- 1College of Animal Science and Technology, Jilin Agricultural University, Changchun, China
- 2College of Chinese Medicinal Materials, Jilin Agricultural University, Changchun, China
- 3Jilin Province Sika Deer Efficient Breeding and Product Development Technology Engineering Research Center, Jilin Agricultural University, Changchun, China
- 4The Ministry of Education Key Laboratory of Animal Production and the Product Quality and Safety, Jilin Agricultural University, Changchun, China
Previously, we researched that bovine viral diarrhea virus (BVDV) induced a very significant increase in fibroblast growth factor 21 (FGF21) expression in mouse liver and that FGF21 was increased in the peripheral blood of BVD cattle and BVD mice. To determine the role of FGF21 in relieving clinical symptoms and inhibiting the intestinal damage caused by BVDV in BVD development in mice, BALB/c mice were intraperitoneally injected with cytopathic biotype (cp) BVDV-LS01 (isolated and identified by our group) to establish a BVD mouse model. The role of FGF21 in the BVD mouse model was investigated by injecting the mice with FGF21. The animals were divided into control, BVDV challenge, BVDV + FGF21, BVDV + FGF21Ab (anti-FGF21 antibody), and BVDV + IgG (immunoglobulin G) groups. The stool consistency, the degree of bloody diarrhea, histopathological changes, inflammatory cell infiltration, weight loss percentage, and detection of BVDV in the feces of the mice were examined, and the pathological changes and inflammatory cytokine expression were analyzed. The results showed that after BVDV challenge, the average BVD mouse model score of the BVDV mice was 11.6 points. In addition to mild diarrhea and tissue damage, BVDV was detected in the stools of 13 BVDV mice. Only two mice in the control group had scores (both, 1 point each). The comprehensive scoring results demonstrated the successful establishment of the BVD mouse model. FGF21 alleviated the clinical symptoms in the BVD mice and significantly improved weight loss. Furthermore, FGF21 inhibited the BVDV-induced leukocyte, platelet, and lymphocyte reduction while inhibiting the expression of BVDV-induced inflammatory factors. In the BVD mice, FGF21 promoted duodenal epithelial cell proliferation, thereby significantly improving the damage to the cells. In conclusion, FGF21 exerted a good therapeutic effect on the BVD mouse model.
1. Introduction
Bovine viral diarrhea (BVD) is an acute, highly contagious disease caused by BVD virus (BVDV). BVD is widely prevalent worldwide and causes huge economic losses to the global livestock industry. The main clinical symptoms of BVD are subclinical infection, reproductive failure, respiratory and intestinal diseases due to immunosuppression, thrombocytopenia, and bleeding, and fatal mucosal disease, which is one of the most severe clinical forms of BVDV infection, with mortality rates as high as 100% (1). In addition to mainly infecting cattle, pigs, sheep, goats, and deer, camels and other wild animals are also susceptible hosts for BVDV, which causes serious harm to them (2, 3). Along with border disease virus and classical swine fever virus, BVDV is a single-stranded RNA virus that belongs to the family Flaviviridae, genus Pestivirus. BVDV genotypes are classified into BVDV1, BVDV2, and HoBi-like viruses based on differences in their 5′ untranslated region, Npro, or E2 genes (HoBi-like viruses are listed as BVDV3, but the International Committee on Taxonomy of Viruses has not confirmed the BVDV3 viral typing) (4). Each genotype is further divided into multiple sub-genotypes. At least 21 BVDV1 sub-genotypes (1a−1u), four BVDV2 sub-genotypes (2a−2d), and four HoBi-like sub-genotypes have been identified (3, 5, 6). The main prevalent BVDV types in China are BVDV2 and BVDV1, of which the main subtypes are BVDV1a and BVDV1b (7). BVDV is divided into the cytopathic biotype (cp) and the non-cp (ncp) based on whether it can cause cellular pathological changes (8–10).
Fibroblast growth factor 21 (FGF21) is a secreted protein with multiple biological functions. Nobuyuki Itoh's team successfully cloned the FGF21 gene in 2000 (11). In 2005, Kharitonenkov et al., scientists at the Lilly laboratory in the United States, reported the role of FGF21 in diabetes treatment and described the biological significance of FGF21 protein as a new metabolic regulator (12). FGF21 contains 210 amino acids, is highly conserved in mammals, and has a molecular weight of −22.3 kDa. Human FGF21 contains 209 amino acids and its coding sequence is very similar to that of mice (75% homology) (13). FGF21 is mainly expressed in liver tissue; it does not bind to heparan sulfate protein glycans or has a very low affinity for them, which enables its circulatory transport and endocrine action. Research on FGF21 in animal and human metabolic diseases such as obesity and diabetes are relatively mature (14) and FGF21 is closely related to diseases or pathological processes such as metabolic syndrome and non-alcoholic fatty liver (15, 16). Recent studies reported that FGF21 expression is significantly increased in diverse inflammatory diseases (17–19). These findings suggested that, as a biomarker, FGF21 is becoming a research hotspot for targeted drugs for various metabolic diseases. Many current studies reported that FGF21 is significantly elevated in the peripheral blood of patients with hepatitis B, hepatitis C, and HIV (20). FGF21 is closely correlated with disease severity, suggesting that it has an antiviral effect, but its mechanism has not been thoroughly explored.
Previously, we researched that BVDV induced a very significant increase in FGF21 expression in mouse liver and that it played an endocrine role in blood circulation. Furthermore, FGF21 expression was increased in the peripheral blood of BVD cattle and BVD mice. Currently, few studies have reported on FGF21 in viral diseases. Therefore, to determine the role of FGF21 in relieving the clinical symptoms and inhibiting the intestinal damage caused by BVDV in BVD development in mice, we established a BVD mouse model by infecting BALB/c mice with the BVDV-LS01 strain we previously isolated and identified, and injected the BVD mice with recombinant FGF21 protein. The mechanism of FGF21 in the BVD mice was identified based on changes in the clinical symptoms, body weight, histopathology, complete blood count (CBC), and cellular inflammatory factors. This study provided evidence for new research directions regarding the role of FGF21 in viral diseases and its application in BVD adjuvant therapy and prevention and control.
2. Materials and methods
2.1. Experimental design and BVD mouse model establishment
This study was conducted after receiving Jilin Agriculture University Institutional Animal Care and Use Committee approval (JLAU08201409). The experimental procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8,023).
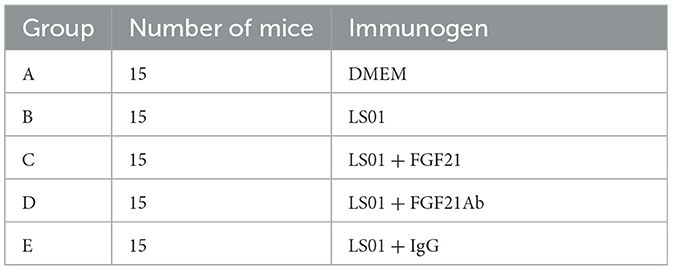
The model objects were 6–8-week-old male BALB/c mice [animal license number: SCXK (Jing) 2019–0008, purchased from Beijing Huafukang Biotechnology Co., Ltd., Beijing, China] (21). In total, 75 mice were used (15 mice per group). The mice were randomly divided into five groups. Group A mice were injected with Dulbecco's modified Eagle's medium (DMEM) as the negative control group. Group B–E mice were injected intraperitoneally with 0.3 mL tissue culture fluid [median tissue culture infective dose (TCID50) = 106.6/0.1 mL containing BVDV-LS01]. Group B (BVDV challenge, BVDV) comprised BVD mice. Group C (BVDV + FGF21) mice were injected intraperitoneally with mouse recombinant FGF21 protein [injection dose, 4 mg/kg/day (22)] donated by the Jilin Institute of Agricultural Science and Technology on day 6 of BVDV challenge. From day 4 of the experiment onwards, group D (BVDV + FGF21Ab) mice were intraperitoneally injected with 5 mg/kg anti-FGF21 antibody once every other day, while group E (BVDV + IgG) mice were intraperitoneally injected with 5 mg/kg immunoglobulin G (IgG) antibody every other day (23).
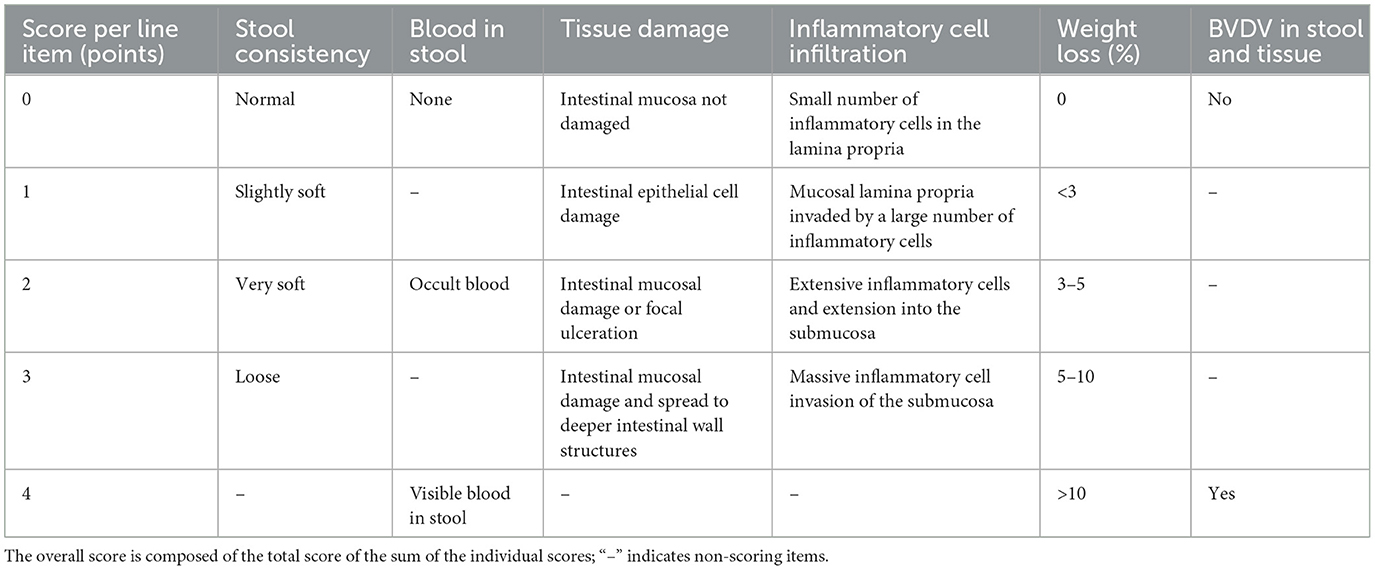
The clinical status of the mice was observed every day after the challenge. The consistency of the mouse feces, presence of bloody stool, tissue damage, inflammatory cell infiltration, and fecal virus detection were recorded. The observations were scored according to the disease scoring standard, where a comprehensive score ≥ 8 points (including BVDV detection) indicated that the mouse qualified as a BVD mouse model. Tables 1, 2 depict the groupings and disease scoring criteria, respectively. The mice were culled when they exhibited obvious clinical symptoms, and the role of FGF21 in the mice was subsequently analyzed from the clinical symptom, tissue change, and inflammatory factor aspects.
2.2. Clinical symptoms and body weight changes in BVD mouse model
The diet, metabolism, and mental state of the mice were observed daily after BVDV challenge, and the mouse body weight changes were recorded. Whether FGF21 played a role in BVD development was evaluated based on the clinical symptoms and body weight changes.
2.3. BVDV detection in BVD mouse tissues and organs
The mice were observed daily for clinical symptoms and were killed when they exhibited listlessness, rough coat, decreased diet, shapeless feces, or obvious diarrhea. Subsequently, blood was collected from the eyes, and the kidney, spleen, liver, duodenum, and colon tissues were collected.
The fresh feces (500 mg) was obtained from the mouse rectum and soaked in 200 μL pre-cooled diethylpyrocarbonate (DEPC) water for 15 min to dissolve the virus contained in the feces. The DEPC water was centrifuged at 4°C at 10,000 × g for 5 min and the supernatant was stored for subsequent use.
The tissue samples (100 mg) was ground into powder with liquid nitrogen, and 1 mL pre-cooled saline was added. After repeated freeze-thawing, the sample was centrifuged at 10,000 × g at 4°C for 5 min, and the supernatant was obtained.
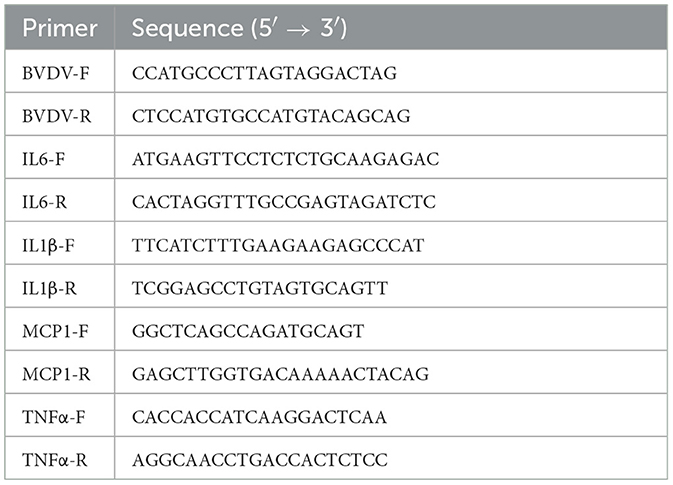
Total RNA was extracted from the fecal and tissues samples with an RNA extraction kit (Takara Bio, Shiga, Japan). Complementary DNA (cDNA) was synthesized by reverse transcription and the BVDV content was analyzed by qRT-PCR using a Prime Direct™ Probe RT-qPCR Mix (Takara Bio). The relative BVDV content was calculated in relation to that of the normal control. Table 3 shows the primers used in this study.
2.4. Histopathological examination of the duodenum
The BVD mouse model exhibited obvious duodenal lesions and relatively severe tissue damage. Therefore, we performed an in-depth study focusing on the duodenal lesions after FGF21 injection. Part of the duodenum was fixed in 4% formaldehyde, paraffin-embedded, and sectioned to 6-μm thickness. The tissue sections were stained with hematoxylin–eosin (H&E) and for E-cadherin for pathological analysis.
2.5. CBC testing
The peripheral blood was collected from the inferior vena cava and anticoagulant (trisodium citrate) was added to the collection tube. The platelets, leukocytes, and lymphocytes were analyzed with a CBC analytical instrument.
2.6. Analysis of inflammatory cytokines in the blood
Peripheral blood that had been collected in non-anticoagulant-containing blood collection tubes was centrifuged at 1,500 × g for 30 min at 4°C to obtain the plasma. The cytokines IL-6, TNF-α, and MCP1 were measured from the cell-free supernatants using enzyme-linked immunosorbent assay kits (Becton, Dickinson and Company, America).
2.7. In vitro culture of duodenal tissue and analysis of inflammatory cytokines
The intestinal contents were cleaned from 2-cm long mouse duodenum samples, the tissue opened longitudinally, soaked in phosphate-buffered saline (PBS, containing streptomycin and penicillin) and washed for 1–2 min, then washed 5–6 times in antibiotic-free RPMI 1640 medium. Subsequently, the tissue was cut into 1-cm2 pieces, then incubated in antibiotic-free RPMI 1640 medium at 37°C for 24 h.
Total RNA from virus-infected duodenum cells was extracted using an RNA extraction kit following the instructions for specific operations. cDNA was synthesized by reverse transcription according to the PrimeScript™ RT Reagent Kit. The reverse transcription condition was 42°C for 1.5 h while the PCR conditions were 94°C for 30 s (one cycle), followed by 95°C for 15 s and 58°C for 1 min for 40 cycles. Blank control wells were set up for each amplification and each experiment was repeated no less than three times. The internal reference gene was β-actin. The target genes were quantified using the relative quantitative analysis method (comparative threshold cycle [2−ΔΔCt] method). Table 3 lists the sequences of the primers used.
2.8. Analysis of FGF21 promotion of duodenal epithelial cell proliferation
To verify whether FGF21 was involved in duodenal epithelial cell proliferation and regeneration, the BVDV-infected mice were injected intraperitoneally with 30 mg/kg 5-bromodeoxyuridine (BrdU) at 2 h, 24 h, and 48 h before the end of BVDV infection.
2.9. Statistical analysis
The data of each group are expressed as the mean ± SEM. Statistical analysis was performed with one-way analysis of variance using GraphPad Prism 8.0.2 (GraphPad Software Inc., La Jolla, CA, USA). The mean between two groups was compared using the t-test. P < 0.05, p < 0.01, and p < 0.001 indicated a statistically significant difference, highly statistically significant difference, and statistically extremely significant difference, respectively.
Results
3.1. FGF21 injection alleviated clinical symptoms in mice after BVDV challenge
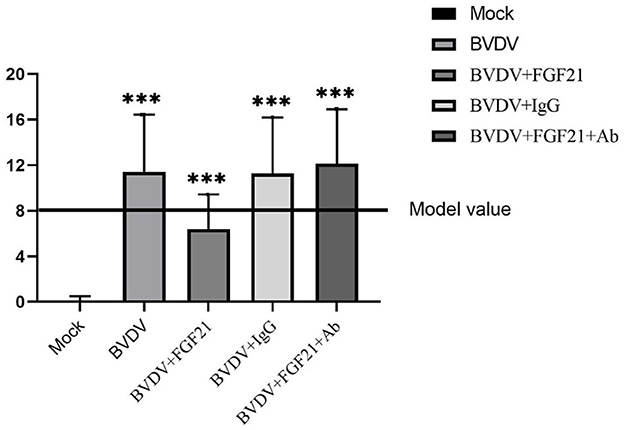
Score statistics were performed according to the BVD mouse model scoring criteria. Figure 1 depicts the results. The mean statistical scores of the BVDV and BVDV + IgG groups were 11.4 and 11.3, respectively. On day 3 after challenge, some mice exhibited signs of lethargy, rough coats, mild diarrhea, eating less, and crowding. The autopsy results of these mice demonstrated a few hemorrhagic spots of different sizes in the liver and lung and obviously enlarged spleen, mesenteric lymph nodes, and paraduodenal Peyer patches.
The BVDV + FGF21Ab mice exhibited more obvious clinical symptoms, where the average statistical score was 12.1 points. Some mice developed obvious diarrhea and lethargy on day 2 after FGF21Ab injection. The autopsy results of the BVDV + FGF21Ab mice revealed a few hemorrhagic spots in the liver and lung and obviously swollen spleen, mesenteric lymph nodes, and paraduodenal Peyer patches.
The BVDV + FGF21 group had an average statistical score of 6.4 points. The BVDV + FGF21 mice had basically normal food intake, smooth fur, no diarrhea symptoms, no hemorrhagic spots in the liver and lungs, and slight swelling of the spleen.
The average statistical score of the control mice was 0.13 points, and they exhibited no obvious clinical symptoms or pathological changes throughout experiment.
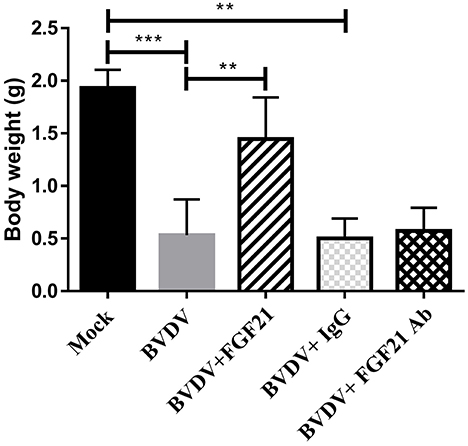
3.2. FGF21 injection slowed BVDV challenge-induced weight loss
The mice were weighed and recorded daily; Figure 2 depicts weight changes. Throughout the experiment, there were extremely significant differences in the body weight changes between the control and BVDV groups and the BVDV + IgG and BVDV + FGF21Ab groups (p < 0.001). The BVDV + FGF21 group recorded significantly different weight gain compared the BVDV and BVDV + IgG groups (p < 0.01). The results indicated that FGF21 attenuated weight loss in the BVD mice during BVD development.
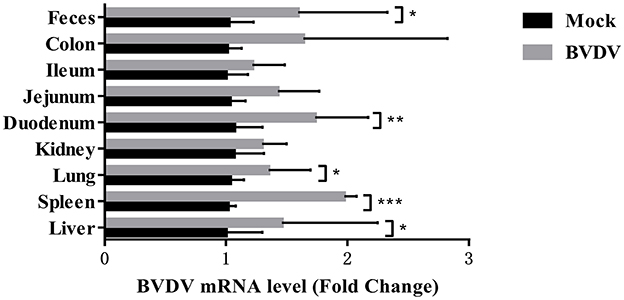
3.3. BVDV detection results in mice after challenge
Figure 3 depicts the BVDV detection results in the mice after challenge. Ten days after BVDV challenge, the BVDV mice exhibited obvious clinical symptoms such as loose stool and diarrhea. BVDV was detected in the mouse liver, lung, spleen, kidney, duodenum, colon, jejunum, ileum, and feces. The BVDV content was highest in the mouse spleen, followed by that in the duodenum, colon, and feces.
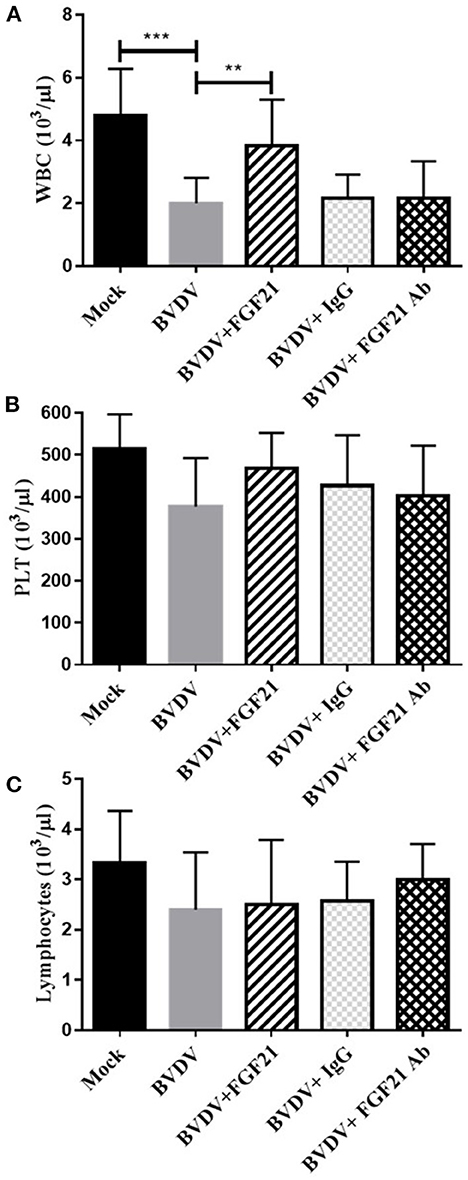
3.4. CBC analysis
Figure 4 depicts the leukocyte, platelet, and lymphocyte levels in the mouse blood after challenge. The BVDV, BVDV + IgG, and BVDV + FGF21Ab mice had significantly decreased leukocyte, platelet, and lymphocyte levels (p < 0.001). The platelet and lymphocyte contents in the BVDV, BVDV + FGF21Ab, and BVDV + IgG groups were not significantly changed, while the leukocyte contents increased significantly after FGF21 injection (p < 0.01).
3.5. Pathological changes of the duodenum
Figure 5 depicts the histopathological observations of the H&E-stained mouse duodenal tissue. The control group demonstrated intact duodenal epithelial phenotype, good crypt structure, and no lymphocyte infiltration in the mucosa. The duodenal tissue of the BVDV and BVDV + FGF21Ab mice exhibited edema, local epithelial ulcers, submucosal inflammatory cell infiltration, and duodenal villus epithelium shedding after challenge. However, injection of the FGF21 recombinant protein was followed by subsiding duodenal intestinal wall edema, significantly relieved ulcer, and the epithelium was significantly improved and tended to be complete.
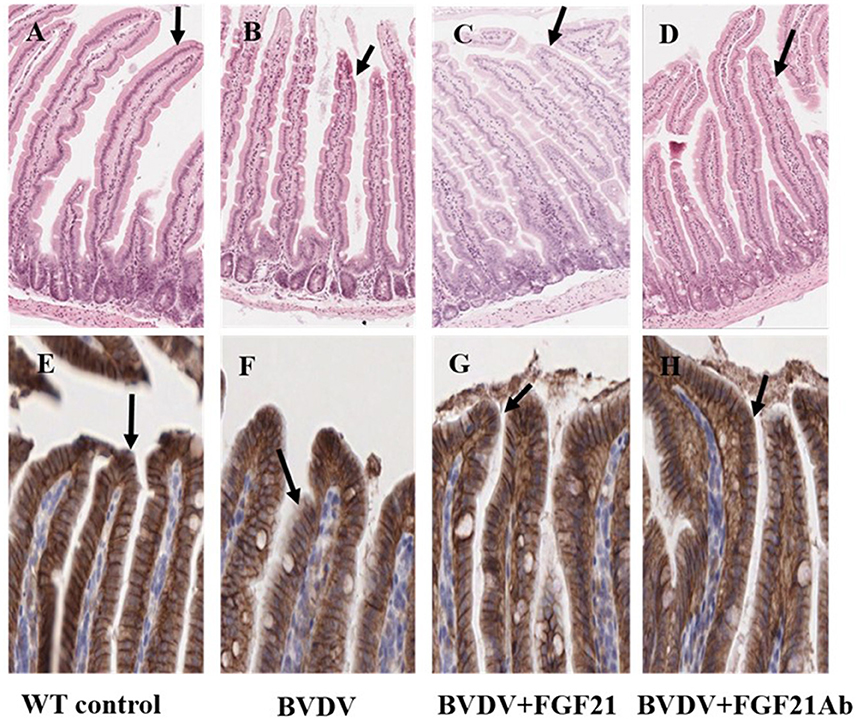
Figure 5. Main pathological changes in the mice (H&E staining, × 100; E-cadherin staining, × 200). (A–D) H&E staining of duodenal epithelial cells in the control (A), BVDV (B), FGF21 (C), and BVDV+FGF21Ab groups (D). (E–H) E-cadherin staining of duodenum in the control (E), BVDV (F), FGF21 (G), and BVDV+FGF21Ab groups (H).
The changes in the mouse duodenal injury were observed using E-cadherin staining, which demonstrated that E-cadherin expression was reduced. The duodenal villous epithelium of the BVDV and BVDV + FGF21Ab mice was damaged and shed after challenge, and the intestinal epithelium was seriously damaged after FGF21Ab injection. After FGF21 recombinant protein injection, the intestinal tract was obviously improved and tended to be complete.
3.6. FGF21 inhibited the expression of BVDV-induced inflammatory factors
Figure 6 depicts the results of FGF21 inhibition of BVDV-induced inflammatory factor expression. After challenge, the peripheral blood of the BVDV, BVDV + FGF21Ab, and BVDV + IgG mice contained significantly increased TNF-α, MCP1 (both, p < 0.01), and IL-6 levels (p < 0.001). However, after FGF21 injection, the peripheral blood IL-6 and TNF-α levels were significantly decreased (p < 0.01 and p < 0.05, respectively), while MCP1 levels were decreased but not significantly (Figure 6A).
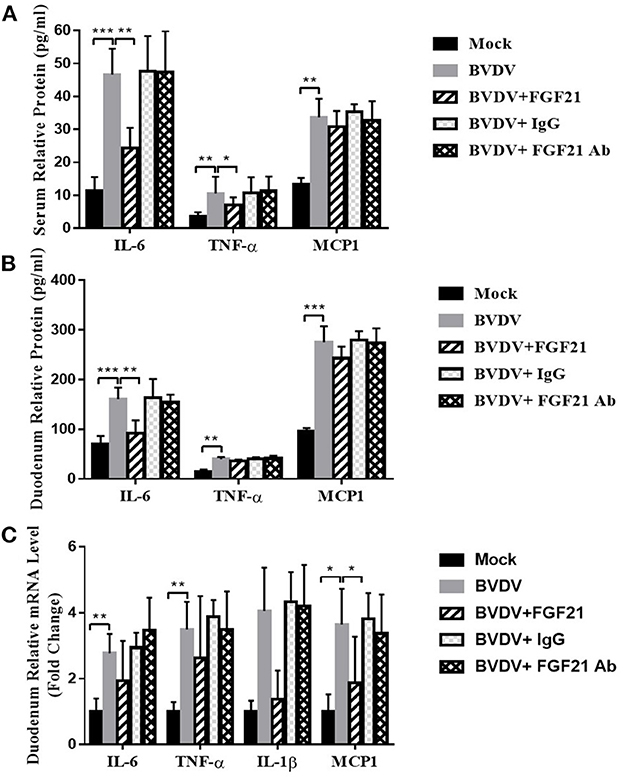
Figure 6. Mouse plasma and duodenum levels of inflammatory cytokines. (A, B) Inflammatory cytokine levels in the plasma (A) and the duodenum (B). (C) Inflammatory cytokine mRNA levels in the duodenum.
After the duodenal tissue had been cultured in vitro for 24 h, the proinflammatory cytokine content in the culture supernatant was detected by ELISA. The results demonstrated that the BVDV, BVDV + FGF21Ab, and BVDV + IgG mice produced significantly more IL-6, TNF-α, and MCP1 than the control group (p < 0.001). However, after the FGF21 injection, the BVDV + FGF21 mice had significantly decreased duodenal IL-6 content (p < 0.01) while the TNF-α and MCP1 contents were not significantly decreased (Figure 6B).
Similar to the results of the peripheral blood and duodenal tissue in vitro culture, the duodenal IL-6, TNF-α, and IL-1β mRNA levels in the BVD, BVDV + FGF21Ab, and BVDV + IgG mice were significantly increased (p < 0.01), as was MCP1 (p < 0.05). After the FGF21 injection, the IL-6, TNF-α, IL-1β, and MCP1 mRNA levels were all decreased in the BVDV + FGF21 group, but only the MCP1 decrease was significantly different (p < 0.05) (Figure 6C).
3.7. FGF21 promoted duodenal epithelial cell proliferation
Figure 7 depicts the results of FGF21 promotion of duodenal epithelial cell proliferation. BVD mice injected with FGF21 recombinant protein demonstrated a significantly larger number of BrdU-positive duodenal intestinal epithelial cells than the control mice.
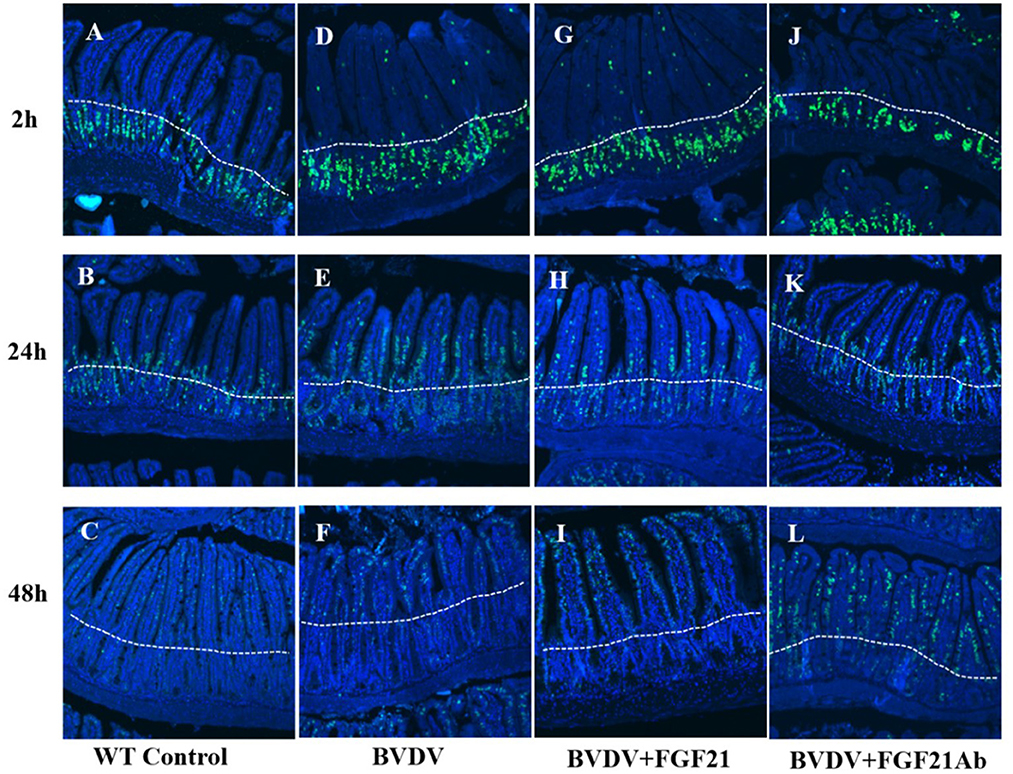
Figure 7. BrdU staining of mouse duodenal epithelial cells. (A–C) BrdU staining results at 2 h (A), 24 h (B), and 48 h (C) in the control group. (D–F) BrdU staining results at 2 h (D), 24 h (E), and 48 h (F) in the BVDV group. (G–I) BrdU staining results at 2 h (G), 24 h (H), and 48 h (I) in the FGF21 group. (J–L) BrdU staining results at 2 h (J), 24 h (K), and 48 h (L) in the BVDV+FGF21Ab group.
4. Discussion
Previously, we determined that FGF21 expression in BVD bovine and BVD mouse peripheral blood exhibited the same upward trend, indicating that BVDV infection in animals stimulates high FGF21 expression. These results were similar to that of Xia et al. regarding FGF21 and ulcerative colitis mice (24). To determine how FGF21 functions in BVD mice, we performed animal experiments based on a BVD mouse model, where BVD mice were injected intraperitoneally with mouse FGF21Ab, IgG, or recombinant FGF21 protein. The results demonstrated that after BVDV challenge, the BVDV and BVDV + IgG groups essentially exhibited the same clinical symptoms and necropsy changes. The results revealed FGF21 delayed or alleviated the clinical symptoms in the BVD mice and significantly improved weight loss, which provided a characterization basis for the role of FGF21.
Diarrhea is one of the main clinical symptoms of BVD. The main pathological changes are hemorrhage spots of different sizes in the intestinal mucosa; swollen and bleeding mesenteric lymph nodes (25–29); intestinal wall thickening; intestinal lymph node enlargement; necrosis and shedding of mucosal epithelial cells in the small intestine, cecum, and colon; lymphocytic infiltration of the mucosal lamina propria; hyaline degeneration and fibrinoid necrosis of the submucosal arteries; crypt epithelial shedding; and vacuolization (28, 29). The histopathological observation revealed that the BVD mice had hemorrhage spots in the intestinal mucosa and lymphocyte infiltration in the mucosal lamina propria, especially duodenal epithelial cell necrosis and shedding, which was consistent with the previous study (30). Gumbiner (31) reported that E-cadherin is a calcium-dependent cell adhesion protein expressed in epithelial cells that is critical for maintaining intestinal epithelial integrity and is indirectly involved in defense against enteropathogens. Stephane et al. (32) reported that the loss of E-cadherin expression led to the loss of adherens junctions and desmosomes, thereby resulting in apoptosis and cell shedding. E-cadherin function in the gut has been implicated in pathological processes. The E-cadherin staining results in this study demonstrated that E-cadherin expression was decreased in the duodenal epithelial cells of the BVDV and BVDV + FGF21Ab mice. FGF21 injection promoted duodenal epithelial cell proliferation and significantly improved the damage to the BVD mouse duodenal epithelial cells.
Proinflammatory cytokines such as IL-6, TNF-α, and MCP1 are key in the pathogenesis of intestinal inflammation. Diarrhea caused by intestinal inflammation is one of the main clinical symptoms of BVD. The autopsy results of the BVD mice demonstrated that duodenal inflammation was the most serious symptom. Therefore, the cytokine levels in the peripheral blood and duodenal tissue of the mice in all groups were detected, where IL-6, TNF-α, and MCP1 in the peripheral blood and duodenum of the BVD mice were significantly increased (p < 0.01). Specifically, IL-6 was extremely significantly increased (p < 0.001). The IL-6 and TNF-α mRNA levels were highly significantly increased in the duodenum of BVD mice (p < 0.01). Furthermore, the leukocyte, platelet, and lymphocyte numbers in the peripheral blood of the BVD mice were decreased, and the leukopenia was highly significantly different (p < 0.01), which indicated that BVDV caused intestinal-related inflammation in the mice, which resulted in epithelial cell damage.
However, after FGF21 injection, the peripheral blood leukocytes of the BVD mice were significantly increased to normal levels. Notably, IL-6 in the peripheral blood and duodenum was highly significantly decreased (p < 0.01). While TNF-α and MCP1 were not significantly different, MCP1 mRNA levels in the duodenum were significantly different (p < 0.05). The results proved that FGF21 inhibited the BVDV-induced inflammatory factor expression. This result was similar to that of Singhal et al. (33) and Johnson et al. (34), who reported that FGF21 might be important in inhibiting and regulating inflammation, respectively.
Our CBC test results were consistent with those of a previous study that reported that different BVDV strains can cause leukocyte, lymphocyte, and platelet reduction in mouse peripheral blood (35). Generally, virus infections cause leukocyte reduction in the peripheral blood. In our experiment, the leukocyte levels returned to the normal range after FGF21 injection.
Although we only focused on the related inflammatory factors in the duodenum with obvious tissue lesions and analysis of the clinical symptoms, body weight changes, CBC testing, histopathology, and changes of inflammatory factors in the BVD mice, the results proved that FGF21 is involved in the improvement and treatment of BVD occurrence and development in mice. The results provided new ideas for BVD prevention and treatment and further research.
5. Conclusions
We successfully established a BVD mouse model. FGF21 injection alleviated the clinical symptoms of BVD in the mice and significantly improved the weight loss in the BVD mice. FGF21 inhibited BVDV and reduced leukocyte, platelet, and lymphocyte levels in the peripheral blood. Furthermore, FGF21 inhibited the expression of BVDV-induced inflammatory factors. Moreover, FGF21 promoted duodenal epithelial cell proliferation and significantly improved the damage to the duodenal epithelial cells in the BVD mice. In conclusion, FGF21 exerted a good therapeutic effect on the BVD mouse model.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was conducted following recommendations of the Jilin Agriculture University Institutional Animal Care and Use Committee (JLAU08201409) and the experimental procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023).
Author contributions
DZ, Y-HS, and J-MS designed the study. KS, J-ML, N-CD, and YZ assisted with data analysis. DZ, F-LZ, and RD performed animal tests, interpreted the results, and wrote the manuscript. All authors have read and approved the final version of manuscript.
Funding
This work was financially supported by grants from the Major Science and Technology Special Project of Jilin Provincial Department of Science and Technology (Grant Number 20220304003YY) and the Key R&D Project of Science and Technology Development Plan of Jilin Provincial Department of Science and Technology (Grant Number 20210204038YY).
Acknowledgments
The authors thank the Jilin Province Sika Deer Efficient Breeding and Product Development Technology Engineering Research Center of Jilin Agricultural University for their technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BVD, bovine viral diarrhea; BVDV, bovine viral diarrhea virus; CBC, complete blood count; CSFV, classical swine fever virus; CPE, cell pathological changes; ELISA, enzyme-linked immunosorbent assay; FGF21, fibroblast growth factor 21; MD, mucosal disease.
References
1. Yesilbag, Foerster K, Ozyigit K, Alpay MO, Tuncer G, Thiel P, et al. Characterisation of bovine viral diarrhea virus (BVDV) isolates from an outbreak with haemorrhagic enteritis and severe pneumonia. Vet Microbiol. (2014) 169:42–9. doi: 10.1016/j.vetmic.2013.12.005
2. Jones LR, Zandomeni R, Weber L. Genetic typing of bovine viral diarrhea virus isolates from Argentina. Vet Microbiol. (2001) 81:367–75. doi: 10.1016/S0378-1135(01)00367-4
3. Rohrer T, Domenico R, Reinhard B, Gabriele E, Alain DG, Christoph R. Combined treatment with zidovudine, lamivudine, nelfinavir and ganciclovir in an infant with human immunodeficiency virus type 1 infection and cytomegalovirus encephalitis: case report and review of the literature. Pediatr Infect Dis J. (1999) 18:382–6. doi: 10.1097/00006454-199904000-00017
4. Bauermann FV, Ridpath JF, Weiblen R, Flores EF. HoBi-like viruses: an emerging group of pestiviruses. J Vet Diagn Invest. (2013) 25:6–15. doi: 10.1177/1040638712473103
5. Neill JD, Workman AM, Hesse R, Bai JF, Porter EP, Meadors B, et al. Identification of BVDV2b and 2c subgenotypes in the United States: genetic and antigenic characterization. Virology. (2019) 528:19–29. doi: 10.1016/j.virol.2018.12.002
6. Yeşilbag K, Alpay G, Becher P. Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses. (2017) 9:128. doi: 10.3390/v9060128
7. Gao YG, Du R, Wang QK, Zheng Q, Wang N, Wang SZ. Isolation of a strain of BVDV from livers of an aborted fetus of sika deer. J Jilin Agricult Univ. (2005) 01:97–99 + 103. doi: 10.13327/j.jjlau.2005.01.024
8. Saliki JT, Dubovi EJ. Laboratory diagnosis of bovine viral diarrhea virus infections veterinary clinics of North America. Vet Clin North Am Food Anim Pract. (2004) 20:69–83. doi: 10.1016/j.cvfa.2003.11.005
9. Lee KM, Gillespie JH. Propagation of virus diarrhea virus of cattle in tissue culture. Am J Vet Res. (1957) 18:952–3.
10. Gillespie JH, Baker JA, McEntee K. A cytopathogenic strain of virus diarrhea virus. Cornell Vet. (1960) 50:73–9.
11. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. (2000) 1492:203–6. doi: 10.1016/S0167-4781(00)00067-1
12. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Investig. (2005) 115:1627–35. doi: 10.1172/JCI23606
13. Ryden M. Fibroblast growth factor 21: an overview from a clinical perspective. Cellular Mol Life Sci. (2009) 66:2067–73. doi: 10.1007/s00018-009-0003-9
14. Gimeno RE, Moller DE. FGF21-based pharmacotherapy: potential utility for metabolic disorders. Trends Endocrinol Metab. (2014) 25:303–11. doi: 10.1016/j.tem.2014.03.001
15. Cheung BM, Deng HB. Fibroblast growth factor 21: a promising therapeutic target in obesity-related diseases. Expert Rev Cardiovasc Ther. (2014) 12:659–66. doi: 10.1586/14779072.2014.904745
16. Zhu SL, Wu YZ, Ye XL, Ma L, Qi JY, Yu D, et al. FGF21 ameliorates non-alcoholic fatty liver disease by inducing autophagy. Mol Cell Biochem. (2016) 420:107–19. doi: 10.1007/s11010-016-2774-2
17. Holm MR, Christensen H, Rasmussen J, Johansen ML, Schou M, Faber J, et al. Fibroblast growth factor 21 in patients with cardiac cachexia: a possible role of chronic inflammation. Esc Heart Fail. (2019) 6:983–91. doi: 10.1002/ehf2.12502
18. Shen Y, Zhang XL, Pan XP, Xu YT, Xiong Q, Lu ZG, et al. Contribution of serum FGF21 level to the identification of left ventricular systolic dysfunction and cardiac death. Cardiovasc Diabetol. (2017) 16:106–12. doi: 10.1186/s12933-017-0588-5
19. Gariani K, Drifte G, Dunn-Siegrist I, Pugin J, Jornayvaz FR. Increased FGF21 plasma levels in humans with sepsis and SIRS. Endocr Connect. (2013) 2:146–53. doi: 10.1530/EC-13-0040
20. Kukla M, Berdowska A, Styga D, Gabriel A, Mazur W, Logiewa-Bazger B, et al. Serum FGF21 and RBP4 levels in patients with chronic hepatitis C. Scand J Gastroenterol. (2012) 47:1037–47. doi: 10.3109/00365521.2012.694901
21. Seong GY, Oem JK, Lee KH, Choi KS. Experimental infection of mice with bovine viral diarrhea virus. Arch Virol. (2015) 160:1565–71. doi: 10.1007/s00705-015-2412-4
22. Liu YL, Zhao CQ, Xiao J, Zhang M, Wang CL, Wu GC, et al. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Scientific Rep. (2016) 6:1–13. doi: 10.1038/srep31026
23. Liu LM, Zhao CQ, Yang Y, Kong XX, Shao T, Ren L, et al. Fibroblast growth factor 21 deficiency attenuates experimental colitis-induced adipose tissue lipolysis. Gastroenterol Res Pract. (2017) 5:1–9. doi: 10.1155/2017/3089378
24. Xia XX, Ni M, Lu YT, Zhao CQ, Wang PR, Jin NY, et al. Correlation between FGF21 and ulcerative colitis in mice. Mod J Anim Husbandr Vet Med. (2020) 4:8–13.
25. Lunardi M, Headley SA, Lisboa JA, Amudea A.A. Outbreak of acute bovine viral diarrhea in Brazilian beef cattle: clinicopathological findings and molecular characterization of a wild-type BVDV strain subtype 1b. Res Vet Sci. (2008) 85:599–604. doi: 10.1016/j.rvsc.2008.01.002
26. Liebler-Tenorio EM, Kenklies S, Greiser-Wilke I, Makoschey B, Pohlenz JF. Incidence of BVDV1 and BVDV2 infections in cattle submitted for necropsy in Northern Germany. J Vet Med B Infect Dis Vet Public Health. (2006) 53:363–69. doi: 10.1111/j.1439-0450.2006.00992.x
27. Campbell JR. Effect of bovine viral diarrhea virus in the feedlot. Vet Clin North Am Food Anim Pract. (2004) 20:39–50. doi: 10.1016/j.cvfa.2003.11.003
28. Cheng ZL. Epidemiological Investigation of BVD, IBR and PR on Cattle Farms in Shandong Province during 2017-2019 and Study on Pathogenic Mechanism of Co-infection of BVDV and BHV-1. Tai-an, Shandong: Agricultural University (2020).
29. Khodakaram-Tafti A, Farjanikish GH, Mohammadi G. Histopathological and immunohistochemical findings from bovine viral diarrhea virus infection in cattle. Online J Vet Res Ojvr. (2015) 19:317–21.
30. Zhao D. Study on effect of Fibroblast Growth Factor 21 in the mice model of bovine viral diarrhea. Changchun: Jilin Agricultural University (2021).
31. Gumbiner, B.M Regulation of cadherin adhesive activity. J Cell Biol. (2000) 148:399–404. doi: 10.1083/jcb.148.3.399
32. Fouquet S, Lugo-Martinez VH, Faussatll AM, Renaud F, Cardot F, Chambaz J, et al. Early loss of e-cadherin from cell-cell contacts is involved in the onset of anoikis in enterocytes. J Biologic Chemistr. (2004) 279:43061–69. doi: 10.1074/jbc.M405095200
33. Singhal G, Fisher FM, Chee MJ, Tan TG, Ouaamari AE, Adams AC, et al. Fibroblast growth factor 21 (FGF21) protects against high fat diet induced inflammation and islet hyperplasia in pancreas. PLoS One. (2016) 11:e0148252. doi: 10.1371/journal.pone.0148252
34. Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. J Gastroenterol. (2009) 137:1795–804. doi: 10.1053/j.gastro.2009.07.064
Keywords: bovine viral diarrhea, fibroblast growth factor 21, bovine viral diarrheal virus, duodenal epithelial cells, mouse model
Citation: Zhao D, Song Y-H, Song J-M, Shi K, Li J-M, Diao N-C, Zong Y, Zeng F-L and Du R (2023) The effect of fibroblast growth factor 21 on a mouse model of bovine viral diarrhea. Front. Vet. Sci. 10:1104779. doi: 10.3389/fvets.2023.1104779
Received: 22 November 2022; Accepted: 20 February 2023;
Published: 09 March 2023.
Edited by:
Yuan Wanzhe, Hebei Agricultural University, ChinaReviewed by:
Chuang Xu, China Agricultural University, ChinaHouqiang Luo, Wenzhou Vocational College of Science and Technology, China
Copyright © 2023 Zhao, Song, Song, Shi, Li, Diao, Zong, Zeng and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan-Li Zeng, emVuZ2ZhbmxpQGpsYXUuZWR1LmNu; Rui Du, ZHVydWlAamxhdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Dan Zhao1†
Dan Zhao1† Yu-Hao Song
Yu-Hao Song Jin-Ming Song
Jin-Ming Song Kun Shi
Kun Shi Nai-Chao Diao
Nai-Chao Diao Ying Zong
Ying Zong Fan-Li Zeng
Fan-Li Zeng