- 1Institute of Animal Health, Guangdong Academy of Agricultural Sciences, Guangzhou, China
- 2Key Laboratory of Livestock Disease Prevention of Guangdong Province, Guangzhou, China
- 3Scientific Observation and Experiment Station of Veterinary Drugs and Diagnostic Techniques of Guangdong Province, Ministry of Agriculture and Rural Affairs, Guangzhou, China
- 4South China Agricultural University Library, Guangzhou, China
Porcine deltacoronavirus (PDCoV) is a newly emerging and important porcine enteropathogenic coronavirus that seriously threatens the swine industry in China and worldwide. We conducted a systematic review and meta-analysis to access the prevalence of PDCoV infection in pig population from mainland China. Electronic databases were reviewed for PDCoV infection in pig population, and meta-analysis was performed to calculate the overall estimated prevalence using random-effect models. Thirty-nine studies were included (including data from 31,015 pigs). The overall estimated prevalence of PDCoV infection in pigs in China was 12.2% [95% confidence interval (CI), 10.2–14.2%], and that in Central China was 24.5% (95%CI, 16.1–32.9%), which was higher than those in other regions. During 2014–2021, the estimated prevalence of PDCoV infection was the highest in 2015 at 20.5% (95%CI, 10.1–31.0%) and the lowest in 2021 at 4.8% (95%CI, 2.3–7.3%). The prevalence of PDCoV infection in sows was 23.6% (95%CI, 15.8–31.4%), which was higher than those in suckling piglets, nursery piglets, and finishing pigs. The prevalence of PDCoV infection was significantly associated with sampling region, sampling year, pig stage, and clinical signs (diarrhea). This study systematically evaluated the epidemiology of PDCoV infection in Chinese pig population. The findings provide us with a comprehensive understanding of PDCoV infection and are beneficial for establishing new controlling strategies worldwide.
Introduction
Coronaviruses (CoVs) cause respiratory and gastrointestinal diseases in humans and animals. Porcine deltacoronavirus (PDCoV) is a newly emerging and important porcine enteropathogenic coronavirus that causes severe enteritis with acute diarrhea and dehydration in pigs. PDCoV infection can occur in pigs of all ages but mainly affects suckling piglets with mortality rate as high as 30–40% (1). Different from other enteric CoVs, PDCoV causes not only extensive intestinal lesions but also significant gastric lesions and mild pulmonary lesions (2). Aminopeptidase N (APN) is considered as an entry receptor of PDCoV, which is widely distributed in various tissues of multi-species, leading to presence of cross-species transmissibility (3). PDCoV can infect calves, turkeys, poultry, and mice and has independently infected children, proving its potential cross-species transmission capacity (4–6). Its spread seriously threatens the global pig industry and public health.
CoVs belong to the subfamily Coronavirinae, family Coronaviridae of the order Nidovirales. These positive-sense, single-stranded RNA viruses have the largest genome size among known RNA viruses. CoVs are genetically classified into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus (DCoV) (7, 8). PDCoV belongs to the genus DCoV and has a size of approximately 25.4 kb (8, 9). Each genus of CoVs usually infects hosts in a specie-specific manner. Alphacoronavirus and Betacoronavirus infect mammals, and Gammacoronavirus primarily infect birds. DCoV can infect birds and mammals and is composed of nine avian DCoVs (White-eye Coronavirus; Sparrow Coronavirus, SpCoV; Magpie robin Coronavirus; Night heron Coronavirus; Wigeon Coronavirus; Common Moorhen Coronavirus; Bulbul Coronavirus; Thrush Coronavirus; and Munia Coronavirus) and three mammal DCoVs (Asian Leopard Cats Coronavirus, Chinese ferretbadger Coronavirus, and PDCoV) (9). The genome of PDCoV is similar to that of SpCoV in the same genus, indicating that the interspecific transmission of DCoV from birds to pigs may have occurred recently. The PDCoV genome organization is in the following order: 5′untranslated region (UTR), replicase open reading frame 1ab (ORF 1ab), spike (S), envelope (E), membrane (M), nucleocapsid (N), and 3′UTR, with two open reading frames encoding accessory genes nonstructural protein 6 (NS6) and nonstructural protein 7 (NS7) between M and N gene and within N gene (10–13). According to phylogenetic and comparative sequence analysis, PDCoV could be divided into four lineages: Early China, China, Thailand, USA (14). Early China and China lineages include strains from China. Thailand lineage includes strains from Laos, Vietnam and Thailand. USA lineage includes strains from USA, Mexico, Peru, Japan, Korea, and China. USA and China lineages are the major genotypes globally, and Thailand and China lineages have higher intra- and inter-lineage recombination and genetic diversity than USA lineage (14–16). Most recombination breakpoints occur in the S and ORF1ab genes, and recombination in ORF1a may result in the porcine innate immune evasion. Recombination of the S gene is a common phenomenon among CoVs; the S gene of PDCoV evolves at a lower rate than porcine epidemic diarrhea virus (PEDV) in pigs (17–22).
PDCoV was first identified in Hongkong, China in 2012. The first PDCoV strain (HKU15) was detected from rectal swabs of healthy pigs by the coronavirus diversity molecule monitoring in Hongkong (9). However, its pathogenic potential was not recognized until the first PDCoV-related diarrhea epidemic was reported in Ohio, USA in February 2014 (1). Since then, many Asian countries (Korea, China, Japan, Thailand, Laos, and Vietnam) and American countries (United States, Canada, Mexico, and Peru) have reported the PDCoV epidemic, causing a widespread concern (15, 23–28). In mainland China, since first report of PDCoV in 2015, it has quickly spread over the country. A large number of studies on PDCoV infection have been conducted in China (17, 23, 29–40). Therefore, we conducted a meta-analysis to systematically assess the prevalence and distribution characteristics of PDCoV infection in China. The findings would provide us with a comprehensive understanding of PDCoV infection and are beneficial for establishing new controlling strategies worldwide.
Materials and methods
Search strategy and selection criteria
This meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (41). A search was conducted on PubMed, Web of Knowledge, CNKI, Wanfang, and Chongqing VIP databases between January 1, 2015 and October 31, 2022 for all studies that possibly contained data for PDCoV infection in pig populations. The databases were searched using MeSH terms and variants: “PDCoV,” “epidemiology or incidence or prevalence or investigation or surveillance or rate,” and “China or Chinese.” Studies without language limitation were included.
The eligibility for inclusion of all studies identified from the database search was independently assessed and compared by two authors. All retrieved articles were manually selected based on the relevance of publication titles and abstracts to PDCoV epidemiology. The full texts of articles considered potentially relevant based on titles and abstracts were independently reviewed by two authors. Exclusion criteria were as follows: retrospective studies, repeated studies, or nonpig studies; providing final results without sample information, such as sampling time and sample size; and sample size was <60.
Data extraction and quality assessment
We extracted the following information from each study: first author, publication year, province of the study, administrative region, positive sample size/sample size, detection method, target gene, coinfection, and study design. The data were extracted by two authors independently, who reached a consensus through a discussion on the controversial information. The quality of included studies was evaluated according to the Grading of Recommendations Assessment, Development, and Evaluation method (42). We assigned a score to each publication. Study was awarded 1 point each when the research objective was defined, the detection method was described, the sampling method was described, subjects were classified into different subgroups, and the risk factors were determined. The publication quality was defined as low (1 point), moderate (2–3 points), or high (4–5 points). High scores indicated high quality.
Statistical analysis
We estimated the prevalence of PDCoV infection by pooling data from included studies. We used the DerSimonian–Laird random-effect model to analyze the data (43, 44), and compared the differences using Wilcoxon two-sample test or t-test. A forest plot was used to present combined estimates with 95% CIs.
We evaluated statistical heterogeneity using p and I2 statistics, and it was considered insignificant only when p > 0.1 and I2 < 50%. The fixed-effect model was adopted in the absence of publication heterogeneity; otherwise, the random-effect model was used. Potential publication bias was assessed via a funnel plot, Egger’s regression test, and Begg’s test. Sensitivity analysis was conducted by modifying the inclusion criteria of this meta-analysis. The investigated factors were sampling region, sampling year, and pig stage. All the analysis was conducted using the Stata software (version 12.0, Stata Corporation, College Station, Texas).
Results
Literature search
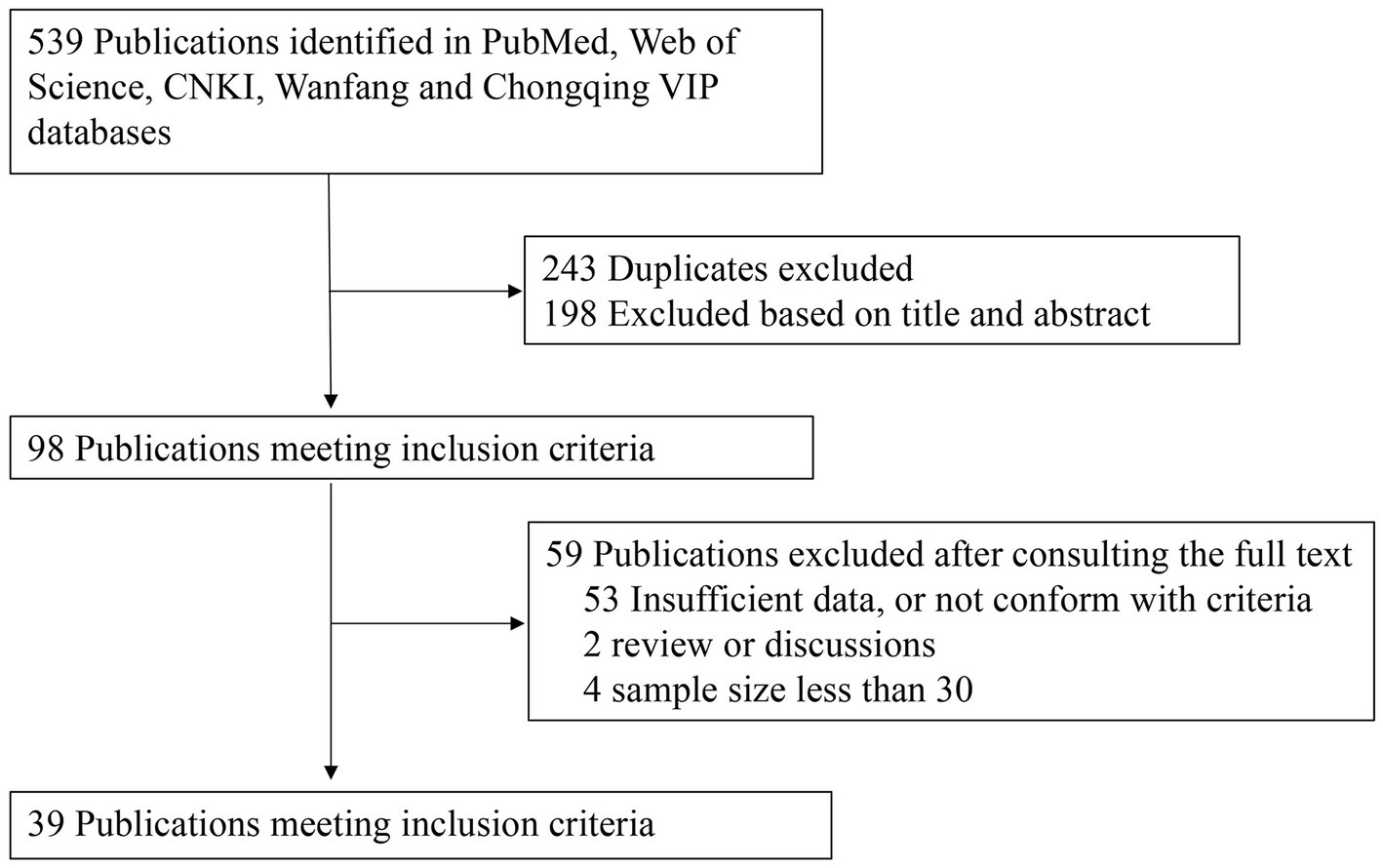
As shown in Figure 1, the literature search yielded 539 relevant studies (226 studies in English and 313 studies in Chinese), of which 243 were duplicates. After the title and abstract of each article were carefully reviewed, 98 articles were considered potentially valuable, and their full texts were retrieved for detailed evaluation. After the full text was reviewed, 59 potentially relevant articles were excluded from this meta-analysis. Among them, 53 articles did not provide required sufficient data or did not meet the inclusion criteria; 4 articles had a sample size of <60; and two articles were review papers. Finally, 39 publications were included for our meta-analysis.
Characteristics of included studies
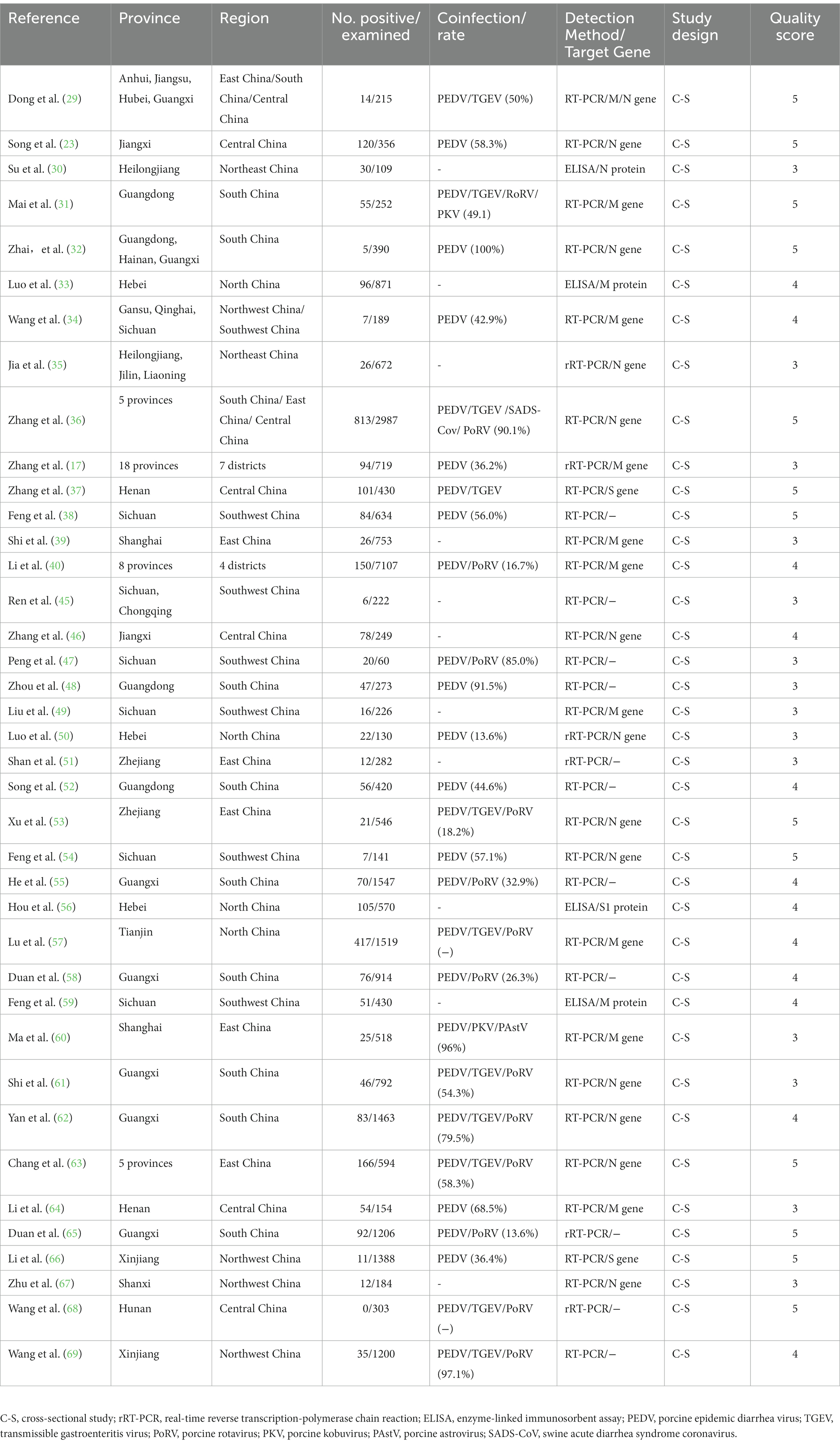
The characteristics of the included studies are listed in Table 1. The articles were published between January 1, 2015 and October 31, 2022 and covered 25 provinces in China. A total of 31,015 pig samples and 3,149 PDCoV-positive cases were included in the meta-analysis. In terms of epidemiological design, all 39 publications were cross-sectional studies and calculated period prevalence. Among them, 14 papers were written in English and 25 in Chinese. According to the established criteria, 25 publications were of high quality (4 or 5 points) and 14 publications were of moderate quality (2 or 3 points).
Prevalence of PDCoV infection in administrative regions of China
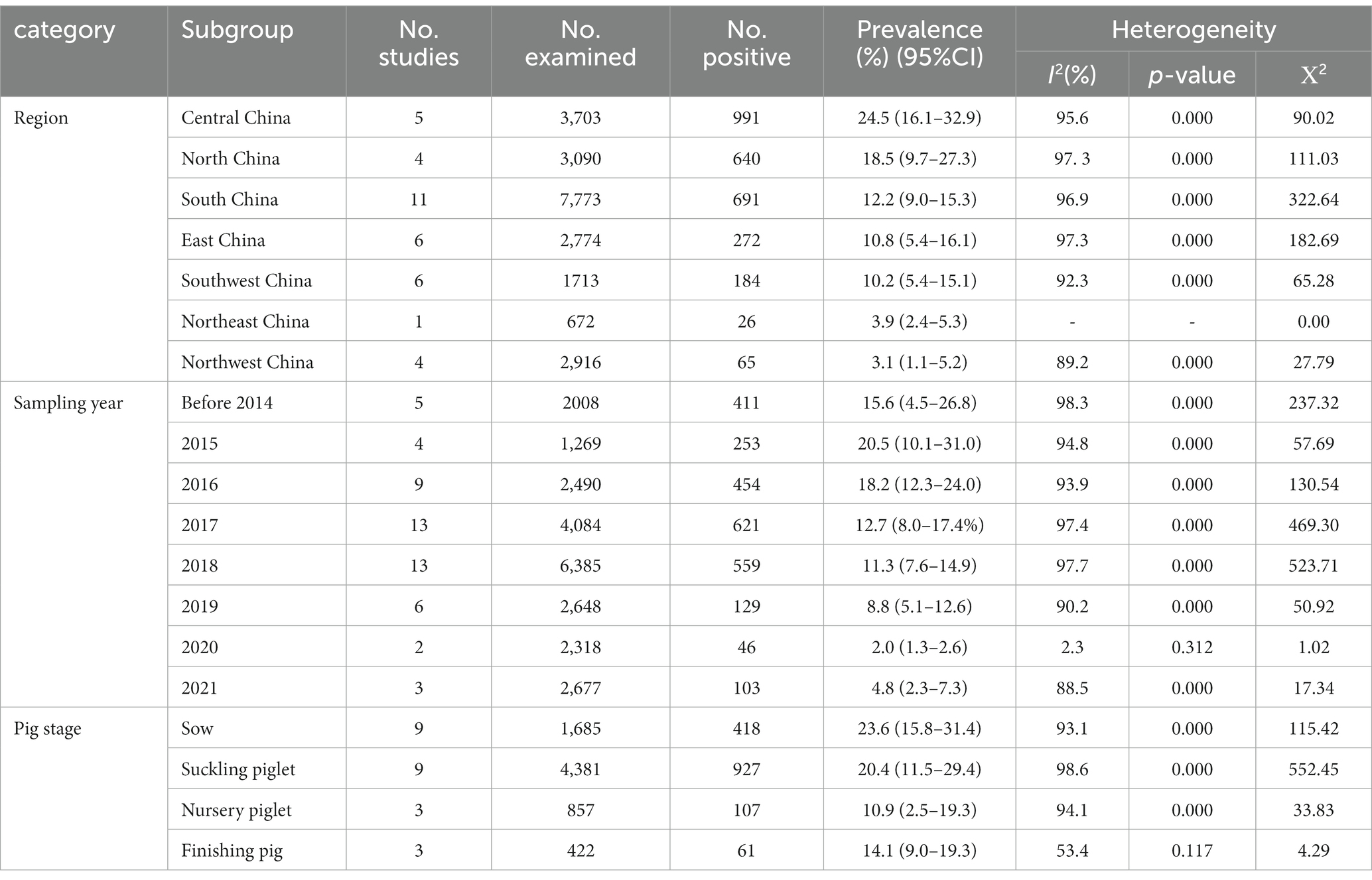
The estimated pooled prevalence of PDCoV infection in pig population from mainland China was 12.2% (95%CI, 10.2–14.2%; Table 1; Figure 2). The prevalence rates of PDCoV infection in Central China, North China, and South China were 24.5% (95%CI, 16.1–32.9%), 18.5% (95%CI, 9.7–27.3%), and 12.2% (95%CI, 9.0–15.3%), respectively. These rates were higher than those in other administrative regions (Figure 3; Table 2). By contrast, the PDCoV positive rates in Northeast China and Northwest China regions were low with percentages of 3.9% (95% CI, 2.4–5.3%) and 3.1% (95% CI, 1.1–5.2%), respectively (Figure 3). Among the 39 studies, 28 reported coinfections. Coinfection diarrhea viruses included PEDV, transmissible gastroenteritis virus (TGEV), porcine rotavirus (PoRV), porcine kobuvirus, swine acute diarrhea syndrome coronavirus, and porcine astrovirus; the coinfection rate accounted for 13.6–100% of the PDCoV infection rate (Table 1).
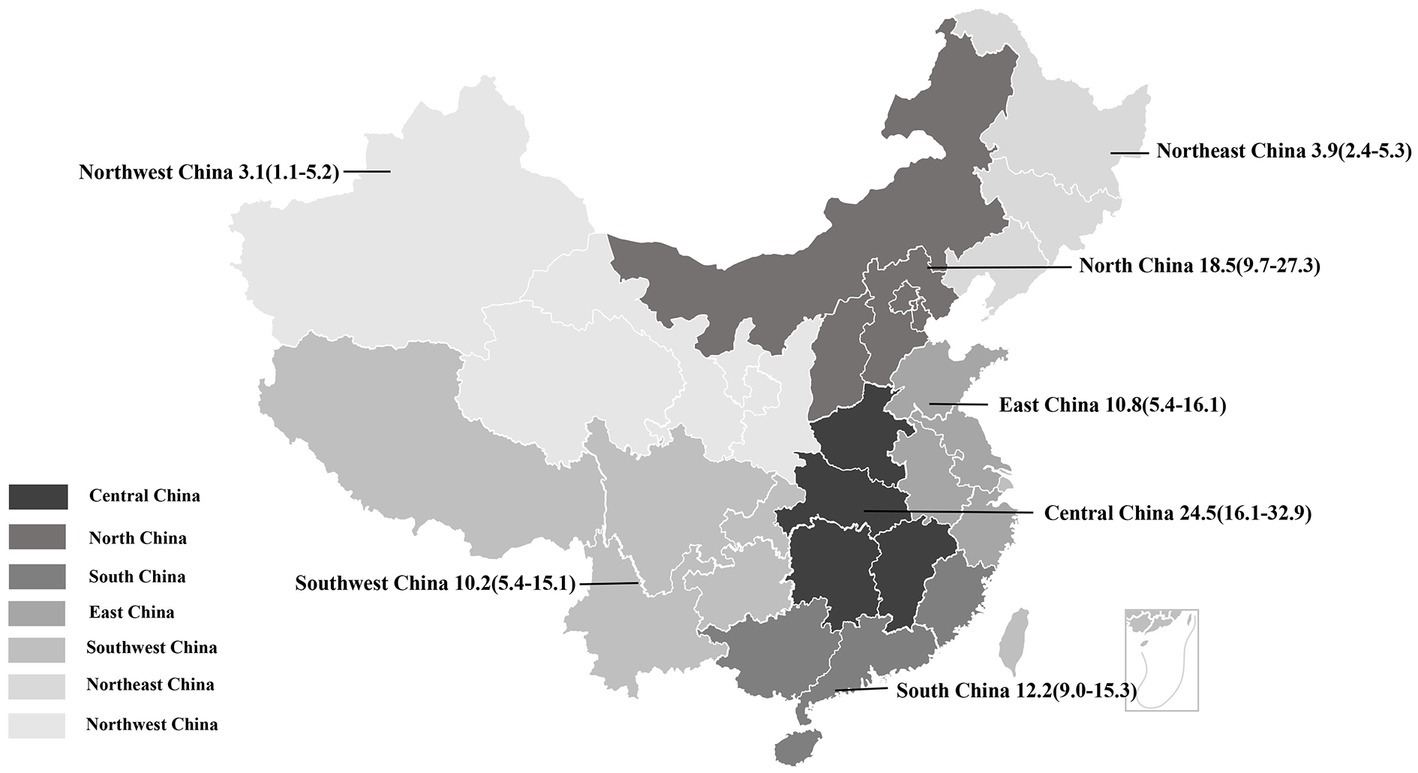
Figure 3. Geographical distribution of PDCoV infection among pigs in China. Pooled prevalence rate (%) and 95%CI are shown for each district.
Subgroup analysis
All subgroup analyses included sampling region, sampling date, pig stage, and clinical signs (diarrhea). Among the seven administrative regions of China, the estimated prevalence of PDCoV infection in pigs in Central China was the highest at 24.5% (95%CI, 16.1–32.9%), and that of Northwest region was the lowest at 3.1% (95% CI, 1.1–5.2%; Table 2; Figure 3). During 2014–2021, the estimated prevalence of PDCoV infection was the highest in 2015 at 20.5% (95%CI, 10.1–31.0%) and the lowest in 2021 at 4.8% (95%CI, 2.3–7.3%), showing a downward trend (Table 2). The prevalence rates of PDCoV infection in sows and suckling piglets were 23.6% (95%CI, 15.8–31.4%) and 20.4% (95%CI, 11.5–29.4%), respectively, which were significantly higher than those in nursery piglets and finishing pigs (Table 2). The prevalence of PDCoV infection was significantly associated with sampling region, sampling date, pig stage, and clinical signs (diarrhea) but was insignificantly associated with detection method and target gene.
Publication bias and sensitivity analysis
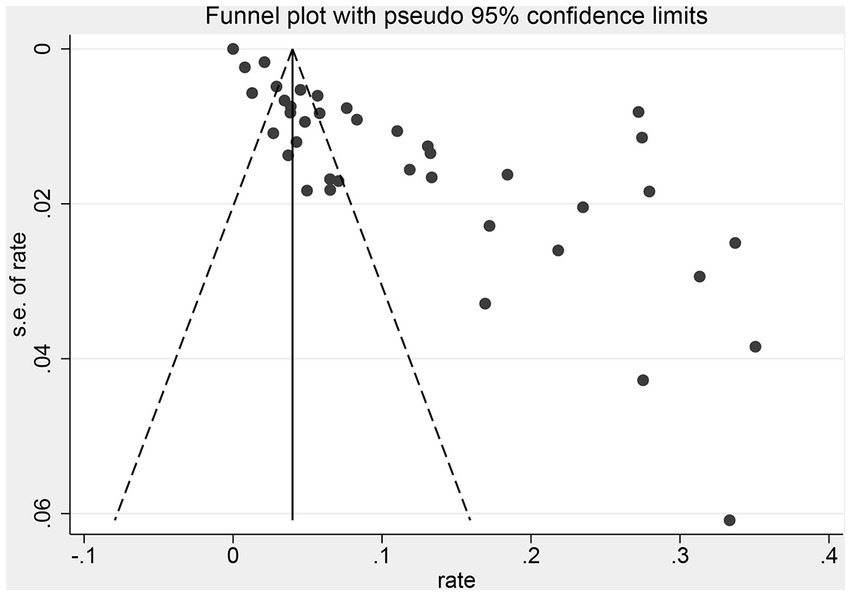
The funnel forest plot was used to measure and illustrate the degree of publication bias of selected studies. The funnel plot was asymmetrical to the overall prevalence (Figure 4), suggesting significant bias in the studies selected for our analysis. A sensitivity analysis was conducted by excluding one study each time to determine whether modification of the inclusion criteria for the meta-analysis would affect the final results. All results were insignificantly changed (data not shown).
Discussion
Coronavirus endangers human and animal health and thus causes serious public health problems and huge economic losses. PEDV and TGEV, which belong to the genus Alphacoronavirus, are two major diarrheal pathogens endangering the pig industry. Severe acute respiratory syndrome coronavirus (SARS), middle east respiratory syndrome coronavirus, and SARS-CoV-2, which belong to the genus Betacoronavirus, have caused three pandemics in human history (70–72). Infectious bronchitis virus, which belongs to the genus Gammacoronavirus, is the main pathogen of respiratory diseases in poultry industry. DCoV is the fourth coronavirus genus formally classified by the International Committee on Taxonomy of Viruses in 2012. PDCoV diarrhea broke out in the USA for the first time in 2014, causing significant economic losses in the American swine industry, and then spread across many countries of Asia and America.
This study is the first meta-analysis and systematic review of PDCoV infection in pig herds in China. Studies on PDCoV infection in pigs from 25 provinces in China were included, all of which were cross-sectional. The pooled prevalence of PDCoV in China reached 12.2%, which indicated that PDCoV occurs extensively in Chinese pig herds. Coinfection with other enteric pathogens was common among PDCoV-positive samples. Among these pathogens, PEDV, TGEV, and PoRV had the highest frequency of coinfection. This situation implied that the current causes of diarrhea among Chinese pig populations are complex and diverse, and coinfection may cause severe clinical symptoms.
Several molecular and immunological methods have been developed to detect PDCoV. Among the molecular methods, specific RT-PCR remains the ideal choice for detection of PDCoV. Immunological methods can determine previous exposure to PDCoV and define antibody responses to infection and vaccination. Among the included papers, 35 used RT-PCR method (5 papers used rRT-PCR) and 4 utilized ELISA method. In clinical diagnostic testing, S, M, and N genes are the most commonly used diagnostic targets for PDCoV infection.
Subgroup analyzes were performed by sampling region, sampling date, and pig stage. From the perspective of geographical distribution, PDCoV was ubiquitous in pig populations in China and has large regional differences. The prevalence rates of PDCoV infection in Northeast China and Northwest China were comparatively low. On the contrary, the prevalence rates in Central China, North China, and South China were high possibly due to the large amount of pig production, high frequency of pig transport, and high humidity of climate in these regions. From the perspective of time distribution, epidemic reports have been available every year since the first report of the epidemic in mainland China in 2015. In the 1st year of the initial outbreak of the epidemic, the prevalence of PDCoV was the highest at 20.5%. Thereafter, it gradually stabilized and reached 4.8% in 2021. This situation showed that PDCoV is still prevalent in pigs in China and remains an important pathogen of porcine diarrheal disease. In terms of infected pigs, PDCoV can infect pigs of all ages. However, the clinical condition is severe in piglets. Our review found that the prevalence of PDCoV infection was significantly higher in sows (23.6%) and suckling pigs (20.4%) than in nursery (10.9%) and finishing pigs (14.1%). These results suggested that piglets are at greater infection risk, leading to high mortality from PDCoV than those of adult pigs. Moreover, the transmission of presence of virus in sows cannot be ignored.
In summary, this review reflects the trend of PDCoV infection prevalence in swine populations in China. However, this meta-analysis has certain limitations. For example, sample sizes were low in some regions (or low sample sizes were reported in certain cases). Analysis was also limited to date of sampling, geographic location, gene of interest, pig stage, and clinical signs. Other potentially influential factors, such as farm size, breed, and sampling season of pigs, were not analyzed. All data were from pigs with diarrhea. Additional samples of healthy pigs are suggested to be included to assess the infection of PDCoV in pigs in China. The abovementioned factors should be considered when conducting epidemiological studies in the future.
Conclusion
Our meta-analysis shows a high prevalence (12.4%) of PDCoV infection in Chinese pig herds. The prevalence rate is significantly associated with sampling region, sampling year, pig stage, and clinical signs in pigs (diarrhea). Therefore, biosecurity prevention and control should be strengthened to reduce the spread of PDCoV between regions. Climate, such as humidity and temperature, correlates with the breakout of PDCoV, Thus, this study recommends to keep pig house dry and warm. Surveillance of PDCoV and detection of other diarrhea pathogens should be strengthened in suckling piglets and sows due to high morbidity in suckling piglets and high virus-carrying rate in sows. The prevalence of PDCoV shows a downward trend; however, consideration of susceptibility of coronavirus to mutation, recombination, and cross-species transmission and continuous surveillance studies in swine remain essential (including non-diarrheal swine) to monitor the geographical spread and incidence trend of PDCoV and detect the genetic evolution.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JS conceptualized the paper and wrote the manuscript. JS, QZ and JZ collected and analyzed the data. CZ and ZL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2022YFD1800801-02); the Science and Technology Planning Project of Guangzhou (grant numbers 202002030456 and 20212100050); the Science and Technology Planning Project of Guangdong Province (grant number 2021B1212050021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, L, Byrum, B, and Zhang, Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg Infect Dis. (2014) 20:1227–30. doi: 10.3201/eid2007.140296
2. Ma, Y, Zhang, Y, Liang, X, Lou, F, Oglesbee, M, Krakowka, S, et al. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio. (2015) 6:e00064. doi: 10.1128/mBio.00064-15
3. Li, W, Hulswit, R, Kenney, SP, Widjaja, I, Jung, K, Alhamo, MA, et al. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc Natl Acad Sci U S A. (2018) 115:E5135–43. doi: 10.1073/pnas.1802879115
4. Jung, K, Hu, H, and Saif, LJ. Calves are susceptible to infection with the newly emerged porcine deltacoronavirus, but not with the swine enteric alphacoronavirus, porcine epidemic diarrhea virus. Arch Virol. (2017) 162:2357–62. doi: 10.1007/s00705-017-3351-z
5. Boley, PA, Alhamo, MA, Lossie, G, Yadav, KK, Vasquez-Lee, M, Saif, LJ, et al. Porcine Deltacoronavirus infection and transmission in poultry, United States(1). Emerg Infect Dis. (2020) 26:255–65. doi: 10.3201/eid2602.190346
6. Lednicky, JA, Tagliamonte, MS, White, SK, Elbadry, MA, Alam, MM, Stephenson, CJ, et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature. (2021) 600:133–7. doi: 10.1038/s41586-021-04111-z
7. González, JM, Gomez-Puertas, P, Cavanagh, D, Gorbalenya, AE, and Enjuanes, L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch Virol. (2003) 148:2207–35. doi: 10.1007/s00705-003-0162-1
8. Adams, MJ, and Carstens, EB. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2012). Arch Virol. (2012) 157:1411–22. doi: 10.1007/s00705-012-1299-6
9. Woo, PC, Lau, SK, Lam, CS, Lau, CC, Tsang, AK, Lau, JH, et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. (2012) 86:3995–4008. doi: 10.1128/JVI.06540-11
10. Li, G, Chen, Q, Harmon, KM, Yoon, KJ, Schwartz, KJ, Hoogland, MJ, et al. Full-length genome sequence of porcine Deltacoronavirus strain USA/IA/2014/8734. Genome Announc. (2014) 2:e00278–14. doi: 10.1128/genomeA.00278-14
11. Lee, S, and Lee, C. Complete genome characterization of Korean porcine Deltacoronavirus strain KOR/KNU14-04/2014. Genome Announc. (2014) 2:e01191–14. doi: 10.1128/genomeA.01191-14
12. Fang, P, Fang, L, Liu, X, Hong, Y, Wang, Y, Dong, N, et al. Identification and subcellular localization of porcine deltacoronavirus accessory protein NS6. Virology. (2016) 499:170–7. doi: 10.1016/j.virol.2016.09.015
13. Choi, S, and Lee, C. Functional characterization and proteomic analysis of porcine Deltacoronavirus accessory protein NS7. J Microbiol Biotechnol. (2019) 29:1817–29. doi: 10.4014/jmb.1908.08013
14. He, WT, Ji, X, He, W, Dellicour, S, Wang, S, Li, G, et al. Genomic epidemiology, evolution, and transmission dynamics of porcine Deltacoronavirus. Mol Biol Evol. (2020) 37:2641–54. doi: 10.1093/molbev/msaa117
15. Saeng-chuto, K, Lorsirigool, A, Temeeyasen, G, Vui, DT, Stott, CJ, Madapong, A, et al. Different lineage of porcine Deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound Emerg Dis. (2017) 64:3–10. doi: 10.1111/tbed.12585
16. Duan, C . An updated review of porcine Deltacoronavirus in terms of prevalence, pathogenicity, pathogenesis and antiviral strategy. Front Vet Sci. (2021) 8:811187. doi: 10.3389/fvets.2021.811187
17. Zhang, Y, Cheng, Y, Xing, G, Yu, J, Liao, A, du, L, et al. Detection and spike gene characterization in porcine deltacoronavirus in China during 2016-2018. Infect Genet Evol. (2019) 73:151–8. doi: 10.1016/j.meegid.2019.04.023
18. Forni, D, Cagliani, R, Clerici, M, and Sironi, M. Molecular evolution of human coronavirus genomes. Trends Microbiol. (2017) 25:35–48. doi: 10.1016/j.tim.2016.09.001
19. Guo, J, Fang, L, Ye, X, Chen, J, Xu, S, Zhu, X, et al. Evolutionary and genotypic analyses of global porcine epidemic diarrhea virus strains. Transbound Emerg Dis. (2019) 66:111–8. doi: 10.1111/tbed.12991
20. Lau, S, Wong, E, Tsang, CC, Ahmed, SS, Au-Yeung, RKH, Yuen, K-Y, et al. Discovery and sequence analysis of four deltacoronaviruses from birds in the middle east reveal interspecies jumping with recombination as a potential mechanism for avian-to-avian and avian-to-mammalian transmission. J Virol. (2018) 92:e00265–18. doi: 10.1128/JVI.00265-18
21. Tao, Y, Shi, M, Chommanard, C, Queen, K, Zhang, J, Markotter, W, et al. Surveillance of bat coronaviruses in Kenya identifies relatives of human coronaviruses NL63 and 229E and their recombination history. J Virol. (2017) 91:e01953–16. doi: 10.1128/JVI.01953-16
22. Sung, MH, Deng, MC, Chung, YH, Huang, YL, Chang, CY, Lan, YC, et al. Evolutionary characterization of the emerging porcine epidemic diarrhea virus worldwide and 2014 epidemic in Taiwan. Infect Genet Evol. (2015) 36:108–15. doi: 10.1016/j.meegid.2015.09.011
23. Song, D, Zhou, X, Peng, Q, Chen, Y, Zhang, F, Huang, T, et al. Newly emerged porcine Deltacoronavirus associated with diarrhoea in swine in China: identification, prevalence and full-length genome sequence analysis. Transbound Emerg Dis. (2015) 62:575–80. doi: 10.1111/tbed.12399
24. Suzuki, T, Shibahara, T, Imai, N, Yamamoto, T, and Ohashi, S. Genetic characterization and pathogenicity of Japanese porcine deltacoronavirus. Infect Genet Evol. (2018) 61:176–82. doi: 10.1016/j.meegid.2018.03.030
25. Pérez-Rivera, C, Ramírez-Mendoza, H, Mendoza-Elvira, S, Segura-Velázquez, R, and Sánchez-Betancourt, JI. First report and phylogenetic analysis of porcine deltacoronavirus in Mexico. Transbound Emerg Dis. (2019) 66:1436–41. doi: 10.1111/tbed.13193
26. Jang, G, Lee, KK, Kim, SH, and Lee, C. Prevalence, complete genome sequencing and phylogenetic analysis of porcine deltacoronavirus in South Korea, 2014-2016. Transbound Emerg Dis. (2017) 64:1364–70. doi: 10.1111/tbed.12690
27. Vicente-Huaman, J, and Gomez-Quispe, OE. Evaluation of a porcine deltacoronavirus eradication program in a full-cycle pig farm in Peru. J Adv Vet Anim Res. (2021) 8:300–6. doi: 10.5455/javar.2021.h515
28. Ajayi, T, Dara, R, Misener, M, Pasma, T, Moser, L, and Poljak, Z. Herd-level prevalence and incidence of porcine epidemic diarrhoea virus (PEDV) and porcine deltacoronavirus (PDCoV) in swine herds in Ontario, Canada. Transbound Emerg Dis. (2018) 65:1197–207. doi: 10.1111/tbed.12858
29. Dong, N, Fang, L, Zeng, S, Sun, Q, Chen, H, and Xiao, S. Porcine Deltacoronavirus in mainland China. Emerg Infect Dis. (2015) 21:2254–5. doi: 10.3201/eid2112.150283
30. Su, M, Li, C, Guo, D, Wei, S, Wang, X, Geng, Y, et al. A recombinant nucleocapsid protein-based indirect enzyme-linked immunosorbent assay to detect antibodies against porcine deltacoronavirus. J Vet Med Sci. (2016) 78:601–6. doi: 10.1292/jvms.15-0533
31. Mai, K, Feng, J, Chen, G, Li, D, Zhou, L, Bai, Y, et al. The detection and phylogenetic analysis of porcine deltacoronavirus from Guangdong Province in southern China. Transbound Emerg Dis. (2018) 65:166–73. doi: 10.1111/tbed.12644
32. Zhai, SL, Wei, WK, Li, XP, Wen, XH, Zhou, X, Zhang, H, et al. Occurrence and sequence analysis of porcine deltacoronaviruses in southern China. Virol J. (2016) 13:136. doi: 10.1186/s12985-016-0591-6
33. Luo, SX, Fan, JH, Opriessnig, T, di, JM, Liu, BJ, and Zuo, YZ. Development and application of a recombinant M protein-based indirect ELISA for the detection of porcine deltacoronavirus IgG antibodies. J Virol Methods. (2017) 249:76–8. doi: 10.1016/j.jviromet.2017.08.020
34. Wang, M, Wang, Y, Baloch, AR, Pan, Y, Tian, L, Xu, F, et al. Detection and genetic characterization of porcine deltacoronavirus in Tibetan pigs surrounding the Qinghai-Tibet plateau of China. Transbound Emerg Dis. (2018) 65:363–9. doi: 10.1111/tbed.12819
35. Jia, S, Feng, B, Wang, Z, Ma, Y, Gao, X, Jiang, Y, et al. Dual priming oligonucleotide (DPO)-based real-time RT-PCR assay for accurate differentiation of four major viruses causing porcine viral diarrhea. Mol Cell Probes. (2019) 47:101435. doi: 10.1016/j.mcp.2019.101435
36. Zhang, F, Luo, S, Gu, J, Li, Z, Li, K, Yuan, W, et al. Prevalence and phylogenetic analysis of porcine diarrhea associated viruses in southern China from 2012 to 2018. BMC Vet Res. (2019) 15:470. doi: 10.1186/s12917-019-2212-2
37. Zhang, H, Liang, Q, Li, B, Cui, X, Wei, X, Ding, Q, et al. Prevalence, phylogenetic and evolutionary analysis of porcine deltacoronavirus in Henan province, China. Prev Vet Med. (2019) 166:8–15. doi: 10.1016/j.prevetmed.2019.02.017
38. Feng, Y, Xu, Z, and Zhu, L. Prevalence and phylogenetic analysis of porcine deltacoronavirus in Sichuan province, China. Arch Virol. (2020) 165:2883–9. doi: 10.1007/s00705-020-04796-z
39. Shi, Y, Li, B, Tao, J, Cheng, J, and Liu, H. The complex co-infections of multiple porcine Diarrhea viruses in local area based on the Luminex xTAG multiplex detection method. Front Vet Sci. (2021) 8:602866. doi: 10.3389/fvets.2021.602866
40. Li, C, Lu, H, Geng, C, Yang, K, Liu, W, Liu, Z, et al. Epidemic and evolutionary characteristics of swine enteric viruses in south-Central China from 2018 to 2021. Viruses. (2022) 14:1420. doi: 10.3390/v14071420
41. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
42. Atkins, D, Best, D, Briss, PA, Eccles, M, Falck-Ytter, Y, Flottorp, S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
43. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
44. Borenstein, M, Hedges, LV, Higgins, JP, and Rothstein, HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
45. Ren, Y, Zhang, B, Tang, C, Cao, GM, and Yue, H. Development and application of a multiplex RT-PCR for detecting PEDV, TGEV and PDCoV. Chin Vet Sci. (2016) 46:756–62. doi: 10.16656/j.issn.1673-4696.2016.06.014 (In Chinese).
46. Zhang, F, Song, D, Zhou, X, Huang, D, Li, A, Peng, Q, et al. Establishment and application of a RT-PCR assay for detection of newly emerged porcine deltacoronavirus. Sci Agric Sin. (2016) 49:1408–16. doi: 10.3864/j.issn.0578-1752.2016.07.016 (In Chinese).
47. Peng, Y, Li, H, Zhang, B, Zhang, B, Li, J, Shen, S, et al. Infection detection of PEDV, PDCoV and GARV in pig farms of Liangshan prefecture. Sichuan An Vet Sci. (2017) 323:18–22. (In Chinese)
48. Zhou, L, Chen, G, Wu, Z, Mai, K, Li, D, Wang, S, et al. Investigation on porcine diarrhea pathogens in Guangdong province from 2016 to 2017. Chin J Vet Med. (2017) 53:3–10. (In Chinese)
49. Liu, H, Huang, X, Li, C, ZhiPeng, L, YingYing, W, YuJia, Z, et al. Establishment and application of RT-PCR detecting porcine deltacoronavirus (PDCoV). J Agric Biotechnol. (2018) 26:1631–8. doi: 10.3969/j.issn.1674-7968.2018.09.017 (In Chinese).
50. Luo, S, Fan, J, Liu, B, QianKai, S, LinShan, H, and YuZhu, Z. Establishment and application of the real-time reverse transcription quantitative PCR assay for porcine epidemic diarrhea virus and porcine deltacoronavirus. Acta Vet Zoo Sinica. (2018) 49:852–8. doi: 10.11843/j.issn.0366-6964.2018.04.025 (In Chinese).
51. Shan, Y, Liu, Z, Shi, X, Guowei, L, Cong, C, Hao, L, et al. Molecular characteristic analysis of porcine epidemic diarrhea virus S gene in Zhejiang and surrounding areas. J Zhejiang Univ. (2018) 44:610–8. doi: 10.3785/j.issn.1008-9209.2017.06.231 (In Chinese).
52. Song, Y, Xu, S, Su, D, Jian, S, and DongSheng, H. Epidemiological survey and analysis of porcine deltacoronavirus in Guangdong province from 2012 to 2016. Chin J Prevent Vet Med. (2018) 40:886–90. doi: 10.3969/j.issn.1008-0589.201703051 (In Chinese).
53. Xu, L, Li, J, Su, F, Bin, Y, Sai, W, RuiYu, T, et al. Epidemiological investigation on the viral diarrhea in pigs in Zhejiang Province during 2011-2017. Chin Vet Sci. (2018) 48:625–30. doi: 10.16656/j.issn.1673-4696.2018.0085 (In Chinese).
54. Feng, Y, Yin, X, Xu, L, XiaoYu, Y, LingHua, L, ZhiWen, X, et al. Detection and genetic analysis of PDCoV in Sichuan from 2017 to 2018. Chinese Journal of Preventive Veterinary Medicine. (2019) 41:1059–71. doi: 10.3969/j.issn.1008-0589.201901042 (In Chinese).
55. He, Y, Qin, Y, Duan, Q, Zhang, Y, Lu, B, Hu, T, et al. Epidemiological investigation of major viral diarrhea diseases during 2013-2018 on some scale pig farms in Guangxi province. Chin J An Infect Dis. (2019) 27:86–93. (In Chinese)
56. Hou, L, Jia, J, Gu, W, Baojing, L, Qiankai, S, Guangfu, Y, et al. Establishment and application of an indirect ELISA based on recombinant S1 protein for the detection of antibodies against porcine deltacoronavirus. Acta Vet Zoo Sinica. (2019) 50:1642–8. doi: 10.11843/j.issn.0366-6964.2019.08.013 (In Chinese).
57. Lu, C, Tian, X, Li, F, Ren, W, Zheng, L, Zhang, L, et al. Epidemiological investigation of major viral diarrhea pathogens during 2016-2018 on scale pig farms in Tianjin district. Heilongjiang An Sci Vet Med. (2019) 22:90–4. (In Chinese)
58. Duan, Q, Li, X, Zhao, S, Yibin, Q, Bingxia, L, Bin, L, et al. Epidemiological investigation on Diarrhea viral disease of piglets in some pig farms in Guangxi during 2016 to 2019. China An Husb Vet Med. (2020) 47:564–74. doi: 10.16431/j.cnki.1671-7236.2020.02.028 (In Chinese).
59. Feng, Y, Yin, X, Yang, X, Lei, X, Zhiwen, X, and Ling, Z. Establishment and application of an indirect ELISA based on recombinant M protein against porcine Deltacoronavirus. Acta Vet Zoo Sinica. (2020) 51:1710–8. doi: 10.11843/j.issn.0366-6964.2020.07.023 (In Chinese).
60. Ma, Y, Li, B, Tao, J, Chen, J, Liu, L, Liu, H, et al. Epidemiological investigation and M gene sequence analysis of porcine deltacoronavirus. Acta Agric Shanghai. (2020) 36:71–6. doi: 10.15955/j.issn1000-3924.2020.01.12 (In Chinese).
61. Shi, K, Yin, Y, Wang, X, Shouyu, X, Yongsheng, S, Hongmei, L, et al. Epidemiological investigation of major porcine viral diseases in Guangxi during 2018. China An Husb Vet Med. (2020) 47:174–81. doi: 10.16431/j.cnki.1671-7236.2020.01.021 (In Chinese).
62. Yan, J, Shi, K, Liu, H, Xie, S, Qin, Y, Li, Z, et al. Epidemiological investigation of major porcine viral Diarrhea pathogens in Guangxi from 2017 to 2019. J Guangxi Agric. (2020) 35:20–5. (In Chinese)
63. Chang, X, Zhou, J, Yin, J, Beibei, N, Baochao, F, Rongli, G, et al. Investigation on pathogens of major viral diarrhea in pig farms in East China from 2017 to 2019. Acta Vet Zoo Sinica. (2020) 51:3141–50. doi: 10.11843/j.issn.0366-6964.2020.12.023 (In Chinese).
64. Li, B, Ding, Q, Wei, X, Zheng, LL, and Wei, ZY. Detection and analysis of PEDV and PDCoV infections in piglets with diarrhea in some areas of Henan province. Progress Vet Med. (2020) 41:17–21. doi: 10.16437/j.cnki.1007-5038.2020.04.004 (In Chinese).
65. Duan, Q, Qin, Y, He, W, Zhao, W, Lu, B, Zhou, Y, et al. Detection of major pathogens leading to porcine viral diarrhea in Guangxi from 2017 to 2021. China An Health Inspection. (2021) 38:1–8. doi: 10.3969/j.issn.1005-944X.2021.10.001 (In Chinese).
66. Li, Y, Cheng, L, Zhang, H, Zhipeng, Z, Du Jiubin, HF, et al. The detection and analysis of porcine viral diarrhea in Xinjiang province during 2018-2019. Chinese Journal of Preventive Veterinary Medicine. (2021) 43:477–500. doi: 10.3969/j.issn.1008-0589.202008014 (In Chinese).
67. Zhu, X, and Wu, X. Establishment of RT-nPCR detection method for porcine deltacoronavirus and analysis of N gene variation. Chin J An Infect Dis. (2021). doi: 10.19958/j.cnki.cn31-2031/s.20210824.007 (In Chinese).
68. Wang, C, Wang, R, Sun, M, Huang, X, Lei, Z, Wang, P, et al. Epidemiological investigation and analysis of four swine diarrhea related viruses in some regions of xiangxi prefecture in the spring of 2021. Swine Prod. (2022). 2:119–121. doi: 10.13257/j.cnki.21-1104/s.2022.02.022 (In Chinese).
69. Wang, C, Mi, Q, and Pi, Z. Detection and analysis of porcine viral diarrhea diseases on scale pig farms in Yilihegu district of Xinjiang province. China Swine Indust. (2022). doi: 10.16174/j.issn.1673-4645.2022.02.016 (In Chinese).
70. Liu, Q., Wang, H., and Zhang, H,, Sui, L., Li, L., Xu, W., du, S., Hao, P., Jiang, Y., Chen, J., Qu, X., Tian, M., Zhao, Y., Guo, X., Wang, X., Song, W., Song, G., Wei, Z., Hou, Z., Wang, G., Sun, M., Li, X., Lu, H., Zhuang, X., Jin, N., Zhao, Y., Li, C., and Liao, M. Proc Natl Acad Sci 2022, 119,:e2123065119, The global succinylation of SARS-CoV-2–infected host cells reveals drug targets, doi: 10.1073/pnas.2123065119
71. Zhao, Y, Sui, L, Wu, P, Wang, W, Wang, Z, Yu, Y, et al. A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Trans Target Ther. (2021) 6:331. doi: 10.1038/s41392-021-00742-w
Keywords: PDCoV, epidemiology, systematic review, meta-analysis, Chinese pig population
Citation: Sun J, Zhang Q, Zhang C, Liu Z and Zhang J (2023) Epidemiology of porcine deltacoronavirus among Chinese pig populations in China: systematic review and meta-analysis. Front. Vet. Sci. 10:1198593. doi: 10.3389/fvets.2023.1198593
Edited by:
Satoshi Ito, Complutense University of Madrid, SpainReviewed by:
Kang Ouyang, Guangxi University, ChinaDeping Song, Jiangxi Agricultural University, China
Copyright © 2023 Sun, Zhang, Zhang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfeng Zhang, MTM2Njg5MzkyOThAMTM5LmNvbQ==
 Junying Sun
Junying Sun Qin Zhang4
Qin Zhang4