- 1Key Laboratory for Animal Disease Resistance Nutrition of the Ministry of Education, Institute of Animal Nutrition, Sichuan Agricultural University, Chengdu, China
- 2Sichuan Dekon Livestock Foodstuff Group, Chengdu, China
- 3College of Food Science, Sichuan Agricultural University, Ya'an, China
The dietary inclusion of phytogenic feed additives to improve the performance and health of sows is considered to be safe, effective and environmentally friendly, thus gaining growing popularity among new strategies. This study was designed with three trials aimed to determine the effective supplemental levels of Scutellaria baicalensis and Lonicera japonica mixed extracts (SLE) in sow diets based on production performance and explore its related mechanisms of action based on serum metabolites, antioxidant capacity, and immune profile of sows and nursing piglets. Trials 1 and 2 were conducted to determine the effective dose and ratio of SLE by supplementation of various proportions and doses of SLE to sows diets from the late pregnancy to weaning, with litter performance at farrowing and weaning and disease conditions being evaluated. Trial 3 was conducted to further explore the mechanisms of action of SLE as evaluated by serum immunity and antioxidants indices in late gestation and lactation sows. The results of trials 1 and 2 showed that dietary supplementation of 1.0 g/kg SLE (50% S. baicalensis extract, 30% L. japonica extract, and 20% wheat bran fiber as carrier) enhanced the number of piglets born alive, litter birth weight, litter weight gain, and average daily feed intake of sows during lactation, while decreased diarrhea of suckling piglets. In Trial 3, compared with the control group, dietary SLE supplementation increased (P < 0.05) sow serum glucose (GLU), triglyceride (TG), total cholesterol (TC), prolactin (PRL) and interleukin-10 (IL-10) concentrations, and total superoxide dismutase (T-SOD) activities at the farrowing, and increased (P < 0.05) sow serum prolactin, leptin, and insulin concentrations at d 14 of lactation. Fat concentrations in sow colostrum and in milk on day 14 of lactation, both IgA and IgG concentrations in colostrum, and both IL-10 and IgA concentrations in piglet serum at d 14 of lactation were all increased (P < 0.05) following dietary SLE supplementation. Altogether, dietary supplementation with the appropriate levels of SLE promoted health and growth of suckling piglets, which was associated with the improvement of maternal metabolism and transmission of antibodies.
Introduction
Milk yield is one of the most important factors limiting neonatal piglet growth (1). Poor milk yield is the most frequent cause of breastfeeding failure (2). For sow-reared piglets, maximum weight gain is limited to as early as seven days after farrowing (3, 4). Besides, genetic selection for highly prolific sows has increased nutrient requirements during gestation and lactation to allow an increased milk yield to support large piglets (4). However, the vigorous metabolism of high-yielding sows during lactation generated extensive reactive oxygen species (ROS) to cause oxidative stress in the body, which resulted in reduced feed intake and milk yield of sows. The growth rate of suckling piglets largely depends on sow milk yield, but the amount of milk produced by sows did not improve significantly, resulting in the slow growth of piglets and the increase of weak and dead piglets (5). The limited nutrient intake would leave sows under severe catabolic status and reduce reproductive performance concurrently (6). One way to increase sow milk yield would be to stimulate mammary development. But sows enter a critical period of mammary gland development and rapid fetal growth at 75 days later in gestation. Maternal metabolic intensity increases and ROS accumulate in the body, which results in increased maternal oxidative stress and immunosuppression, and increased stillbirth and postpartum inflammation. In addition, maternal oxidative stress and low immune status will reduce the content of immune factors in colostrum and change the milk composition, increase the risk of diarrhea in piglets, and cause poor growth and development and even death of piglets. Therefore, optimizing feed intake during lactation, slowing down progressive oxidative stress, and improving immune function are the keys to improving the reproductive performance of sows. It is becoming more and more urgent to find an additive that can improve the milking ability and immune function of sows.
Several herbs and spices are assumed to have beneficial effects on milk secretion. Many herbal products are currently used in the European Union and elsewhere by the feed industry as feed additives (7). Traditional Chinese medicine (TCM), after a long period for being screened, is considered natural, low/non-toxic, showing short resistance and less residue. And its extracts have more functions including antibacterial and antioxidant activities, improving immunity, and regulating hormone secretion (8). Scutellaria baicalensis (S. baicalensis) Georgi and Lonicera japonica (L. japonica) Thunb. are two widely used traditional Chinese herbal medicines, and are officially listed in the Chinese Pharmacopeia. S. baicalensis roots have been used as anti-inflammatory and anticancer agent, for the treatment of bacterial and viral infections of the respiratory and the gastrointestinal tract, and because of its cholagogic, diuretic, and detoxifying properties. Baicalin is the most abundant component of S. baicalensis extracts, which could alleviate the adverse effect of heat stress and showed anti-allergic, anti-tumor, anti-inflammatory, and antioxidant activities (9, 10). Lonicera japonica contains a variety of organic acids, essential oils, flavones, saponins, and iridoids. Among them, chlorogenic acid and essential oils are the primary pharmacological compounds in L. japonica. Modern pharmacological studies showed that L. japonica and its extracts possessed wide pharmacological actions, such as antibacterial, anti-inflammatory, antiviral, antiendotoxin, antioxidant, antipyretic, and excitation of nerve centers (11–13). In clinical practice, the L. japonica extracts have been used for the treatment of fever, heatstroke, bloody flux, sores, carbuncles, furunculosis, headache, and some infectious diseases (11).
In weaning and growing-finishing pigs, many positive reports are available on the herbal extract supplementation diets (14–17). However, there is little information on responses of gestation and lactation sows to dietary herbal extract supplementation. Therefore, the objectives of the present study were to determine the effect of dietary herbal supplements on performance of sows and their progeny, and explore the underlying mechanisms as evaluated by serum metabolites, antioxidant capacity, and immune function of sows and suckling piglets.
Materials and methods
Ethics approval
The protocol of this study was approved by the Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University (Ethics Approval Code: SCAUAC201606-6), and was carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Animals, diets, and experimental design
The study was designed with three trials. Trials 1 and 2 were conducted to determine the effective dose and ratio of S. baicalensis (SBE) and L. japonica (LJE) mixed extracts (SLE) by supplementation of various proportions and doses of SLE to sow diets from the late pregnancy to weaning, with litter performance at farrowing and weaning and disease conditions being evaluated. Trial 3 was conducted to further explore the mechanisms of action of SLE as evaluated by immunity and antioxidants indices during the late gestation and lactation period. Basal diets for all sows contained corn as a cardinal energy source and soybean meal as a cardinal protein source. Diets for sows during the late pregnancy (from day 85 of gestation to farrowing) and lactation were formulated to meet or exceed the NRC (2012) recommendations (Table 1). The herbal extract from S. baicalensis and L. japonica was extracted by a combination of ultrasound and microwave systems. The SLE products were provided by Beijing Centre Biology Co., Ltd., (Beijing, China).
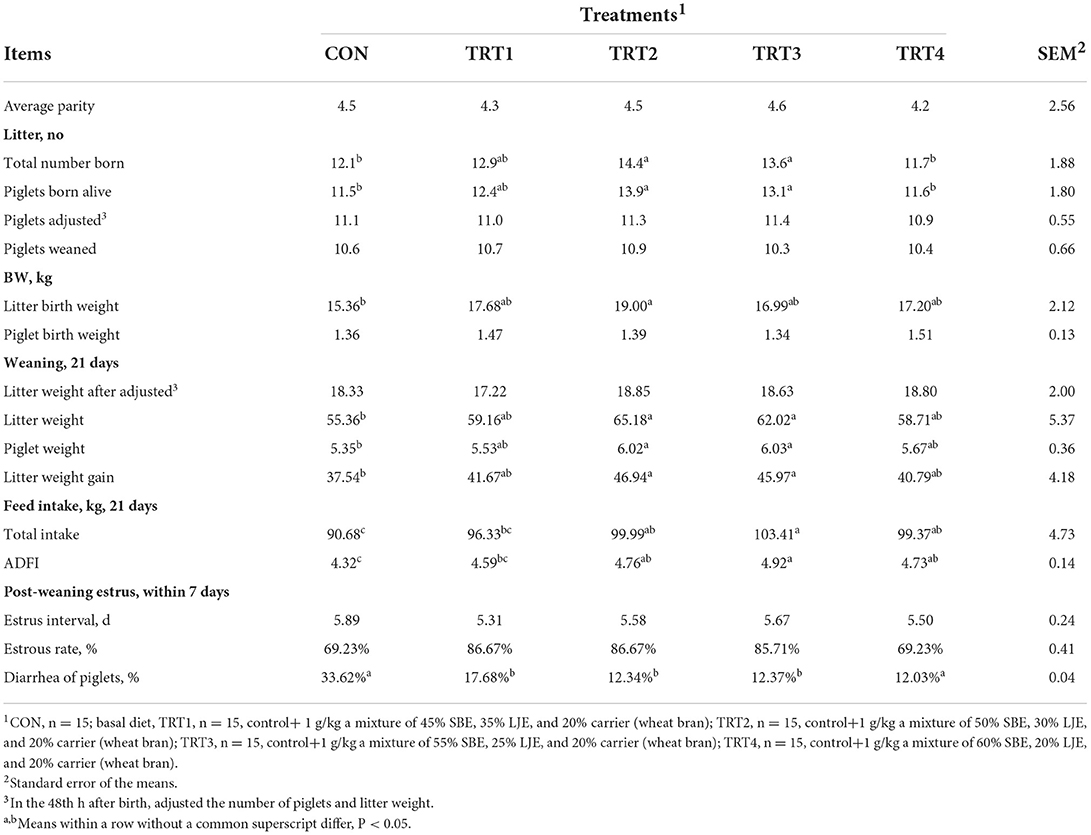
The objective of Trial 1 was to determine the optimal proportion of the two extracts (SBE and LJE) in the SLE mixture as evaluated by the farrowing and lactation performance. Trial 1 was conducted in a pig farm in Fujian province from July to August, with an average room temperature being 28–30°C. According to a completely randomized block design, a total of 75 Yorkshire × Landrace sows (weighing 275.55 ± 20.97 kg, mean parity 4.44 ± 1.84) on the 85th day of pregnancy were assigned based on genetic background, parity and body weight to five experiment groups: control (CON, n = 15; basal diet, Table 1), treatment group 1 (TRT1, n = 15, basal diet + 1 g/kg SLE powder product consisting of 45% SBE, 35% LJE and 20% wheat bran as carrier); treatment group 2 (TRT2, n = 15, basal diet + 1 g/kg a mixture of 50% SBE, 30% LJE, and 20% carrier); treatment group 3 (TRT3, n = 15, basal diet + 1 g/kg a mixture of 55% SBE, 25% LJE, and 20% carrier); and treatment group 4 (TRT4, basal diet +1 g/kg a mixture of 60% SBE, 20% LJE, and 20% carrier).
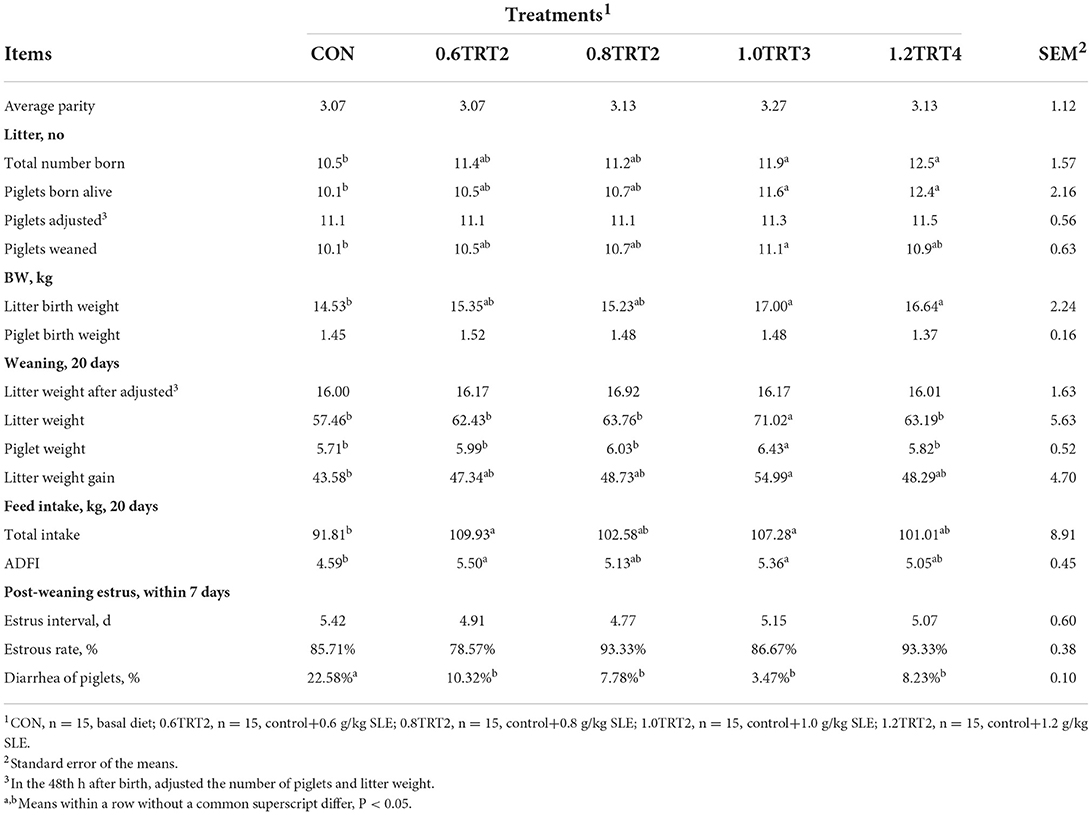
The objective of Trial 2 was to determine the optimal dietary supplementation level of the SLE mixture (50% SBE, 30% LJE, and 20% carrier) that was observed to be effective in improving sow performance in Trial 1. Trial 2 was carried out on a pig farm in Sichuan province from November to December. A total of 75 Yorkshire × Landrace sows (weighing 261.42 ± 25.74 kg, mean parity 3.13 ± 1.87) on the 85th day of pregnancy were assigned based on parity and body weight to five experiment groups: Control (CON, n = 15; basal diet, Table 1), 0.6TRT2 (basal diet + 0.6 g/kg SLE), 0.8TRT2 (basal diet + 0.8 g/kg SLE), 1.0TRT2 (basal diet + 1.0 g/kg SLE), and 1.2TRT2 (basal diet + 1.2 g/kg SLE). The basal diet was isoenergy but 1.5% lower in CP than diet shown in Table 1.
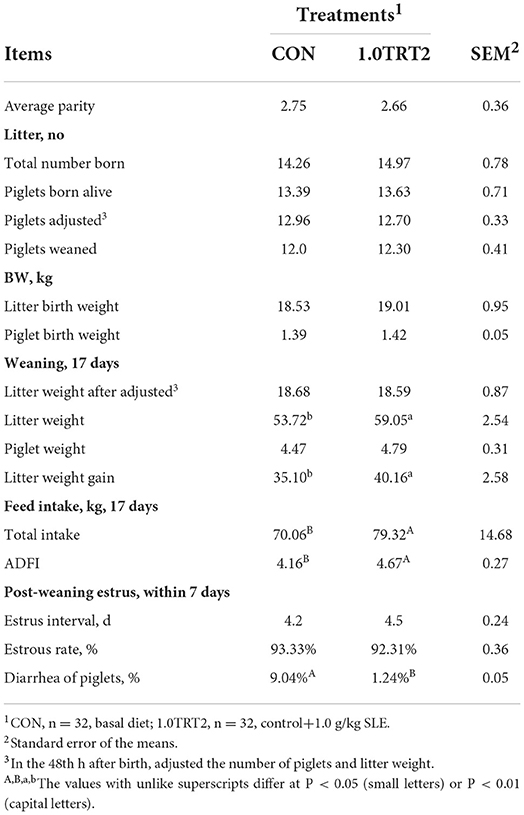
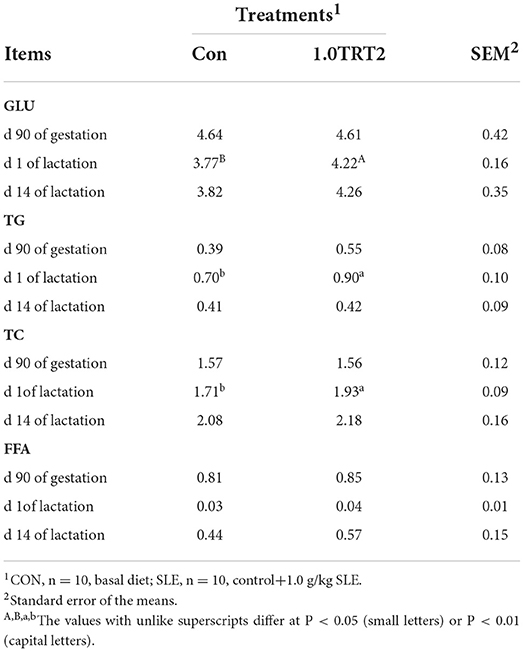
The objective of Trial 3 was to further verify the positive role of dietary SLE mixture supplementation at 1.0 g/kg diet (Table 1) on farrowing and lactation performance, and at the same time explore the mechanisms of action of SLE as evaluated by immunity and antioxidants indices based on blood and milk samples. Trial 3 was carried out on a pig farm in Sichuan province from May to July. A total of 64 Yorkshire × Landrace sows (mean weight 250.15 ± 10.35 kg, mean parity 2.70 ± 1.43) on the 85th day of pregnancy were assigned based on genetic background, parity and body weight to two experiment groups: Control (CON, n = 32; basal diet) and 1.0 TRT2 (SLE, n = 32, basal diet + 1 g/kg of SLE).
Feeding management
Sows were fed 3.0 kg/d from d 85 to 112 of pregnancy, and then fed 2.0 kg/d from d 113 of pregnancy to farrowing. After farrowing, sows were fed 2.0 kg of diet, which was increased by 1 kg/d in the following 3 days, and then sows had free access to feed from d 5 of lactation until weaning. During the gestation period, sows were housed in individual cages with concrete floors and equipped with automated drop feeders and nipple drinkers. On d 109 of gestation, sows were transported to the farrowing facility, where they were housed in individual farrowing crates (2.4 × 2.2 m) with creep area and nipple drinkers. Litters were standardized to be 12–14 piglets within 48 h after birth. Voluntary feed intake was measured daily throughout the lactation period. According to the practical situation of the pig farm in each trial, piglets were weaned at 21, 20, and 17 d postnatal, respectively, in trials 1, 2, and 3.
The feed consumption during lactation was recorded for each sow to calculate the average daily feed intake (ADFI). During the experimental period, numbers of piglets alive and dead per litter were recorded to calculate the survival ratio. Piglet body weight (BW) was recorded on d 1 (within 12 h of birth) and weaning day. In the 48th h after birth, the number of piglets in sows was adjusted so that litter piglets of sows were consistent among groups, and the number of piglets and litter weight were recorded. No creep feed was offered to piglets throughout the lactation period. The health status of sows and piglets were recorded daily during the lactation period. After weaning, the estrus interval (within 7 days) was recorded for each sow.
Sample collection
In Trial 3, blood samples of sows were collected from the ear vein using sterile vacuum tubes from 20 randomly chosen sows on d 90 of gestation and d 1, d 14 of lactation before the morning feeding. At d 14 of lactation, blood samples were taken by jugular venipuncture using sterile vacuum tubes from 40 piglets selected from the 20 sows used for sow blood collection, with one male and one female piglet selected from each sow. After blood collection, serum were separated by centrifugation at 3,000 rpm for 5 min and then serum samples were collected and stored in sterile tubes at −20°C until analysis.
Colostrum was collected from 20 randomly chosen sows on d 1 of lactation within 4 h after initiation of farrowing. Milk was collected on d 14 of lactation after intramuscular injection of 10 IU of oxytocin behind the ear. After collection, milk (5 ml) for determination of hormone and immune indices was centrifuged (3,500 rpm) for 20 min to remove the fats and then the supernatant was collected and stored at −20°C until analysis.
Chemical analysis
The glucose (Glu), triglyceride (TG), total cholesterol (TC), and free fatty acid (FFA) were measured using an auto-analyzer (BMD/Hitachi 7050 Auto Analyser; Japan). Whole milk was analyzed for dry matter, protein, fat, and lactose contents by a Milkyway-cp2 rapid milk composition analyzer (Institute of Food Science and Fermentation Engineering, Zhejiang University). The cytokines (IL-8, IL-10, TNF-α) and immunoglobulin (IgG, IgA, IgM) concentrations in colostrum, milk, and serum samples were determined using specific pig-ELISA quantification kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to instructions of their respective manufacturers, respectively. The serum concentrations of insulin (INS), prolactin (PRL), thyroid-stimulating hormone (TSH), leptin, and growth hormone (GH) in sows were measured by respective ELISA quantification kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Assessment of total antioxidant capacity (T-AOC) and total superoxide dismutase (T-SOD) in serum from sows and piglets were performed using specific assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analyses
All data were checked for normality using the univariate procedure of Statistical Analysis Software (Version 9.4, SAS Institute Inc., Cary, NC, USA) and transformed, if required. The data of litter weight at weaning, litter weight gain and the number of weaned piglets were assessed by analysis of covariance using the general linear model (GLM) procedure of SAS, and the adjusted litter weight and litter number were used as covariates. The data of piglets born alive, litter birth weight, and piglet birth weight were assessed using the total number born as covariate by covariance analysis. The data of diarrhea of piglets and estrous rate were analyzed by χ2-test. The data of the estrus interval was analyzed by rank-sum test. The other data were analyzed with one-way analysis of variance (ANOVA) using the GLM procedure of SAS software. CONTRAST was used to compare differences with the control group. Differences were considered significant when P ≤ 0.05, and trends were noted when 0.05 < P < 0.10. Quadratic effects were not significant, and hence their P-values were omitted from the tables.
Results
Sows and piglets performance
As presented in Table 2, the performance of sows and piglets was affected by diet. Sows in the TRT2 showed significantly increased total number born, number born alive, litter birth weight, litter weight and piglet weight of weaning at 21 days, litter weight gain, total intake and ADFI of sows at day 21 of lactation, and significantly decreased piglets diarrhea compared with sows in the CON treatment (P < 0.05). However, the number of weaned piglets, piglet birth weight, weaning-estrous interval, and estrous rate within 7 days were not affected (P > 0.05) by SLE dietary supplementation. TRT3 and TRT2 had the same trend, but no difference (P > 0.05) in reproductive performance was observed between other treatments. Therefore, we would choose TRT2 [50% SBE, 30% LJE, and 20% carrier (wheat bran), SLE] as the optimized mixed proportion for Trial 2.
As presented in Table 3, compared with sows in the CON treatment, sows in the 1.0TRT2 significantly increase the total number born, number born alive, number of weaned piglets, litter birth weight, litter weight and piglet weight of weaning at 20 days, litter weight gain, total intake and ADFI of sows at day 20 of lactation (P < 0.05) and significantly decreased piglets' diarrhea (P < 0.05). There was no effect (P > 0.05) on piglet weight of birth, weaning-estrous interval, and estrous rate. In addition, 0.6TRT2 sows had higher total feed intake and ADFI (20 days) (P < 0.05) compared with CON sows. No difference (P > 0.05) in reproductive performance was observed among treatments. Therefore, the 1.0TRT2 (1.0 g/kg SLE) was selected as the optimum content for Trial 3.
As presented in Table 4, the lactation ADFI and total intake (17 days) were significantly increased (P < 0.01) by dietary supplementation of 1.0 g/kg SLE. In addition, compared with the CON group, the 1.0TRT2 group had significantly increased (P < 0.05) litter weight at weaning and litter weight gain and decreased (P < 0.05) diarrhea of piglets during lactation. The number of piglets born alive, litter birth weight, number of weaned piglets, and weaning-estrous interval were not affected (P > 0.05) by SLE supplementation.
Blood profiles of sows
As presented in Table 5, there were no differences (P > 0.05) in serum concentrations of GLU, TG, TC, and FFA between groups at d 90 of gestation and d 14 of lactation. Compared with the CON group, dietary SLE supplementation significantly increased serum GLU (P < 0.01) and TG and TC (P < 0.05) concentrations at the farrowing day (Table 5).
Serum hormones of sows
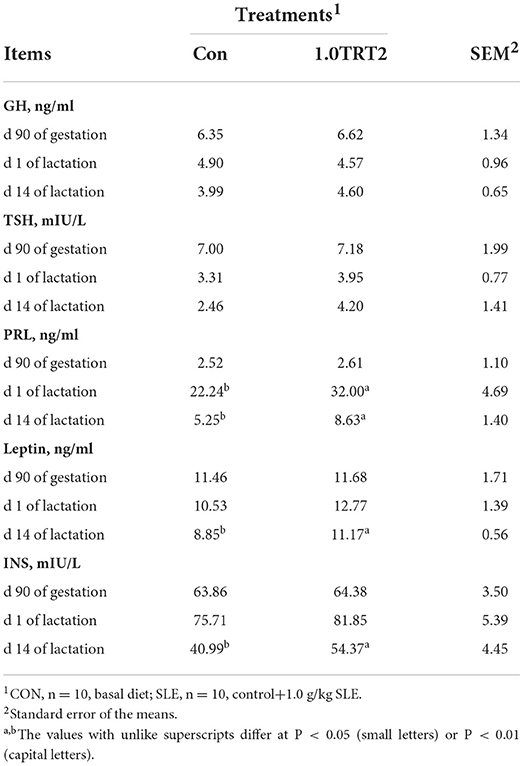
As presented in Table 6, there were no differences (P > 0.05) in the concentrations of GH, TSH, PRL, INS, and leptin in sow serum between groups at d 90 of gestation. Compared with the CON group, dietary SLE supplementation significantly increased sow serum PRL concentrations at the farrowing day and significantly increased serum PRL, leptin, and INS concentrations at d 14 of lactation (P < 0.05). However, the concentrations of GH and TSH in sow serum on days 1 and 14 of lactation were not affected (P > 0.05) by SLE diet treatments.
Serum immunization index of sows
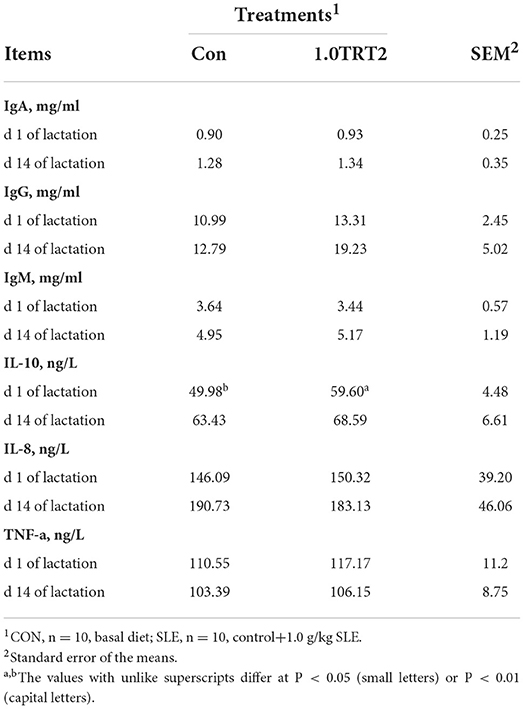
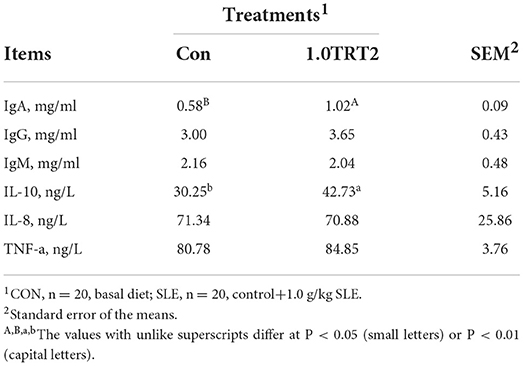
As presented in Table 7, compared with the CON group, dietary SLE supplementation significantly increased serum IL-10 (P < 0.05) concentrations at farrowing (Table 7). But the humoral immunity factor contents of IgA, IgG, IgM, IL-8, and TNF-α in sow serum were not affected (P > 0.05) by dietary SLE supplementation.
Colostrum and milk conventional ingredients
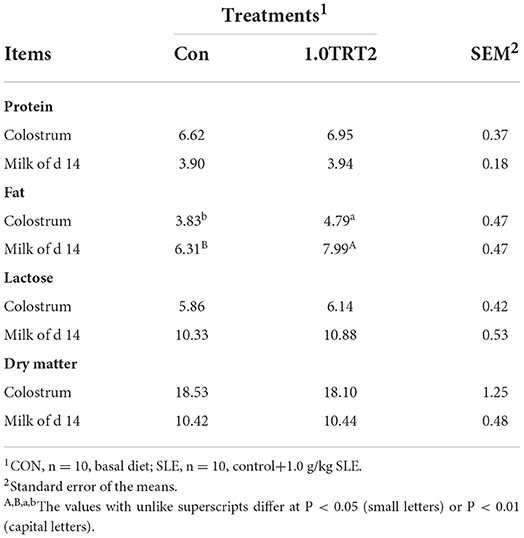
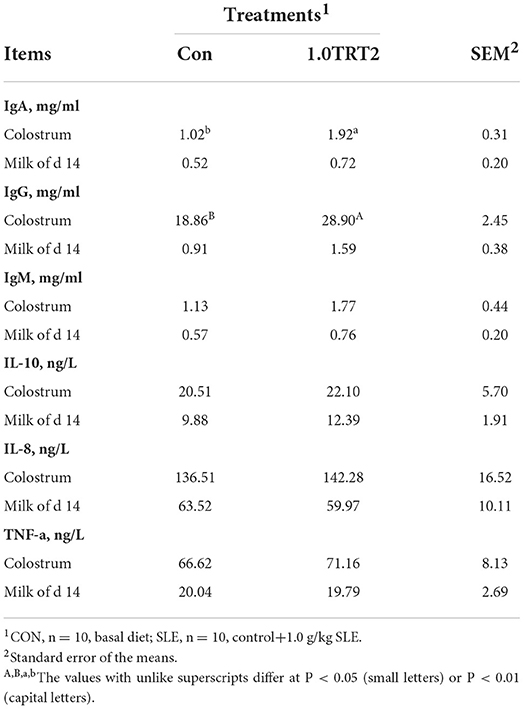
Colostrum and milk composition (i.e., dry matter, protein, and lactose) was not affected by dietary treatment (P > 0.05). Compared with the CON group, dietary SLE supplementation significantly increased fat concentrations in sow colostrum and milk on day 14 of lactation (P < 0.05; Table 8). As presented in Table 9, sows receiving SLE had significantly higher colostrum concentrations of IgA (P < 0.05) and IgG (P < 0.01) than the CON sows. However, no significant difference (P > 0.05) was observed in concentrations of IL-10, IgM, IL-8, and TNF-a in colostrum and milk on day 14 of lactation between the two groups.
Serum immunization index of piglets
As presented in Table 10, compared with the CON group, dietary SLE supplementation significantly increased concentrations of IL-10 (P < 0.05) and IgA (P < 0.01) in serum at d 14 of piglets. There were no differences (P > 0.05) in concentrations of IgG, IgM, IL-8, and TNF-α in serum between groups at d 14 of piglets.
Serum SOD and T-AOC activity levels of sows and piglets
The impact of dietary SLE supplementation on serum antioxidant indexes is shown in Figure 1. The T-SOD activity in sow serum on d 1 of lactation were significantly increased compared with the CON group (P < 0.05; Figure 1A). However, there were no differences (P > 0.05) in sow serum T-AOC activity between groups at farrowing and d 14 of lactation (Figure 1B). The activities of T-AOC and T-SOD in piglet serum were not affected by dietary SLE supplementation (Figures 1C,D).
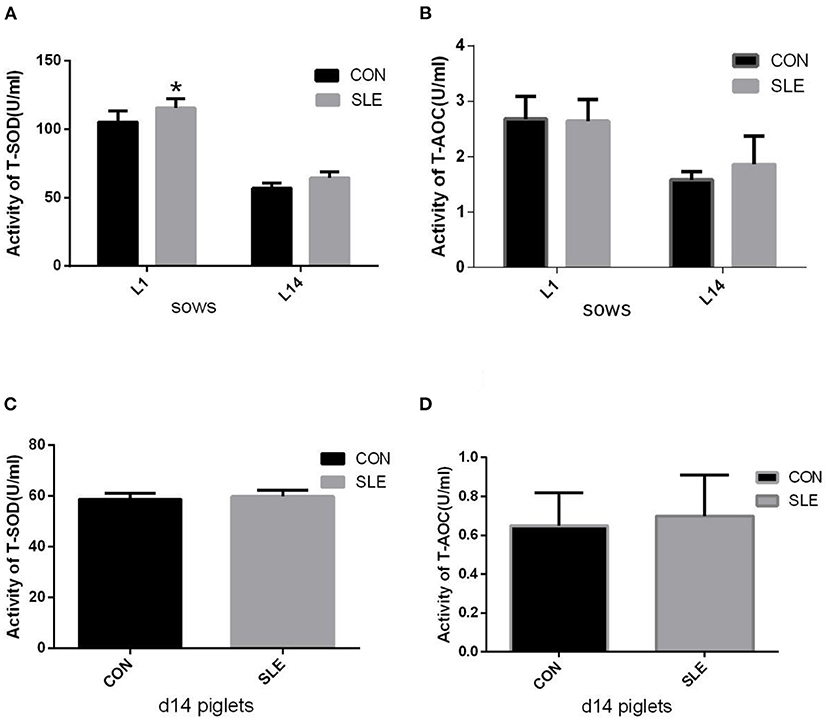
Figure 1. The effects of dietary S. baicalensis and L. japonica extract supplementation on serum anti-oxidative capacity of sows and piglets. (A,B) Total superoxide dismutase (T-SOD) and total antioxidant capacity (T-AOC) activities in serum of sows (n = 10); (C,D) T-SOD and T-AOC activities in serum of d14 piglets (n = 20). *Indicating a significant difference (P < 0.05). Data show the means ± standard deviation (SD).
Discussion
Herbal medicines have been tested extensively in swine diets as potential alternatives to antibiotics growth promoters owing to antiviral, antibacterial, and antioxidant properties, stimulation of the immune system, and improvement of digestibility and absorption of nutrients (18–20). However, the clinical efficacy has huge differences with different compatibility ratios and dosages of herbs and extracts (21). According to the “Chinese Pharmacopeia” records, the proportion of L. japonica (chlorogenic acid 12 g/tube) and S. baicalensis (baicalin 24 g/tube) is 1:2 in the Yinhuang Oral Liquid, which is beneficial for fever, cough, hemoptysis, jaundice, dysentery, acute conjunctivitis, carbuncle, and furuncle, and prevents abnormal fetal movements (22). But despite these advantages, information available in the previous literature on the response of supplementing L. japonica and S. baicalensis in swine is scarce. In the present studies, dietary herbs extract mixture supplementation exerting positive effects on production performance in sows and nursing piglets were observed in all three trials, and the effective dose of SLE was observed to be 1.0 g/kg diet with the ratio of SBE and LJE in the SLE mixture being 50 and 30%, respectively. The feed intake during lactation period is a key factor to limit the production performance of sows, and maximum sow milk output requires that feed intake was maximized (4). Previous studies have confirmed that dietary herb extract supplementation could contribute to the desired organoleptic qualities of the diets and stimulate the appetite, as well as improve digestive tract function by increasing hydrochloric acid and enzyme secretion, thus improving the feed intake and lactation yield of sows (8, 23). Likewise, there were many reports on improved feed intake through herb extract additives in sows or pigs diets (15–18, 24–27). In addition, the herbal ingredients' metabolites can improve growth performance and reduce diarrhea in nursing piglets through breast milk (18, 28). Liu et al. indicated that dietary supplementation of herbal extract mixture (55% S. baicalensis and 25% L. japonica) at 0.5 and 1.0 g/d could alleviate heat stress, improve the feed intake and dry matter digestibility of sows during lactation, and reduce diarrhea and enhance daily gain in piglets (29), which were similar to observations in the current studies. However, the differences in the total number born may not be related to the SLE in this study because the SLE mixture added to the sow feed was started on the 85th day of pregnancy.
A high feed intake of lactating sows can increase piglets' growth performance and positively influence subsequent reproduction (30). Furthermore, Oliviero et al. demonstrated that a sow diet has a profound effect on the robustness of piglets (31). In the current studies, piglets weaned from SLE-supplemented sows had a greater weaning weight and overall ADG than the CON group, which suggested that SLE supplementation improved sow milk production. This improvement in milk output may be due to enhanced feed intake and nutrient digestibility. Similarly, the inclusion of herbal extract blends in lactation diet were shown to enhance piglet performance and result in higher weight at weaning (32). Zhong et al. also observed that supplementation of 0.04% phytogenic additive to sows positively affected feed intake and milk production of sows and litter performance (33). In addition, Liu et al. demonstrated that the antibacterial activity and anti-inflammatory properties of S. baicalensis and L. japonica provided a beneficial effect on the immune system and even reduced subclinical or clinical infections, subsequently benefiting the health of the pigs, which could account for enhanced growth performance (18). Watson also confirmed that the inclusion of herbs could lead to an improvement in antibody levels of colostrum and increase milk quality (34). Redoy et al. suggested that dietary supplementation with herbs (Plantago lanceolata L. and Allium sativum) could prevent the undesirable microorganisms' reproduction and stimulate the secretion of antibodies, thus positively influencing the colostral concentration of IgA and IgG, serum immunocompetence, and growth performance of animals (35). On the other hand, the beneficial properties of herbal extract such as antimicrobial, antiviral, and stimulation of the immune system could improve uterine involution and protect the sow from possible postpartum urogenital infections (36). As expected, the current study indicated that SLE supplementation decreased piglets' diarrhea, which also could account for the enhanced growth performance in this study.
Notably, relative to control sows, sows fed SLE had increased serum concentrations of triglycerides, glucose, and cholesterol on farrowing. Modern genotype sows' farrowing is a long process, and yet prolonged labor could lead to dystocia and even stillbirth. And part of the reason for the prolonged labor is insufficient energy supply (18). Glucose in serum is the main source of energy for tissue cells in the sow. In this regard, improving serum concentrations of glucose and triglycerides could help to reduce sows' stress in farrowing, which is conducive to the progress of farrowing and reduces labor. The increased triglycerides and glucose concentrations in the serum of sows receiving herbal extracts indicate the latter's homeostasis-promoting effects (18, 37). Meng et al. and Ruan et al. also indicated that chlorogenic acid could regulate glucose metabolism, and improve lipids and enzymes involved in lipid metabolism in the organism (38, 39). More than 95% of the fatty acids in the cream were in the form of triglycerides, and the ingredients needed for the synthesis of fat in the milk were derived from blood fats (1). The content of triglycerides in serum of farrowing sows was significantly increased, suggesting that more triglycerides in plasma may be absorbed by the body from the peripheral circulation of the blood and used for the synthesis of milk fat (1). However, Yan et al. suggested that the increased concentrations of serum cholesterol and triglycerides might be resulted from homeostasis and the promotion of intestinal lipid absorption, respectively (16, 27). Myer et al. indicated that the formation of fat deposits depended on the level of serum triglyceride, which is accompanied by increased triglyceride and cholesterol levels. The possible reason was the increase of fatty acid synthase and the outcome of combined action of hormone sensitive enzyme activity (40). However, contrary to the results of this experiment, chlorogenic acid and baicalein had a certain regulating effect on body fat metabolism. Chlorogenic acid seemed to be more potent for bodyweight reduction and regulation of lipid metabolism than caffeic acid (41, 42). Baicalein reduces body cholesterol content by inhibiting cholesterol acyltransferase activity and cholesterol absorption, which was also clarified in earlier studies (18, 43–45).
Interleukin 10 (IL-10) is a pleiotropic cytokine with an extensive spectrum of biological effects in immunoregulation and inflammation (46). As previously reported, chlorogenic acid had a certain regulation effect on organism immunity and adaptive immunity in the regulation of inflammation and immunity, which can regulate the number of white blood cells, the function of macrophages, expression of cytokines secretion, and immune cell activation factors (47, 48). Baicalin and baicalein not only have obvious anti-inflammatory and immunosuppressive effects but also can improve the functions of macrophages and NK cells (49, 50). In the present study, serum IL-10 levels in sows and piglets increased, showing an improvement in cellular and humoral immunity of offspring in response to SLE supplementation of sows during late gestation and nursing period.
Besides providing energy and nutrients for piglets, sow colostrum's most important function was to activate the immune system and equip piglets with specific and non-specific immunity protection functions (46, 51). Feeding lactating sows a diet supplemented with SLE increased colostrum IgG and IgA concentrations in sows and serum IgA concentrations in piglets, indicating that the active compounds in SLE were deposited in sow milk. Wang et al. reported dietary herbal extracts supplementation increased colostrum IgG and IgA concentrations (52). Thus, these findings substantiate that dietary supplementation of sows with SLE during late gestation and lactation could significantly improve serum IgA and IL-10 of piglets. These may be because active substances in SLE from colostrum and milk effectively strengthen the provision of resources for supporting cells and the immune system of piglets by regulating lipid and protein metabolism (12, 13, 38). These results indicate that dietary SLE may improve weaned piglets' immune function and resistance to pathogenic microorganisms infection and attenuate stress injury on the organism.
The antioxidant status of an organism is critical for maintaining animal health and can be affected by nutrients (53, 54). Due to the antioxidant properties contained in herbs, the use of herbs as additives is important for the antioxidant system and stress tolerance of animals. Previous studies have stated that S. baicalensis inhibited lipid peroxidation in rat liver homogenate (55). Su et al. also clarified the antioxidant effect of L. japonica extract in rats (56). Shang et al. demonstrated that SLE supplementation generated a decrease in serum cortisol that could be attributed to the anti-stress and sedative properties (11). Consistent with the antioxidant function, the current study stated that supplementation of SLE mixture in sow diets significantly increased T-SOD activities in sow serum on day one of lactation. Wang et al. and Huang et al. elucidated that the antioxidant roles of S. baicalensis have been traced to several of its flavones, which include wogonin, baicalin, baicalein, and the skullcap flavone (57, 58). Choi et al. clarified that the anti-oxidative activity of L. japonica was attributed to polyphenols, flavones, iridoids, and saponins, which exhibit various antioxidant properties (59). Taking into account that increased systemic oxidative stress is observed throughout lactation in sows and that high energy metabolic demands in the lactation process accelerate mitochondrial oxidative stress and reactive oxygen species production (60, 61), it is necessary to prevent oxidative stress by SLE supplementation during lactation.
Conclusion
In summary, this study demonstrated that supplementation of 1.0 g/kg SLE [50% S. baicalensis, 30% L. japonica extract mixture, and 20% carrier (wheat bran)] in sow diet in late gestation and during lactation was the optimum content and optimized mixed-proportion that could improve immunity and production performance of sows and nursing piglets. The SLE supplementation increased immune molecules in sows' serum and milk, which is beneficial to piglet health and growth through a transmission effect.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University.
Author contributions
ZF and DW designed the study. LW and BH performed experiments. LW, BH, LH, LC, BF, YL, and SX performed data analysis. BH, LW, and ZF wrote the draft and revised the manuscript. All authors have read and approved the manuscript.
Funding
This work was supported by Beijing Centre Biology Co. Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boyd RD, Kensinger RS. Metabolic Precursors for Milk Synthesis. The Lactating Sow (1998). Available online at: https://www.researchgate.net/publication/283276946 (accessed January 1, 1998).
2. Zuppa AA, Sindico P, Orchi C, Carducci C, Cardiello V, Romagnoli C, et al. Safety and efficacy of galactogogues: substances that induce, maintain and increase breast milk production. J Pharm Pharm Sci. (2010) 13:162–74. doi: 10.18433/J3DS3R
3. NRC. Nutrient Requirements of Swine, 11th ed. National Research Council, Academic Press, Washington, DC (2012).
4. Ball RO, Samuel RS, Moehn S. Nutrient requirements of prolific sows. In: Proceedings of the Advances in Pork Production: Proceedings of the Banff Pork Seminar. Edmonton (2008). Available online at: https://www.researchgate.net/publication/285264007
5. Miller YJ, Collins AM, Smits RJ, Thomson PC, Holyoake PK. Providing supplemental milk to piglets preweaning improves the growth but not survival of gilt progeny compared with sow progeny. J Anim Sci. (2012) 90:5078–85. doi: 10.2527/jas.2011-4272
6. Sulabo RC, Tokach MD, Dritz SS, Goodband RD, DeRouchey JM, Nelssen JL. Effects of varying creep feeding duration on the proportion of pigs consuming creep feed and neonatal pig performance. J Anim Sci. (2010) 88:3154–62. doi: 10.2527/jas.2009-2134
7. Maenner K, Vahjen W, Simon O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. J Anim Sci. (2011) 89:2106–12. doi: 10.2527/jas.2010-2950
8. Wenk C. Herbs and botanicals as feed additives in monogastric animals. Asian Austral J Anim Sci. (2003) 16:282–9. doi: 10.5713/ajas.2003.282
9. Liao HF, Ye J, Gao LL, Liu YL. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: a comprehensive review. Biomed Pharmacother. (2021) 133:110917. doi: 10.1016/j.biopha.2020.110917
10. Li J, Khan IA. The advanced bioactivity studies of scutellaria baicalensis georgi and its phenolic compounds. Acta Hortic. (2006) 720:157–70. doi: 10.17660/ActaHortic.2006.720.15
11. Shang XF, Pan H, Li MX, Miao XL, Ding H. Lonicera japonica Thunb: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. (2011) 138:1–21. doi: 10.1016/j.jep.2011.08.016
12. Ma FT, Shan Q, Jin YH, Gao D, Li HY, Chang MN, et al. Effect of Lonicera japonica extract on lactation performance, antioxidant status, and endocrine and immune function in heat-stressed mid-lactation dairy cows. J Dairy Sci. (2020) 103:10074–82. doi: 10.3168/jds.2020-18504
13. Shinomiya K, Omichi J, Ohnishi R, Ito H, Yoshida T, Kamei C. Effects of chlorogenic acid and its metabolites on the sleep-wakefulness cycle in rats. Eur J Pharmacol. (2004) 504:185–9. doi: 10.1016/j.ejphar.2004.09.054
14. Tsai CH, Lin FM, Yang YC, Lee MT, Cha TL, Wu GJ. Herbal extract of wedelia chinensis attenuates androgen receptor activity and orthotopic growth of prostate cancer in nude mice. Clin Cancer Res. (2009) 15:5435–44. doi: 10.1158/1078-0432.CCR-09-0298
15. Yan L, Meng QW, Kim IH. The effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics and fecal noxious gas content in growing pigs. Livest Sci. (2011) 141:143–7. doi: 10.1016/j.livsci.2011.05.011
16. Yan L, Meng QW, Kim IH. The effects of dietary houttuynia cordata and taraxacum officinale extract powder on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. Livest Sci. (2011) 141:188–93. doi: 10.1016/j.livsci.2011.05.017
17. Huang CW, Lee TT, Shih YC, Yu B. Effects of dietary supplementation of chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J Anim Physiol An N. (2012) 96:285–94. doi: 10.1111/j.1439-0396.2011.01151.x
18. Liu WC, Pi SH, Kim IH. Effects of Scutellaria baicalensis and Lonicera japonicaextract mixture supplementation on growth performance, nutrient digestibility, blood profiles and meat quality in finishing pigs. Ital J Anim Sci. (2016) 15:446–52. doi: 10.1080/1828051X.2016.1202736
19. Costa LB, Luciano FB, Miyada VS, Gois FD. Herbal extracts and organic acids as natural feed additives in pig diets. S Afr J Anim Sci. (2013) 43:181–93. doi: 10.4314/sajas.v43i2.9
20. Oetting LL, Utiyama CE, Giani PA, Ruiz UDS, Miyada VS. Effects of herbal extracts and antimicrobials on apparent digestibility, performance, organs morphometry and intestinal histology of weanling pigs. Rev Bras Zootecn. (2006) 35:2013–7. doi: 10.1590/S1516-35982006000700019
21. Ewa H, Małgorzata S, Eugeniusz RG. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. (2015) 108:61–6. doi: 10.1016/j.meatsci.2015.05.020
22. Chinese Pharmacopeia Commission. Pharmacopoeia of the People's Republic of China (I Volumes). Peking: China Medical Science and Technology Press (2010).
23. Frankic T, Voljč M, Salobir J, Rezar V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric Slov. (2009) 94:95–102. http://aas.bf.uni-lj.si/zootehnika/94-2009/PDF/94-2009-2-95-102.pdf
24. Peeters E, Driessen B, Steegmans R, Henot D, Geers R. Effect of supplemental tryptophan, vitamin e, and a herbal product on responses by pigs to vibration. J Anim Sci. (2004) 82:2410–20. doi: 10.2527/2004.8282410x
25. Szewczyk A, Hanczakowska E, Swiatkiewicz M. The effect of nettle (Urtica Dioica) extract on fattening performance and fatty acid profile in the meat and serum lipids of pigs. J Anim Feed Sci. (2006) 15:81–84. doi: 10.22358/jafs/70148/2006
26. Wang Y, Chen YJ, Cho JH, Yoo JS, Wang Q, Huang Y. The effects of dietary herbs and coral mineral complex on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. J Anim Feed Sci. (2007) 16:395–8. doi: 10.22358/jafs/66796/2007
27. Yan L, Meng QW, Kim IH. Effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics, and fecal microbial shedding in weanling pigs. Livest Sci. (2012) 145:189–95. doi: 10.1016/j.livsci.2012.02.001
28. Namkung H, Li M, Gong J, Yu H, Cottrill M, Lange CD. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. (2004) 84:697–704. doi: 10.4141/A04-005
29. Liu WC, Yun HM, Pi SH, Kim IH. Supplementing lactation diets with herbal extract mixture during summer improves the performance of sows and nursing piglets. Ann Anim Sci. (2017) 17:835–47. doi: 10.1515/aoas-2016-0084
30. Strathe AV, Bruun TS, Hansen CF. Sows with high milk production had both a high feed intake and high body mobilization. Animal. (2017) 11:1913–21. doi: 10.1017/S1751731117000155
31. Oliviero C, Kokkonen T, Heinonen M, Sankari S, Peltoniemi O. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res Vet Sci. (2009) 86:314–9. doi: 10.1016/j.rvsc.2008.07.007
32. Ilsley S, Miller H, Greathead H, Kamel C. Herbal sow diets boost preweaning growth. Feed Mix. (2002) 10:24–5. doi: 10.1017/S1752756200006797
33. Zhong MD, Wu D, Lin Y, Fang ZF. Phytogenic feed additive for sows: effects on sow feed intake, serum metabolite concentrations, IgG level, lysozyme activity and milk quality. J Agr Sci Tech-iran. (2011) 1:802–10. http://qikan.cqvip.com/Qikan/Article/Detail?id=40018923
34. Watson DL. Immunological functions of the mammary gland and its secretion–comparative review. Aust J Biol Sci. (1980) 33:403–22. doi: 10.1071/BI9800403
35. Redoy MRA, Shuvo AAS, Cheng L, Al-Mamun M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal. (2020) 14:2433–41. doi: 10.1017/S1751731120001196
36. Shokoh P, Anousheh ZK, Hamid RBR, Hadi N, Ahmad FI, Safian S, et al. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants. (2020) 9:1309–45. doi: 10.3390/antiox9121309
37. Yan SQ, Wang YR, Liu PP, Chen A, Chen MY, Yao D, et al. Baicalin attenuates hypoxia-induced pulmonary arterial hypertension to improve hypoxic cor pulmonale by reducing the activity of the p38 MAPK signaling pathway and MMP-9. Evid Based Complement Alternat Med. (2016) 2546402:1–9. doi: 10.1155/2016/2546402
38. Meng S, Cao XJM, Feng Q, Peng JH, Hu YY. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med. (2013) 2013:801457. doi: 10.1155/2013/801457
39. Ruan Z, Yang YH, Zhou Y, Wen YM, Ding S, Liu G, et al. Metabolomic analysis of amino acid and energy metabolism in rats supplemented with chlorogenic acid. Amino Acids. (2014) 46:2219–29. doi: 10.1007/s00726-014-1762-7
40. Myer RO, Johnson DD, Knauft DA, Gorbet DW, Brendemuhl JH, Walker WR. Effect of feeding high-oleic-acid peanuts to growing-finishing swine on resulting carcass fatty acid profile and on carcass and meat quality characteristics. J Anim Sci. (1992) 70:3734–41. doi: 10.2527/1992.70123734x
41. Delcy V, Rodriguez DS, Hadley M. Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J Nutr Biochem. (2002) 13:717–26. doi: 10.1016/S0955-2863(02)00231-0
42. Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. (2010) 48:937–43. doi: 10.1016/j.fct.2010.01.003
43. Yotsumoto H, Yanagita T, Yamamoto K, Ogaw Y, Cha J, Mori Y. Inhibitory effects of Oren-gedoku-to and its components on cholesteryl ester synthesis in cultured human hepatocyte GepG2 cells: Evidence from the cultured HepG2 cells and in vitro assay of ACAT. Planta Med. (1997) 63:141–5. doi: 10.1055/s-2006-957631
44. Hanczakowska E, Witkiewicz M, Grela ER. Effect of dietary supplement of herbal extract from hop (Humulus lupulus) on pig performance and meat quality. Czech J Anim Sci. (2017) 62:287–95. doi: 10.17221/49/2016-CJAS
45. Fermont L, Gozzelino MT, Linard A. Response of plasma lipids to dietary cholesterol and wine polyphenols in rats fed polyunsaturated fat diets. Lipids. (2000) 35:991–9. doi: 10.1007/s11745-000-0610-2
46. Huo B, He J, Shen XY. Effects of selenium-deprived habitat on the immune index and antioxidant capacity of Przewalski's Gazelle. Biol Trace Elem Res. (2020) 198:149–56. doi: 10.1007/s12011-020-02070-6
47. Chen WP, Tang JL, Bao JP, Hu PF, Shi ZL, Wu LD. Anti-arthritic effects of chlorogenic acid in interleukin-1β-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int Immunopharmacol. (2011) 11:23–8. doi: 10.1016/j.intimp.2010.09.021
48. Kim HR, Lee DM, Lee SH, Seong AR, Gin DW, Hwang JA, et al. Chlorogenic acid suppresses pulmonary eosinophilia, ige production, and th2-type cytokine production in an ovalbumin-induced allergic asthma: activation of stat-6 and jnk is inhibited by chlorogenic acid. Int Immunopharmacol. (2010) 10:1242–8. doi: 10.1016/j.intimp.2010.07.005
49. Liu LL, Gong LK, Wang H, Xiao Y, Wu XF, Zhang YH, et al. Baicalin inhibits macrophage activation by lipopolysaccharide and protects mice from endotoxin shock. Biochem Pharmacol. (2008) 75:914–22. doi: 10.1016/j.bcp.2007.10.009
50. Park SW, Lee CH, Kim YS, Kang SS, Jeon SJ, Son KH, et al. Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. J Pharmacol Sci. (2008) 106:136–43. doi: 10.1254/jphs.FP0071392
51. Kielland C, Rootwelt V, Reksen O, Framstad T. The association between immunoglobulin G in sow colostrum and piglet plasma. J Anim Sci. (2015) 93:4453–62. doi: 10.2527/jas.2014-8713
52. Wang Q, Kim HJ, Cho JH, Chen YJ, Yoo JS, Wang Y. Effects of phytogenic substances on growth performance, digestibility of nutrients, faecal noxious gas content, blood and milk characteristics and reproduction in sows and litter performance. J Anim Feed Sci. (2008) 17:362–78. doi: 10.22358/jafs/66469/2008
53. Huo B, Wu T, Song CJ, Shen XY. Studies of selenium defciency in the Wumeng semi-fine wool sheep. Biol Trace Elem Res. (2020) 194:152–8. doi: 10.1007/s12011-019-01751-1
54. Liu YH, Huo B, Chen ZP, Wang K, Huang LJ, Che LQ, et al. Effects of organic chromium yeast on performance, meat quality, and serum parameters of grow-finish pigs. Biol Trace Elem Res. (2022) 200:1–9. doi: 10.1007/s12011-022-03237-z
55. Kimura Y, Kubo M, Tani T, Arichi S, Okuda H. Studies on Scutellariae Radix. IV Effect on lipid peroxidation in rat liver. Chem Pharm Bull. (1981) 29:2610–7. doi: 10.1248/cpb.29.2610
56. Su DY, Li S, Zhang W, Wang J, Wang JJ, Lv MH. Structural elucidation of a polysaccharide from Lonicera japonica flowers, and its neuroprotective effect on cerebral ischemia-reperfusion injury in rat. Int J Biol Macromol. (2017) 99:350–7. doi: 10.1016/j.ijbiomac.2017.02.096
57. Wang ZL, Wang S, Kuang Y, Hu ZM. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. (2018) 56:465–84. doi: 10.1080/13880209.2018.1492620
58. Huang WH, Lee AR, Yang CH. Antioxidative and anti-Inflammatory activities of polyhydroxyflavonoids of Scutellaria Baicalensis GEORGI. Biosci Biotech Bioch. (2006) 70:2371–80. doi: 10.1271/bbb.50698
59. Choi CW, Jung HA, Kang SS, Choi JS. Antioxidant constituents and a new triterpenoid glycoside from Flos Lonicerae. Arch Pharm Res. (2007) 30:1–7. doi: 10.1007/BF02977770
60. Hoving LL, Soede NM, Feitsma H, Kemp B. Lactation weight loss in primiparous sows: consequences for embryo survival and progesterone and relations with metabolic profiles. Reprod Domest Anim. (2012) 47:1009–16. doi: 10.1111/j.1439-0531.2012.02007.x
Keywords: scutellaria baicalensis, lonicera japonica, sows, performance, immunity
Citation: Wang L, Huo B, Huang L, Che L, Feng B, Lin Y, Xu S, Wu D and Fang Z (2022) Dietary supplementation with a mixture of herbal extracts during late gestation and lactation improves performance of sows and nursing piglets through regulation of maternal metabolism and transmission of antibodies. Front. Vet. Sci. 9:1026088. doi: 10.3389/fvets.2022.1026088
Received: 23 August 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Wen-Chao Liu, Guangdong Ocean University, ChinaReviewed by:
Hongkui Wei, Huazhong Agricultural University, ChinaMarcin Barszcz, Polish Academy of Sciences, Poland
Copyright © 2022 Wang, Huo, Huang, Che, Feng, Lin, Xu, Wu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengfeng Fang, emZhbmdAc2ljYXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Li Wang1,2†
Li Wang1,2† Bin Feng
Bin Feng Yan Lin
Yan Lin De Wu
De Wu Zhengfeng Fang
Zhengfeng Fang