- Department of Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul, South Korea
The objective of this study was to compare two different bivalent vaccines containing porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae. One vaccine contained PCV2a and the other contained PCV2b, and both were administered on a farm suffering from subclinical PCV2d infection and enzootic pneumonia. A total of 180 pigs were randomly divided into 3 groups (60 pigs per group; male pigs = 30 and female pigs = 30). Bivalent vaccination significantly improved growth performance in both vaccinated groups as compared to the unvaccinated (UnVac) group. Growth performance measured by body weight and average daily weight gain (ADWG) was not significantly different between the two bivalent-vaccinated groups (VacA and VacB). Both bivalent vaccines elicited high levels of neutralizing antibodies and interferon-γ secreting cells (IFN-γ-SC) against PCV2d, leading to a reduction in the levels of PCV2d blood viral load as compared to unvaccinated animals. Similarly, both bivalent vaccines elicited high levels of IFN-γ-SC against M. hyopneumoniae that reduced the level of M. hyopneumoniae laryngeal viral loads as compared to unvaccinated animals. Significant differences in severity of lung and lymphoid lesions were observed in both vaccinated groups as compared to the UnVac group. These comparative field data demonstrated that both bivalent vaccines are good candidates for controlling subclinical PCV2d infection and enzootic pneumonia in swine farms suffering from an existing infection.
Introduction
Porcine circovirus type 2 (PCV2) is the main etiological agent of porcine circovirus-associated disease (PCVAD) (1, 2). Since the introduction of PCV2 vaccines to the market, the clinical form of PCVAD has dramatically decreased, but subclinical PCV2 infection remains the most identified problem in the field (3). This subclinical PCV2 infection is measured and observed through only one clinical sign; growth retardation (4, 5). Meanwhile, Mycoplasma hyopneumoniae is recognized as the primary causative agent of the so-called enzootic pneumonia. The disease causes significant economic loss, also due to growth retardation and the increased cost of antimicrobial medication (6, 7).
Complications from both subclinical PCV2 infection and enzootic pneumonia were involved in the porcine respiratory disease complex (PRDC). PRDC devastates swine herds through decreased growth rate and poor feed efficiency results in an extended time to market (8, 9). Vaccination with a combination product is a great option in the simultaneous control of these two pathogens as it reduces both labor involved and animal stress during vaccination. Therefore, a single-dose-combined vaccine of PCV2 and M. hyopneumoniae is a better choice in the control of PRDC in herds suffering from severe respiratory disease.
A homologous vaccination and challenge (matched genotype) may offer better protection than a heterologous (non-matched genotype) vaccination and challenge for PCV2 (10). However, there is strong evidence of a higher immunogenicity of PCV2a when compared with PCV2b. Therefore, it is misleading to consider vaccine performance only in terms of similarity between field and vaccine PCV2 strains (11, 12). In global field situations, PCV2d has become the predominant genotype in pig populations over both PCV2a and PCV2b (13–16). A new genotype in the field may be more pathogenic, but it is likely to be properly controlled by the present vaccines if properly adopted and in the context of good farming practices (17). Nevertheless, only PCV2a- and PCV2b-based bivalent vaccines also containing M. hyopneumoniae are commercially available (18, 19). For this reason, PCV2b-based bivalent vaccines are of particular interest for swine producers and practitioners as PCV2b is genetically closely related to PCV2d (formerly called “mutant PCV2b”) (20). To date, a comparison between PCV2a- and PCV2b-based bivalent vaccines that also contain M. hyopneumoniae has not been conducted under field conditions. The objective of this study, therefore, was to compare PCV2a- and PCV2b-based bivalent vaccines containing PCV2 and M. hyopneumoniae for each of these clinical, immunological, microbiological, and pathological outcomes in a farm suffering from subclinical PCV2d infection and enzootic pneumonia.
Materials and Methods
Ethical Statement
All of the methods were approved by the Seoul National University Institutional Animal Care and Use and Ethics Committee (SNU-210518-3).
Farm History
The clinical field trial was performed on an 800-sow, farrow-to-finish swine farm that carries out an all-in-all-out production system. A stable status of the porcine reproductive and respiratory syndrome (PRRS) was maintained with the absence of active porcine reproductive and respiratory syndrome virus (PRRSV) circulation (only multiple parity sows were seropositive animals). Sows were not previously immunized against PCV2 and M. hyopneumoniae. The farm was selected on the basis of subclinical PCV2 infection and enzootic pneumonia. The farm consistently had respiratory problems due to poor growth in the late post-weaning and growing stages. Clinical signs first appeared at approximately 8–11 weeks of age and reached peak mortality (~3–5%) between 10 and 15 weeks of age. PCV2d was detected in serum from 3 pigs with poor growth, where log10 DNA copies/ml ranged from 2.46 to 3.34. These values were consistent with the definition of subclinical PCV2 infection (21, 22). Through pre-trial investigations, a PCV2 serological profile was identified that presented an increase in antibody titers starting around 8 weeks of age; 7–16-week-old pigs with poor growth were also PCV2 PCR-positive in tested blood samples. M. hyopneumoniae serology was positive in 8–16-week-old pigs with respiratory signs. Moreover, the laryngeal swabs of the same pigs were PCR-positive for M. hyopneumoniae on the farm. Histologically, mycoplasmal lung lesions characterized by peribronchiolar and peribronchial lymphoid tissue hyperplasia were observed in 4 out of 5 submitted pigs. Lymphoid depletion without granulomatous inflammation was also observed in 3 out of 5 submitted pigs. Pre-trial diagnostic results indicated subclinical PCV2 infection and enzootic pneumonia.
Experimental Design
To reduce sow variation, six pigs (21-day-old) per sow were randomly selected by the random number generator function (Excel, Microsoft Corporation, Redmond, WA, USA) and evenly allocated to each of the three groups. A total of 180 pigs were randomly divided into 3 groups (60 pigs per group; male pigs = 30 and female pigs = 30) within the same software and function (Table 1).
At 0 day post-vaccination (dpv, 21 days of age), pigs in the VacA group were intramuscularly vaccinated with a 2.0 ml dose of the bivalent vaccine containing PCV2a and M. hyopneumoniae (Porcilis® PCV M Hyo, lot no. A114A01, expiration date: 23 June 2022, MSD Animal Health, Boxmeer, Netherlands) at the right side of the neck in accordance with the manufacturer's directions. According to the manufacturer's instruction, pigs in the VacB group were intramuscularly vaccinated with a 1.0 ml dose of the bivalent vaccine containing PCV2b and M. hyopneumoniae (Circo/MycoGard®, serial no: CMG-21006, expiration date: 20 January 2023, Pharmgate Animal Health, Wilmington, NC, USA) in the same anatomical location. Pigs in the unvaccinated (UnVac) group were injected with 2.0 ml of phosphate-buffered saline (PBS, 0.01 M, pH 7.4) in the same anatomical location. Sample collection of blood and laryngeal swabs were performed at 0 (21 days old), 28 (49 days old), 49 (70 days old), and 91 (112 days old) dpv.
Clinical Observations
The pigs were daily monitored for abnormal clinical signs and weekly scored using scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (23). The mortality rate was calculated as the number of dead pigs by the number of pigs initially assigned to the group within the batch. Necropsy was performed on dead or culled pigs throughout the study. Including palpation, injection site reaction was evaluated 24 h post-vaccination. Observers were blinded to the type of vaccine status and vaccination.
Average Daily Weight Gain
The live weight of each pig was measured at 21 (0 dpv), 70 (49 dpv), and 175 (154 dpv) days of age. Over two time periods, (i) between 21 and 70 days old and (ii) between 70 and 175 days old, the ADWG (grams/pig/day) was analyzed. During the different production stages, ADWG was calculated as the difference between the starting and final weight divided by the duration of the stage. Data for culled or dead pigs were also included in the calculation.
Quantification of PCV2d DNA in Blood
For DNA extraction from collected serum samples, the commercial kit was used (QIAamp DNA Mini Kit, QIAGEN). An extracted DNA sample was used for the quantification of PCV2d genomic DNA copy numbers by real-time PCR (24). The forward and reverse primers (5′-GTA TTC AAA GGG CAC AGT GAG G-3′ and 5′-GCA CCA TCG GTT ATA CTG TCA AGA AA-3′) and probe specific for PCV2d (5′-FAMTM-CAT CAT GTC CAC ATT CCA G-3′ Black Hole Quencher) were designed for detecting specific Capsid-coding region of PCV2d only (24).
Quantification of M. hyopneumoniae DNA in the Larynx
For DNA extraction from collected laryngeal swabs, the commercial kit was used (QIAamp DNA Mini Kit, QIAGEN). An extracted DNA sample was used for the quantification of M. hyopneumoniae genomic DNA copy numbers by real-time PCR (25). The forward and reverse primers (5′-TTG ACT GCT ATC TTT GCA CGA TAA G-3′ and 5′- ACA ATA ATT GCT GAC CGT GGC-3′) and probe (5′-FAM-TGT CCA CTG CTG CAA ATA TTC GAT TTC TTG AA-TAMRA-3′) were used to detect M. hyopneumoniae (25).
Serology
Enzyme-linked immunosorbent assay (ELISA) was performed to measure antibodies against M. hyopneumoniae (M. hyo. Ab test, IDEXX Laboratories Inc.) and PCV2 (Ingezim CIRCO IgG, Ingenasa, Madrid, Spain). According to the instruction of the manufacturer for each kit, serum samples were recorded as positive for M. hyopneumoniae antibody if the S/P ratio (sample-to-positive ratio) was ≥0.4. For anti-PCV2 antibodies, samples were recorded as positive if the reciprocal ELISA titer was >350. A serum virus neutralization test against PCV2d was used to test the serum sample (26–28).
Enzyme-Linked Immunospot Assay
The number of PCV2d-specific and M. hyopneumoniae-specific interferon-γ secreting cells (IFN-γ-SC) in peripheral blood mononuclear cells (PBMCs) was measured by ELISPOT assay (24, 29). Briefly, 100 ml containing 2 × 106 PBMCs in Gibco Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc., SelectScience, Bath, UK) were seeded onto plates and precoated overnight with anti-porcine IFN-γ monoclonal antibody (5 μg/ml, Mabtech, Mariemont, OH, USA) and incubated with PCV2d (20 mg/ml), M. hyopneumopniaee (4 mg/ml), phytohemagglutinin (10 mg/ml, Roche Diagnostics GmbH, Mannheim, Germany) as a positive control, or PBS as a negative control for 20 h at 37°C in a 5% humidified CO2 atmosphere. The wells were washed five times with PBS (200 ml per well) and thereafter, the procedure followed instructions of the manufacturer using a commercial ELISpot assay kit (Mabtech). The spots on the membranes were read by an automated ELISpot reader (AID ELISpot Reader, AID GmbH, Strassberg, Germany). The results were expressed as the number of responding cells/million PBMC.
Pathology
To estimate the percentage of the lung affected by pneumonia, two pathologists (Chae and one graduate student) at the Seoul National University (Seoul, Republic of Korea) scored the severity of macroscopic lung lesions as previously described (23). The collected lung and lymphoid tissue sections were examined and scored by two blinded veterinary pathologists; the severity of peribronchiolar and perivascular lymphoid tissue hyperplasia by mycoplasmal pneumonia lesions, on a scale of 0–6 (30). Based on lymphoid depletion and granulomatous inflammation, lymphoid lesion severity was also scored on a scale of 0–5 (31).
Statistical Analysis
Real-time PCR and neutralizing antibody data were recalculated to log10 and log2 values, prior to statistical analysis. The collected data were assessed by the Shapiro-Wilk test for a normal distribution. Then, one-way ANOVA was performed to determine if there were statistically significant differences between different groups at each time point. For further evaluation, a post-hoc test for a pairwise comparison with Tukey's adjustment was conducted with a statistical significance result from a one-way ANOVA test. In case of the normality, assumption was not met and the Kruskal-Wallis test was performed. Results, which showed a statistical significance from the Kruskal-Wallis test, were further evaluated with the Mann-Whitney test to include Tukey's adjustment to compare the differences among the groups. The value of p < 0.05 was considered significant and reported in p-values.
Results
Clinical Signs
Two vaccinated animals from the VacA and VacB groups had significantly (p < 0.05) lower respiratory signs than those of unvaccinated animals from the UnVacA group at 14–112 dpv. There were no differences in respiratory signs between the two vaccinated groups (VacA and VacB).
Average Daily Weight Gain
No difference in mean body weight was observed between the vaccinated and unvaccinated animals at the time of vaccination. During the growing period (70–175 days of age), the ADWG of the vaccinated groups (VacA and VacB) was significantly (p < 0.05) higher than that of the unvaccinated group (UnVac). Overall (3–175 days of age), the ADWG of the vaccinated groups (VacA and VacB) was significantly (p < 0.05) higher than that of the unvaccinated group (UnVac) (Table 1).
Mortality
A total of 2 pigs died in the VacA group of severe pneumonia as determined by a combination of M. hyopneumoniae and PCV2d that was detected with PCR testing, and Pasteurella multocida was isolated from the lungs at 77 and 85 days of age. One pig from the VacB group died of pleuropneumonia caused by coinfection with Actinobacillus pleuropneumoniae and Glaesserella parasuis that was determined through isolation from the lungs at 81 days of age. A total of 4 pigs were died in the UnVac group; two pigs were died of bronchopneumonia as determined by a combination of M. hyopneumoniae that was detected with PCR, and P. multocida and Trueperella pyogenes that were isolated from the lungs at 69 and 85 days of age. The other two pigs were died from bronchopneumonia as determined by a combination of M. hyopneumoniae and PCV2d that were detected with PCR, and G. parasuis that was isolated from the lungs at 81 and 86 days of age.
Quantification of PCV2 in Blood
The PCV2 DNA blood loads from vaccinated groups (VacA and VacB) were significantly (p < 0.05) lower than that of unvaccinated groups (UnVac) at 28, 49, and 91 dpv (Figure 1). Two of the vaccinated groups (VacA and VacB) had comparable PCV2 DNA loads in their blood throughout the entire field trial.
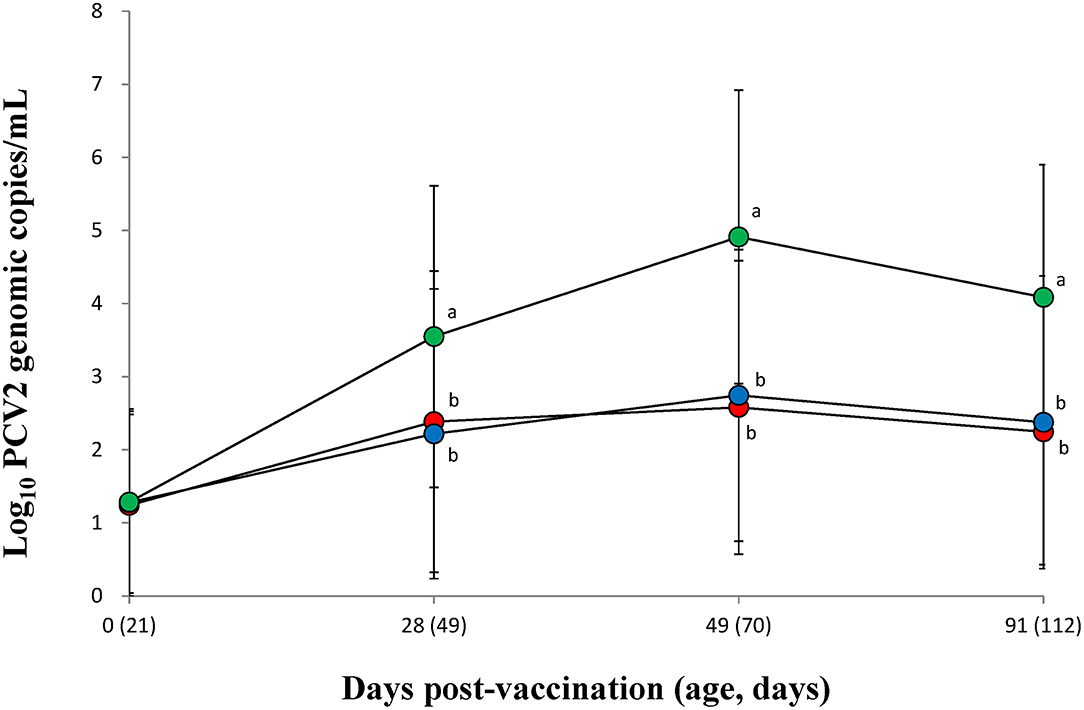
Figure 1. Mean values of the genomic copy number of PCV2d DNA in serum of pigs from the VacA ( ), VacB (
), VacB ( ), and UnVac (
), and UnVac ( ) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
Quantification of M. hyopneumoniae DNA in Larynx
The amount of M. hyopneumoniae DNA loads in the larynx was significantly (p < 0.05) lower in the vaccinated groups (VacA and VacB) than in those of the unvaccinated group (UnVac) between 28 and 91 dpv (Figure 2). Throughout the entire field trial, two vaccinated groups (VacA and VacB) had comparable M. hyopneumoniae DNA loads in their larynx.
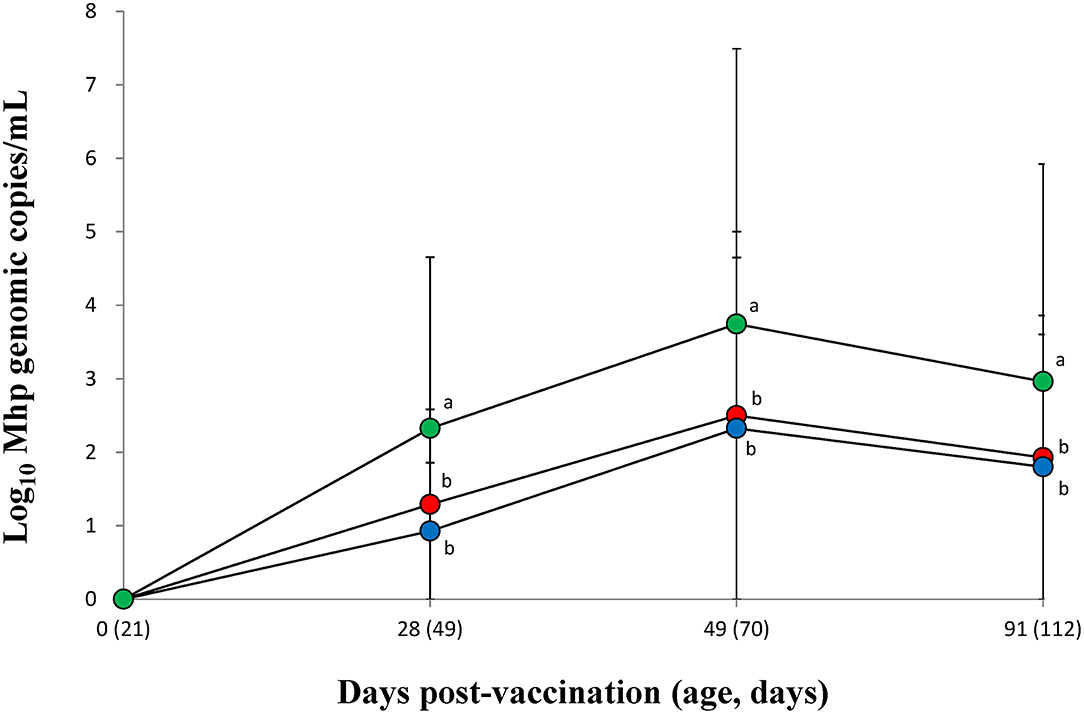
Figure 2. Mean values of the genomic copy number of Mycoplasma hyopneumoniae DNA in the larynx of pigs from the VacA ( ), VacB (
), VacB ( ), and UnVac (
), and UnVac ( ) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
Immune Responses Against PCV2
The vaccinated groups (VacA and VacB) were measured significantly (p < 0.05) higher in their PCV2 ELISA titers (Figure 3A), neutralizing antibody titers (Figure 3B), and IFN-γ-SC (Figure 3C) than that of the unvaccinated (UnVac) group at 28, 49, and 91 dpv. No significant differences in PCV2 ELISA titers, neutralizing antibody titers, or IFN-γ-SC were observed in the two vaccinated (VacA and VacB) groups throughout the entire field trial.
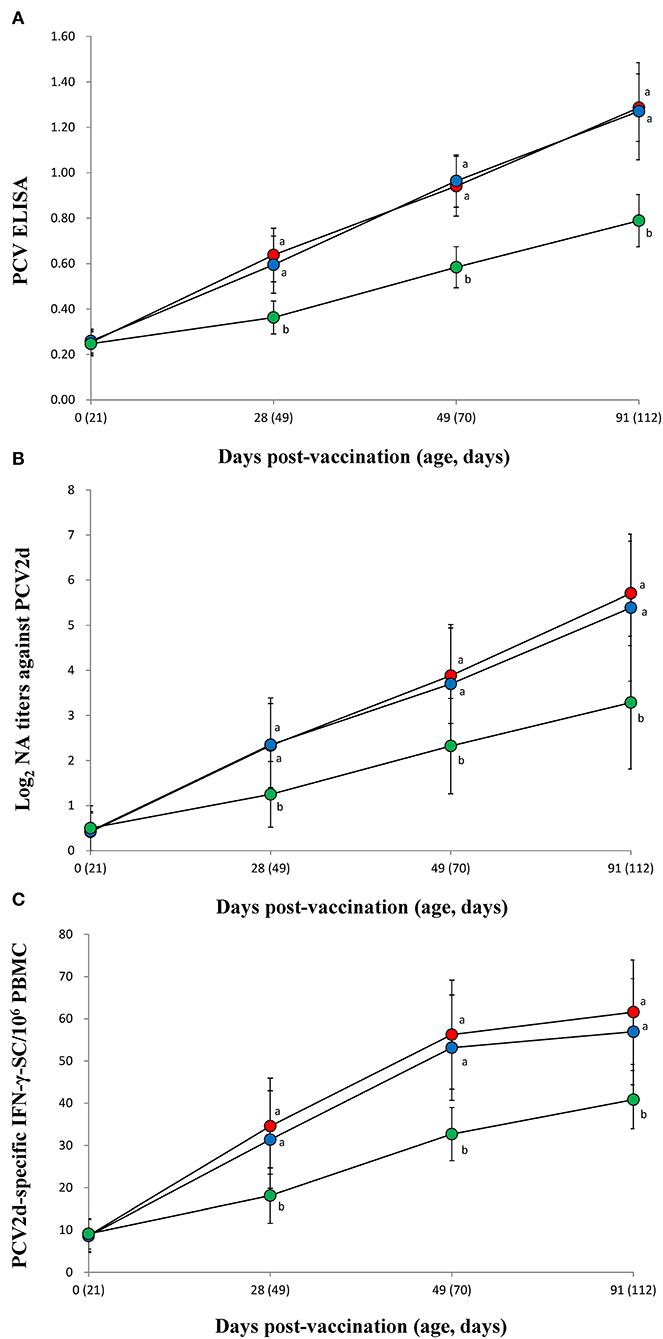
Figure 3. Immune responses against porcine circovirus type 2 (PCV2). (A) Mean values of the ELISA anti-PCV2 antibodies. (B) Mean values of the neutralizing antibody (NA) titers. (C) Frequency of PCV2d-specific interferon-γ secreting cells (IFN-γ-SC) from the VacA ( ), VacB (
), VacB ( ), and UnVac (
), and UnVac ( ) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
Immune Responses Against M. hyopneumoniae
Both vaccinated groups (VacA and VacB) were measured significantly (p < 0.05) higher in their M. hyopneumoniae ELISA S/P ratios (Figure 4A) and IFN-γ-SC levels (Figure 4B) than animals from the UnVac group at 28, 49, and 91 dpv. No significant differences in M. hyopneumoniae ELISA S/P ratios or IFN-γ-SC were observed in the two vaccinated (VacA and VacB) groups throughout the entire field trial.
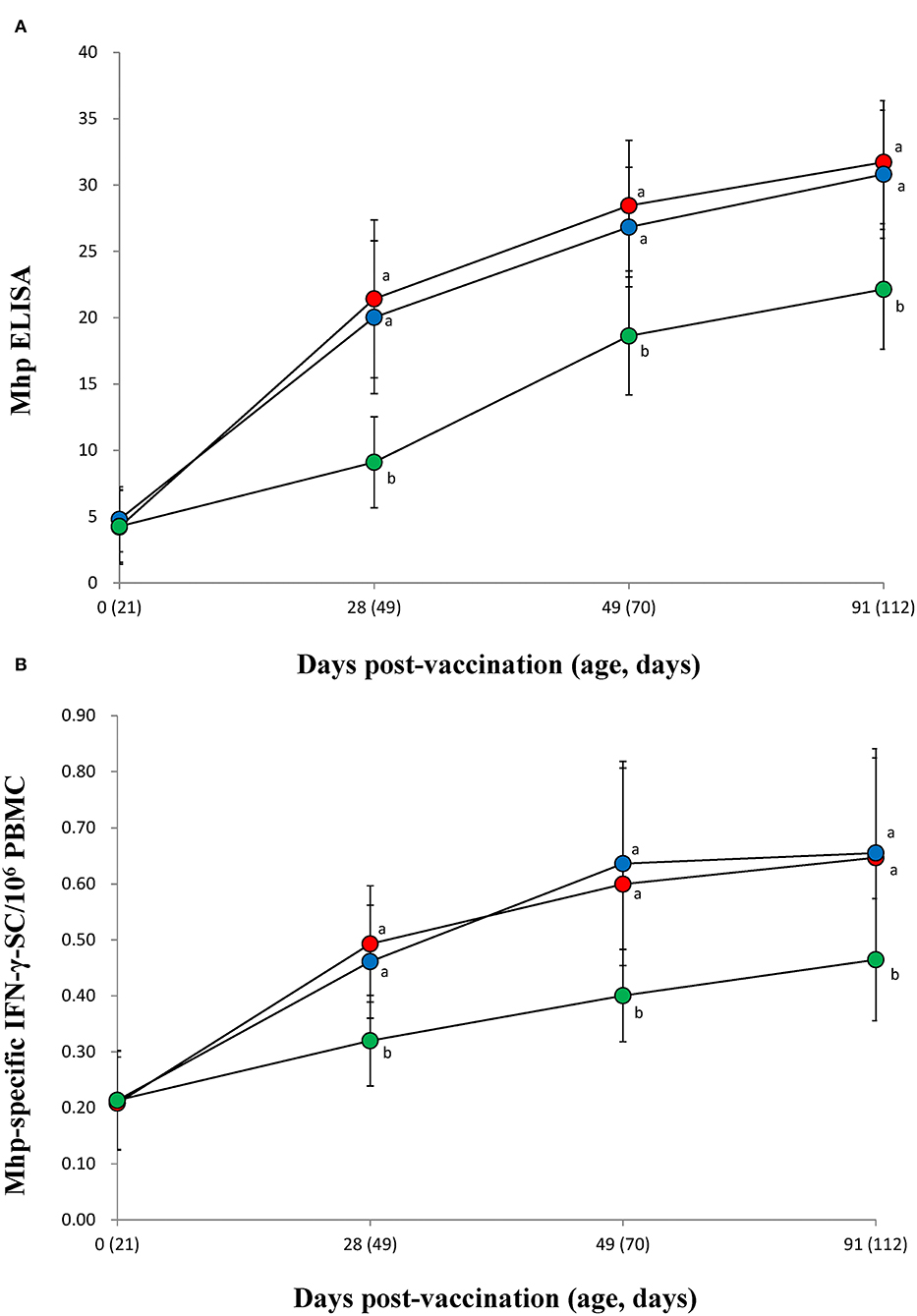
Figure 4. Immune responses against Mycoplasma hyopneumoniae. (A) Mean values of the anti-M. hyopneumoniae antibodies. (B) Frequency of M. hyopneumoniae-specific interferon-γ secreting cells (IFN-γ-SC) of pigs from the VacA ( ), VacB (
), VacB ( ), and UnVac (
), and UnVac ( ) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
) groups. Variation is expressed as the standard deviation (SD). Different superscripts (a and b) indicate a significant (p < 0.05) difference between vaccinated (VacA and VacB) and unvaccinated (UnVac) groups.
Pathology
Vaccination of animals from both vaccinated groups (VacA and VacB) effectively reduced macroscopic lung lesion scores, microscopic lung, lymphoid lesion scores, and the numbers of lymphoid PCV2-positive cells when compared to the unvaccinated group (UnVac) at 154 dpv (Table 2). Throughout the entire field trial, there were no significant differences in overall scores for macroscopic and microscopic lung lesions, microscopic lymphoid lesions, and the numbers of lymphoid PCV2-positive cells between the two vaccinated groups (VacA and VacB).
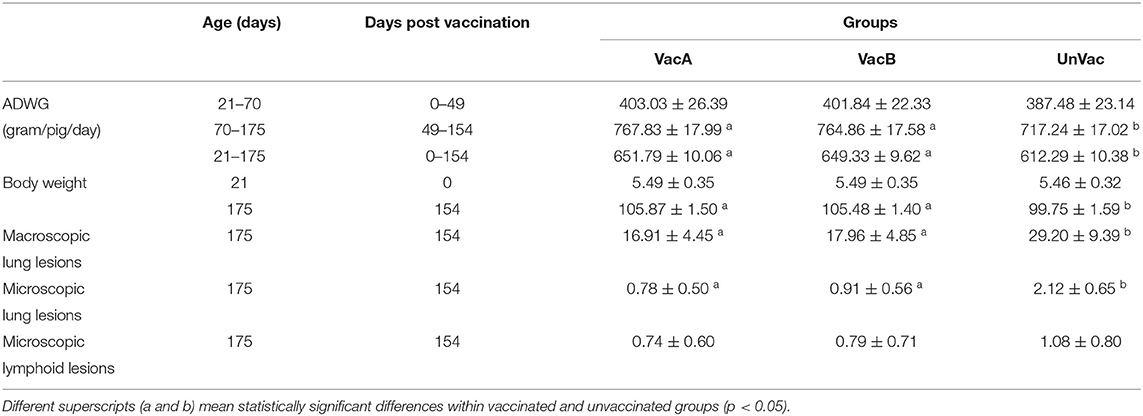
Table 2. Growth performance with average daily weight gain (ADWG) and pathology between vaccinated and unvaccinated animals.
Discussion
The results of this comparative field trial demonstrate that PCV2a- and PCV2b-based bivalent vaccines that also contain M. hyopneumoniae provide equal protection for pigs in herds suffering from subclinical PCV2d infection and enzootic pneumonia. The common denominator of PCV2d and M. hyopneumoniae infection is weight loss, so it was important to evaluate the improvement of growth performance in the comparative field trial. Vaccination of pigs with both evaluated bivalent vaccines (two groups) resulted in a significantly improved growth performance as compared to the pigs in the unvaccinated group. Significant differences in growth performance as measured by body weight and ADWG were not found between the two bivalent-vaccinated groups.
In general, both PCV2/M. hyopneumoniae combination vaccines induced protective immunity by reducing PCV2 blood viral load and M. hyopneumoniae laryngeal load while simultaneously reducing lung and lymphoid lesions, thereby controlling these two diseases (32–34). A PCV2b-based bivalent vaccine may provide better protection in theory against PCV2d than PCV2a-based bivalent vaccines as PCV2b is closely related to PCV2d genetically (10, 20). In the present study, both bivalent vaccines elicited equal levels of neutralizing antibodies and IFN-γ-SC against PCV2d while simultaneously reducing the level of PCV2d blood viral load. Genetic similarity therefore does not guarantee that one bivalent vaccine can offer superior protection over the other. Like the PCV2 response with vaccination, both bivalent vaccines elicited an equal level of IFN-γ-SC against M. hyopneumoniae while simultaneously equally reducing the levels of M. hyopneumoniae laryngeal load. Besides antigen genotype, several other factors, such as adjuvant, vaccine formulation, and route of administration, also influence the immune responses and efficacy of a vaccine.
The immunological and microbiological findings in this study were consistent with the pathological findings. The pathological analysis is critical in the evaluation and comparison of bivalent vaccine efficacy because pathological lesion severity is the critical criterion for the diagnosis of PCVAD. The reduction in lung and lymphoid lesions due to M. hyopneumoniae and PCV2d infection is correlated with growth performance (35–38). Both bivalent vaccines reduced lung and lymphoid lesions without any clear advantages of one vaccine over the other. Therefore, it was confirmed that both vaccines efficiently reduced lung lesions caused by M. hyopneumoniae and lymphoid lesions caused by PCV2d (35–38).
Porcine circovirus type 2 is a virus that has the highest mutation rate among all DNA viruses (39). The high mutation rate of PCV2 results in the emergence of new genotypes. Currently, PCV2d is the most prevalent genotype found in all major pigs rearing Asian countries (13–16). To date, a commercial bivalent vaccine containing PCV2d and M. hyopneumoniae is not yet available. Alternatively, it is necessary to compare the differences in clinical, immunological, microbiological, and pathological results between PCV2a- and PCV2b-based bivalent vaccines and how they result in farm outbreaks with subclinical PCV2d infection and enzootic pneumonia. These comparative field data indicate that both bivalent vaccines are good candidates for controlling disease in swine farms suffering from subclinical PCV2d infection and enzootic pneumonia.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Seoul National University Institutional Animal Care and Use Committee (SNU-210518-3).
Author Contributions
HC and TO contributed to the performance of the experimental trials, data analysis, and writing of the manuscript. HC, TO, and JS contributed to the preparation of the inoculum and lab analysis. CC contributed to the development of the protocol, design of the study, review of the final manuscript, and the approval for publication. All authors read and approved the final manuscript.
Funding
The author's research was supported by contract research funds (Grant No. 550-20190068) of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine and by the BK 21 Plus Program (Grant No. 5260-20150100) for Creative Veterinary Science Research.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Afolabi KO, Iweriebor BC, Okoh AI, Obi LC. Global status of Porcine circovirus type 2 and its associated diseases in Sub-Saharan Africa. Adv Virol. (2017) 2017:6807964. doi: 10.1155/2017/6807964
2. Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. (2005) 169:326–36. doi: 10.1016/j.tvjl.2004.01.012
3. Segalés J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. (2012) 164:10–9. doi: 10.1016/j.virusres.2011.10.007
4. Alarcon P, Rushton J, Nathues H, Wieland B. Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical infection in 3-weekly batch system farms. Prev Vet Med. (2013) 110:103–18. doi: 10.1016/j.prevetmed.2012.12.006
5. Kurmann J, Sydler T, Brugnera E, Buergi E, Haessig M, Suter M, et al. Vaccination of dams increases antibody titer and improves growth parameters in finisher pigs subclinically infected with porcine circovirus type 2. Clin Vaccine Immunol. (2011) 18:1644–9. doi: 10.1128/CVI.05183-11
6. Maes D, Verdonck M, Deluyker H, de Kruif A. Enzootic pneumonia in pigs. Vet Q. (1996) 18:104–9. doi: 10.1080/01652176.1996.9694628
7. Maes D, Segales J, Meyns T, Sibila M, Pieters M, Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet Microbiol. (2008) 126:297–309. doi: 10.1016/j.vetmic.2007.09.008
8. Kim J, Chung H-K, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. (2003) 166:251–6. doi: 10.1016/S1090-0233(02)00257-5
9. Hansen MS, Pors SE, Jensen HE, Bille-Hansen V, Bisgaard M, Flachs EM, et al. An investigation of the pathology and pathogens associated with porcine respiratory disease complex in Denmark. J Comp Pathol. (2010) 143:120–31. doi: 10.1016/j.jcpa.2010.01.012
10. Karuppannan AK, Opriessnig T. Porcine circovirus type 2 (PCV2) vaccines in the context of current molecular epidemiology. Viruses. (2017) 9:99. doi: 10.3390/v9050099
11. Guarneri F, Tresoldi ET, Sarli G, Boniotti MB, Lelli D, Barbieri I, et al. Protective immunity in swine induced by porcine circovirus 2b inactivated vaccines with different antigen payload. Vet Microbiol. (2021) 252:108887. doi: 10.1016/j.vetmic.2020.108887
12. Huang L, Wang Y, Wei Y, Chen D, Liu D, Du W, et al. Capsid proteins from PCV2a genotype confer greater protection against a PCV2b strain than those from PCV2b genotype in pigs: evidence for PCV2b strains becoming more predominant than PCV2a strains from 2000 and 2010s. Appl Microbiol Biotechnol. (2016) 100:5933–43. doi: 10.1007/s00253-016-7459-y
13. Dinh PX, Nguyen MN, Nguyen HT, Tran VH, Tran QD, Dang KH, et al. Porcine circovirus genotypes and their copathogens in pigs with respiratory disease in southern provinces of Vietnam. Arch Virol. (2021) 166:403–11. doi: 10.1007/s00705-020-04878-y
14. Thangthamniyom N, Sangthong P, Poolperm P, Thanantong N, Boonsoongnern A, Hansoongnern P, et al. Genetic diversity of porcine circovirus type 2 (PCV2) in Thailand during 2009-2015. Vet Microbiol. (2017) 208:239–46. doi: 10.1016/j.vetmic.2017.08.006
15. Tsai GT, Lin YC, Lin WH, Lin JH, Chiou MT, Liu HF, et al. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001-2017. Sci Rep. (2019) 9:10782. doi: 10.1038/s41598-019-47209-1
16. Yang S, Yin S, Shang Y, Liu B, Yuan L, Zafar Khan MU, et al. Phylogenetic and genetic variation analyses of porcine circovirus type 2 isolated from China. Transbound Emerg Dis. (2018) 65:e383–92. doi: 10.1111/tbed.12768
17. Franzo G, Segalés J. Porcine circovirus 2 genotypes, immunity and vaccines: Multiple genotypes but one single serotype. Pathogens. (2020) 9:1049. doi: 10.3390/pathogens9121049
18. Yang S, Oh T, Park KW, Cho H, Chae C, A dual swine challenge with porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae used to compare a combination of mixable monovalent PCV2 and monovalent M. hyopneumoniae vaccines with a ready-to use PCV2 and M hyopneumoniae bivalent vaccine. Front Vet Sci. (2020) 7:579. doi: 10.3389/fvets.2020.00579
19. Yang S, Ahn Y, Oh T, Cho H, Park KH, Chae C. Field evaluation of a single-dose bivalent vaccine of porcine circovirus type 2b and Mycoplasma hyopneumoniae. Vet Med Sci. (2021) 7:755–65. doi: 10.1002/vms3.420
20. Xiao CT, Halbur PG, Opriessnig T. Complete genome sequence of a novel porcine circovirus type 2b variant present in cases of vaccine failures in the United States. J Virol. (2012) 86:12469–73. doi: 10.1128/JVI.02345-12
21. Darwich L, Segalés J, Resendes A, Balasch M, Plana-Duran J, Mateu E. Transient correlation between viremia levels and IL-10 expression in pigs subclinically infected with porcine circovirus type 2 (PCV2). Res Vet Sci. (2008) 84:194–98. doi: 10.1016/j.rvsc.2007.04.005
22. Segalés J, Calsamiglia M, Olvera A, Sibila M, Badiella L, Domingo M. Quatification of porcine circovirus type 2 (PCV2) DNA in serum and tonsillar, nasal, trachea-bronchial, urinary and faecal swabs of pigs with and without postweaning multisystemic wasting syndrome (PMWS). Vet Microbiol. (2005) 111:223–29. doi: 10.1016/j.vetmic.2005.10.008
23. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, et al. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. (1995) 32:648–60. doi: 10.1177/030098589503200606
24. Jeong J, Park C, Choi K, Chae C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet Microbiol. (2015) 177:43–52. doi: 10.1016/j.vetmic.2015.02.027
25. Dubosson CR, Conzelmann C, Miserez R, Boerlin P, Frey J, Zimmermann W, et al. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet Microbiol. (2004) 102:55–65. doi: 10.1016/j.vetmic.2004.05.007
26. Pogranichnyy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. (2000) 13:143–53. doi: 10.1089/vim.2000.13.143
27. Fort M, Sibila M, Pérez-Martín E, Nofrarías M, Mateu E, Segalés J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine. (2009) 27:4031–7. doi: 10.1016/j.vaccine.2009.04.028
28. Shen HG, Beach NM, Huang YW, Halbur PG, Meng XJ, Opriessnig T. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV). Vaccine. (2010) 28:5960–6. doi: 10.1016/j.vaccine.2010.07.002
29. Jeong J, Kang I, Kim S, Park KH, Park C, Chae C. Comparison of 3 vaccination strategies against porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, and porcine circovirus type 2 on 3 pathogen challenge model. Can J Vet Res. (2018) 82:39–47.
30. Opriessnig T, Thacker EL Yu S, Fenaux M, Meng X-J, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. (2004) 41:624–40. doi: 10.1354/vp.41-6-624
31. Kim J, Chae C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J Comp Pathol. (2004) 131:121–6. doi: 10.1016/j.jcpa.2004.02.001
32. Kim SH, Oh T, Yang S, Cho H, Chae C. Experimental evaluation of Mycoplasma hyopneumoniae bacterin against a Korean M. hyopneumoniae challenge. Can J Vet Res. (2021) 85:77–81.
33. Meerts P, Van-Gucht S, Cox E, Vandebosch A, Nauwynck HJ. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. (2005) 18:333–41. doi: 10.1089/vim.2005.18.333
34. Meerts P, Misinzo G, Lefebvre D, Nielsen J, Bøtner A, Kristensen CS, et al. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res. (2006) 2:6. doi: 10.1186/1746-6148-2-6
35. Jensen CS, Ersboll AK, Nielsen JP. A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev Vet Med. (2002) 54:265–78. doi: 10.1016/S0167-5877(02)00005-3
36. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, et al. The effect of vaccination against Mycoplasma hypopneumoniae in pig herds with a continuous production system. Zentralbl Veterinarmed B. (1998) 45:495–505. doi: 10.1111/j.1439-0450.1998.tb00820.x
37. Martelli P, Ferrari L, Morganti M, Angelis DE, Bonilauri P, Guazzetti S, et al. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet Microbiol. (2011) 149:339–51. doi: 10.1016/j.vetmic.2010.12.008
38. Segalés J, Urniza A, Alegre A, Bru T, Crisci E, Nofrarias M, et al. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine. (2009) 27:7313–21. doi: 10.1016/j.vaccine.2009.09.084
Keywords: Mycoplasma hyopneumoniae, porcine circovirus type 2, porcine circovirus type 2a (PCV-2a), porcine circovirus type 2b (PCV-2b), bivalent vaccine
Citation: Cho H, Oh T, Suh J and Chae C (2022) A Comparative Field Evaluation of the Effect of Growth Performance Between Porcine Circovirus Type 2a (PCV2a)- and PCV2b-Based Bivalent Vaccines Containing PCV2 and Mycoplasma hyopneumoniae. Front. Vet. Sci. 9:859344. doi: 10.3389/fvets.2022.859344
Received: 21 January 2022; Accepted: 16 May 2022;
Published: 24 June 2022.
Edited by:
Constantinos S. Kyriakis, Auburn University, United StatesReviewed by:
Anbu K. Karuppannan, Tamil Nadu Veterinary and Animal Sciences University, IndiaMassimo Amadori, Italian Network of Veterinary Immunology, Italy
Copyright © 2022 Cho, Oh, Suh and Chae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanhee Chae, c3dpbmVAc251LmFjLmty
 Hyejean Cho
Hyejean Cho Taehwan Oh
Taehwan Oh Jeongmin Suh
Jeongmin Suh Chanhee Chae
Chanhee Chae