- 1Animal Sciences Division, Nuclear Institute for Agriculture and Biology College, Pakistan Institute of Engineering and Applied Sciences (NIAB-C, PIEAS), Faisalabad, Pakistan
- 2Animal Sciences Institute, National Agricultural Research Centre, Islamabad, Pakistan
- 3Nuclear Institute for Agriculture and Biology College, Pakistan Institute of Engineering and Applied Sciences (NIAB-C, PIEAS), Faisalabad, Pakistan
- 4Department of Reproduction and Artificial Insemination, Faculty of Veterinary Medicine, Ataturk University, Erzurum, Türkiye
- 5Livestock and Fisheries Department, Government of Sindh, Karachi, Pakistan
This study investigated the antioxidant effect of quercetin-treated semen on frozen–thawed spermatozoa quality and in-vivo fertility in crossbred Kamori goats. In total, 32 ejaculates from four fertile bucks were diluted in Tris-based egg yolk extender with varying levels of quercetin (0, 1, 5, 10, and 15 μM). Qualified semen samples were pooled and frozen in French straws. The results revealed that the addition of quercetin in the semen extender increased (p < 0.05) frozen–thawed sperm total motility (TM), progressive motility (PM), rapid velocity (RV), average path velocity (VAP), straight line velocity (VSL), curvilinear velocity (VCL), and amplitude of lateral head (ALH) displacement in contrast to the control group. Quercetin supplementation had no effect on beat cross frequency (BCF), straightness (STR), and linearity (LIN) (p > 0.05). Quercetin showed significantly higher (p < 0.05) plasma membrane and acrosome integrity and viability (p < 0.05) of spermatozoa in contrast to the control group. Quercetin in the semen extender significantly increased (p < 0.05) superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and total antioxidant capacity (TAC) levels while reduced (p < 0.05) the contents of total oxidant status (TOS) and malondialdehyde (MDA), which were in contrast to the control group. Ultrasound results revealed that 24 out of 30 (80%) goats were found pregnant when semen was treated with 5 μM quercetin while the control group showed 18 out of 30 (60%) animals were pregnant. Thus, the study concluded that 5 μM quercetin-treated semen was found to be efficient, showed increased antioxidant status, and reduced oxidant production, leading to improved spermatozoa quality and in-vivo fertility in goats.
Introduction
Artificial insemination (AI) is a reproductive biotechnology tool used worldwide for improving animal production. However, it is not being practiced in small ruminants in Pakistan due to factors such as the lower conception rate, the unavailability of goat semen in the market, and a lack of awareness of AI (1, 2). The reluctance to adopt AI is because of the semen cryopreservation process, which reduces sperm viability due to the oxidative attack, resulting in a poor conception rate (3). Oxidative stress can cause deleterious effects on sperm concentration, sperm motility, sperm morphology, and sperm DNA damage, eventually resulting in apoptosis (4, 5). The sperm plasma membrane is a rich source of lipids and is more vulnerable to lipid peroxidation (6). The sperm cells consist of both enzymatic and non-enzymatic antioxidant enzymes, which aid them to protect against oxidative damage. Depleted antioxidant capacity is a presumed indicator of sperm infertility, resulting in a lower conception rate in animals (7). The addition of antioxidants or cryoprotectant media in semen extender improves sperm viability and the motility of fresh and cryopreserved ram spermatozoa (7, 8). The antioxidant capacity of frozen–thawed semen is not sufficient to overcome oxidative damage during the cryopreservation process. However, semen cryopreservation delivers successful results but declines sperm motility by 50% (9).
The cryopreservation process induces temperature alteration in spermatozoa, which makes them vulnerable to cryoinjuries such as ice crystal formation, oxidative stress, and cold shock (10–12). Egg yolks, which help prevent cold shock, are an essential energy source for semen extenders (13). However, the low-freezing potential of buck semen influences the pregnancy rate of artificial insemination (14). Buck sperms have been reported to be vulnerable to cryo-damages due to changes in seminal plasma across different species (15). The presence of phospholipase A causes the breakdown of fatty acids in buck seminal plasma, resulting in sperm lipid peroxidation (16) and the production of oxidants (17). Hence, centrifugation can be performed to eliminate the detrimental effects of seminal plasma interaction with egg yolks (18).
Quercetin is a flavonoid compound, which is well-known for its antioxidant properties, and is present in fruits and vegetables (19). Quercetin is a potent antioxidant that enables scavenging ROS and hydroxyl radicals (19). Moreover, quercetin has exhibited protective effects on fresh and frozen–thawed sperm parameters in diverse species (20–23) and is believed to be involved with α-tocopherol to delay oxidation and activate gene expressions of detoxifying enzymes (24). Antioxidants play a crucial role in the protection of spermatozoa from damage caused by oxidants, bacteria, leukocytes, and abnormal oxidants produced by sperm (25). Hence, cryoprotective media are subjected to be included in different antioxidants to optimize sperm cryo-survival (26, 27). Therefore, antioxidants, such as those with non-enzymatic and scavenging activities, are routinely employed in cryo-protective media (28, 29). The addition of quercetin to the semen extender provides a beneficial antioxidant effect on post-thawed semen attributes in rams (30) and bucks (31).
The application of estrus synchronization protocols during the on/off seasons improves fertility and allows kidding in goats at the desired period of the year (32, 33). In Pakistan, goats are bred through natural breeding due to the unavailability of goat semen units. Furthermore, AI services utilizing superior bucks are also negligible in government farms (34). The short and long-term protocols of estrus synchronization have been employed in cycling and non-cycling goats (35) with 40–80% fertility rates. Our locally developed polyurethane-based medroxyprogesterone acetate (MAP) sponges are cost-effective and offer precise estrus synchronization. Our previous studies on MAP sponges have exhibited promising results of MAP sponges in buffaloes, goats, and sheep (36–38). This study was designed to investigate the different levels of quercetin in semen extender on frozen–thawed spermatozoa quality and in-vivo fertility in crossbred Kamori goats.
Materials and methods
Ethical statement
The experiment was conducted following the guidelines of the Institutional Animal Care and Use Committee of the Animal Sciences Division, Nuclear Institute for Agriculture and Biology, Faisalabad, Pakistan, under the number NIAB-C-PIEAS-0023.
Chemicals
The chemicals used in this study were procured from Sigma-Aldrich Chemical Co., USA and Merck, Darmstadt, Germany.
Animal selection and management
The study was carried out at an animal Farm in the Nuclear Institute for Agriculture and Biology (NIAB), located nearly 7 Km from the center of Faisalabad city, Pakistan (longitude 73.0791° E and latitude 31.4287° N) with an altitude of 184 m above the sea level. The average temperature and rainfall were 26°C and 19.66 mm, respectively.
Four adult, fertile, healthy bucks of the Kamori breed, aged 8–12 months, weighing about 38 ± 0.8 kg were selected from Nawabshah, Sanghar, and Matiari districts of Sindh province, Pakistan. After purchasing, fertile bucks were kept in quarantine for 30 days to acclimatize and potentially identify any underlying diseases. Bucks were provided free grazing pasture for a period of 3–5 h/day, and green fodder was mixed with wheat bran, berseem, and concentrates (barley, wheat, oat, sorghum, rice, wheat bran, polished rice, molasses, and soybean meal) and supplemented at 250 grams/buck based on their body weight. Proper vaccination and deworming were done before the initiation of the experiment. Animals had ad libitum access to water.
Semen collection, dilution, and freezing
Before the initiation of the experiment, bucks were properly trained with a teaser, and semen collection was carried out at an animal farm in the Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan. Ejaculations (n = 32) from four active bucks (8 ejaculations/buck) were obtained through a caprine artificial vagina (IMV Technologies, France) during October and November 2023. Semen collection was performed every week for consecutive 4 weeks. After collection, the semen was brought to the laboratory and maintained at 37°C before evaluation. The motility (%) was assessed under a phase-contrast microscope (CX43, Olympus, Japan) at a magnification of X 40. The semen samples that had 3–4 × 109 sperm/ml determined through the Neubauer chamber (Germany) and showed greater than 75% motility were processed for further experimentation. The centrifugation was performed at 315 g for 3 min for the removal of seminal plasma (39). Thereafter, semen was divided into five aliquots and diluted with a Tris-egg yolk extender composed of tris of 3.07 g, citric acid of 1.64 g, fructose of 1.26 g, streptomycin of 100 mg, fresh egg yolk of 15% and glycerol of 5% to make the final volume of 100 mL, pH 6.8–7.2, and 425 mOsm (40). Then different levels of quercetin (0, 1, 5, 10, and 15 μM) were used in an extender to make five aliquots of semen conceded in a single step at 37°C and adjusted up to 50 × 106 motile sperm/0.5 mL. The sperm concentration was evaluated using a hemocytometer (Neubauer chamber, Germany). Consequently, antioxidant-treated diluted semen was cooled at 5°C. The semen was aspirated into 0.5 mL French straws, sealed with straw sealer, and then equilibrated at 5°C for 4 h. Following equilibration, the semen straws were frozen in liquid nitrogen vapor (4 cm above the liquid nitrogen) for 15 min and then kept in liquid nitrogen for storage. Frozen semen was thawed using a digital thawing jug at 37°C for 30 s for freeze–thaw evaluation.
Freeze–thaw sperm evaluation
Sperm kinematics analysis
Cell Motion Analyzer (CEROS, Hamilton Thorne Biosciences, Beverly, USA) was used to evaluate the characteristics of sperm motion. The CASA system was programmed to the following settings: frames acquired, 30; frame rate, 60 Hz; minimum contrast, 56; minimum cell size, 5 pixels; minimum static contrast, 30; straightness threshold, 80%; average path velocity cutoff, 20 μm/s; progressive minimum average path velocity, 80 μm/s; curvilinear velocity cutoff, 0 μm/s; cell size, 2 pixels; cell intensity, 50; static head size, 0.71–10.00, and static head intensity, 0.79–1.41. A preheated slide (37°C) was loaded with 5 μL of semen sample and microscopic fields with the best visual motility. The microscopic image showed at least a count of 200 spermatozoa under magnification of × 910 in two to five fields. The motility parameters were the percentage (%) of motile and progressive motile spermatozoa. Velocity parameters of average path velocity (1 μm/s), straight line velocity (straight line velocity; 1 μm/s), and curvilinear velocity (1 μm/s) and frequency parameters of the amplitude of lateral head displacement (μm), beat cross frequency (Hz), straightness (STR, %), and linearity (LIN, %) were recorded. A description of these parameters of CASA was described in the previous publication (41).
Functional plasma membrane integrity (PMI)
The sperm-vital plasma membrane integrity was analyzed following the protocol of Khalil Ur Rehman et al. (42). Approximately, 200 spermatozoa were measured using a phase-contrast microscope (magnification of x 400; BH2 Olympus, Japan). The intact spermatozoa had a white head and coiled tails; however, stained spermatozoa had uncoiled tails and showed a pinkish appearance in the sperm head.
Acrosomal integrity
Trypan blue-Geimsa staining assay was conducted to examine the sperm viability and acrosome integrity (43, 44). Nearly, 200 spermatozoa per sample were examined under a phase-contrast microscope (magnification, x 400). The spermatozoa with an un-stained head and the purple acrosomal region for viable with intact acrosome and the blue head region and the pale lavender acrosomal region for non-viable and non-intact acrosome were observed.
Viability
A 5 μL of frozen–thawed semen samples was employed and mixed with 5 μL of eosin-nigrosin stain using a micropipette tip. A smear was made, air dried, and examined by using a phase-contrast microscope with X 40 (CX 43, Olympus, Japan) magnification. The image showed that sperms with pink heads were dead, and sperms with white heads were alive.
DNA integrity (DI)
The DI was determined by the Acridine Orange (AO) assay. Briefly, a smear of 100 μL of semen sample was prepared using a clean glass slide and air dried. The smear was submersed in fresh Carnoy’s solution (methanol and glacial acetic acid in a 3:1 ratio) for 2 h. After the fixation, the dried smear was incubated in tampon solution (0.1 M of citric acid and 0.3 M of Na2HPO4 were added in the ratio of 16:1; pH 2.5) for 7 min at 60°C. Furthermore, the smear was rinsed by using water and stained (in the dark) with AO stain (1,000 μg/mL stock solution using distilled water; Sigma Aldrich, St. Louis, MO, USA) for 5 min. Then the slide was rinsed, and the coverslip was placed on the wet slide. The stained slides were evaluated immediately under a fluorescent microscope (magnification × 400; Nikon Optiphot, Japan; 480/550 nm excitation/barrier filter) and with an excitation filter (460–570 nm) and an emission filter (460–610 nm). Each microscopic field was observed for a maximum period of 25 s. Approximately, 350 sperm per smear were examined for DNA integrity. Sperm having intact DNA emitted green fluorescence, while fragmented DNA emitted yellowish-orange-red fluorescence.
The detection of antioxidant/oxidant enzymes
Four straws per group, each having 0.5 mL French straws (50 × 106 sperm concentration), were subjected to thawing at 37°C for 30 s and centrifuged at 1000 x g at 4°C for 10 min. The pellet was centrifuged again at 1000 x g for 10 min after being washed in 50 mM potassium phosphate buffer (pH 7.0) to a concentration of 1 × 108 spermatozoa/ml. Then, samples were sonicated at a 15 s interval for 2 min (Elma E 30 (H), D-78224 Singen, Germany) and subjected to the following parameters.
Superoxide dismutase
The assay solution for the inhibition of nitroblue tetrazolium (NBT) consists of 50 mM phosphate buffer (45) of pH 7.8, 13 mM of L-methionine, 57 μM of NBT, 0.004% of riboflavin, 0.025% of triton, and 50 μL of sample to make up the volume of 3 mL. The photoreaction was conceded out under the 15 W and the absorbance was recorded at 560 nm in the spectrophotometer. SOD activity was measured in one unit as the amount of enzyme required to inhibit a 50% reduction of NBT.
Peroxidase activity
The guaiacol peroxidase (POD) measurement was conducted using sperm cells homogenized in a 50 mM potassium phosphate buffer with pH 7.0, 0.1 mM of ethylenediaminetetraacetic acid (EDTA), and 1 mM of dithiothreitol (DTT). The POD activity was estimated using the protocol of Hameed et al. (46) with minor modifications. The 3 mL solution consisted of 50 mM phosphate buffer (7.0 pH, 20 mM of guaiacol, 40 mM of H2O2, and 0.1 mL of enzyme extract). Then, the reaction was initiated by the inclusion of enzyme extract. Enhanced absorbance of the reaction mixture at 470 nm was measured every 20 s for 1 min. The POD activity in one unit was referred to as an absorbance change of 0.01 units/min.
Catalase
The catalase activity of samples was estimated using 50 mM potassium phosphate buffer (pH 7.0) and dithiothreitol (1 mM) by referring to earlier protocols (47). The reaction mixture comprised 59 mM of H2O2, 50 mM of phosphate buffer (pH 7), and 100 μL of sample. The reduction pattern of absorbance was assessed every 20 s for 1 min at 240 nm and a unit of CAT activity was described as a change in absorbance in 0.01 min.
Malondialdehyde (MDA)
The MDA levels in semen samples were estimated calorimetrically using MDA as standard (48). The sample (25 μL) was homogenized in 0.1% trichloroacetic acid and centrifuged for 5 min at 14,000 × g. Then, trichloroacetic acid (20%) consisting of thiobarbituric acid (0.05%) was added to 1 mL aliquot of supernatants and heated for 30 min in a water bath. The reaction mixture was cooled and centrifuged for 10 min at 14,000 × g. The absorbance of supernatants was computed at 535 nm, and the value of non-specific absorbance (600 nm) was subtracted from it. The MDA contents were measured by a coefficient of extinction of 155/mM/cm.
Total oxidant status
The procedure of evaluating total oxidant status (TOS) is based on the oxidation of iron and iron complex in an acidic medium (48). Ferric ions interact with xylenol orange and form a particular colored complex. The intensity of the color is linked with the quantity of the oxidant molecule and can be measured using a spectrophotometer. The mixture for the analysis of TOS consisted of reagent xylenol (0.38 g in 500 μL of 25 Mm H2SO4), o-dianisidine 0.0317 g, ferrous ammonium sulfate 0.0196 g, NaCl 0.4 g, and glycerol 500 μL) mixed in sample. The absorbance was recorded at 520 nm after 5 min. The standards used were 5, 10, 15, 20, 25, and 30 μM/mL hydrogen peroxide equivalents. The calibration curve of H2O2 was used to calculate TOS and was denoted in μmol H2O2 equivalent mL−1.
Total antioxidant capacity (TAC)
The TAC samples were measured based on the reduction of a blue radical cation (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) (ABTS∙+) to its original colorless form ABTS by antioxidants (49). The solution to detect TAC consisted of reagent R1 (CH3COONa buffer and glacial acetic acid), reagent R2 (H2O2, Na3PO4 buffer, glacial acetic acid, and ABTS), and the sample. The reacted solution was incubated for 6 min at room temperature, and the absorbance was taken at 734 nm. The standards used were 0, 0.075, 0.125, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2.0). The TAC was calculated using ascorbic acid as a calibration curve and represented as ascorbic acid (μM) equivalent per milliliter of sample.
In-vivo fertility trials
Statistical data showed that 5 μM quercetin was found to be effective in terms of CASA parameters, structural semen parameters, and antioxidant/oxidant indices. Approximately, 60 clinically fit multiparous crossed breed Kamori goats also known as Pateri goats, which are the cross of (Kamori x Gulabi), were assigned in the surrounding area of Sabu Rahu, Sindh, Pakistan. Deworming and vaccination schedules were followed prior to the start of the experiment. Estrus synchronization was performed using two methods: Ovsynch, a fixed-time artificial insemination protocol (50), and medroxyprogesterone acetate (MAP) sponges (51, 52). To rectify the in-vivo fertility results, the MAP sponges group (n = 30) was inseminated with 5 μM quercetin, while the Ovsynch group (n = 30) was inseminated with non-antioxidant treated semen, serving as the control group. The double insemination was performed with each insemination having 50 × 106 sperms/0.5 mL French straw.
Ultrasonography for pregnancy detection
Pregnancy was further confirmed through trans-rectal ultrasound scanning on day 40 post-insemination using an ALOKA ECHO CAMERA Model SSD-500 equipped with a 3.5 MHz linear array transducer. Clinical pregnancy was examined when the gestational sac was observed under trans-rectal ultrasonography.
Statistical analysis
Data were expressed as mean ± standard error, and the Shapiro–Wilk test was used to check whether the data were normal. Data of different variables were analyzed using one-way analysis of variance (ANOVA), and the Tukey test was performed to compare the means of different groups and Pearson correlation by using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). The chi-square statistical model was applied for in-vivo fertility data. The probability values of < 0.05 were taken to indicate statistical significance.
Results
Sperm kinematics parameters
The different values of post-thaw semen parameters using the CASA system are shown in Table 1. Quercetin at 0, 1, 5, 10, and 15 μM significantly improved total motility (p < 0.001), progressive motility (p < 0.004), and rapid velocity (p < 0.001), in contrast to the control group. Similarly, average path velocity (p < 0.009), straight line velocity (p < 0.038), curvilinear velocity (p < 0.001), and lateral head displacement (p < 0.047) were significantly higher in the semen groups consisting of 0, 5, 10, and 15 μM quercetin.
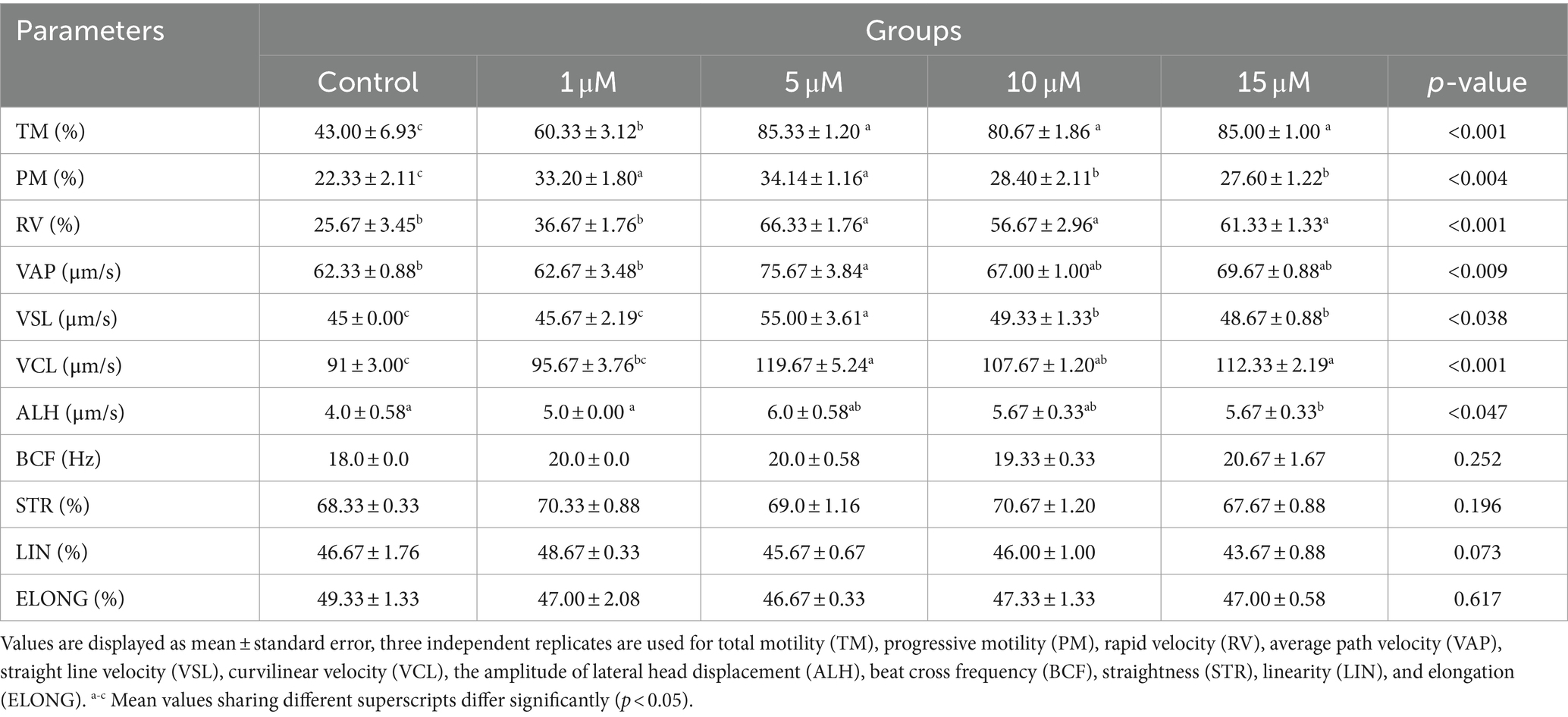
Table 1. Effect of adding quercetin in semen extender on post-thawed spermatozoa quality parameters.
Acrosomal integrity, plasma membrane integrity, viability, and DNA integrity
The plasma membrane and acrosome activity, the viable percentage of spermatozoa, and the DNA integrity of quercetin-treated spermatozoa are illustrated in Table 2. The membrane integrity was reported to be higher (p < 0.001) in the group treated with 5, 10, and 15 μM of quercetin than in other groups. Conversely, significantly increased viable sperm with intact acrosome (V/IACR) (p < 0.001) and percentage of viable spermatozoa (p < 0.013) were observed in 5, 10, and 15 μM levels of quercetin, as opposed to other groups. All treated groups of quercetin have failed to provide a significant effect (p > 0.275) on the DNA integrity of spermatozoa.
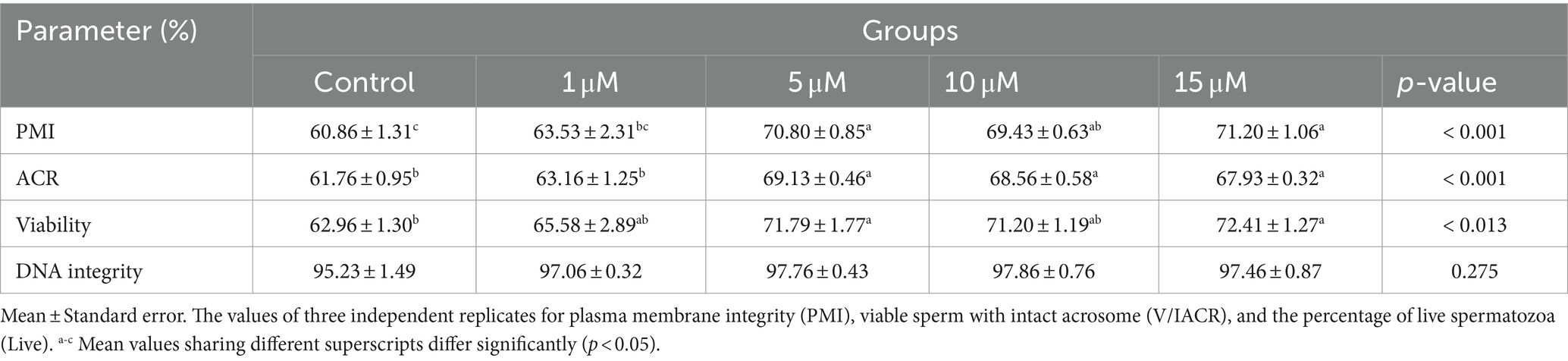
Table 2. Effect of quercetin on frozen–thawing plasma membrane integrity, viable sperm with intact acrosome, viability, and DNA integrity of spermatozoa.
Antioxidant/oxidant sperm parameters
The results of freeze-thawing oxidant/antioxidant status of Kamori bucks are summarized in Table 3. The SOD, CAT, POD, and APX were increased (p < 0.05) with the inclusion of quercetin in treated groups compared to the control group. However, quercetin also significantly reduced (p < 0.05) TOS and MDA levels while enhancing TAC levels (p < 0.05) in treated groups compared to the control group. Among the treated groups, quercetin at 5 μM was found to be an effective concentration with increased antioxidant and reduced oxidant status.
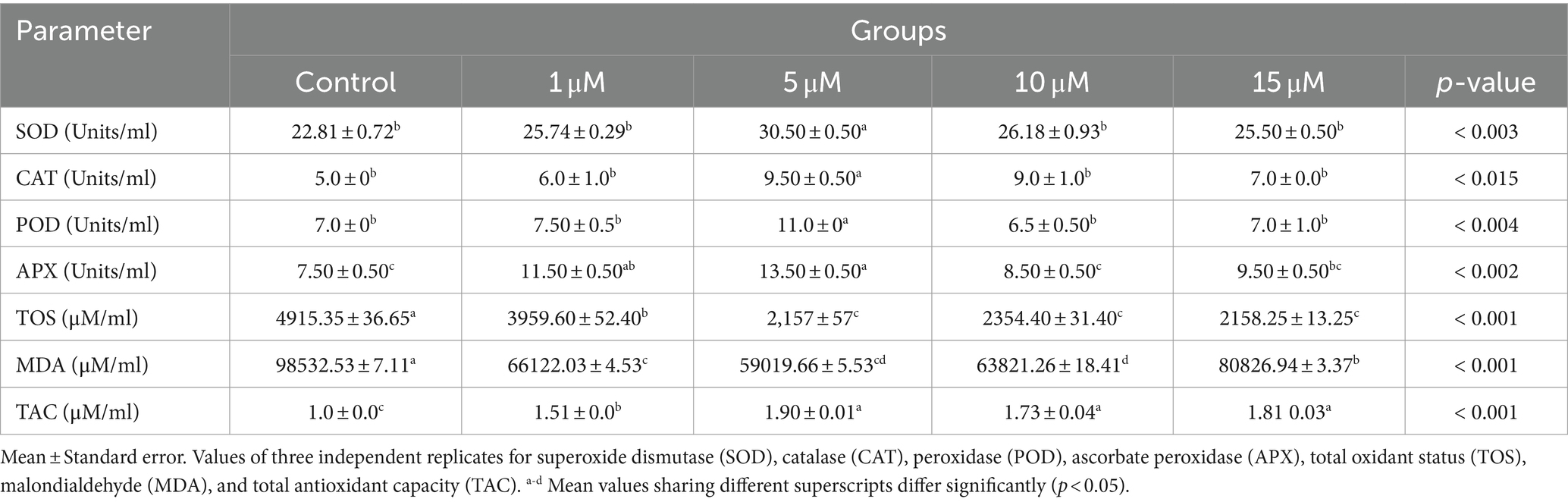
Table 3. Impact of adding quercetin in semen extender on freeze-thawing antioxidant/oxidant status in Kamori bucks.
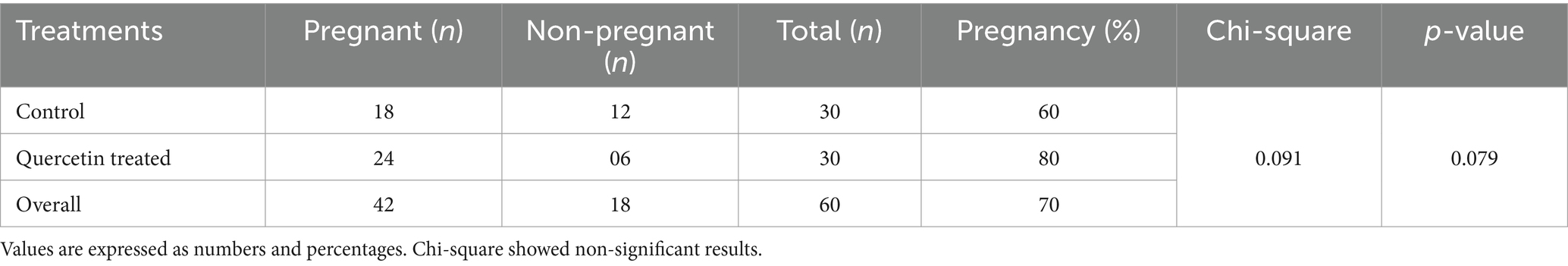
Pregnancy rate through AI
The results of pregnancy confirmation through artificial insemination are shown in Table 4. Ultrasound results showed that 18 out of 30 (60%) animals and 24 out of 30 (80%) animals from the control group and the quercetin-treated semen group, respectively, were found to be pregnant. The pregnancy rate tended to be higher (p > 0.079) for the quercetin-treated semen group compared to the control group.
Discussion
The ability of spermatozoa to assess the sperm kinematics parameters and other sperm structural attributes, such as functional plasma membrane integrity, acrosome integrity, DNA integrity, and viability, are important entities for successful fertilization (53, 54). In our study, total motility, progressive motility, rapid velocity, average path velocity, straight line velocity, curvilinear velocity, and amplitude of lateral head displacement were significantly increased in the semen extender group treated with quercetin compared with other groups. Among all treated groups, quercetin at 5 μM concentration provided the best motility and velocity parameters. Previous studies showed that adding quercetin with other compounds or alone promoted different frozen–thawed sperm motion kinetic parameters in goat semen (55), sheep (56), and bull semen (57). Quercetin is a potent antioxidant compound that acts as a scavenger of reactive oxygen species (RNS) (58). It can preserve frozen–thawed sperm viability, DNA integrity, and functionality in bovine (59), ram (30), and humans (60). Quercetin, when combined with other compounds, increases antioxidant activity, thereby defending the plasma membrane against the detrimental effect of ROS and hence supporting sperm motion kinetic parameters (55).
The plasma membrane and acrosomal integrity are the key components of spermatozoa that play important roles in penetrating the ovum and are responsible for sperm survival. When these two structures are damaged during the cryopreservation process, they cause detrimental effects on sperm cells and eventually compromise sperm survival, resulting in poor fertilizing potential (61). Our study showed that 5, 10, and 15 μM of quercetin produced a beneficial effect on plasma membrane and acrosome integrity. Our results are in line with previous studies that showed improved intactness of the plasma membrane and acrosome integrity due to quercetin in post-thawed Egyptian buffalo bulls (62). Oxidative stress influences membrane integrity and acrosome integrity, while the addition of quercetin in semen ameliorated oxidant production and conferred a protective effect on the plasma membrane and acrosome integrity in jungle fowl (63). The DNA integrity of spermatozoa is a pivotal variable for assessing semen quality and is known to be related to fertility in bull (64), stallion (65), bucks (66), and buffalo (57). We used acridine orange assay for the estimation of DNA integrity; green fluorescence indicates the normal DNA pattern while red fluorescence shows DNA damage (67). In this study, we found non-significant differences between sperm DNA integrity among control and all quercetin-treated groups. Our results are in agreement with previous studies in which egg yolk consisted of ovine and Red, Triladyl®, and Biladyl® in frozen–thawed white-tail deer spermatozoa (68). It is well-established that abundant ROS causes DNA fragmentation during the freeze-thawing process (69). Furthermore, a non-significant effect was observed in sperm DNA damage among the control group and the 0.040 mg/mL quercetin-treated group in post-thaw rooster semen (70).
The viability of spermatozoa is a fertility predictor in which live and dead sperm cells are counted using eosin and nigrosin dyes (71). We found that all levels of quercetin showed a significant effect on sperm viability compared to the control group. The freezing–thawing process reduces sperm viability, normal morphology, and motility but enhances DNA damage (72). Our results corroborated Mahmoud et al.’s study (73), in which Moringa oleifera increased sperm quality parameters including the viability of spermatozoa. The addition of organic zinc and copper (antioxidant properties) in the buck’s diet improved sperm viability, plasma membrane and acrosome integrities, and CASA parameters, which were compromised due to oxidative stress (74).
Spermatozoa are naturally embedded with an antioxidant defense that encompasses superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase (75). Overproduction of ROS significantly impairs the structural and functional intactness of sperm and eventually causes reduced fertility (54). In our study, different levels of quercetin significantly increased SOD, CAT, POD, and APX levels by reducing TOS and MDA levels and thus improved TAC activity, suggesting the protective effects of quercetin against ROS-triggered cryo-damage. Our results are in line with previous studies on rooster and bovine semen in which varying levels of quercetin significantly increased SOD, CAT, and GPx and reduced MDA and ROS concentrations (70, 76). Enzymatic antioxidants, such as SOD, CAT, and GPx, are naturally localized within cells to remove excess ROS [10]. The addition of quercetin increased the levels of SOD, CAT, GSH, and GPx, which can inhibit oxidative stress (70, 77, 78). Quercetin, an antioxidant compound, has shown beneficial effects on fresh and frozen–thawed sperm parameters in different species (20–23). It is involved in neutralizing and suppressing free radical production via interacting with α-tocopherol and eventually declining lipid peroxidation (24). In other studies, quercetin enhanced TAC contents that improved semen parameters in humans and goats (79, 80). Barranco et al. (81) revealed that high contents of TAC in boar seminal plasma were related to sperm survival and fertility and can be used as a marker for predicting fertility.
From our findings, the 5 μM quercetin-treated semen group reported a higher (80%) conception rate than the control (60%) group, showing that both control and treated groups are suitable for artificial insemination in field conditions. Perhaps this favorable effect might be due to the addition of quercetin in the semen extender, optimizing temperature shocks, season, and skills of AI technicians and management of the animals that ultimately improved conception rate. Al-Faruque et al. (82) reported that 39 out of 48 goats conceived using frozen semen through AI. CASA and other semen parameters play a crucial role in determining sperm quality with biological significance during cryopreservation (83) and fertility. Additionally, sperm motility and motion parameters significantly impact both in-vivo and in-vitro fertilization potential (53). Based on our findings, it can be conferred that cryo-damages were minimal in the 5 μM quercetin treated group compared to the control group. The purpose of our study was fulfilled by quercetin serving as the best option for improving the conception rate in the caprine artificial insemination program. However, further studies are required for using varying levels of quercetin and conducting large-scale in-vivo field trials of artificial insemination in goats during different seasons and with semen extenders.
Conclusion
This study concludes that 5 μM quercetin to semen extender increased antioxidant status and attenuated oxidative stress, which, in turn, improved sperm kinematic parameters and sperm structural variables that ultimately promoted in-vivo fertility in crossbred Kamori goats. Further studies are warranted on a large-scale basis in-vivo fertility trials in field conditions in the consideration of other factors such as season, management conditions, and parity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the experiment was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Animal Sciences Division, Nuclear Institute for Agriculture and Biology in Faisalabad, Pakistan, according to number of (NIAB-C-PIEAS-0023). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
IB: Data curation, Methodology, Writing – original draft. MF: Methodology, Validation, Writing – review & editing. AH: Data curation, Formal analysis, Methodology, Writing – review & editing. SA: Supervision, Validation, Writing – review & editing. RK: Supervision, Validation, Writing – review & editing. MS: Investigation, Supervision, Validation, Writing – review & editing. YM: Supervision, Validation, Writing – review & editing. AO: Investigation, Supervision, Validation, Writing – review & editing. GM: Investigation, Methodology, Validation, Writing – review & editing. AD: Formal analysis, Software, Writing – review & editing. TH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors are thankful to PSF-NSLP-785 for their financial grant.
Acknowledgments
We are thankful to PSF-NSLP-785 for their financial support. We are grateful to Mr. Dildar Hussain Rahu for his great help in purchasing Kamori bucks from different districts of Sindh province. Moreover, we express our gratitude to Muhammad Aslam and Saleem Abbas for their assistance in training the bucks and collecting semen. We also thank Ali Asghar Rahu, Farhatullah Rahu, and Moro Khan Rahu in animal selection, estrus synchronization protocols, and artificial insemination in goats.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors did not show any conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Andrabi, SMH, Lal, C, Haider, MS, Khan, MFU, and Ghaffar, A. Artificial insemination in beetal and jattal goats: preliminary results In: NA Siddiky , editor. Sustainable goat farming for livelihood improvement in South Asia. Bangladesh: SAARC Agriculture Centre (SAC) (2018). 177–81.
2. Kumar Patel, G, Haque, N, Madhavatar, M, Kumar Chaudhari, A, Kumar Patel, D, Bhalakiya, N, et al. Artificial insemination: a tool to improve livestock productivity. J Pharmacogn Phytochem. (2017) 6:307–13.
3. Gharagozloo, P, and Aitken, RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. (2011) 26:1628–40. doi: 10.1093/humrep/der132
4. Agarwal, A, Tvrda, E, and Sharma, R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. (2014) 12:45. doi: 10.1186/1477-7827-12-45
5. Du Plessis, SS, Agarwal, A, Halabi, J, and Tvurda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. (2015) 32:509–20. doi: 10.1007/s10815-014-0425-7
6. Aitken, RJ, Harkiss, D, and Buckingham, DW. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol Reprod Dev. (1993) 35:302–15. doi: 10.1002/mrd.1080350313
7. Budai, C, Egerszegi, I, Olah, J, Javor, A, and Kovacs, A. The protective effect of antioxidants on liquid and frozen stored ram semen – review. Anim Sci Biotechnol. (2014) 47:46–52.
8. Azawi, OI, and Hussein, EK. Effect of vitamins C or E supplementation to Tris diluent on the semen quality of Awassi rams preserved at 5 °C. Vet Res Forum. (2013) 4:157–60.
9. Lessard, C, Danielson, J, Rajapaksha, K, and Adams, GR. McCorkell banking north American buffalo semen. Theriogenology. (2009) 71:1112–9. doi: 10.1016/j.theriogenology.2008.12.004
10. Gangwar, C, Saxena, A, Patel, A, Singh, SP, Yadav, S, Kumar, R, et al. Effect of reduced glutathione supplementation on cryopreservation induced sperm cryoinjuries in Murrah bull semen. Anim Reprod Sci. (2018) 192:171–8. doi: 10.1016/j.anireprosci.2018.03.005
11. Januskauskas, A, Johannisson, A, and Rodriguez-Martinez, H. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility. Theriogenology. (2003) 60:743–58. doi: 10.1016/S0093-691X(03)00050-5
12. Soltanpour, F, and Moghaddam, G. Effects of frozen diluents on storage of ram sperm. Int J Adv Biol Bio Res. (2013) 1:1698–704.
13. Hahn, K, Failing, K, and Wehrend, A. Effect of temperature and time after collection on buck sperm quality. BMC Vet Res. (2019) 15:1–7. doi: 10.1186/s12917-019-2135-y
14. Agossou, DJ, and Koluman, N. The effects of natural mating and artificial insemination using cryopreserved buck semen on reproductive performance in Alpine goats. Arch Anim Breed. (2018) 61:459–461. doi: 10.5194/aab-61-459-2018
15. Zou, J, Wei, L, Li, D, Zhang, Y, Wang, G, Zhang, L, et al. Study on cryopreservation of Guanzhong dairy goat semen with bovine semen seminal plasma. Theriogenology. (2022) 189:113–7. doi: 10.1016/j.theriogenology.2022.05.027
16. Purdy, PH . A review on goat sperm cryopreservation. Small Rumin Res. (2006) 63:215–25. doi: 10.1016/j.smallrumres.2005.02.015
17. Leboeuf, B, Restall, B, and Salamon, S. Production and storage of goat semen for artificial insemination. Anim Reprod Sci. (2003) 62:113–41. doi: 10.1016/S0378-4320(00)00156-1
18. Ustuner, B, Gunay, U, and Nur, ZE. Effect of seminal plasma, egg yolk, and season on the freezability of Saanen buck semen. Bull Vet Inst Pulawy. (2009) 53:369–74.
19. Boots, AV, Haenen, GR, and Bast, A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. (2008) 585:325–37. doi: 10.1016/j.ejphar.2008.03.008
20. Ben Abdallah, F, Zribi, N, and Ammar-Keskes, L. Antioxidative potential of quercetin against hydrogen peroxide induced oxidative stress in spermatozoa in vitro. Andrologia. (2011) 43:261–5. doi: 10.1111/j.1439-0272.2010.01063.x
21. Gibb, Z, Butler, TJ, Morris, LHA, Maxwell, WMC, and Grupen, CG. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology. (2013) 79:1001–9. doi: 10.1016/j.theriogenology.2012.06.032
22. Khaki, A, Fathiazad, F, Nouri, M, Khaki, A, Maleki, NA, Khamnei, HJ, et al. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother Res. (2010) 24:1285–91. doi: 10.1002/ptr.3100
23. McNiven, MA, and Richardson, GF. Effect of quercetin on capacitation status and lipid peroxidation of stallion spermatozoa. Cell Preserv Technol. (2006) 4:169–77. doi: 10.1089/cpt.2006.4.169
24. Wang, W, Sun, C, Mao, L, Ma, P, Liu, F, Yang, J, et al. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol. (2016) 56:21–38. doi: 10.1016/j.tifs.2016.07.004
25. Bansal, AK, and Bilaspuri, GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. (2011) 2011. doi: 10.4061/2011/686137
26. Li, Z, Qionglin, L, Rongju, L, Wanfen, X, and Wanmin, L. Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J Androl. (2010) 31:437–444. doi: 10.2164/jandrol.109.007849
27. Roca, J, Rodríguez, MJ, Gil, MA, Carvajal, G, Garcia, EM, Cuello, C, et al. Survival and in vitro fertility of boar spermatozoa frozen in the presence of superoxide dismutase and/or catalase. J Androl. (2005) 26:15–24.
28. Gadea, J, Molla, M, Selles, E, Marco, MA, Garcia-Vazquez, FA, and Gardon, JC. Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology (2011) 62:40–6. doi: 10.1016/j.cryobiol.2010.12.001
29. Kalthur, G, Raj, S, Thiyagarajan, A, Kumar, S, Kumar, P, and Adiga, SK. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril. (2011) 95:1149–51. doi: 10.1016/j.fertnstert.2010.10.005
30. Silva, EC, Cajueiro, JF, Silva, SV, Soares, PC, and Guerra, MM. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology. (2012) 77:1722–6. doi: 10.1016/j.theriogenology.2011.11.023
31. Silva, EC, Arruda, LCP, Silva, SV, Souza, HM, and Guerra, MM. High resveratrol or quercetin concentrations reduce the oscillation index of frozen goat semen. Arq Bras Med Vet Zootec. (2016) 68:1237–43. doi: 10.1590/1678-4162-8670
32. Andrabi, SMH, Anwar, M, and Mehmood, A. Efficacy of short-term estrus synchronization protocol and timed artificial insemination in subtropical goats. J Anim Plant Sci. (2015) 25:298–300.
33. Mehmood, A, Andrabi, SMH, Anwar, M, and Rafiq, M. Estrus synchronization and artificial insemination in goats during low breeding season-A preliminary study. Pak Vet J. (2010) 31:157–9.
34. Khan, MS, Khan, MA, and Mahmood, S. Genetic resources and diversity in Pakistani goats. Int J Agric Biol. (2008) 10:227–31.
35. Amoah, EA, Gelay, S, Guthrie, P, and Rexroad, CE Jr. Breeding season and aspects of reproduction of female goats. J Anim Sci. (1996) 74:723–8. doi: 10.2527/1996.744723x
36. Kausar, R, Khanum, SA, Hussain, M, Hussain, T, Ahmad, N, Ahmad, L, et al. Estrus synchronization and conception rates using locally prepared methylacetoxyprogesterone sponges in cyclic and acyclic Nili-Ravi buffaloes (Bubalus bubalis). (2013) 433–437.
37. Afzal, A, Hussain, T, and Hameed, A. Moringa oleifera supplementation improves antioxidant status and biochemical indices by attenuating early pregnancy stress in Beetal goats. Front Nutr. (2021) 8:700957.
38. Yaseen, A, Hussain, T, and Hameed, A. Association of faecal volatiles and steroids with behavioural expression during oestrous cycle of sheep (Ovis aries). Reprod Domest Anim. (2023) 58:708–16. doi: 10.1111/rda.14340
39. Andrabi, SH, Mehmood, A, Anwar, M, and Khan, MF. In vitro viability and longevity of cooled Beetal buck spermatozoa extended in skimmed milk and Tris-citric acid based extenders. Small Rumin Res. (2016) 143:61–6. doi: 10.1016/j.smallrumres.2016.09.002
40. Naijian, HR, Kohram, H, Shahneh, AZ, Sharafi, M, and Bucak, MN. Effects of different concentrations of BHT on microscopic and oxidative parameters of Mahabadi goat semen following the freeze–thaw process. Cryobiology. (2013) 66:151–5. doi: 10.1016/j.cryobiol.2012.12.010
41. Mortimer, ST . CASA—practical aspects. J Androl. (2000) 21:515–24. doi: 10.1002/j.1939-4640.2000.tb02116.x
42. Khalil Ur Rehman, H, Andrabi, SMH, Ahmed, H, and Shah, SAH. Programmable fast-freezing method improves the post-thaw motion dynamics, integrities of plasmalemma, mitochondrial transmembrane, DNA and, acrosome, and in vivo fertility of water buffalo (Bubalus bubalis) spermatozoa. Andrologia. (2017) 49:e12733. doi: 10.1111/and.12733
43. Shah, SAH, Andrabi, SMH, Ahmed, H, and Qureshi, IZ. Cryoprotection synergism between glycerol and dimethyl sulfoxide improves the mitochondrial transmembrane potential, plasmalemma, acrosomal and DNA integrities, and in vivo fertility of water buffalo (Bubalus bubalis) spermatozoa. Cytotechnology. (2016) 68:2335–44. doi: 10.1007/s10616-016-0027-6
44. Shah, SAH, Andrabi, SMH, and Qureshi, IZ. Effect of equilibration times, freezing, and thawing rates on post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. Andrology. (2016) 4:972–6. doi: 10.1111/andr.12214
45. Beyer, WF Jr, and Fridovich, I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. (1987) 161:559–66. doi: 10.1016/0003-2697(87)90489-1
46. Hameed, A, Shah, TM, Atta, BM, Haq, MA, and Sayed, HINA. Gamma irradiation effects on seed germination and growth, protein content, peroxidase and protease activity, lipid peroxidation in desi and kabuli chickpea. Pak J Bot. (2008) 40:1033–41.
47. Beers, RF, and Sizer, IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. (1952) 195:133–40. doi: 10.1016/S0021-9258(19)50881-X
48. Dhindsa, RS, Plumb-Dhindsa, PA, and Thorpe, TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. (1981) 32:93–101. doi: 10.1093/jxb/32.1.93
49. Dikilitas, M, Guldur, ME, Deryaoglu, A, and Ozcan, ER. Antioxidant and oxidant levels of pepper (Capsicum annuum cv. â€~ Charlee’) infected with pepper mild mottle virus. Not Bot Horti Agrobo. (2011) 39:58–63. doi: 10.15835/nbha3925881
50. Holtz, W, Sohnrey, B, Gerland, M, and Driancourt, M-A. Ovsynch synchronization and fixed-time insemination in goats. Theriogenology (2008) 69:785–792. doi: 10.1016/j.theriogenology.2007.10.004
51. Robinson, TJ . Use of progestagen-impregnated sponges inserted intravaginally or subcutaneously for the control of the oestrous cycle in the sheep. Nature (1965) 206:39–41. doi: 10.1038/206039a0
52. Afzal, A, Hussain, T, and Hameed, A. Moringa oleifera supplementation improves antioxidant status and biochemical indices by attenuating early pregnancy stress in Beetal goats. Front nutr. (2021) 8:700957. doi: 10.3389/fnut.2021.700957
53. Ahmed, H, Andrabi, SMH, and Jahan, S. Semen quality parameters as fertility predictors of water buffalo bull spermatozoa during low-breeding season. Theriogenology. (2016) 86:1516–22. doi: 10.1016/j.theriogenology.2016.05.010
54. Nazif, MS, Khan, H, Khan, FA, Hussain, T, Ahmad, A, Husnain, A, et al. Glycine improved cryopreserved spermatozoa quality in Achai bull. Biomed Res Int. (2022) 2022:1–9. doi: 10.1155/2022/8282387
55. Seifi-Jamadi, A, Ahmad, E, Ansari, M, and Kohram, H. Antioxidant effect of quercetin in an extender containing DMA or glycerol on freezing capacity of goat semen. Cryobiology. (2017) 75:15–20. doi: 10.1016/j.cryobiol.2017.03.002
56. Jiménez-Aguilar, E, Quezada-Casasola, A, Prieto-Caraveo, M, Orozco-Lucero, E, Itzá-Ortiz, M, and Carrera-Chávez, J. Evaluation of the quercetin and vitamin E addition to the cryopreservation medium of sheep semen on in vivo fertility. Abanico Vet. (2021) 11:36. doi: 10.21929/abavet2021.36
57. Ahmed, H, Jahan, S, Salman, MM, and Ullah, F. Stimulating effects of quercetin (QUE) in tris citric acid extender on post thaw quality and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology. (2019) 134:18–23. doi: 10.1016/j.theriogenology.2019.05.012
58. Cushnie, TT, and Lamb, AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. (2005) 26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002
59. Satorre, MM, and Córdoba, M. Involvement of intracellular calcium and src tyrosine-kinase in capacitation of cryopreserved bovine spermatozoa. InVet. (2010) 12:75–83.
60. Zribi, N, Chakroun, NF, Abdallah, FB, Elleuch, H, Sellami, A, Gargouri, J, et al. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology. (2012) 65:326–31. doi: 10.1016/j.cryobiol.2012.09.003
61. Stival, C, Puga Molina, LDC, Paudel, B, Buffone, MG, Visconti, PE, and Krapf, D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol. (2016) 220:93–106. doi: 10.1007/978-3-319-30567-7_5
62. El-Khawagah, AR, Kandiel, MM, and Samir, H. Effect of quercetin supplementation in extender on sperm kinematics, extracellular enzymes release, and oxidative stress of Egyptian buffalo bulls frozen–thawed semen. Front Vet Sci. (2020) 7:604460. doi: 10.3389/fvets.2020.604460
63. Rakha, BA, Qurrat-ul-Ain, A, Ansari, MS, Akhter, S, Akhter, A, Awan, MA, et al. Effect of quercetin on oxidative stress, mitochondrial activity, and quality of Indian red jungle fowl (Gallus gallus murghi) sperm. Biopreserv Biobank. (2020) 18:311–20. doi: 10.1089/bio.2020.0007
64. Ballachey, BE, Evenson, DP, and Saacke, RG. The sperm chromatin structure assay relationship with alternate tests of semen quality and heterospermic performance of bulls. J Androl. (1988) 9:109–15. doi: 10.1002/j.1939-4640.1988.tb01020.x
65. Love, CC, and Kenney, RM. The relationship of increased susceptibility of sperm DNA to denaturation and fertility in the stallion. Theriogenology. (1998) 50:955–72. doi: 10.1016/S0093-691X(98)00199-X
66. Khalique, MA, Andrabi, SMH, Majeed, KA, Yousaf, MS, Ahmad, N, Tahir, SK, et al. Cerium oxide nanoparticles improve the post-thaw quality and in-vivo fertility of Beetal buck spermatozoa. Theriogenology. (2024) 214:166–72. doi: 10.1016/j.theriogenology.2023.10.022
67. Martins, C, Oliveira, NG, Pingarilho, M, Gamboa da Costa, G, Martins, V, Marques, MM, et al. Cytogenetic damage induced by acrylamide and glycidamide in mammalian cells: correlation with specific glycidamide-DNA adducts. Toxicol Sci. (2007) 95:383–90.
68. Thundathil, J, Meyer, R, Palasz, AT, Barth, AD, and Mapletoft, RJ. Effect of the knobbed acrosome defect in bovine sperm on IVF and embryo production. Theriogenology. (2000) 54:921–34. doi: 10.1016/S0093-691X(00)00402-7
69. Tran, M, Uriondo, H, Nodar, F, and Sedó, CA. Cryopreservation promotes sperm DNA damage through oxidative stress [38N]. Obstet Gynecol. (2018) 131:162S. doi: 10.1097/01.AOG.0000533134.61739.52
70. Appiah, MO, Li, W, Zhao, J, Liu, H, Dong, Y, Xiang, J, et al. Quercetin supplemented casein-based extender improves the post-thaw quality of rooster semen. Cryobiology. (2020) 94:57–65. doi: 10.1016/j.cryobiol.2020.04.010
71. Moskovtsev, SI, and Librach, CL. Methods of sperm vitality assessment. Methods Mol Biol. (2013) 927:13–9. doi: 10.1007/978-1-62703-038-0_2
72. Asturiano, J, Marco-Jiménez, F, Peñaranda, D, Garzón, D, Pérez, L, Vicente, J, et al. Effect of sperm cryopreservation on the European eel sperm viability and spermatozoa morphology. Reprod Domest Anim. (2007) 42:162–6. doi: 10.1111/j.1439-0531.2006.00746.x
73. Mahmoud, R, Gabr, SA, Yousif, AIA, El-Sherbieny, MA, El-Hayes, AM, and Abdel-Khalek, AE. Visual examination and computer assessed sperm analysis (Casa) of goat semen diluted by Tris-extender supplemented with aqueous extract of Moringa Oleifera leaves. J Sust Agric Enviro Sci. (2023) 2:81–90. doi: 10.21608/jsaes.2023.246737.1061
74. Arangasamy, A, Krishnaiah, MV, Manohar, N, Selvaraju, S, Rani, GP, Soren, NM, et al. Cryoprotective role of organic Zn and cu supplementation in goats (Capra hircus) diet. Cryobiology. (2018) 81:117–24. doi: 10.1016/j.cryobiol.2018.02.001
75. Ismail, AA, Abdel-Khalek, AKE, Khalil, WA, Yousif, AI, Saadeldin, IM, Abomughaid, MM, et al. Effects of mint, thyme, and curcumin extract nanoformulations on the sperm quality, apoptosis, chromatin decondensation, enzyme activity, and oxidative status of cryopreserved goat semen. Cryobiology (2020) 97:144–152. doi: 10.1016/j.cryobiol.2020.09.002
76. Tvrdá, E, Tušimová, E, Kováčik, A, Paál, D, Libová, Ľ, and Lukáč, N. Protective effects of quercetin on selected oxidative biomarkers in bovine spermatozoa subjected to ferrous ascorbate. Reprod Domest Anim. (2016) 51:524–37. doi: 10.1111/rda.12714
77. Ghaniei, A, Eslami, M, Zadeh Hashem, E, Rezapour, R, and Talebi, A. Quercetin attenuates H2O2-induced toxicity of rooster semen during liquid storage at 4 C. J Anim Physiol Anim Nutr. (2019) 103:713–22. doi: 10.1111/jpn.13056
78. Zhang, YMC . Protective effect of quercetin on Aroclor 1254–induced oxidative damage in cultured chicken spermatogonial cells. Toxicol Sci. (2005) 88:545–50. doi: 10.1093/toxsci/kfi333
79. Cheraghi, E, Sajadi, SMS, and Soleimani Mehranjani, M. The effect of quercetin on the quality of sperm parameters in frozen-thawed semen of patients with Asthenospermia. Andrologia. (2021) 53:e14167. doi: 10.1111/and.14167
80. Hassan, SA, Khalil, WA, Hassan, MA, Yousif, AI, Sabry, OM, Wink, M, et al. Antioxidant and antiapoptotic effects of a turraea fischeri leaf extract on cryopreserved goat sperm. Animals. (2021) 11:2840. doi: 10.3390/ani11102840
81. Barranco, I, Tvarijonaviciute, A, Perez-Patiño, C, Parrilla, I, Ceron, JJ, and Martinez, EA. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci Rep. (2015) 5:18538. doi: 10.1038/srep18538
82. Al-Faruque, MH, Bari, FY, Siddiqui, MAR, and Shamsuddin, M. Fertilizing capacity of buck (Capra hircus) semen frozen with different concentrations of egg yolk. J Bangladesh Agric Univ. (2007) 5:95–104.
Keywords: quercetin, antioxidants/oxidants, spermatozoa quality and in-vivo fertility, CASA parameters, crossbred Kamori goats
Citation: Batool I, Fayyaz MH, Hameed A, Andrabi SMH, Kausar R, Shahzad M, Mubashir Y, Omur AD, Murtaza G, Ditta A and Hussain T (2024) Quercetin in semen extender improves frozen-thawed spermatozoa quality and in-vivo fertility in crossbred Kamori goats. Front. Vet. Sci. 11:1385642. doi: 10.3389/fvets.2024.1385642
Edited by:
Amal M. Aboelmaaty, National Research Centre, EgyptReviewed by:
Valeria Pasciu, University of Sassari, ItalyFarooz Ahmad Lone, Sher-e-Kashmir University of Agricultural Sciences and Technology, India
Copyright © 2024 Batool, Fayyaz, Hameed, Andrabi, Kausar, Shahzad, Mubashir, Omur, Murtaza, Ditta and Hussain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tarique Hussain, ZHJ0YXJpcXVlcmFob29AZ21haWwuY29t
 Iqra Batool
Iqra Batool Muhammad Hammad Fayyaz
Muhammad Hammad Fayyaz Amjad Hameed
Amjad Hameed Syed Murtaza Hassan Andrabi
Syed Murtaza Hassan Andrabi Rehana Kausar
Rehana Kausar Muhammad Shahzad
Muhammad Shahzad Yasin Mubashir
Yasin Mubashir Ali Dogan Omur
Ali Dogan Omur Ghulam Murtaza5
Ghulam Murtaza5 Allah Ditta
Allah Ditta Tarique Hussain
Tarique Hussain