- 1College of Life Science and Food Engineering, Hebei University of Engineering, Handan, China
- 2Breeding Branch, Muyuan Foods Co., Ltd., Nanyang, China
The purpose of the present study was to investigate the effects of different levels of a Chinese herbal medicine formulation combined with JM113 (CHM-JM113) on growth performance, antioxidant capacity, organ index, and intestinal health of AA broilers. The AA broiler chicks were randomly allocated to 5 treatments as follows: a basic diet for the control group, the basic diet supplemented with 0.25% CHM-JM113, 0.5% CHM-JM113, 1% CHM-JM113 and 2% CHM-JM113 for the treatment group, respectively. The results showed that the addition of CHM-JM113 to the diet significantly reduced the mortality (p < 0.01) and improved the European Broiler Index (EBI) (p < 0.05), whereas it had no significance on growth performance of AA broilers (p > 0.05). Comparing the control group, 0.5 and 1% CHM-JM113 group significantly improved the organ index of liver, spleen and bursa (p < 0.05). In terms of intestinal morphology and structure, the addition of different levels of CHM-JM113 increased VH and VH/CD ratio, decreased CD in the small intestine compared to the control group, with 1 and 2% of the additive dose being more effective (p < 0.05). Chinese herbal medicine and probiotics as natural antioxidants also significantly increased the content of SOD in serum of 21-day-old broilers (p < 0.01), and significantly decreased the content of MDA in serum (p < 0.01). At 42 days of age, the addition of 1 and 2% CHM-JM113 significantly increased the content of SOD (p < 0.01) and significantly decreased the content of MDA in the organism (p < 0.01), accompanied by a significant increase in T-AOC and CAT content. In the study of the effect of CHM-JM113 on intestinal immunity, compared with the control group, we found that 1% or 2% CHM-JM113 had a better effect on the expression of occludin and claudin-1 in the intestinal segments of broilers (p < 0.05). For the expression of GATA-3, 0.5% CHM-JM113 may have a better effect (p < 0.05). CHM-JM113 may be used as an antibiotic alternative in broiler production.
1 Introduction
Antibiotics paly an essential role in animal husbandry and are generally used to prevent, treating diseases and enhancing growth (1, 2). However, the extensive use of antibiotics will lead to drug resistance of the harmful strains (3, 4), negatively impact the quality of livestock products (5, 6), and even destroy the ecological environment (7–9), endangering human health (5, 10–12). For agriculture, there is an urgent need to find alternatives to antibiotics in edible animals, especially poultry and livestock (1, 13). Probiotics, prebiotics, synbiotics, organic acids, essential oils, enzymes, immunostimulants, and plant-based (plant-derived) substances, including herbs, botanicals, essential oils, and oleoresins, are the most common feed additives for substituting antibiotics (13, 14).
Chinese herbal medicine and its extracts are natural and effective, with few side effects and the risk of inducing bacterial resistance is minimal (15), and have gradually become the preferred alternative to antibiotics (16). Chinese herbal medicine contains a variety of active ingredients, such as plant polysaccharides, chlorogenic acid, alkaloids, essential oils, flavonoids, plant polyphenols, sterols and so on. These active substances enhance the antioxidant capacity and immunity of animal bodies and limit the formation of dangerous bacteria (17–19). Thyme, Astragalus, tangerine peel and dandelion are traditional Chinese medicinal materials, which play a certain role in bacteriostasis, promoting animal growth and development, and enhancing animal immunity. The addition of thyme could improve the activity of SOD and GSH-Px in the blood of rats, reduce the level of malondialdehyde and boost the antioxidant capacity of rats (20). Astragalus polysaccharide in Astragalus has the advantages of minimal residue and no tolerance, which increases animal immunity and stimulates animal growth (21). Flavonoids (active ingredients in tangerine peel) contain phenolic hydroxyl structure, which could be combined with free radicals to form complex free radicals, reduce the production of free radicals and reduce the damage of free radicals to the body, so as to achieve the effect of anti-oxidation (22, 23). Adding 500 mg/kg dandelion tannins increased the ADG of Wenchang chickens, stimulated intestinal development, and increased the absorption rate of nutrients in the small intestine (24). It also enhance the daily feed intake (ADFI) of AA broilers, reduced the feed/gain ratio (F/G), and promoted the expression of claudin and occludin-1 in the intestine (25). The effects of Chinese herbal medicine mixtures on animal body vary with its unique circumstances. Herbal mixtures composed of numerous herbs contain a variety of active ingredients, which may demonstrate higher biological efficacy than single herb extracts (26).
In addition, probiotics also play a crucial role in resistance. Probiotics colonize in the digestive tract of animals, minimize the harm of pathogenic bacteria to the intestinal tract, protect the integrity of the intestinal structure, and stimulate the intestinal development of the animals (27). Lactobacillus plantarum JM113 has high antioxidant activity, which could enhance the digestion, absorption and metabolism of intestinal tract under DON pollution by reducing the damage of intestinal morphology and intestinal barrier, increasing the abundance of beneficial bacteria and regulating the balance of intestinal flora (28). Li et al. (29) found that the combination of non-antibiotic growth promoters (NAGPCs) had superior effect than the usage of non-antibiotic growth promoter alone. However, the combination of the same type of NAGPC may limit the beneficial benefits of synergy.
Based on the above background, it is considered whether the combined use of Chinese herbal medicine and probiotics may have a cumulative effect on the alternative use of antibiotics. This study was conducted to study the effects of Chinese herbal medicine compound preparation and probiotics preparation on growth performance, antioxidant capacity, organ index and intestinal health of broilers, and to provide potential alternatives to antibiotics for use in broiler production.
2 Materials and methods
2.1 Parameters experimental design, characteristics of objects and conditions of research
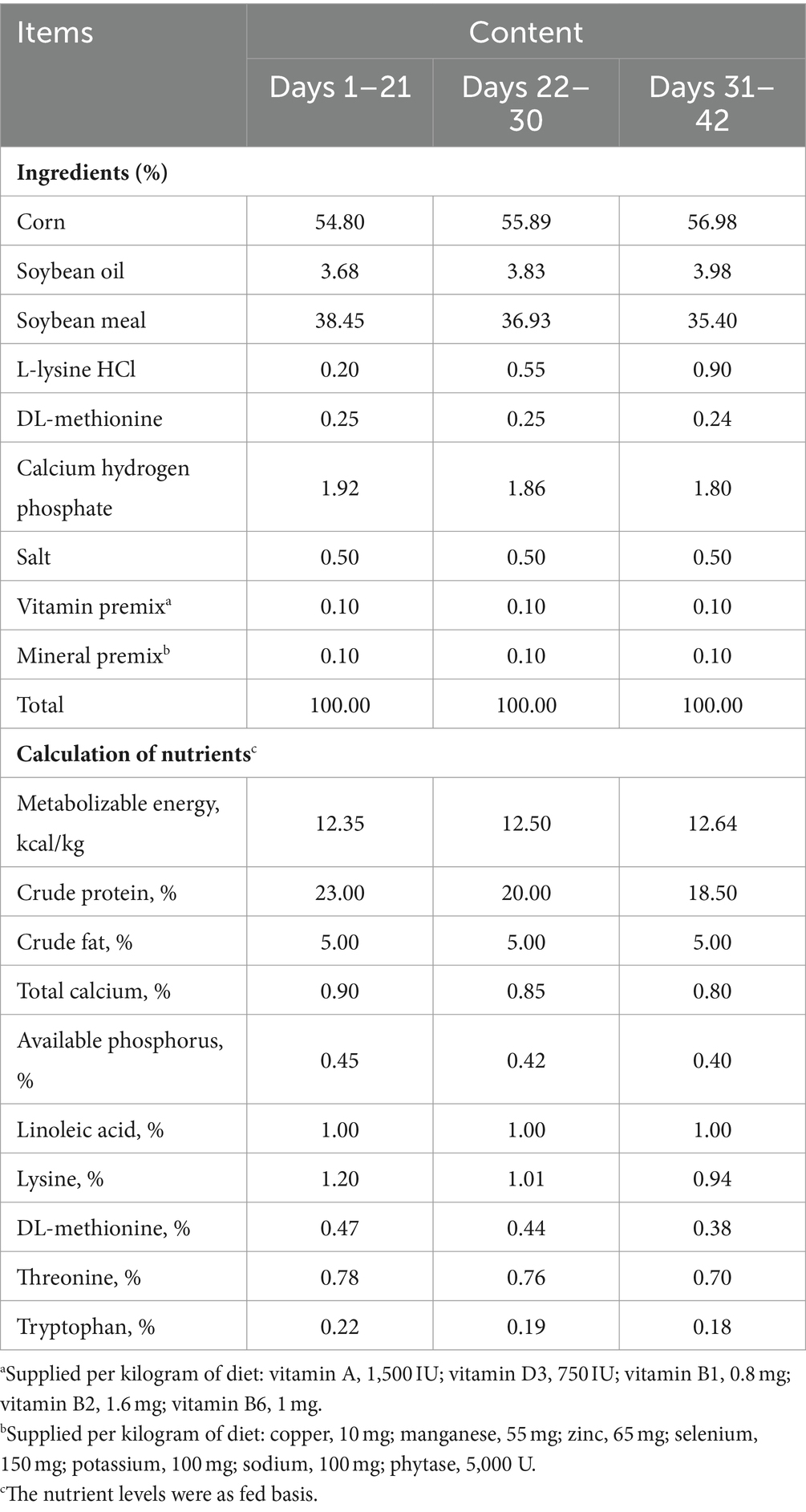
A total of 450 healthy 1-day-old AA broilers with similar body weight (40.0 ± 5.0 g) were randomly divided into 5 groups, with 6 replicates per group and 15 broilers per replicate. The animal protocols used in this study have been evaluated and approved by the Institutional Animal Care and Use Committee of Hebei University of Engineering (Identification code: HBUE2022, Date of approval: 16 March 2022). The control group was provided a basic diet, while the experimental groups were fed the basic diet supplemented with 0.25, 0.5, 1 and 2% CHM-JM113 (a Chinese herbal medicine formulation combined with JM113), respectively. During the feeding phase, all experimental groups were supplemented with JM113 powder (1 g/kg in the meal). The experiment lasted for 42 days, which was divided into an early stage (1–21 days old) and a late stage (22–42 days old). The basic diet composition was developed and granulated according to the Chinese broiler nutrition standard (NY/T 33-2004). The food composition and nutritional composition are shown in Table 1. During a 42 day test period, animals were fed in Gaobai Family Farm in Linzhang County, Handan City. Chinese herbal medicine was composed of Astragalus, tangerine peel, dandelion and thyme (brought from Anhui Bozhou Wuhua Food Co., Ltd.) in a ratio of 1:1:1:1. The viable count of Lactobacillus plantarum JM113 was 4.4 × 109 CFU/g.
During the experiment, free diet, drinking water and artificial light were supplemented. The feeding method was imprisoned in the house. The illumination time was 24 h per day from 1 to 7 days of age, and subsequently the illumination was reduced by 1 h per week. After 4 weeks of age, the illumination time was switched to a standard illumination time of 20 h. The room temperature was 33°C on the first day of the experiment, and then decreased by approximately 2.5°C every week until it reached 19°C, while maintaining a humidity level of 60–70%.
2.2 Sample collection
On the 21st and 42nd days of the experiment, one AA broiler was randomly selected from each replicate to collect wing vein serum, which was then stored at −20°C for further use. After bloodletting the broiler’s neck, the neck and abdominal cavity were quickly opened, and the cardiac, liver, spleen, bursa of Fabricius, and other organs were completely removed. The fascia and adipose tissue adhered to the surface of the organs were removed, and the blood on the surface was sucked with filter paper. Accurately weigh and calculate organ index.
2.3 Intestinal morphology
The duodenum, jejunum and ileum were excised approximately 2 cm and preserved in 4% paraformaldehyde. The intestinal tissue was dehydrated with ethanol, washed with xylene, soaked in wax, embedded, sectioned (μm), and stained with hematoxylin-eosin (H&E) for morphological measurement. The villus height (VH; measured from the villus-crypt junction to the villus tip) and the crypt depth (CD; measured from the crypt base to the villus-crypt junction) were estimated by the Image-Pro Plus 6.0 software, and the ratio of villus height to crypt depth (VH/CD) was calculated.
2.4 Serum antioxidant capacity
According to the manufacturer’s plan (Suzhou Grace Biotechnology Co., Ltd.), superoxide dismutase (SOD) detection kit (G0101W), catalase (CAT) detection kit (G0105W), total antioxidant capacity (T-AOC) detection kit (G0142W) and malondialdehyde (MDA) detection kit (G0109W) were used to determine the levels of SOD (U/mL), CAT (U/mL), T-AOC (U/mL) and MDA (nmol/mL) in serum of AA broilers.
2.5 The level of claudin-1, occludin and GATA-3 gene expression in intestinal mucosa
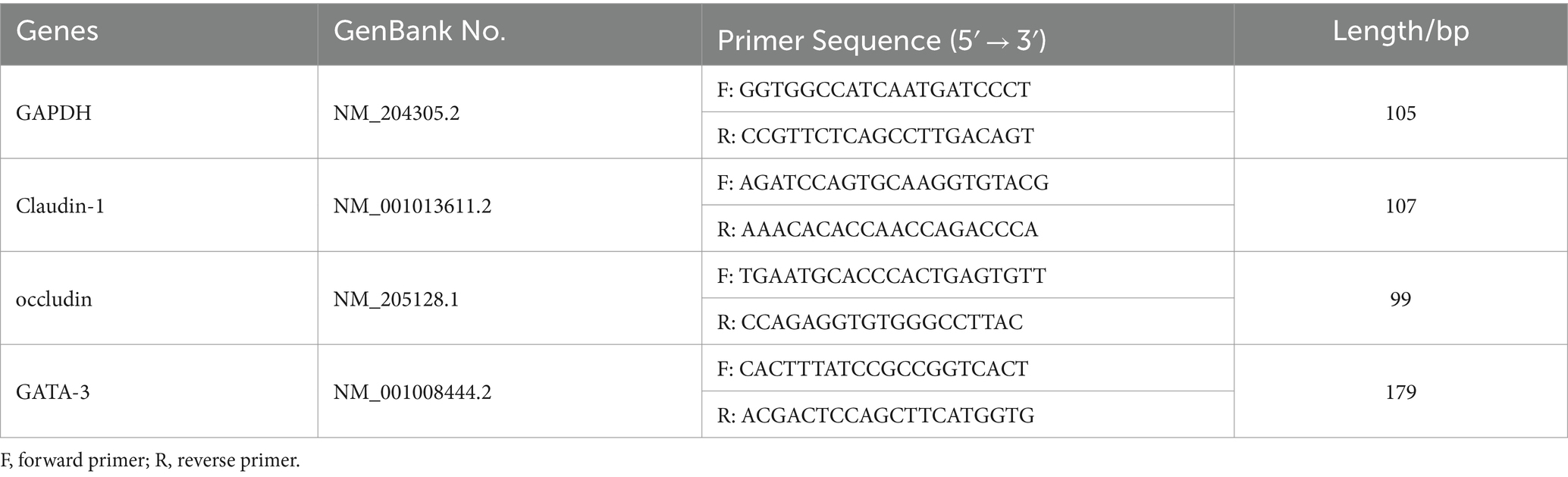
Primers were designed according to the gene sequences of GADPH, claudin-1, occludin and GATA-3 from the GenBank database. Primers for quantitative reverse transcription polymerase chain reaction (qRT-PCR) were designed using Primer 5.0 and the National Center for Biotechnology Information (NCBI). Primer-related information is shown in Table 2. Total RNA was extracted from each segment of the small intestine, and the integrity of the RNA was confirmed through agarose gel electrophoresis. The entire RNA was selected for reverse transcription, and the expression levels of claudin-1, occludin and GATA-3 genes in the intestinal segments of AA broilers were detected by RT-PCR. The expression of the target gene relative to the internal reference gene was calculated by 2−ΔΔCt method.
2.6 Statistical analysis
Data were analyzed using SPSS 20.0 (SPSS, Inc., Chicago, Illinois, United States) and compared using one-way analysis of variance (ANOVA) and Duncan’s multiple comparisons. The data is expressed as mean ± standard deviation (SD). p < 0.05 was considered significant.
3 Results
If there is no specific explanation, the following results were analyzed in comparison with the control group.
3.1 Growth performance
As shown in Table 3, dietary CHM-JM113 supplementation had no statistically significant impact (p > 0.05) on the ADG, ADFI and F/G during days 1 to 21, days 22 to 42, and during the whole period (days 1 to 42). Dietary supplementation with 0.25, 0.5, 1 and 2% CHM-JM113 significantly reduced (p < 0.01) mortality from days 1 to 42, and linearly (p < 0.01) and quadratically (p < 0.01) decreased the mortality. Additionally, dietary supplementation with 0.5, 1 and 2% CHM-JM113 increased the EBI (p < 0.05) for AA broiler breeding from days 1 to 42.
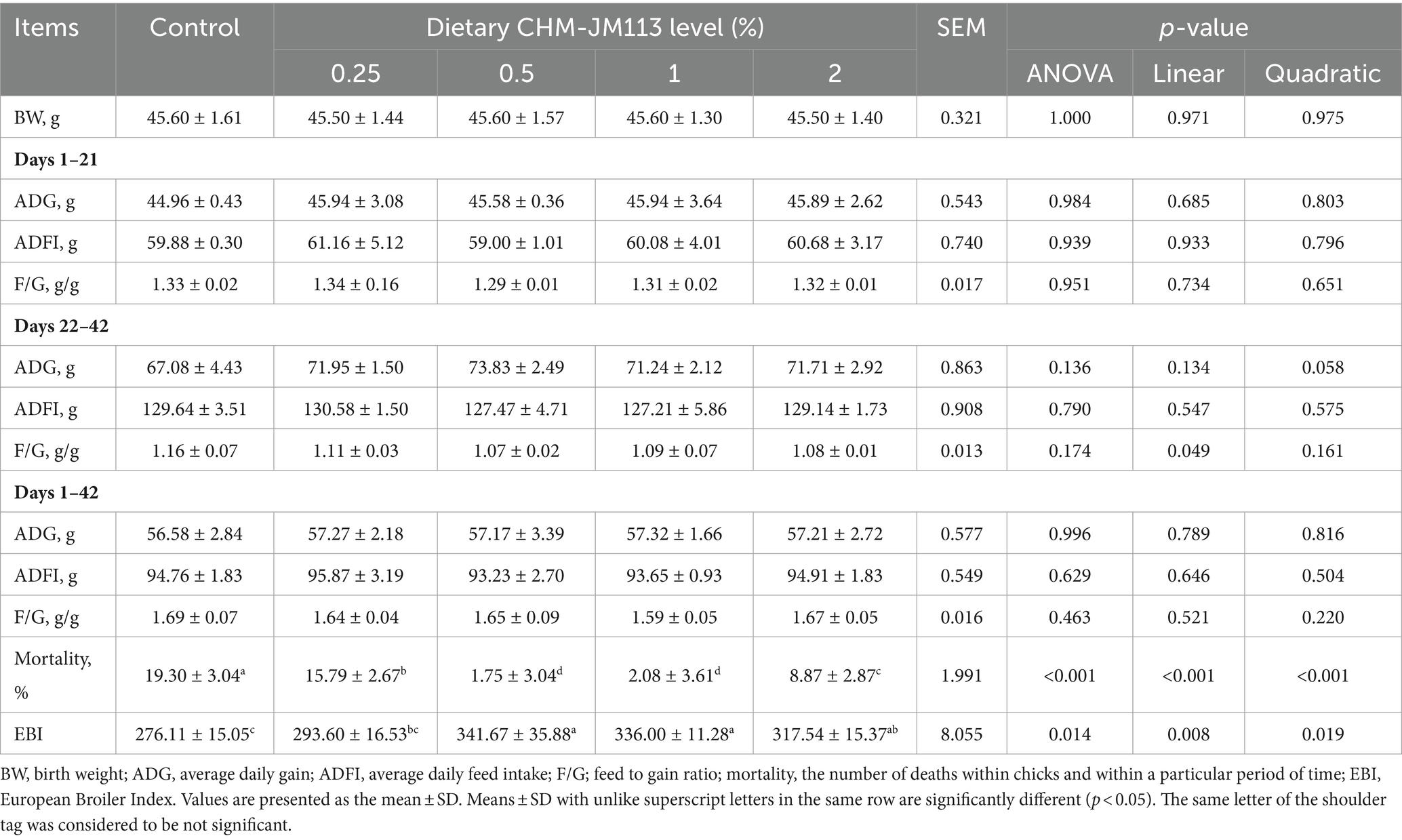
Table 3. Effect of experimental treatments on growth performances of broilers during 1 to 42 days of age.
3.2 Organ index
As shown in Table 4, at 21 days of age, the cardiac index of broilers was significantly increased (p < 0.05) in groups supplemented with 0.5 and 1% CHM-JM113. The liver index of 0.25, 0.5 and 2% CHM-JM113 was significantly higher than that of control group (p < 0.01), and the addition of 0.5% CHM-JM113 was found to be the most effective. The spleen index of the 0.5 and 1% CHM-JM113 groups was significantly higher than that of the control group (p < 0.01). The broilers fed with diet containing 1 and 2% CHM-JM113 also had a higher bursa of Fabricius index than those fed with basal diet (p < 0.05). Dietary supplementation with CHM-JM113 linearly increased the bursa index (p < 0.05) and quadratically increased the cardiac index, liver index, and spleen index of broilers at 21 days of age (p < 0.05). At 42 days of age, the cardiac index of 1% CHM-JM113 was significantly higher than that of the 0.5% CHM-JM113 group (p < 0.05). The 0.5% supplementation level of CHM-JM113 could significantly (p < 0.01) decrease the liver index of broilers, while 0.25 and 2% CHM-JM113 could also significantly (p < 0.05) increase the spleen index of broilers. The dietary addition of CHM-JM113 linearly increased the spleen index and quadratically increased the liver index of 42-day-old broilers.
3.3 Histomorphology of small intestine
The light microscope images of the intestinal morphology are shown in Figures 1, 2. The effects of CHM-JM113 on the intestinal morphology of broilers are shown on Tables 5, 6.
Figure 1. The duodenum, jejunum and ileum histological morphology of 21 days of broilers (hematoxylin and eosin). CON, broilers were fed with a basal diet; 0.25, 0.5, 1 and 2, broilers were fed with a basal diet supplemented with 0.25, 0.5, 1 and 2% CHM-JM113, respectively. Scale bars = 200 μm.
Figure 2. The duodenum, jejunum and ileum histological morphology of 42 days of broilers (hematoxylin and eosin). CON, broilers were fed with a basal diet; 0.25, 0.5, 1 and 2, broilers were fed with a basal diet supplemented with 0.25, 0.5, 1 and 2% CHM-JM113, respectively. Scale bars = 200 μm.
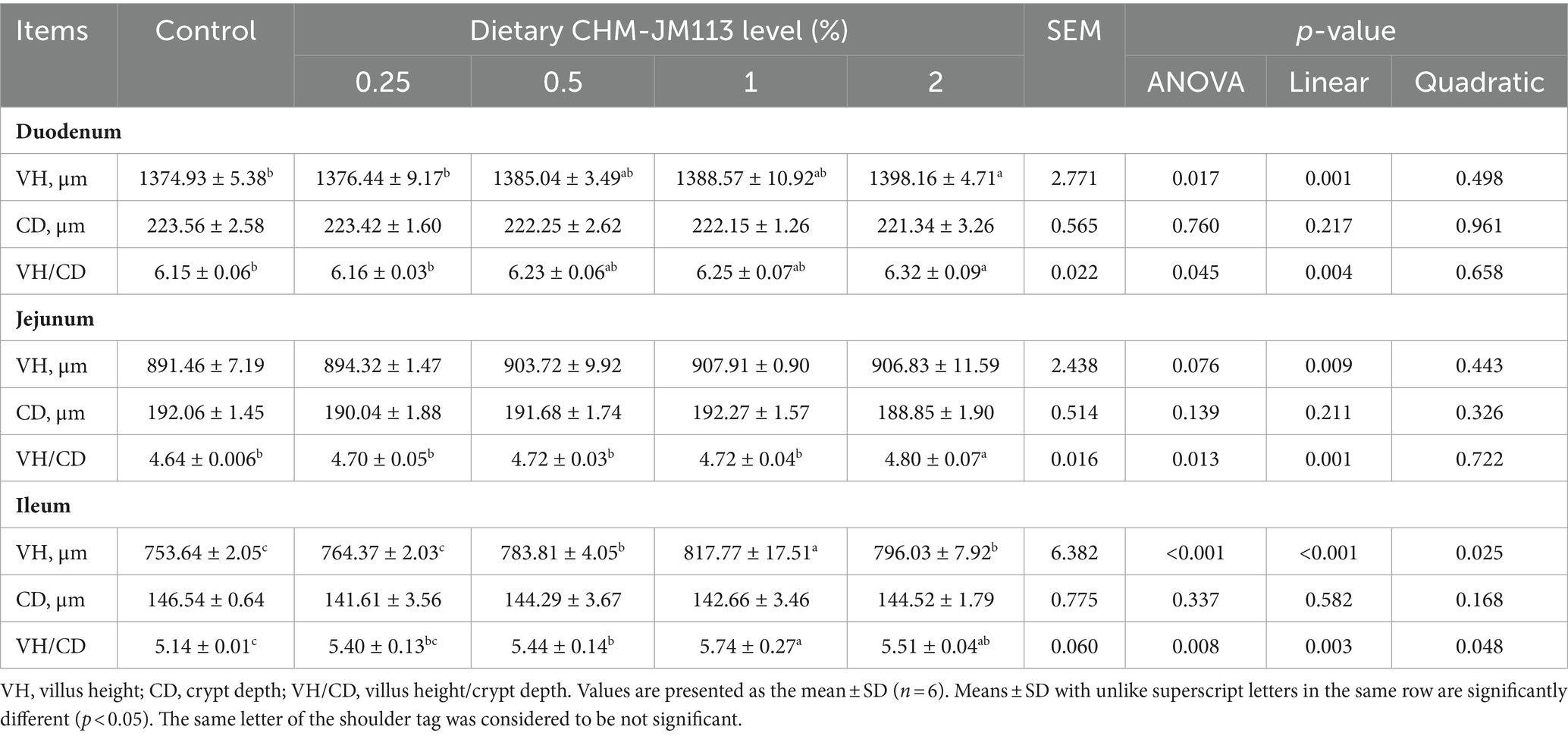
Table 5. Effects of different experimental treatments on intestinal morphology of 21-day-old broilers.
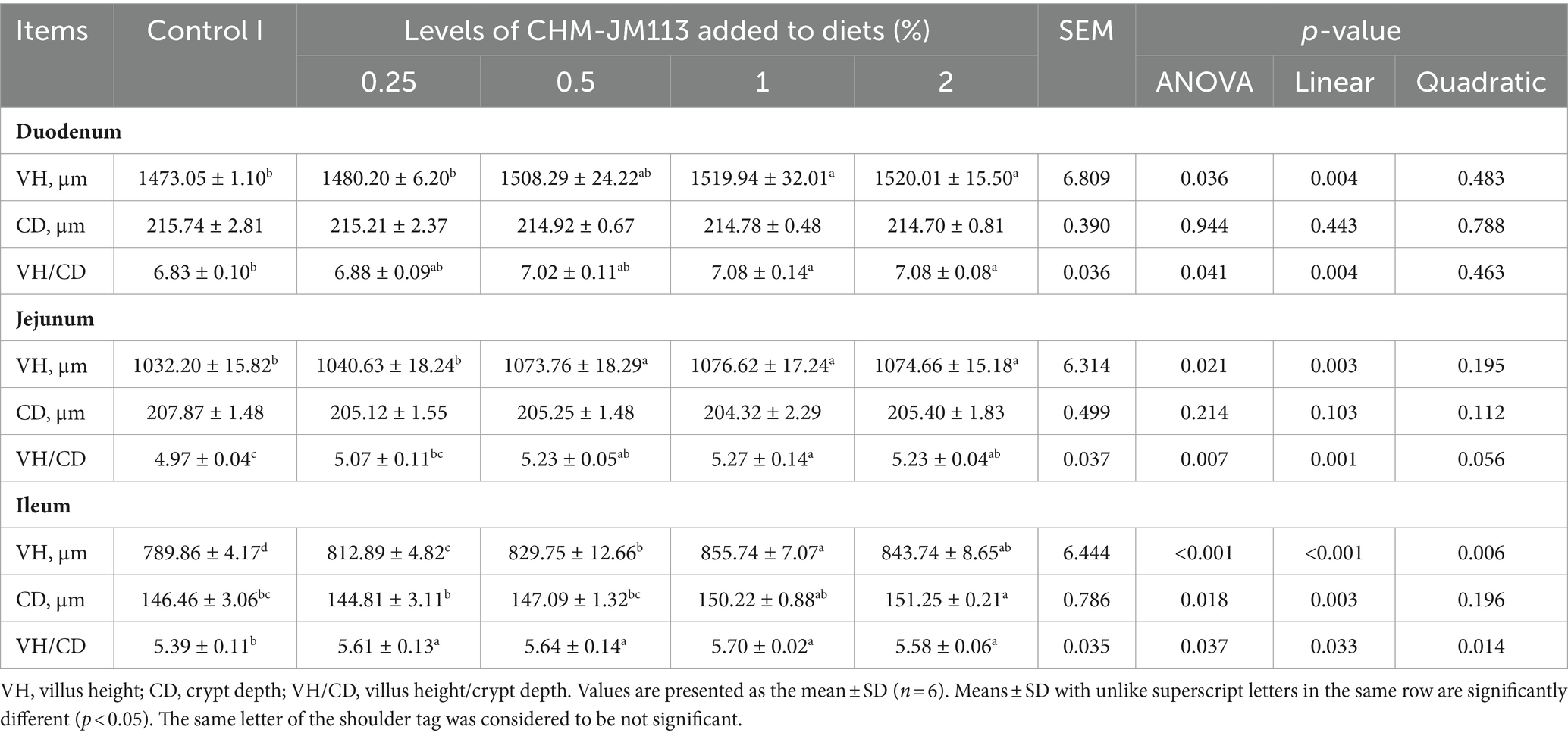
Table 6. Effects of different experimental treatments on intestinal morphology of 42-day-old broilers.
Table 5 demonstrates that in 21-day-old broilers, 2% CHM-JM113 resulted in increased VH and VH/CD ratio in the duodenum, as well as an increased VH/CD ratio in the jejunum (p < 0.05). These indices were also linearly elevated in comparison to the control group (p < 0.01). VH and VH/CD ratio in the ileum were significantly, linearly and quadratically enhanced (p < 0.01) when 0.5, 1, and 2% CHM-JM113 were added to the basal diet.
As observed in Table 6, at 42 days of age, 1 and 2% CHM-JM113 significantly (p < 0.05) increased VH and VH/CD ratio in the duodenum of broilers, while showing a linear effect (p < 0.01). The VH and VH/CD ratio in the jejunum of the 0.5, 1, and 2% CHM-JM113 groups was significantly higher than that of the control group. Additionally, the jejunal VH and VH/CD ratio values showed a linear increase with the addition of CHM-JM113 (p < 0.01). Meanwhile, the VH and VH/CD ratio of the ileum in CHM-JM113 treated groups with different levels of supplementation were significantly greater than those of the control group, and the addition of CHM-JM113 enhanced these indices in both quadratically and linearly (p < 0.05). Notably, the addition of 2% CHM-JM113 treatment group significantly and linearly increased CD values in the ileum of broilers (p < 0.05).
3.4 Serum antioxidant capacity
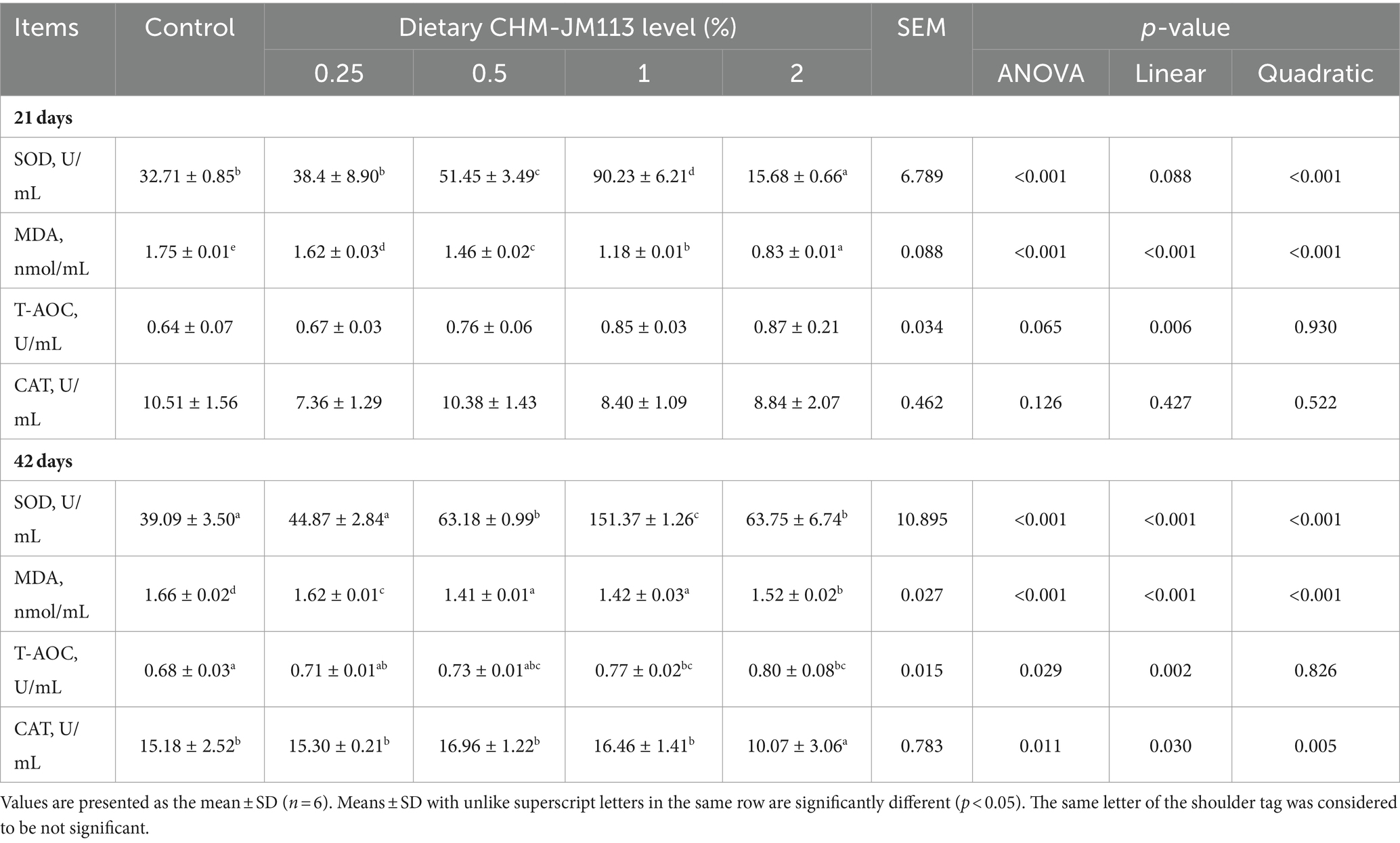
The impact of CHM-JM113 on the antioxidant properties of broilers is presented in Table 7. Compared with the control group, at 21 days of age, 0.5 and 1% CHM-JM113 significantly increased the serum SOD concentration of broilers (p < 0.01). Conversely, the addition of different levels of CHM-JM113 highly significantly decreased the serum MDA concentration (p < 0.01), and this reduction effect was dose-dependent. At 42 days of age, the SOD concentrations of 0.5, 1 and 2% CHM-JM113 were significantly higher than those of the control group (p < 0.01). The highest SOD concentration was found in 1% CHM-JM113. Additionally, the MDA concentrations in broiler serum were significantly reduced by different levels of CHM-JM113 (p < 0.01), and the best reduction observed in 0.5% CHM-JM113. The inclusion of 1 and 2% levels of CHM-JM113 notably increased the T-AOC level of broilers (p < 0.05). Furthermore, 2% CHM-JM113 significantly reduced the CAT level of broilers (p < 0.05).
3.5 Gene expression
As shown in Figure 3, adding 1% of CHM-JM113 to the duodenum of broiler chickens was found to significantly increase the level of occludin (p < 0.05) and to very significantly increase the levels of claudin-1 and GATA-3 (p < 0.01). Adding varying amounts of CHM-JM113 was found to promote the expression of GATA-3 in the duodenum (p < 0.01), with the 0.5% addition having the greatest effect in the duodenum (p < 0.01).
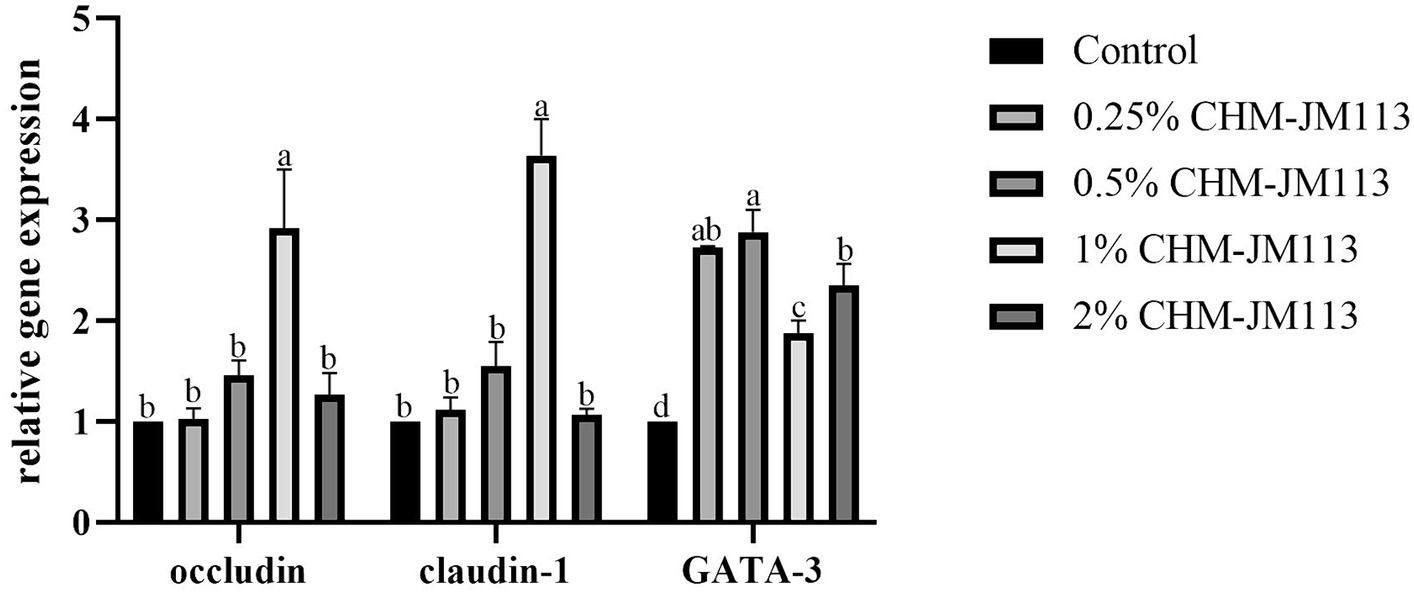
Figure 3. Effects of CHM-JM113 on gene expression levels of occludin, claudin-1 and GATA-3 in duodenum of broilers (day 21).
As can be seen from Figure 4, the addition of 0.25, 0.5 and 1% CHM-JM113 all significantly increased the expression level of occludin in the duodenum of broiler chickens extremely significantly (p < 0.01). The promotion effect increased linearly, but the intestinal occludin expression level of 2% addition level of CHM-JM113 was significantly lower than that of the control group (p < 0.01). The claudin-1 expression level of CHM-JM113 added at 0.25 and 0.5% was significantly higher than that of the control group (p < 0.01), and there was no significant difference in the promotional effect of the two addition levels of CHM-JM113 on claudin-1 expression in the jejunum (p > 0.05). The addition of 0.5% CHM-JM13 significantly increased the 42-day-old GATA-3 levels in broilers (p < 0.01), but the addition of 1 and 2% CHM-JM113 instead decreased GATA-3 levels (p < 0.01).
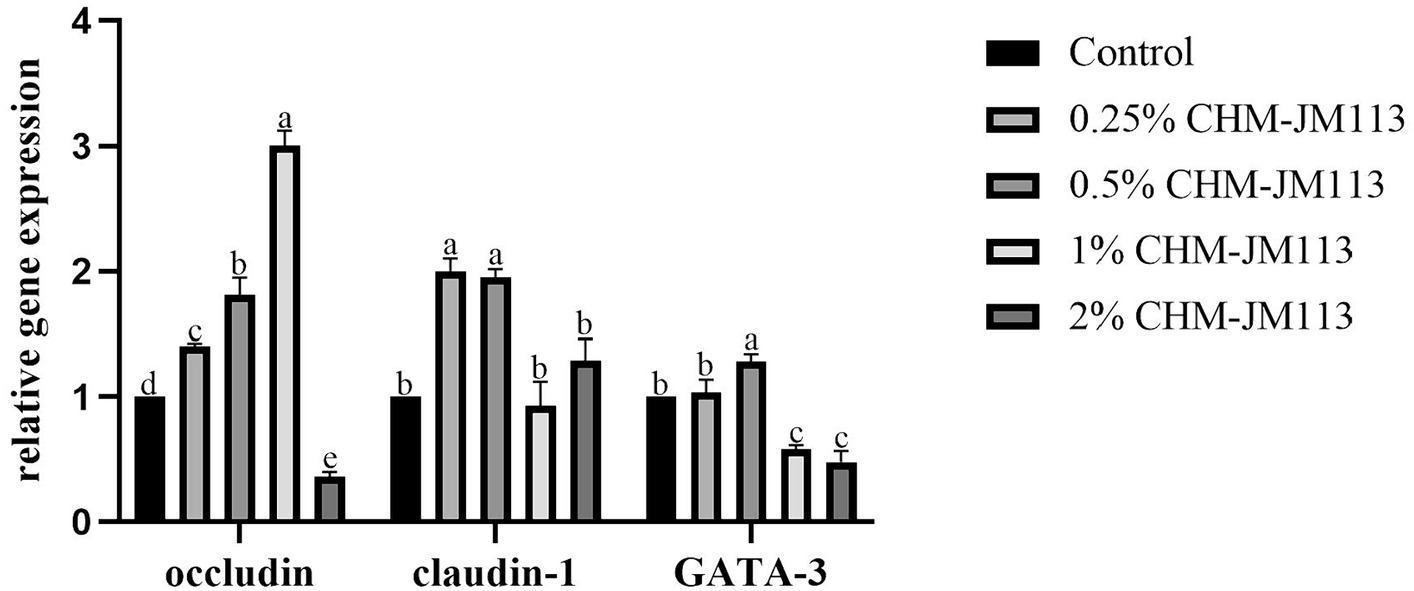
Figure 4. Effects of CHM-JM113 on gene expression levels of occludin, claudin-1 and GATA-3 in duodenum of broilers (day 42).
As can be seen from Figure 5, the adding of different proportions of CHM-JM113 had a linear effect on the expression of occludin in the intestines (p < 0.01). However, only at the addition of 2% CHM-JM113, the expression level was significantly higher than that of the control group (p < 0.01). The addition of 0.25% CHM-JM113 extremely significantly decreased the expression level of claudin-1 in the jejunum of 21-day-old broilers (p < 0.01). On the other hand, 0.5% CHM-JM113 significantly increased the expression of claudin-1 (p < 0.01), and 1 and 2% CHM-JM113 significantly decreased the level of GATA-3 expression in the jejunum (p < 0.05).
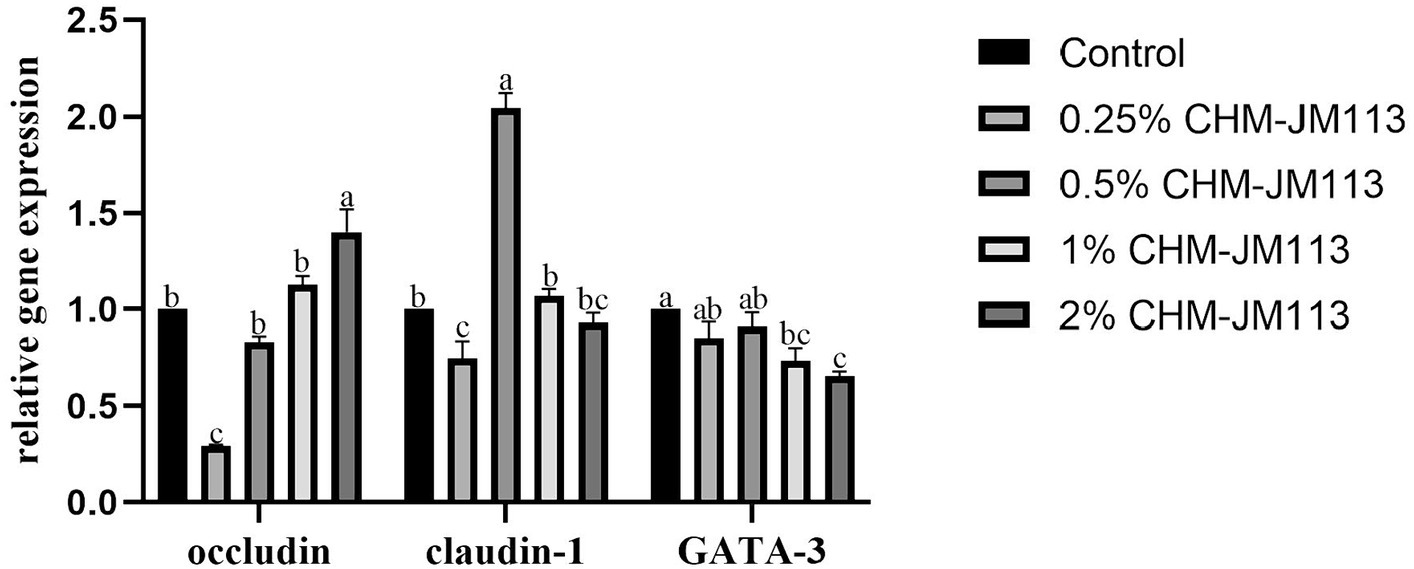
Figure 5. Effects of CHM-JM113 on gene expression levels of occludin, claudin-1 and GATA-3 in jejunum of broilers (day 21).
As can be seen in Figure 6, 0.5% CHM-JM113 significantly increased the occludin expression level in 42-day-old broilers (p < 0.01), while 2% CHM-JM113 significantly decreased its expression level (p < 0.01). Additionally, 0.5% CHM-JM113 significantly increased the claudin-1 expression level (p < 0.01); however, other levels of CHM-JM113 significantly decreased the expression of claudin-1 (p < 0.01). Furthermore, 0.25% CHM-JM113 significantly decreased the expression of GATA-3 in the jejunum of broilers (p < 0.01), and 1% CHM-JM113 highly significantly promoted the expression of GATA-3 (p < 0.01).
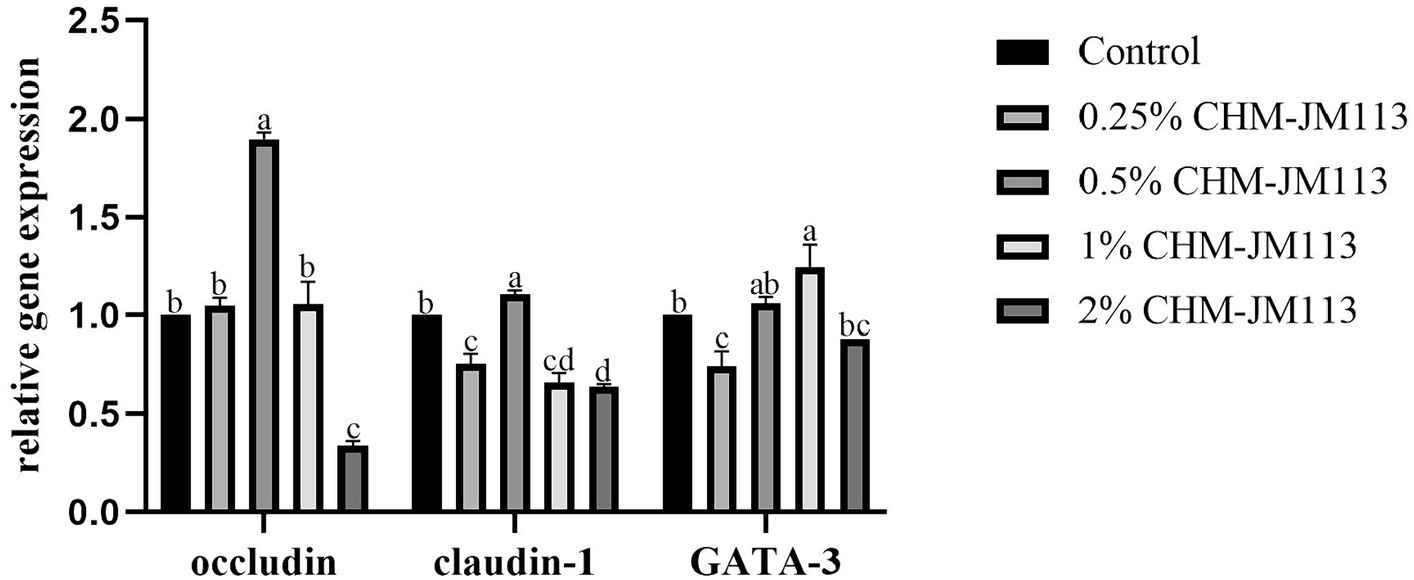
Figure 6. Effects of CHM-JM113 on gene expression levels of occludin, claudin-1 and GATA-3 in jejunum of broilers (day 42).
4 Discussion
4.1 Growth performance
Chinese herbal medicine is able to promote the proliferation of probiotics and has a turnover effect on intestinal flora imbalance. Probiotics also facilitate the dissolution of active ingredients of Chinese herbal medicine, reduce the toxic and side effects of Chinese herbal medicine (30), and improve its efficacy. Wang et al. (31) showed that Lactobacillus plantarum JM113 has the ability to inhibit the growth of Salmonella in broilers, reduce the incidence of broiler chickens, and reduce the mortality. Liang et al. (32) found that the combination of Chinese herbal medicine and lactic acid bacteria could enhance the intestinal sensitivity of broilers, inhibit the growth of Escherichia coli, and reduce the diarrhea rate and mortality of broilers. Consistent with the results of this experiment, the addition of CHM-JM113 in the diet significantly reduced the mortality of broilers. Both herbs and probiotics have been shown to promote growth in animals (33, 34). Theoretically, the growth-promoting effects of herbs and probiotics in broilers should have a cumulative effect, but in fact, in this study, the compound herbs and JM113 did not have a significant growth-promoting effect on broilers (p > 0.05), which was similar to the results of Majekodunmi et al. (35), who found that there was no significant difference in feed intake and weight gain of Ross broilers with sweet citrus peel. Sun et al. (36) found that dietary supplementation with Bacillus amyloliquefaciens CECT 5940 did not significantly promote the growth of broilers fed a corn-soybean meal diet. This may be due to the antibacterial effect of compound Chinese herbal medicine and probiotics (37), rather than their growth-promoting effects. Several studies have demonstrated that thyme, Astragalus, tangerine peel, and dandelion have antioxidant and immune-promoting effects (38–40) and partially growth-promoting effects (24). Furthermore, the intricate and variability of the synergistic potential of plant extract combinations (41) may be a contributing factor to the lack of improvement in broiler growth performance in this experiment. The strain specificity of the host’s immune and gut health response to probiotics (42) may also be a contributing factor to the non-significant differences in growth performance in this experiment.
4.2 Organ index
To a certain extent, organ index could reflect the development of animal organs, body weight changes and indirectly reflect the ability of animals to cope with external stress. The higher the index, the stronger the body’s immunity (43). The bursa of Fabricius is the site where B lymphocytes mature, and the spleen regulates the cellular immunity and humoral immunity of poultry by activating T lymphocytes, B lymphocytes and macrophages (44). Liang et al. (32) found that the combination of Chinese herbal medicine and probiotics improved the spleen index, thymus index and bursa index of broilers. This combined treatment had a more significant effect compared to using Chinese herbal medicine or probiotics alone. Li et al. (45) found that Astragalus polysaccharides significantly improved the thymus index and spleen index in vivo experiments, and were useful in restoring the damaged thymus and spleen tissue structure. In this experiment, the enhancement of the immune organ index in broilers in different degrees verified the previous perspective. The liver is the largest solid gland and an important metabolic organ, plays a vital role in excretion, metabolism, detoxification and the production of various coagulation factors (46). The growth performance of broilers may also decrease to a certain extent after liver damage (47). This experiment found that adding 0.3 g/mL of a compound Chinese herbal medicine with JM113 improved the liver index of broilers, indicating that Chinese herbal medicine is beneficial to liver health, which is consistent with previous studies (48). It is known that the combined use of compound herbs and JM113 promotes the development of the intestinal tract, thereby boosting the absorption of nutrients by the animal organism, which in turn supports the improvement of the organ index of broilers. The addition of CHM-JM113 in this experiment boosted the development of broiler organ indices to varying degrees, but was significantly influenced by the dosage, suggesting that the use of CHM-JM113 had a positive impact on the immunological performance of broilers.
4.3 Histomorphology of small intestine
The integrity of intestinal structure is very important for animals to absorb nutrients. Intestinal villus height (VH), crypt depth (CD) and VH/CD ratio are important indicators of intestinal structural integrity (49). Longer intestinal villus height and shallower crypt depth indicate better healthy digestion and absorption of microorganisms (10), improved intestinal mucosal differentiation, and enhanced capacity for digestion and absorption (50). Previous studies have found that adding probiotics, forsythia extract, natural pepper extract, and ellagic acid is an alternative to antibiotics in increasing the intestinal villus height of broilers (51, 52). This experiment found that compared with the control group, the combination of compound Chinese herbal medicine and JM113 improved the villus height and villus height ratio of the duodenum, jejunum and ileum of broilers, which is consistent with previous studies. This may be due to the fact that lactic acid bacteria, acting as probiotics, convert dietary fiber into short-chain fatty acids. They also promote the development of intestinal villi, improve the integrity of the intestinal barrier, and consequently improve the absorption of nutrients (53). Chinese herbal medicine is abundant in active ingredients, including lipids, unsaturated fatty acids, sugars, trace elements and vitamins, which provide sufficient nutrition for intestinal villus epithelial cells and support intestinal development (26). Studies have shown that there is a two-way communication between the liver and the intestine. Bile acids produced in the liver regulate the microbial composition and intestinal barrier function, while intestinal products regulate bile acid synthesis, as well as glucose and lipid metabolism in the liver. The intestinal barrier serves to restrict the systemic transmission of microorganisms and toxins, while facilitating the passage of nutrients into the portal vein circulation for delivery to the liver (54). Based on the gut-liver relationship, the increase of liver index is also benefits the absorption of nutrients by the intestine. In this experiment, the addition of compound Chinese herbal medicine and JM113 not only increased the liver index but also increased the absorption of nutrients in the small intestine, possibly as a result of the bidirectional communication between the liver and the intestine. The combined use of compound Chinese herbal medicine and JM113 can be observed to promote the development of the intestine, thereby enhancing the absorption of nutrients by the animal body and consequently improving the organ index of broilers.
4.4 Serum antioxidant capacity
Oxidative stress refers to the imbalance between antioxidants and free radicals, which may lead to the production of various reactive oxygen species (ROS) such as hydroxyl radicals and superoxide anions in the body (55). Excess ROS can disrupt animal metabolism, damage cell structure, and accelerate oxidation, leading to tissue damage and various diseases (56). Antioxidant enzyme activity and oxidation product concentration are reliable biomarkers for assessing the antioxidant status of animals (50). SOD catalyzes the disproportionation of superoxide anions into oxygen and H2O2 (57). CAT is a common enzyme that catalyzes the decomposition of H2O2 into water and oxygen. In addition, previous studies have shown that the removal rate of H2O2 removal in cells is partially dependent on GSH levels (58). The reduction reaction of lipid peroxide is catalyzed by glutathione peroxidase (GSH-Px), and the overall antioxidant capacity is indicated by the level of T-AOC (59). MDA is one of the end products of peroxidation derived from membrane lipids, reflecting the extent of lipid peroxidation and damage to antioxidant capacity in animals (60). Yin et al. (59) found that adding LBPs to the diet could increase the serum total antioxidant capacity (T-AOC) and GSH-Px activity, and decrease the MDA level (p < 0.05). Abo-Samaha et al. (61) found that adding licorice (0.4 g/L and 0.8 g/L) to drinking water resulted in antioxidant activity, leading to a reduction in MDA levels and an increase in GSH levels and CAT activity. Various active ingredients in thyme, Astragalus, tangerine peel and dandelion have been shown to reduce the effects of peroxides on animal bodies and enhance the body’s antioxidant capacity (62–64). In this experiment, the concurrent administration of Chinese herbal medicine and JM113 resulted in increased levels of SOD, T-AOC and MDA in 21-day-old broilers. Additionally, the SOD level of 42-day-old broilers was also improved. The liver is the primary site of free radical formation. Shati et al. (65) found that aqueous extracts of thyme and ginger have detoxifying and antioxidant effects on the liver and brain of mice. Antioxidants like thyme and rosemary have been utilized as therapeutic agents to alleviate liver damage (66). Additionally, dandelion root is also believed to have a beneficial effect on the gastrointestinal system by stimulating digestion and liver function (67). The increase in the liver index in this experiment also supports the positive impact of CHM-JM113 on the antioxidant performance of broilers from another perspective.
4.5 Gene expression
Nutrients entering the animal intestine will be selectively digested and absorbed by the intestine. This is due to the physical barrier on the intestinal surface, which prevents harmful substances from entering the bloodstream through the intestinal mucosa and reduces the damage caused by these substances to the body (68). The intestinal physical barrier is composed of tight junction structures (TJ) and mucosal epithelial cells. TJ regulate the permeability of the intestinal barrier by sealing the paracellular space between adjacent epithelial cells, thereby preventing the spread of microorganisms and antigens (69). This is also a key indicator for assessing intestinal health. The regulation of intestinal tight junctions is mediated by related proteins, such as occludin, claundin-1 and MUC2. Occludin and claudin-1 play an important role in maintaining epithelial cell polarity and regulating intestinal barrier permeability (70). The up-regulation of occludin may indicate an increase in cell permeability to nutrients, which facilitates the digestion and absorption of nutrients (71). GATA-3 is a significant regulator of T-cell differentiation and a Th2 cell-specific transcription factor (72). Gene-targeting studies have demonstrated that GATA-3 plays a vital role in the development and function of T cells, B cells, CD1 natural killer cells (NKT cells), natural killer cells (NK), and innate lymphoid cells (ILCs) (73). This experiment revealed that the addition of CHM-JM113 significantly enhanced the expression of occludin and claudin-1 in the intestine and to varying extents, improved the integrity of the intestinal barrier and organ indices. This may be due to the fact that the intestinal mucosal immune system influences the development of immune organs, including the spleen, thymus, and secondary immune organs, as well as immune responses (74), which in turn promotes the expression of occludin, claudin-1, and GATA-3 in the intestine, consistent with previous research findings (75).
In summary, CHM-JM113 may be considered as an alternative to antibiotics for broiler production, but it does not improve the performance of the animal and economic considerations need to be taken into account.
5 Conclusion
In summary, the use of CHM-JM113 has been shown to reduce the mortality of AA broilers, increase the European Broiler Index (EBI), and improve the organ index of broilers, particularly the immunological organ index. In addition, CHM-JM113 promoted the growth of villi in the small intestine, increased the VH/CD ratio of each intestinal segment, enhanced the organism’s antioxidant performance, and up-regulated the expression of occludin, claudin-1 and GATA-3 in the gut. CHM-JM113 can be used as a substitute for antibiotics, but the specific mechanism needs further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Animal Welfare and Ethics Committee of Hebei University of Engineering. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GF: Conceptualization, Investigation, Supervision, Writing – review & editing. MZ: Data curation, Formal analysis, Software, Writing – original draft. LW: Funding acquisition, Project administration, Writing – review & editing. YuaH: Investigation, Conceptualization, Methodology, Resources,Writing – original draft. RH: Validation, Resources, Writing–original draft. KQ: Investigation, Methodology, Writing–original draft. LY: Date curation, Visualization, Writing–review & editing. DZ: Resources, Methodology, Writing–original draft. YueH: Formal analysis, software, Writing–original draft. TM: Visualization, Vvalidation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hebei Natural Science Foundation, China (C2021402036 and C2021402020) and Hebei Science and Technology Agency, China (22327212D).
Acknowledgments
We would like to express our gratitude to Gaobai Family Farm in Linzhang County, Handan City for generously providing a test site for this experiment. We also extend our thanks to the School of Life Sciences and Food Engineering of Hebei University of Engineering for supplying the Lactobacillus plantarum JM113 strain, and to the graduate students of Animal Husbandry at Hebei University of Engineering for their assistance in collecting samples.
Conflict of interest
YuaH was employed by Muyuan Foods Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author note
Chinese herbal medicines used in this experiment, including thyme, Astragalus, tangerine peel and dandelion, do not violate the taboos of “eighteen antagonisms and nineteen antagonisms.” The results indicated that the compound Chinese herbal medicine selected in this experiment had no significant effect on the activity of JM113. The plate count showed that 1 g of JM113 powder contained 4.4 × 109 CFU of viable bacteria.
References
1. Tian, M, He, X, Feng, Y, Wang, W, Chen, H, Gong, M, et al. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics. (2021) 10:539. doi: 10.3390/antibiotics10050539
2. Cully, M. Public health: the politics of antibiotics. Nature. (2014) 509:S16–7. doi: 10.1038/509S16a
3. Aslam, B, Khurshid, M, Arshad, MI, Muzammil, S, Rasool, M, Yasmeen, N, et al. Antibiotic resistance: one health one world outlook. Front Cell Infect Microbiol. (2021) 11:771510. doi: 10.3389/fcimb.2021.771510
4. Lee, S, Fan, P, Liu, T, Yang, A, Boughton, RK, Pepin, KM, et al. Transmission of antibiotic resistance at the wildlife-livestock interface. Commun Biol. (2022) 5:585. doi: 10.1038/s42003-022-03520-8
5. Chen, J, Ying, GG, and Deng, WJ. Antibiotic residues in food: extraction, analysis, and human health concerns. J Agric Food Chem. (2019) 67:7569–86. doi: 10.1021/acs.jafc.9b01334
6. Wan, F, Deng, FL, Chen, L, Zhong, RQ, Wang, MY, Yi, B, et al. Long-term chemically protected sodium butyrate supplementation in broilers as an antibiotic alternative to dynamically modulate gut microbiota. Poult Sci. (2022) 101:102221. doi: 10.1016/j.psj.2022.102221
7. Li, C, Li, Y, Li, X, Ma, X, Ru, S, Qiu, T, et al. Veterinary antibiotics and estrogen hormones in manures from concentrated animal feedlots and their potential ecological risks. Environ Res. (2021) 198:110463. doi: 10.1016/j.envres.2020.110463
8. Xu, L, Wang, W, and Xu, W. Effects of tetracycline antibiotics in chicken manure on soil microbes and antibiotic resistance genes (ARGs). Environ Geochem Health. (2022) 44:273–84. doi: 10.1007/s10653-021-01004-y
9. Gu, Y, Shen, S, Han, B, Tian, X, Yang, F, and Zhang, K. Family livestock waste: an ignored pollutant resource of antibiotic resistance genes. Ecotoxicol Environ Saf. (2020) 197:110567. doi: 10.1016/j.ecoenv.2020.110567
10. Zhou, X, Li, S, Jiang, Y, Deng, J, Yang, C, Kang, L, et al. Use of fermented Chinese medicine residues as a feed additive and effects on growth performance, meat quality, and intestinal health of broilers. Front Vet Sci. (2023) 10:1157935. doi: 10.3389/fvets.2023.1157935
11. Huemer, M, Mairpady Shambat, S, Brugger, SD, and Zinkernagel, AS. Antibiotic resistance and persistence-implications for human health and treatment perspectives. EMBO Rep. (2020) 21:e51034. doi: 10.15252/embr.202051034
12. Bacanlı, M, and Başaran, N. Importance of antibiotic residues in animal food. Food Chem Toxicol. (2019) 125:462–6. doi: 10.1016/j.fct.2019.01.033
13. Lillehoj, H, Liu, Y, Calsamiglia, S, Fernandez-Miyakawa, ME, Chi, F, Cravens, RL, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. (2018) 49:76. doi: 10.1186/s13567-018-0562-6
14. Abd El-Hack, ME, El-Saadony, MT, Salem, HM, El-Tahan, AM, Soliman, MM, Youssef, GBA, et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult Sci. (2022) 101:101696. doi: 10.1016/j.psj.2022.101696
15. Yin, B, Li, W, Qin, H, Yun, J, and Sun, X. The use of Chinese skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals. (2021) 11:1039. doi: 10.3390/ani11041039
16. Rafeeq, M, Bilal, RM, Batool, F, Yameen, K, Farag, MR, Madkour, M, et al. Application of herbs and their derivatives in broiler chickens: a review. Worlds Poult Sci J. (2023) 79:95–117. doi: 10.1080/00439339.2022.2151395
17. Wang, D, Yang, Y, Li, J, Wang, B, and Zhang, A. Enhancing immune responses to inactivated foot-and-mouth virus vaccine by a polysaccharide adjuvant of aqueous extracts from Artemisia rupestris. J Vet Sci. (2021) 22:e30. doi: 10.4142/jvs.2021.22.e30
18. Miao, M, and Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv Pharmacol. (2020) 87:71–88. doi: 10.1016/bs.apha.2019.12.002
19. Speisky, H, Shahidi, F, Costa de Camargo, A, and Fuentes, J. Revisiting the oxidation of flavonoids: loss, conservation or enhancement of their antioxidant properties. Antioxidants. (2022) 11:133. doi: 10.3390/antiox11010133
20. Placha, I, Ocelova, V, Chizzola, R, Battelli, G, Gai, F, Bacova, K, et al. Effect of thymol on the broiler chicken antioxidative defence system after sustained dietary thyme oil application. Br Poult Sci. (2019) 60:589–96. doi: 10.1080/00071668.2019.1631445
21. Li, S, Wang, XF, Ren, LN, Li, JL, Zhu, XD, Xing, T, et al. Protective effects of Γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult Sci. (2019) 98:6400–10. doi: 10.3382/ps/pez478
22. Singh, B, Singh, JP, Kaur, A, and Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res Int. (2020) 132:132. doi: 10.1016/j.foodres.2020.109114
23. Huang, R, Zhang, Y, Shen, S, Zhi, Z, Cheng, H, Chen, S, et al. Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: an in vitro study. Food Chem. (2020) 326:126785. doi: 10.1016/j.foodchem.2020.126785
24. Li, X, Sun, R, Liu, Q, Gong, Y, Ou, Y, Qi, Q, et al. Effects of dietary supplementation with dandelion tannins or soybean isoflavones on growth performance, antioxidant function, intestinal morphology, and microbiota composition in Wenchang chickens. Front Vet Sci. (2022) 9:1073659. doi: 10.3389/fvets.2022.1073659
25. Mao, J, Wang, Y, Wang, W, Duan, T, Yin, N, Guo, T, et al. Effects of Taraxacum mongolicum Hand.-Mazz. (dandelion) on growth performance, expression of genes coding for tight junction protein and mucin, microbiota composition and short chain fatty acids in ileum of broiler chickens. BMC Vet Res. (2022) 18:180. doi: 10.1186/s12917-022-03278-5
26. Liu, B, Ma, R, Yang, Q, Yang, Y, Fang, Y, Sun, Z, et al. Effects of traditional Chinese herbal feed additive on production performance, egg quality, antioxidant capacity, immunity and intestinal health of laying hens. Animals. (2023) 13:2510. doi: 10.3390/ani13152510
27. Cameron, A, and McAllister, TA. Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Benefic Microbes. (2019) 10:773–99. doi: 10.3920/bm2019.0059
28. Wu, S, Liu, Y, Duan, Y, Wang, F, Guo, F, Yan, F, et al. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum Jm113 and consequentially altered gut microbiota in broiler chickens. J Anim Sci Biotechnol. (2018) 9:74. doi: 10.1186/s40104-018-0286-5
29. Li, Z, Zhang, B, Zhu, W, Lin, Y, Chen, J, Zhu, F, et al. Effects of nonantibiotic growth promoter combinations on growth performance, nutrient utilization, digestive enzymes, intestinal morphology, and cecal microflora of broilers. PLoS One. (2023) 18:e0279950. doi: 10.1371/journal.pone.0279950
30. Liu, SH, Chuang, WC, Lam, W, Jiang, Z, and Cheng, YC. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf. (2015) 38:117–28. doi: 10.1007/s40264-014-0250-z
31. Wang, L, Fu, G, Liu, S, Li, L, and Zhao, X. Effects of oxygen levels and a Lactobacillus plantarum strain on mortality and immune response of chickens at high altitude. Sci Rep. (2019) 9:16037. doi: 10.1038/s41598-019-52514-w
32. Liang, W, Li, H, Zhou, H, Wang, M, Zhao, X, Sun, X, et al. Effects of Taraxacum and Astragalus extracts combined with probiotic bacillus subtilis and Lactobacillus on Escherichia coli-infected broiler chickens. Poult Sci. (2021) 100:101007. doi: 10.1016/j.psj.2021.01.030
33. Liu, M, Zhou, J, Li, Y, Ding, Y, Lian, J, Dong, Q, et al. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult Sci. (2023) 102:102714. doi: 10.1016/j.psj.2023.102714
34. Chai, C, Guo, Y, Mohamed, T, Bumbie, GZ, Wang, Y, Zeng, X, et al. Dietary Lactobacillus reuteri Sl001 improves growth performance, health-related parameters, intestinal morphology and microbiota of broiler chickens. Animals. (2023) 13:1690. doi: 10.3390/ani13101690
35. Majekodunmi, BC, Logunleko, MO, Adekunle, EO, Abioja, MO, Akinjute, OF, Owolabi, TO, et al. Evaluation of sweet citrus peel supplement in water on performance and ileal microbial count of broiler chickens. Trop Anim Health Prod. (2021) 53:405. doi: 10.1007/s11250-021-02858-1
36. Sun, Y, Zhang, Y, Liu, M, Li, J, Lai, W, Geng, S, et al. Effects of dietary Bacillus amyloliquefaciens Cect 5940 supplementation on growth performance, antioxidant status, immunity, and digestive enzyme activity of broilers fed corn-wheat-soybean meal diets. Poult Sci. (2022) 101:101585. doi: 10.1016/j.psj.2021.101585
37. Xie, J, Yu, Q, Nie, S, Fan, S, Xiong, T, and Xie, M. Effects of Lactobacillus plantarum Ncu116 on intestine mucosal immunity in immunosuppressed mice. J Agric Food Chem. (2015) 63:10914–20. doi: 10.1021/acs.jafc.5b04757
38. Gholami-Ahangaran, M, Ahmadi-Dastgerdi, A, Azizi, S, Basiratpour, A, Zokaei, M, and Derakhshan, M. Thymol and Carvacrol supplementation in poultry health and performance. Vet Med Sci. (2022) 8:267–88. doi: 10.1002/vms3.663
39. Shimamura, Y, Sei, S, Nomura, S, and Masuda, S. Protective effects of dried mature Citrus unshiu peel (Chenpi) and hesperidin on aspirin-induced oxidative damage. J Clin Biochem Nutr. (2021) 68:149–55. doi: 10.3164/jcbn.20-83
40. Sultan, MT, Butt, MS, Qayyum, MM, and Suleria, HA. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr. (2014) 54:1298–308. doi: 10.1080/10408398.2011.633249
41. Abdallah, EM, Alhatlani, BY, de Paula, MR, and Martins, CHG. Back to nature: medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants. (2023) 12:3077. doi: 10.3390/plants12173077
42. Bilal, M, Si, W, Barbe, F, Chevaux, E, Sienkiewicz, O, and Zhao, X. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health, and immunity of broiler chickens raised under suboptimal conditions. Poult Sci. (2021) 100:100871. doi: 10.1016/j.psj.2020.11.048
43. Wu, QJ, Zheng, XC, Wang, T, and Zhang, TY. Effects of dietary supplementation with oridonin on the growth performance, relative organ weight, lymphocyte proliferation, and cytokine concentration in broiler chickens. BMC Vet Res. (2018) 14:34. doi: 10.1186/s12917-018-1359-6
44. Choi, WJ, Kim, JH, Han, GP, Kwon, CH, and Kil, DY. Effects of dietary hatchery by-products on growth performance, relative organ weight, plasma measurements, immune organ index, meat quality, and tibia characteristics of broiler chickens. Anim Biosci. (2021) 34:1181–92. doi: 10.5713/ab.20.0755
45. Li, W, Hu, X, Wang, S, Jiao, Z, Sun, T, Liu, T, et al. Characterization and anti-tumor bioactivity of Astragalus polysaccharides by immunomodulation. Int J Biol Macromol. (2020) 145:985–97. doi: 10.1016/j.ijbiomac.2019.09.189
46. Liu, Y, Li, Y, Niu, J, Liu, H, Jiao, N, Huang, L, et al. Effects of dietary Macleaya cordata extract containing isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth performance and liver health of broiler chickens. Front Vet Sci. (2022) 9:950174. doi: 10.3389/fvets.2022.950174
47. Liu, YL, Ding, KN, Shen, XL, Liu, HX, Zhang, YA, Liu, YQ, et al. Chronic heat stress promotes liver inflammation in broilers via enhancing Nf-Κb and Nlrp3 signaling pathway. BMC Vet Res. (2022) 18:289. doi: 10.1186/s12917-022-03388-0
48. Yuan, P, Xu, H, Ma, Y, Niu, J, Liu, Y, Huang, L, et al. Effects of dietary Galla chinensis tannin supplementation on immune function and liver health in broiler chickens challenged with lipopolysaccharide. Front Vet Sci. (2023) 10:1126911. doi: 10.3389/fvets.2023.1126911
49. Paiva, D, Walk, C, and McElroy, A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult Sci. (2014) 93:2752–62. doi: 10.3382/ps.2014-04148
50. Li, Z, Long, L, Jin, X, Li, Y, Wu, Q, Chen, X, et al. Effects of Clostridium butyricum on growth performance, meat quality, and intestinal health of broilers. Front Vet Sci. (2023) 10:1107798. doi: 10.3389/fvets.2023.1107798
51. Wang, F, Chen, J, Yin, Y, Yang, M, Xiao, Y, Cheng, Y, et al. The effects of dietary ellagic acid supplementation on growth performance, immune response, antioxidant activity, digestive enzyme activities, and intestinal functions in yellow-feathered broilers. J Anim Sci. (2022) 100:skac301. doi: 10.1093/jas/skac301
52. Long, SF, He, TF, Wu, D, Yang, M, and Piao, XS. Forsythia suspensa extract enhances performance via the improvement of nutrient digestibility, antioxidant status, anti-inflammatory function, and gut morphology in broilers. Poult Sci. (2020) 99:4217–26. doi: 10.1016/j.psj.2020.05.011
53. Liao, X, Shao, Y, Sun, G, Yang, Y, Zhang, L, Guo, Y, et al. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult Sci. (2020) 99:5883–95. doi: 10.1016/j.psj.2020.08.033
54. Li, Z, Fang, X, Hu, X, Li, C, Wan, Y, and Yu, D. Amelioration of alcohol-induced acute liver injury in C57bl/6 mice by a mixture of TCM phytochemicals and probiotics with antioxidative and anti-inflammatory effects. Front Nutr. (2023) 10:1144589. doi: 10.3389/fnut.2023.1144589
55. Xu, Y, Yu, Y, Shen, Y, Li, Q, Lan, J, Wu, Y, et al. Effects of bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult Sci. (2021) 100:101358. doi: 10.1016/j.psj.2021.101358
56. Jiang, S, Yang, C, Xiao, Y, Zheng, S, Jiang, Q, and Chen, J. Effects of polysaccharides-rich extract from Gracilaria lemaneiformis on growth performance, antioxidant capacity, immune function, and meat quality in broiler chickens. J Poult Sci. (2023) 60:2023018. doi: 10.2141/jpsa.2023018
57. Zhao, XC, Zhang, L, Yu, HX, Sun, Z, Lin, XF, Tan, C, et al. Curcumin protects mouse neuroblastoma neuro-2a cells against hydrogen-peroxide-induced oxidative stress. Food Chem. (2011) 129:387–94. doi: 10.1016/j.foodchem.2011.04.089
58. Ng, CF, Schafer, FQ, Buettner, GR, and Rodgers, VG. The rate of cellular hydrogen peroxide removal shows dependency on GSH: mathematical insight into in vivo H2O2 and Gpx concentrations. Free Radic Res. (2007) 41:1201–11. doi: 10.1080/10715760701625075
59. Yin, Y, Wang, F, Yang, M, Tan, B, Yin, Y, Chen, J, et al. Lycium barbarum polysaccharides as antibiotic substitutes improve growth performance, serum immunity, antioxidant status, and intestinal health for weaned piglets. Front Microbiol. (2021) 12:819993. doi: 10.3389/fmicb.2021.819993
60. Xu, Q, Cheng, M, Jiang, R, Zhao, X, Zhu, J, Liu, M, et al. Effects of dietary supplement with a Chinese herbal mixture on growth performance, antioxidant capacity, and gut microbiota in weaned pigs. Front Vet Sci. (2022) 9:971647. doi: 10.3389/fvets.2022.971647
61. Abo-Samaha, MI, Alghamdi, YS, el-Shobokshy, SA, Albogami, S, El-Maksoud, EMA, Farrag, F, et al. Licorice extract supplementation affects antioxidant activity, growth-related genes, lipid metabolism, and immune markers in broiler chickens. Life. (2022) 12:914. doi: 10.3390/life12060914
62. Gu, T, Lu, L, Xu, W, Zeng, T, Tian, Y, Chen, B, et al. Immunopotentiators improve the antioxidant defense, apoptosis, and immune response in Shaoxing ducklings. Poult Sci. (2022) 101:101641. doi: 10.1016/j.psj.2021.101641
63. Li, CX, Liu, Y, Zhang, YZ, Li, JA-O, and Lai, J. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch Pharm Res. (2022) 45:367–89. doi: 10.1007/s12272-022-01393-3
64. Liu, H, Li, X, Shi, S, Zhou, Y, Zhang, K, Wang, Y, et al. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult Sci. (2022) 101:102036. doi: 10.1016/j.psj.2022.102036
65. Shati, AA, and Elsaid, FG. Effects of water extracts of thyme (Thymus vulgaris) and ginger (Zingiber officinale roscoe) on alcohol abuse. Food Chem Toxicol. (2009) 47:1945–9. doi: 10.1016/j.fct.2009.05.007
66. Vitaglione, P, Morisco, F, Caporaso, N, and Fogliano, V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr. (2004) 44:575–86. doi: 10.1080/10408690490911701
67. Balenović, M, Savić, V, Janjecic, Z, Popović, M, Šimpraga, B, Carović-Stanko, K, et al. Immunomodulatory and antimicrobial effects of selected herbs on laying hens. Vet Arh. (2018) 88:673–86. doi: 10.24099/vet.arhiv.0104
68. Duangnumsawang, Y, Zentek, J, and Goodarzi, BF. Development and functional properties of intestinal mucus layer in poultry. Front Immunol. (2021) 12:745849. doi: 10.3389/fimmu.2021.745849
69. Li, E, Horn, N, and Ajuwon, KM. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch Toxicol. (2021) 95:2065–79. doi: 10.1007/s00204-021-03044-w
70. Luo, C, Wang, L, and Yuan, J. Supplemental enzymes and probiotics on the gut health of broilers fed with a newly harvested corn diet. Poult Sci. (2023) 102:102740. doi: 10.1016/j.psj.2023.102740
71. Zhou, HJ, Kong, LL, Zhu, LX, Hu, XY, Busye, J, and Song, ZG. Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal. (2021) 15:100138. doi: 10.1016/j.animal.2020.100138
72. Ho, IC, Tai, TS, and Pai, SY. Gata3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. (2009) 9:125–35. doi: 10.1038/nri2476
73. Wan, YY. Gata3: a master of many trades in immune regulation. Trends Immunol. (2014) 35:233–42. doi: 10.1016/j.it.2014.04.002
74. Li, Y, Lei, X, Yin, Z, Guo, W, Wu, S, and Yang, X. Transgenerational effects of paternal dietary Astragalus polysaccharides on spleen immunity of broilers. Int J Biol Macromol. (2018) 115:90–7. doi: 10.1016/j.ijbiomac.2018.04.009
Keywords: Chinese herbal medicine, JM113, growth performance, antioxidant capacity, organ index, intestinal health, AA broiler
Citation: Fu G, Zhang M, Huang Y, Han R, Qi K, Yin L, Zhao D, Huang Y, Ma T and Wang L (2024) Effects of different addition levels of CHM-JM113 on growth performance, antioxidant capacity, organ index, and intestinal health of AA broilers. Front. Vet. Sci. 11:1388173. doi: 10.3389/fvets.2024.1388173
Edited by:
Tugay Ayasan, Osmaniye Korkut Ata University, TürkiyeReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceMilena Milojević, Academy of Professional Studies Sabac, Serbia
Memiş Bolacali, Ahi Evran University, Türkiye
Copyright © 2024 Fu, Zhang, Huang, Han, Qi, Yin, Zhao, Huang, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Wang, MTg3MTAzNTQzMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Guanhua Fu1†
Guanhua Fu1† Mengyu Zhang
Mengyu Zhang