- 1Orthopaedic Research Center, C. Wayne McIlwraith Translational Medicine Institute, College of Veterinary Medicine, Colorado State University, Fort Collins, CO, United States
- 2Gene Therapy Center, University of North Carolina, Chapel Hill, NC, United States
With an intrinsically low ability for self-repair, articular cartilage injuries often progress to cartilage loss and joint degeneration resulting in osteoarthritis (OA). Osteoarthritis and the associated articular cartilage changes can be debilitating, resulting in lameness and functional disability both in human and equine patients. While articular cartilage damage plays a central role in the pathogenesis of OA, the contribution of other joint tissues to the pathogenesis of OA has increasingly been recognized thus prompting a whole organ approach for therapeutic strategies. Gene therapy methods have generated significant interest in OA therapy in recent years. These utilize viral or non-viral vectors to deliver therapeutic molecules directly into the joint space with the goal of reprogramming the cells' machinery to secrete high levels of the target protein at the site of injection. Several viral vector-based approaches have demonstrated successful gene transfer with persistent therapeutic levels of transgene expression in the equine joint. As an experimental model, horses represent the pathology of human OA more accurately compared to other animal models. The anatomical and biomechanical similarities between equine and human joints also allow for the use of similar imaging and diagnostic methods as used in humans. In addition, horses experience naturally occurring OA and undergo similar therapies as human patients and, therefore, are a clinically relevant patient population. Thus, further studies utilizing this equine model would not only help advance the field of human OA therapy but also benefit the clinical equine patients with naturally occurring joint disease. In this review, we discuss the advancements in gene therapeutic approaches for the treatment of OA with the horse as a relevant patient population as well as an effective and commonly utilized species as a translational model.
Introduction
Osteoarthritis (OA) is a debilitating, painful and often chronic degenerative condition, negatively impacting a significant percentage of the human population. It is the most common form of arthritis affecting nearly 30 million Americans and causing an economic loss of $186 billion annually (1, 2). It is also a significant clinical problem in horses with OA-associated lameness being the predominant factor contributing to diminished athletic function, inability to race, and perform sport horse activities (3–5). A U.S Department of Agriculture survey performed in horses attributed 60% of lameness to be related to OA which translates to millions of horses being impacted by this performance-limiting musculoskeletal condition (6). OA has a considerable economic impact on the equine industry, with the annual direct and indirect costs amounting to over $1 billion per year in the United States (7, 8). There is no cure for OA and treatment primarily revolves around managing symptoms by systemic and local pharmacological therapies including analgesics and non-steroidal anti-inflammatory agents (NSAIDs) (9), surgical approaches such as microfracture and chondroplasty (10–13), and regenerative medicine strategies using blood derived (ACS, APS, and PRP) (14–17) or cell-based approaches (ACI/MACI) (18–22). However, these are only effective at providing short-term relief and do not alter disease progression nor do they completely restore cartilage structure and function (4, 23). Therefore, the restoration of articular cartilage remains an unmet clinical need.
One of the challenges to studying osteoarthritis is the difficulty in finding experimental subjects to accurately mimic the pathology of human OA. Laboratory animal models, although inexpensive and easy to use, do not truly represent most aspects of human OA due to their differences in cartilage thickness and small joint size (24, 25). Dogs are reasonably good animal models for studying human OA due to their similarity in joint anatomy (26, 27); however, their companion animal status and associated ethical challenges have precluded their widespread use as an animal model (26, 27). Caprine models are more commonly used to study human OA; however, limitations such as variability in cartilage thickness and defect volume leads to inconsistencies in drawing experimental conclusions (26, 28). Minipigs (a smaller version of traditional pigs) are another suitable animal model for human OA studies (29). They have comparable cartilage thickness allowing for the creation of partial thickness (cartilage-only) defects; however, handling and housing challenges have limited their active use as an animal model (26, 30, 31).
In this regard, horses have been shown to be an ideal model due to the similarities in structural and functional anatomy of the synovial joints. The overall cartilage thickness (1.75–2 mm), subchondral bone characteristics and joint anatomy are similar between horses and humans (30, 32). In particular, the carpal and metacarpal joints of the equine forelimb have comparable size, tissue structure and biomechanical loading to human joints. Moreover, as these equine joints are responsible for 60–65% of weight bearing, they are the most susceptible to post traumatic OA induced by athletic training and secondary trauma (4, 5). As an experimental model, the large size of the equine system also allows for the use of similar imaging and diagnostic modalities as used in humans unlike traditional small animal models. Moreover, horses are amenable to controlled exercise such as treadmilling, analysis of joint function (lameness and joint effusion scoring), examination of internal structures using imaging such as radiographic, CT, MRI analysis, and minimally invasive arthroscopy (4, 33–35). Synovial fluid analysis offers a relatively non-invasive measure of therapeutic drug levels after intra-articular administration. However, this is challenging in small animal models due to the small joint size and proportionally small synovial fluid volume. In this regard, the ease of harvesting large volumes of synovial fluid under sedation is a significant benefit of using the equine model in OA studies (36, 37). Furthermore, horses sustain naturally occurring OA, frequently undergo arthroscopic procedures and commonly have their joints aspirated and treated and, therefore, are a clinically relevant patient population, further justifying the use of horses for preclinical analyses since the results from those studies would benefit clinical equine patients with naturally occurring disease in parallel. Limitations of the equine model including cost, handling/housing, long time to maturity and ethical concerns must be taken into consideration with the use of this large animal model.
Principles of gene therapy
OA is a degenerative joint disorder and as joints are discrete enclosed spaces, the effects tend to be largely localized to the joints. While an imbalance between cartilage degradation and new matrix synthesis leading to cartilage damage is considered central to the pathogenesis of OA, the involvement of an inflammatory component has now been well-recognized. Recent literature suggests that an innate immune response mediated by the joint components such as synovial membrane, joint capsule, subchondral bone and ligaments is responsible for initiating and sustaining an immune-mediated inflammation in the diseased joint (38–41). It is important to recognize that early inflammation, in response to joint injury is beneficial for the repair process. However, progressive damage to the joint and failed tissue repair results in activation of stress signaling pathways which initiate and perpetuate a low-grade chronic inflammation leading to clinical OA (42–45). Anti-inflammatory agents can help in inhibiting a chronic inflammatory response to protect the cartilage from further damage. In recent years, targeted approaches to retain the beneficial effects of acute inflammation and prevent the progression to low-grade, chronic inflammation have been adopted using inducible transcription factors which regulate several genes of the inflammatory cascade. An example for this approach is the use of specific inhibitors of Nuclear factor-κB (NF-κB), a central inflammatory mediator which responds to a large variety of inflammatory and immune receptors (46, 47). However, as NF-κB is also involved in normal immune responses and cell survival (48, 49), a global inhibition strategy is not ideal and methods to selectively manipulate NF-κB to achieve safe and effective anti-inflammatory effects need to be explored (47).
With OA having a targetable inflammatory component, the intra-articular route is particularly well-suited for the delivery of anti-inflammatory therapeutics into the joint. As this involves direct delivery of the drug into the joint space, it has the potential advantage of reducing off-target and systemic adverse effects (50, 51). Although this seems straightforward theoretically, achieving adequate and long-lasting concentration of the therapeutic agent using this method poses certain challenges. The synovial fluid which fills the joint space and acts as a lubricant and a nutrient source for chondrocytes, is a dialysate of blood plasma. The increased pressure from the intra articular injections prompts rapid turnover and clearing of the drug molecules from the joint space, often within a half-life of 4–5 h (50, 52, 53). Thus, it is difficult to maintain adequate levels of the delivered drug long-term in the synovial fluid using intra-articular delivery. Gene therapy approaches were developed in an effort to address these drawbacks (54).
Gene therapy methods were designed with the goal of delivering therapeutic molecules directly into the joint space using vectors such that the cells' own machinery is programmed to endogenously and continuously produce high levels of the target protein at the site of injection (36, 55). This can be achieved either by using a plasmid/vector encoding a target protein (in vivo gene therapy) or modifying cells outside the body to produce the target protein (transgene) which can be reintroduced into the body (ex vivo gene therapy). The therapeutic targets for gene therapy approaches are either anti-catabolic (anti-inflammatory), anabolic, or both. Anti-catabolic factors primarily act by halting the inflammation-mediated degradation of cartilage while anabolic factors are geared towards chondrocyte proliferation and new matrix synthesis. While this approach has clear advantages compared to the delivery of recombinant proteins, the practical application has certain challenges. Firstly, for efficient transduction, the host cell needs to be metabolically active. A significant percentage of the cells need to be successfully transduced to produce detectable levels of protein (53, 56). Although this is a potential caveat, it is important to point out the possibility that the therapeutic levels of the target protein necessary to alter a disease state might be much lower than those needed for detection by routine assays. Further, the vectors and the modified cells must have low immunogenicity to evade detection by the host immune system to allow for prolonged transgene expression in the joint or to allow for periodic redosing without adverse immune responses (57–59). The cytotoxicity and immunogenicity associated with viral vectors also raises important safety concerns with using direct viral delivery systems. Achieving lasting clinically relevant levels of the therapeutic agent is vital for the successful treatment of a chronic condition such as osteoarthritis (60).
Ex vivo gene therapy
A common method of delivering target drugs to the joint is using ex vivo gene therapy approaches where the cells harvested from the joint are transduced in culture and then transplanted back into the joint. Under ideal conditions, the transduced cells become an intrinsic site of protein production. Ex vivo approaches are relatively safe as it allows for rigorous quality control of the modified cells before reintroduction into the body. There is extensive literature to demonstrate the use of ex vivo delivery approaches using various vectors derived from retrovirus (61, 62), foamy virus (63), and adenovirus (64, 65) to transduce a variety of cell types. A study where autologous synovial fibroblasts modified with a recombinant retroviral vector to overexpress IL-1Ra was the first to use an ex vivo approach in the field of cartilage repair (66). Since then, there have been several studies investigating the feasibility of using modified cells to overexpress therapeutic gene products in joint tissues (67, 68). Ex vivo approaches are well-suited for use in the equine model. Autologous cell therapy, where cells are isolated from joint tissues, expanded in culture and administered back into the joints, is commonly used in horses (18, 19) and therefore, they are a particularly amenable system to model ex vivo gene therapy methods. The first such studies in horses utilized allogenic chondrocytes which were adenovirally transduced to overexpress IGF-1(69) and BMP7 (70), both of which resulted in improved cartilage healing when transplanted into cartilage defects. A more recent study has reported improved healing and defect filling when autologous chondrocytes transduced with AAVIGF-1 were evaluated in chondral defects (71). However, ex vivo gene delivery methods are time and labor intensive and therefore not ideal for clinical application. Another major disadvantage is the rapidity with which intra-articularly injected cells are cleared from the joint, often within 1–2 weeks, thus affecting the long-term efficacy of the approach (72, 73).
In vivo gene therapy
Owing to the limitations of ex vivo gene delivery, direct in vivo delivery approaches have been extensively explored in the field of gene therapy (36, 74–83). Viral vector-based systems utilize the natural tendency of viruses to efficiently penetrate and translocate their genetic material into the host cell as part of the disease process. To create a viral vector system, the viral genome responsible for virulence is replaced by the gene of interest along with their regulatory sequences. This ensures that the virus retains their infectivity while limiting their pathogenicity and the possibility of integrating with the host genome which could lead to insertional mutagenesis and cancer (84). Although there are different viral vectors that are appropriate, there are several safety and efficacy criteria that must be met before a viral vector can be successfully used in gene therapy. Important safety issues centered around the use of viral vectors include the probability of integration of the vector genome with the host cell and the degree of immunogenicity of the vector. An important reminder of the safety risks of viral gene therapy was the death of a participant in a gene therapy clinical trial due to an immune reaction to a systemically administered adenoviral vector (85). Another instance of serious adverse effects to viral vectors was the development of leukemia in several subjects successfully treated for X-linked severe combined immunodeficiency using retroviral vectors (86, 87).
Direct in vivo approaches have been utilized extensively in the equine OA model. The relative ease of intra-articular injections and synovial fluid analysis make it a beneficial animal model for direct viral mediated gene therapy. Several viral vectors have been investigated in the equine system for their safety, transduction efficiency and long-term transgene expression including adenovirus (36, 79, 80), lentivirus (77, 88, 89), and adeno-associated virus (56, 81, 82, 90, 91). Adenoviral mediated delivery of IGF-1 into normal equine joints was shown to result in elevated and persistent synovial fluid levels without any adverse effects (80). Further, AAV mediated delivery of IL-1Ra in an equine model demonstrated sustained therapeutic transgene levels for at least 8 months post-injection. In addition, efficienct transduction of in situ equine chondrocytes and synoviocytes was observed up to 4 months following AAV mediated intra-articular delivery of GFP (82). Consistent with these findings, studies conducted by others have demonstrated consistent and stable transduction of equine joint tissues using viral vectors up to 12 weeks (91) and 24 weeks post injection (56), which speaks to the suitability of the equine model for direct gene therapy methods.
Viral vectors
Several viral vectors have been explored for the intra-articular delivery of therapeutics for cartilage repair. Adenoviral vectors were tested extensively in early studies in the equine model (36, 55, 92). Adenoviral vectors generate significantly elevated transgene expression; however, they are unable to achieve sustained expression longer than 4 weeks. In addition, adenoviral vectors have high immunogenicity and can lead to adverse tissue reactions (4, 36, 55). Retroviral vectors are also able to achieve high levels of target protein in the tissues, but these vectors need actively dividing cells to achieve efficient transduction and are therefore, are not feasible for use with chondrocytes, a cell with low turnover rate (66). Other vectors include herpes viruses which are highly cytotoxic in joints (93, 94), and lentiviruses which have been utilized in equine in vitro models (88, 95) and small animal models (89, 96, 97). However, lentiviruses show a high tendency for host integration and potential mutational adverse effects.
A significant improvement in adenoviral gene therapy was the development of replication defective E1-deleted first-generation adenoviral vectors (FGAds) (98–102). However, deletion of the E1 replication gene left the majority of the viral genome intact resulting in leaky expression of viral genes consequently leading to destruction of the transduced cells, chronic cytotoxicity and transient transgene expression. Subsequent deletion of replication genes E2 (second generation) and E3 (third generation) has progressed to the generation of helper-dependent adenoviral vectors (HDAds, gutted, gutless, or high capacity) with all of the viral coding sequences removed. Due to the complete absence of a viral genome, HDAds are capable of long-term high-level transgene expression without acute cytotoxicity. In addition, the lack of a viral genome offers additional advantages such as large cloning capacity (~37 kb) and a limited risk of insertional mutagenesis (103–105).
HDAds have been investigated extensively for liver-directed gene therapy in small animal models [reviewed in Brunetti-Pierri and Ng (104)]. These studies have demonstrated HDAds to provide long-term transgene expression with minimal cytotoxicity (106). The safety and efficacy of HDAd mediated therapy has also been explored in large animal models of liver disease including dogs (107–109) and non-human primates (110–112). In the non-human primate model, preferential delivery of HDAd vector into the liver resulted in transgene expression up to 7 years although with a gradual decline toward the end of the study (113). Further, HDAd vectors have been employed to deliver genes intra-articularly in mouse (114–116) and equine models of OA (114). In the horse OA model, treatment with HDAd-IL-1Ra resulted in a significant improvement in lameness scores, and cartilage and synovial membrane parameters suggesting an effective symptom and disease-modifying capacity as demonstrated in Figure 1. However, studies in large animal models have also revealed that HDAds show a dose-dependent acute cytotoxicity with the systemic route to administration (117). This cytopathic effect is not caused by viral gene expression, instead is an innate immune response triggered by the viral capsid. At high viral doses which is required for efficient transduction, acute cytotoxicity is observed, the level of which increases with dose. Therefore, strategies to block this host response or achieve efficient transduction at a sub-toxic dose need to be explored to overcome these challenges to clinical translation. In addition, the host inflammatory response against the viral capsid proteins is limited to high-dose systemic injections (104). This is not a limitation when using non-systemic routes of administration where HDAds can be directly delivered to isolated closed spaces thus minimizing the systemic cytotoxic effects. This is relevant in the context of OA therapy where HDAds can be administered intra-articularly (115) to reduce systemic cytopathic effects.
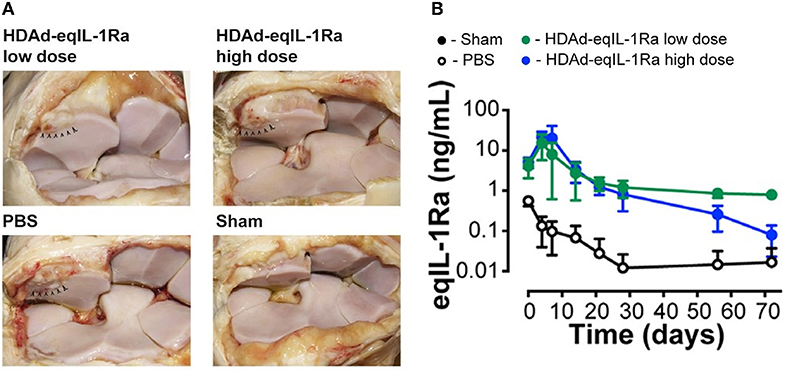
Figure 1. (A) Synovial membrane of OA joints injected with helper-dependent adenoviral vector encoding equine IL-1Ra (HDAd-eqIL-1Ra) at a low dose (2 × 1011 vp) or high dose (2 × 1012 vp) were comparable to sham-operated joints, whereas PBS-treated control joints appeared more hemorrhagic. Arrowheads indicate the site of osteochondral fragmentation. (B) Equine IL-1Ra levels in the synovial fluid peaked at 4 days after injection and declined to 1 ng/ml and 0.1 ng/ml in the low- and high-dose HDAd-eqIL-1Ra groups towards the end of the study. Values are represented as mean ± SEM. Reproduced with permission from Nixon et al., 2018 (114).
Adeno-associated virus (AAV) offers significant advantages over other viral vectors. It is a DNA parvovirus with a 4.68-kb genome composed of a linear single strand of DNA (118). AAV is unique in that it is naturally defective for replication and requires co-infection with a helper virus, most commonly adenovirus, to induce and support replication (119). This dependency on the helper virus for co-infection makes AAV non-pathogenic to humans (120, 121). Its recombinant form does not encode any viral proteins and thus has low chances of being recognized by the host immune system. Further, AAV vectors do not integrate into the genome of the host like lentiviruses. Various preclinical studies have demonstrated extended and successful transgene expression with AAV serotypes 2, 2.5, 5, and 8 (83, 122, 123). Unlike other viral vectors, AAV can transduce chondrocytes in addition to synoviocytes with a high level of transduction (83). Although the proportion of transduced cells are lower in cartilage than synovium, this is a major advantage considering that most viral vectors are unable to penetrate the extracellular matrix and efficiently transduce chondrocytes (53, 124–126). As chondrocytes are at the center of the OA pathogenesis, this is a major advantage of using AAV vectors for OA therapy. Importantly, AAV serotypes 2 and 2.5 use heparan sulfate, a vital ECM component of cartilage, as the primary binding receptor. This was found to be an important determinant of serotype dependent AAV transduction efficiency between cartilage explants and monolayer cultures which differ in their heparan sulfate content (127). The small particle size of the AAV vector allows it to enter and diffuse through the cartilage matrix to achieve effective transduction. However, this small capsid size also limits the transgene payload of AAV vectors to 4,100–4,900 bp, and this poses a problem for genes with large coding sequences, such as cystic fibrosis transmembrane conductance regulator gene (128, 129).
AAV vector is composed of a single stranded DNA (ssDNA) genome, and the synthesis of the complementary strand occurs using the host's cellular replication factors by virtue of a palindromic terminal repeat (TR) structure which serves as a primer for synthesis of the complementary DNA strand (130–132). Therefore, transduction efficiency and onset of transgene expression is dependent on the conversion from the single to double strand DNA by the host cell and this is a major limiting factor of AAV vectors. This disadvantage has been overcome by the development of self-complimentary AAV vectors (scAAV) which are designed to self-generate both the + and –strand viral genomes without depending on the host cell (133, 134). scAAV vectors are composed of two halves of the ssDNA packaged separately such that they fold and base pair to form the dsDNA of half the length. While this reduces the average packaging capacity of scAAV vectors (~2.2 kb) to half of that of AAVs, this eliminates the need for host cell-dependent DNA synthesis which translates into higher efficiency and faster onset of transgene expression (135). scAAV vectors have demonstrated ~25-fold higher transduction efficiencies compared to the conventional AAV vectors as demonstrated in Figure 2 (136) and faster onset of protein production (81, 82, 133, 134, 136, 137). As packaging size is even more restricted in scAAVs compared to AAVs, optimization of transcriptional and post-transcriptional regulatory elements, as well as codon optimization, is vital to achieving high levels of transduction (82). The field of gene therapy has the potential to advance significantly with the recent development of CRISPR/Cas9 technology. In fact, a recent study demonstrated the feasibility of this approach using an adeno-associated virus, which expressed CRISPR/Cas9 components to target multiple genes simultaneously in an induced OA mouse model (138).
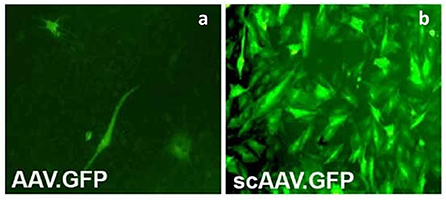
Figure 2. Primary articular fibroblasts from rabbits transduced with the same dose of conventional AAV encoding GFP (AAVGFP) (a) or double-stranded, self-complementary AAV encoding GFP (scAAVGFP) (b). scAAV vectors produced a ~25-fold greater transduction compared to the conventional AAV vector. Reproduced with permission from Kay et al. (136).
Non-viral vectors
Viral vector-based gene therapy offers the advantage of high transduction efficiency, however, it has not garnered unanimous acceptance due to the drawbacks of immunogenicity and cytotoxicity. In this context, non-viral vectors have been actively explored as an alternative. Non-viral vectors include naked-DNA and liposomes both of which comprise a plasmid, a circular closed DNA strand. The transgene is inserted into the plasmid directly followed by delivery into the cells. Non-viral gene delivery systems can be categorized into physical methods such as electroporation, sonoporation, photoporation, hydroporation, and magnetofection or chemical carriers such as inorganic particles and synthetic/biodegradables (139, 140). Compared to viral vectors, plasmids are relatively safe as there is no risk of integrating with the host genome which also allows for potential re-dosing with low immunogenicity. In addition, they are easier and cheaper to manufacture and have a longer shelf life. Owing to these advantages, plasmids have been used extensively in non-viral gene therapy approaches (141–143). A landmark study in the field of OA therapy involved the use of a plasmid to deliver a long-acting human interleukin-10 variant into the joints of companion dogs with naturally occurring osteoarthritis (144). This resulted in an improvement in pain measurements without any adverse effects in the double blinded placebo-controlled study. However, a major challenge with the existing non-viral methodologies is their inability to match the efficiency of viral vector-based systems.
Challenges in gene therapy
The field of viral vector-based gene therapy has expanded significantly in recent years which translates to over 2000 clinical trials initiated since the 1990s and a drastic rise in commercial initiatives (145). However, this acceleration in the field has been accompanied by challenges associated with viral vector manufacturing capacity, vector characterization and increased regulatory scrutiny (146). Designing and manufacturing viral vectors successfully and consistently is expensive, and requires experienced scientists and high quality control (QC). These challenges have limited the widespread clinical application of gene therapy approaches (147). Viral vector manufacturing involves a variety of approaches, typically using mammalian cells in adherent or suspension systems. However, these systems are challenging to scale up due to the increased supply costs, processing time and batch to batch variation (148, 149). An alternative to overcome these limitations is the use of larger single-use culture systems and bioreactors which is increasingly being adopted in the field (150–154). Another source of variation in vector manufacturing arises from the use of transfection-based methods to generate vectors. Efficient transfection is highly dependent on the appropriate combination of transfection reagents, pH and plasmid DNA, and is highly susceptible to batch-to-batch variation. When scaling up for clinical manufacturing, this poses a significant barrier to producing consistent results with low lot-to-lot variability (155–158). Moreover, the transition of gene therapy approaches from pre-clinical animal studies to human clinical studies has prompted extensive characterization and QC testing of recombinant viral vectors to ensure batch-to-batch consistency. However, one of the limitations to characterizing and QC testing of viral vectors is their inherent degree of complexity. AAV, one of the smallest and simplest of recombinant viral vectors is significantly more complex than the most complex recombinant protein. Retroviral vectors are more complex with a double-stranded genome and a lipid bilayer encapsulating the capsid (146, 159). Thus, methods for characterizing viral vectors need to be customized for each different viral vector and each serotype.
Cell-based (ex vivo) gene therapy offers a different set of problems such as high sensitivity to environmental factors and intrinsic biological variability. Unlike conventional therapeutics, ex vivo gene therapy products are composed of live cells from the starting material to the final product. As the cells cannot be filtered or sterilized at the end of culture before use in the patient, the entire manufacturing process must be carefully designed and enforced with excellent QC strategies in place to ensure the safety and efficacy of the final product (160–162). The existing manufacturing systems for gene therapy are largely manual involving planar culture systems, which are difficult to scale-up and are riddled with batch-to-batch variability due to human error (163, 164). Cell based gene therapies typically use the patient's own cells as starting material which is also a source of variation owing to the inherent biological differences between donors. This variability can be considerably reduced when using allogenic or “off-the-shelf” therapies where cells from one individual can be used for multiple treatments. In contrast, autologous cell-based therapies, where cells taken from a patient are reintroduced into the same patient, present additional manufacturing challenges to control for the biological variation in the input material and inconsistent storage and preservation methods across samples (164–167). To add to these challenges, commercial gene therapy products are tightly regulated and are required to be produced in accordance with good manufacturing practice (GMP). However, this becomes challenging in the field of patient-specific (autologous) therapies where the cells are often sourced from a diseased joint and therefore, may not meet the required GMP standard (163, 168). In this context, gene therapy manufacturing processes would benefit from a more adaptive strategy that accounts for the inherent biological variation in the input material, differences in product quality and the complexity of viral systems. Technological improvements in viral manufacturing in future years is also likely to help in overcoming obstacles in large scale vector manufacturing and facilitating easier clinical translation.
Horse as a translational OA model
Pre-clinical studies using viral vectors
As illustrated in previous sections, horses offer an ideal model system for human OA. Numerous studies have demonstrated the advantages of viral gene delivery for the treatment of osteoarthritis in animal models. The purpose of this review article is not to provide a comprehensive review of all of these studies. Instead, examples of particular significance or interest are highlighted. Preclinical gene therapy studies in horses were performed by our group in 2002 with a first-generation adenoviral mediated delivery of Interleukin-1 receptor antagonist (IL-1Ra) into healthy equine joints. The study was successful in demonstrating a dose-dependent increase in IL-1Ra levels in synovial fluid, however, an acute leukocytosis was observed in the synovial fluid at the highest concentration tested (5 × 1011 viral particles) (36). Further, the efficacy of IL-1Ra to ameliorate the symptoms of OA was investigated in an induced equine OA model using an osteochondral chip fragment created in the intercarpal joint. Viral delivery of IL-1Ra resulted in increased intra-articular expression of IL-1Ra for ~28 days with a peak at 7 days. Moreover, the elevated IL-1Ra expression reduced joint pain and had a protective effect on the joint tissues over the course of the 90 day study as summarized in Figure 3 (169). While these were hallmark studies illustrating the success of viral mediated gene delivery into large animal joints, these studies used first-generation adenovirus vectors. An improvement in the field was the discovery of single-stranded adeno-associated viruses (AAVs) and subsequently of scAAV vectors generated using half-genome sized vector plasmids, or those containing a mutation in one of the terminal resolution sequences of the AAV ITRs which demonstrated greater transduction efficiencies and prolonged transgene expression compared to AAVs (81, 82, 133–135).

Figure 3. Effect of osteoarthritis and gene transfer on cartilage erosion. Photographs of the intercarpal joint illustrating extensive full-thickness articular cartilage erosions in OA joints of both untreated (a) and adenoviral vector encoding equine IL-1Ra (Ad-EqIL-1Ra) (b) treated horses. Erosions are evident in the untreated joint especially in areas of the third carpal bone (2) not adjacent to the osteochondral fragment (1). (3) shows the area of aseptic harvest of cartilage from the intermediate carpal bone. (c) presents cartilage erosion scores by treatment group. Sections of articular cartilage stained with SOFG demonstrating little or no stain uptake in the OA joint of an untreated horse (d) vs. moderate stain uptake from an OA joint with Ad-EqIL-1ra treatment (e). Plot of SOFG scores by treatment group (f). Different letters associated with bars indicate a statistical difference (P < 0.05) between bars. Lines with an asterisk (*) linking treatment groups indicate a statistical difference between treatment groups. Reproduced with permission from Frisbie et al. (36).
scAAV vector-based gene therapy approaches were successfully tried in laboratory animals (136, 170) before being tested in normal equine joints (56, 81, 82, 171, 172) and in an induced OA model (91). Goodrich et al. were the first to demonstrate via a novel fluorescent arthroscopic imaging system that in situ chondrocytes (in addition to synoviocytes) can be efficiently transduced with intra articular injection of scAAV GFP in equine joints (82). Watson et al. used scAAV containing the cDNAs for human IL-1Ra (scAAVhIL-1Ra) and green fluorescent protein (scAAVGFP) to transduce both equine and human synovial fibroblasts in culture. Of the AAV serotypes tested, AAV1, 2 and 5 were able to transduce both cell types at high efficiency with the equine cells showing a 10-fold higher viral uptake compared to the human cells. Further, delivery of these scAAV containing human IL-1Ra into the joints of equine forelimbs revealed biologically relevant levels of transgene expression mainly in the synovial cells and weakly in the articular chondrocytes. However, transgene expression steadily declined over a period of 5 weeks potentially due to detection and clearing of cells expressing the xenogenic human IL-1Ra protein by the host immune system (172). The human cDNA was later replaced with the homologous equine IL-1Ra, subsequent codon optimization, and optimizing the promoter for joint tissues resulting in a scAAV equine IL-1Ra vector which induced significant and therapeutic protein production in equine joints (82). This was followed by several dosing studies to identify dosing regimens to achieve prolonged and therapeutic levels of transgene expression (56, 81). In our study (81), scAAVIL-1ra, scAAV2GFP or saline at a dose ranging from 5 × 1010 to 5 × 1012 viral particles were delivered into the middle carpal space of six healthy horses. The dose of 5 × 1012 achieved therapeutic levels of IL-1Ra for at least 8 months following injection without any adverse effects (Figure 4). Moreover, re-dosing of the low dose groups with an alternate serotype demonstrated a rescue of IL-1Ra expression. Interestingly, one horse that was redosed with a scAAV6 IL-1Ra demonstrated a rescue of IL-1Ra levels at 2 weeks post injection but a rapid drop at 4 weeks suggestive of an immune response (Figure 5) while the other horse also redosed with the same serotype showed high levels of IL-1Ra which was sustained for over 100 days followed by a gradual decline. The results of this study suggest the ability to re-dose a patient with an alternate serotype and rescue IL-1Ra expression. Chondrocytes could be efficiently transduced using the dosing protocol tested in this study although the response to repeated dosing needs to be investigated further.
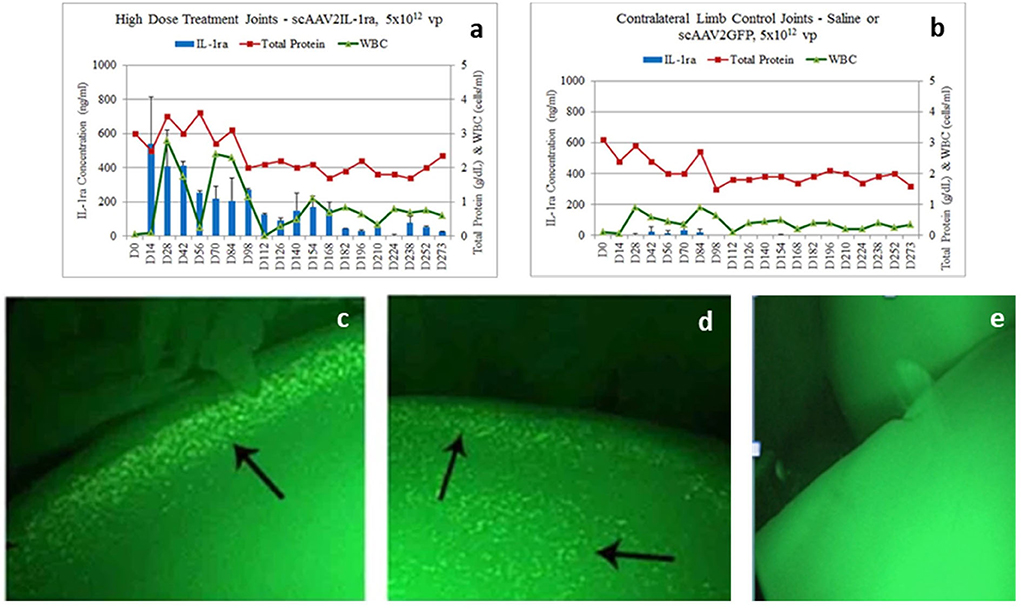
Figure 4. (a,b) IL-1Ra levels (blue bars), total protein levels (red line) and white blood cell (WBC) levels (green line) in the joints of horses in a scAAVIL-1Ra dosing trial. IL-1Ra levels remain elevated in joints injected with the highest dose of scAAVIL-1Ra (a) compared to the control joints (saline or scAAVGFP) (b). Transduced joints produced very high levels of IL-1Ra (over 100 ng/ml) for over 168 days after which, levels of IL-1Ra continued to be produced between 50–100 ng/ml out to 273 days. WBC counts did not rise above normal (1,000 cells/ml) for joints injected with scAAVIL-1Ra and saline or scAAVGFP. (c–e) Arthroscopic image of in situ vector encoded-GFP transduced chondrocytes (arrows in left and middle images) from joints injected with 5 × 1012 vg of scAAVGFP (left) or saline (right image). The arthroscopic images were taken 4 months following intra-articular injection of scAAVGFP suggesting chondrocytes have been stably transduced to produce protein. The arrows point to fluorescent chondrocytes on the edge (c) or edge and surface of cartilage (d). Some green autofluorescence (of the cartilage) surrounds the cells and can be seen in the saline injected joint (e). scAAV, self-complementary AAV. Reproduced with permission from Goodrich et al. (81).

Figure 5. (A–D) IL-1Ra (ng/ml), total protein (g/dl) and WBC (cells/ml) levels in low dosed and control limb synovial fluid samples after redosing at Day 154. One horse (A) whose joint was re-injected had IL-1Ra level sharply increase to 675 ng/ml and decline rapidly while the other horse (C) that was redosed showed elevated IL-1Ra levels which remained elevated until Day 273 compared to the control joints (B,D). Reproduced with permission from Goodrich et al., 2015 (81).
Similar patterns were observed in a 6-month study done by Watson Levings et al. (56). The viral dose of 5 × 1012 viral particles was found to result in stable therapeutic levels of IL-1Ra (over 35 ng/ml) with no adverse effects up to 24 weeks after intra-articular administration in healthy equine joints. In a related study, the same group of investigators tracked the expression of IL-1Ra in healthy vs. diseased joints. Interestingly, the vector activity appeared to be significantly higher in the joints with OA pathologies compared to healthy joints as demonstrated by a concentration of GFP fluorescence in the areas of articular cartilage with distinct damage. The investigators postulate that the increase in transgene expression could be a result of proliferation and increased metabolism of the diseased chondrocytes as well as stress induced activation of the cytomegalovirus (CMV) promoter which is normally activated by stress-activated protein kinases (173–175). These observations present a unique opportunity to target transgene expression to specific diseased regions of the joint and direct site-specific repair. Further, in a 12-week study, the same dose of 5 × 1012 of scAAV.eqIL-1Ra administered to joints of an induced equine OA model resulted in significantly elevated IL-1Ra expression levels in synovial fluid with a significant functional outcome of reduction in lameness, inflammatory responses and a chondroprotective effect at the site of injury (Figure 6) (91). While no adverse effects were observed in response to the viral dose tested in healthy horses (56, 81) and in a 12-week study in an induced OA model (91) summarized above, it is unclear how a diseased joint will behave long-term to this dose.

Figure 6. Changes in tissue pathology associated with scAAV.eqIL-1Ra treatment in an equine model of OA (A) showing a significant decrease in total arthroscopy scores for treated joints compared to the control joints (B) Representative arthroscopic images of the osteochondral lesions in treated and control horses at the time of OA induction (week -2) and at the endpoint (week 12) (C) showing a significant decrease in total histologic scores for treated joints compared to controls consistent with the microscopic appearance of bone repair tissue (H&E) and cartilage (toluidine blue) in treated and control joints (D) Reproduced with permission from R. S. Watson Levings et. al, 2018 (91). An asterisk (*) linking treatment groups indicate a statistical difference (p < 0.05) between treatment groups.
Gene therapy strategies in the realm of OA therapy can be broadly grouped into two–one aimed at delivering anti-catabolic gene products which halt the activity of inflammatory cytokines responsible for inflammation and breakdown of the cartilage ECM and the other directed toward delivering anabolic products that stimulate chondrocytes to proliferate and increase new matrix synthesis thus promoting cartilage regeneration.
Of the several anti-inflammatory factors tested for their efficacy in OA therapy, IL-1Ra is by far the most extensively investigated (36, 81, 93, 114, 176–179). IL-1Ra is the natural inhibitor of IL-1β and competitively inhibits IL-1β by binding to the surface receptors thereby preventing the cellular effects of IL-1β. As a potent mediator of the inflammation, IL-1β is responsible for the production of the major effectors of the inflammatory cascade including cyclooxygenases I and II, nitric oxide, phospholipase A2, prostaglandin E2, reactive oxygen species as well as inflammatory cytokines and chemokines which trigger degenerative changes in the cartilage matrix (180, 181). IL-1Ra, being a small protein [with a cDNA 1.6 kb in length], is ideal for gene therapy approaches using scAAV vectors which have packaging size limitations of ~2.5 kb (136, 182). IL-1Ra is being used clinically as a recombinant protein, Anakinra/Kineret® in human and animal patients (183). This is administered as a daily subcutaneous injection, therefore the risk of toxic levels intra-articularly is low however, it does pose systemic side effects such as local reaction at the injection site and upper respiratory tract infections (184–186).
Insulin-like growth factor-1 (IGF-1) is a critical anabolic growth factor for maintaining cartilage health and integrity and for this reason, IGF-1 offers another useful target in OA therapy as a chondroprotective factor. In vitro studies provide strong support for the use of IGF-1 in promoting cartilage repair. IGF-1 has been shown to increase the metabolism and proliferation of chondrocytes in culture in several studies (187–189). The effect of IGF-1 was tested in an antigen-induced arthritis rabbit model using adenoviral-mediated delivery. Although IGF-1 levels were elevated in the joints which resulted in enhanced proteoglycan synthesis, this did not translate into a significant chondroprotective effect against OA pathology (190). IL-1Ra and IGF-1 have also been used in combination with the goal of simultaneously blocking cartilage breakdown and effecting cartilage regeneration, respectively (187). Synovial fibroblasts transduced with both IL-1Ra and IGF-1 were cocultured with normal equine articular cartilage and cartilage damaged by exposure to IL-1. The transgene expression from the synovial fibroblasts was able to increase matrix synthesis in normal cartilage as well as partially reverse the IL-1 induced cartilage matrix depletion in the damaged cartilage. Among large animal models, Goodrich et. al demonstrated that direct adenoviral mediated delivery of IGF-1 to the synovium of healthy equine joints was able to provide sustained high levels of IGF-I in the synovial fluid with minimal detrimental effects (80). Further, the chondroprotective effect of IGF-I was illustrated in an equine cartilage repair model (67). Chondrocytes genetically modified by an adenovirus vector encoding equine IGF-1 (AdIGF-1) administered into surgically induced cartilage defects resulted in improved repair of the cartilage defect with increased defect filling and elevated type II collagen expression compared to control defects.
Although IL-1Ra and IGF-1 have been the focus of OA gene therapy systems, significant advances in the field of viral vector design and generation opens the door for other potential gene targets known to play a role in cartilage development and regeneration. In fact, several of these have been investigated in in vitro studies and laboratory animal studies. SRY-Box Transcription Factor 9 (SOX9), a chondrocyte-specific transcription factor was tested in human and rabbit cell lines as well as in a rabbit in vivo model for degenerative disc disease using recombinant adenoviruses (191) and AAVs (192). Both studies demonstrated successful upregulation in SOX9 and Type 2 collagen levels and an associated protective effect on the architecture of the nucleus pulposus in vivo. Similar observations were made in another study investigating the effect of a recombinant adeno-associated viral (rAAV) vector mediated delivery of SOX9 in modulating the osteoarthritic phenotype of chondrocytes in a three-dimensional in vitro model (193). SOX9 has also been investigated in combinatorial gene delivery approaches in the field of cartilage regeneration (194).
Interleukin 10 (IL-10), an anti-inflammatory cytokine, which downregulates proinflammatory cytokines and its receptors, is another molecule that the gene therapy field has targeted. Over expression of this protein has been investigated in arthritis (195–200) and neural disorders (201–203). Human IL-10 gene carrying plasmids were designed by Watkins et. al. to test the safety and efficacy of IL-10 in a 6-month study in dogs. The therapy was well-tolerated without any adverse effects in the toxicology study. Subsequent testing in a translational model of OA in companion (pet) dogs with naturally occurring OA showed a significant improvement in pain measurements based on clinical assessments without any side-effects (144). Direct viral mediated delivery of IL-10 has also been examined in both in vitro and in vivo studies. Transduction of equine chondrocytes with AAV5 overexpressing IL-10 was able to mitigate the IL-1β mediated pro-inflammatory cascade in pellet cultures with a reduction in IL-1β and Prostaglandin E2 levels (204). In a related study, equine bone marrow-derived MSCs overexpressing IL-10 were able to provide anti-inflammatory effects in a stimulated, co-culture OA model. However, this did not translate to a protective effect on the extracellular matrix (ECM) or a rescue of ECM loss in the transduced co-cultures (90). Further study in an equine model demonstrated that an AAV5 vector overexpressing IL-10 provided rapid and sustained IL-10 expression following direct intra-articular delivery. Importantly, IL-10 levels could be detected in plasma, synovial fluid, and synovial membrane of treated joints until day 84 compared to PBS injected controls without any adverse synovial response (205). These studies demonstrate the feasibility of delivering IL-10 into diseased joints and provide support for further in vivo investigations into the chondroprotective effects of IL-10 for OA therapy.
Proteoglycan 4 (PRG4), a secreted protein has also been explored as a potent chondroprotective factor in blocking the pathogenesis of osteoarthritis (116, 206). PRG4 or lubricin, produced by superficial zone chondrocytes and the synovial lining cells, is an important component of synovial fluid. It provides synovial fluid with the ability to disperse strain energy under biomechanical loading thus contributing to the lubrication and protection of articular ccartilage surfaces. Recombinant PRG4 has been reported to protect against progression of OA in rodent models (207–209). Intra-articular expression of PRG4 using a helper-dependant adenoviral vector has been reported to provide protection against the development of both age-related and post traumatic OA in a mouse model (210). Further, transcriptional profiling studies revealed that PRG4 overexpression inhibits the transcriptional networks associated with cartilage catabolism and hypertrophy through the up-regulation of hypoxia inducible factor 3α (HIF3α), thus protecting against cartilage degradation and development of OA. Bone morphogenetic proteins (BMPs) are a class of proteins that have generated interest in recent years for their role in musculoskeletal repair. Recombinant protein injections of BMP2 and BMP7 has shown improved cartilage healing in previous studies (211, 212). BMP7 has been shown to enhance cartilage matrix synthesis and chondrogenic ability of chondrocytes modified by an adenovirus vector encoding BMP-7 in a bovine ex vivo model (213) and an equine model (70). However, more extensive studies are needed to identify and establish these additional therapeutic targets for their potential role in OA therapy.
Future directions and conclusions
This review summarizes the current state of the field of gene therapy with emphasis on preclinical studies using the horse as an experimental model for human OA. We outline the recent advancements in viral vector-based delivery systems and potential therapeutic targets for OA therapy. The field of gene therapy has weathered many setbacks, however, technological advancements resulting in the development of safe and effective vectors and delivery methods have paved the way for increased acceptance and renewed interest in the field. There have been several successful clinical trials in human medicine (Luxterna® for congenital retinal degeneration and Zolgensma® for spinal muscular atrophy) which resulted in positive effects on the quality of life of the patients. The preclinical studies outlined in this review demonstrate the feasibility and relevance of using the equine joint as a translational model to explore treatment strategies for OA using gene therapy. However, high manufacturing costs associated with vector production and the inherent expensive nature of equine research pose significant challenges to undertaking large preclinical studies using this model. Improvements in vector development technology would likely lead to decreased production costs in the future, however, a growth in resources available for equine research would be vital in moving the field forward.
Author contributions
PT and LRG were responsible for drafting the review and revising it critically. PT, JNP, JCG, RJS, CWM, and LRG were involved in revisions and approval of the final version. All authors have read and approved the final submitted manuscript.
Funding
This work was supported by internal funding from the Orthopaedic Research Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-Pocket costs of osteoarthritis in the us: evidence from national survey data. Arthritis Rheum. (2009) 60:3546–53. doi: 10.1002/art.24984
2. Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a us population-based survey. Arthritis Care Res. (2016) 68:574–80. doi: 10.1002/acr.22721
3. Dyson SJ, Ross MW. Diagnosis and Management of Lameness in the Horse. Philadelphia, PA: WB Saunders Company (2011).
4. Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse - a review. Vet J. (2006) 171:51–69. doi: 10.1016/j.tvjl.2004.07.008
5. McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. (2012) 1:297–309. doi: 10.1302/2046-3758.111.2000132
6. USDA L, Lameness V. Laminitis in US Horses. Fort Collins, CO: USDA; APHIS; VS, CEAH, National Animal Health Monitoring System (2000).
7. Schlueter AE, Orth MW. Equine osteoarthritis: a brief review of the disease and its causes. Equine Comp Exerc Physiol. (2004) 1:221–31. doi: 10.1079/ECP200428
8. Keegan KG. Evidence-Based lameness detection and quantification. Vet Clin N Am Equine Prac. (2007) 23:403–23. doi: 10.1016/j.cveq.2007.04.008
9. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. Oarsi guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. (2014) 22:363–88. doi: 10.1016/j.joca.2014.01.003
10. Gobbi A, Karnatzikos G, Kumar A. Long-Term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. (2014) 22:1986–96. doi: 10.1007/s00167-013-2676-8
11. Hangody L, Kish G, Kárpáti Z, Udvarhelyi I, Szigeti I, Bély M. Mosaicplasty for the Treatment of Articular Cartilage Defects: Application in Clinical Practice. Thorofare, NJ: SLACK Incorporated (1998). doi: 10.3928/0147-7447-19980701-04
12. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. (2001) 391:S362–9. doi: 10.1097/00003086-200110001-00033
13. Torrie AM, Kesler WW, Elkin J, Gallo RA. Osteochondral allograft. Curr Rev Musculoskelet Med. (2015) 8:413–22. doi: 10.1007/s12178-015-9298-3
14. Camargo Garbin L, Morris MJ. A comparative review of autologous conditioned serum and autologous protein solution for treatment of osteoarthritis in horses. Front Vet Sci. (2021) 8:602978. doi: 10.3389/fvets.2021.602978
15. Garbin LC, Olver CS. Platelet-Rich products and their application to osteoarthritis. J Equine Vet Sci. (2020) 86:102820. doi: 10.1016/j.jevs.2019.102820
16. Khurana A, Goyal A, Kirubakaran P, Akhand G, Gupta R, Goel N. Efficacy of autologous conditioned serum (Acs), platelet-rich plasma (Prp), hyaluronic acid (Ha) and steroid for early osteoarthritis knee: a comparative analysis. Indian J Orthop. (2021) 55 (Suppl. 1):217–27. doi: 10.1007/s43465-020-00274-5
17. Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-Rich plasma. Clin Sports Med. (2019) 38:17–44. doi: 10.1016/j.csm.2018.08.001
18. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. (1994) 331:889–95. doi: 10.1056/NEJM199410063311401
19. Brittberg M, Recker D, Ilgenfritz J, Saris DB, Group SES. Matrix-Applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. (2018) 46:1343–51. doi: 10.1177/0363546518756976
20. Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. (2017) 24:508–17. doi: 10.1016/j.knee.2016.12.002
21. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-Based status of second-and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. (2013) 29:1872–8. doi: 10.1016/j.arthro.2013.07.271
22. Jacobi M, Villa V, Magnussen RA, Neyret P. Maci-a new era? Sports Med Arthrosc Rehabil Ther Technol. (2011) 3:1–7. doi: 10.1186/1758-2555-3-10
23. Baek S-H, Kim S-Y. Pharmacologic treatment of osteoarthritis. J Korean Med Assoc. (2013) 56:1123–31. doi: 10.5124/jkma.2013.56.12.1123
24. Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. (2016) 11:19. doi: 10.1186/s13018-016-0346-5
25. Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. (2013) 15:438–46. doi: 10.1208/s12248-013-9454-x
26. Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. (2009) 17:705–13. doi: 10.1016/j.joca.2008.11.008
27. Breinan HA, Minas T, Hsu H-P, Nehrer S, Shortkroff S, Spector M. Autologous chondrocyte implantation in a canine model: change in composition of reparative tissue with time. J Orthop Res. (2001) 19:482–92. doi: 10.1016/S0736-0266(00)90015-9
28. Hurtig MB, Buschmann MD, Fortier LA, Hoemann CD, Hunziker EB, Jurvelin JS, et al. Preclinical studies for cartilage repair: recommendations from the international cartilage repair society. Cartilage. (2011) 2:137–52. doi: 10.1177/1947603511401905
29. Stricker-Krongrad A, Shoemake CR, Bouchard GF. The miniature swine as a model in experimental and translational medicine. Toxicol Pathol. (2016) 44:612–23. doi: 10.1177/0192623316641784
30. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. (2006) 19:142–6. doi: 10.1055/s-0038-1632990
31. Hembry RM, Dyce J, Driesang I, Hunziker EB, Fosang AJ, Tyler JA, et al. Immunolocalization of matrix metalloproteinases in partial-thickness defects in pig articular cartilage. A preliminary report. J Bone Joint Surg Am. (2001) 83:826–38. doi: 10.2106/00004623-200106000-00003
32. Malda J, Benders KE, Klein TJ, de Grauw JC, Kik MJ, Hutmacher DW, et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage. (2012) 20:1147–51. doi: 10.1016/j.joca.2012.06.005
33. McIlwraith CW, editor. From arthroscopy to gene therapy-−30 years of looking in joints. In: Proceedings of the Annual Convention Seattle, WA (2005).
34. Nelson BB, Goodrich LR, Barrett MF, Grinstaff MW, Kawcak CE. Use of contrast media in computed tomography and magnetic resonance imaging in horses: techniques, adverse events and opportunities. Equine Vet J. (2017) 49:410–24. doi: 10.1111/evj.12689
35. Nelson BB, Kawcak CE, Barrett MF, McIlwraith CW, Grinstaff MW, Goodrich LR. Recent advances in articular cartilage evaluation using computed tomography and magnetic resonance imaging. Equine Vet J. (2018) 50:564–79. doi: 10.1111/evj.12808
36. Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. (2002) 9:12–20. doi: 10.1038/sj.gt.3301608
37. Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. (2007) 25:913–25. doi: 10.1002/jor.20382
38. Estrada McDermott J, Pezzanite L, Goodrich L, Santangelo K, Chow L, Dow S, et al. Role of innate immunity in initiation and progression of osteoarthritis, with emphasis on horses. Animals. (2021) 11:3247. doi: 10.3390/ani11113247
39. Frisbie DD. Synovial joint biology and pathobiology. In: Auer JA, Stick JA, editors. Equine Surgery. 4th ed. Saint Louis, MO: W.B. Saunders Company (2012). p. 1096–114.
40. McIlwraith CW. Traumatic arthritis and posttraumatic osteoarthritis in the horse. In Mcllwraith CW, Frisbie DD, Kawcak CE, van Weeran PR, editors. Joint Disease in the Horse. 2nd ed. Edinburgh: W. B. Saunders Company (2016). p. 33–48.
41. Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. the role of macrophages in osteoarthritis and cartilage repair. Osteoarthritis Cartilage. (2020) 28:544–54. doi: 10.1016/j.joca.2019.12.007
42. Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res. (2020) 38:253–7. doi: 10.1002/jor.24457
43. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-Grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:580–92. doi: 10.1038/nrrheum.2016.136
44. Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. (2017) 29:79–85. doi: 10.1097/BOR.0000000000000353
45. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. (2013) 5:77–94. doi: 10.1177/1759720X12467868
46. Choi MC, Jo J, Park J, Kang HK, Park Y. Nf-Kb signaling pathways in osteoarthritic cartilage destruction. Cells. (2019) 8:734. doi: 10.3390/cells8070734
47. Tas SW, Vervoordeldonk MJ, Tak PP. Gene therapy targeting nuclear factor-kappab: towards clinical application in inflammatory diseases and cancer. Curr Gene Ther. (2009) 9:160–70. doi: 10.2174/156652309788488569
48. Roman-Blas JA, Jimenez SA. Nf-Kb as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. (2006) 14:839–48. doi: 10.1016/j.joca.2006.04.008
49. Liu T, Zhang L, Joo D, Sun S-C. Nf-Kb signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. doi: 10.1038/sigtrans.2017.23
50. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. (2014) 10:11–22. doi: 10.1038/nrrheum.2013.159
51. Simkin PA. Synovial perfusion and synovial fluid solutes. Ann Rheum Dis. (1995) 54:424–8. doi: 10.1136/ard.54.5.424
52. Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, et al. Intra-Articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. (2008) 97:4622–54. doi: 10.1002/jps.21346
53. Levings R, Smith A, Levings PP, Palmer GD, Dacanay A, Colahan P, et al. Gene therapy for the treatment of equine osteoarthritis. In: Rutland C, Rizvanov A, editors. Equine Science. London: IntechOpen (2020). doi: 10.5772/intechopen.93000
54. Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. (1992) 11:227–31. doi: 10.1089/dna.1992.11.227
55. Nixon AJ, Goodrich LR, Scimeca MS, Witte TH, Schnabel LV, Watts AE, et al. Gene therapy in musculoskeletal repair. Ann N Y Acad Sci. (2007) 1117:310–27. doi: 10.1196/annals.1402.065
56. Watson Levings RS, Broome TA, Smith AD, Rice BL, Gibbs EP, Myara DA, et al. Gene therapy for osteoarthritis: pharmacokinetics of intra-articular self-complementary adeno-associated virus interleukin-1 receptor antagonist delivery in an equine model. Hum Gene Ther Clin Dev. (2018) 29:90–100. doi: 10.1089/humc.2017.142
57. Kotani H, Newton III PB, Zhang S, Chiang YL, Otto E, Weaver L, et al. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. (1994) 5:19–28. doi: 10.1089/hum.1994.5.1-19
58. Mulligan RC. The basic science of gene therapy. Science. (1993) 260:926–32. doi: 10.1126/science.8493530
59. Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. (2000) 1:91–9. doi: 10.1038/35038533
60. Evans CH. Gene therapies for osteoarthritis. Curr Rheumatol Rep. (2004) 6:31–40. doi: 10.1007/s11926-004-0081-5
61. Kim SH, Lechman ER, Kim S, Nash J, Oligino TJ, Robbins PD. Ex vivo gene delivery of Il-1ra and soluble Tnf receptor confers a distal synergistic therapeutic effect in antigen-induced arthritis. Mol Ther. (2002) 6:591–600. doi: 10.1006/mthe.2002.0711
62. Makarov SS, Olsen JC, Johnston WN, Anderle SK, Brown RR, Baldwin AS, et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist Cdna. Proc Natl Acad Sci USA. (1996) 93:402–6. doi: 10.1073/pnas.93.1.402
63. Armbruster N, Weber C, Wictorowicz T, Rethwilm A, Scheller C, Steinert AF. Ex vivo gene delivery to synovium using foamy viral vectors. J Gene Med. (2014) 16:166–78. doi: 10.1002/jgm.2774
64. Gelse K, Jiang QJ, Aigner T, Ritter T, Wagner K, Pöschl E, et al. Fibroblast-mediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum. (2001) 44:1943–53. 10.1002/1529-0131(200108)44:8< 1943::AID-ART332>3.0.CO;2-Z
65. Day CS, Kasemkijwattana C, Menetrey J, Floyd SS Jr, Booth D, Moreland MS, et al. Myoblast-Mediated gene transfer to the joint. J Orthop Res. (1997) 15:894–903. doi: 10.1002/jor.1100150616
66. Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci USA. (1993) 90:10764–8. doi: 10.1073/pnas.90.22.10764
67. Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. (2007) 89:672–85. doi: 10.1302/0301-620X.89B5.18343
68. Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. (2005) 52:164–70. doi: 10.1002/art.20739
69. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. (2002) 84:276–88. doi: 10.1302/0301-620x.84b2.11167
70. Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. (2003) 21:573–83. doi: 10.1016/S0736-0266(02)00264-4
71. Ortved KF, Begum L, Mohammed HO, Nixon AJ. Implantation of Raav5-Igf-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol Ther. (2015) 23:363–73. doi: 10.1038/mt.2014.198
72. Ozeki N, Muneta T, Koga H, Nakagawa Y, Mizuno M, Tsuji K, et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. (2016) 24:1061–70. doi: 10.1016/j.joca.2015.12.018
73. Horie M, Choi H, Lee RH, Reger RL, Ylostalo J, Muneta T, et al. Intra-Articular injection of human mesenchymal stem cells (Mscs) promote rat meniscal regeneration by being activated to express indian hedgehog that enhances expression of type II collagen. Osteoarthritis Cartilage. (2012) 20:1197–207. doi: 10.1016/j.joca.2012.06.002
74. Nita I, Ghivizzani S, Galea-Lauri J, Bandara G, Georgescu H, Robbins P, et al. Direct gene delivery to synovium: an evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. (1996) 39:820–8. doi: 10.1002/art.1780390515
75. Roessler BJ, Allen ED, Wilson JM, Hartman JW, Davidson BL. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest. (1993) 92:1085–92. doi: 10.1172/JCI116614
76. Ghivizzani SC, Lechman ER, Kang R, Tio C, Kolls J, Evans CH, et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor α soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci USA. (1998) 95:4613–8. doi: 10.1073/pnas.95.8.4613
77. Gouze E, Pawliuk R, Pilapil C, Gouze J-N, Fleet C, Palmer GD, et al. In vivo gene delivery to synovium by lentiviral vectors. Mol Ther. (2002) 5:397–404. doi: 10.1006/mthe.2002.0562
78. Adriaansen J, Tas SW, Klarenbeek PL, Bakker AC, Apparailly F, Firestein GS, et al. Enhanced gene transfer to arthritic joints using adeno-associated virus type 5: implications for intra-articular gene therapy. Ann Rheum Dis. (2005) 64:1677–84. doi: 10.1136/ard.2004.035063
79. Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. (2001) 12:117–29. doi: 10.1089/104303401750061186
80. Goodrich LR, Brower-Toland BD, Warnick L, Robbins PD, Evans CH, Nixon AJ. Direct adenovirus-mediated Igf-I gene transduction of synovium induces persisting synovial fluid Igf-I ligand elevations. Gene Ther. (2006) 13:1253–62. doi: 10.1038/sj.gt.3302757
81. Goodrich LR, Grieger JC, Phillips JN, Khan N, Gray SJ, McIlwraith CW, et al. Scaavil-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther. (2015) 22:536–45. doi: 10.1038/gt.2015.21
82. Goodrich LR, Phillips JN, McIlwraith CW, Foti SB, Grieger JC, Gray SJ, et al. Optimization of scaavil-1ra in vitro and in vivo to deliver high levels of therapeutic protein for treatment of osteoarthritis. Mol Ther Nucleic Acids. (2013) 2:e70. doi: 10.1038/mtna.2012.61
83. Hemphill D, McIlwraith C, Slayden R, Samulski R, Goodrich L. Adeno-Associated virus gene therapy vector scaavigf-I for transduction of equine articular chondrocytes and Rna-Seq analysis. Osteoarthritis Cartilage. (2016) 24:902–11. doi: 10.1016/j.joca.2015.12.001
84. Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. Lmo2-Associated clonal t cell proliferation in two patients after gene therapy for scid-X1. Science. (2003) 302:415–9. doi: 10.1126/science.1088547
85. Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. (2003) 80:148–58. doi: 10.1016/j.ymgme.2003.08.016
86. Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (Scid)-X1 disease. Science. (2000) 288:669–72. doi: 10.1126/science.288.5466.669
87. Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for x-linked severe combined immunodeficiency. N Engl J Med. (2003) 348:255–6. doi: 10.1056/NEJM200301163480314
88. Gabner S, Ertl R, Velde K, Renner M, Jenner F, Egerbacher M, et al. Cytokine-Induced interleukin-1 receptor antagonist protein expression in genetically engineered equine mesenchymal stem cells for osteoarthritis treatment. J Gene Med. (2018) 20:e3021. doi: 10.1002/jgm.3021
89. Gouze E, Pawliuk R, Gouze J-N, Pilapil C, Fleet C, Palmer GD, et al. Lentiviral-Mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. (2003) 7:460–6. doi: 10.1016/S1525-0016(03)00024-8
90. Cameron AD, Even KM, Linardi RL, Berglund AK, Schnabel LV, Engiles JB, et al. Adeno-Associated virus-mediated overexpression of interleukin-10 affects the immunomodulatory properties of equine bone marrow-derived mesenchymal stem cells. Hum Gene Ther. (2021) 32:907–18. doi: 10.1089/hum.2020.319
91. Watson Levings RS, Smith AD, Broome TA, Rice BL, Gibbs EP, Myara DA, et al. Self-Complementary adeno-associated virus-mediated interleukin-1 receptor antagonist gene delivery for the treatment of osteoarthritis: test of efficacy in an equine model. Hum Gene Ther Clin Dev. (2018) 29:101–12. doi: 10.1089/humc.2017.143
92. Frisbie DD, McIlwraith CW. Gene therapy: future therapies in osteoarthritis. Vet Clin N Am Equine Prac. (2001) 17:233–43. doi: 10.1016/S0749-0739(17)30059-7
93. Oligino T, Ghivizzani S, Wolfe D, Lechman E, Krisky D, Mi Z, et al. Intra-Articular delivery of a herpes simplex virus Il-1ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. (1999) 6:1713–20. doi: 10.1038/sj.gt.3301014
94. Glorioso JC, Krisky D, Marconi P, Oligino T, Ghivizzani SC, Robbins PD, et al. Progress in development of herpes simplex virus gene vectors for treatment of rheumatoid arthritis. Adv Drug Deliv Rev. (1997) 27:41–57. doi: 10.1016/S0169-409X(97)00021-5
95. Petersen GF, Hilbert B, Trope G, Kalle W, Strappe P. Efficient transduction of equine adipose-derived mesenchymal stem cells by Vsv-G pseudotyped lentiviral vectors. Res Vet Sci. (2014) 97:616–22. doi: 10.1016/j.rvsc.2014.09.004
96. Chen S, Shiau A, Li Y, Lin Y, Lee C, Wu C, et al. Suppression of collagen-induced arthritis by intra-articular lentiviral vector-mediated delivery of toll-like receptor 7 short hairpin Rna gene. Gene Ther. (2012) 19:752–60. doi: 10.1038/gt.2011.173
97. Liu S, Kiyoi T, Takemasa E, Maeyama K. Intra-Articular lentivirus-mediated gene therapy targeting cracm1 for the treatment of collagen-induced arthritis. J Pharmacol Sci. (2017) 133:130–8. doi: 10.1016/j.jphs.2017.02.001
98. Schaack J. Adenovirus vectors deleted for genes essential for viral DNA replication. Front Biosci. (2005) 10:1146–55. doi: 10.2741/1607
99. Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. (1998) 72:926–33. doi: 10.1128/JVI.72.2.926-933.1998
100. Räty JK, Lesch HP, Wirth T, Ylä-Herttuala S. Improving safety of gene therapy. Curr Drug Saf. (2008) 3:46–53. doi: 10.2174/157488608783333925
101. Wang H, Georgakopoulou A, Li C, Liu Z, Gil S, Bashyam A, et al. Curative in vivo hematopoietic stem cell gene therapy of murine thalassemia using large regulatory elements. JCI Insight. (2020) 5:e139538. doi: 10.1172/jci.insight.139538
102. Khoja S, Nitzahn M, Hermann K, Truong B, Borzone R, Willis B, et al. Conditional disruption of hepatic carbamoyl phosphate synthetase 1 in mice results in hyperammonemia without orotic aciduria and can be corrected by liver-directed gene therapy. Mol Genet Metab. (2018) 124:243–53. doi: 10.1016/j.ymgme.2018.04.001
103. Brunetti-Pierri N, Ng P. 17—helper-dependent adenoviral vectors. In: Curiel DT, editor. Adenoviral Vectors for Gene Therapy. 2nd edition. San Diego, CA: Academic Press (2016). p. 423–50. doi: 10.1016/B978-0-12-800276-6.00017-6
104. Brunetti-Pierri N, Ng P. Helper-Dependent adenoviral vectors for liver-directed gene therapy. Hum Mol Genet. (2011) 20:R7–13. doi: 10.1093/hmg/ddr143
105. Rosewell A, Vetrini F, Ng P. Helper-Dependent adenoviral vectors. J Genet Syndr Gene Ther. (2011) Suppl 5:001. doi: 10.4172/2157-7412.S5-001
106. Brunetti-Pierri N, Ng P. Gene therapy with helper-dependent adenoviral vectors: lessons from studies in large animal models. Virus Genes. (2017) 53:684–91. doi: 10.1007/s11262-017-1471-x
107. Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, et al. Sustained phenotypic correction of canine hemophilia b after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. (2005) 16:811–20. doi: 10.1089/hum.2005.16.811
108. Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-Dependent adenoviral vectors mediate therapeutic factor viii expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. (2004) 103:804–10. doi: 10.1182/blood-2003-05-1426
109. Mc CW, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P, et al. Helper-Dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia a model. J Thromb Haemost. (2006) 4:1218–25. doi: 10.1111/j.1538-7836.2006.01901.x
110. Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. (1999) 96:12816–21. doi: 10.1073/pnas.96.22.12816
111. Brunetti-Pierri N, Ng T, Iannitti DA, Palmer DJ, Beaudet AL, Finegold MJ, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. (2006) 17:391–404. doi: 10.1089/hum.2006.17.391
112. Unzu C, Melero I, Hervás-Stubbs S, Sampedro A, Mancheño U, Morales-Kastresana A, et al. Helper-Dependent adenovirus achieve more efficient and persistent liver transgene expression in non-human primates under immunosuppression. Gene Therapy. (2015) 22:856–65. doi: 10.1038/gt.2015.64
113. Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther. (2013) 24:761–5. doi: 10.1089/hum.2013.071
114. Nixon AJ, Grol MW, Lang HM, Ruan MZC, Stone A, Begum L, et al. Disease-Modifying osteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol. (2018) 70:1757–68. doi: 10.1002/art.40668
115. Ruan MZ, Cerullo V, Cela R, Clarke C, Lundgren-Akerlund E, Barry MA, et al. Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol Ther Methods Clin Dev. (2016) 3:16008. doi: 10.1038/mtm.2016.8
116. Stone A, Grol MW, Ruan MZC, Dawson B, Chen Y, Jiang MM, et al. Combinatorial Prg4 and Il-1ra gene therapy protects against hyperalgesia and cartilage degeneration in post-traumatic osteoarthritis. Hum Gene Ther. (2019) 30:225–35. doi: 10.1089/hum.2018.106
117. Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. (2004) 15:35–46. doi: 10.1089/10430340460732445
118. Samulski RJ, Muzyczka N. Aav-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. (2014) 1:427–51. doi: 10.1146/annurev-virology-031413-085355
119. Meier AF, Fraefel C, Seyffert M. The interplay between adeno-associated virus and its helper viruses. Viruses. (2020) 12:662. doi: 10.3390/v12060662
120. Weitzman MD, Fisher KJ, Wilson JM. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J Virol. (1996) 70:1845–54. doi: 10.1128/jvi.70.3.1845-1854.1996
121. Martini S, Rocco P, Morales M. Adeno-Associated virus for cystic fibrosis gene therapy. Braz J Med Biol Res. (2011) 44:1097–104. doi: 10.1590/S0100-879X2011007500123
122. Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intraarticular factor Ix protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor Ix. Blood. (2008) 112:4532–41. doi: 10.1182/blood-2008-01-131417
123. Goodrich LR, Choi VW, Carbone BA, McIlwraith CW, Samulski RJ. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum Gene Ther. (2009) 20:1697–702. doi: 10.1089/hum.2009.030
124. Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C, et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. (2011) 32:6324–32. doi: 10.1016/j.biomaterials.2011.05.017
125. Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther. (2018) 29:2–14. doi: 10.1089/hum.2017.181
126. Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. (2003) 14:393–402. doi: 10.1089/104303403321208998
127. Hemphill DD, McIlwraith CW, Samulski RJ, Goodrich LR. Adeno-Associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured monolayers. Sci Rep. (2014) 4:5861. doi: 10.1038/srep05861
128. Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. (1993) 90:10613–7. doi: 10.1073/pnas.90.22.10613
129. Guggino WB, Cebotaru L. Adeno-Associated virus (Aav) gene therapy for cystic fibrosis: current barriers and recent developments. Expert Opin Biol Ther. (2017) 17:1265–73. doi: 10.1080/14712598.2017.1347630
130. Berns KI, Linden RM. The cryptic life style of adenoassociated virus. Bioessays. (1995) 17:237–45. doi: 10.1002/bies.950170310
131. Salganik M, Hirsch ML, Samulski RJ. Adeno-associated virus as a mammalian DNA vector. Microbiol Spectr. (2015) 3:10.1128/microbiolspec.MDNA3-0052-2014. doi: 10.1128/microbiolspec.MDNA3-0052-2014
132. Grieger JC, Samulski RJ. Adeno-Associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. (2012) 507:229–54. doi: 10.1016/B978-0-12-386509-0.00012-0
133. McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-Associated virus terminal repeat (Tr) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. (2003) 10:2112–8. doi: 10.1038/sj.gt.3302134
134. McCarty DM, Monahan PE, Samulski RJ. Self-Complementary recombinant adeno-associated virus (scaav) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. (2001) 8:1248–54. doi: 10.1038/sj.gt.3301514
135. McCarty DM. Self-Complementary aav vectors; advances and applications. Mol Ther. (2008) 16:1648–56. doi: 10.1038/mt.2008.171
136. Kay JD, Gouze E, Oligino TJ, Gouze JN, Watson RS, Levings PP, et al. Intra-Articular gene delivery and expression of interleukin-1ra mediated by self-complementary adeno-associated virus. J Gene Med. (2009) 11:605–14. doi: 10.1002/jgm.1334
137. Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker S, Smith A, et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. (2008) 15:463–7. doi: 10.1038/sj.gt.3303074
138. Zhao L, Huang J, Fan Y, Li J, You T, He S, et al. Exploration of Crispr/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis. (2019) 78:676–82. doi: 10.1136/annrheumdis-2018-214724
139. Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. (2015) 9:Ge01–6. doi: 10.7860/JCDR/2015/10443.5394
140. Uzieliene I, Kalvaityte U, Bernotiene E, Mobasheri A. Non-Viral gene therapy for osteoarthritis. Front Bioeng Biotechnol. (2021) 8:618399. doi: 10.3389/fbioe.2020.618399
141. Clanchy FIL, Williams RO. Plasmid DNA as a safe gene delivery vehicle for treatment of chronic inflammatory disease. Exp Opin Biol Ther. (2008) 8:1507–19. doi: 10.1517/14712598.8.10.1507
142. Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. (2017) 8:65. doi: 10.3390/genes8020065
143. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-Viral vectors for gene-based therapy. Nat Rev Genet. (2014) 15:541–55. doi: 10.1038/nrg3763
144. Watkins LR, Chavez RA, Landry R, Fry M, Green-Fulgham SM, Coulson JD, et al. Targeted interleukin-10 plasmid DNA therapy in the treatment of osteoarthritis: toxicology and pain efficacy assessments. Brain Behav Immun. (2020) 90:155–66. doi: 10.1016/j.bbi.2020.08.005
145. Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. (2018) 20:e3015. doi: 10.1002/jgm.3015
146. van der Loo JCM, Wright JF. Progress and challenges in viral vector manufacturing. Hum Mol Genet. (2015) 25:R42–52. doi: 10.1093/hmg/ddv451
147. Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. (2015) 11:234–42. doi: 10.1038/nrrheum.2015.28
148. Merten O-W, Schweizer M, Chahal P, Kamen AA. Manufacturing of viral vectors for gene therapy: part I. Upstream processing. Pharm Bioprocess. (2014) 2:183–203. doi: 10.4155/pbp.14.16
149. Merten OW. State-of-the-Art of the production of retroviral vectors. J Gene Med. (2004) 6:S105–S24. doi: 10.1002/jgm.499
150. Emmerling VV, Pegel A, Milian EG, Venereo-Sanchez A, Kunz M, Wegele J, et al. Rational plasmid design and bioprocess optimization to enhance recombinant adeno-associated virus (aav) productivity in mammalian Cells. Biotechnol J. (2016) 11:290–7. doi: 10.1002/biot.201500176
151. Kutner RH, Puthli S, Marino MP, Reiser J. Simplified production and concentration of Hiv-1-based lentiviral vectors using hyperflask vessels and anion exchange membrane chromatography. BMC Biotechnol. (2009) 9:61. doi: 10.1186/1472-6750-9-10
152. Lesch HP, Heikkilä KM, Lipponen EM, Valonen P, Müller A, Räsänen E, et al. Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum Gene Ther. (2015) 26:560–71. doi: 10.1089/hum.2015.081
153. Merten OW, Cruz P, Rochette C, Geny-Fiamma C, Bouquet C, Goncalves D, et al. Comparison of different bioreactor systems for the production of high titer retroviral vectors. Biotechnol Prog. (2001) 17:326–35. doi: 10.1021/bp000162z
154. Wang X, Olszewska M, Qu J, Wasielewska T, Bartido S, Hermetet G, et al. Large-Scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J Immunother. (2015) 38:127. doi: 10.1097/CJI.0000000000000072
155. Galibert L, Merten O-W. Latest developments in the large-scale production of adeno-associated virus vectors in insect cells toward the treatment of neuromuscular diseases. J Invertebr Pathol. (2011) 107:S80–93. doi: 10.1016/j.jip.2011.05.008
156. Lee J, Welsh M. Enhancement of calcium phosphate-mediated transfection by inclusion of adenovirus in coprecipitates. Gene Ther. (1999) 6:676–82. doi: 10.1038/sj.gt.3300857
157. Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. (1993) 90:8392–6. doi: 10.1073/pnas.90.18.8392
158. Segura MM, Mangion M, Gaillet B, Garnier A. New developments in lentiviral vector design, production and purification. Exp Opin Biol Ther. (2013) 13:987–1011. doi: 10.1517/14712598.2013.779249
159. Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, et al. The atomic structure of adeno-associated virus (Aav-2), a vector for human gene therapy. Proc Natl Acad Sci USA. (2002) 99:10405–10. doi: 10.1073/pnas.162250899
160. Elverum K, Whitman M. Delivering cellular and gene therapies to patients: solutions for realizing the potential of the next generation of medicine. Gene Ther. (2020) 27:537–44. doi: 10.1038/s41434-019-0074-7
161. Gee AP. Gmp car-T cell production. Best Pract Res Clin Haematol. (2018) 31:126–34. doi: 10.1016/j.beha.2018.01.002
162. Iancu EM, Kandalaft LE. Challenges and advantages of cell therapy manufacturing under good manufacturing practices within the hospital setting. Curr Opin Biotechnol. (2020) 65:233–41. doi: 10.1016/j.copbio.2020.05.005
163. Moutsatsou P, Ochs J, Schmitt RH, Hewitt CJ, Hanga MP. Automation in cell and gene therapy manufacturing: from past to future. Biotechnol Lett. (2019) 41:1245–53. doi: 10.1007/s10529-019-02732-z
164. Heathman TR, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. (2015) 10:49–64. doi: 10.2217/rme.14.73
165. Liu Y, Hourd P, Chandra A, Williams DJ. Human cell culture process capability: a comparison of manual and automated production. J Tissue Eng Regen Med. (2010) 4:45–54. doi: 10.1002/term.217
166. Thomas RJ, Chandra A, Liu Y, Hourd PC, Conway PP, Williams DJ. Manufacture of a human mesenchymal stem cell population using an automated cell culture platform. Cytotechnology. (2007) 55:31–9. doi: 10.1007/s10616-007-9091-2
167. Williams DJ, Thomas RJ, Hourd PC, Chandra A, Ratcliffe E, Liu Y, et al. Precision manufacturing for clinical-quality regenerative medicines. Philos Trans R Soc A Math Phys Eng Sci. (2012) 370:3924–49. doi: 10.1098/rsta.2011.0049
168. Mason C, Dunnill P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regener Med. (2009) 4:835–53. doi: 10.2217/rme.09.64
169. Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage. (2008) 16:1196–204. doi: 10.1016/j.joca.2008.03.008
170. Izal I, Acosta CA, Ripalda P, Zaratiegui M, Ruiz J, Forriol F. Igf-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. (2008) 128:239–47. doi: 10.1007/s00402-007-0407-7
171. Ishihara A, Bartlett JS, Bertone AL. Inflammation and immune response of intra-articular serotype 2 adeno-associated virus or adenovirus vectors in a large animal model. Arthritis. (2012) 2012:735472. doi: 10.1155/2012/735472
172. Watson RS, Broome TA, Levings PP, Rice BL, Kay JD, Smith AD, et al. Scaav-Mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene Ther. (2013) 20:670–7. doi: 10.1038/gt.2012.81
173. Bruening W, Giasson B, Mushynski W, Durham HD. Activation of stress-activated map protein kinases up-regulates expression of transgenes driven by the cytomegalovirus immediate/early promoter. Nucleic Acids Res. (1998) 26:486–9. doi: 10.1093/nar/26.2.486
174. Svensson RU, Barnes JM, Rokhlin OW, Cohen MB, Henry MD. Chemotherapeutic agents up-regulate the cytomegalovirus promoter: implications for bioluminescence imaging of tumor response to therapy. Cancer Res. (2007) 67:10445–54. doi: 10.1158/0008-5472.CAN-07-1955
175. Ramanathan M, Haskó G, Leibovich SJ. Analysis of signal transduction pathways in macrophages using expression vectors with cmv promoters: a cautionary tale. Inflammation. (2005) 29:94–102. doi: 10.1007/s10753-006-9005-z
176. Jacques C, Gosset M, Berenbaum F, Gabay C. The role of Il-1 and Il-1ra in joint inflammation and cartilage degradation. Vitam Horm. (2006) 74:371–403. doi: 10.1016/S0083-6729(06)74016-X
177. Joosten LA, Helsen MM, Saxne T, van de Loo FA, Heinegård D, van den Berg WB. Il-1αβ blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas Tnf-α blockade only ameliorates joint inflammation. J Immunol. (1999) 163:5049–55.
178. Hung G, Galea-Lauri J, Mueller G, Georgescu H, Larkin L, Suchanek M, et al. Suppression of Intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. (1994) 1:64–9.
179. Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. (2000) 43:289–97. 10.1002/1529-0131(200002)43:2< 289::AID-ANR8>3.0.CO;2-H
180. Geng Y, Blanco FJ, Cornelisson M, Lotz M. Regulation of cyclooxygenase-2 expression in normal human articular chondrocytes. J Immunol. (1995) 155:796–801.
181. Stadler J, Stefanovic-Racic M, Billiar T, Curran R, McIntyre L, Georgescu H, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. (1991) 147:3915–20.
182. Howard RD, McIlwraith CW, Trotter GW, Nyborg JK. Cloning of equine interleukin 1 receptor antagonist and determination of its full-length Cdna sequence. Am J Vet Res. (1998) 59:712–6.
183. Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. (2009) 61:344–52. doi: 10.1002/art.24096
184. Cohen SB, Woolley JM, Chan W, Group AS. Interleukin 1 receptor antagonist anakinra improves functional status in patients with rheumatoid arthritis. J Rheumatol. (2003) 30:225–31.
185. Kary S, Burmester G. Anakinra: the first interleukin-1 inhibitor in the treatment of rheumatoid arthritis. Int J Clin Pract. (2003) 57:231–4.
186. Fleischmann R. Addressing the safety of anakinra in patients with rheumatoid arthritis. Rheumatology. (2003) 42:ii29–35. doi: 10.1093/rheumatology/keg330
187. Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-Mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. (2005) 12:177–86. doi: 10.1038/sj.gt.3302396
188. Fortier LA, Nixon AJ, Lust G. Phenotypic expression of equine articular chondrocytes grown in three-dimensional cultures supplemented with supraphysiologic concentrations of insulin-like growth factor-I. J Am Vet Med Assoc. (2002) 63:301–5. doi: 10.2460/ajvr.2002.63.301
189. Tyler JA. Insulin-Like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. (1989) 260:543–8. doi: 10.1042/bj2600543
190. Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH, et al. Adenovirus-Mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum. (2000) 43:2563–70. 10.1002/1529-0131(200011)43:11< 2563::AID-ANR25>3.0.CO;2-8
191. Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. (2003) 28:755–63. doi: 10.1097/01.BRS.0000058946.64222.92
192. Ren S, Liu Y, Ma J, Liu Y, Diao Z, Yang D, et al. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated Sox9 and osteogenic protein-1 double genes in vivo. Int J Mol Med. (2013) 32:1063–8. doi: 10.3892/ijmm.2013.1497
193. Daniels O, Frisch J, Venkatesan JK, Rey-Rico A, Schmitt G, Cucchiarini M. Effects of raav-mediated Sox9 overexpression on the biological activities of human osteoarthritic articular chondrocytes in their intrinsic three-dimensional environment. J Clin Med. (2019) 8:1637. doi: 10.3390/jcm8101637
194. Tao K, Rey-Rico A, Frisch J, Venkatesan JK, Schmitt G, Madry H, et al. Raav-Mediated combined gene transfer and overexpression of Tgf-β and sox9 remodels human osteoarthritic articular cartilage. J Orthop Res. (2016) 34:2181–90. doi: 10.1002/jor.23228
195. Neumann E, Judex M, Kullmann F, Grifka J, Robbins PD, Pap T, et al. Inhibition of cartilage destruction by double gene transfer of Il-1ra and Il-10 involves the activin pathway. Gene Therapy. (2002) 9:1508–19. doi: 10.1038/sj.gt.3301811
196. Fellowes R, Etheridge CJ, Coade S, Cooper RG, Stewart L, Miller AD, et al. Amelioration of Established collagen induced arthritis by systemic Il-10 gene delivery. Gene Ther. (2000) 7:967–77. doi: 10.1038/sj.gt.3301165
197. Vermeij EA, Broeren MG, Bennink MB, Arntz OJ, Gjertsson I, van Lent PL, et al. Disease-Regulated local Il-10 gene therapy diminishes synovitis and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis. (2015) 74:2084–91. doi: 10.1136/annrheumdis-2014-205223
198. Ma Y, Thornton S, Duwel LE, Boivin GP, Giannini EH, Leiden JM, et al. Inhibition of collagen-induced arthritis in mice by viral Il-10 gene transfer. J Immunol. (1998) 161:1516–24.
199. Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral Il-10 gene inhibits murine collagen-induced arthritis. J Immunol. (1998) 160:5213–20.
200. Whalen JD, Lechman EL, Carlos CA, Weiss K, Kovesdi I, Glorioso JC, et al. Adenoviral transfer of the viral Il-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol. (1999) 162:3625–32.
201. Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. (2005) 14:166–74. doi: 10.1159/000087655
202. Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. (2015) 96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020
203. Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immunity. (2007) 21:686–98. doi: 10.1016/j.bbi.2006.10.012
204. Ortved KF, Begum L, Stefanovski D, Nixon AJ. Aav-mediated overexpression of Il-10 mitigates the inflammatory cascade in stimulated equine chondrocyte pellets. Current Gene Therapy. (2018) 18:171–9. doi: 10.2174/1566523218666180510165123
205. Moss KL, Jiang Z, Dodson ME, Linardi RL, Haughan J, Gale AL, et al. Sustained Interleukin-10 transgene expression following intra-articular Aav5-Il-10 administration to horses. Hum Gene Ther. (2020) 31:110–8. doi: 10.1089/hum.2019.195
206. Reesink HL, Watts AE, Mohammed HO, Jay GD, Nixon AJ. Lubricin/Proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthritis Cartilage. (2017) 25:128–37. doi: 10.1016/j.joca.2016.07.021
207. Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA. (2007) 104:6194–9. doi: 10.1073/pnas.0608558104
208. Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermúdez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. (2009) 60:840–7. doi: 10.1002/art.24304
209. Chavez RD, Sohn P, Serra R. Prg4 prevents osteoarthritis induced by dominant-negative interference of Tgf-ß signaling in mice. PLoS ONE. (2019) 14:e0210601. doi: 10.1371/journal.pone.0210601
210. Ruan MZC, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. (2013) 5:176ra34. doi: 10.1126/scitranslmed.3005409
211. Sellers RS, Zhang R, Glasson SS, Kim HD, Peluso D, D'Augusta DA, et al. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (Rhbmp-2). J Bone Joint Surg Am. (2000) 82:151–60. doi: 10.2106/00004623-200002000-00001
212. Louwerse RT, Heyligers IC, Klein-Nulend J, Sugihara S, van Kampen GP, Semeins CM, et al. Use of recombinant human osteogenic protein-1 for the repair of subchondral defects in articular cartilage in goats. J Biomed Mater Res. (2000) 49:506–16. 10.1002/(SICI)1097-4636(20000315)49:4< 506::AID-JBM9>3.0.CO;2-A
Keywords: osteoarthiritis, gene therapy, cartilage, adeno-associated viral vectors, translational
Citation: Thampi P, Samulski RJ, Grieger JC, Phillips JN, McIlwraith CW and Goodrich LR (2022) Gene therapy approaches for equine osteoarthritis. Front. Vet. Sci. 9:962898. doi: 10.3389/fvets.2022.962898
Received: 06 June 2022; Accepted: 08 August 2022;
Published: 28 September 2022.
Edited by:
Cristina Esteves, University of Edinburgh, United KingdomReviewed by:
Heidi Reesink, Cornell University, United StatesKyla Ortved, University of Pennsylvania, United States
Katja Duesterdieck-Zellmer, Oregon State University, United States
Copyright © 2022 Thampi, Samulski, Grieger, Phillips, McIlwraith and Goodrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurie R. Goodrich, bGF1cmllLmdvb2RyaWNoQGNvbG9zdGF0ZS5lZHU=
 Parvathy Thampi
Parvathy Thampi R. Jude Samulski
R. Jude Samulski Joshua C. Grieger
Joshua C. Grieger Jennifer N. Phillips
Jennifer N. Phillips C. Wayne McIlwraith
C. Wayne McIlwraith Laurie R. Goodrich
Laurie R. Goodrich