- 1Department of Agriculture, Faculty of Environmental Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Poultry Production Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt
Productive traits and immunity in laying hens decrease sharply during the late phase of laying due to aging, which negatively affects the metabolism and hormonal status of the animals. The influence of Ca levels (3.5, 4.0, and 4.5%) and/or cholecalciferol [Vitamin D3 (VD3)] supplementation (800-, 1,000-, and 1,200-IU/kg diet or as total of 3,800, 4,000, and 4,200 IC VD3) on performance, egg quality, blood biochemistry, and immunity of brown egg layers was investigated. Three hundred and sixty H&N Brown egg layers (60 weeks old) were allocated at random into nine nutritional treatments of five replications (cages) of eight hens each. The control diet in this experiment contained a 3.5% Ca level with 800 IU VD3. The addition of VD3 at 1,000 and 1,200 IU to 3.5 and 4% Ca diets significantly (P ≤ 0.05) increased the rate of laying, egg mass, and feed conversion ratio (FCR) compared to the control diet on 3.5% and 800 U of VD3. Besides this, the addition of VD3 at 800 and 1,200 IU to 3.5% Ca level diets enhanced the Haugh unit score. Similar results were observed in eggshell quality measurements and tibia ash. Increasing the Ca concentration from 3.5 to 4 and 4.5% and increasing VD3 levels from 800 to 1,000 or 1,200 IU significantly and similarly increased serum total protein and globulin. In addition, VD3 at 1,000 IU increased serum albumin, compared to 800 IU. Increasing Ca level increased IgA, and 4 and 4.5% Ca levels similarly increased IgG and α-2 globulin compared to the 3.5% Ca diet. VD3 addition at 1,200 IU to the 4% Ca diet significantly increased γ-globulin compared to 1,000 IU, but decreased β-globulin. Increasing the Ca level to 4% significantly reduced serum triglycerides, and the very low-density lipoprotein and the triglyceride/high-density lipoprotein ratio were both decreased with 4 and 4.5% Ca level diets. Increasing the Ca level caused a stepwise increase in catalase, which was markedly increased with VD3 supplementation at 1,200 IU. Plasma estrogen was increased considerably with VD3 supplementation at 3.5% Ca, but parathyroid hormone levels were not affected. In conclusion, increasing Ca levels in the diet of laying hens to 4% during the late production phase could be a useful tool to improve laying performance, eggshell quality, Haugh unit score, and physiological and immunological status. Besides, VD3 at a 1,000 IU/kg diet to 3.5% Ca improved performance of hens fed 3.5% Ca, showing that the potential impact of VD3 depends on Ca concentrations.
Introduction
Globally, both eggs and meat are acceptable and economical sources of protein for human nutrition (1). Modern hybrids of laying hens produce more than 320 eggs per year. The eggs contain 10% shell, and eggshell contains 40% Ca; thus, hens require a considerable amount of Ca for eggshell formation. Eggshell protects the eggs and is responsible for maintaining their inner quality from oviposition until use by the consumers (2). Obviosuly, egg quality and particularly shell quality decline with the age of laying hens during the late phase (60 wk or older) of production (3, 4). The decrease in eggshell quality during the late stage of production could be determined by an increase in egg size, reductions in nutrient metabolism, mainly of Ca, and in reproductive hormones, especially estrogens (5). Calcium is a critical constituent in the nutrition of laying hens, and its availability depends on dietary intestinal absorption. Still, the skeleton is an essential Ca source during the night when intestinal absorption has ceased (5, 6). Increased breakage and loss of eggs are the greatest problems in the table egg industry, causing substantial economic loss, possibly due to low egg shell quality (6, 7). Not only low eggshell quality but also loss of bone strength occurs during the late laying period (5, 8). Recent evidence has shown that proteins are integrated in the regulation of proteins driving the calcification of shell quality and function in laying hens (9, 10).
Adequate Ca consumption by laying hens is critical for ensuring reliable eggshell quality. However, the impact of dietary Ca level on egg production and quality are contradictory in the literature, and some data were reported several decades ago. Besides, there has been a significant improvement in the laying rate due to genetic progress and the use of biotechnical tools in animal breeding, together with consumers' awareness of egg quality, that needs further attention when producing functional eggs (5, 11). In the literature, Ca requirements for laying hens depend on age, production phase, environmental temperature, strain, and the concentrations of Ca, P, and vitamin D (VD) in the diet (5). For example, qualitative traits of egg and tibia weight of laying hens fed 3.5% Ca (3.77 g/d) were higher than those fed 3.0% (3.29 g/d), but increasing Ca level to 4% (4.31 g/d) did not affect eggshell quality or tibia weight (12). Besides, a daily Ca requirement of 3.75 g was recommended more than two decades ago (13, 14). On the other hand, the optimum percentage of Ca for eggshell formation was cited to be 4.73% (15). Recent guidelines of breeder companies recommend a daily Ca intake of 4.10 g during the early egg production phase (19 wk), and this gradually increases with age to reach 4.4 g% for Lohaman Brown-Classic layers in the late phase of production at 65 wk or later (16). On the other hand, the NRC (17) recommends a daily intake of 3.75 g for white and brown eggshell layers.
Cholecalciferol (VD3) and its two metabolites are essential for the calcification of eggshell and bone and have both hormonal and immune influences (3, 4, 18). VD3 can be formed in the skin of the dermis and epidermis from 7-dihydrocholesterol under ultraviolet light or can be offered as a dietary component (3, 19). Nowadays, laying hens are reared under the extensive system and housed in closed-housing in cages for different perspectives, such as the increasing intensity of production, farming profits, production of clean eggs, and improving rearing conditions. Hens housed under these conditions are not exposed to adequate natural light to transform 7-dihydrocholesterol at levels appropriate for the sufficient synthesis of VD3. Thus, VD3 is usually added to layer feeds; these are critical for the homeostasis of calcium and to maintain laying performance, bone calcification, and eggshell formation (5, 14, 20). The biosynthesis of the active form 1, 25-dihydroxycholecalciferol (1,25-(OH)2D3) from VD3 was reviewed by Geng et al. (20). This occurred in the liver and kidney in two steps, mediated by 25-hydroxylase1 and α-hydroxylase enzymes (5).
The common level of VD3 in layer diets is about 2,200 IU/kg (21, 22), while the commercial egg breeders' guides recommend 2,500 IU/kg in diets (14), and 3,000–5,000 IU/kg is recommended by commercial producers (18). The Ca-binding protein implicated in the active transport of Ca across the intestinal wall requires VD3 (18, 22). In the literature, VD3 has many health, immunological, and physiological benefits (5), and it is a vital vitamin that plays a considerable role in the development of muscle, skeletal health, and in sustaining the homeostasis of calcium and phosphorus (18, 23, 24). Formation of eggshell and health of bone in laying hens is essential and involves the integration between the metabolism of Ca, P, and VD3 (5, 18, 25). Rodriguez-Lecompte et al. (26) and Manolagas et al. (27) have reported that VD3 or the active form of VD3, 25-(OH)D, both have strong immunomodulatory properties with a gradually ultimate help of T cells (Th2).
The commercial hybrid laying hens produce eggs at a higher rate due to increasing genetic potential and improvement in farming and nutrition strategies. Consequently, taken together, factors affecting the requirements of Ca and VD3 of laying hens need further investigation, particularly during the late phase of production, due to a decline in laying performance, the quality of eggs and shells, and physiological and immunological adaption. Hence, we hypothesized that the Ca and VD3 requirements of laying hens increased during late stage of production and increasing Ca and/or VD3 may improve production and quality of eggs and profits for laying hens farmers. Thus, this work examines the integration between different concentrations of Ca (3.5, 4.0, and 4.5%) and a (VD3) 800-, 1,000-, 1,200-IU/kg diet on the productive traits, blood biochemistry, and immune response of laying hens during the late phase of laying brown eggs.
Materials and Methods
The scientific committee of the Poultry Production Department, Faculty of Agriculture, Mansoura University, approved the present experiment. The protocol number was DF-715-155-1441 H. The committee recommended that care and handling of the animal maintained rights and welfare and minimized stress (Directive 2010/63/EU).
Laying Hens and Husbandry
The research was performed at the Poultry Research Unit, Qalabsho Center of Agricultural Researches and Experiments, Faculty of Agriculture, Mansour University, Egypt, from July to September 2018. This is the hottest period of the year in Egypt; the relative humidity and ambient temperature during the experimental period ranged between 54 and 70% and 22.8 and 35.6°C, respectively. The birds were exposed to hot weather conditions, and hens showed symptoms of high ambient temperatures, such as lying down in cages, wing flapping, and panting. Nonetheless, we did not discuss the heat stress in the Results and Discussion section, due to a lack of a control group for heat stress that was kept under optimum temperature (25°C).
360 H&N Brown Nick layers (60-wk-old) were assigned randomly into nine treatments, consisting of five replicates of eight hens. Hens were reared in open-sided laying cages (eight hens/cage with one feeder and two nipples); the size of each cage was 60 × 120 × 50 cm. The hens were submitted to a light program of 16:8 hrs light/dark cycle and received mash feed and tap water ad libitum.
Experimental Diets
Nine experimental diets, consisting of three levels of Ca (3.5, 4.0, and 4.5%) with three levels of supplemented vitamin D3 at the dosage of an 800-, 1,000-, 1,200-IU/kg diet or as total of 3,800, 4,000, and 4,200 IC VD3 were fed during the experimental period from 60 to 72 wk of age (Table 1). The control diet in this experiment was that contained 3.5% Ca level with 800 IU VD3.
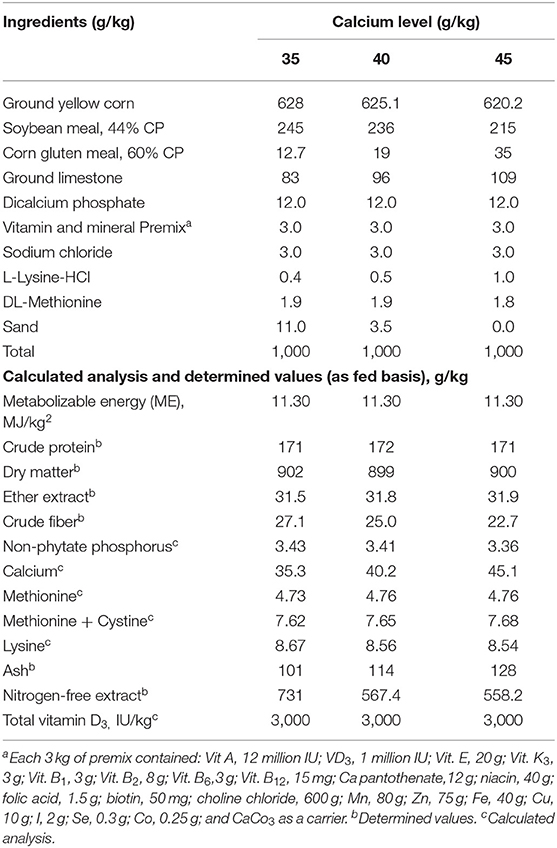
Table 1. Formulation and proximate analyses of the experimental diets (g/kg) on dry matter basis fed to Brown egg layers strain from 60 to 72 week of age.
The total VD3 amounts in the tested diets were 3,800, 4,000, and 4,200 IU/kg. The VD in feedstuffs is found in the form of VD2, which has a small activity for poultry (17); thus, it was not considered in the calculation of the total VD3 in the diet. The cholecalciferol VD3 was of powder feed grade, a product of Polifar Group Limited, Nanjing, Jiangsu, China. The diets were formulated according to NRC (17). The diets met or surpassed the nutrient needs specified in the H&N new management guide (28). The experimental diets were analyzed using the official methods of analysis (29) dry matter, crude protein, ether extract, crude fiber, and ash.
Laying Performance
Daily records of laying rate (LR), feed intake (DFI), egg weight (EW), egg mass (EM), and mortality rate were recorded and used to calculate the production indexes in a 28-day period as follows:
Bodyweight change (BWC) was also estimated from the differences between the initial and final body weights during the testing period.
Egg Quality Measurements
Eggs (30 eggs per treatment) were selected randomly at 72 wk of age to represent equally all replicates and used in the determination of the exterior and interior egg quality characteristics, as cited by Burke and Attia (30) and Attia et al. (31, 32). These characteristics involved EW and its comparative constituents (albumen, yolk, and shell), Haugh units, yolk color score (YCS), yolk index (YI), shell thickness (ST), and egg-shape index (ESI). The eggshell quality was measured at three different points of the shell in the mid-point and at the two ends of eggs, and the values was used for the calculation of the mean value of egg shell thickness. Shell weight per unit surface area (SWUSA) was determined by dividing shell weight by the egg surface area (ESA; cm2). These measurements were done as reported by Burke and Attia (30) and Attia et al. (31, 32).
Scanning electronic microscopy (SEM) images of the eggshells were taken according to Stefanello et al. (33) using two samples of the eggshell of each egg collected at 72 wk of age. The number of eggshells was one per replicate of each of the nine treatments. Eggshell free of shell membrane was obtained after breaking open the eggs. The shell membrane was removed by immersion of the samples in a solution of 0.15% sodium hydroxide, 4.12% sodium chloride, and 6% sodium hypochlorite. Tap water was used to wash the shells, which were then air-dried at room temperature (27°C). The images were made using 0.5 cm2 of the membrane-free eggshell from each replicate of each treatment using a Shimadzu SS-550 Super Scan instrument (Shimadzu Corporation, EVISA, Kyoto, Japan).
Blood Parameters
Five milliliter blood samples were collected from wing vein of six hens per group of 72-wk-old in two blood tubes, with and without heparin. The hens used for measuring blood constituents were selected with hard shell eggs in the uterus. Blood samples were centrifugated at 1,716 g for 15 min to separate the plasma and serum, which were kept at 20°C until analysis. Serum total protein, albumin, globulin, triglycerides (Trig.), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL-C) were determined. Plasma calcium (Ca) and inorganic P (Pi), total antioxidant capacity (TAC), malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were measured. Aspartate aminotransferase (AST); Alanine aminotransferase (ALT); alkaline phosphate (AlkP); α-, β-, and γ-globulin; immunoglobulin IgG, IgM, IgA, estrogen, and parathyroid hormone (PTH) were also determined. The measurements were made using commercial diagnostic kits (34) as cited by Attia et al. (35, 36). The antibody titer for avian influenza and Newcastle disease virus were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits as cited by Attia et al. (35, 36).
Reproductive Inner Organs
Five hens were randomly selected from each treatment and slaughtered according to Islamic methods (35). After complete bleeding, the hens were opened, and the ovary and reproductive tract were dissected. Then the weight of the ovary and oviduct parts (infundibulum, magnum, isthmus, and uterus) were recorded, and their proportions relative to the live bodyweight of the hens were estimated.
Statistical Analysis
The analytical processing of results was performed by using a two-way analysis of variance (Ca, VD3, and their interaction) of the GLM procedure of the Statistical Analysis System (37) using the replicate as the experimental unit. Data were transformed to arcsine to normalize the distribution. Differences between means were tested using the Student–Newman–Keuls test at P < 0.05 (37). The P-values between 0.10 and <0.05 were considered as a trend.
Results and Discussion
Laying Hens Performance
Table 2 shows the influence of Ca and/or VD3 level on the production traits of laying hens during the late phase. Initial BW of laying hens was not different between the experimental groups, indicating a random distribution of hens; thus, data were not presented. The results suggest that Ca and/or VD3 levels had a significant effect on most laying performance traits, except for feed intake. EW was gradually increased with increasing Ca levels. The interaction effect indicates that increasing Ca level from 3.5 to 4 or 4.5% similarly improved LR, EM, FCR, EPI, and BWG. Increasing the Ca level from 3.5 to 4% did not influence EW, but a further increase to 4.5% increased EW; thus, the difference between 3.5 and 4.5% Ca groups was significant. These results reveal that 4% Ca was adequate for the performance of layers of 60–72 wk of age and confirmed the suggested Ca requirements (4.1% during 45–70 wk of age) in the H&N management guide (28) and those by Rao and Roland (38) and Zhang and Coon (39). These may differ due to the role of Ca in regulating the reproductive hormones and ovary growth (5). Nascimento et al. (40) found that there was no interaction between the different sources of vitamin D at a 2,000-IU/kg diet from VD3, 25-(OH)D3, 1,25-(OH)2D3, and four calcium levels from 2.85 to 5.25%, with intervals of 0.80% in all egg production traits. However, a significant quadratic component of the contrast analysis for the level of calcium in LR and FCR was observed, showing better productive characteristics at 4.12 and 4.09% Ca, respectively. In contrast, the level of Ca did not affect the EW. Nonetheless, the source of VD3 influenced (P < 0.05) LR, FCR, and EW, showing improved results of laying hens with 25-(OH)D3 and cholecalciferol (40).
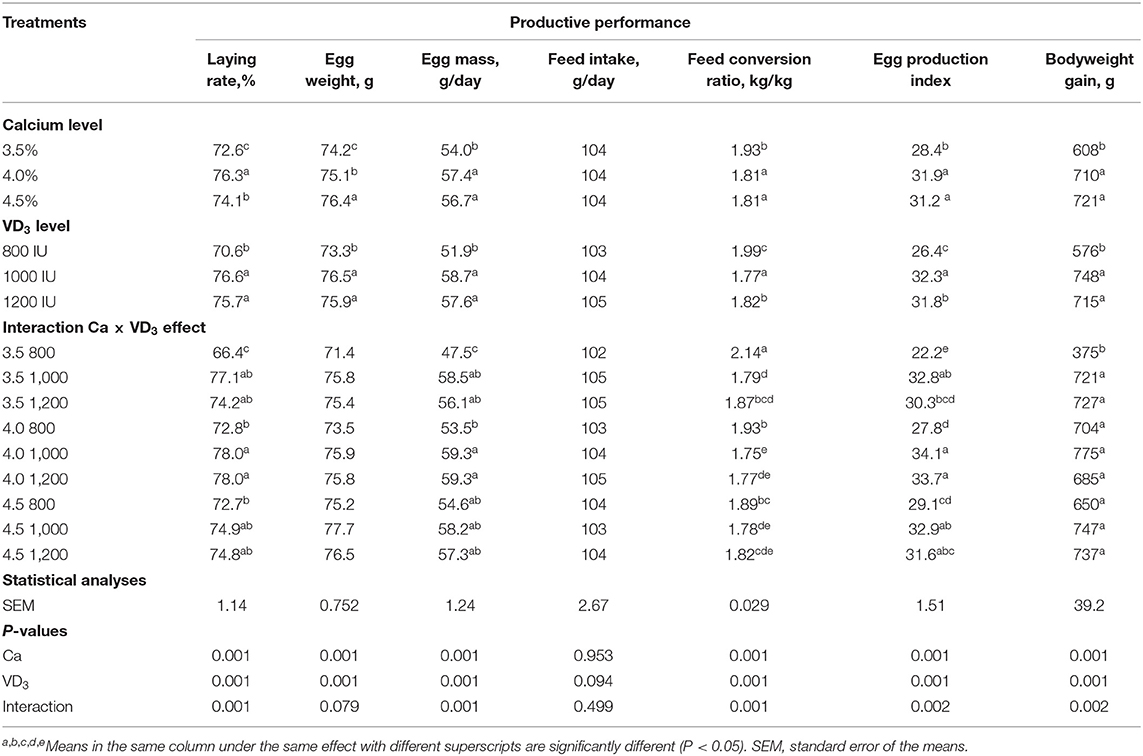
Table 2. Productive performance of brown egg layers fed three levels of Ca, supplemented with three levels of VD3 from 60 to 72 wk of age.
It was observed that of VD3 at a 1,000- and 1,200-IU/kg diet to 3.5 and 4% Ca diets significantly increased LR, EM, and improved FCR. In addition, VD3 supplementation at a 1,000- and 1,200-IU/kg diet to 3.5% Ca markedly increased the BWG of laying hens (Table 2). Increasing VD3 to 1,000 IU significantly increased EW, and the effect was saturated at this level. The addition of VD3 at 1,000 and 1,200 IU to different Ca levels increased the EPI of hens on different Ca levels except for hens fed 4.5% Ca supplemented with a 1,200-IU/kg diet. These results reveal that supplementation with 1,000 IU of VD3/kg diet or a total of 4,000 IU/kg is adequate for egg production traits of hens fed 3.5% Ca level.
The present observations indicate that VD3 content in the diet containing 3.5% with a total of VD3 at 3,000 IU/kg (Table 1) was not adequate for H&N Brown laying hens in the late phase. It should be mentioned that the H&N Brown nutrition management guide recommends 2,500 IU for laying during the laying period with no specific recommendations and/or considerations for each stage of laying. In addition, 3,000–5,000 IU of VD3 for laying hens during different stages of production was recommended (18). It is evident that as the metabolism of nutrients decreases with age (5, 41), the VD3 requirements should be reinvestigated in relation to stage of laying considering the impact of aging on VD3 requirements and due to VD3 and its metabolites playing a vital role in the uptake, deposition, and excretion of Ca (5, 21, 42). Likewise, other authors (5, 24, 27) showed similar findings. The VD3 is essential for the formation of Ca-bound proteins, which are involved in the active transport of Ca across the intestine (28), and in lipoprotein, the precursor of yolk formation (5, 20, 21).
Egg Quality Traits
The results indicate that Ca and the interaction between Ca and VD3 levels has significant effects on egg quality traits (Table 3).
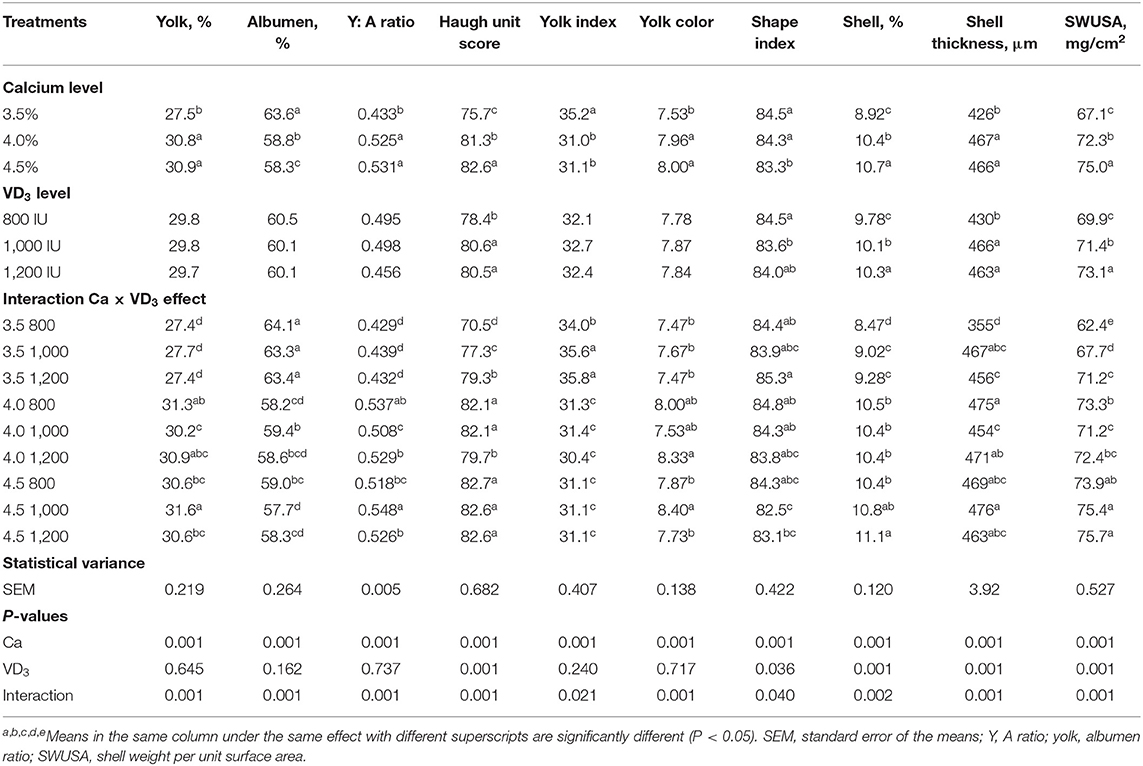
Table 3. Exterior and interior egg quality traits of brown Egg layers fed three levels of Ca supplemented with three levels of VD3 from 60 to 72 wk of age.
Substantial effects of VD3 on only the Haugh unit score, shape index, and shell quality traits were recorded. Similarly, VD sources such as VD3, and 25-(OH) D3 at 2,000 IU increased the Haugh unit score, following Nascimento et al. (40).
The interaction between Ca level and VD3 reveals that increasing the Ca level from 3.5 to 4 or 4.5% markedly and similarly increased the percentage of yolk, yolk:albumen ratio, Haugh unit score, shell percentage, ST, and SWUSA. The results indicated that 4% Ca was adequate for improving egg quality characteristics during the late stage of egg production and increasing the Ca level to 4.5% had no positive effects. The improvement in egg and shell quality traits of hens fed 4% Ca suggests an increased Ca availability for eggshell formation, in accordance with other researchers in this field (16, 40). This may be due to the role of Ca in regulating the reproductive hormones and ovary growth and its vital role in the eggshell formation and as a critical agent for preserving egg quality (5, 16, 21). It was cited that every increase in the quantity of available Ca may improve shell quality and bone-breaking strength, with the effect being linear (40, 43).
The supplementation with VD3 at 1,000 IU to 4% Ca diet substantially reduced the yolk percentage of hens on 4% Ca, compared to the same Ca group with 800 IU of VD3, and yolk:albumen ratio, compared to 800 and 1,200 IU. However, 1,000 IU of VD3 increased percentage of yolk, yolk:albumen ratio, and yolk color of those fed 4.5% Ca compared to the other groups at the same Ca level. The yolk index was significantly increased due to supplementation of 3.5% Ca with 1,000 and 1,200 IU VD3 compared to 800 IU added to the same level of Ca.
Albumen percentage showed a marked increase due to VD3 supplementation at 1,000 IU to 4.0% Ca, compared to the 800 IU of VD3 supplemented to the same level of Ca. On the other hand, 1,000 IU of VD3 significantly decreased albumen percentage of hens fed 4.5% Ca, compared to 800 IU of VD3 added to the same level of Ca. These changes are contrary to the changes showed in yolk percentage.
Haugh unit, the mirror of albumen quality, showed a considerable increase stepwise, with increasing VD3 addition to 3.5% Ca diet, but 1,200 IU of VD3 to 4% Ca diet significantly decreasing Haugh unit, compared to the other levels of VD3 supplemented to the same level of Ca. The effect of VD3 is dependent on concentrations of Ca level. The highest egg shape index was from hens fed 3.5% Ca level supplemented with 1,200 IU while the smallest was from hens fed 4.5% Ca supplemented with 1,000 IU of VD3. The fortification with VD3 at 1,000 and 1,200 IU to 3.5% Ca feed substantially increased the percentage of the shell and shell thickness similarly compared to those on 800 IU of VD3. The increase in the SWUSA was linear, maybe due to the correction for egg surface area (31, 32).
The effect of VD3 on eggshell quality of hens fed adequate Ca levels (4 and 4.5%) was less pronounced and depended on the type of measurements. These results demonstrated that the impact of VD3 on eggshell quality depends on dietary Ca level being more pronounced at inadequate Ca intake, according to other research (5, 21, 27). VD metabolites are essential for Ca-binding protein that involved in the active transport of Ca across the intestinal wall, which is essential for eggshell formation (5, 21, 23). The formation of Ca-binding proteins in different tissues (intestine, kidney, and uterus) required VD metabolites at both stages of transcriptional and post-transcriptional. Calcium-binding proteins enhance the absorption of calcium in the gut, recovery from the urine, and shell deposition (17, 20).
The electronic microscope images (Figures 1–9) showed an increase in ultra-structure of eggshell due to increasing Ca level and VD3 supplementation, as manifested by the different distribution of mammillary buttons, which became larger as the dietary levels of Ca and VD3 increased (Figures 2–9). The eggshell, evaluated by electronic microscopy, consists of several morphologically different regions and also contains thousands of gas exchange pores. The outermost layer is the cuticle that acts as a physical barrier to water and bacterial contamination. The specific nucleation sites on the outer surface of the outer shell membrane attract calcium salts for the formation of the calcified layers (cone or mammillary layer), which may be influenced by the enzymatic activity, and trace minerals act as cofactors of such a process. Each palisade column grows from one mammillary button, and as the calcification mechanism proceeds, they provide greater resistance to the shell (44).
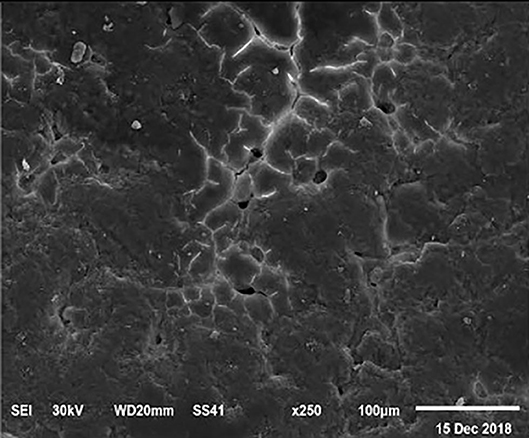
Figure 1. SEM photo of the eggshell of laying hens fed the control diet of 3.5% Ca with 800-IU VD3/kg diet. The figure shows a low density of mammillary buttons, which results in a reduction of strength, according to the significantly lowest eggshell thickness and SWUSA (P < 0.05) recorded in this experimental group. It is important to underline the great relative interstitial area between mammillary formations, which makes the egg more susceptible to breaking along these cracks.
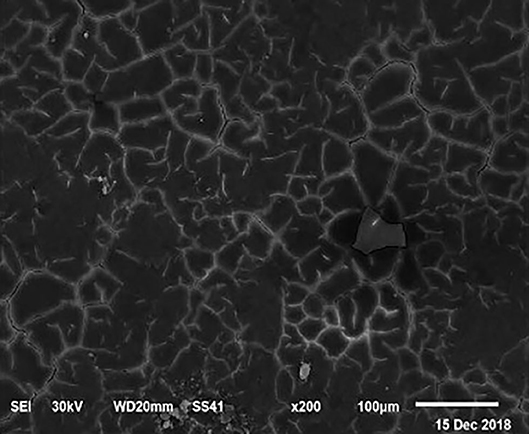
Figure 2. SEM photo of the eggshell of laying hens fed a diet containing 4.0% Ca with 800-IU VD3/kg diet. Compared to Figure 1, a slight increase in the number of mammillary buttons associated with an increase of the larger size can be detected.
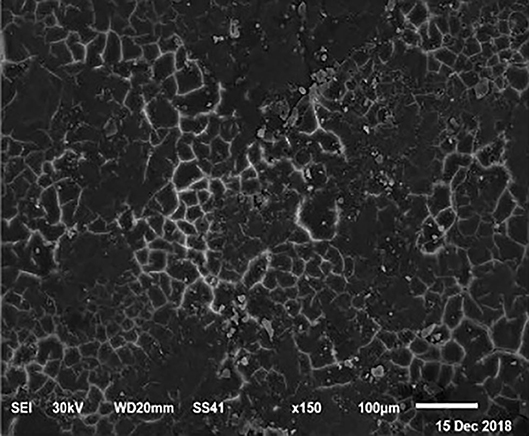
Figure 3. SEM photo of the eggshell of laying hens fed a diet containing 4.5% Ca with 800-IU VD3/kg diet. It is possible to observe an increase in the number of mammillary buttons, compared to Figure 1, by increasing only the levels of Ca.
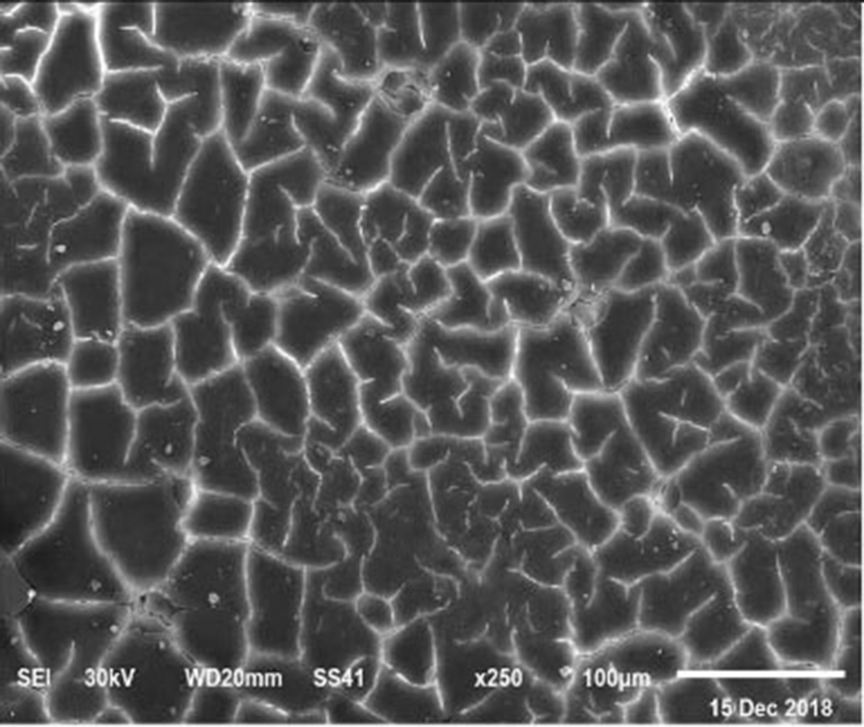
Figure 4. SEM photo of the eggshell of laying hens fed a diet containing 3.5% Ca with 1,000-IU VD3/kg diet. This figure shows a similar mammillary buttons distribution, but the interstitial area seems less evident than in Figure 1.

Figure 5. SEM photo of the eggshell of laying hens fed a diet containing 4.0% Ca with 1,000-IU VD3/kg diet. In this figure, it is possible to observe a greater relative interstitial area between mammillary formations that negatively influences the strength of the eggshell. The higher interstitial area is an indicator of the degree of elasticity of the eggshell.
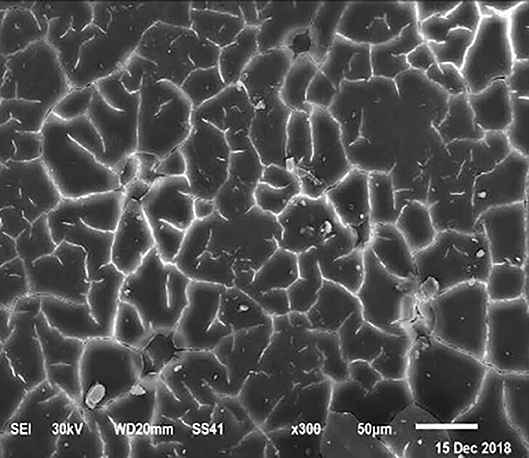
Figure 6. SEM photo of the eggshell of laying hens fed a diet containing 4.5% Ca with 1,000-IU VD3/kg diet. Compared to Figure 5, a reduction of the interstitial area between mammillary buttons was observed, but no differences were detected concerning the mammillary buttons density.
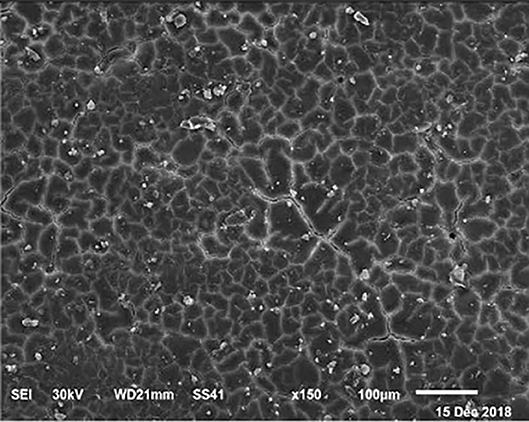
Figure 7. SEM photo of the eggshell of laying hens fed a diet containing 3.5% Ca with 1,200-IU VD3/kg diet. In this figure, it is possible to observe that rather than Ca percentage, VD3 is responsible for the size of mammillary buttons.
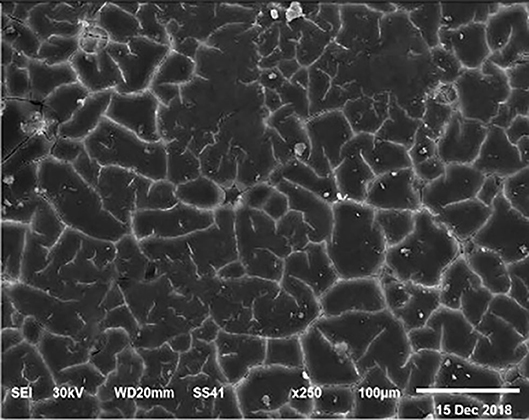
Figure 8. SEM photo of the eggshell of laying hens fed a diet containing 4.0% Ca with 1,200 IU-VD3/kg diet. In this figure, it is possible to observe the highest density and percentage of mammillary buttons in the eggs from laying hens fed the diet with the 4.0 and 4.5% Ca and 1,200 IU VD3, according to the highest (P < 0.05) shell thickness and SWUSA.
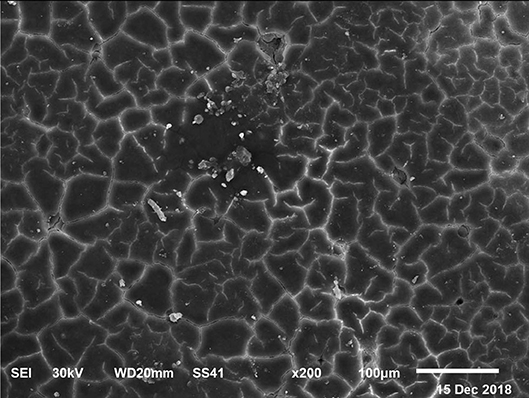
Figure 9. SEM photo of the eggshell of laying hens fed a diet containing 4.5% Ca with 1,200-IU VD3/kg diet. In this figure, it is possible to observe the highest density and percentage of mammillary buttons in the eggs from laying hens fed the diet with the 4.0 and 4.5% Ca and 1,200 IU VD3, according to the highest (P < 0.05) shell thickness and SWUSA.
The increased strength of shells, as well as the reduction in the egg loss, is an important goal that has economic importance in commercial terms. The quality of the eggshell has been considered to be affected by organic components particularly protein (9, 10) and the inorganic components (45). Therefore, the palisade layer comprises approximately two-thirds of the entire thickness of the shell (46). In this regard, the palisade layer showed a linear reduction in the number of mammillary buttons in the eggshell of hens fed, increasing Mn, Zn, and Cu levels (33). As reported by Stefanello et al. (33), supplementation with organic trace elements can exert an influence on the formation of the palisade layer, resulting in larger mammillary buttons. Our results showed that the group fed a diet containing 3.5% Ca supplemented with 800 IU VD3 had a clutter on the distribution of mammillary buttons on the inner surface of the shell (Figure 1). From these results, we can speculate that the palisade layer and the number of mammillary buttons present in the shell may also influence the quality of the shell.
Plasma and Tibia Minerals Content
Table 4 shows the influence of dietary Ca and VD3 on plasma minerals and tibia characteristics. Dietary Ca and/or VD3 levels did not affect plasma Ca, Pi, and Ca:Pi levels.
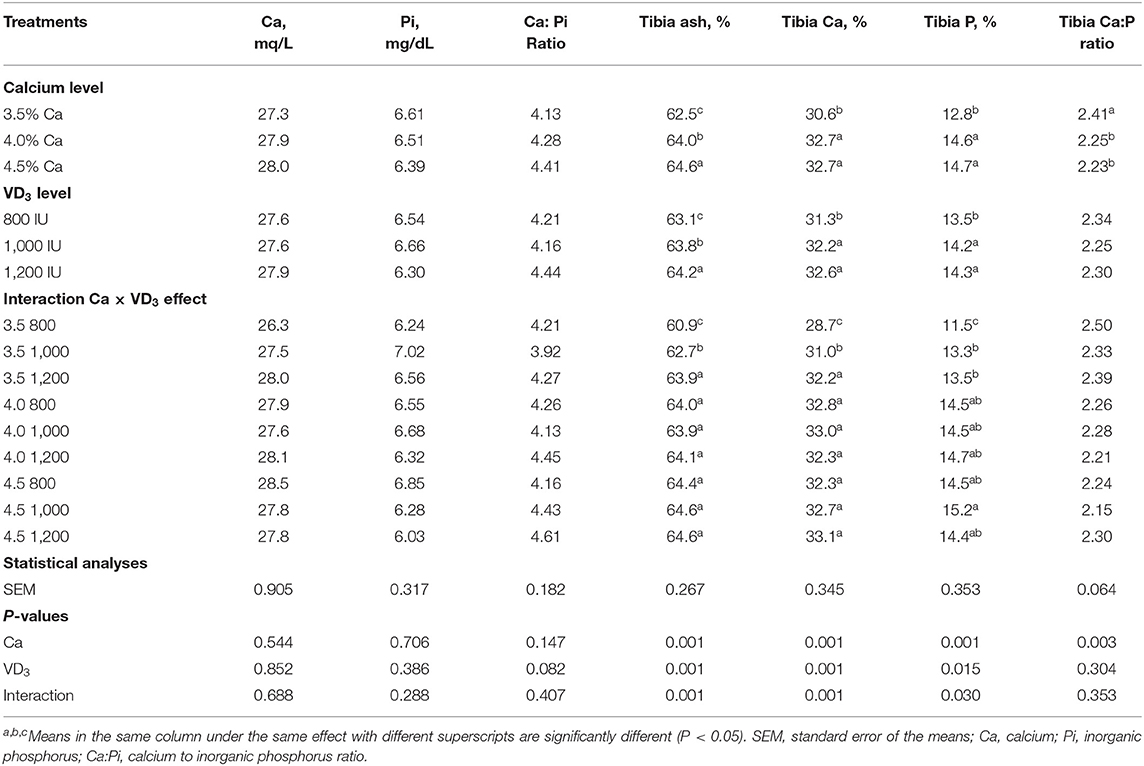
Table 4. Blood plasma minerals and tibia characteristics of 72-wk-old brown egg layers fed three levels of Ca supplemented with three levels of VD3 from 60 to 72 wk of age.
This indicates that Ca and VD3 in the control diets (3.5% Ca and 800 IU of supplemented VD3 or as a total VD3 at a 3,800-IU/kg diet) were adequate to maintain plasma Ca and Pi concentation at normal levels. Also, Nascimento et al. (20, 40) demonstrated that there were no effects of VD sources at 2,000 IU from VD3, 25- (OH) D3, and 1, 25 (OH)2 D3 on plasma Ca. Also, Susanna et al. (47) observed that dietary VD3 at 3,000 IU from a single source or at 1,500 IU of each VD3 and 25 (OH) D3 did not affect plasma Ca level, but decreased plasma Pi of 34-wk-old laying hens. This may be due to hormonal control of Ca and Pi via parathyroid and calcitonin (21, 41, 48).
It was found that ash, Ca, and Pi were affected significantly by dietary Ca and VD3 levels, but the impact interfered with the interaction between the two variables. Also, tibia Ca: Pi was affected only by nutritional Ca levels, showing that the Ca:Pi ratio was significantly similarly decreased due to Ca increasing above 3.5%. Increasing Ca level from 3.5 to 4 and 4.5% within the unsupplemented diets significantly and similarly increased tibia ash, Ca, and Pi, showing response saturation at 4% Ca. This suggests that 4% Ca was adequate for bone calcification (5, 37–41). The supplementation of the 3.5% Ca level with VD3 at 1,000 and 1,200 IU significantly increased tibia ash and Ca in a stepwise manner and similarly increased Pi. This indicates that supplementation with VD3 is beneficial for laying hens from 60 wk of age on for bone calcification (16, 20). These may be due to the role of VD3 and its metabolites in the bone matrix (20, 41). It was observed that increasing the VD3 supplementation to 1,000 and 1,200 IU within 4 and 4.5% Ca levels did not affect tibia ash and Ca and Pi contents. These results indicate that the response to VD3 depends on the dietary Ca levels (5, 14).
Serum Lipid Metabolites
The effect of different dietary Ca and/or VD3 levels on most of the blood serum lipid metabolites was not significant, except for triglycerides, vLDL, and triglyceride:HDL ratio and approached significant for HDL (Table 5).
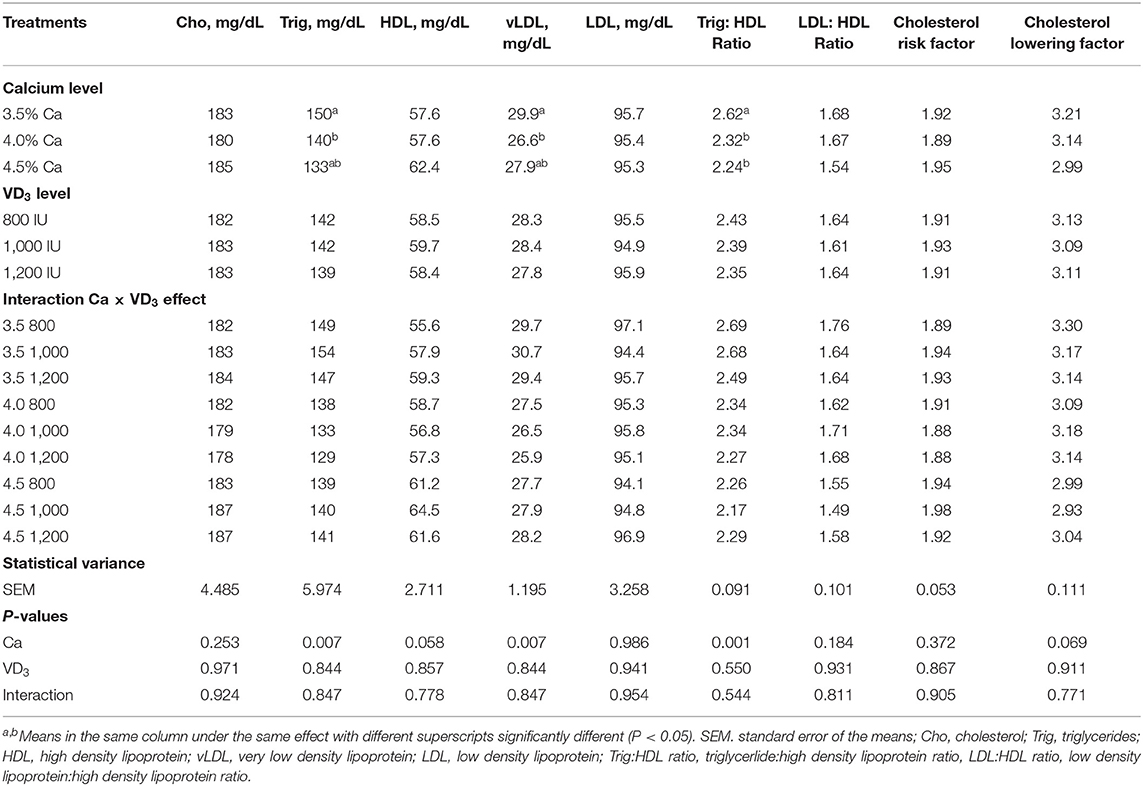
Table 5. Blood serum lipid metabolites of brown egg layers fed three levels of Ca supplemented with three levels of VD3 from 60 to 72 wk of age.
The results demonstrated that Ca levels at 4% considerably decreased triglycerides and vLDL compared to 3.5% Ca. Besides, 4 and 4.5% Ca concentrations substantially decreased serum triglyceride:HDL, compared to 3.5% Ca. Also, this associated with an increase in HDL with increasing Ca level. These decreases could be attributed to the increase in the daily egg mass output of hens fed 4 and 4.5% Ca, due to the use lipoproteins for yolk formation. Lipoproteins, particularly very low density lipoprotein yolk (VLDLy) were formatted under the influence of estrogen. The yolk precursor is synthesized in the liver and transported to the ovary for yolk formation, which would be increased with increasing LR (1, 26, 49).
Serum Protein Fractions and Liver Index Enzymes
Table 6 displays the impact of different Ca and VD3 levels on serum protein fractions and liver indices for leakage enzymes. There were no effects of different Ca levels on serum albumin, AST, ALT, and alkaline phosphatase. The increase in Ca level induced a similar increase in the total serum protein, globulin (specific immune protein) and α-2-globulin compared to 3.5% Ca, but similarly decreased the serum albumin (non-specific immune agent)/globulin ratio and the AST:ALT ratio. These results indicate that Ca is an essential element for the immunity of laying hens, as manifested by the increase in serum total protein and globulin (50–52). Ca deficiency in laying hens can cause bone diseases such as cage layer fatigue and osteoporosis (1, 5, 18, 26).
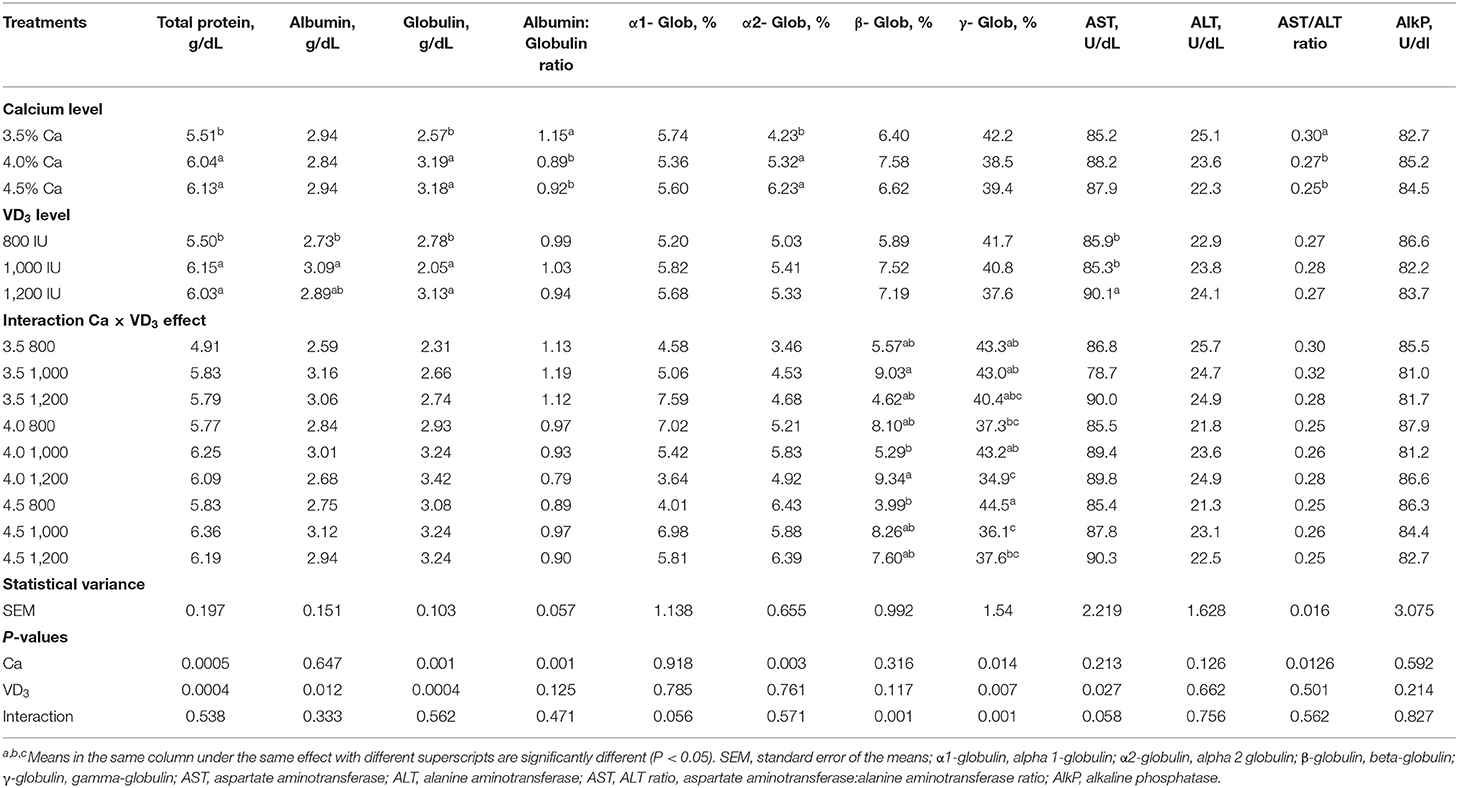
Table 6. Blood serum protein fractions and indices of liver leakage enzymes of brown egg layers fed three levels of Ca, supplemented with three levels of VD3 from 60 to 72 wk of age.
Dietary VD3 did not influence the albumin/globulin ratio, α-1- and α-2-globulin, β-globulin, γ-globulin, ALT levels, the AST:ALT ratio, and alkaline phosphatase level. Besides, the serum protein fractions and liver index leakage enzymes are not affected by the interaction between the two variables.
It was found that increasing VD3 to a 1,000 and 1,200-IU/kg diet substantially increased total protein and globulin, compared to an 800 and 1,000 IU/kg diet, increased serum albumin compared to an 800 IU/kg diet. The immunomodulatory, anti-inflammatory, and anti-coccidia activity of VD3 or its metabolites have been reported in chickens (20, 23–26, 51, 52) and chicken cells (25, 27, 28). The antibody is proteomic in nature, and the increase in total protein and globulin levels supported the hypothesis that increasing Ca, and VD3 improves the immunity of laying hens, perhaps due to the protection from bone disease [rickets, osteoporosis, and cage layer fatigue (20, 41, 53)]. VD has an essential task in maintaining immunity and communication between the adaptive and innate immunity systems by affecting vitamin receptors of VD and activating enzymes (20, 54, 55).
The interaction effect indicates that the highest β-globulin was seen in groups fed 3.5 and 4% Ca, supplemented with 1,000 and 1,200 IU VD3, respectively, while the lowest values were from hens fed 4 and 4.5% Ca, supplemented with 1,000 and 800 IU VD3, respectively.
The γ-globulin, the material antibody, was significantly higher in hens fed 4.5% Ca than in hens fed 4% Ca when the unsupplemented groups were compared. Besides, supplementation of 4% Ca with 1,200 IU VD3 significantly decreased γ-globulin, compared to 1,000 IU VD3 supplementing the same Ca concentration. Also, supplementation of 4.5% Ca with 1,000 and 1,200 IU VD3 significantly decreased serum γ-globulin compared to 800 IU supplementation of the same Ca level.
Immunoglobulin and Antibody Titer
Table 7 shows the effect of different dietary Ca and/or VD3 on blood plasma antioxidant enzymes and antibody titer. There was no effect of Ca level on most of the evaluated immune responses and antioxidant indices, except for serum IgG and IgA, plasma estrogen, and catalase (CAT). The results indicate that increasing Ca levels to 4 and 4.5% similarly increased IgG compared to 3.5% and raising the Ca level caused a gradual increase in serum IgA. Dietary Ca concentration affects plasma estrogen and Ca and VD3 influence CAT, but the effect was confounded by the significant interaction between dietary Ca and VD3 levels.
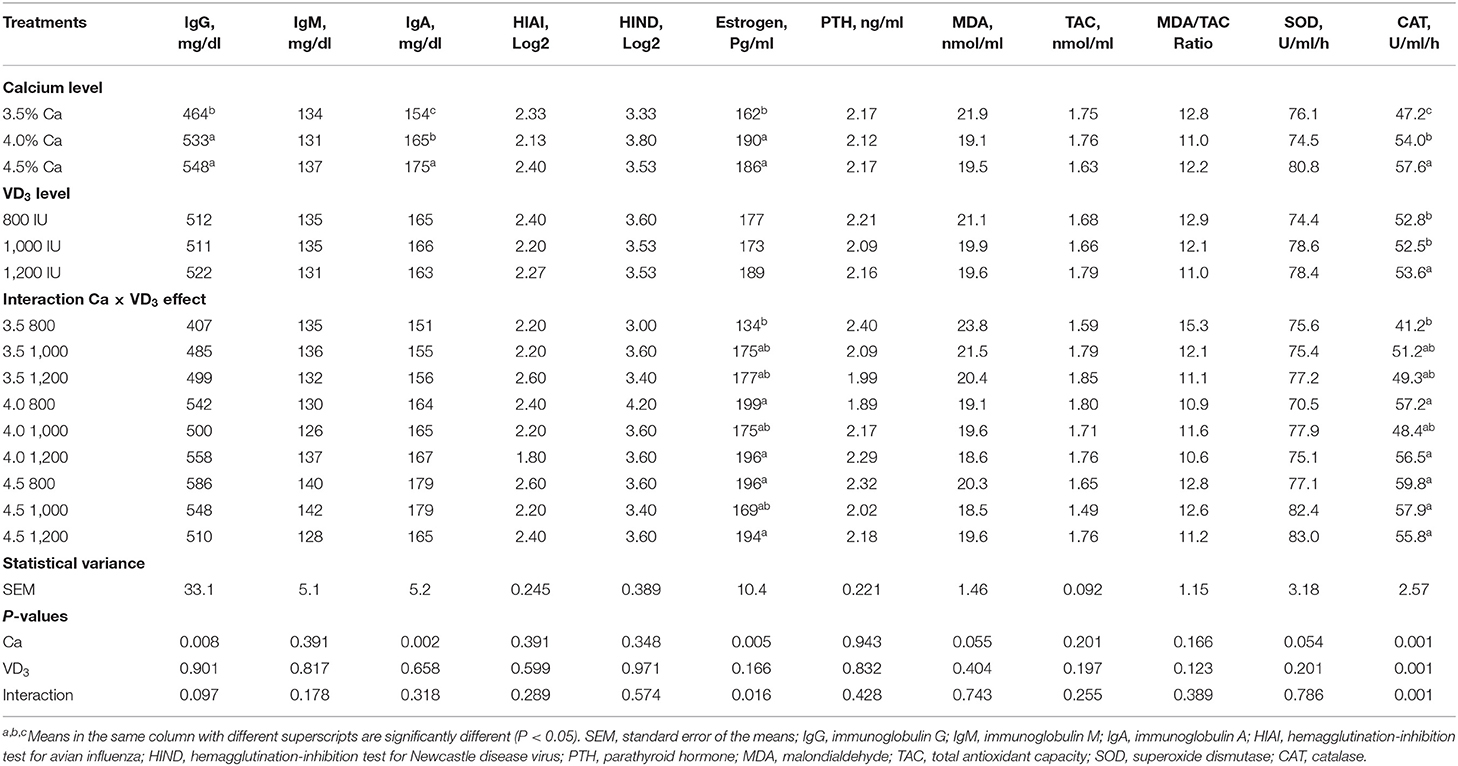
Table 7. Blood serum immunity parameters and some hormones of brown egg layers fed three levels of Ca supplemented with three levels of VD3 from 60 to 72 wk of age.
The lack of significant effects of VD3 on most immune responses (the type of globulin, immunoglobulins, and antibody titer), rather than total serum protein, albumin, and globulin, may indicate that the control diet supplemented with 800 IU, or a total VD3 of 3,800 IU, contains adequate VD3 to maintain the basic immune function. This may offset the effect of supplemented cholecalciferol on cellular and humoral immunity. This was further established by the lack of bone disease such as osteoporosis and cage layer fatigue in laying hens kept in cages under hot weather conditions. Such disorders indicate inadequate dietary VD3 (1, 20, 26).
In the literature, VD3 is reported to have many properties, such as antioxidant, immunomodulatory, anti-inflammatory, antiviral, antibacterial, anti-allergy, and cancer prevention activities (10–12). In poultry, the role of VD3 in Ca and Pi metabolism is fundamental for the development of bone and eggshell formation. In this regard, Rodriguez-Lecompte et al. (26) demonstrated that both VD3 and 25-(OH) D have strong immunomodulatory effects, such as increased positive helper T cell (Th2) response and autophagy. Laying hens kept under high environmental temperatures, such as those observed herein, are highly susceptible to infectious diseases, due to low immunity (49). Aslam et al. (56) found that VD deficiency decreases the cellular immune response in broilers. The literature reports that VD3 or 25-hydroxycholecalciferol has an anti-inflammatory activity in bird immune cells following the administration of lipo-oligosaccharides [LPSs (20, 57–60)]. Despite this, little attention has been paid to the influence of dietary VD3 supplementation or its deficiency on immune response and blood biochemistry of laying hens challenged with LPSs, or the mechanisms of action, although the anti-cancer and anti-inflammatory effects of VD3 are well-documented (17, 61–64).
Plasma Hormones
The effect of Ca level and interaction between Ca level and VD3 was seen on levels of plasma estrogen (Table 7). It is clear that increasing Ca level from 3.5 to 4 or 4.5% increased plasma estrogen. Plasma PTH was not influenced by dietary Ca and/or VD3 concentrations. This indicates that the control diet (3.5% Ca and 3,800 IU VD3) contains adequate Ca and VD3 to maintain the normal level of PTH, which, together with 1,25-(OH)2D3, and controls calcium absorption in the digestive canal and calcium resorption from the bones and excretion to maintain Ca balance (1, 21, 26).
It was found that increasing Ca levels to 4 and 4.5% within the unsupplemented groups significantly, and similarly, increased plasma E2 compared to the 3.5% level. There were no significant changes in plasma estrogen within each Ca level or across different levels of Ca as a result of supplementation with VD3 when the corresponding levels were compared. The lowest E2 level was in hens fed 3.5% Ca supplemented with 800 IU of VD3, while the highest was from hens fed 4 and 4.5% Ca, supplemented with 800 and 1,200 IU of VD3. These findings demonstrate that a Ca level of 4% was adequate when supplemented with 800 IU of VD3 to enhance E2. The metabolism of Ca in laying hens is controlled by E2, PTH, and calcitonin (5, 21), and the increase in E2 was associated with increasing performance and eggshell quality (Table 7).
Antioxidant Status
No effect was found on most of the antioxidant indices (TAC, MDA, and SOD) with Ca levels, except for CAT. Dietary Ca and VD3 concentration affected plasma CAT, but the effect was confounded by the significant interaction between dietary Ca and VD3 levels (Table 7). It was found that increasing the VD3 level to 1,000 and 1,200 IU in the 3.5% Ca group increased CAT to some extent. There no significant difference in CAT levels among different VD3 levels or within or between different Ca levels. These results show that the impact of VD3 on CAT depends on the dietary Ca concentration. Hence, antioxidant enzymes such as CAT need an adequate quantity of Ca and VD3 to be maintained for laying hens of 60–72 wk of age.
Ovary and Reproductive Organs
The results of the relative weight of ovary and reproductive organs as affected by different Ca and VD3 concentrations are presented in Table 8.
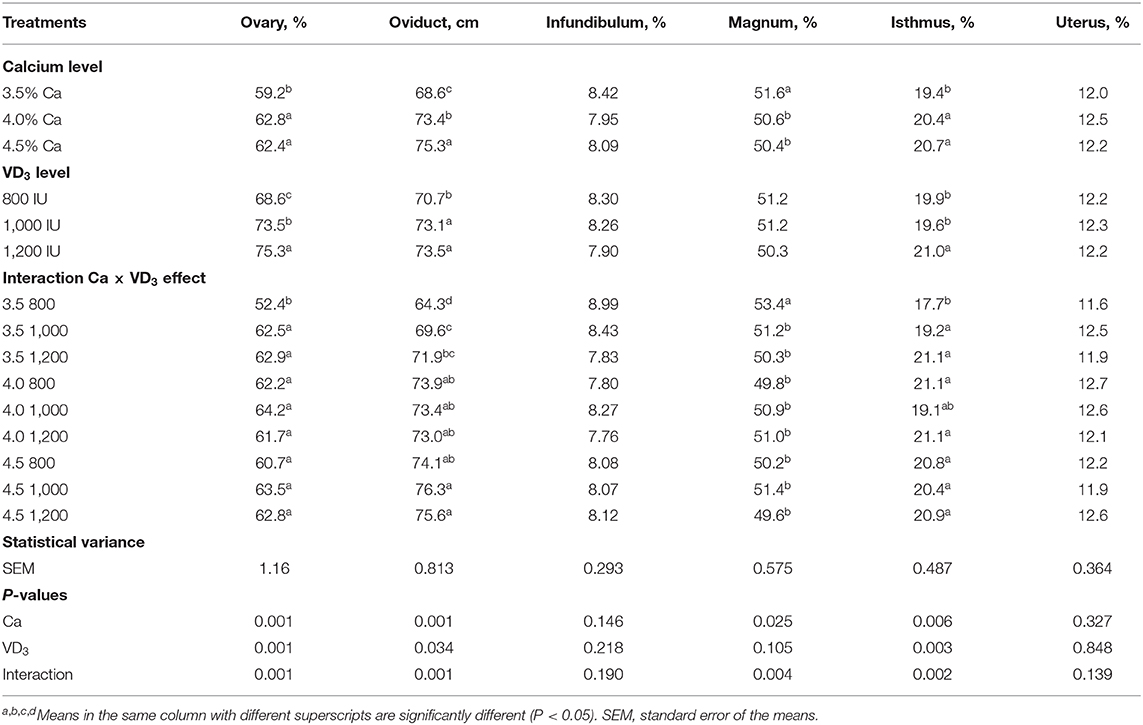
Table 8. The length of parts of the female reproductive system of brown egg layers fed three levels of Ca supplemented with three levels of VD3 from 60 to 72 wk of age.
The Ca level in diet had an impact (P < 0.05) on the percentage of the ovary, magnum, the isthmus, and the absolute oviduct length (cm), and VD3 concentrations showed a marked influence on the relative weight of the ovary and isthmus and the oviduct length. These impacts were confounded by the interaction between Ca and VD3.
The results of the interaction indicate that increasing Ca levels with the groups supplemented with 800 IU of VD3 significantly increased the relative weight of ovary and isthmus and absolute length of the oviduct, compared to the control diet containing 3.5% Ca and 3,800 IU of total VD3, but decreased the magnum percentage. It was found that elevating the VD3 concentrations to 1,000 (4,000 IU as total VD3) and 1200 IU (4,200 IU as total VD3) within the 3.5% Ca level similarly elevated the relative weights of ovary and isthmus and the absolute oviduct length, but decreased the relative weight of the magnum. These changes in ovary and oviduct, except for the magnum, reflected the positive changes in E2 and enhanced the laying performance of hens fed 3.5% Ca when supplemented with 1,000 or 1,200 IU of VD3.
In conclusion, increasing dietary Ca levels in laying hens up to 4% during the late production phase could be a useful tool to improve laying performance, eggshell quality, Haugh unit, and physiological and immunological status. Besides, supplementing 3.5% Ca diets with 1,000 IU of VD3 or a total 4,000 IU/kg diet VD3 improved performance of hens fed 3.5% Ca level during late stage of production (60–72 wk of age), showing that the impact of VD3 depends on dietary Ca concentrations.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The experimental procedures were approved by King Abdulaziz University, Jeddah, Saudi Arabia under protocol number (DF-715-155-1441H) that recommends animal rights, welfare, and minimal stress and did not cause any harm or suffering to animals according to the Royal Decree number M59 in 14/9/1431H.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant No. (DF-715-155-1441 H).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully acknowledge the DSR, King Abdulaziz University for technical and financial support.
References
1. Oluyemi JA, Roberts FA. Poultry Production in Warm Wet Climates. 2nd ed. Ibadan: Spectrum Books Limited (2000).
2. Pizzolante CC, Saldanha ESPB, Lagana C, Kakimoto SK, Toghashi CK. Effect of calcium levels and limestone pareticle size on the egg quality of semi-heavy layers in their second produc-tion cycle. Braz J Poult Sci. (2009) 11:79–86. doi: 10.1590/S1516-635X2009000200002
3. Saunders-Blades JL, Korver DR. Effect of hen age and maternal vitamin D source on performance, hatchability, bone mineral density, and progeny in vitro early innate immune function. Poult Sci. (2015) 94:1233–46. doi: 10.3382/ps/pev002
4. Keshavarz KA. Comparison between cholecalciferol and 25-OH-cholecalciferol on performance and eggshell quality of hens fed different levels of calcium and phosphorus. Poult Sci. (2003) 82:1415–22. doi: 10.1093/ps/82.9.1415
5. Roland, DA. Egg shell quality III: calcium and phosphorus requirements of commercial Leghorns. Worlds Poult Sci J. (1986) 42:154–65. doi: 10.1079/WPS19860012
6. Al-Batshan HA, Scheideler SE, Black BL, Garlich JD, Anderson KE. Duodenal calcium-uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult Sci. (1994) 73:1590–6. doi: 10.3382/ps.0731590
7. Saunders-Blades JL, MacIsaac JL, Korver DR, Anderson DM. The effect of calcium source and particle size on the production performance and bone quality of laying hens. Poult Sci. (2009) 88:338–53. doi: 10.3382/ps.2008-00278
8. Gregory NG, Wilkins LJ. Broken bones in domestic fowl: handling and processing damage in end-of-lay battery hens. Br Poult Sci. (1989) 30:555–62. doi: 10.1080/00071668908417179
9. Marie P, Labas V, Brionne A, Harichaux G, Hennequet-Antier C, Nys Y, et al. Quantitative proteomics and bioinformatic analysis provide new insight into protein function during avian eggshell biomineralization. J Proteomics. (2015) 113:178–93. doi: 10.1016/j.jprot.2014.09.024
10. Marie P, Labas V, Brionne A, Harichaux G, Hennequet-Antier C, Rodriguez-Navarro AB, et al. Quantitative proteomics provides new insights into chicken eggshell matrix protein functions during the primary events of mineralisation and the active calcification phase. J Proteomics. (2015) 126:140–54. doi: 10.1016/j.jprot.2015.05.034
11. Attia YA, Al-Harthi MA, Shiboob MM. Evaluation of quality and nutrient contents of table eggs from different sources in the retail market. Ital J Anim Sci. (2014) 13:3269. doi: 10.4081/ijas.2014.3294
12. Keshavarz K, Scott ML, Blanchard J. The effect of solubility and particle size of calcium sources on shell quality and bone mineralization. J Appl Poult Res. (1993) 2:259–67. doi: 10.1093/japr/2.3.259
13. Clunies M, Park D, Leeson S. Calcium and phosphorus metabolism and eggshell formation of hens fed different amounts of calcium. Poult Sci. (1992) 71:482–9. doi: 10.3382/ps.0710482
14. Wallner-Pendleton E, Scheideler SE. The Influence of Varying Levels of Calcium and Vitamin D3 in the Mature Laying Hen's Diet: Effect on Egg Production, Shell Quality, Bone Ash and Urolithiasis. The Nebraska Poultry Report. University of Nebr Coop Ext EC, Lincoln (1996).
15. Roush WB, Mylet M, Rosenberger JL, Derr J. Investigation of calcium and available phosphorus requirement of laying hens by response surface methodology. Poult Sci. (1986) 65:964–70. doi: 10.3382/ps.0650964
16. Tierzucht L, Layers LBC. Lohaman-Brown-Classic Layers Management–Guide Cage Housing. (2016). Available online at: https://www.ltz.de/ (accessed March 20, 2018).
17. Dale N. National Research Council Nutrient Requirements of Poultry 9th Revised Edition. Washington, DC: National Academy Press (1994).
18. Guidelines 2016 for Domestic Animals Health Nutrition Materials. DSM Vitamin Supplementation Guidelines 2016 for Animal Nutrition. (2016). p. 1–26. Available online at: https://www.dsm.com/content/dam/dsm/anh/en_US/documents/Vitamin_Supp_Guidelinespdf.?download=a1debc5c-f2e3-43c5-b782-64b7257f55b21584479483674 (accessed March 25, 2019).
19. Stevens VI, Blair R, Salmon RE. Effects of VD3, calcium, and phosphorus on growth and bone development of market turkeys. Poult Sci. (1984) 63:1571–85. doi: 10.3382/ps.0631571
20. Geng Y, Ma Q, Wang ZD, Guo Y. Dietary vitamin D3 supplementation protects laying hens against lipopolysaccharide-induced immunological stress. Nutr Metab. (2018) 15:58. doi: 10.1186/s12986-018-0293-8
21. Scott ML, Nesheim MC, Young RJ. Nutrition of the Chicken. Ithaca, NY: Scott ML and Associates (1982).
22. Soares JH Jr, Kerr JM, Gray RW. 25-Hydroxycholicalciferol in poultry nutrition. Poult Sci. (1995) 74:1919–34. doi: 10.3382/ps.0741919
23. Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J, et al. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin Exp Immunol. (2000) 120:139–46. doi: 10.1046/j.1365-2249.2000.01204.x
24. Gorman S, Buckley AG, Ling KM, Berry LJ, Fear VS, Stick SM, et al. Vitamin D supplementation of initially vitamin D-deficient mice diminishes lung inflammation with limited effects on pulmonary epithelial integrity. Physiol Rep. (2017) 15:e13371. doi: 10.14814/phy2.13371
26. Rodriguez-Lecompte JC, Yitbarek A, Cuperus T, Echeverry H, van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult Sci. (2016) 95:2547–56. doi: 10.3382/ps/pew186
27. Manolagas SC, Provvedini DM, Tsoukas CD. Interactions of 1, 25-dihydroxy VD3 and the immune system. Mol Cell Endocrinol. (1985) 43:113–22. doi: 10.1016/0303-7207(85)90074-7
28. H & N International Brown Nick, Brown Egg Layers. New Management Guide – H&N International GmbH. (2016). p. 76. Available online at: https://www.hn-int.com/eng-wAssets/docs/managementguides/Layers-englisch/HN_MG_Brown-Nick_EN.pdf (accessed May 25, 2019).
29. AOAC. Official Methods of Analysis. Washington, DC: Association of Official Analytical Chemists (2004).
30. Burke, WH, Attia YA. Molting single comb white leghorns with the use of the lupron depot formulation of leuprolide acetate. Poult Sci. (1995) 73:1226–32. doi: 10.3382/ps.0731226
31. Attia YA, Burke WH, Yamani KA. Response of broiler breeder hens to forced molting by hormonal and dietary manipulations. Poult Sci. (1994) 73:245–58. doi: 10.3382/ps.0730245
32. Attia YA, Burke WH, Yamani KA, Jensen LS. Energy allotments and performance of broiler breeders 2- females. Poult Sci. (1995) 74:261–70. doi: 10.3382/ps.0740261
33. Stefanello C, Santos TC, Murakami AE, Martins EN, Carneiro TC. Productive performance, eggshell quality, and eggshell ultrastructure of laying hens fed diets supplemented with organic trace minerals. Poult Sci. (2014) 93:104–13. doi: 10.3382/ps.2013-03190
34. Spectrum, Corp for Biotech SAE, Egypt Spectrum Diagnostic Kits. (2016) Available online at: http://www.spectrum-diagnostics.com/new/
35. Attia YA, Al-Khalaifah HS, Abd El-Hamid HE, Al-Harthi MA, El-shafey AA. Effect of different levels of multi-enzymes on immune response, blood hematology and biochemistry, antioxidants status and organs histology of broiler chicks fed standard and low-density diets. Front Vet Sci. (2020) 6:510. doi: 10.3389/fvets.2019.00510
36. Attia YA, Al-Khalifa H, Ibrahim MS, Abd Al-Hamid AE, Al-Harthi MA, El-Naggar Asmaa. Blood hematological and biochemical constituents, antioxidant enzymes, immunity and lymphoid organs of broiler chicks supplemented with propolis, bee pollen and mannan oligosaccharides continuously or intermittently. Poult Sci. (2017) 96:4182–92. doi: 10.3382/ps/pex173
38. Rao KS, Roland DA. Influence of dietary calcium level and particle size of calcium source on in vivo calcium solubilization by commercial leghorns. Poult Sci. (1989) 68:1499–505. doi: 10.3382/ps.0681499
39. Zhang B, Coon CN. The relationship of calcium intake, source, size, solubility in vitro and in vivo, and gizzard limestone retention in laying hens. Poult Sci. (1997) 76:1702–6. doi: 10.1093/ps/76.12.1702
40. Nascimento GR, Murakami AE, Guerra AFQM, Ospinas-Rojas IC, Ferreira MFZ, Fanhani JC. Effect of different vitamin D sources and calcium levels in the diet of layers in the second laying cycle. Braz J Poult Sci. (2014) 16:37–42. doi: 10.1590/1516-635x160237-42
41. Elaroussi MA, Forte LR, Eber SL, Biellier HV. Calcium homeostasis in the laying hen 1 age and dietary calcium effects. Poult Sci. (1994) 73:1581–9. doi: 10.3382/ps.0731581
42. Duran MR, Chen C, Kim WK. Effects of vitamin d and calcium for the prevention of osteoporosis at various stages of life of laying hens-review. Inter J Poult Sci. (2018) 17:405–9. doi: 10.3923/ijps.2018.405.409
43. Sauveur B. Lésions osseuses et articulaires des pattes des volailles: rôles de l'alimentation. Prod Anim. (1988) 11:35–45.
44. Nys Y, Gautron J. Structure and formation of the eggshell. In: Huopalahti R, López-Fandiño R, Anton M, Schade R, editors. Bioactive Egg Compounds. Berlin: Springer (2007). p. 99–102.
45. El-Safty SA. Stepwise regression analysis for ultrastructural measurements of eggshell quality in two local breeds of chicken. Egypt Poult Sci. (2004) 24:189–203.
46. Fathi MM, Zein El-Dein A, El-Safty SA, Radwan LM. Using scanning electron microscopy to detect the ultrastructural variations in eggshell quality of Fayoumi and Dandarawi chicken breeds. Int J Poult Sci. (2007) 6:236–41. doi: 10.3923/ijps.2007.236.241
47. Susanna K, Fröhlich E, Gebhardt-Henrich GS, Schäublin H, Pfulg A, Zweifel R et al. Effects of dietary supplementation with synthetic vitamin D3 and 25-hydroxycholecalciferol on blood calcium and phosphate levels and performance in laying hens. ArchGeflügelk. (2011) 75:179–84.
48. Baimbridg KG, Taylor TG. The role of calcitonin in controlling hypercalcaemia in the domestic fowl (Gallus domesticus). Comp. Bioch. Phys. Part A Phys. (1981) 68:647–51. doi: 10.1016/0300-9629(81)90372-8
49. Rosemary L, Walzem RJ, Hansen DL, Williams RL. Hamilton estrogen induction of VLDLy assembly in egg-laying hens. J Nut. (1999) 129:467S−72S. doi: 10.1093/jn/129.2.467S
50. Garlich J, Brake J, Parkhurst CR, Thaxton JP, Morgan GW. Physiological profile of caged layers during one production year, molt, and postmolt: egg production, egg shell quality, liver, femur, and blood parameters. Poult Sci. (1984) 63:339–33. doi: 10.3382/ps.0630339
51. Barreda DR, Konowalchuk JD, Rieger AM, Wong ME, Havixbeck JJ. Triennial growth symposium–novel roles for vitamin D in animal immunity and health. J Anim Sci. (2014) 92:930–8. doi: 10.2527/jas.2013-7341
52. Attia YA, Abd El-Hamid AE, Abedalla AA, Berika MA, El-Gandy MF, Sahin K, et al. Effect of betaine, vitamin C, and vitamin E on egg quality, hatchability, and markers of liver and renal functions in dual-purpose breeding hens exposed to chronic heat stress. Europ Poult Sci. (2018) 82. doi: 10.1399/eps2017171
53. Suaini NH, Zhang Y, Vuillermin PJ, Allen KJ, Harrison LC. Immune modulation by vitamin D and its relevance to food allergy. Nutrition. (2015) 7:6088–108 doi: 10.3390/nu7085271
54. Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. (2008) 4:80–90. doi: 10.1038/ncpendmet0716
55. Li YC, Chen YZ, Liu WC, Thadhani R. MicroRNA-mediated mechanism of vitamin D regulation of innate immune response. J Steroid Biochem Mol Biol. (2014) 144PA:81–6. doi: 10.1016/j.jsbmb.2013.09.014
56. Aslam SM, Garlich JD, Qureshi MA. Vitamin D deficiency alters the immune responses of broiler chicks. Poult Sci. (1998) 77:842–9. doi: 10.1093/ps/77.6.842
57. Lu L, Li SM, Zhang L, Liu XQ, Li DY, Zhao XL, et al. Expression of beta defensins in intestines of chickens injected with vitamin D3 and lipopolysaccharide. Genet Mol Res. (2015) 14:3330–7. doi: 10.4238/2015.April.13.12
58. Morris A, Shanmugasundaram R, Lilburn MS, Selvaraj RK. 25- hydroxycholecalciferol supplementation improves growth performance and decreases inflammation during an experimental lipopolysaccharide injection. Poult Sci. (2014) 93:1951–6. doi: 10.3382/ps.2014-03939
59. Morris A, Shanmugasundaram R, McDonald J, Selvaraj RK. Effect of in vitro and in vivo 25-hydroxyvitamin D treatment on macrophages, T cells, and layer chickens during a coccidia challenge. J Anim Sci. (2015) 93:2894–903. doi: 10.2527/jas.2014-8866
60. Shojadoost B, Behboudi S, Villanueva AI, Brisbin JT, Ashkar AA, Sharif S. Vitamin D3 modulates the function of chicken macrophages. Res Vet Sci. (2015) 100:45–51. doi: 10.1016/j.rvsc.2015.03.009
61. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. (2011) 51:311–36. doi: 10.1146/annurev-pharmtox-010510-100611
62. Xu S, Chen YH, Tan ZX, Xie DD, Zhang C, Xia MZ, et al. Vitamin D3 pretreatment alleviates renal oxidative stress in lipopolysaccharide-induced acute kidney injury. J Steroid Biochem Mol Biol. (2015) 152:133–41. doi: 10.1016/j.jsbmb.2015.05.009
63. Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. (2011) 134:123–39. doi: 10.1111/j.1365-2567.2011.03482.x
Keywords: laying hens, calcium, VD3, egg quality, immunological responses, blood biochemistry, electron microscope
Citation: Attia YA, Al-Harthi MA and Abo El-Maaty HM (2020) Calcium and Cholecalciferol Levels in Late-Phase Laying Hens: Effects on Productive Traits, Egg Quality, Blood Biochemistry, and Immune Responses. Front. Vet. Sci. 7:389. doi: 10.3389/fvets.2020.00389
Received: 30 March 2020; Accepted: 01 June 2020;
Published: 30 July 2020.
Edited by:
Andrea Serra, University of Pisa, ItalyReviewed by:
Sebastian Knaga, University of Life Sciences of Lublin, PolandMohamed E. Abd El-Hack, Zagazig University, Egypt
Copyright © 2020 Attia, Al-Harthi and Abo El-Maaty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youssef A. Attia, eWFhdHRpYUBrYXUuZWR1LnNh; Hayam M. Abo El-Maaty, aGF5YW0xNTFAeWFob28uY29t
 Youssef A. Attia
Youssef A. Attia Mohammed A. Al-Harthi
Mohammed A. Al-Harthi Hayam M. Abo El-Maaty
Hayam M. Abo El-Maaty