology profile of pastured horses may be more dynamic than stabled horses, and that one-time BALF cytology may be insufficient to characterize the complete picture of the airway immune response in horses on pasture.
Our study had several limitations. First, because our herd was small, further investigations will be needed to determine whether these results extrapolate to other population of horses. Second, disease classification in this study was solely based on BALF cytology, and further analyses such as pulmonary function testing and/or endoscopy were not available. Therefore, it is unclear how BALF cytology in our study population correlates with lung function or airway hyperreactivity in these horses. Finally, we do not know if any of the horses in our population have inducible, reversible bronchoconstriction, as antigen challenge was not performed. Further research will be needed to determine whether individual horse variability in BAL cytology correlates with disease status as determined by pulmonary function testing or antigen challenge. Despite these limitations, our study findings provide important data on the prevalence of EAS in a different type of horse population than those previously examined. We hope this data will provide more context for equine practitioners who perform BALF cytology and are required to interpret their findings without the aid of advanced diagnostics.
While the prevalence of severe EAS in our study population is similar to previous reports, we were surprised by the 60–87% prevalence of mild/moderate EAS in pastured horses with no history of respiratory disease. We also did not expect an increase in mild/moderate neutrophilic inflammation in the winter. To the authors' knowledge, this is the first study to describe the prevalence of EAS, as well as seasonal changes in BALF cytology profiles, in pastured horses. We expect these results to help inform equine clinicians and researchers regarding expectations and interpretation of BALF cytology for horses in different environments and during different seasons. In future studies, we will utilize more advanced diagnostics (i.e., conventional pulmonary function testing) to determine whether classification by BALF cytology correlates with inducible bronchoconstriction in our herd. Additionally, we will determine whether lower airway inflammation in these horses is responsive to environmental management (i.e., removal of round bale-hay).
Data Availability
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Author Contributions
KD was responsible for study design, experimental execution, and preparing the manuscript. MS was responsible for overseeing all aspects of the study, including study design, and critically reviewing the manuscript.
Funding
Funding for this study was provided by the Morris Animal Foundation (grant # D17EQ-029).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Jenna Schirmer for assistance with the BAL procedures.
References
1. Couëtil LL, Cardwell JM, Gerber V, Lavoie JP, Léguillette R, Richard EA. Inflammatory airway disease of horses–revised consensus statement. J Vet Intern Med. (2016) 30:503–15. doi: 10.1111/jvim.13824
2. Bedenice D, Mazan MR, Hoffman AM. Association between cough and cytology of bronchoalveolar lavage fluid and pulmonary function in horses diagnosed with inflammatory airway disease. J Vet Intern Med. (2008) 22:1022–8. doi: 10.1111/j.1939-1676.2008.0109.x
3. Richard EA, Fortier GD, Denoix JM, Art T, Lekeux PM, Van Erck E. Influence of subclinical inflammatory airway disease on equine respiratory function evaluated by impulse oscillometry. Equine Vet J. (2009) 41:384–9. doi: 10.2746/042516409X366121
4. Beekman L, Tohver T, Léguillette R. Comparison of cytokine mRNA expression in the bronchoalveolar lavage fluid of horses with inflammatory airway disease and bronchoalveolar lavage mastocytosis or neutrophilia using REST software analysis. J Vet Intern Med. (2012) 26:153–61. doi: 10.1111/j.1939-1676.2011.00847.x
5. Hughes KJ, Nicolson L, Da Costa N, Franklin SH, Allen KJ, Dunham SP. Evaluation of cytokine mRNA expression in bronchoalveolar lavage cells from horses with inflammatory airway disease. Vet Immunol Immunopathol. (2011) 140:82–9. doi: 10.1016/j.vetimm.2010.11.018
6. Pacholewska A, Jagannathan V, Drögemüller M, Klukowska-Rötzler J, Lanz S, Hamza E, et al. Impaired cell cycle regulation in a natural equine model of asthma. PLoS ONE. (2015) 10:e0136103. doi: 10.1371/journal.pone.0136103
7. Ramery E, Fraipont A, Richard EA, Art T, Pirottin D, van Delm W, et al. Expression microarray as a tool to identify differentially expressed genes in horses suffering from inflammatory airway disease. Vet Clin Pathol. (2015) 44:37–46. doi: 10.1111/vcp.12216
8. Bullone M, Murcia RY, Lavoie JP. Environmental heat and airborne pollen concentration are associated with increased asthma severity in horses. Equine Vet J. (2016) 48:479–84. doi: 10.1111/evj.12559
9. Ivester KM, Couëtil LL, Moore GE, Zimmerman NJ, Raskin RE. Environmental exposures and airway inflammation in young thoroughbred horses. J Vet Intern Med. (2014) 28:918–24. doi: 10.1111/jvim.12333
10. Ivester KM, Couëtil LL, Zimmerman NJ. Investigating the link between particulate exposure and airway inflammation in the horse. J Vet Intern Med. (2014) 28:1653–65. doi: 10.1111/jvim.12458
11. Riihimäki M, Raine A, Elfman L, Pringle J. Markers of respiratory inflammation in horses in relation to seasonal changes in air quality in a conventional racing stable. Can J Vet Res. (2008) 72:432–9
12. Doubli-Bounoua N, Richard EA, Léon A, Pitel PH, Pronost S, Fortier G. Multiple molecular detection of respiratory viruses and associated signs of airway inflammation in racehorses. Virol J. (2016) 13:197. doi: 10.1186/s12985-016-0657-5
13. Houtsma A, Bedenice D, Pusterla N, Pugliese B, Mapes S, Hoffman AM, et al. Association between inflammatory airway disease of horses and exposure to respiratory viruses: a case control study. Multidisc Respir Med. (2015) 10:33. doi: 10.1186/s40248-015-0030-3
14. Holcombe SJ, Jackson C, Gerber V, Jefcoat A, Berney C, Eberhardt S, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet J. (2001) 33:244–9. doi: 10.2746/042516401776249606
15. Ivester KM, Couëtil LL, Moore GE. An observational study of environmental exposures, airway cytology, and performance in racing thoroughbreds. J Vet Intern Med. (2018) 32:1754–62. doi: 10.1111/jvim.1522
16. Niedźwiedź. Equine recurrent airway obstruction. Macedon Vet Rev. (2014). 37:6. doi: 10.14432/j.macvetrev.2014.08.019
17. Lavoie JP, Cesarini C, Lavoie-Lamoureux A, Moran K, Lutz S, Picandet V, et al. Bronchoalveolar lavage fluid cytology and cytokine messenger ribonucleic Acid expression of racehorses with exercise intolerance and lower airway inflammation. J Vet Intern Med. (2011) 25:322–9. doi: 10.1111/j.1939-1676.2010.0664.x
18. Hare JE, Viel L. Pulmonary eosinophilia associated with increased airway responsiveness in young racing horses. J Vet Intern Med. (1998) 12:163–70
19. Hotchkiss JW, Reid SW, Christley RM. A survey of horse owners in Great Britain regarding horses in their care. Part 2: risk factors for recurrent airway obstruction. Equine Vet J. (2007) 39:301–8. doi: 10.2746/042516407X180129
20. Leclere M, Lavoie-Lamoureux A, Lavoie JP. Heaves, an asthma-like disease of horses. Respirology. (2011) 16:1027–46. doi: 10.1111/j.1440-1843.2011.02033.x
21. Clements JM, Pirie RS. Respirable dust concentrations in equine stables. Part 1: validation of equipment and effect of various management systems. Res Vet Sci. (2007) 83:256–62. doi: 10.1016/j.rvsc.2006.12.002
22. Whittaker AG, Hughes KJ, Parkin TD, Love S. Concentrations of dust and endotoxin in equine stabling. Vet Rec. (2009) 165:293–5. doi: 10.1136/vr.165.10.293
23. Larson J. The comparison of airway responses of normal horses fed round bale versus square bale hay. In: Biomedical and Veterinary Sciences. Blacksberg: Virginia Polytechnic Institute and State University (2012)
24. Robinson NE, Karmaus W, Holcombe SJ, Carr EA, Derksen FJ. Airway inflammation in Michigan pleasure horses: prevalence and risk factors. Equine Vet J. (2006) 38:293–9. doi: 10.2746/042516406777749281
25. Ranalli G, Grazia L, Roggeri A. The influence of hay-packing techniques on the presence of saccharopolyspora rectivirgula. J Appl Microbiol. (1999) 87:359–65
26. Choudhury AH, Gordian ME, Morris SS. Associations between respiratory illness and PM10 air pollution. Arch Environ Health. (1997) 52:113–7. doi: 10.1080/00039899709602873
27. Brønnum-Hansen H, Bender AM, Andersen ZJ, Sørensen J, Bønløkke JH, Boshuizen H, et al. Assessment of impact of traffic-related air pollution on morbidity and mortality in copenhagen municipality and the health gain of reduced exposure. Environ Int. (2018) 121:973–980. doi: 10.1016/j.envint.2018.09.050
28. Peters A, Goldstein IF, Beyer U, Franke K, Heinrich J, Dockery DW, et al. Acute health effects of exposure to high levels of air pollution in eastern Europe. Am J Epidemiol. (1996) 144:570–81
29. Gerber V, Robinson NE, Luethi S, Marti E, Wampfler B, Straub R. Airway inflammation and mucus in two age groups of asymptomatic well-performing sport horses. Equine Vet J. (2003) 35:491–5. doi: 10.2746/042516403775600424
30. Sanni H, Louise HM, Miia R, John PAAH, Julie F. Seasonal variation in tracheal mucous and bronchoalveolar lavage cytology for adult clinically healthy stabled horses. J. Equine Vet. Sci. (2018) 71:5. doi: 10.1016/j.jevs.2018.09.001
31. Sue-Chu M, Larsson L, Bjermer L. Prevalence of asthma in young cross-country skiers in central Scandinavia: differences between Norway and Sweden. Respir Med. (1996) 90:99–105.
32. Sue-Chu M, Larsson L, Moen T, Rennard SI, Bjermer L. Bronchoscopy and bronchoalveolar lavage findings in cross-country skiers with and without “ski asthma”. Eur Respir J. (1999) 13:626–32.
33. Loughlin CE, Esther CR, Lazarowski ER, Alexis NE, Peden DB. Neutrophilic inflammation is associated with altered airway hydration in stable asthmatics. Respir Med. (2010) 104:29–33. doi: 10.1016/j.rmed.2009.07.002
34. Davis MS, Freed AN. Repeated hyperventilation causes peripheral airways inflammation, hyperreactivity, and impaired bronchodilation in dogs. Am J Respir Crit Care Med. (2001) 164:785–9. doi: 10.1164/ajrccm.164.5.2003081
35. Davis MS, Malayer JR, Vandeventer L, Royer CM, McKenzie EC, Williamson KK. Cold weather exercise and airway cytokine expression. J Appl Physiol. (2005) 98:2132–6. doi: 10.1152/japplphysiol.01218.2004
36. Davis MS, Williams CC, Meinkoth JH, Malayer JR, Royer CM, Williamson KK, et al. Influx of neutrophils and persistence of cytokine expression in airways of horses after performing exercise while breathing cold air. Am J Vet Res. (2007) 68:185–9. doi: 10.2460/ajvr.68.2.185
37. Thomas JS, Gray PR, Tobey JC. Bronchoalveolar lavage in ponies with heaves during disease remission. Vet Clin Pathol. (1993) 22:49–53
38. Jean D, Vrins A, Beauchamp G, Lavoie JP. Evaluation of variations in bronchoalveolar lavage fluid in horses with recurrent airway obstruction. Am J Vet Res. (2011) 72:838–42. doi: 10.2460/ajvr.72.6.838
39. Sweeney CR, Rossier Y, Ziemer EL, Lindborg S. Effects of lung site and fluid volume on results of bronchoalveolar lavage fluid analysis in horses. Am J Vet Res. (1992) 53:1376–9
40. Pickles K, Pirie RS, Rhind S, Dixon PM, McGorum BC. Cytological analysis of equine bronchoalveolar lavage fluid. Part 1: comparison of sequential and pooled aliquots. Equine Vet J. (2002) 34:288–91. doi: 10.2746/042516402776186137
41. Depecker M, Richard EA, Pitel PH, Fortier G, Leleu C, Couroucé-Malblanc A. Bronchoalveolar lavage fluid in standardbred racehorses: influence of unilateral/bilateral profiles and cut-off values on lower airway disease diagnosis. Vet J. (2014) 199:150–6. doi: 10.1016/j.tvjl.2013.10.013
42. Pickles K, Pirie RS, Rhind S, Dixon PM, McGorum BC. Cytological analysis of equine bronchoalveolar lavage fluid. Part 2: comparison of smear and cytocentrifuged preparations. Equine Vet J. (2002) 34:292–6.
Keywords: equine asthma syndrome, heaves, inflammatory airway disease, recurrent airway obstruction, asthma phenotype, round bale hay
Citation: Davis KU and Sheats MK (2019) Bronchoalveolar Lavage Cytology Characteristics and Seasonal Changes in a Herd of Pastured Teaching Horses. Front. Vet. Sci. 6:74. doi: 10.3389/fvets.2019.00074
Received: 08 January 2019; Accepted: 21 February 2019;
Published: 14 March 2019.
Edited by:
Peter James O'Brien, University College Dublin, IrelandReviewed by:
Francisco Ruben Carvallo, University of California, Davis, United StatesMichela Pugliese, University of Messina, Italy
Copyright © 2019 Davis and Sheats. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary Katherine Sheats, bWtwZWVkQG5jc3UuZWR1
 Kaori Uchiumi Davis
Kaori Uchiumi Davis Mary Katherine Sheats
Mary Katherine Sheats