- 1Department of Parasitology, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China
- 2Institute of Veterinary Pharmacology, Sichuan Animal Science Academy, Chengdu, China
- 3Animal Breeding and Genetics Key Laboratory of Sichuan Province, Sichuan Animal Science Academy, Chengdu, China
- 4Institute of Animal Genetics and Breeding, College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, China
Psoroptes ovis var. cuniculi is a common ectoparasite of the wild and domestic rabbits worldwide, which causes significant economic losses in commercial rabbit breeding. In China, the diagnosis of rabbits infested with P. ovis var. cuniculi currently relies on detection of clinical signs and Psoroptes mites in skin scrapings by microscopy examination. However, this method is not very efficient in detection of the low mite loads and/or sub-clinical infections. In the present study, we cloned and expressed an arginine kinase homolog gene from P. ovis var. cuniculi (Poc-AK) and used its recombinant protein rPoc-AK to develop an indirect enzyme-linked immunosorbent assay (iELISA) method for diagnosis of P. ovis var. cuniculi infestation in rabbits. The results showed that the rPoc-AK protein was ~61 kDa and had no signal peptide. The rPoc-AK-based iELISA achieved a 94.4% sensitivity and a 88.2% specificity, and was able to detect P. ovis var. cuniculi infection as early as the 1st week post-infection, prior to the appearance of clinical signs. Further field study showed 24.94% (66.33/266) clinically normal rabbits were seropositive with the highest and lowest seropositive rates for California (35.71%) and Belgian (15.14%), respectively. These results suggested that the rPoc-AK has potential as a diagnostic antigen for early P. ovis var. cuniculi infestation in rabbits.
Introduction
Psoroptes ovis var. cuniculi (also known as Psoroptes cuniculi) is a common ectoparasite of the wild and domestic rabbits worldwide and causes over 70% incidence rate in China (1–3). Rabbits infestations with P. ovis var. cuniculi are characterized by dermatitis, itching, hair loss, a thick crust, and a considerable negative impact on weight (2, 4–7). In China, the diagnosis of rabbits infested with P. ovis var. cuniculi currently relies on detection of clinical signs and further confirmation by observation of Psoroptes mites in skin scrapings using microscopy examination. This method is highly sensitive in severely affected rabbits, but low sensitive in the low mite loads and/or sub-clinical infections. Moreover, the collection and examination of skin-scrapings from rabbits are time-consuming, tedious, and impractical. Therefore, serological methods would be more convenient and efficient. The crude extracts of Psoroptes mites were reported to detect P. ovis var. cuniculi infestation in rabbits (8), however, the lacking in vitro propagation systems for Psoroptes mites caused the mass production problems. Thus, the recombinant antigens would be more practical for diagnosis of the psoroptic mange in rabbits. However, until now only one recombinant protein troponin C of P. ovis var. cuniculi has been evaluated and its potential as serological antigen for diagnosis of Psoroptes infestation was disappointing because of the extremely low specificity (9). So, there are still lacking of effective antigens for serodiagnosis of P. ovis var. cuniculi infestation in rabbits.
Arginine kinase (AK; EC 2.7.3.3), a highly conserved member of the phosphoprotokinase (PK) family, has only been observed in invertebrates (10) and is involved in energy metabolism, cell growth, immune modulation, and environmental adaptation (10–12). More importantly, the AK protein has been considered a potential diagnostic antigen for the zoonotic toxocariasis (13). In P. ovis, the AK has been reported to exist across all lifecycle stages of this mite (14), however, until now no other researches on P. ovis AK haven been reported. Herein, we cloned and expressed a novel AK homolog from P. ovis var. cuniculi (Poc-AK) and assessed its potential as a seroantigen in diagnosis of P. ovis var. cuniculi infestation in rabbits by indirect ELISA (iELISA), and then this rPoc-AK-based iELISA was further used to investigate seroprevalence of P. ovis var. cuniculi infestation in a rabbit farm.
Materials and Methods
Mite Source
Psoroptes ovis var. cuniculi mites were collected from a clinically infected New Zealand White rabbit (NZW), provided by the Department of Parasitology, Sichuan Agricultural University (Sichuan, China). About 300 mites, a mixture of adults, nymphs, and larvae, were harvested and stored in liquid nitrogen for subsequent RNA extraction.
Rabbit Sera
Thirty-six P. ovis var. cuniculi-positive serum samples were collected from naturally infected rabbits at two rabbit farms located in Chengdu, Sichuan, China. P. ovis var. cuniculi infections were examined according to two gold standards, i.e., skin lesion condition and identification of P. ovis var. cuniculi mites in skin scrapings (15). Forty-seven negative serum samples from rabbits clinically and parasitologically free of P. ovis var. cuniculi were collected from rabbits raised in a farm without a history of psoroptic mange. Another 38 serum samples for cross-reactions included Sarcoptes scabiei-positive (n = 10), Cysticercus pisiformis-positive (n = 10), and Eimeria-positive (n = 18) sera, provided by the Department of Parasitology, Sichuan Agricultural University.
Cloning, Expression, and Purification of Poc-AK
Total RNA was extracted from P. ovis var. cuniculi mites using a MiniBest universal RNA extraction kit (TaKaRa, Dalian, China) and reverse-transcribed into cDNA using a PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa). According to the annotated P. ovis var. cuniculi transcriptome sequence (GenBank No. PRJNA317241) (16), the full-length sequence encoding Poc-AK was amplified from cDNA using primers 5′-CGGGATCCCCAATGCCTTCAGGTG-3′ (forward; BamHI restriction site underlined) and 5′-CCCTCGAGTCACATTGTTTTTTCCATT-3′ (reverse; XhoI restriction site underlined). The PCR product was ligated into the pET32a (+) expression vector (Invitrogen, Beijing, China), and the resulting construct was transformed into Escherichia coli BL21 (DE3). The recombinant protein expression was induced by 0.5 mM isopropyl-β-D-thiogalactoside (IPTG) at 37°C for 12 h. The recombinant Poc-AK (rPoc-AK) was harvested in form of the inclusion body, solubilized in 8 M urea, and purified by a Ni-NTA His-tag affinity kit (Bio-Rad, California, USA) using a step-wise elution with 20, 50, and 100 mM imidazole. The purified protein was further dialyzed against phosphate buffered saline (PBS) and concentrated using Amicon Ultra Centrifugal Filter devices (Millipore, Billerica, MA, USA) according to the manufacturer's protocol.
Sequence Analysis
The complete gene sequence of Poc-AK was obtained and the corresponding amino acid sequence was deduced by ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi). DNAMAN version 7.0 was used to calculate identities between homologous genes. SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) was used to predict signal peptides. Transmembrane regions and subcellular localization were analyzed using the online Transmembrane Prediction Server (http://www.sbc.su.se/miklos/DAS/). The theoretical isoelectric point (pI) and molecular weight were predicted using the ExPASy server (http://web.expasy.org/protparam/).
Recombinant Poc-AK Polyclonal Antibody Preparation
Polyclonal antibody recognizing rPoc-AK was raised as previously described (17). In brief, rabbits were immunized four times by subcutaneous injection (2 weeks apart) with 200 μg purified rPoc-AK. Sera were collected before immunization and 3 days after the final injection, then purified using HiTrap ProteinA affinity chromatography (Bio-Rad).
Western Blotting Analysis
The purified rPoc-AK protein was separated by 12% SDS-PAGE and then transferred to a nitrocellulose membrane by a trans-blot SD semi-dry transfer cell (Bio-Rad). The membrane was blocked using 5% skim milk powder for 2 h at room temperature, then incubated with rabbit anti-P. ovis var. cuniculi serum or anti-rPC-AK IgG (1:200 v/v) overnight at 4°C. Non-infected rabbit serum was used as a negative control. After washing with TBST (0.02 M Tris-HCl, pH 7.6, 0.15 M NaCl, 0.05% Tween-20), the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:1,000 dilution; Boster Bio-project Co. Dalian, China) for 2 h at room temperature. Finally, the signal was measured using an Enhanced HRP-DAB Chromogenic Substrate Kit (Tiangen, Beijing, China).
Establishment of an Indirect ELISA (iELISA)
An indirect ELISA was performed essentially by following Crowther and Walker's protocol (18). The checkerboard titration test was performed to ascertain the optimal concentrations of antigen and serum for the rPoc-AK-based iELISA. The 96-well plates were coated with 100 μL of two-fold dilutions of rPoc-AK protein (diluted in 0.1 M carbonate buffer pH 9.6 at 12, 6, 3, 1.5, 0.75, and 0.375 μg/well) and incubated overnight at 4°C. After three 5-min washes with phosphate-buffered saline (PBS) containing Tween-20 (PBST), plates were incubated with blocking buffer (5% skim milk diluted in PBS) for 90 min at 37°C, then with 100 μL of two-fold dilutions of P. ovis var. cuniculi-infested positive or negative sera (pooled samples from six animals; diluted in PBS at 1:20, 1:40, 1:80, 1:160, 1:320, and 1:640) for 60 min at 37°C. After washing three times, the plates were incubated with the recommended working concentration of HRP-conjugated goat anti-rabbit IgG (1:3,400; diluted in 0.01 M PBS) (Boster Bio-project Co., Wuhan, China) at 37°C for 60 min. Following five washes in PBST for 5 min each time, 100 μL of TMB chromogenic substrate solution (Tiangen) was added and the plates were placed in the dark at 37°C for 20 min. Subsequently, 100 μL of 2 M H2SO4 was added to stop the reaction, and the optical density (OD) was determined at 450 nm using a microplate reader (Thermo Scientific, Pittsburgh, PA, USA). The optimal working concentrations of antigen and sera were determined when the highest P/N value was obtained between positive and negative sera.
Under optimal conditions, 47 negative serum samples from healthy rabbits free of P. ovis var. cuniculi were used to determine the cut-off value for indirect ELISA, which serves as an identity standard for negative and positive sera. The cut-off value was calculated as the arithmetic mean OD450 value plus three standard deviations (SD) for Psoroptes-free rabbits (19).
Sensitivity and Specificity of the rPoc-AK Indirect ELISA
Thirty-six serum samples from rabbits infected with P. ovis var. cuniculi were used for determination of sensitivity according to the following formula: sensitivity = (ELISA positive/true positive) × 100%. Cross-reactions were investigated with sera from rabbits infected with S. scabiei (n = 10), C. pisiformis (n = 10), and Eimeria (n = 18), respectively. In addition, 47 negative serum samples were collected from P. ovis var. cuniculi-free rabbits without a history of psoroptic mange and use to determine the specificity of the iELISA. In summary, specificity was determined using the following formula: specificity = (ELISA negative/true negative) × 100%.
Repeatability and Reproducibility of the rPoc-AK Indirect ELISA
Five positive serum samples collected from rabbits infested with P. ovis var. cuniculi were used to evaluate repeatability and reproducibility. Repeatability (intra-assay variability) was assessed from OD450 values with triplicates in one coated ELISA plate. The same five serum samples were also applied to assess reproducibility (inter-assay variability) in another three different coated plates. These experiments were performed under the optimal conditions for the rPoc-AK indirect ELISA. The coefficients of variation (CV) for intra- and inter-assay variability were calculated according to the OD450 values and standard deviation (SD) from all samples.
Experimental Infestation of Rabbits With P. ovis var. cuniculi and Diagnosis Testing Using the rPoc-AK Indirect ELISA
Experimental infestation of rabbits with P. ovis var. cuniculi was carried out essentially following a previously described protocol (8). In brief, 10 3-month-old naive NZW rabbits (half females and half males) were infested by placing ~200 different life-cycle stages P. ovis var. cuniculi mites deeply into the external auditory canal and closing with cotton for 1 day. All rabbit serum samples were taken weekly from week 0 until week 4 post-infection (p.i.). All 50 serum samples from rabbits experimentally infested with P. ovis var. cuniculi were then examined using the rPoc-AK indirect ELISA. Each serum sample was analyzed in triplicate, and positive and negative controls were included in all plates.
Field Investigation of the rPoc-AK Indirect ELISA
The field investigation using the rPoc-AK indirect ELISA was carried out for analysis of 266 serum samples collected from a rabbit farm in Chengdu City, Sichuan Province, China, in October 2018. These sera were collected from clinically unaffected individual rabbits, but these subjects were from a rabbit farm with a previous history of psoroptic mange. Serum samples were collected from five breeds of rabbits (Table 1) as follows: California (n = 84), Hyla (n = 77), Belgian (n = 35), Qixing crossbreds (France White rabbit × Sichuan White Rabbit, n = 22), ZIKA × NZW crossbreed (n = 48). Each serum sample was tested three times.
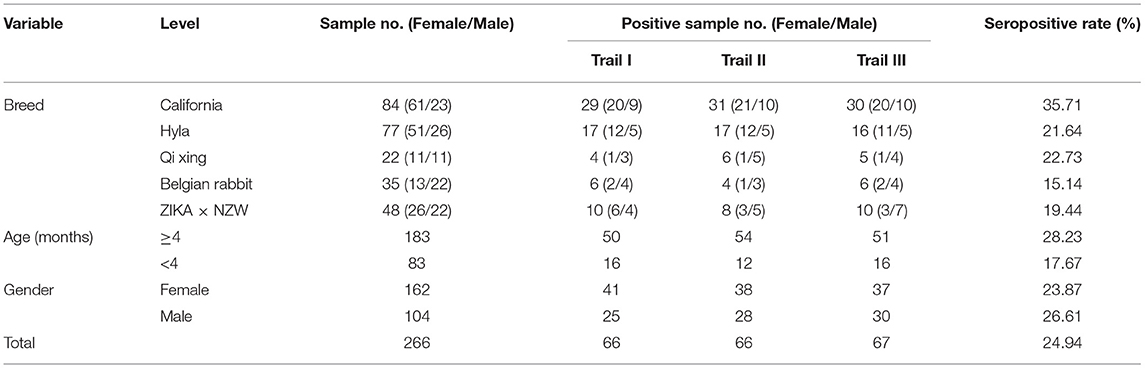
Table 1. Two hundred and sixty six rabbit serum samples collected and the results of three replicates detections using rPoc-AK-based indirect ELISA.
Statistical Analysis
All data are presented as mean ± standard deviation (SD). The significance of differences between groups were tested using Mann-Whitney U-tests in SPSS software v.17.0 (SPSS, Inc., Chicago, Illinois, USA), and p < 0.05 were considered statistically significant.
Results
Recombinant Poc-AK and Sequence Analysis
The full-length P. ovis var. cuniculi arginine kinase gene includes an open reading frame of 1,116 bp encoding a 371 amino acid polypeptide. The expected molecular weight of the protein is 42.9 kDa and the theoretical isoelectric point (pI) is 9.10. The Poc-AK protein has no signal peptide, no transmembrane regions, and is hydrophilic. We identified six highly conserved motifs in the deduced amino acid sequence of Poc-AK, including an arginine binding site, an ADP binding site, a substrate binding site, a synergistic substrate binding site (D78, Y84, R208, and P287) and an arginine enzyme active site residue essential for kinase activity of C286. The nucleotide sequence of Poc-AK reported in this study was identical to those reported for an arginine kinase from P. ovis var. cuniculi (PRJNA317241) (16). The amino acid alignment showed Poc-AK had identities of 52.15–82.46% with other mites including Dermatophagoides pteronyssinus, Tetranychus urticae, Dermanyssus gallinae, and S. scabiei. The full-length coding sequence of Poc-AK was successfully ligated into the pET32a (+) vector for recombinant expression of the Poc-AK in E. coli BL21 (DE3) cells (Figure 1, lane 1), and the fusion protein was mainly present in insoluble inclusion bodies (Figure 1, lanes 2 and 3). The purified rPoc-AK protein was of the expected molecular size (61 kDa), including an extra 20 kDa for the attached His-tag fusion peptide (Figure 1, lane 4).
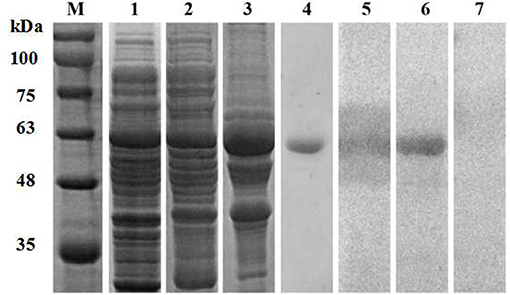
Figure 1. SDS-PAGE and western blotting of P. ovis var. cuniculi AK. Lanes: M, protein molecular weight markers (kDa); 1, non-purified recombinant AK; 2,3, protein solubility analysis (supernatant and inclusion bodies); 4, purified recombinant AK; 5, purified recombinant AK detected in serum from a rabbit naturally infested with P. ovis var. cuniculi; 6, purified recombinant AK detected in rabbit anti-AK serum; 7, purified recombinant AK detected in negative serum.
Western Blotting
Western blotting confirmed that the purified rPoc-AK ~61 kDa protein was recognized by rabbit anti-P. ovis var. cuniculi serum and anti-rPoc-AK serum IgG (Figure 1, lanes 5 and 6), which revealed this recombined Poc-AK protein with strong reactivity and antigenicity. Meanwhile, negative sera from naive rabbits did not react with the purified rPoc-AK protein (Figure 1, lane 7).
Establishment of an Indirect ELISA (iELISA) Using rPoc-AK
In accordance with the checkerboard titration procedure, optimal conditions for iELISA were determined from the highest positive to negative ratios and then used in subsequent experiments. Optimal conditions for iELISA were 13.3 μg/mL for the coated rPoc-AK antigen per well, and a 1:320 dilution of rabbit primary serum. The cut-off value was 0.401 according to the mean OD450 value plus 3SD from 47 sera from naive rabbits using optimal conditions. Therefore, serum with OD450 ≥ 0.401 was judged positive, while OD450 <0.401 was judged negative.
Using optimal conditions, 34/36 serum samples from rabbits naturally infected with P. ovis var. cuniculi were determined as positive (OD450 > 0.401), equating to a sensitivity of 94.4% (Figure 2, panel 2). In cross-reactivity tests, there were three S. scabiei-positive sera, four C. pisiformis-positive sera, and three Eimeria-positive sera giving cross-reactivity with Poc-AK, representing a specificity of 88.2% (75/85; Figure 2, panels 1, 3–5).
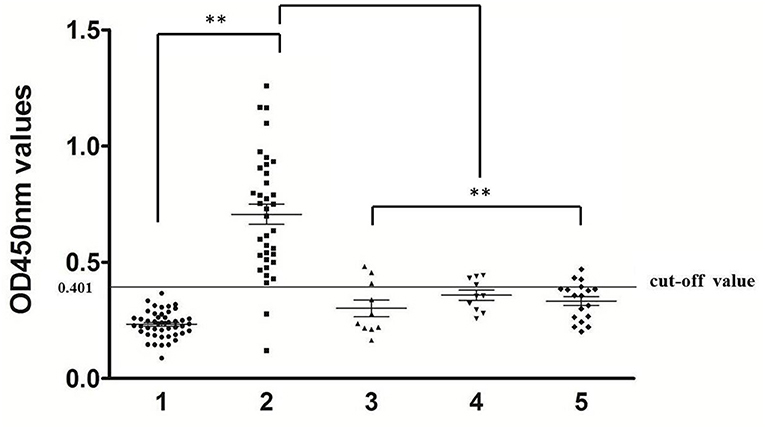
Figure 2. Sensitivity and specificity of indirect ELISA. 1, rabbit serum samples of uninfected P. ovis var. cuniculi (n = 47), 2, rabbit serum samples of infected P. ovis var. cuniculi (n = 36), 3, S. scabiei (n = 10), 4, C. pisiformis (n = 10), 5, Eimeria spp. (n = 18). The symbol (**) indicates a very significant difference (P < 0.01) between P. ovis var. cuniculi-positive sera and other serum samples according to the Mann-Whitney U-tests.
The coefficients of variation (CV) for intra- and inter-assay variability ranged from 0.34 to 2.13% (average = 1.13%) and from 2.13 to 6.45% (average = 4.31%), respectively. Both intra- and inter-assay CVs were below 10%, confirming that the rPoc-AK-based iELISA was stable and reproducible.
Diagnosis Testing
At 2 weeks p.i., a slight crust started to form at the inoculation site in all 10 experimentally-infested rabbits, and this tend increased after 3–4 weeks p.i. (Figure 3, lower lane). Changes in anti-Poc-AK antibody levels in rabbits during artificial infection with P. ovis var. cuniculi are shown in Figure 3. A higher anti-Poc-AK antibody value than cut-off value (0.401) was first detected at 1 week p.i., with 8/10 rabbit sera identified as positive (Figure 3, lane 2). Positive serum antibodies against Poc-AK gradually increased from 2 to 4 weeks p.i. (Figure 3, lanes 3–5), suggested that the rPoc-AK-based iELISA developed in this study could be used for early diagnosis of psoroptic mange in rabbits.
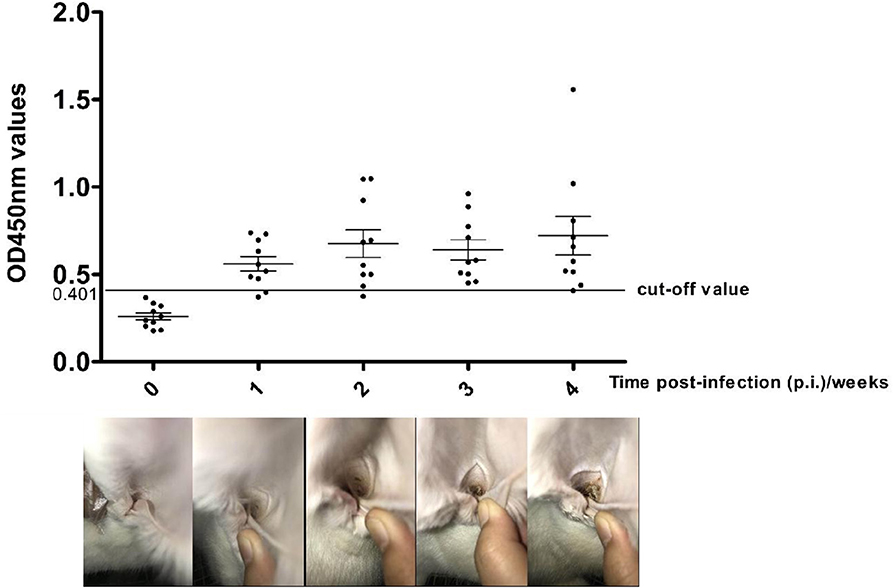
Figure 3. Serum antibody profiles probed by P. ovis arginine kinase (rPoc-AK)-based indirect ELISA in rabbits experimental infections of P. ovis var. cuniculi. The horizontal line shows the cut-off value of 0.410.
Field Investigation of Seroprevalence
A panel of 266 serum samples from a rabbit farm were included in a field study analysis of the rPoc-AK-based iELISA (Table 1). The iELISA achieved an overall seropositive rate of 24.94% (66.33/266). Among the five rabbit breeds, the highest seropositive rate was observed for California (35.71%), followed by Qixing (22.73%), Hyla (21.64%), ZIKA × NZW crossbreed (19.44%), and Belgian (15.14%; Table 1). Moreover, the California breed yielded a significantly higher seropositive rate than that of other four rabbit breeds (p < 0.01), while the value for the Belgian breed was significantly lower than that of Qixing and Hyla (p < 0.05; Figure 4). No significant differences were observed between Qixing and Hyla (p > 0.05), or between Hyla and the ZIKA × NZW crossbreed (p > 0.05; Figure 4).
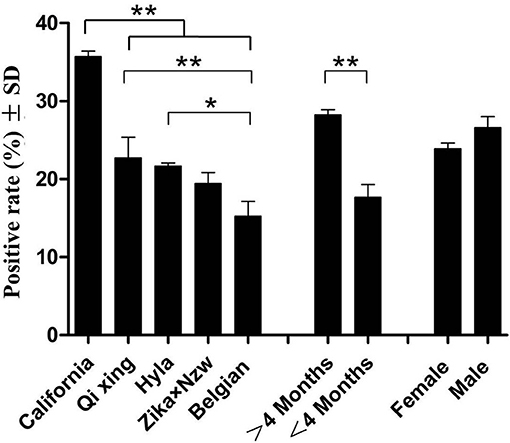
Figure 4. Clinical detections using rPoc-AK-based indirect ELISA. * and ** indicate statistical significance at p < 0.05 and p < 0.01, respectively.
Comparison of seropositive rates of rabbits of different ages revealed that ≥4-months rabbits yielded higher seropositive rate than that of rabbits under 4 months of age (Figure 4). However, the difference in seropositive rates between sex was not statistically significant (p > 0.05; Figure 4).
Discussion
To our knowledge, the arginine kinase (AK) has been reported to exist in all P. ovis lifecycle stages (14), however, no other research on P. ovis var. cuniculi AK has been conducted so far. In the present study, we cloned and expressed AK from P. ovis var. cuniculi and evaluated its potential application for the serodiagnosis of the mite infection in rabbits.
AK, a member of the highly conserved phosphoprotokinase (PK) family present in invertebrates, reversibly catalyzes the transphosphorylation reaction between arginine and ATP, yielding ADP and phosphagen (20). It is known to play a crucial role in cellular energy metabolism and maintaining constant ATP levels in invertebrate cells. Data on AKs in parasites are limited to a few reports on Trypanosoma brucei (21), T. cruzi (22), Toxocara canis (23), Haemonchus contortus (24), Teladorsagia circumcincta (25), Ascaris suum (26), Lucilia cuprina, and Ctenocephalides felis (27, 28). Thus, there remain large gaps in our knowledge of AK from parasitic mites. In the present study, AK from mite parasite P. ovis var. cuniculi was cloned and expressed. Comparison of deduced amino acid sequences revealed conserved regions in mite AKs and suggested all AKs possess identical substrate and arginine binding sites, i.e., a central AK region consisting of seven amino acid residues (CPTNLGT) and an essential arginine enzyme active site C276 residue (29–31).
The clinical signs of Psoroptes infestation in the field can be observed over a long period of time, sometimes several months (32, 33). Although microscopy examination is able to detect P. ovis infection with minimal clinical signs, the sensitivity is as low as 18% (15). Therefore, other approaches, especially serodiagnostic methods are urgently needed. Previous serological diagnostics based on crude extracts were reported to detect P. ovis infestation in sheep (34), elk (35), cattle and rabbit (8, 36). However, because of lacking in vitro propagation systems for Psoroptes mites, the recombinant proteins that can be recognized by serum antibodies raised in P. ovis-infested animals would be more practical for serodiagnosis of psoroptic mange. To date, the recombinant P. ovis allergen, Pso o 2, is an ideal antigen in serodiagnosis of subclinical P. ovis infection in sheep with the high sensitivity and specificity (37–39), however, its potential serodiagnosis in detect P. ovis var. cuniculi infestation in rabbits is unknown. Zheng et al. showed that the recombinant Troponin C was not a suitable antigen candidate for detection of the P. ovis var. cuniculi infestation in rabbits because of the low specificity (25.0%) (9). In the present study, we developed a rPoc-AK-based iELISA and showed that this serological approach can achieve a 94.4% sensitivity and a 88.2% specificity. Three serum samples from S. scabiei-infection showed positive. Similar cross-reactions between Psoroptes and Sarcoptes infestations were also observed in other studies and have been verified as a common problem (9, 34, 40). Psoroptes and Sarcoptes mites are sometimes present in a same rabbit in nature and same acaricidal drugs are used for control of these two mite species (41, 42). In addition, seven serum samples from rabbits infection with C. pisiformis and Eimeria also showed weak cross-reactions with rPoc-AK but their OD values were significantly lower than those in the P. ovis var. cuniculi group (p < 0.01) and all were close to the cut-off value. These results suggested that the recombinant Poc-AK could be a potential antigen for diagnosis of the rabbit infestation of P. ovis var. cuniculi in practice.
In our study, the ELISA test showed that rPoc-AK detected anti-P. ovis var. cuniculi antibodies as early as the 1st week p.i. in the overwhelming majority of rabbits (80%) which was 1 week earlier than a slight crust emergence and also earlier than previously reported rPso o2-ELISA and crude Psoroptes extract-based ELISA (15, 37). The finding indicated that rPoc-AK has great potential as an antigen in diagnosing the early stages of P. ovis var. cuniculi infestation in rabbits.
In our field investigation, our rPoc-AK-based iELISA revealed a 24.94% seropositive rate in 266 clinically normal rabbits. This result indicated that our iELISA can detect asymptomatic animals during the early infestation stages of P. ovis var. cuniculi. In nature, such asymptomatic animals may be a main source of infection but are neglected when developing control strategies. Moreover, there were significant differences in seropositive rates found between different rabbit breeds in this study. The California breed yielded the highest seropositive rate (35.71%), implying its susceptibility to Psoroptes mites; whilst the Belgian breed displayed the lowest seropositive rate (15.14%), to certain extent indicating its resistance. However, both speculations still require further researches. In addition, seropositive rates of ≥4 months rabbit were higher in rabbits than those <4 months (28.23% vs. 17.67%, p < 0.01), and higher in males than females (23.87% vs. 26.61%, p > 0.05). Similar infection differences between age and sex were observed for elk scabies (35).
Conclusion
Taken together, in the present study we firstly identified a homolog of arginine kinase of P. ovis var. cuniculi and developed an indirect ELISA method with high sensitivity and specificity using its recombinant protein rPoc-AK. The iELISA method can detect P. ovis var. cuniculi infestation on 1 week p.i., prior to observable disease symptoms, revealing its potential for early diagnosis of P. ovis var. cuniculi in rabbits.
Data Availability Statement
The nucleotide sequence of arginine kinase gene from P. ovis var. cuniculi in this article is available in the GenBank database under the accession no. MN013016.
Ethics Statement
All rabbits in this study were managed in strict accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Bethesda, MD, USA) and the recommendations in the ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines). The experiment protocol was approved by the Animal Care and Use Committee of Sichuan Agricultural University (SYXK 2014-187).
Author Contributions
XG, JG, YR, YZ, GY, and XZ performed the experiments, analyzed the data, and drafted parts of the manuscript. XG and YX conceived and funded the study, revised, and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (Grant No. 2019YFN0155).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Xiaoxiao Yin, Yuhang Cheng, Ce Wang, and Chongyang Zhang for their help and suggestions.
References
1. OConnor BM, Klimov PB. Review and resolution of some nomenclatural issues regarding the genus Psoroptes (Acari: Psoroptidae). Exp Appl Acarol. (2015) 66:337–45. doi: 10.1007/s10493-015-9916-1
2. Jiang A, Yuan Y, Yang R, Zhang N, Xie Y, Lai W, et al. Beauveria bassiana is a potential effective biological agent against Psoroptes ovis var. cuniculi mites. Biol control. (2019) 131:43–8. doi: 10.1016/j.biocontrol.2019.01.010
3. Rehbein S, Visser M, Winter R, Trommer B, Matthes HF, Maciel A, et al. Productivity effects of bovine mange and control with ivermectin. Vet Parasitol. (2003) 114:267–84. doi: 10.1016/S0304-4017(03)00140-7
4. Bo KR. Investigation on psoroptic mange of in Gangou township, Minhe county. Qinghai J Animal Husbandry Vet Med. (2012) 42:12. doi: 10.3969/j.issn.1003-7950.2012.03.012
5. Shang X, Wang D, Miao X, Wang X, Li J, Yang Z, et al. The oxidative status and inflammatory level of the peripheral blood of rabbits infested with Psoroptes cuniculi. Parasit Vectors. (2014) 7:124. doi: 10.1186/1756-3305-7-124
6. Ilić T, Stepanović P, Nenadović K, Dimitrijević S. Improving agricultural production of domestic rabbits in Serbia by follow-up study of their parasitic infections. Iran J Vet Res. (2018) 19:290–7.
7. Shang XF, Dai LX, Liu YQ, Zhao ZM, Li JC, Yang GZ, et al. Acaricidal activity and enzyme inhibitory activity of active compounds of essential oils against Psoroptes cuniculi. Vet Parasitol. (2019) 267:54–9. doi: 10.1016/j.vetpar.2019.01.013
8. Siegfried E, Ochs H, Deplazes P. Clinical development and serological antibody responses in sheep and rabbits experimentally infested with Psoroptes ovis and Psoroptes cuniculi. Vet Parasitol. (2004) 124:109–24. doi: 10.1016/j.vetpar.2004.06.014
9. Zheng W, Zhang R, Wu X, Ren Y, Nong X, Gu X, et al. Evaluating troponin C from Psoroptes cuniculi as a diagnostic antigen for a dot-ELISA assay to diagnose mite infestations in rabbits. Parasit Immunol. (2014) 36:53–9. doi: 10.1111/pim.12076
10. Liu F, Wang XD, Zhao YY, Li YJ, Liu YC, Sun J. Silencing the HaAK gene by transgenic plant-mediated RNAi impairs larval growth of Helicoverpa armigera. Int J Biol Sci. (2015) 11:67–74. doi: 10.7150/ijbs.10468
11. Tanaka K, Ichinari S, Iwanami K, Yoshimatsu S, Suzuki T. Arginine kinase from the beetle Cissites cephalotes (Olivier). Molecular cloning, phylogenetic analysis and enzymatic properties. Insect Biochem Mol Biol. (2007) 37:338–45. doi: 10.1016/j.ibmb.2006.12.006
12. Sonobe H, Yamada R. Ecdysteroids during early embryonic development in silkworm Bombyx mori: metabolism and functions. Zool Sci. (2004) 21:503–17. doi: 10.2108/zsj.21.503
13. Wickramasinghe S, Yatawara L, Nagataki M, Takamoto M, Watanabe Y, Rajapakse R, et al. Development of a highly sensitive IgG-ELISA based on recombinant arginine kinase of Toxocara canis for serodiagnosis of visceral larva migrans in the murine model. Parasitol Res. (2008) 103:853–8. doi: 10.1007/s00436-008-1067-4
14. Burgess ST, Marr EJ, Bartley K, Nunn FG, Down RE, Weaver RJ, et al. A genomic analysis and transcriptomic atlas of gene expression in Psoroptes ovis reveals feeding-and stage-specific patterns of allergen expression. BioRxiv. (2019) 578120. doi: 10.1101/578120
15. Ochs H, Lonneux JF, Losson B, Deplazes P. Diagnosis of psoroptic sheep scab with an improved enzyme-linked immunosorbent assay. Vet Parasitol. (2001) 96:233–42. doi: 10.1016/S0304-4017(00)00444-1
16. He ML, Xu J, He R, Shen NX, Gu XB, Peng XR, et al. Preliminary analysis of Psoroptes ovis transcriptome in different developmental stages. Parasit Vector. (2016) 9:570. doi: 10.1186/s13071-016-1856-z
17. Sun Y, Wang Y, Huang X, Gu X, Lai W, Peng X, et al. Characterization of glutathione S-transferase and its immunodiagnostic potential for detecting Taenia multiceps. Vet Parasitol. (2017) 242:31–7. doi: 10.1016/j.vetpar.2017.05.013
18. Crowther J, Walker J. The ELISA Guidebook. 2nd ed. New Jersey, NJ: Humana Press (2009). doi: 10.1007/978-1-60327-254-4
19. Jacobson R. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech Oie. (1998) 17:469–86. doi: 10.20506/rst.17.2.1119
20. Wang HR, Zhu WJ, Wang XY. Mechanism of inhibition of arginine kinase by flavonoids consistent with thermodynamics of docking simulation. Int J Biol Macromol. (2011) 49:985–91. doi: 10.1016/j.ijbiomac.2011.08.017
21. Voncken F, Gao F, Wadforth C, Harley M, Colasante C. The phosphoarginine energy-buffering system of Trypanosoma brucei involves multiple arginine kinase isoforms with different subcellular locations. PLoS ONE. (2013) 8:e65908. doi: 10.1371/journal.pone.0065908
22. Fernandez P, Haouz A, Pereira CA, Aguilar C, Alzari PM. The crystal structure of Trypanosoma cruzi arginine kinase. Proteins. (2007) 69:209–12. doi: 10.1002/prot.21557
23. Kulathunga D, Wickramasinghe S, Rajapakse R, Yatawara L, Jayaweera W, Agatsuma T. Immunolocalization of arginine kinase (AK) in Toxocara canis, Toxocara vitulorum, and Ascaris lumbricoides. Parasitol Res. (2012) 111:663–71. doi: 10.1007/s00436-012-2884-z
24. Ehsan M, Gao W, Gadahi JA, Lu M, Liu X, Wang Y, et al. Arginine kinase from Haemonchus contortus decreased the proliferation and increased the apoptosis of goat PBMCs in vitro. Parasit Vector. (2017) 10:311. doi: 10.1186/s13071-017-2244-z
25. Umair S, Knight J, Bland R, Simpson H. Molecular and biochemical characterisation of arginine kinases in Haemonchus contortus and Teladorsagia circumcincta. Exp Parasitol. (2013) 134:362–67. doi: 10.1016/j.exppara.2013.03.011
26. Nagataki M, Uda K, Jarilla BR, Tokuhiro S, Wickramasinghe S, Suzuki T, et al. Molecular and catalytic properties of an arginine kinase from the nematode Ascaris suum. J Helminthol. (2012) 86:276–86. doi: 10.1017/S0022149X11000381
27. Ilg T, Werr M. Arginine kinase of the sheep blowfly Lucilia cuprina:Gene identification and characterization of the native and recombinant enzyme. Pestic Biochem Phys. (2012)102:115–23. doi: 10.1016/j.pestbp.2011.12.001
28. Werr M, Cramer J, Ilg T. Identification and characterization of two arginine kinases from the parasitic insect Ctenocephalides felis. Insect Biochem Mol Biol. (2009) 39:634–45. doi: 10.1016/j.ibmb.2009.07.001
29. Naka F, Kumiko T, Tomohiko S. Amino acid residues 62 and 193 play the key role in regulating the synergism of substrate binding in oyster arginine kinase. Febs Lett. (2005) 579:1688–92. doi: 10.1016/j.febslet.2005.02.026
30. Brown AE, France RM, Grossman SH. Purification and characterization of arginine kinase from the American cockroach (Periplaneta americana). Arch Insect Biochem. (2004) 56:51–60. doi: 10.1002/arch.10143
31. Uda K, Suzuki T. Role of amino acid residues on the GS region of stichopus arginine kinase and danio creatine kinase. Protein J. (2004) 23:53–64. doi: 10.1023/B:JOPC.0000016258.55991.8a
32. Wells B, Innocent GT, Eckersall PD, Mcculloch E, Nisbet AJ, Burgess ST. Two major ruminant acute phase proteins, haptoglobin and serum amyloid A, as serum biomarkers during active sheep scab infestation. Vet Res. (2013) 44:103. doi: 10.1186/1297-9716-44-103
33. Wells B, Burgess STG, Nisbet AJ. Characterization of the ovine complement 4 binding protein-beta (C4BPB) chain as a serum biomarker for enhanced diagnosis of sheep scab. Mol Cell Probe. (2013) 27:158–63. doi: 10.1016/j.mcp.2013.03.003
34. Matthes HF, Harrison GBL, Shaw RJ, Heath ACG, Pfeffer A, Hiepe T H. Cross-reacting antibodies to Sarcoptes suis, Chorioptes bovis and Notoedres cati and anti- P. ovis IgE in sera from sheep infested naturally with Psoroptes ovis. Int J Parasitol. (1996) 26:437–44. doi: 10.1016/0020-7519(96)00005-7
35. Ziccardi MH, Boyce WM, Gardner IA. Seroprevalence of Psoroptes sp. mites in free-ranging elk (Cervus elaphus) as determined by kinetic ELISA. J Wildlife Dis. (1996) 32:51–6. doi: 10.7589/0090-3558-32.1.51
36. Fisher WF, Wilson GI. Precipitating antibodies in cattle infested by Psoroptes ovis (Acarina: Psoroptidae). J Med Entomol. (1997) 14:146–51. doi: 10.1093/jmedent/14.2.146
37. Nunn FG, Burgess ST, Innocent G, Nisbet AJ, Bates P, Huntley JF. Development of a serodiagnostic test for sheep scab using recombinant protein Pso o 2. Mol Cell Probe. (2011) 25:212–18. doi: 10.1016/j.mcp.2011.09.002
38. Temeyer KB, Soileau LC, Pruett JH. Cloning and sequence analysis of a cDNA encoding Pso o II, a mite group II allergen of the sheep scab mite (Acari:Psoroptidae). J Med Entomol. (2002) 39:384–91. doi: 10.1603/0022-2585-39.2.384
39. Hamer K, Burgess S, Busin V, Sargison ND. Performance of the Psoroptes ovis antibody enzyme-linked immunosorbent assay in the face of low-level mite infestation. Vet Rec. (2019) 185:107. doi: 10.1136/vr.105304
40. Xu J, Huang X, Dong XW, Ren YJ, Wu MD, Shen NX, et al. Serodiagnostic potential of alpha-enolase from Sarcoptes scabiei and its possible role in host-mite interactions. Front Microbiol. (2018) 9:1024. doi: 10.3389/fmicb.2018.01024
41. Panigrahi PN, Gupta A. Therapeutic management of concurrent sarcoptic and psoroptic acariosis in rabbits. Intas Polivet. (2013) 14:319–21. Available online at: https://www.researchgate.net/publication/261214449_Therapeutic_Management_of_concurrent_Sarcoptic_and_Psoroptic_Acariosis_in_Rabbits
42. Elshahawy I, Goniemy AE, Esraa A. Epidemiological survey on mange mite of rabbits in the southern regions of Egypt. Sains Malaysiana. (2016) 45:745–51. Available online at: http://journalarticle.ukm.my/9878/1/10_Ismail_Elshahawy.pdf
Keywords: Psoroptes ovis var. cuniculi, arginine kinase, indirect ELISA, early serodiagnosis, rabbit
Citation: Gu X, Gu J, Ren Y, Zheng Y, Yang G, Zhou X and Xie Y (2019) Evaluation of an Indirect ELISA Using Recombinant Arginine Kinase for Serodiagnosis of Psoroptes ovis var. cuniculi Infestation in Rabbits. Front. Vet. Sci. 6:411. doi: 10.3389/fvets.2019.00411
Received: 26 August 2019; Accepted: 05 November 2019;
Published: 20 November 2019.
Edited by:
Edwin Claerebout, Ghent University, BelgiumReviewed by:
Kate Mounsey, University of the Sunshine Coast, AustraliaAntti Oksanen, Finnish Food Safety Authority Evira, Finland
Copyright © 2019 Gu, Gu, Ren, Zheng, Yang, Zhou and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobin Gu, Z3V4aWFvYmluMTk4MjI1QDEyNi5jb20=; Yue Xie, eHl1ZTE5ODVAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Xiaobin Gu
Xiaobin Gu Jiang Gu
Jiang Gu Yongjun Ren
Yongjun Ren Youle Zheng1
Youle Zheng1 Guangyou Yang
Guangyou Yang Xuan Zhou
Xuan Zhou Yue Xie
Yue Xie