- 1Royal Veterinary College, Pathobiology and Population Health, London University, London, United Kingdom
- 2Royal Veterinary College, Clinical Science and Services, London University, London, United Kingdom
- 3Royal Veterinary College, Comparative Biomedical Science, London University, London, United Kingdom
- 4Zoetis, Outcomes Research, Parsippany, NJ, United States
- 5Zoetis, Outcomes Research, Dublin, Ireland
Objectives: Systemic glucocorticoids are widely used in companion animals. This study aimed to estimate the frequency, describe the characteristics and to evaluate risk factors for common side effects to systemic glucocorticoid therapy in dogs under primary veterinary care in the UK.
Methods: A cohort study using VetCompass™ data from 455,557 dogs under primary veterinary care during 2013 estimated the frequency of side effects to systemic glucocorticoid therapy occurring within 31 days of therapy. Risk factors for the most common side effects, polyuria and polydipsia (PUPD), were evaluated using multivariable logistic regression modeling (P < 0.05).
Results: During 2013, 28,472 study dogs received systemic glucocorticoids (6.2%, 95% CI 6.2–6.3). Review of the records of 3,000 randomly selected treated dogs identified 148 (4.9%, 95% CI 4.2–5.7%) dogs with at least one side effect recorded within 31 days of therapy. The most frequent side effects were polydipsia (39.2% of total presenting signs), polyuria (28.4%), vomiting (16.2%) and diarrhea (14.9%), dogs receiving only oral systemic glucocorticoids (odds ratio, OR: 3.72) and dogs receiving both oral and injectable systemic glucocorticoid (OR: 10.71) had increased odds of PUPD compared with dogs receiving only injectable systemic glucocorticoid. Focusing on the active substance used, treatment with prednisolone tablets only (OR: 3.53) and treatment with both prednisolone tablets and injectable dexamethasone sodium phosphate (OR: 7.62) showed increased odds of PUPD compared to treatment with injectable dexamethasone sodium phosphate only.
Brief: These results can assist veterinarians to optimize therapeutic selection for reduced side effect, to inform owners on common side effects, and help protect the welfare of pets and their owners.
Introduction
Glucocorticoids are commonly used in companion animal veterinary practice as anti-inflammatory and immunosuppressive agents (1, 2). However, glucocorticoids have been associated with various side effects, including vomiting, diarrhea, bodyweight gain or loss, polyuria, polydipsia, delayed wound healing, behavioral problems, immunosuppression and predisposition to infection (3, 4).
There is some published evidence on side effects that may occur following systemic glucocorticoid usage. Systemic glucocorticoid treatment has been associated with predisposition to infection (5, 6) immunosuppression and decreased urine osmolality (1, 2, 7). Glucocorticoid treatment has been associated with increased risk of urinary tract infection among dogs with skin disease (5, 6, 8). Dogs administered long-term hydrocortisone showed side effects including thinning of skin, PUPD (9). Another study reported increased surgical complications, diarrhea and urinary tract infection in dogs with acute thoracolumbar intervertebral disk herniation treated with dexamethasone before surgery compared with dogs treated with other glucocorticoids (10). Dogs treated with dexamethasone and methylprednisolone acetate have also been reported to have an increased risk of hepatopathy (11). Despite this qualitative evidence, there is little published quantitative evidence on the frequency or risk factors for side effects following systemic glucocorticoids therapy.
Anonymised clinical data from primary-care veterinary practices offers potential to investigate the frequency of side effects following glucocorticoid therapy (12). Previous studies within the VetCompass™ Programme used data collected from primary-care small animal practices to quantify the usage of glucocorticoids in dogs but did not report on side effects (13, 14). The current study aimed to estimate the frequency and describe the characteristics of side effects to systemic glucocorticoid therapy recorded in the electronic clinical records from a large population of dogs under primary veterinary care in the UK. The study further aimed to evaluate risk factors for PUPD as side effects in dogs treated with systemic glucocorticoids.
Methods
The VetCompass Programme collates de-identified electronic patient record (EPR) data from primary-care veterinary practices in the UK (15). VetCompass collects information fields with relevant dates that include species, breed, date of birth, sex, neuter status, insurance status, bodyweight, clinical notes and treatment. The EPR data were extracted from practice management systems using integrated clinical queries and uploaded to the VetCompass database (16).
A retrospective cohort study of dogs attending VetCompass practices was used to estimate the frequency and risk factors for PUPD after systemic glucocorticoid therapy. The sampling frame for the study included dogs under veterinary care during 2013 that had at least one EPR within the VetCompass database from January 1st 2013 to December 31st 2013 and/or at least one EPR both before and after this period. Sample size calculations estimated that ~3,000 dogs would be required to be studied in order to detect a 0.5% side effects frequency (with a precision of ± 0.25% confidence limit and 95% confidence level). Ethical approval was granted by the RVC Ethics and Welfare Committee (URN 2015 1369).
A systemic glucocorticoid treatment was defined as any glucocorticoid preparation administered by injection, dispensed as oral tablets or an oral suspension. Inclusion criteria required that a systemic glucocorticoid was either administered or dispensed during 2013. Animals receiving only topical glucocorticoid therapy were excluded from consideration. A comprehensive list of chemical and brand names for systemic glucocorticoids was generated by searching the Veterinary Medicines Directorate (17), British Small Animal Veterinary Association (BSAVA) formulary (18) and National Office for Animal Heath (NOAH) veterinary products databases (19). A list of search terms was derived from these chemical and brand names to identify all systemic glucocorticoids within the treatment field of the VetCompass database. Case-finding involved initial screening of the treatment fields of all dogs within the study population for any treatment record that may have been a systemic glucocorticoid based on these search terms. A list of animal unique identification codes for these candidate cases for systemic glucocorticoid usage was generated and treatment data (date, drug, volume of injectable/number of tablets, dosage instructions) on all treatments during 2013 were extracted from the VetCompass database to an Excel format. Duplicates were removed and all remaining dispensing terms used were manually coded as systemic glucocorticoid (yes/no) to create a master list of systemic glucocorticoid dispensing terms. Data were extracted to describe the name of the active substance, dosage and route of administration of glucocorticoids used.
For the current study, a side effect case was defined as a dog with evidence in the clinical notes of a side effect within 31 days of a new episode of systemic glucocorticoid usage during 2013. This period was selected to allow for manifestation of potentially delayed onset side-effects whilst reducing the inclusion of potential side-effect events unrelated to glucocorticoid therapy. It was required that there was no evidence in the clinical notes of the observed potential side effect(s) in the 31 days prior to the new systemic glucocorticoid episode. A new episode was defined as systemic glucocorticoid usage that was not preceded by systemic glucocorticoid therapy during the previous 31 days. Individual treatment episodes could include the usage of multiple systemic glucocorticoid products and dogs could have multiple treatment episodes during 2013. All dogs that received at least one systemic glucocorticoid during 2013 were randomly ordered and the EPRs of a random sample of 3,000 of these dogs were manually reviewed in detail to identify all dogs that met the side effects case definition. Data were extracted for identified side effects cases to describe the dates of the start of the episode of systemic glucocorticoid usage and the first reported side effect, the clinical sign(s) of the side effect, the clinical action(s) taken in response to the side effect and the main clinical indication for the systemic glucocorticoid originally prescribed in the side effects cases.
The period (days) of glucocorticoid administration prior to the side effect was calculated from the glucocorticoid episode start date to the first reported side effect. Initial duration of systemic glucocorticoid prescribed for side effects cases was extracted from the treatment data field. The initial daily systemic glucocorticoid dosage (mg/kg/day) was calculated from the total daily dose for each glucocorticoid from the treatment data field and dividing by the bodyweight.
A breed variable included individual breeds with 60 or more systemic glucocorticoid cases in the random sample of 3,000 dogs, a grouped category of all remaining purebreds and a general grouping of crossbred dogs. This approach was taken to allow focus on commonly affected breeds and to facilitate statistical power for the individual breed analyses (20). A purebred variable categorized all dogs of recognizable breeds as “purebred” and the remaining dogs as “crossbred” (21). Age (years) was calculated for side effect cases at the date of first administration of the systemic glucocorticoid episode for the relevant side effects and for non-case dogs at the date of administration of a randomly selected systemic glucocorticoid event during 2013. An age variable categorized age (years) into four groups (≤ 2.0, > 2.0 to ≤ 5.0, > 5.0 to ≤ 8.0, > 8.0 years). Bodyweight (kg) described the nearest bodyweight recorded during 2013 to the date of the systemic glucocorticoid usage episode for dogs ≥ 18 months and the nearest bodyweight recorded within 1 month to the systemic glucocorticoid usage episode date for dogs ≤ 18 month. A bodyweight variable categorized bodyweight into six groups (0.0–10.0, > 10.0–20, > 20.0–30, > 30.0–40 kg, ≥ 40.0 kg, unavailable). Route of administration and active substance variables were defined for side effects cases on all systemic glucocorticoid products used within a maximum of 31 days from the start of the systemic glucocorticoid episode up to the onset of a side effect. For non-case dogs, these data were extracted for treatment within 31 days of the start of a systemic glucocorticoid episode that was randomly selected from all systemic glucocorticoid episodes for that dog. A season variable defined the season of treatment based on the age date defined above and categorized into winter (December to February), summer (June to August), autumn (September to November) and spring (March to May).
Following data checking and cleaning in Excel (Microsoft Office Excel 2013, Microsoft Corp.), analyses were conducted using Stata Version 14 (Stata Corporation). Numerical data were assessed graphically for normality and summarized with median (interquartile range, IQR) or mean (standard deviation) as appropriate (22). Risk factor analysis evaluated the 3,000 randomly selected dogs treated with at least one systemic glucocorticoid to compare PUPD cases with the non- PUPD dogs. These two side effects were considered closely related therefore any dog with at least one of these PUPD side effects was included in the study. Logistic regression modeling was used to evaluate univariable associations between risk factors and PUPD outcome. Risk factors with liberal associations in univariable modeling (P < 0.3) were taken forward for mixed effects multivariable logistic regression modeling using a manual backwards-stepwise elimination method. Residual interclass correlation coefficients were assessed, clinic was evaluated as a random effect and pair-wise interaction effects were evaluated for the final model variables. Two multivariable models were developed to alternatively evaluate two strongly biologically correlated variables of interest separately: route of administration of the steroids and active substances used. The Hosmer-Lemeshow test for goodness of fit was conducted for assessment of the fit of the final fixed effects multivariable model (23). Final statistical significance was set at P < 0.05.
Results
The study population included 455,557 dogs from 304 clinics in the VetCompass database under veterinary care during 2013. Of these, 28,472 dogs received at least one systemic glucocorticoid during 2013 giving an overall one-year period prevalence for systemic glucocorticoid usage of 6.2% (95% CI 6.2–6.3). From a random sample of 3,000 dogs receiving systemic glucocorticoids during 2013 that were reviewed in detail, 148 dogs were identified with at least one side effect within 31 days of administration, giving a side effects occurrence among dogs receiving systemic glucocorticoids of 4.9% (95% CI 4.2–5.7%). Of these 148 side effects reported, 97 (65%) occurred within the first 14 days after initiation of therapy. None of the side effects cases had more than one event of side effect during 2013.
The most common clinical categories leading to prescribing of systemic glucocorticoid to the 148 side effects cases were skin conditions (n = 50, 32.5%), ear problems (33, 21.4%) and gastro-intestinal tract problems (20, 13.0%) (Table 1). The most common clinical indications were dermatitis (38, 24.7%) and ear infections (28, 18.2%). Prednisolone 5 mg was the most common oral systemic glucocorticoid prescribed for dogs with a side effect without a preceding systemic glucocorticoid injection, with a median initial daily dosage of 0.67 mg/kg/day (Table 2). Similarly, prednisolone 5 mg was the most common oral systemic glucocorticoid prescribed for side effect cases with a preceding systemic glucocorticoid injection (median initial daily dosage of 0.48 mg/kg/day). Dexamethasone sodium phosphate was the most common injectable systemic glucocorticoid used in side effects cases (median initial daily dosage 0.10 mg/kg/day). The duration of dispensed treatments for side effects cases varied with active substance and formulation type though was generally for a median duration of ~2–3 weeks (Table 3).
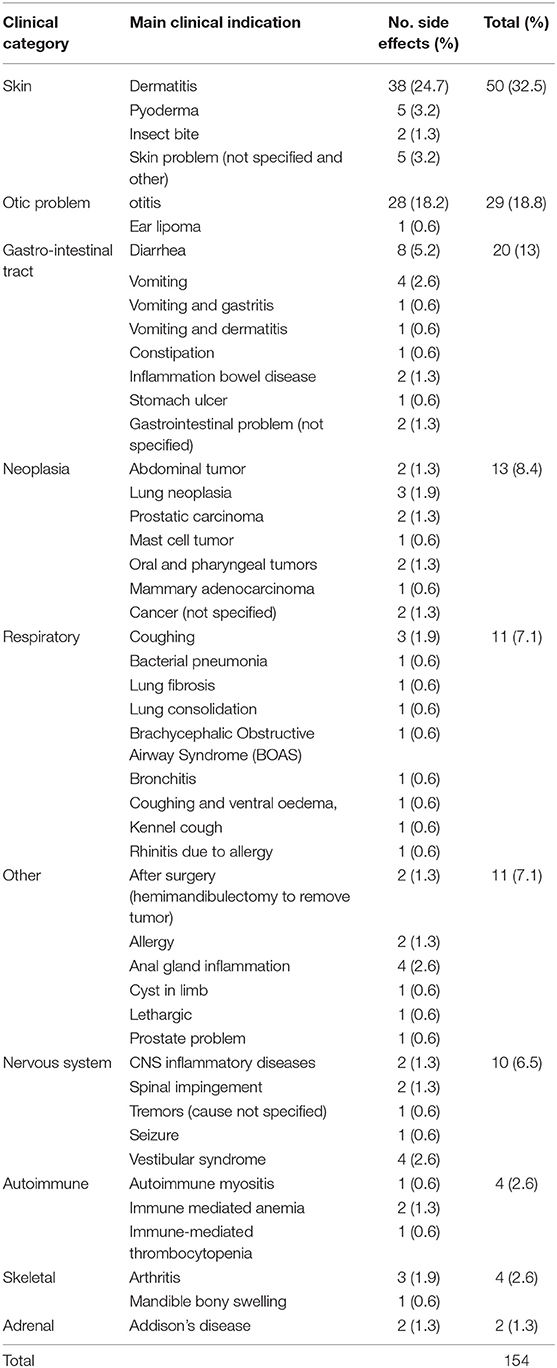
Table 1. Main clinical indications for systemic glucocorticoid usage in 148 side effects cases recorded within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK. Some cases had multiple indications.
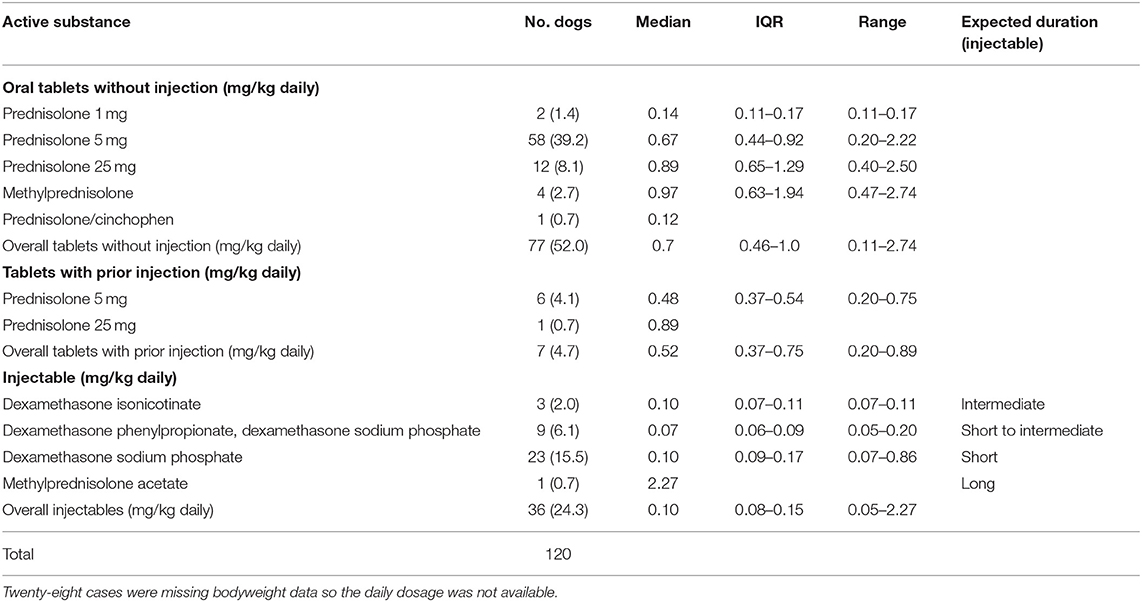
Table 2. Initial dosages of systemic glucocorticoid active substances that had a side effect recorded within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK (n = 120).
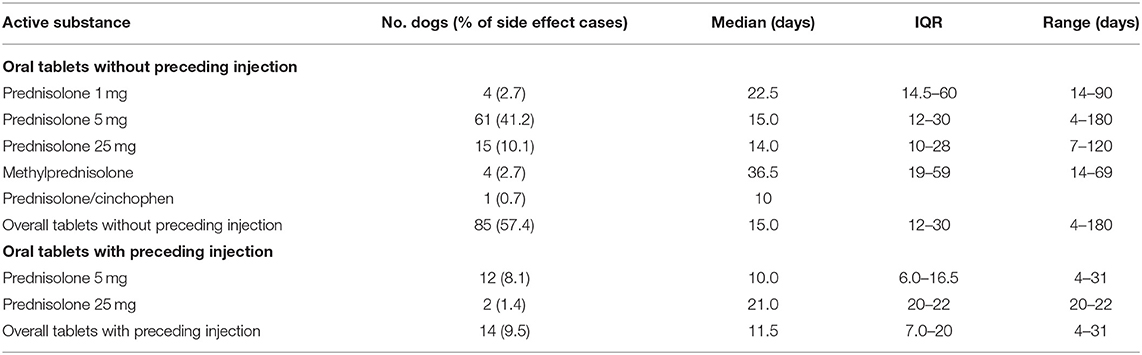
Table 3. Duration of systemic glucocorticoid active substances dispensed for oral treatments that had a side effect recorded within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK (n = 148).
The period to onset of the side effects varied across side effect cases. For prednisolone administrations without preceding injection, the median duration between start of glucocorticoid treatment and the development of side effects varied from 10.5 to 13 days, whilst methylprednisolone had a median onset of 4.5 days, though this was based on only 4 events (Table 4). Dexamethasone sodium phosphate, the most commonly administered injectable systemic glucocorticoid, had a median onset to side effects of 7 days based on a single injection course for all these events.
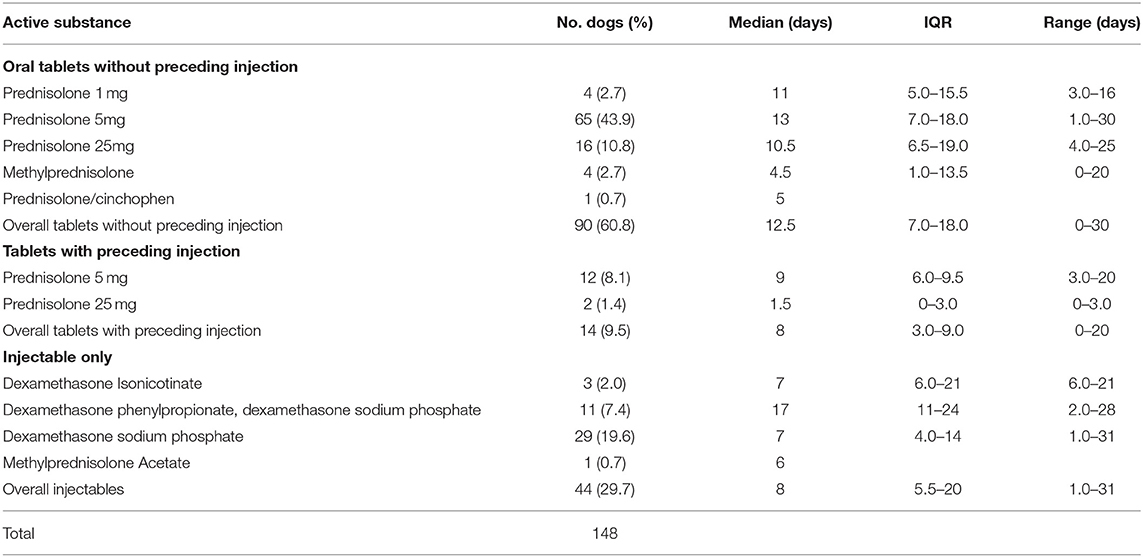
Table 4. Period (days) from commencing systemic glucocorticoid treatment to developing a side effect for dogs under primary veterinary care in the UK (n = 148).
There were 241 clinical signs recorded in the EPR within the 148 side effects cases (Table 5). Some side effects cases had more than one clinical sign recorded. No fatality was reported. The most frequent clinical signs reported for side effects cases were polydipsia (58 events, 39.2% of the side effects cases), polyuria (42, 28.4%), vomiting (24, 16.2%), diarrhea (22, 14.9%) and polyphagia (20, 13.5%). The dose of the systemic glucocorticoid was subsequently reduced in 43 (29.1%) side effects cases whilst therapy was discontinued in 25 (16.9%) side effects cases.
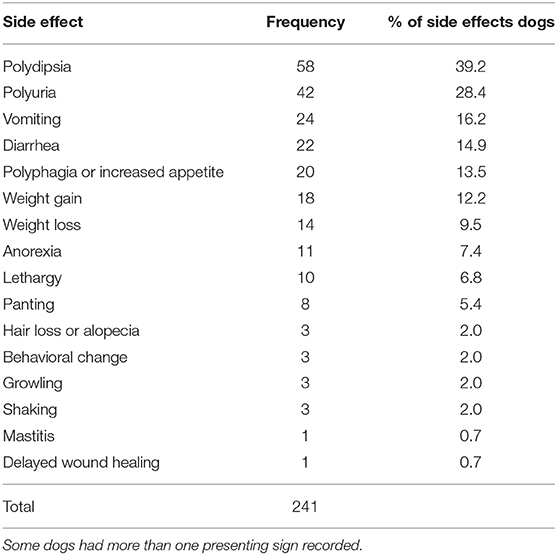
Table 5. Frequency (%) of presenting signs among 148 dogs under primary veterinary care with side effects recorded within 31 days after receiving systemic glucocorticoid therapy.
Seventy-one dogs had either PUPD. Of these, 53 (75.71%) were purebred and 40 (56.34%) were male. PUPD cases had a median bodyweight of 19.1 kg (IQR 10.8–30.4) and median age was 6.9 years (IQR 4.2–10.2), (Table 6). The most common breeds among the PUPD cases were Labrador Retriever (8/71, 11.27%), West Highland White Terrier (4/71, 5.63%), Boxer (4/71, 5.63%), along with crossbred dogs (17/71, 23.94%) (Table 6). Of the non-cases dogs, 2266 (77.55%) were purebred, 1630 (55.65%) were male. The median bodyweight for non-cases was 14.3 kg (IQR 8.6–25.0) and the median age was 4.9 years (IQR 2.1–8.3). The most common breeds among the non-case dogs were Staffordshire Bull Terrier 23 0 (7.85%), Labrador Retriever (179, 6.11%), Jack Russell Terrier (178, 6.08%) as well as crossbreds 593 (20.25%).
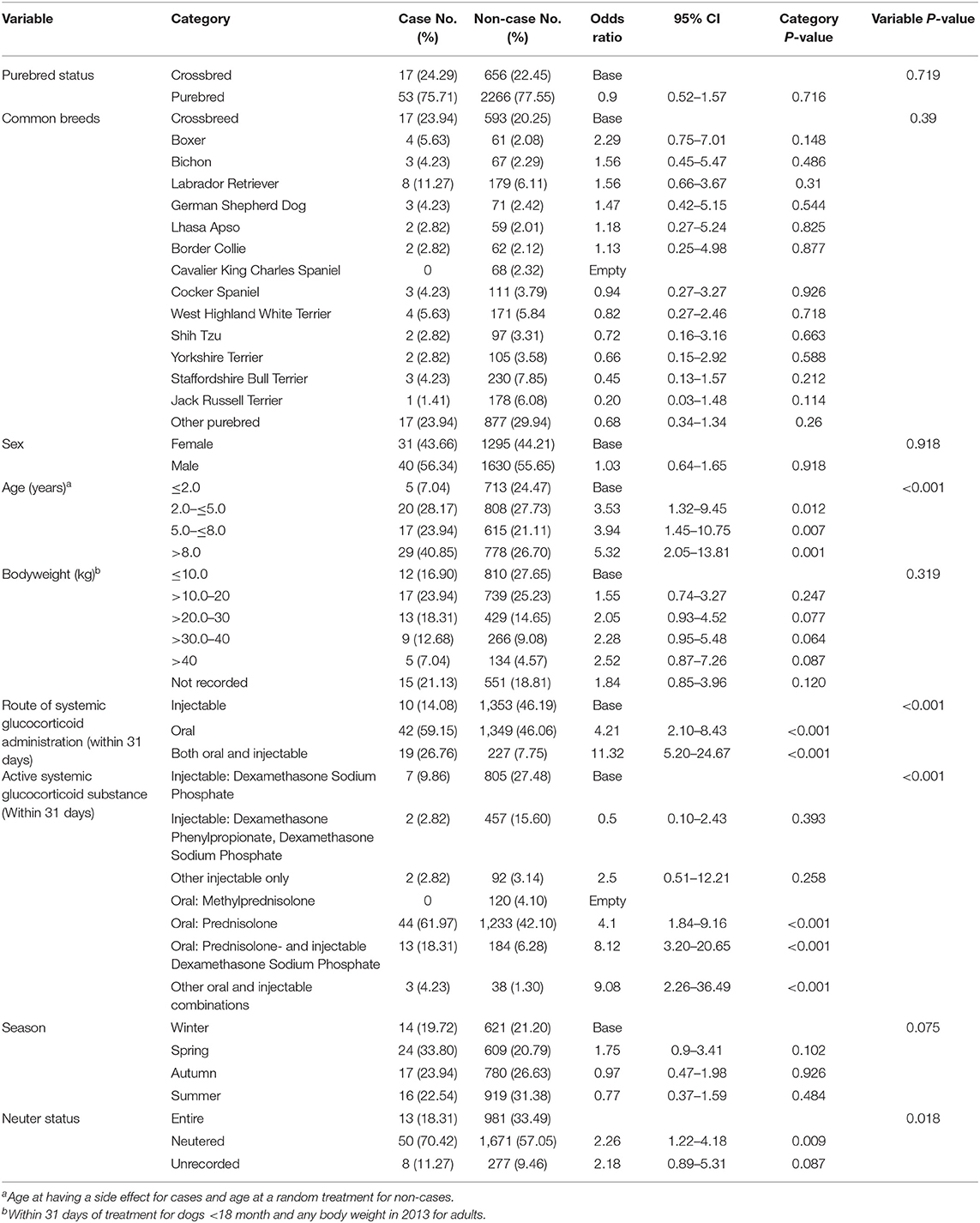
Table 6. Descriptive analysis and univariable logistic regression results for risk factors for polyuria and/or polydipsia within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK.
Univariable logistic regression identified four of nine tested variables that were liberally associated with PUPD and were further evaluated using multivariable logistic regression modeling: age, route of administration, active substance and neuter status. Add in the ones that were dropped also. There is strong biological correlation between route of administration and active substance so these were evaluated in separate multivariable modeling that resulted in two final models.
The final model that focused on route of administration retained 2 variables: route of administration and age. After accounting for the effects of the age, dogs that had both oral and injectable systemic glucocorticoids (OR: 10.40 95% CI 4.76–22.75) and dogs that had oral systemic glucocorticoids (OR: 3.69 95% CI 1.84–7.42) showed increased odds of PUPD compared with dogs that had injectable systemic glucocorticoids only (Table 7). Dogs aged > 8 years had 4.24 times the odds (95% CI 1.62–11.09) of PUPD compared with dogs aged ≤ 2 years. No biologically significant interactions were identified. Clinic was not significant as a random effect (P-value = 0.252). There was no evidence of poor fit in the final fixed effect multivariable model (Hosmer-Lemeshow P-value = 0.789).
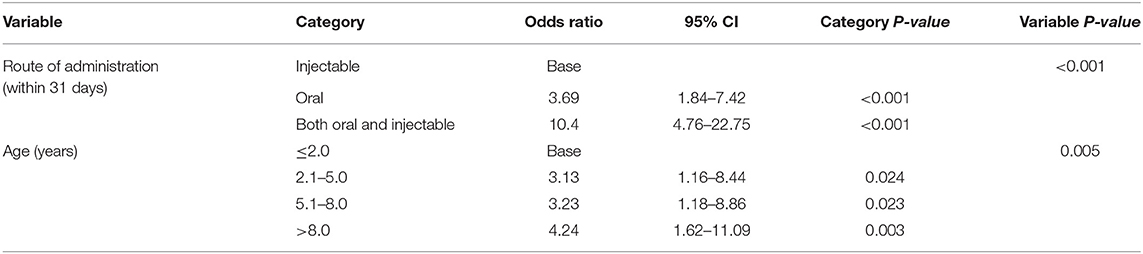
Table 7. Final model 1: Multivariable logistic regression results with route of administration as the focus for risk factors for polyuria and polydipsia within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK.
The final model that focused on active substance also retained two variables: active substance and age. Dogs treated with prednisolone tablets only had 3.44 (95% CI 1.54–7.73) times the odds of a PUPD compared with dogs that had injectable dexamethasone sodium phosphate only (Table 8). Dogs treated with both prednisolone tablets and injectable dexamethasone sodium phosphate had 7.29 (95% CI 2.86–18.60) times the odds of PUPD compared with dogs that had injectable dexamethasone sodium phosphate alone. Dogs aged > 8 years had 4.38 times the odds (95% CI 1.67–11.47) of PUPD compared with dogs aged ≤ 2 years. Clinic was not significant as a random effect (P-value = 0.198). No biologically significant interactions were identified and there was no evidence of poor fit in the final fixed effect multivariable model (Hosmer-Lemeshow P-value = 0.579).
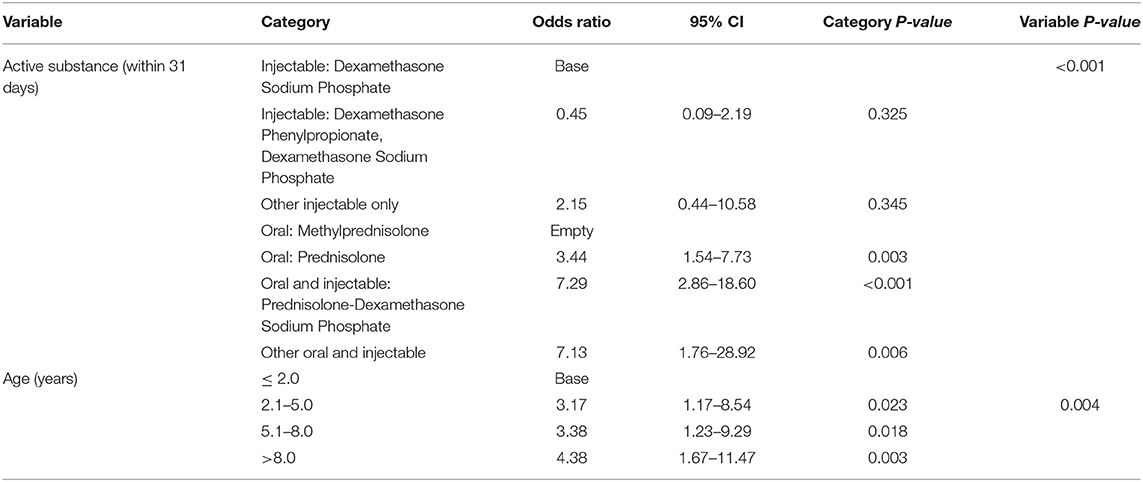
Table 8. Final model 2: Multivariable logistic regression results with active substance as the focus for risk factors for polyuria and polydipsia within 31 days after receiving systemic glucocorticoid therapy among dogs under primary veterinary care in the UK.
Discussion
This is the first study to explore side effects to systemic glucocorticoids in dogs attending primary-care practices by analyzing clinical records from a multi-center primary-care research database. The study identified that nearly 5% of dogs receiving a systemic glucocorticoid had evidence of a side effect recorded in the EPR within 31 days of onset of therapy.
Of the 148 side effects cases identified, none had more than one side effect event during 2013, suggesting that veterinarians may have taken steps to avoid recurrent side effect events to systemic glucocorticoids in known susceptible animals. Immediate clinical actions in response to side effects identified in the current study, included reduction of the glucocorticoid dose (29.1% of side effect events) and discontinuation of therapy (16.9% of side effect events). For consistency of the study, cases were only evaluated for the first course where side-effects were reported, though it would have been interesting to evaluate the number of these dogs that had subsequent courses within the study period.
Skin (32.5%) and ear (21.4%) conditions were the most common indications for prescribing systemic glucocorticoids where a side effect was subsequently observed. However, these findings are not conclusive evidence for a side effect predisposition by condition because the clinical indication for the non-side effect dogs was not studied, but it does suggest that this may be a useful future line of research. Many skin and ear disorders have an inflammatory or immune-mediated component that may benefit from systemic glucocorticoid therapy and consequently systemic glucocorticoids are routinely recommended for these conditions by dermatologists (24). A study using primary-care practice data in the UK reported that 20% of dermatology cases were prescribed systemic glucocorticoid therapy (25).
Prednisolone was the most commonly prescribed oral systemic glucocorticoid among the side effects cases. A previous study for the whole population of dogs under primary veterinary care in 2013 using VetCompass data reported similar results (14). The median prednisolone initial daily dosage was between 0.14 and 0.89 mg/kg/day, depending on tablet size. These were within the recommended manufacturer dosages of 0.1–2.0 mg/kg/day (2017). The short-acting agent, dexamethasone sodium phosphate, was the most commonly administered injectable systemic glucocorticoids among the side effects cases with a median daily dose of 0.10 mg/kg/day. Other injectable glucocorticoids, dexamethasone isonicotinate, dexamethasone phenylpropionate with dexamethasone sodium phosphate and methylprednisolone acetate were also used and are considered intermediate, short to intermediate and long acting respectively, so are less suitable for initiation of a combination therapy “injectable followed by oral treatment” plan. However, just under 5% of side effects occurred after injectable combined with oral glucocorticoid treatment were administered.
The median time to side effect after initiation of oral glucocorticoid therapy alone was 12.5 days, whilst side effects appeared to occur sooner when an injectable agent was administered either alone or with oral therapy, at a median of 8 days after onset of therapy. Injectable glucocorticoid therapy is likely to reach a higher plasma concentration sooner and hence any side effects might be expected to become evident sooner (3). However, there was a wide range in onset across agents and this reflects the variable nature of the side effects reported, such that certain side effects may occur more immediately after administration of the systemic glucocorticoids (e.g., PUPD) whilst others require prolonged exposure to occur (e.g., hair loss/alopecia) (26).
The two most frequent side effects to systemic glucocorticoids recorded in the current study were polydipsia (39.2%) and polyuria (28.4%). For this reason, factors associated with these side effects were explored more deeply in the analysis. These side effects likely result from the effect of glucocorticoids on the antidiuretic hormone (ADH) leading to excessive loss of fluids in urine and enhancing feeling of thirst to replace fluid loss (26). Polydipsia and polyuria can promote urinary incontinence by increasing bladder fill beyond the physiological thresholds of the urinary sphincter (27). Urinary incontinence has been considered to negatively impact on the welfare of affected dogs (28). Within the 31 day period PUPD were frequently reported however, urinary incontinence was not observed in the records.
The next most frequent side effect in the current study were vomiting (16.2%) and diarrhea (14.9%). Vomiting is associated with unpleasant nausea sensations, while profuse or prolonged vomiting can cause dehydration, electrolyte and acid-base imbalances that may require hospitalization (29, 30). Many human surgical patients report nausea and vomiting to be more distressing than the postsurgical pain (31). Behavioral changes (3 cases) and growling (3 cases) contributed 4% to the side effects cases in the current study. Previous study found an association between corticosteroids therapy and dog behavioral problems (4). In this study aggression toward people was the most recorded behavioral side effect. Therefore, as well as side effects to systemic glucocorticoids being relatively common, the results of the current study suggest that the clinical signs induced by these side effects can have substantial negative impacts for both owners and their dogs.
Dogs over 8 years old were more than 4 times more likely to have PUPD following systemic glucocorticoid treatment compared to dogs aged under 2 years. Increasing predisposition to incontinence (32) and chronic kidney disease (27) as dogs age may promote increased PUPD in older dogs. An experimental study reported higher liver enzymes and pathological hepatic changes in older dogs after glucocorticoids therapy compared to young dogs (33). Overall, these findings suggest that veterinarians should be especially vigilant about prescribing systemic glucocorticoids to older dogs. Sex did not appear to be associated with side-effects in the current study. A previous study that reported increased susceptibility of acquired urinary incontinence in neutered bitches compared to male dogs and this may suggest increased risk of PUPD in female dogs (28). However, the small number of cases may have underpowered the current study in identifying this association.
Dogs treated with oral systemic glucocorticoids had 3.69 times the odds, and dogs treated with both oral and injectable systemic glucocorticoids had 10.40 times the odds, of PUPD compared to dogs treated only with an injectable systemic glucocorticoid. Similarly, dogs receiving oral prednisolone alone had 3.44 times the odds, and dogs receiving a combination of oral prednisolone with injectable dexamethasone sodium phosphate had 7.29 times the odds of PUPD than dogs that received injectable dexamethasone sodium phosphate alone. The current results suggest prolonged and combination therapy compared to single injectable glucocorticoid administration may result in increased probability of PUPD. In humans, oral therapies are associated with prolonged exposure and maintenance of blood levels of these drugs and therefore suggest that the cumulative effect over time may be a strong contributor to PUPD risk (32, 34).
The data resource used for this study had some limitations as previously reported (13, 35). These EPR data were not recorded primarily for research purposes and thus were limited by reliance on accurate and thorough record-keeping by the clinicians. It was assumed all side effects would be recorded by the attending veterinarians, though it is possible that common side effects may not have been recorded, due to the anticipation of such side effects, suggesting that the current results may underestimate the frequency of complications. Owners' previous experience of expected side effects from using glucocorticoids may additionally have led to reduced reporting to the veterinarian and additional under-reporting of side effects. Furthermore, chronic side effects that may have happened after 31 days of receiving systemic glucocorticoid treatment were likely to be underreported. However, it was considered evaluation of a shorter period after therapy/acute side effects here would reduce the risk that side-effects reported were unrelated to the glucocorticoid therapy administered.
This study was limited by the low power in identifying PUPD due to small number of cases in some categories leading to data sparsity. The study did not analyse data on concurrently administered medications (for example drugs with ulcerogenic or nephrotoxic potential such as NSAIDs) due to the variability in co-therapies used, the range of associated potential side effects and the limited sample size for the PUPD risk factor study undertaken. Nor did the study attempt to extract data on the overall health status of the dogs due to challenges identifying this information consistently within the clinical records of all 3,000 dogs. It is acknowledged these factors in particular could have confounded in part the associations reported and further work would ideally incorporate these variables within the analyses. Clinical indications for the use of systemic glucocorticoids were extracted only for the side effects cases and not for the overall population of dogs treated with systemic glucocorticoids. Knowledge of the prescribed daily doses (mg/kg) could have helped to define the intended therapeutic effect (high “shock” dose, immunosuppression, anti-inflammatory daily, or alternate days) as well as allow evaluation of an association with dose. However, dosage instructions frequently varied within each animal over time (e.g., start one tablet twice a day reduce to one daily then to one every other day) making extraction of these data difficult. Finally, given the study was based on data from 2013 it is possible current available systemic glucocorticoids have changed. Nonetheless, the range of most common systemic glucocorticoids would appear to be broadly comparable, suggesting the study remains relevant (18).
In conclusion, this is the first study to estimate the frequency of side effects and risk factors for PUPD to systemic glucocorticoids occurring with 31 days of treatment in a large general population of dogs attending primary-care practices in UK. The results highlight that combination systemic glucocorticoid therapy and prolonged treatments were associated with increased risk of recording PUPD. Older dogs were at most risk of PUPD. These results can help veterinarians to optimize decision-making when prescribing systemic glucocorticoids for dogs in order to reduce the risks of side effects and to protect the welfare of both dogs and their owners.
Data Availability Statement
The raw data supporting the conclusions of this article is available on the RVC repository through the following link http://researchonline.rvc.ac.uk/id/eprint/12566/.
Ethics Statement
Ethical approval was granted by the RVC Ethics and Welfare Committee (URN 2015 1369).
Author Contributions
DB, DO'N, DE, AW, KM, and LP contributed to the design of the study and the data analysis plan. DO'N and DE organized the database. DE performed the statistical analysis and wrote the manuscript. All authors contributed to the manuscript revision.
Funding
Zoetis funded the research assistant position to investigate glucocorticoids usage. RVC and Zoetis funded the open access publication fees.
Conflict of Interest
The authors declare that this study received funding from Zoetis. KM and AW were employed by Zoetis and contributed to the design of the study and revision of the manuscript. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Noel Kennedy (RVC) for VetCompass software and programming development. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Goddard Veterinary Group, Independent Vet Care, Beaumont Sainsbury Animal Hospital, Vets Now, CVS and the other UK practices who collaborate in VetCompass. We are especially grateful to Zoetis Ltd. for supporting this study.
Abbreviations
BSAVA, British Small Animal Veterinary Association; EPR, electronic patient record; IQR, interquartile range; NOAH, National Office for Animal Heath; OR, odds ratio; PUPD, polyuria and polydipsia; VMD, Veterinary Medicines Directorate.
References
1. Ferguson DC, Dirikolu L, Hoenig M. Glucocorticoids, mineralocorticoids and adrenolytic drugs. In: Riviere JE Papich MG, editors. Veterinary Pharmacology and Therapeutics. 10th ed. Iowa, IA: Wiley-Blackwell (2017). p. 729–62.
2. Riviere JE, Papich MG. Veterinary Pharmacology and Therapeutics, 10th ed. Wiley-Blackwell (2017).
3. Barbara DM Jr, Bubrick MP, Jacobs DM, Timmerman WR, Onstad GR. The effects of methylprednisolone on postoperative bowel motility and propulsion in dogs. Dis Colon Rectum. (1986) 29:18–21. doi: 10.1007/BF02555278
4. Notari L, Burman O, Mills DS. Is there a link between treatments with exogenous corticosteroids and dog behaviour problems? Vet Rec. (2016) 179:462. doi: 10.1136/vr.103768
5. Ihrke PJ, Norton AL, Ling GV, Stannard AA. Urinary tract infection associated with long-term corticosteroid administration in dogs with chronic skin diseases. J Am Vet Med Assoc. (1985) 186:43–6.
6. Torres SM, Diaz SF, Nogueira SA, Jessen C, Polzin DJ, Gilbert SM, et al. Frequency of urinary tract infection among dogs with pruritic disorders receiving long-term glucocorticoid treatment. J Am Vet Med Assoc. (2005) 227:239–43. doi: 10.2460/javma.2005.227.239
7. Mckay Li CJ. Physiologic and pharmacologic effects of corticosteroids. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th ed. Hamilton, ON: BC Decker (2003). p. 34–67.
8. Freshman JL, Reif JS, Allen TA, Jones RL. Risk factors associated with urinary tract infection in female dogs. Prevent Vet Med. (1989) 7:59–67. doi: 10.1016/0167-5877(89)90037-8
9. Schellenberg S, Mettler M, Gentilini F, Portmann R, Glaus TM, Reusch CE. The effects of hydrocortisone on systemic arterial blood pressure and urinary protein excretion in dogs. J Vet Int Med. (2008) 22:273–81. doi: 10.1111/j.1939-1676.2007.0039.x
10. Levine JM, Levine GJ, Boozer L, Schatzberg SJ, Platt SR, Kent M, et al. Adverse effects and outcome associated with dexamethasone administration in dogs with acute thoracolumbar intervertebral disk herniation: 161 cases (2000-2006). J Am Vet Med Assoc. (2008) 232:411–7. doi: 10.2460/javma.232.3.411
11. Kondratjeva J, Birgele E. Corticosteroid-induced hepatopathy in dogs. Lativia Univ Agric. (2014) 21:179–82.
12. O'Neill D, Church D, Mcgreevy P, Thomson P, Brodbelt D. Approaches to canine health surveillance. Canine Genet Epidemiol. (2014) 1:2. doi: 10.1186/2052-6687-1-2
13. O'Neill D, Hendricks A, Summers J, Brodbelt D. Primary care veterinary usage of systemic glucocorticoids in cats and dogs in three UK practices. J Small Anim Pract. (2012) 53:217–22. doi: 10.1111/j.1748-5827.2011.01190.x
14. Elkholly DA, O'Neill D, Wright AK, Mwacalimba K, Nolan LS, Pavlock A, et al. Systemic glucocorticoid usage in dogs under primary veterinary care in the UK: prevalence and risk factors. Vet Rec. (2019) 185:108. doi: 10.1136/vr.105220
15. O'Neill DG, Church DB, Mcgreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE. (2014) 9:e90501. doi: 10.1371/journal.pone.0090501
16. O'Neill DG, Scudder C, Faire JM, Church DB, Mcgreevy PD, Thomson PC, et al. Epidemiology of hyperadrenocorticism among 210,824 dogs attending primary-care veterinary practices in the UK from 2009 to 2014. J Small Anim Pract. (2016) 57:365–73. doi: 10.1111/jsap.12523
18. BSAVA. Small animal formulary. 8th ed. Gloucester: Gloucester British Small Animal Veterinary Association (2014).
20. Scott M, Flaherty D, Currall J. Statistics: how many? J Small Anim Pract. (2012) 53:372–6. doi: 10.1111/j.1748-5827.2012.01231.x
21. Irion DN, Schaffer AL, Famula TR, Eggleston ML, Hughes SS, Pedersen NC. Analysis of genetic variation in 28 dog breed populations with 100 microsatellite markers. J Hered. (2003) 94:81–7. doi: 10.1093/jhered/esg004
22. Kirkwood B, Sterne J. Analysis of numerical outcomes. In: Goodgame F, Pinder V, Moore K, editors. Essential Medical Statistics. Oxford: Blakwell Science (2003). p. 33–118.
23. Hosmer DW, Lemeshow S. Multiple logistic regression. In: Shwhart WA, Wilks SS, editors. Applied Logistic Regression. John Wiley and Sons, Inc (2005). p. 31–46. doi: 10.1002/0471722146.ch2
24. Scott DW, Miller WH, Griffin CE. Parasitic skin disease. In: Scott DW, Miller WH, Griffin CE, Muller, GH, editors. Muller and Kirk's Small Animal Dermatology, 6th ed. Philadelphia, PA: Saunders (2001). p. 423–516. doi: 10.1016/B978-0-7216-7618-0.50010-9
25. Hill PB, Lo A, Eden CA, Huntley S, Morey V, Ramsey S, et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet Rec. (2006) 158:533–9. doi: 10.1136/vr.158.16.533
26. Osbaldiston GW. Renal effects of long term administration of triamcinolone acetonide in normal dogs. Can J Comp Med. (1971) 35:28–35.
27. O'Neill DG, Elliott J, Church DB, Mcgreevy PD, Thomson PC, Brodbelt DC. Chronic kidney disease in dogs in UK veterinary practices: prevalence, risk factors, and survival. J Vet Int Med. (2013) 27:814–21. doi: 10.1111/jvim.12090
28. Coit VA, Gibson IF, Evans NP, Dowell FJ. Neutering affects urinary bladder function by different mechanisms in male and female dogs. Eur J Pharmacol. (2008) 584:153–8. doi: 10.1016/j.ejphar.2008.02.037
29. Rau SE, Barber LG, Burgess KE. Efficacy of maropitant in the prevention of delayed vomiting associated with administration of doxorubicin to dogs. J Vet Int Med. (2010) 24:1452–7. doi: 10.1111/j.1939-1676.2010.0611.x
30. Hay Kraus BL. Effect of dosing interval on efficacy of maropitant for prevention of hydromorphone-induced vomiting and signs of nausea in dogs. J Am Vet Med Assoc. (2014) 245:1015–20. doi: 10.2460/javma.245.9.1015
31. Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. (1999) 89:652. doi: 10.1213/00000539-199905000-00023
32. O'Neill DG, Riddell A, Church DB, Owen L, Brodbelt DC, Hall JL. Urinary incontinence in bitches under primary veterinary care in England: prevalence and risk factors. J Small Anim Pract. (2017) 58:685–93. doi: 10.1111/jsap.12731
33. Syakalima M, Takiguchi M, Yasuda J, Hashimoto A. The age dependent levels of serum ALP isoenzymes and the diagnostic significance of corticosteroid-induced ALP during long-term glucocorticoid treatment. J Vet Med Sci. (1997) 59:905–9. doi: 10.1292/jvms.59.905
34. Zhao R, Wang H, Wang X, Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporosis Int. (2016) 28:1027–34.doi: 10.1007/s00198-016-3824-z
Keywords: glucocorticoids, side effects, dogs, veterinary care, VetCompass, corticosteroid, polyuria, polydipsia
Citation: Elkholly DA, Brodbelt DC, Church DB, Pelligand L, Mwacalimba K, Wright AK and O'Neill DG (2020) Side Effects to Systemic Glucocorticoid Therapy in Dogs Under Primary Veterinary Care in the UK. Front. Vet. Sci. 7:515. doi: 10.3389/fvets.2020.00515
Received: 07 May 2020; Accepted: 06 July 2020;
Published: 14 August 2020.
Edited by:
Chi-Chung Chou, National Chung Hsing University, TaiwanReviewed by:
Cengiz Gokbulut, Balikesir University, TurkeyBenito Soto-Blanco, Federal University of Minas Gerais, Brazil
William Whitehouse, Kansas State University, United States
Copyright © 2020 Elkholly, Brodbelt, Church, Pelligand, Mwacalimba, Wright and O'Neill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doaa A. Elkholly, ZGVsa2hvbGx5QHJ2Yy5hYy51aw==
 Doaa A. Elkholly
Doaa A. Elkholly Dave C. Brodbelt
Dave C. Brodbelt David B. Church2
David B. Church2 Ludo Pelligand
Ludo Pelligand Kennedy Mwacalimba
Kennedy Mwacalimba Andrea K. Wright
Andrea K. Wright Dan G. O'Neill
Dan G. O'Neill