- 1School of Biodiversity, One Health and Veterinary Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom
- 2Dundas Veterinary Group Limited, Edinburgh, United Kingdom
Over the last two decades, vector-borne pathogens (VBPs) have changed their distribution across the globe as a consequence of a variety of environmental, socioeconomic and geopolitical factors. Dirofilaria immitis and Dirofilaria repens are perfect exemplars of European VBPs of One Health concern that have undergone profound changes in their distribution, with new hotspots of infection appearing in previously non-endemic countries. Some areas, such as the United Kingdom, are still considered non-endemic. However, a combination of climate change and the potential spread of invasive mosquito species may change this scenario, exposing the country to the risk of outbreaks of filarial infections. Only a limited number of non-autochthonous cases have been recorded in the United Kingdom to date. These infections remain a diagnostic challenge for clinicians unfamiliar with these “exotic” parasites, which in turn complicates the approach to treatment and management. Therefore, this review aims to (i) describe the first case of D. repens infection in a dog currently resident in Scotland, (ii) summarise the available literature on Dirofilaria spp. infections in both humans and animals in the United Kingdom and (iii) assess the suitability of the United Kingdom for the establishment of these new VBPs.
1. Introduction
The zoonotic mosquito-borne filarial nematodes Dirofilaria immitis (Leidy, 1856) and Dirofilaria repens, Railliet and Henry, 1911, are among the most important canine vector-borne pathogens (VBPs) of One Health concern in mainland Europe (1, 2). Both Dirofilaria species are widely distributed in tropical and temperate regions (3, 4) and can infect a wide range of animals and humans. Dirofilaria immitis is the causative agent of canine heartworm disease (HWD), a severe syndrome that can be fatal if not promptly diagnosed and properly treated. The closely related parasite, D. repens, is the causative agent of subcutaneous dirofilariosis (SCD), which is generally asymptomatic or paucisymptomatic, and is often only reported as an incidental finding during unrelated surgical or clinical procedures (3). Although humans are not the definitive host of these mosquito-borne filaroids, they may still develop symptoms. In fact, D. repens causes subcutaneous and/or ocular nodules while D. immitis infection results in the development of characteristic ‘coin’ lesions in the lung parenchyma (3). In some cases, D. immitis human infections may be misdiagnosed as cancer or pulmonary disease and thus infected individuals may undergo unnecessary and painful surgical procedures and/or be prescribed inappropriate treatment before receiving the correct diagnosis (5, 6). Despite humans occasionally experiencing microfilaraemia due to D. repens infection (7), dogs remain the main reservoir of both infections for humans and animals and play a central role in disease epidemiology, especially when competent mosquito vectors and other susceptible hosts coexist in the same environment (8). Almost 70 mosquito species belonging to the genera Aedes/Ochlerotatus, Anopheles and Culex are known to be susceptible to these filarial worms. However, only a proportion of these are considered competent vectors and only a handful of species, including Aedes albopictus and Culex pipiens, are understood to play a major role in disease transmission (9, 10). Mosquitoes become infected during the blood meal, after ingesting the microfilariae (mfs) released by the adult female in the bloodstream of the mammalian host. Once inside the Malpighian tubules of the invertebrate host, the mfs moult several times before developing into the infective L3 stage larvae which migrate to the mosquito’s labium. In this location, they are ready to actively penetrate the skin of the next host during the mosquito’s blood meal (3). The entire process requires from 8 to 21 days depending on ambient temperature and filarial species involved (10, 11). Filarial nematodes are considered to be a re-emerging threat for both humans and animals in the majority of European countries due to an increasing number of cases and the extension of their geographical range (4, 12). Certain areas, such as the United Kingdom, are not considered to be endemic and thus it may be argued that they are exposed to the risk of unexpected outbreaks of infection, as has been documented in other previously non-endemic countries (2, 13).
1.1. Dirofilaria spp. diagnosis as a distribution and prevalence study-bias
The prevalence of Dirofilaria spp. infection in humans and animals is often underestimated and clinical cases can be misdiagnosed, particularly in non-endemic countries (14, 15). This may be explained, in part, by the lack of a single definitive diagnostic method for the efficient, simultaneous detection of both filarial species. This is compounded by the fact that infected individuals may be either asymptomatic or paucisymptomatic, in particular for D. repens in dogs and D. immitis in humans, and that symptoms/clinical signs, when present, may overlap with other illnesses. This may be further exacerbated by a knowledge gap, on the part of clinicians, on the ever-changing geographical distribution of both Dirofilaria species and thus filarial infection may not even be considered as a differential diagnosis in areas where the parasite is usually absent (13, 15, 16). In endemic countries or in geographical areas where at least a low/moderate prevalence has been recorded and consequently there is appreciable awareness in the veterinary community, effective treatment and control measures can result in an observable decrease in incidence of both diseases (4, 12). Several methods have been developed to diagnose dirofilariosis and are currently in use. These may be divided into parasitological techniques for mfs detection in the host’s blood (e.g., fresh blood smear, buffy coat examination, filtration or modified Knott’s test), serological (i.e., enzyme-linked immunosorbent assays (ELISA), rapid immunochromatographic tests (ICT) and molecular approaches (i.e., probe-based quantitative polymerase chain reaction (qPCR), conventional PCR (cPCR), duplex qPCR for the simultaneous detection of both Dirofilaria spp.)), together with fine-needle aspirate (FNA) and histological examination of nodules when present (3, 12, 17, 18). Serological tests are available as reference laboratory assays and also point-of care (POC) tests (3, 19–21). While for D. immitis, one can rely on rapid and straightforward in-clinic test kits together with several serological laboratory tools, no similar tests are available for D. repens (12). Encouragingly, epitopes of D. repens have recently been identified by Pękacz and colleagues (22), using phage display technology in combination with a 12-mer peptide library. These antigenic peptides have been shown to be strongly recognised by IgG from sera of infected dogs (22) and these may form the basis of a new generation of serological tests for Dirofilaria spp.
According to several studies, antigen tests may be effective in determining infection status earlier than the parasitological concentration assays; the former may be accurate as early as 5 months post infection (p.i.), while the latter require testing at least 7 months p.i. (23, 24). The sensitivity of these tests when used in the field may not be as impeccable as the product sheets imply (23), as it has been recognised since the 1980s that the presence of immune complexes may negatively impact antigen test reliability (23). Consequently, it has been determined that test sensitivity may be improved by subjecting samples to immune complex dissociation (ICD) prior to testing (25, 26). Indeed, ICD has been shown to improve antigen detection in both experimental and natural D. immitis infections by releasing HW antigens that are bound to host antibodies (27–29). ICD methods are based broadly on two alternate technical approaches, namely heat-and acid-treatment. Although heat-treatment is the one most commonly employed (29), acid-treatment with trichloroacetic acid (TCA) has shown similar efficacy without decreasing specificity, a recognised drawback of heat-treatment (23, 29–32). According to the American Heartworm Society (AHS) and the European Society of Dirofilariosis and Angiostrongylosis (ESDA), the diagnosis of D. immitis should be demonstrated by the presence of circulating mfs and/or adult antigens (24, 33). However, each diagnostic method has different sensitivity and specificity, and each of them has its own advantages and disadvantages which may lead to false positive or negative results (22–25, 31–33). Indeed, studies on the incidence and/or prevalence of Dirofilaria spp. infection in the same canine population have shown differing results according to the diagnostic method adopted (15, 18, 19, 30). For this reason, diagnosis of animal dirofilariosis must rely on the integration of results from at least two methods in order to reduce the risk of false positives and negatives, as recommended by the AHS (24). In human medicine, the diagnostic approach places particular emphasis on clinical examination, anamnesis and travel history of the patient, while additional laboratory methods may include morphological identification of the worm extracted from the nodules, direct detection of DNA of the parasite or of its endosymbiont Wolbachia and on other serological tests for detecting antibodies against the filarial antigens (7, 34). In addition to this, modified Knott’s method can be applied also in humans, but it is only helpful in D. repens cases when microfilaraemia is present (7). Unfortunately, the combination of a non-specific clinical presentation, the potential delay in seeking medical assistance and frequent initial misdiagnosis, conspires to hamper disease diagnosis and management and thus dirofilariosis is currently considered to be an emerging zoonosis in Europe (34).
1.2. Dirofilaria spp. in Europe: the latest trend
The epidemiology of vector-borne pathogens (VBPs) has dramatically changed across the globe over the last two decades as a consequence of a variety of environmental, socioeconomic and geopolitical factors (35–39). These changes are, to a large extent, associated with alterations in the distribution and biology of their arthropod vectors. Taken together, this reshaping of VBPs and vector epidemiology has far-reaching implications for both animal and human health (40, 41). Dirofilaria immitis and D. repens are perfect exemplars of European VBPs that have undergone profound changes in their distribution which in turn have precipitated striking changes in disease epidemiology (1, 2). Several studies have investigated the influence of a global temperature rise on the development time of Dirofilaria spp. in the mosquito vector and the impact of this on human and animal health (11, 42). In particular, it has been estimated that the threshold temperature for the extrinsic incubation of the filarial larval stages within the arthropod-vector is 14°C, below which larval development stops (11). Climate change, therefore, represents a clear and present threat which may both directly and indirectly affect the distribution, persistence and spread of these filarial species, exposing new naïve animal and human populations to the risk of infection (2, 13, 42). According to the Intergovernmental Panel on Climate Change (IPCC), there has been a documented recent rise in world temperature of 1.1°C in recent years (43). This has already affected VBP transmission and the further predicted increase in global temperature will only serve to worsen the situation (11). Indeed, all projected distribution studies on Dirofilaria spp. employ a climate-based approach to mathematically model the capacity of different geographical areas to support the extrinsic development of the parasite. A geographical area may be classed as suitable for extrinsic development if the following criteria are met: a minimum total environmental heat of 1°C in excess of the threshold of 14°C, for a total of 130 Heartworm Development Units (HDU), evaluated over a mosquito life span of a maximum of 30 days (a longer period being incompatible with mosquito survival) (11, 44). Along with a rise in global temperatures which affects the arthropod vectors, the change in distribution has been ascribed also to an increased movement of animals among non-and endemic countries and to the lack of chemoprophylaxis treatments in non-endemic areas (2). All these factors have increased the numbers of new clinical cases in geographical areas previously described as non-endemic, which has led to the establishment of new hotspot of infections, such as those in southern Europe. For example, in southern Italy, prevalences between 56 and 78% have been recorded, the highest in the Mediterranean regions (2, 13). Dirofilaria repens, known to be endemic in the temperate regions, is now extending its range across Central, Eastern and Northern Europe (45, 46). While D. immitis is endemic in Southern and Western Europe, it is extending its range into the cooler regions of Central, Eastern and Northern Europe, where it is not yet endemic (4, 46, 47).
2. Dirofilaria spp. infections in the United Kingdom: the state of the art
The United Kingdom has always been considered a non-endemic country for the presence of Dirofilaria spp. (12, 16, 48) and the limited number of human and canine cases of dirofilariosis reported in the United Kingdom have been contracted while in endemic geographical areas such as Italy, Greece, Romania, Spain and Sri Lanka (12, 16, 48, 49). The few case reports recorded only D. immitis infection, with no mention of D. repens infection in the United Kingdom (3, 16, 50). However, in the United Kingdom, as in the rest of Europe, environmental factors such as climate change, which may facilitate the introduction of new invasive mosquito vectors, and an increased movement of animals and humans across the national borders have affected the parasite incidence dynamic, with an increased number of HW reports over the last two decades (11, 51). Several authors have argued that the progressive relaxation of the Pet Travel Scheme 2000 (PETS) in recent years (i.e., since 2012) is one of the major factors responsible for the spread of VBPs (45, 52). In particular, in the United Kingdom, this relaxation has been accompanied by a huge influx of dogs from abroad (i.e., 300,000 imported dogs), principally from Romania and Spain (12, 16, 53). Nonetheless, the United Kingdom has not been a member of the PETS since the first of January 2021, and it is currently considered a ‘Part II listed non-EU country’. Thus, pets travelling from or to the United Kingdom may be subject to differing health requirements compared to EU countries (54, 55). During the first epidemiological survey conducted in 2010, the filarial-specific antibody prevalence was recorded as 0% over a total of 1,028 dogs tested across the United Kingdom (56). A later European study performed between 2016 and 2020 revealed a canine HW seroprevalence of 5–7% in central/southern England, with no seropositive dogs detected in other parts of the country (57). While the overall seroprevalence of HW infection remains very low (56, 57), there have been two reports of clinical D. repens infection in United Kingdom dogs in recent years in the midlands of England (12, 58, 59). Both studies reported infection in imported dogs, the first originating from Romania (59, 60) and the second from Corfu (58). The first report documents a dog living in the United Kingdom for several months before, in 2014, a diagnosis of filarial infection being made, and treatment being instigated. The diagnosis was based on molecular and serological analyses although it was not stated whether mfs were present in the peripheral blood (59). The second report recounts a dog which had recently been relocated to the United Kingdom and in which a 17 cm female nematode was discovered as an incidental finding during routine castration. Again, no information was reported on the presence of mfs (58). Similarly, to the best of our knowledge, only a very limited number of human cases of Dirofilaria spp. infection have been recorded in the United Kingdom and these have been attributed to people contracting the disease while living abroad in endemic countries before returning to the United Kingdom (12). For example, a case of subcutaneous dirofilariasis in a 32-year old man was reported by Ahmed and colleagues in 2010 (49). The patient presented with a parotid duct obstruction and a plum-sized swelling on the same side, anterior to his masseter muscle. Initially misdiagnosed as a tumour of the accessory parotid gland, histological examination revealed the presence of an adult Dirofilaria spp. nematode and it was established that the man had a history of travelling in Sri Lanka (1, 49). More recently, subcutaneous dirofilariasis was detected in a 67-year-old English man (48). This individual presented with a six-month history of a small painless lump developing on the right side of his abdomen which initially appeared 2 years after a fortnight’s travel in Tuscany, Italy (48). Currently, the United Kingdom is still considered a non-endemic country, however in this age of frequent foreign travel, an awareness of clinical dirofilariasis is required among veterinarians and medical practitioners alike.
3. Case report in Scotland
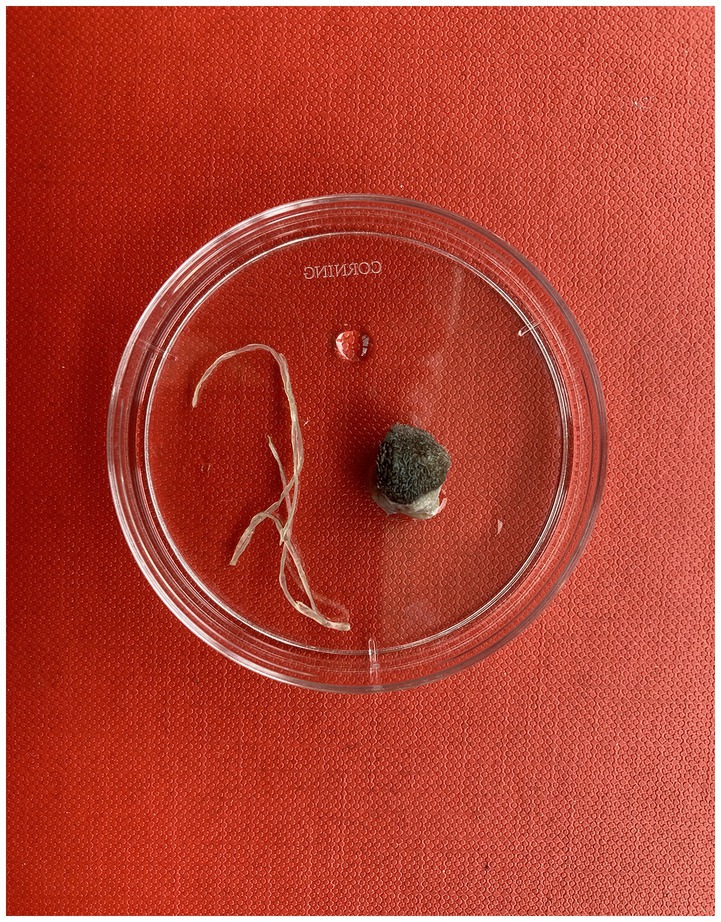
On the 24th of August 2020, a 4-year-old female neutered crossbreed dog was presented at a veterinary clinic in Edinburgh, Scotland with a small, raised lesion on its nasal dorsum (Figure 1). The dog had arrived in the United Kingdom on the 7th of July 2020, having been born in Romania, where it had been kept in a dog shelter since it was a puppy. Apart from treatment for Echinococcus multilocularis, which is necessary for entry into the United Kingdom (i.e., praziquantel, (61)), the dog arrived with no additional record of endo-or ectoparasitic treatments. At first examination, the dog was found to be generally in good health. The nodule was initially described as being a small and firm fluctuating mass on the left side of the dog’s face, above the premolar teeth of the maxillary arch, 206 and 207, and did not appear to be causing any pain. It was recommended that the owner monitor the nodule over the time and report any change in its dimensions.
About 1 month after the first clinical examination, the dog was treated with Milbemax® (12.5 mg milbemycin oxime and 125 mg praziquantel, Elanco United Kingdom AH Limited) and Bravecto® (500 mg fluralaner, MSD Animal Health S.r.l.), a standard prophylactic protocol for endo-and ectoparasites. It subsequently received the same anti-parasitic treatment every three-months. Over the following 2 years, the dog was seen by the practice for problems related to anxiety and weight loss (1 kg over 4 months) with the latter being mostly attributed to the dog being a fussy eater. Subsequent biochemical analyses of the dog’s blood revealed no anomalies and therefore the owner decided against further investigation.
On the 25th of July 2022, the dog was again presented to the clinic as the owner had become concerned about the nodule on the dog’s nose. This had grown since the initial consultation and was now approximately 2 cm in diameter and, thus, a FNA was performed under sedation. It was observed that the material inside the nodule was mucopurulent in nature and the dog was treated with a broad spectrum antibiotic (250 mg Clavaseptin®) and an anti-histamine (4 mg chlorphenamine) for 1 week. Cytological analysis of the FNA confirmed a severe neutrophilic inflammation, but no microorganisms or neoplastic cells were observed. Still considering the remote possibility of a neoplastic origin of the nodule or the presence of a fistulous tract originating from a tooth root abscess, the dog underwent another week of treatment. Following radiography, which produced no evidence of a dental fistula, the mass was removed and found to be a series of cysts which were well-organised around a nematode-like body. The structure, tentatively suspected of being an aberrantly located Thelazia callipaeda, was stored in a 70% ethanol solution and referred to the Veterinary Diagnostic Service (VDS) at the University of Glasgow School of Veterinary Medicine, for parasitological and molecular analyses together with a 2 ml sample of blood in sodium citrate.
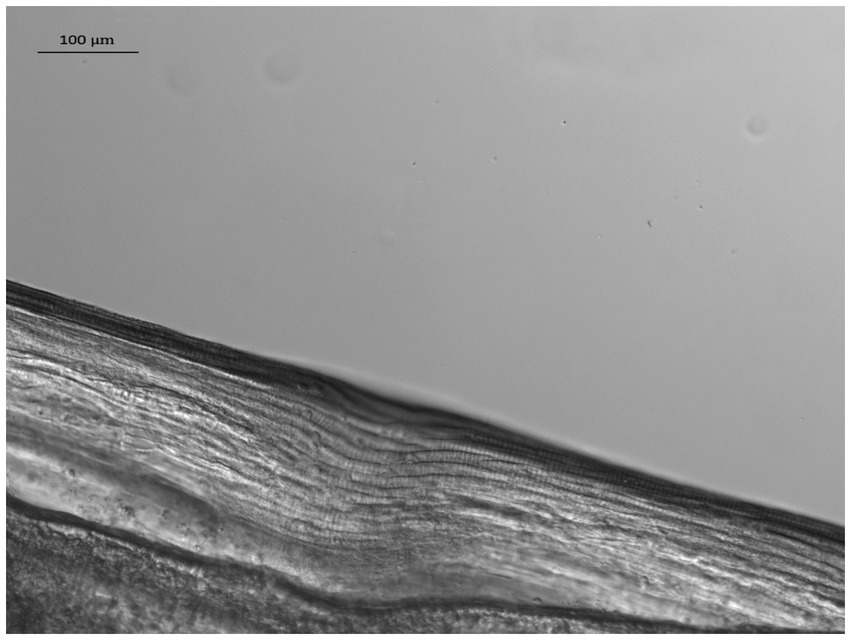
At the VDS laboratory, following suspension in 10% glycerol solution, the nematode’s length was measured and its sex determined using light microscopy. Measurements and photographs were taken using a Zeiss Axioskop 2 microscope, with Zeiss Mrm camera and Axioscope software, while the identification was carried out using previously published morphological keys (62, 63). The nematode had a whitish color, narrow rounded ends and a total length of 13.0 mm. It was highly dehydrated with the anterior extremity showing evidence of decomposition and crushing. It was therefore difficult to accurately measure the width of the anterior end and its distance from the nerve ring and the vulva. However, characteristic fine longitudinal cuticular ridges (Figure 2) visible along the whole body allowed its identification as D. repens. In addition, although no mfs were present in the uterus, it was identified as a female specimen due to the absence of the spicula and the length of its body.
The dog’s blood sample was processed using a modified Knott’s test for the detection of potentially circulating mfs (64). In order to confirm the nematode’s identity and to exclude the occurrence of other filarial infections, it was analysed by polymerase chain reaction (PCR). Genomic DNA was extracted from a portion of its body and from 200 μl of whole blood using a Blood & Tissue® nucleic acid extraction kit (Qiagen, Germany) and screened by conventional PCR using generic primers targeting the 12S rDNA and COX1 locus of filarial nematodes (17, 65, 66). The amplicons were gel purified using a QIAquick® Gel extraction kit (Qiagen, Germany), then sequenced and compared with the GenBank non-redundant nucleotide database using the Basic Local Alignment Search Tool (BLAST).1 The highest ranking ‘hits’ for both amplicons were D. repens reference sequences, with 99% identity over 586 nucleotides for COX1 (MT345575) and 100% identity over 237 nucleotides for the 12S locus (KY828984), thus confirming the morphological identification. Finally, the dog was found to be negative for the presence of mfs using the modified Knott’s test and no filarial nematode DNA was detected in the blood by PCR. The dog made an uneventful recovery from surgery and no further health issues have been reported.
4. Discussion
This review reports the current status of dirofilariosis in the United Kingdom, presenting the first non-autochthonous D. repens case reported in a dog resident in Scotland, which follows on from two previous cases in the English midlands (58, 59). The dog discussed herein received a definitive diagnosis of dirofilariasis 2 years after its arrival from Romania, where it likely has contracted the infection. If the dog had been microfilaraemic and competent mosquito vectors had been present in the locality, local transmission would have been feasible. Fortunately, mfs were not detected in this case and this was fully anticipated, as the dog had been treated on a three-monthly basis with macrocyclic lactones since its arrival in the United Kingdom (67). Despite this anthelminthic regime being prescribed on a prophylactic basis with a relatively low drug dosage, it would still have acted as a “soft-kill” drug against any Dirofilaria species present (68). While the use of milbemycin oxime for “soft-killing” may be recommended in some cases, it is considered the least safe choice among the various macrocyclic lactones employed for treating positive animals (68). In other case scenarios, the lack of a prompt diagnosis for HW disease or a sub-optimal treatment plan may have dramatic consequences on the infected dog’s health. According to Nolan and colleagues, treatment with macrocyclic lactones can have a safe and off-label effect also on D. repens adults (69), which would explain the death of the nematode prior the surgery in this particular case. Macrocyclic lactones also affect D. repens mfs, as demonstrated in previous studies (67, 70). Although milbemycin oxime acts against D. repens microfilariae and prevents any further D. repens infection (71, 72), its adulticidal activity has not yet been confirmed. Nonetheless, in a previous study conducted by Giudice and colleagues, a correlation was noted between the inflammatory response of a dog infected with multiple subcutaneous dirofilariosis nodules and milbemycin oxime administration, which presumably prevented the nematodes from further development (71). Additional investigations are needed to fully understand this process and its impact on management of this VBP.
According to the European Scientific Counsel on Companion Animal Parasites (ESCCAP) guidelines, before travelling from endemic to non-endemic areas, dogs should be examined and, if the infection is detected, treated for both dirofilarial infections. Conversely, for travel from non-endemic to endemic areas, it is recommended to start a monthly preventive treatment of dogs and cats with macrocyclic lactones 30 days prior to entering the risk area. For long stays in endemic areas (i.e., more than 1 month), the administration should occur every month or every 12-weeks for cats with an extended duration spot-on macrocyclic lactone, with the last dose given after return to a non-endemic country (73). Furthermore, animals with unknown history should receive prophylactic treatment for 2 months in order to kill any migrating L3 and L4 and should be retested after 6 and 12 months after arrival in the new country (73, 74). As reported by the Animal and Plant Health Agency (APHA), more than 66,000 dogs were commercially imported into the United Kingdom in 2020, with a concomitant rise in ‘low-welfare’ importation practices and smuggling activities (61). Rising imports and falling standards of husbandry can only serve to increase the prevalence of exotic parasite infections in dogs resident in the United Kingdom. As for the Dirofilaria case reported by Agapito and colleagues, this nematode was initially misdiagnosed as T. callipaeda in an aberrant location (59). Interestingly, the two adult nematodes species are very different in their morphometric characteristics and in the clinical signs they cause (75). Similar to Dirofilaria, T. callipaeda is a European VBP which is undergoing a shift in its epidemiology (76). Only imported cases of thelaziosis have been recorded to date in the United Kingdom, but there appears to be a better awareness of this parasite, its life cycle and its distribution among United Kingdom veterinarians compared to Dirofilaria spp. Thus, there exists a clear need to reinforce awareness of dirofilariasis in the United Kingdom in both veterinary and medical fields. This should be accompanied by the ongoing publication of bulletins reporting parasite and disease distribution together with the creation of ‘easy to access’ resources documenting new clinical cases of exotic VBP disease together with guidelines on the diagnostic approaches which should be employed. The risk of the establishment of hitherto exotic filarial nematodes in the United Kingdom is a realistic and ongoing concern. New dirofilarial ‘hot spots’ have emerged in central Europe in recent years (2, 4, 13) and environmental conditions and vector availability appear more permissive for Dirofilaria spp. to encroach into northern European countries than in the past (11, 42, 77). In fact, as reported by Medlock and colleagues, modelling studies have predicted that certain areas as the United Kingdom are becoming sufficiently warm for the survival of invasive mosquitoes such as Aedes albopictus, known to be a competent vector of Dirofilaria spp. (42, 78). Therefore, in 2010 the Health Protection Agency (HPA) and colleagues started an intensive surveillance programme to investigate the presence of invasive mosquito species in England, Wales, Scotland and Northern Ireland (42). Ae. albopictus eggs were detected in the United Kingdom for the first time in 2016 and then again in 2017 and 2018, although no Ae. albopictus adult mosquitoes have been captured to date (77). However, Ae. albopictus is not the only potential vector of Dirofilaria spp.; several other Aedes and Anopheles mosquitoes have established in the United Kingdom which may transmit the infection when environmental conditions allow and these include Aedes vexans, Aedes cinereus, Ochlerotatus detritus, Ochlerotatus caspius, Ochlerotatus punctor, Ochlerotatus sticticus, Finlaya geniculatus, Anopheles atroparvus, Anopheles claviger and An. plumbeus (79). Other potential vectors such as Culex pipiens and Culiseta annulata have also been recently identified in this country (13, 42).
It is difficult to contend with the ecological changes extending vector and pathogen distributions and these will ultimately determine whether Dirofilaria spp. can definitely establish in the United Kingdom. However, it is prescient for veterinarians, physicians and pet owners to be more aware of these exotic parasites, so that they may be properly considered in the course of differential diagnosis in suspected human and animal cases.
Author contributions
RP and WW contributed to conception and design of the study and wrote sections of the manuscript. RM referred the clinical case. RP, EM, AP, and MM performed the laboratory analyses. RP wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
RM was employed by Dundas Veterinary Group Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Otranto, D, Capelli, G, and Genchi, C. Changing distribution patterns of canine vector borne diseases in Italy: leishmaniosis vs. dirofilariosis Parasit Vectors. (2009) 2:1–8. doi: 10.1186/1756-3305-2-S1-S2
2. Brianti, E, Panarese, R, Napoli, E, De Benedetto, G, Gaglio, G, Bezerra-Santos, MA, et al. Dirofilaria immitis infection in the Pelagie archipelago: the southernmost hyperendemic focus in Europe. Transbound Emerg Dis. (2022) 69:1274–80. doi: 10.1111/tbed.14089
3. Simón, F, Siles-Lucas, M, Morchón, R, González-Miguel, J, Mellado, I, Carretón, E, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microb Rev. (2012) 25:507–44. doi: 10.1128/CMR.00012-12
4. Genchi, C, and Kramer, LH. The prevalence of Dirofilaria immitis and D. repens in the old world. Vet Parasitol. (2020) 280:108995. doi: 10.1016/j.vetpar.2019.108995
5. Mimori, T, Tada, I, and Takeuchi, T. Dirofilaria infection in the breast of a woman in Japan. Southeast Asian J Trop Med Publ Hlth. (1986) 17:165–7.
6. Maltezos, E, Sivridis, E, Giatromanolaki, A, and Simopoulos, C. Human subcutaneous dirofilariasis: a report of three cases manifesting as breast or axillary nodules. Scott Med J. (2002) 47:86–8. doi: 10.1177/003693300204700404
7. Pupić-Bakrač, A, Pupić-Bakrač, J, Beck, A, Jurković, D, Polkinghorne, A, and Beck, R. Dirofilaria repens Microfilaremia in humans: case description and literature review. One Health. (2021) 13:100306. doi: 10.1016/j.onehlt.2021.100306
8. Otranto, D, Brianti, E, Gaglio, G, Dantas-Torres, F, Azzaro, S, and Giannetto, S. Human ocular infection with Dirofilaria repens (Railliet and Henry, 1911) in an area endemic for canine dirofilariasis. Am J Trop Med Hyg. (2011) 84:1002–4. doi: 10.4269/ajtmh.2011.10-0719
9. Eldridge, BF, and Edman, JD. Introduction to medical entomology In: Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods. eds. BF Eldridge and JD Edman Dordrecht: Springer (2000). 1–12.
10. Cancrini, G, and Gabrielli, S. Vectors of Dirofilaria nematodes: biology, behaviour and host/parasite relationships. In: Dirofilaria immitis and D. repens in Dog and Cat and Human Infections, ed. G. Cringoli (2007): 48–58.
11. Genchi, C, Rinaldi, L, Mortarino, M, Genchi, M, and Cringoli, G. Climate and Dirofilaria infection in Europe. Vet Parasitol. (2009) 163:286–92. doi: 10.1016/j.vetpar.2009.03.026
12. Capelli, G, Genchi, C, Baneth, G, Bourdeau, P, Brianti, E, Cardoso, L, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors. (2018) 11:1–21. doi: 10.1186/s13071-018-3205-x
13. Panarese, R, Iatta, R, Latrofa, MS, Zatelli, A, Ćupina, AI, Montarsi, F, et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: an expanding threat in the Mediterranean region. Int J Parasitol. (2020) 50:555–9. doi: 10.1016/j.ijpara.2020.04.002
14. Archer, J, LaCourse, JE, Webster, BL, and Stothard, JR. An update on non-invasive urine diagnostics for human-infecting parasitic helminths: what more could be done and how? Parasitology. (2020) 147:873–88. doi: 10.1017/S0031182019001732
15. Panarese, R, Iatta, R, Mendoza-Roldan, JA, Szlosek, D, Braff, J, Liu, J, et al. Comparison of diagnostic tools for the detection of Dirofilaria immitis infection in dogs. Pathogens. (2020) 9:499. doi: 10.3390/pathogens9060499
16. Genchi, C, Bowman, D, and Drake, J. Canine heartworm disease (Dirofilaria immitis) in Western Europe: survey of veterinary awareness and perceptions. Parasit Vectors. (2014) 7:206. doi: 10.1186/1756-3305-7-206
17. Latrofa, MS, Montarsi, F, Ciocchetta, S, Annoscia, G, Dantas-Torres, F, Ravagnan, S, et al. Molecular xenomonitoring of Dirofilaria immitis and Dirofilaria repens in mosquitoes from north-eastern Italy by real-time PCR coupled with melting curve analysis. Parasit Vectors. (2012) 5:1–8. doi: 10.1186/1756-3305-5-76
18. Negron, V, Saleh, MN, Sobotyk, C, Luksovsky, JL, Harvey, TV, and Verocai, GG. Probe-based QPCR as an alternative to modified Knott’s test when screening dogs for heartworm (Dirofilaria immitis) infection in combination with antigen detection tests. Parasit Vectors. (2022) 15:1–7. doi: 10.1186/s13071-022-05372-x
19. Miterpáková, M, Antolová, D, Rampalová, J, Undesser, M, Krajčovič, T, and Víchová, B. Dirofilaria immitis pulmonary dirofilariasis, Slovakia. Emerg Infect Dis. (2022) 28:482–5. doi: 10.3201/eid2802.211963
20. Newton, WL, and Wright, WH. The occurrence of a dog Filariid other than Dirofilaria immitis in the United States. J Parasitol. (1956) 42:246–58. doi: 10.2307/3274849
21. Lee, ACY, Bowman, DD, Lucio-Forster, A, Beall, MJ, Liotta, JL, and Dillon, R. Evaluation of a new in-clinic method for the detection of canine heartworm antigen. Vet Parasitol. (2011) 177:387–91. doi: 10.1016/j.vetpar.2010.11.050
22. Pękacz, M, Basałaj, K, Kalinowska, A, Klockiewicz, M, Stopka, D, Bąska, P, et al. Selection of new diagnostic markers for Dirofilaria repens infections with the use of phage display technology. Sci Rep. (2022) 12:2288. doi: 10.1038/s41598-022-06116-8
23. Little, S, Saleh, M, Wohltjen, M, and Nagamori, Y. Prime detection of Dirofilaria immitis: understanding the influence of blocked antigen on heartworm test performance. Parasit Vectors. (2018) 11:1–10. doi: 10.1186/s13071-018-2736-5
24. AHS. Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria immitis) Infection in Dogs; (2018). Available at: https://www.heartwormsociety.org/images/pdf/2018-AHS-Canine-Guidelines.pdf. (Accessed November 22, 2022)
25. Venco, L, Manzocchi, S, Genchi, M, and Kramer, LH. Heat treatment and false-positive heartworm antigen testing in ex vivo parasites and dogs naturally infected by Dirofilaria repens and Angiostrongylus vasorum. Parasit Vectors. (2017) 10:476. doi: 10.1186/s13071-017-2444-6
26. Henry, LG, Brunson, KJ, Walden, HS, Wenzlow, N, Beachboard, SE, Barr, L, et al. Comparison of six commercial antigen kits for detection of Dirofilaria immitis infections in canines with necropsy-confirmed heartworm status. Vet Parasitol. (2018) 254:178–82. doi: 10.1016/j.vetpar.2018.02.037
27. Weil, GJ, Malane, MS, Powers, KG, and Blair, LS. Monoclonal antibodies to parasite antigens found in the serum of Dirofilaria immitis-infected dogs. J Immun. (1985) 134:1185–91. doi: 10.4049/jimmunol.134.2.1185
28. Little, SE, Raymond, MR, Thomas, JE, Gruntmeir, J, Hostetler, JA, Meinkoth, JH, et al. Heat treatment prior to testing allows detection of antigen of Dirofilaria immitis in feline serum. Parasit Vectors. (2014) 7:1. doi: 10.1186/1756-3305-7-1
29. Beall, MJ, Arguello-Marin, A, Drexel, J, Liu, J, Chandrashekar, R, and Alleman, AR. Validation of immune complex dissociation methods for use with heartworm antigen tests. Parasit Vectors. (2017) 10:481. doi: 10.1186/s13071-017-2442-8
30. Starkey, L, Bowles, J, and Blagburn, B. Comparison of acid-versus heat-treatment for immune complex dissociation and detection of Dirofilaria immitis antigen in canine plasma. Vet Parasitol. (2020) 282:109134. doi: 10.1016/j.vetpar.2020.109134
31. Velasquez, L, Blagburn, BL, Duncan-Decoq, R, Johnson, EM, Allen, KE, Meinkoth, J, et al. Increased prevalence of Dirofilaria immitis antigen in canine samples after heat treatment. Vet Parasitol. (2014) 206:67–70. doi: 10.1016/j.vetpar.2014.03.021
32. Venco, L, Genchi, C, and Simón, F. La Filariosis Cardiopulmonar (Dirofilaria immitis) En El Perro. La Filariosis en las Especies Domésticas y en el Hombre Barcelona, Spain: Merial Laboratorios (2011):19–60.
33. ESDA. Guidelines for Clinical Management of Canine Heartworm; (2017). Available at: https://www.esda.vet/media/attachments/2021/08/19/canine-heartworm-disease.pdf. (Accessed November 22, 2022)
34. Simón, F, Diosdado, A, Siles-Lucas, M, Kartashev, V, and González-Miguel, J. Human Dirofilariosis in the 21st century: a scoping review of clinical cases reported in the literature. Transbound Emerg Dis. (2022) 69:2424–39. doi: 10.1111/tbed.14210
35. Parham, PE, Waldock, J, Christophides, GK, Hemming, D, Agusto, F, Evans, KJ, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos Trans R Soc B Biol Sci. (2015) 370:20130551. doi: 10.1098/rstb.2013.0551
36. Semenza, JC ed. Vector-Borne Disease Emergence and Spread in the European Union Forum on Microbial Threats; Board on Global Health; Health and Medicine Division (2016) Washington, DC: National Academies Press.
37. Grillet, ME, Hernández-Villena, JV, Llewellyn, MS, Paniz-Mondolfi, AE, Tami, A, Vincenti-Gonzalez, MF, et al. Venezuela's humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect Dis. (2019) 19:e149–61. doi: 10.1016/S1473-3099(18)30757-6
38. Tarnas, MC, Desai, AN, Lassmann, B, and Abbara, A. Increase in vector-borne disease reporting affecting humans and animals in Syria and neighboring countries after the onset of conflict: a Promed analysis 2003–2018. Int J Infect Dis. (2021) 102:103–9. doi: 10.1016/j.ijid.2020.09.1453
39. Stufano, A, Iatta, R, Sgroi, G, Jahantigh, HR, Cagnazzo, F, Flöel, A, et al. Seroprevalence of vector-borne pathogens in outdoor workers from southern Italy and associated occupational risk factors. Parasit Vectors. (2022) 15:1–9. doi: 10.1186/s13071-022-05385-6
40. Wilke, AB, Beier, JC, and Benelli, G. Complexity of the relationship between global warming and urbanization–an obscure future for predicting increases in vector-borne infectious diseases. Curr Opin Insect Sci. (2019) 35:1–9. doi: 10.1016/j.cois.2019.06.002
41. O’Lear, S. Environmental Geopolitics: An Introduction to Questions and Research Approaches. A Research Agenda for Environmental Geopolitics. United Kingdom: Edward Elgar Publishing (2020). p. 1–14.
42. Medlock, JM, Hansford, KM, Vaux, AG, Cull, B, Gillingham, E, and Leach, S. Assessment of the Public Health Threats Posed by Vector-Borne Disease in the United Kingdom (UK). Int J Environ Res Public Health. (2018) 15:2145. doi: 10.3390/ijerph15102145
43. Pörtner, H-O, Roberts, DC, Adams, H, Adler, C, Aldunce, P, Ali, E, et al. Climate change 2022: Impacts, Adaptation and Vulnerability. Geneva, Switzerland: IPCC (2022).
44. Fortin, J, and Slocombe, J. Temperature requirements for the development of Dirofilaria immitis in aedes Triseriatus and ae. Vexans Mosquito News. (1981) 41:625–33.
45. Alsarraf, M, Levytska, V, Mierzejewska, EJ, Poliukhovych, V, Rodo, A, Alsarraf, M, et al. Emerging risk of Dirofilaria Spp. infection in northeastern Europe: high prevalence of Dirofilaria repens in sled dog kennels from the Baltic countries. Sci Rep. (2021) 11:1–1068. doi: 10.1038/s41598-020-80208-1
46. Manev, I. Prevalence of Dirofilaria immitis in stray dogs from Sofia, Bulgaria. Int J Vet Sci Anim Husb. (2020) 5:40–3.
47. Fontes-Sousa, A, Silvestre-Ferreira, A, Carretón, E, Esteves-Guimarães, J, Maia-Rocha, C, Oliveira, P, et al. Exposure of humans to the zoonotic nematode Dirofilaria immitis in northern Portugal. Epidemiol Infect. (2019) 147:e282. doi: 10.1017/S0950268819001687
48. Coelho, RR, Tsigka, A, Lee, KY, and Grattan, CEH. A case of subcutaneous dirofilariasis presenting in the UK: an unexpected finding. Clin Experim Dermatol. (2015) 40:449–51. doi: 10.1111/ced.12518
49. Ahmed, ACL, Goodman, A, and Tadrous, P. Cutaneous dirofilariasis affecting the parotid duct: a case report. Maxillofacial. (2010) 10:107–9. doi: 10.1102/1470-5206.2010.0023
50. Thomas, RE. A case of canine heartworm disease (Dirofilaria immitis) in the UK. Vet Rec. (1985) 117:14–5. Epub 1985/07/06. doi: 10.1136/vr.117.1.14
51. Trotz-William, LA, and Trees, AJ. Systematic review of the distribution of the major vector-borne parasitic infections in dogs and cats in Europe. Vet Rec. (2003) 152:97–105. doi: 10.1136/vr.152.4.97
52. Curry, E, Traversa, D, Cárreton, E, Kramer, L, Sager, H, Young, L, et al. The use of molecular markers to investigate possible resistance to heartworm preventives in Dirofilaria immitis samples from heartworm positive dogs in Europe. Research Square [preprint] (2021). Available at: https://doi.org/10.21203/rs.3.rs-864621/v1 (Accessed April 17, 2023).
53. Norman, C, Stavisky, J, and Westgarth, C. Importing rescue dogs into the UK: reasons, methods and welfare considerations. Vet Rec. (2020) 186:248. doi: 10.1136/vr.105380
54. Regulation (EU) no 576/2013 of the European Parliament and of the Council of 12 June 2013 on the non-commercial movement of pet animals and repealing Regulation (EC) No 998/2003 Text with EEA relevance. OJ (2013) 178:1–26.
55. Department of Agriculture EaRAD. Travelling with Pets; (2022). Available at: https://www.daera-ni.gov.uk/articles/travelling-pets#skip-link. (Accessed September 30, 2022)
56. Fehr, JE, Schnyder, M, Joekel, DE, Pantchev, N, Sarkunas, M, Torgerson, P, et al. Estimated specific antibody-based true Sero-Prevalences of canine Filariosis in dogs in Central Europe and the UK. Parasitol Res. (2022) 121:3671–80. doi: 10.1007/s00436-022-07695-1
57. Miró, G, Wright, I, Michael, H, Burton, W, Hegarty, E, Rodón, J, et al. Seropositivity of Main vector-borne pathogens in dogs across Europe. Parasit Vectors. (2022) 15:189. doi: 10.1186/s13071-022-05316-5
58. Wright, I. Case report: Dirofilaria repens in a canine castrate incision. Companion Animal. (2017) 22:316–8. doi: 10.12968/coan.2017.22.6.316
59. Agapito, D, Aziz, N-AA, Wang, T, Morgan, ER, and Wright, I. Subconjunctival Dirofilaria repens infection in a dog resident in the UK. J Small Anim Pract. (2018) 59:50–2. doi: 10.1111/jsap.12795
60. Agapito, D, Aziz, NA, Wang, T, Morgan, E, and Wright, I. Subconjunctival Dirofilaria repens infection in a dog resident in the UK. J Small Anim Pract. (2018) 59:50–2. doi: 10.1111/jsap.12795
61. DEFRA. Commercial and Non-Commercial Movements of Pets into Great Britain. Consultation Document (2021). [cited 2022 20 November]. Available from: https://consult.defra.gov.uk/pet-travel-and-imports-team/pet-travel/
63. Bain, O, and Chabaud, A. Atlas of infective larvae of Filariae. Trop Med Parasitol. (1986) 37:301–40.
64. Knott, J. A method for making microfilarial surveys on day blood. Trans R Soc Trop Med Hyg. (1939) 33:191–6. doi: 10.1016/s0035-9203(39)90101-x
65. Casiraghi, M, Anderson, TJC, Bandi, C, Bazzocchi, C, and Genchi, C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. (2001) 122:93–103. Epub 2002/01/11. doi: 10.1017/S0031182000007149
66. Casiraghi, M, Bain, O, Guerrero, R, Martin, C, Pocacqua, V, Gardner, SL, et al. Mapping the presence of Wolbachia Pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. (2004) 34:191–203. doi: 10.1016/j.ijpara.2003.10.004
67. Giannelli, A, Ramos, RAN, Traversa, D, Brianti, E, Annoscia, G, Bastelli, F, et al. Treatment of Dirofilaria repens Microfilariaemia with a combination of doxycycline Hyclate and Ivermectin. Vet Parasitol. (2013) 197:702–4. doi: 10.1016/j.vetpar.2013.05.012
68. McCall, JW. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet Parasitol. (2005) 133:197–206. doi: 10.1016/j.vetpar.2005.04.005
69. Nolan, J, T, B, and Lok, J. Macrocyclic lactones in the treatment and control of parasitism in small companion animals. Curr Pharm Biotechnol. (2012) 13:1078–94. doi: 10.2174/138920112800399167
70. Petry, G, Genchi, M, Schmidt, H, Schaper, R, Lawrenz, B, and Genchi, C. Evaluation of the Adulticidal efficacy of Imidacloprid 10%/Moxidectin 2.5%(W/V) spot-on (advocate®, advantage® multi) against Dirofilaria repens in experimentally infected dogs. Parasitol Res. (2015) 114:131–44. doi: 10.1007/s00436-015-4519-7
71. Giudice, E, Di Pietro, S, Gaglio, G, Di Giacomo, L, Bazzano, M, and Mazzullo, G. Adult of Dirofilaria repens in a dog with recurrent multiple subcutaneous nodular lesions. Parasitol Res. (2014) 113:711–6. doi: 10.1007/s00436-013-3699-2
72. Di Cesare, A, Braun, G, Di Giulio, E, Paoletti, B, Aquilino, V, Bartolini, R, et al. Field clinical study evaluating the efficacy and safety of an Oral formulation containing Milbemycin oxime/Praziquantel (Milbemax®, Novartis animal health) in the chemoprevention of the zoonotic canine infection by Dirofilaria repens. Parasit Vectors. (2014) 7:347. doi: 10.1186/1756-3305-7-347
73. ESCCAP. Control of Vector-Borne 5 Diseases in Dogs and Cat; (2019). Available at: https://yhwdhifq_0775_ESCCAP_Guideline_GL5_v10_1p.pdf. (Accessed November 23, 2022)
74. APHA. Imported Disease Summaries for Dogs and Cats; (2022). Available at: http://apha.defra.gov.uk/documents/surveillance/diseases/imported-dog-disease-for-dog-and-cats.pdf. (Accessed November 20, 2022).
75. Faust, EC. Studies on Thelazia callipaeda Railliet and Henry, 1910. J Parasitol. (1928) 15:75–86. doi: 10.2307/3271341
76. Palfreyman, J, Graham-Brown, J, Caminade, C, Gilmore, P, Otranto, D, and Williams, DJL. Predicting the distribution of Phortica Variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasit Vectors. (2018) 11:272. doi: 10.1186/s13071-018-2842-4
77. Vaux, A, Dallimore, T, Cull, B, Schaffner, F, Strode, C, Pflüger, V, et al. The challenge of invasive mosquito vectors in the UK during 2016–2018: a summary of the surveillance and control of Aedes Albopictus. Med Vet Entomol. (2019) 33:443–52. doi: 10.1111/mve.12396
78. Cancrini, G, Romi, R, Gabrielli, S, Toma, L, Di Paolo, M, and Scaramozzino, P. First finding of Dirofilaria repens in a natural population of Aedes Albopictus. Med Vet Entomol. (2003) 17:448–51. doi: 10.1111/j.1365-2915.2003.00463.x
Keywords: Dirofilaria repens, Dirofilaria immitis, heartworm disease, subcutaneous dirofilariosis, mosquito-borne disease, dirofilariasis, United Kingdom
Citation: Panarese R, Moore R, Page AP, McDonald M, MacDonald E and Weir W (2023) The long-distance relationship between Dirofilaria and the UK: case report and literature review. Front. Vet. Sci. 10:1128188. doi: 10.3389/fvets.2023.1128188
Edited by:
Ettore Napoli, University of Messina, ItalyReviewed by:
Lavinia Ciuca, University of Naples Federico II, ItalyGiovanni De Benedetto, University of Messina, Italy
Copyright © 2023 Panarese, Moore, Page, McDonald, MacDonald and Weir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossella Panarese, Um9zc2VsbGEuUGFuYXJlc2VAZ2xhc2dvdy5hYy51aw==
 Rossella Panarese
Rossella Panarese Rhiannon Moore2
Rhiannon Moore2 Antony P. Page
Antony P. Page