- 1College of Acupuncture and Massage, Changchun University of Chinese Medicine, Changchun, Jilin, China
- 2Department of Gynecology, The Third Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, Jilin, China
- 3Department of Tuina, The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan, China
- 4Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China
- 5Department of Acupuncture, Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, Jilin, China
The purpose of this review is to evaluate the effectiveness of large animal models in gynecology research and provide future perspectives. Gynecological diseases are diverse and pose a serious threat to women’s physical and mental health. In addition to the commonly used small animal models, large animal models have gradually entered the field of gynecological research. Results suggest that large animal models offer significant advantages in simulating human physiological processes, despite ethical and practical challenges. This paper reviews the application of large animal models in the study of gynecological diseases, provides a summary of the research characteristics of large animal models, analyses the advantages and challenges of these models in disease research, and compares the research differences between large and small animal models. It also discusses the relationship between these models and new alternative models, with a view to providing more new ideas for the selection of animal models in the study of gynecological diseases.
1 Introduction
As society develops and the pressures of modern life increase, the number of female diseases derived from a lack of education, sexual violence, lack of contraception and psychological problems is rising (1). According to recent studies, it is estimated that around 1 in 4 women suffer from gynecological disorders, with menstrual disorders, infertility, and gynecological cancers being the most prevalent (2). These conditions not only cause significant physical pain but also contribute to emotional distress, reduced quality of life, and long-term mental health challenges. For example, approximately 10–15% of women globally suffer from endometriosis, and nearly 30% of couples experience infertility, affecting both physical and mental health (3). These ailments present challenges due to their high prevalence, intricate pathophysiological mechanisms, and the critical need for early detection and treatment in medical research (4). It is therefore imperative that experimental models are developed to simulate human physiological processes in order to conduct more research, explore disease mechanisms, and develop novel medical diagnostics and treatments. However, animal models are selected not only for their ability to simulate human physiology but also for ethical considerations, availability, cost-effectiveness, and their relevance to specific research questions. These factors, including the feasibility of model selection and the alignment with research objectives, play a crucial role in determining the appropriate model for studying complex diseases. New alternative models, such as organ-on-a-chip systems and computational models, have been introduced to enhance the selection of animal models for gynecological diseases. These models allow for more ethical, efficient, and accurate simulations of human physiological processes, providing novel insights into disease mechanisms, drug testing, and patient-specific therapies. Such models are gaining recognition for their potential to complement traditional animal models and reduce reliance on animal use, addressing both scientific and ethical considerations.
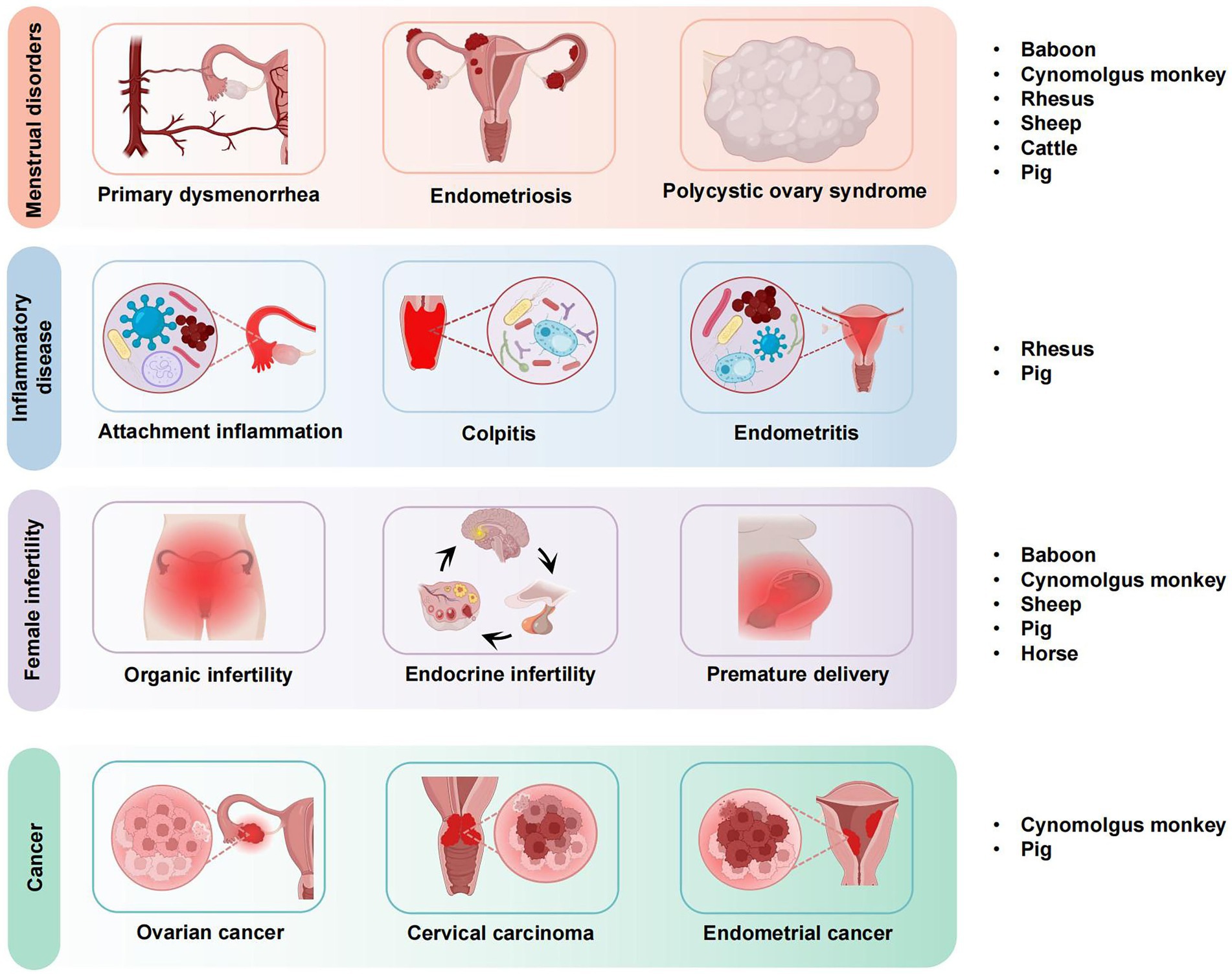
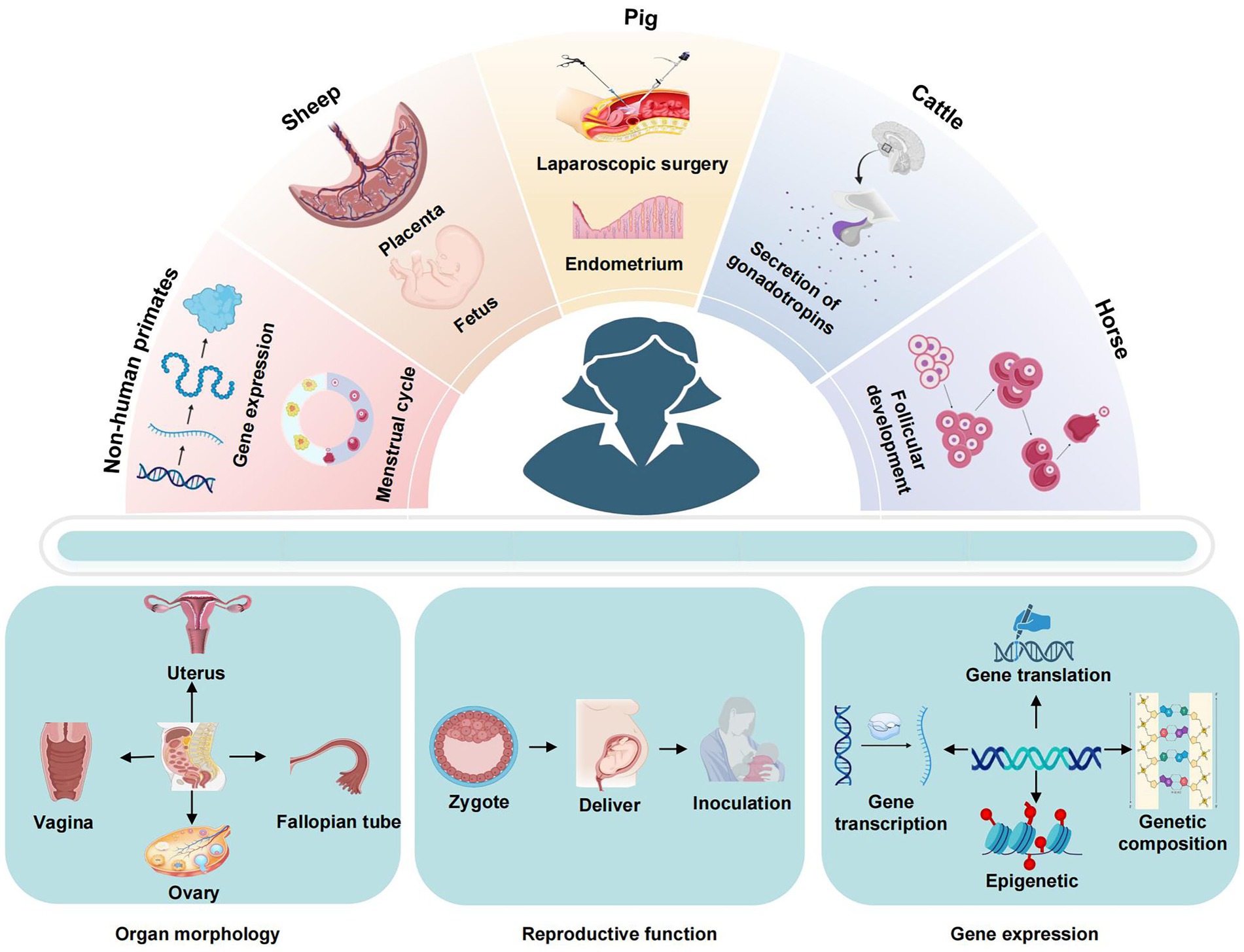
Large animal models, such as pigs, sheep, and non-human primates, are crucial in gynecologic research due to their physiological similarities to humans. Unlike small animal models, which often lack the complexity of human reproductive systems, large animals exhibit more accurate representations of human physiology, including hormonal regulation, organ structure, and response to disease (5). For example, pigs have a reproductive cycle similar to that of humans, and non-human primates share many genetic and anatomical traits with humans, making them valuable models for studying menstrual disorders, infertility, and cancer (6). For instance, a 2021 report by Makowska (7) indicated that rodents, particularly mice and rats, account for over 70% of laboratory animal use globally. Additionally, studies by Baker and Lind (8, 9) have highlighted the growing use of non-human primates, pigs, and other large animals in specific research fields such as reproductive biology and disease modeling. Of these, rodents, notably mice and rats, have historically dominated the market share, accounting for over 70% of laboratory animal use (10). Moreover, in recent years, the development of additional animal models and the occurrence of epidemics have led to a notable increase in the utilization of experimental large animals (11). The data indicate that in 2021, the market share of non-human primates surpassed that of larger strains of laboratory mice for the first time in China, with a demand for experimental non-primate animals, including pigs, reaching 129,200. Additionally, the annual demand for experimental non-primate animals reached 129,200, while the demand for other large animal models, such as experimental pigs, was 66,000. Currently, the primary citation is based on a study involving Chinese women aged 50–70 years. However, to broaden the scope of these findings, additional studies encompassing diverse populations and age groups, both in China and globally, should be cited. For example, studies involving younger women or those from different ethnic backgrounds could provide more comprehensive insights into the topic. Overall, the quantity scale of these animals was approximately equal to that of non-human primates (12). It is evident that in the domain of scientific research and medicine, large animal models, which exhibit considerable similarities to the human body in a multitude of aspects, including genetics, physiology, and structure, have garnered significant attention from researchers, particularly in the fields of reproduction, dermatology, immunology, and metabolism, where they offer distinct advantages. These models offer distinct advantages, such as more accurate simulations of human physiological processes, which is critical for studies in reproductive biology, disease modeling, and drug testing (13). These advantages make them particularly valuable in preclinical trials and in modeling complex human diseases that require a more detailed biological context. This review introduces new ideas for the selection of animal models in the study of gynecological diseases. It presents the application of large animal models in the study of common gynecological diseases (see Figure 1), analyses the current status of their research, clarifies their advantages and challenges in the study of this field, and explores the value of applying large animal models to the preclinical experimental study of gynecological diseases and the significance of their use in the preclinical experimental study of gynecological diseases.
2 Large animal models and gynecological diseases
Large animal models play an important role in gynecologic research due to their physiologic similarity to humans, allowing for more accurate studies of disease and therapy. These models help bridge the gap between small animal models and human clinical trials, enabling better understanding of disease mechanisms and the testing of therapeutic interventions. Methods to create animal models of gynecological diseases include spontaneous disease models, induced disease models, and genetically modified models, which simulate human diseases such as endometriosis, polycystic ovary syndrome, and uterine cancer. Large animal models are a valuable tool in the study of gynecological diseases, particularly in the investigation of pathogenesis, the evaluation of diagnostic techniques and the creation of innovative therapies or drugs (14). Large animal models are important in the study of common gynecological diseases, offering distinct advantages in simulating human conditions and advancing diagnostic and therapeutic research (15). At present, researchers have the option of utilizing a variety of large animal models, employing different modeling techniques, including spontaneous models, induced models, transplanted models and genetic intervention models. As illustrated in Table 1, the pathogenesis and progression of gynecological diseases were modeled to facilitate the generation of novel diagnostic and therapeutic strategies for clinical applications.
2.1 Menstrual disorders
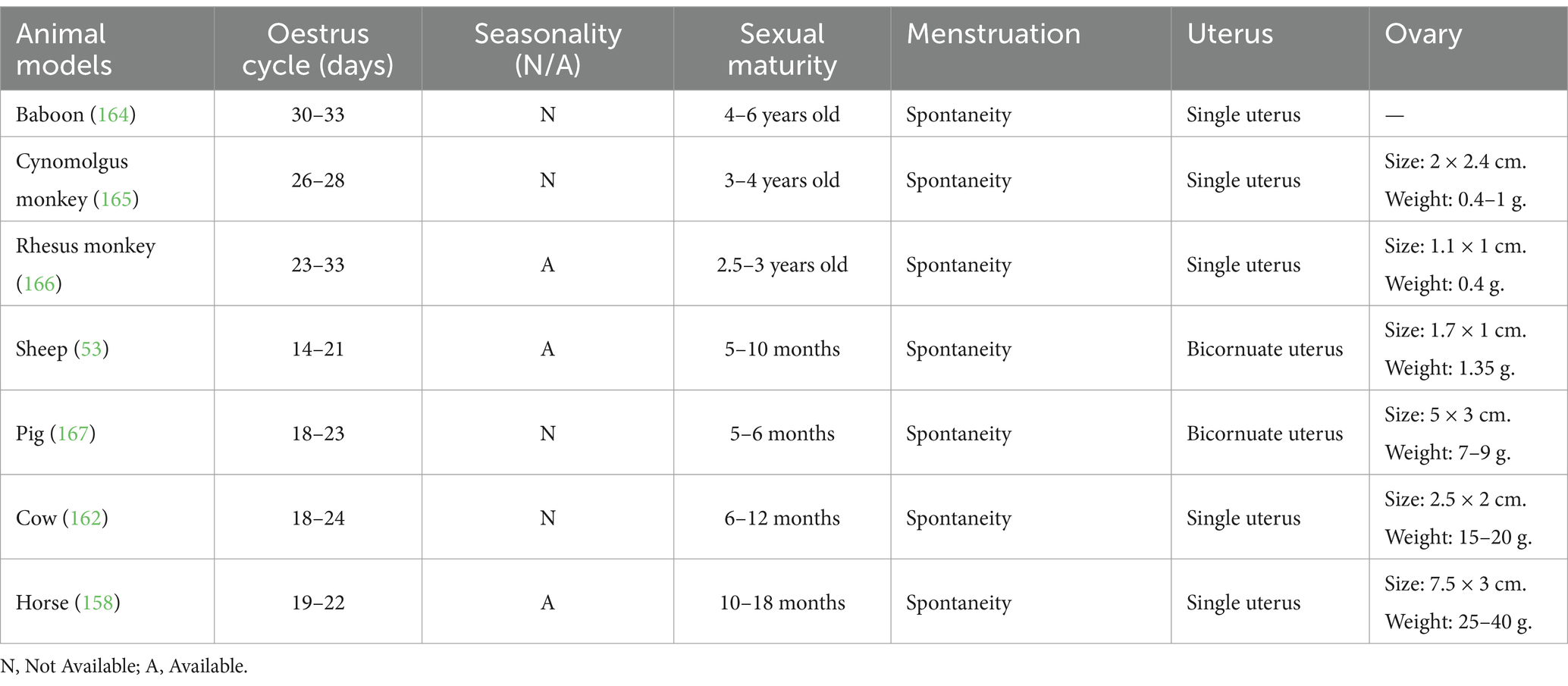
The use of animal models, particularly non-human primates, in the study of menstrual disorders dates to the early 20th century (16). Researchers first observed menstrual-like cycles in non-human primates, which paved the way for understanding the hormonal regulation of menstruation (17, 18). These models have provided invaluable insights into disorders such as endometriosis, polycystic ovary syndrome, and amenorrhea, which were initially characterized and studied through the behavior and physiological changes observed in these animals. Since the International Federation of Gynecology and Obstetrics (FIGO) Working Group on Menstrual Disorders commenced efforts to establish a global consensus in 2005, the subject of women’s menstrual health has begun to attract significant attention from a range of stakeholders (19). Menstruation is a symbol of female fertility and an integral component of women’s health. The fundamental processes of menstruation are regulated by the hormones estrogen and progesterone, which induce cyclic necrosis and exfoliation of the endometrium (20). For example, a study involving rhesus monkeys demonstrated that endometriosis lesions could be induced and observed, with findings similar to those seen in human patients (21). These animal models have helped identify potential biomarkers for early diagnosis and have been used to evaluate the effectiveness of various therapeutic approaches, such as hormonal treatments and surgical interventions. However, the menstrual cycle in rhesus monkeys is most prominently observed in November and December, although it occurs throughout the year (22). Consequently, when selecting animal models for such diseases, researchers have prioritized those that exhibit similarities to female pelvic anatomy and reproductive characteristics.
As demonstrated in Table 2, menstruation was spontaneously initiated in all large animal models, and the estrous cycles exhibited notable similarities. Among these, the reproductive organs of non-human primates are most similar to those of humans. Non-human primates are frequently employed in research pertaining to the diagnosis and treatment of menstrual disorders, including endometriosis, adenomyosis, polycystic ovary syndrome, functional uterine bleeding, and other menstrual disorders. As early as 2007, researchers observed the location of pelvic lesions laparoscopically in non-human primates with endometriosis and found them to be highly comparable to those observed in humans. The baboon is a large non-human primate that allows for repetitive blood sampling and complex surgical operations when used as an animal model (23). It is genetic and protein expression similarity to human’s results in symptomatic manifestations akin to those found in female, evident at both macroscopic and microscopic levels. Additionally, the baboon’s short and straight cervix enables direct access to the uterine cavity for procedures such as endometrial biopsy, embryo transfer, pre-implantation embryo flushing, and hysteroscopy (24). This unique anatomical feature makes the baboon an ideal model for studying endometriosis, offering a non-invasive alternative to conventional invasive procedures. Numerous studies support the baboon as the most suitable and effective experimental animal model for investigating endometriosis (25).
Nishimoto-Kakiuchi and colleagues discovered that adolescent cynomolgus monkeys between the ages of 11 to 20 exhibited heightened menstrual bleeding frequency and robust ovarian activity (26). These monkeys, due to their manageable size and ease of control in experimental settings, offer a more viable option for observing menstrual disease dynamics in comparison to human subjects. However, due to the fact that cynomolgus monkeys are an endangered species and resources are scarce, it has not been feasible to conduct experimental studies with a large data set (27). However, rhesus monkeys from India exhibit a highly regular menstrual cycle, occurring once per month (28). This regularity is mirrored in the human body in numerous ways, including genomics, anatomy and physiology, neurological function, and aging. As a result, rhesus monkeys present a more suitable model for exploring gynecological diseases, particularly in investigating gene function, structure, and epigenetic factors. In populations exhibiting a significant familial predisposition to polycystic ovary syndrome, there exists a minimum of 26 risk genes that govern diverse reproductive functions (29). Rhesus monkeys exhibit similarities to humans in aspects such as physiology, aging, and disease susceptibility (30). Consequently, researchers frequently select rhesus monkeys for the investigation of variant genes and dysfunction in polycystic ovary syndrome, with the objective of identifying potential therapeutic interventions. Moreover, investigations have revealed a 43% incidence of spontaneous endometriosis via laparoscopy at breeding sites of rhesus monkeys (31). Notably, an analysis of familial aggregation of the disease indicated a significantly elevated mean kinship coefficient for endometriosis among rhesus monkeys possessing complete genealogical data compared to unaffected kinship coefficients in a random sample (32). This has spurred the utilization of rhesus monkeys in genetic epidemiology studies on endometriosis, histopathology, and the assessment of drug dosage efficacy (33). It is worth mentioning that both cynomolgus monkeys and rhesus monkeys are classified under macaque monkeys, with the estrus cycle of macaque monkeys displaying distinct seasonality primarily in November and December (34). It is therefore imperative that researchers pay close attention to the specific characteristics of the estrous cycle of rhesus monkeys when selecting them for studies into menstrual diseases.
In addition to non-human primates, a number of large animals, including pigs, sheep and cattle, have contributed to the study of menstrual disorders. For instance, in the context of studying polycystic ovary syndrome through prenatal androgen investigations, manipulating testosterone levels in sheep during the fetal stage has led to the discovery of significant impacts on neuroendocrine feedback mechanisms in adulthood. This disruption has been linked to heightened pituitary response to gonadotropin-releasing hormone (GnRH), elevated luteinizing hormone levels, increased presence of functional androgens, and the development of a multifollicular ovarian morphology (35). Notably, these findings have also been associated with the initiation of preterm labor. Such insights derived from animal models present promising avenues for identifying novel therapeutic targets for polycystic ovary syndrome. In addition, lesions of the ovary are characterized mainly by granulosa cell and oocyte apoptosis, follicular atresia, decreased oocyte quality and embryonic developmental potential, oxidative stress and mitochondrial abnormalities, which ultimately lead to reduced fertility in women (36). Additionally, the physiological characteristics of large animal models with single follicles and multiple cycles are similar to those of women (37). When exploring the fluctuation patterns of follicular dynamics, cows are also often selected as a model to replace human subjects for real-time ultrasonography tests and to analyze the specific waveform characterization of their follicular dynamics, which facilitates the dissection of systemic dysfunctions of ovarian function in humans (38). Pigs, due to their comparable organ size to humans and consistent estrus cycle, have been utilized in magnetic resonance imaging-guided focused ultrasound surgical simulations of adenomyosis. These simulations involve the autologous endometrial implantation technique, circumventing issues related to extended drug-induced cycles and hormonal imbalances. This approach better reflects the characteristics of adenomyosis in the uterus, with a natural affinity to uterine tissue.
The utilization of large animal models in the investigation of menstrual disorders contributes significantly to advancing knowledge on the physiological intricacies of the female reproductive cycle and ovarian processes. Moreover, it offers valuable insights into the malfunctions affecting the uterus, ovaries, and associated reproductive organs, thereby enabling researchers to delve into innovative approaches for diagnosis and treatment.
2.2 Inflammatory diseases
In recent times, there has been a rise in gynecological inflammatory diseases linked to sexual transmission due to increased sexual openness, indicating a trend toward a younger affected demographic (39). Among these, pelvic inflammatory disease, which is caused by bacterial and viral infections, is a highly inflammatory response in the upper reproductive tract of women (including the endometrium, fallopian tubes and ovaries, etc.) (40). The chronic inflammatory state with an immune response often induces the emergence of ovarian cancer. Furthermore, mycoplasma infection-induced inflammation of the reproductive tract frequently results in significant complications, including infertility and preterm delivery, due to its prolonged nature, lasting for months to years (41). It is evident that gynecological inflammation should not be trivialized and may result in further complications if left untreated for an extended period.
The investigation of microbial communities in bacterial pelvic inflammatory disease and vaginitis has presented challenges in conducting analogous experiments in Lactobacillus-dominated mammals (42). A thorough examination utilizing 16SrRNA amplicons and shotgun metagenomic sequencing in rhesus monkeys revealed similarities in physiology and genetic makeup with female counterparts, along with a diverse array of vaginal flora shared with female microbiota. This discovery positions female rhesus monkeys as promising subjects for further research in this domain (43). Additionally, the utilization of non-human primates in the study of salpingitis through animal models is hindered by factors such as high costs, logistical difficulties, and ethical considerations, limiting their applicability (44). Researchers have noted that pigs are a valuable animal model due to their comparable anatomy and abdominal size to humans, making them suitable for simulating clinical conditions in research (45). Pigs have been successfully utilized in modeling endometritis by inducing inflammatory responses and vasodilation in the uterine horns using Escherichia coli, thus affecting the noradrenergic neurons in the caudal mesentery (46). This model has provided a solid foundation for the study of anti-inflammatory drugs. Pigs, particularly when employing endoscopic methods, offer researchers a clearer view of uterine lesions. Moreover, pigs are suitable for pharmacokinetic investigations due to their anisotropic growth patterns, a departure from human applications (47). In a study aimed at developing a novel formulation of the non-steroidal anti-inflammatory drug carprofen, an alternative to individual pig analysis was explored (48). This is also applicable to men, where similar genetic and physiological factors play a role. The study administered carprofen through both intravenous and intramuscular routes to assess the bioavailability of plasma samples pre-and post-intervention. The results revealed that using pigs as an animal model for such experiments not only did not compromise the concurrent analysis of pharmacokinetic profiles in the dataset but also conferred an advantage in data mining due to the longer half-life of pigs compared to humans. Pigs have a longer half-life for certain drugs compared to humans, which can be advantageous in drug testing, but may also present challenges in terms of accurate dosage prediction for human treatments. This extended time frame provides ample opportunity for data mining, showing potential for evaluating the efficacy and determining appropriate dosages of novel drugs for gynecological conditions (49). The findings from large animal models of gynecological inflammation, such as endometritis and pelvic inflammatory disease, have direct clinical relevance. These studies have led to the development of new therapeutic approaches, such as antibiotic and anti-inflammatory drug therapies, as well as diagnostic tools like imaging techniques that improve the detection of inflammatory lesions in the reproductive tract (50).
In recent years, experimental research on gynecological inflammation has been conducted on an ongoing basis, and the selection of animal models has been refined. However, gynecological inflammation has a multitude of potential triggers, and the selection of experimental animal models for this disease must consider not only the findings of etiological research, but also the characteristics of cyclic recurrence.
2.3 Infertility
Infertility represents a significant global health concern that poses a threat to women’s reproductive well-being. It is estimated that approximately 480,000 couples and 18.62 billion people of reproductive age in countries around the world are affected by infertility (51). This statistic refers to women, but similar figures can be observed for men as well. Female reproductive disorders may have a number of causes, including genetic mutations, chromosomal abnormalities, ovulatory dysfunction and genital pathologies such as those affecting the uterus (52). Approximately 25% of cases of female infertility are attributable to dysfunctions in the regulation of the hypothalamic–pituitary-gonadal axis, resulting in an endocrine hormone imbalance that affects normal ovulation, fetal development and reproductive function (53). Furthermore, it is estimated that 4–9% of women experience potential luteal phase dysfunction, which impairs endometrial receptivity, potentially leading to pregnancy difficulties and infertility (54). Further research into this disease is imperative, however, it is not possible to conduct this research directly on humans due to ethical and humanitarian considerations (55). It is therefore imperative to select an appropriate animal model for the study of this disease.
The pertinent reproductive characteristics of the large animal models are presented in tabular form in Table 3. The striking resemblance between non-human primates and humans with regard to reproductive characteristics and reproductive cycles has rendered the non-human primate model a more extensively investigated model for the study of female infertility (56). Furthermore, the model is regarded as a valuable and well-founded tool for investigating female reproductive health and disease.
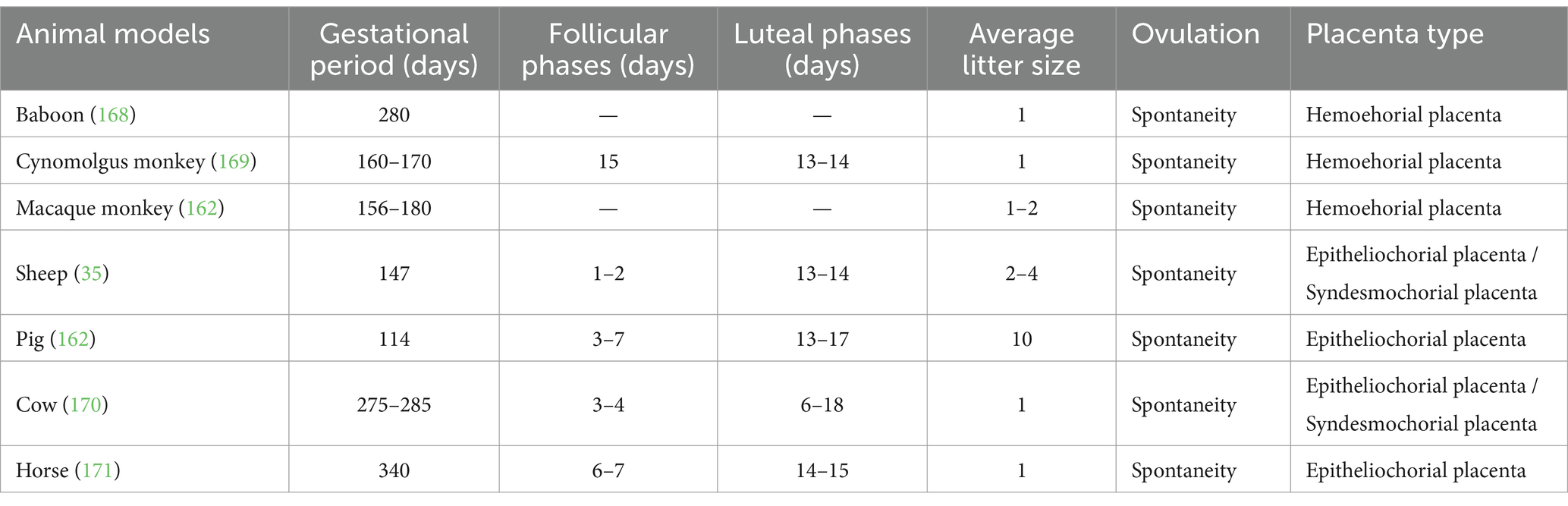
Infertility can result from both genetic and environmental factors, including hormonal imbalances, genetic mutations, and environmental exposures (57). Large animal models, such as pigs and non-human primates, offer a unique opportunity to study these factors in a more controlled and physiologically relevant environment. For example, studies on genetically modified pigs have been used to explore the impact of specific gene mutations on ovarian function and fertility, while environmental factors such as endocrine disruptors have been investigated using rhesus monkeys (58). Therapeutic interventions, such as hormone therapy, assisted reproductive technologies (ART), and ovarian tissue transplantation, have been tested in large animal models. For instance, studies on sheep and pigs have explored the efficacy of ovarian tissue transplantation in restoring fertility in females with ovarian failure, showing promising results with successful pregnancies (59, 60). Infertility resulting from the absence or dysfunction of the uterus remains a significant challenge that has persisted as a research focus. The advent of uterus transplantation technology, involving the retrieval of a uterus from a living or brain-dead donor and its transplantation into an infertile recipient, has emerged as a promising solution. This innovative technique not only supports the healthy development of the fetus post-conception but also offers renewed optimism for individuals facing absolute uterine infertility (61). Drawing on the anatomical and physiological similarities with non-human primates, researchers have successfully transplanted autologous uteri into Cynomolgus monkey. Notably, these transplants led to natural recognition postoperatively, evidenced by three cyclic menstrual periods, culminating in the delivery of a healthy fetus via cesarean section. Conversely, baboons subjected to uterine transplantation through modified vascular anastomosis exhibited a 90% survival rate, with 60% of individuals resuming normal menstruation (62). Furthermore, the distinctive hemochorial placenta found in non-human primates facilitates direct contact between maternal blood and placental tissues, thereby extending gestation and enhancing blood nourishment in comparison to larger animal models (63). The study conducted by researchers on uterine transplantation utilizing bilateral ovarian venous reflux in pigs yielded a favorable outcome (64). The procedure resulted in successful establishment of uterine-ovarian venous reflux, facilitated by four vascular anastomoses performed with the ovarian veins. Notably, the uterine transplantation demonstrated a commendable 83% success rate in immediate reperfusion surgery (65). These findings underscore the cost-effectiveness and proficient tissue anastomosis associated with utilizing pigs in uterine transplantation techniques. Nevertheless, careful consideration is advised in experimental protocols to mitigate risks of ureteral damage.
Furthermore, sheep in large animal models are not only of moderate size, but also exhibit a high degree of similarity to women in terms of gonadal differentiation, meiosis, primordial follicle emergence, placenta development, and nutrient transport (66). Consequently, sheep are frequently employed in hormonal investigations pertaining to reproductive disorders, placental development, ovarian dynamics, and neurotransmitter assay studies. However, owing to their possession of a bicornuate uterus with a shorter length compared to humans, the arterial and venous vasculature, crucial for their blood supply, presents differences that lead to variations such as an extended thermal ischemia time during immunosuppression (67). Horses exhibit similarities to women in terms of follicular recruitment waves and the selection mechanism of dominant follicles, with their larger follicles facilitating easy observation (68). Consequently, horses have been extensively utilized in research pertaining to infertility, including instances of low ovarian reserve function. An illustrative example lies in the investigation of the heterotopic autotransplantation technique of ovarian tissues, where the utilization of horses as an experimental model demonstrated that subjecting ovarian tissues to a cooling process at 4°C for a minimum of 24 h prior to transplantation effectively preserved the quality of the tissues (69). This preservation method led to favorable rates of follicular survival and development.
Animal models utilized in infertility research must meet stringent criteria for their reproductive capabilities and should be effectively employed to mimic hormone levels, gene alterations, and other relevant factors consistent with human physiology. Large animal models have garnered significant interest in infertility studies owing to their reproductive, physiological, and anatomical advantages (70).
2.4 Cancer
Cancer represents a significant challenge within the biomedical field, with breast cancer emerging as the primary cause of cancer-related fatalities among women globally (71). Moreover, cervical, ovarian, and endometrial cancers, identified as the most prevalent malignant tumors, present substantial risks to women’s well-being. The complexity of malignant tumors is frequently influenced by intricate genetic and molecular signaling pathways originating from tumor cells and their surrounding microenvironment (72). Due to the limited availability of data on pathological response interactions in human subjects, experimental animals are frequently employed for in vitro cancer analyses. Delving deeply into cancer pathogenesis and targeted therapies can yield valuable insights for subsequent clinical trials in the field of oncology (73).
Large animal models are used to investigate the molecular mechanisms underlying gynecologic cancers, such as ovarian, cervical, and endometrial cancers. For example, research using non-human primates has provided valuable insights into the signaling pathways involved in tumorigenesis and metastasis (74). These models also allow for the study of the tumor microenvironment, which plays a crucial role in cancer progression and therapeutic response. Innovative therapeutic approaches, such as immunotherapy and gene therapy, are currently being tested using large animal models (75). For example, studies on genetically engineered pigs have been used to evaluate the efficacy of CAR-T cell therapy in treating ovarian cancer (76). Additionally, immunotherapeutic agents targeting the PD-1/PD-L1 pathway have shown promise in preclinical trials involving non-human primates (77). Farletuzumab, a newly developed inhibitor of folate receptor α, exhibited promising efficacy in initial trials targeting epithelial ovarian cancer (78). To investigate the toxicological profile of this compound, a series of four studies were conducted by researchers. These studies included assessments of dosage levels, tolerability, as well as repeat dosing over 28 days and 24 weeks, utilizing Cynomolgus monkeys as experimental subjects (79). The immunohistochemical analysis revealed minimal staining of Farletuzumab in the pancreatic ductal epithelium, oviductal epithelium, and pancreatic ductal epithelium of both cynomolgus monkeys and normal human tissues (80). Consequently, cynomolgus monkeys were identified as a suitable model for toxicological evaluations. Furthermore, a number of studies have indicated that the long noncoding RNA gene (long noncoding RNAs) may serve as a highly promising biomarker for various cancers (81). Among these, MIR503HG, a long-stranded non-coding RNA on the human X chromosome, has been clearly characterized in the early stages of the reproductive system, particularly in ovarian development, with down-regulation of transcripts expressed in gynecological cancers (82). Conversely, MIR503HG is currently observed to be highly conserved in only five non-human primate species. A comparison of human genes with those of selected non-human primates revealed that the exon and intron lengths of MIR503HG were well conserved in the large animal models of chimpanzees and gorillas, with the exception of the small animal model mouse lemur (83). Additionally, the uterine structure of pigs differs from that of humans. It is a bicornuate uterus, with the fetus mainly conceived in the uterine horns, and its uterine wall is relatively thin, with an average thickness of 3.88 ± 1.27 mm for the entire layer and 1.17 ± 0.15 mm for the myometrium (84). Consequently, an experiment utilizing a novel cryoablation balloon probe in a porcine model of endometrial cancer demonstrated that the effective freezing range could encompass a minimum of 2 cm in diameter, and that the procedure did not result in damage to adjacent organs (85). This successfully validated the safety and efficacy of the cryoablation technique.
Animal models have historically served as the foundation for translational research into biochemical and physiological processes, particularly in the context of cancer onset, development, and propagation in organisms (86). Furthermore, the significant heterogeneity of cancer, which differs from the human body in terms of physiology, pathology, genetics, and immunity, has resulted in an urgent need for researchers to develop animal models that can respond to the biological characteristics of humans (87). Consequently, researchers are currently engaged in the development of novel animal models utilizing innovative transgenic and transplantation technologies, which are anticipated to facilitate significant advancements in the research and development of cancer and other diseases (88).
3 Application characteristics of large animal models
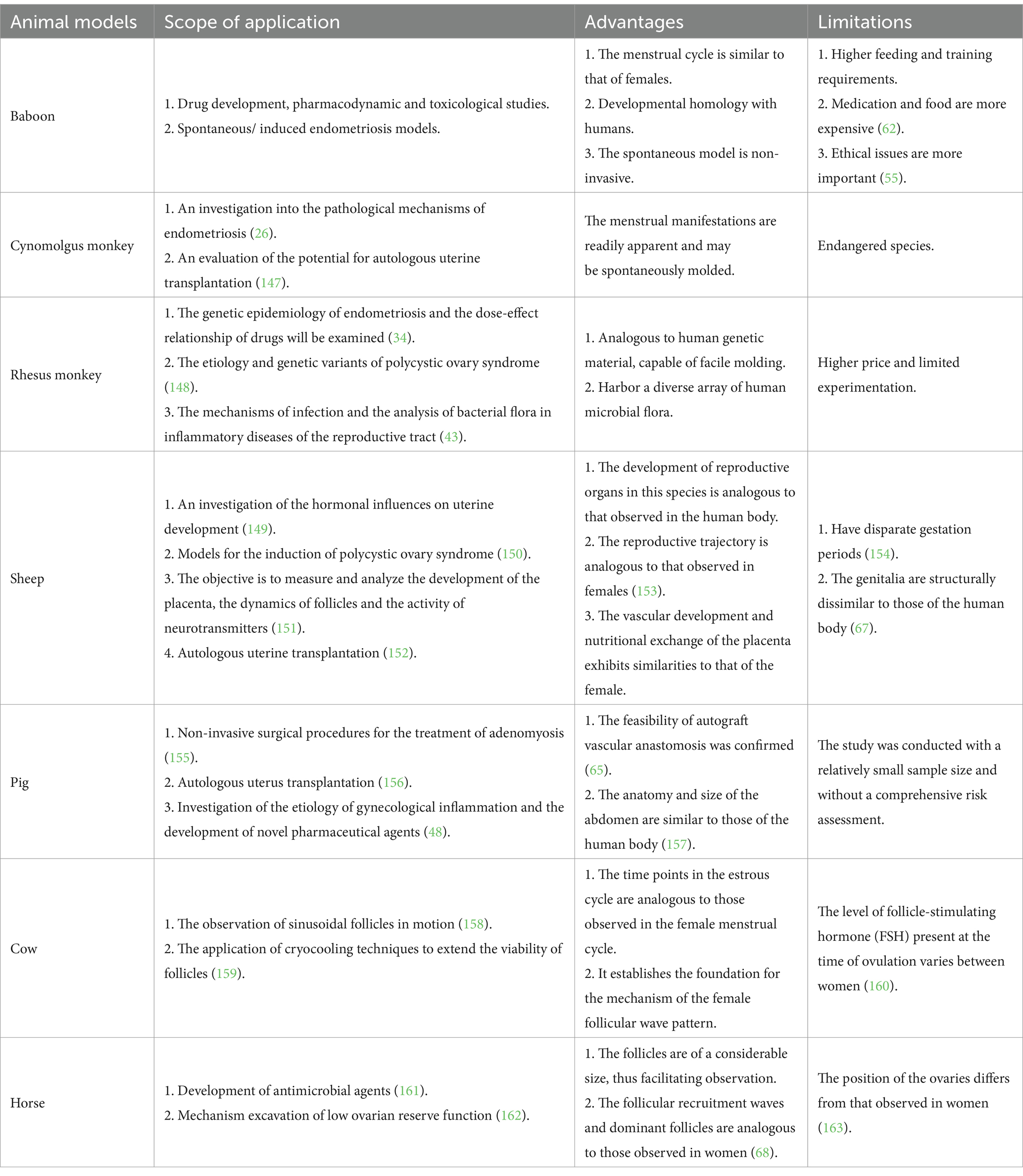
The research of large animal models in the field of gynecology is primarily concerned with the elucidation of disease mechanisms and the advancement of diagnostic and therapeutic techniques. As medical research progresses and science and technology advance, the creation of experimental models is becoming increasingly sophisticated, enabling more accurate and efficient simulation of human disease states, as illustrated in Figure 2 and Table 4. This provides a crucial experimental foundation for investigating disease pathogenesis, developing diagnostic methods and advancing therapeutic measures.
The non-human primate model stands out among large animal models in gynecology for its strong resemblance to humans. Non-human primates exhibit similarities with humans in terms of neuroendocrine regulation of the reproductive system, as well as in circulatory functions of the ovaries and reproductive tract critical for establishing and managing the maternal-fetal-placental connection during pregnancy (89). The most commonly used non-human primate models in laboratory settings are macaques and cynomolgus monkeys, which exhibit approximately 95% genetic homology to humans. Additionally, baboons are regarded as the most suitable models for studying embryonic stem cells and artificial reproductive technology (ART) (90). This makes non-human primates a valuable resource for investigating female infertility, facilitating advancements in uterine transplantation techniques, minimizing damage from organ ischemia and reperfusion, and enhancing postoperative outcomes (91). Furthermore, non-human primates can replicate infertility scenarios such as placental dysplasia and maternal-fetal dysfunction resulting from environmental influences. As such, they are frequently employed in studies related to infertility treatment, hormonal disorders analysis, pharmacokinetics of pregnancy-related compounds, and antiretroviral therapy.
The pig, alongside non-human primates, possesses a unique uterine structure characterized by a bicornuate uterus with prominent lumen uterine horns positioned adjacent to the bladder (92). These uterine horns are deemed suitable for simulating laparoscopic ureteral reconstruction due to their sufficient length, offering an alternative to dilated ureters. Furthermore, the stromal and perivascular regions of the porcine uterus contain marker proteins for mesenchymal stem cells (MSCs), serving as potential sites for stem/progenitor cell residence. Notably, the presence of unevenly distributed stromal CD105 + and CD106 + cells suggests their role as precursors for peripheral vascular growth (93). This similarity in the endometrial MSC phenotype between pigs and humans underscores the utility of pigs as a valuable large animal model for investigating endometrial function in translational research (94). Additionally, pigs are frequently utilized as models for training and experimental purposes in various pelvic surgical procedures. Junior surgeons often practice laparoscopic surgery on pig models using advanced 3D technology, while pigs are also employed as test subjects for suturing techniques in gynecology residency training programs (95).
Sheep serve as vital models in the examination of fetal physiology throughout pregnancy. They are ideal for translational research questions due to their gestational length, the availability of a single fetus for catheter insertion, and the similarity in fetal size to humans, which facilitates delivery using standard clinical techniques (96). Furthermore, fetal sheep preparations provide an effective means of treating preterm labor associated with ischemia-hypoxia or maternal-fetal infections, as evidenced by near-full-term fetal sheep in preclinical studies (97). The use of instrumented fetal sheep in utero presents numerous practical benefits compared to extrauterine models featuring cerebral gyration. The extended gestation period of fetal sheep allows for the strategic selection of an appropriate estrous phase for the induction and evaluation of brain injury, highlighting the susceptibility of preterm fetal sheep to acute and chronic brain damage resembling that observed in humans in terms of histopathological traits.
The utilization of large animal models presents an opportunity to enhance our comprehension of ovarian function, specifically in cattle and horses, due to their possession of sizable antral follicles and growth stages akin to the dominant ovulatory follicle in humans (98). As a result, cattle and horses serve as appropriate subjects for investigating follicle dynamics through the application of ultrasonography. It has been demonstrated that the temporal events that occur in cattle during the menstrual cycle are consistent with findings in women (99). The decline in maternal fertility with age is associated with the initiation of follicular waves, leading to a 4-day cyclic increase in gonadotropin levels (100). The circulating concentrations of gonadotropins are increased in the late reproductive stages, which is accompanied by a decrease in inhibin B secretion (101). Furthermore, the antral follicle count (AFC) has been employed as a marker of reproductive potential and a predictor of ovarian response to hyperstimulation (102). As such, investigations focusing on the depletion of primordial follicles, diminished antral follicle reserve, changes in hormone secretion, and meiotic abnormalities leading to female infertility may benefit from mechanistic exploration using cattle as an animal model (103).
4 Conclusion
As a crucial instrument in translational research within the context of clinical trials for human diseases, the suitability of the animal model to the disease under investigation hinges on the efficacy of the experimental process and the scientific rigor of the resulting data.
4.1 Comparison of differences in gynecological research between large and small animal models
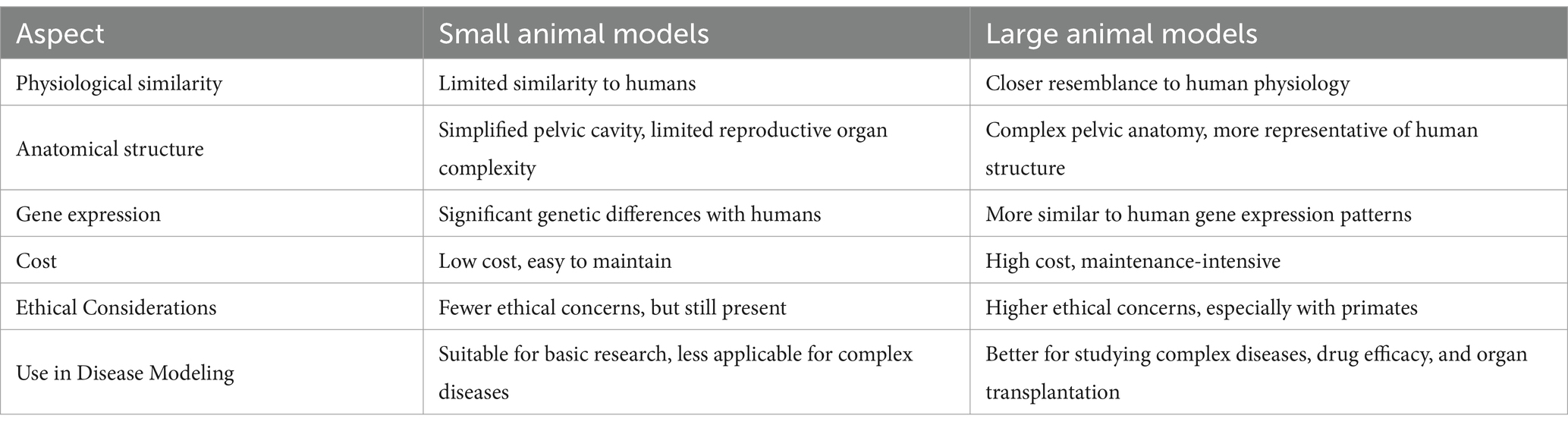
Animal models have emerged as a valuable asset in gynecological research, particularly in light of ethical considerations within the field. Small animal models, notably rodents, are prevalent due to their accessibility and cost-effectiveness. Nonetheless, a comprehensive understanding of gynecological diseases necessitates a thorough characterization of the phenotype and genotype of experimental animals (104). Despite the fact that humans and rodents share a number of fundamental biological processes, the differences between the two species become increasingly apparent as research progresses (105). In terms of anatomical structure, there are significant differences between small animal models and humans, particularly in the pelvic structure and reproductive organs, which exhibit notable species-specific variations (106). Small animals have a relatively uncomplicated pelvic cavity primarily dedicated to housing the reproductive organs. In contrast, the female human pelvis not only accommodates the reproductive organs but also interfaces with the rectum, bladder, and other vital organs, directly influencing the birthing process (107). Additionally, the commonly used ‘Y’-shaped uterus in female mice differs substantially from the inverted ‘pear-shaped’ uterus in humans, highlighting the inadequacy of small animal models in replicating the reproductive system’s morphology (108). With regard to physiological function, no small animal models have been identified to exhibit natural menstruation through cyclic endometrial interstitial metamorphosis, with the exception of the spiny mouse that has been recently discovered. The induction of exogenous hormones or the removal of the ovaries does not fully replicate the cyclical hormonal fluctuations and blood supply to the spiral arteries observed during normal menstruation (109). Conversely, the spiny mouse is distinguished as the sole rodent species to naturally undergo menstruation. Being a native North American species, it is susceptible to environmental factors, and its utility in menstrual research is constrained by its distinct physiology and lack of immune response to antigens and antibodies (110). With regard to gene expression, the utilization of transgenic mice in investigating gynecological malignancies has seen extensive adoption in gene expression studies (111). In addition to their low cost of application, their diverse immunodeficiency strains can be employed in the experimental development of cancer drugs (112). Nonetheless, comparative analysis of mouse and human genes has revealed substantial variances in transcription factor binding sites, ranging from 41 to 89% of instances (113). Such disparities have impeded investigations into the genetic, immunological, and molecular underpinnings of gynecological disorders in murine models.
4.2 Opportunities and challenges faced by large animal models
It is evident that there is a paucity of suitable models for the study of diseases in the field of gynecology. The discrepancies between small animal models and women in terms of anatomy, organ morphology, reproductive function and gene expression have led to a growing interest in the study of large animal models. Large animal models exhibit greater physiological similarity with humans in terms of genetics, structure and function, which renders their experimental results more informative (114). Moreover, due to the closer proximity in body size to humans, large animal models manifest immune and metabolic responses that more closely mirror those in humans (115). As a result, the experimental data derived from large animal models are deemed more dependable and enable more accurate predictions in assessing the safety and effectiveness of drugs for gynecological conditions. In the current era, the advent of genome editing technology (CRISPR-Cas9 system) has propelled the advancement of cellular imaging, gene expression regulation, epigenetic modification, therapeutic drug development, functional gene screening, and genetic diagnosis (116). Non-human primates have emerged as viable translation targets due to their analogous capability to humans in gene editing and expression, showcasing commendable gene modification and delivery efficiency (117). In the field of biomedical research, cloning has also been employed in the replication of genes and the development of immunological agents in large animal models (118). Notably, cloned pigs exhibit genetic sequences more akin to humans, allowing for advances in clinical research on human embryonic development through the selection of cloned pigs for embryo biopsy in conjunction with microproteomics (119). In recent years, the development of molecular biology has also facilitated the rapid advancement of multifunctional stem cell technology (120). Nevertheless, the lack of in vivo data on human development makes it challenging to ascertain the extent to which in vitro models accurately reflect in vivo processes (16). While primate hematopoietic stem cells share an origin with pre-implantation epiblasts, their transcriptomic profile aligns more closely with post-implantation epiblasts than with traditional rodent models (121). Consequently, to address potential artifacts and species discrepancies and gain deeper insights into human development, an integrated approach encompassing analyses across various platforms (in vivo, ex vivo, in vitro) and species (hominids, primates) is warranted (122). Furthermore, the integration of organ bioengineering with the use of large animal models has significantly advanced the field of organ replacement in the context of gynecological disorders (123). Research endeavors focusing on uterine transplantation have been conducted in various large animal species such as pigs, sheep, and non-human primates. This represents a significant advancement in the field of bioengineering of the female reproductive system.
In the realm of gynecological research, the utilization of large animal models serves to better replicate the physiological and pathological manifestations of human gynecological diseases, offering researchers a more authentic experimental setting akin to clinical scenarios. This not only enhances the realism of the studies conducted but also furnishes a crucial foundation for the development and evaluation of pharmaceutical interventions (124). Nevertheless, the exploration of large animal models necessitates comprehensive data support through the acquisition of substantial sample sizes (125). Consequently, it becomes imperative to both optimize cost-effectiveness to mitigate financial constraints and bolster the technical proficiency of personnel in order to elevate the scientific rigor and safety standards of experimental endeavors (126). Ethical considerations pose significant challenges when implementing large animal models for experimental research, particularly in the case of non-human primate models like chimpanzees, which share a closer genetic and evolutionary relationship with humans (127). The utilization of non-human primates in research has sparked intense debates, particularly following the 2015 legislation in the United States that designated captive chimpanzees, including those in laboratory settings, as endangered species (128). This development underscored the necessity for stringent ethical scrutiny in experimental research involving non-human primates, aligning with elevated ethical standards (129). In contrast to countries like the United Kingdom and the United States, Brazil engages in comparatively less research involving non-human primates (130). Nonetheless, Brazil’s National Commission for the Control of Experiments on Animals (CONCEA) has been consistently revising its ethical guidelines pertaining to primate research (131). Notably, the UK Parliament has been a trailblazer in enacting legislation concerning animal usage, with the principles of the 3Rs—Reduce, Replace, and Improve—originating from the UK and now serving as a guiding framework for animal research worldwide. It is evident that ethical and transparent practices are imperative in experimental research involving large animal models to ensure the ethical justification of animal use, particularly in instances of invasive procedures and reproductive studies, and to uphold the strict adherence to animal protection regulations safeguarding the welfare and rights of animals (131).
4.3 The relationship between large animal models and new alternative models
Preclinical large animal models are integral to drug development as they replicate the pharmacokinetic characteristics of the human body, facilitating comprehension of drug absorption, distribution, metabolism, and excretion processes (132). These models establish a foundation for determining appropriate drug doses and dosing schedules, assessing a drug’s therapeutic effectiveness against specific diseases through disease modeling and therapeutic trials, and furnishing data that supports drug marketing efforts. Nonetheless, the utility of large animal models in drug safety evaluations and toxicology studies is constrained by their typically high costs, ethical considerations, unsuitability for high-throughput bioassays and extensive drug screenings, and limited ability to faithfully mirror human responses (133). Consequently, there is a growing imperative to develop preclinical drug testing models with enhanced physiological relevance to more accurately replicate complex human-related conditions and thereby enhance the efficacy of clinical trials.
The emergence of the ‘organ-on-a-chip’ concept in recent years presents a promising avenue for modeling human physiology in vitro. This innovative approach involves replicating the physiological conditions and functions of human organs within a microfluidic chip platform (134). By incorporating strategies for co-culturing with microorganisms and utilizing multi-parameter control approaches, researchers have been able to create a more sophisticated in vitro model (135). The ‘organ-on-a-chip’ technology has been suggested as a viable replacement for animal studies and as a method to address the underrepresentation of female participants in human clinical trials (136). However, existing single-organ microarrays lack systemic dimensions and inter-organ communication (137). To enhance the efficacy of drug development, integrating large animal models with organ-on-a-chip technology has been proposed. By combining preliminary drug screening in large animal models with comprehensive in vitro experiments using organ-on-a-chip systems, researchers can achieve more accurate predictions of drug responses and effects in the human body. Furthermore, AI has shown considerable benefits in various medical domains, including disease diagnosis, precision medicine, and drug development (138). The utilization of AI technology enables the rapid and direct transformation of vast quantities of medical data, such as medical images and gene expression, into actionable experimental parameters. This integration enhances the efficiency of drug development processes, eliminates human intervention and subjective biases, and significantly enhances the accuracy of data analysis (139). By leveraging AI technology in conjunction with large animal models to collect and analyze data from drug experiments and disease simulations, insights into disease progression patterns and drug response kinetics can be expedited (140). Furthermore, the combined application of AI and medical imaging technologies, such as ultrasound, MRI, and CT scans, allows for comprehensive imaging and data analysis of large animal models, thereby enhancing the precision and predictive capabilities of disease diagnostics (141). Additionally, leveraging the genetic background, physiological traits, and other pertinent data of large animal models, personalized diagnosis and treatment strategies can be formulated through the integration of AI technology.
In brief, the significance of employing large animal models in gynecological research is unequivocal, as evidenced by their substantial utility in advancing women’s health. In the forthcoming era, these large animal models are poised to leverage their inherent strengths in conjunction with innovative technologies, thereby catalyzing transformative breakthroughs in medical research and fostering accelerated growth and advancement in the field.
4.4 Ethical considerations and the 3Rs principle in gynecological research
The use of large animal models in gynecological research offers significant scientific advantages, particularly for studying complex reproductive processes. However, it also raises critical ethical concerns, especially regarding the use of non-human primates (NHPs) (16). Recent legislative developments, such as the European Union’s proposed ban on NHP research by 2025, highlight the growing ethical scrutiny surrounding animal research (142). This policy shift underscores the need for alternative approaches that reduce reliance on primates in biomedical studies (143).
The 3Rs principle (Replacement, Reduction, Refinement) provides a framework to address these ethical concerns (144). Replacement encourages the use of alternative models, such as organ-on-a-chip systems and AI-based simulations, which can replicate human physiological processes without the need for animal testing (145). These technologies are particularly promising in gynecological research, offering ethical and scientifically valid alternatives to traditional animal models.
Reduction emphasizes minimizing the number of animals used in experiments by refining study designs and utilizing more efficient models. Large animal models, due to their closer physiological similarity to humans, can reduce the number of animals required in research by providing more relevant data in fewer subjects. Refinement focuses on improving research methodologies to minimize animal suffering, such as using non-invasive imaging techniques like MRI and ultrasound, which allow for detailed observations of reproductive processes without the need for invasive procedures (146).
In conclusion, while large animal models remain essential for advancing gynecological research, it is imperative that ethical considerations are prioritized. By adhering to the 3Rs principle, researchers can ensure that scientific progress is made responsibly, with a continued focus on reducing animal use and improving welfare standards.
Author contributions
DZ: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. XL: Supervision, Writing – original draft, Writing – review & editing. XZ: Supervision, Writing – original draft, Writing – review & editing. XX: Writing – original draft, Writing – review & editing. YZ: Writing – original draft, Writing – review & editing. YL: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jilin Province Science and Technology Development Project in China [grant no. 20210204075YY].
Acknowledgments
The authors would like to express their sincere gratitude to the participants for their invaluable contributions throughout the research process. Special thanks are due to the Jilin Province Science and Technology Development Project in China for providing financial support that made this study possible. We also appreciate BioRender for helping us in our drawing process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nie, M, Luo, Y, Meng, YT, Fan, L, Yue, J, Li, T, et al. Diseases, health behaviors, psychological health and associated factors among women aged 50-70 years: a cross-sectional study in Hunan Province, China. J Women Aging. (2023) 35:210–22. doi: 10.1080/08952841.2022.2026164
2. Zheng, X, Zhao, D, Liu, Y, Jin, Y, Liu, T, Li, H, et al. Regeneration and anti-inflammatory effects of stem cells and their extracellular vesicles in gynecological diseases. Biomed Pharmacother. (2023) 168:115739. doi: 10.1016/j.biopha.2023.115739
3. Rangi, S, Hur, C, Richards, E, and Falcone, T. Fertility preservation in women with endometriosis. J Clin Med. (2023) 12. doi: 10.3390/jcm12134331
4. Pasamba, EC, Orda, MA, Villanueva, BHA, Tsai, PW, and Tayo, LL. Transcriptomic analysis of hub genes reveals associated inflammatory pathways in estrogen-dependent gynecological diseases. Biology (Basel). (2024) 13. doi: 10.3390/biology13060397
5. Cui, M, Liu, Y, Men, X, Li, T, Liu, D, and Deng, Y. Large animal models in the study of gynecological diseases. Front Cell Dev Biol. (2023) 11:1110551. doi: 10.3389/fcell.2023.1110551
6. Pereira, WA, Franco, SM, Reis, IL, Mendonça, CMN, Piazentin, ACM, Azevedo, POS, et al. Beneficial effects of probiotics on the pig production cycle: an overview of clinical impacts and performance. Vet Microbiol. (2022) 269:109431. doi: 10.1016/j.vetmic.2022.109431
7. Makowska, IJ, and Weary, DM. A Good life for laboratory rodents? ILAR J. (2021) 60:373–88. doi: 10.1093/ilar/ilaa001
8. Baker, KC, and Dettmer, AM. The well-being of laboratory non-human primates. Am J Primatol. (2017) 79:1–5. doi: 10.1002/ajp.22520
9. Lind, NM, Moustgaard, A, Jelsing, J, Vajta, G, Cumming, P, and Hansen, AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. (2007) 31:728–51. doi: 10.1016/j.neubiorev.2007.02.003
10. Vodegel, EV, Guler, Z, Ras, L, Mackova, K, Groeneveld, ACHM, Bezuidenhout, D, et al. Vaginal changes after ovariectomy in ewes: a large animal model for genitourinary syndrome of menopause. Int J Gynaecol Obstet. (2023) 162:1042–9. doi: 10.1002/ijgo.14816
11. Harland, N, Knoll, J, Amend, B, Abruzzese, T, Abele, H, Jakubowski, P, et al. Xenogenic application of human placenta-derived mesenchymal stromal cells in a porcine large animal model. Cell Transplant. (2024) 33:9636897241226737. doi: 10.1177/09636897241226737
12. D'Hooghe, TM, Bambra, CS, Xiao, L, Peixe, K, and Hill, JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus). Am J Obstet Gynecol. (2001) 184:917–25. doi: 10.1067/mob.2001.111715
13. Milani-Nejad, N, and Janssen, PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. (2014) 141:235–49. doi: 10.1016/j.pharmthera.2013.10.007
14. Simitsidellis, I, Gibson, DA, and Saunders, PTK. Animal models of endometriosis: replicating the aetiology and symptoms of the human disorder. Best Pract Res Clin Endocrinol Metab. (2018) 32:257–69. doi: 10.1016/j.beem.2018.03.004
15. Kyama, CM, Mihalyi, A, Chai, D, Simsa, P, Mwenda, JM, and D'Hooghe, TM. Baboon model for the study of endometriosis. Womens Health (Lond). (2007) 3:637–46. doi: 10.2217/17455057.3.5.637
16. Nakamura, T, Fujiwara, K, Saitou, M, and Tsukiyama, T. Non-human primates as a model for human development. Stem Cell Reports. (2021) 16:1093–103. doi: 10.1016/j.stemcr.2021.03.021
17. Mayor, P, Pereira, W, Nacher, V, Navarro, M, Monteiro, FOB, el Bizri, HR, et al. Menstrual cycle in four New World primates: Poeppig's woolly monkey (Lagothrix poeppigii), red uakari (Cacajao calvus), large-headed capuchin (Sapajus macrocephalus) and nocturnal monkey (Aotus nancymaae). Theriogenology. (2019) 123:11–21. doi: 10.1016/j.theriogenology.2018.09.019
18. Viganò, P, Caprara, F, Giola, F, Di Stefano, G, Somigliana, E, and Vercellini, P. Is retrograde menstruation a universal, recurrent, physiological phenomenon? A systematic review of the evidence in humans and non-human primates. Hum Reprod Open. (2024) 2024. doi: 10.1093/hropen/hoae045
19. Cassioli, E, Rossi, E, Melani, G, Faldi, M, Rellini, AH, Wyatt, RB, et al. The menstrual distress questionnaire (MEDI-Q): reliability and validity of the English version. Gynecol Endocrinol. (2023) 39:2227275. doi: 10.1080/09513590.2023.2227275
20. Nappi, RE, Tiranini, L, Sacco, S, De Matteis, E, De Icco, R, and Tassorelli, C. Role of estrogens in menstrual migraine. Cells. (2022) 11. doi: 10.3390/cells11081355
21. Slayden, OD, Luo, F, and Martin, DVML. A protocol for creating endometriosis in rhesus macaques (Macaca mulatta). J Med Primatol. (2023) 52:405–13. doi: 10.1111/jmp.12681
22. Critchley, HOD, Babayev, E, Bulun, SE, Clark, S, Garcia-Grau, I, Gregersen, PK, et al. Menstruation: science and society. Am J Obstet Gynecol. (2020) 223:624–64. doi: 10.1016/j.ajog.2020.06.004
23. D'Hooghe, TM, Bambra, CS, Raeymaekers, BM, and Koninckx, PR. Development of spontaneous endometriosis in baboons. Obstet Gynecol. (1996) 88:462–6.
24. Nyachieo, A, Chai, DC, Deprest, J, Mwenda, JM, and D'Hooghe, TM. The baboon as a research model for the study of endometrial biology, uterine receptivity and embryo implantation. Gynecol Obstet Investig. (2007) 64:149–55. doi: 10.1159/000101739
25. D'Hooghe, TM, Kyama, CM, and Chai, D. Nonhuman primate models for translational research in endometriosis. Reprod Sci. (2009) 16:152–61. doi: 10.1177/1933719108322430
26. Nishimoto-Kakiuchi, A, Netsu, S, Okabayashi, S, Taniguchi, K, Tanimura, H, Kato, A, et al. Spontaneous endometriosis in cynomolgus monkeys as a clinically relevant experimental model. Hum Reprod. (2018) 33:1228–36. doi: 10.1093/humrep/dey095
27. Sakai, T, Hata, J, Ohta, H, Shintaku, Y, Kimura, N, Ogawa, Y, et al. The Japan monkey Centre Primates brain imaging repository for comparative neuroscience: an archive of digital records including records for endangered species. Primates. (2018) 59:553–70. doi: 10.1007/s10329-018-0694-3
28. Bimber, BN, Yan, MY, Peterson, SM, and Ferguson, B. mGAP: the macaque genotype and phenotype resource, a framework for accessing and interpreting macaque variant data, and identifying new models of human disease. BMC Genomics. (2019) 20:176. doi: 10.1186/s12864-019-5559-7
29. Dapas, M, Sisk, R, Legro, RS, Urbanek, M, Dunaif, A, and Hayes, MG. Family-based quantitative trait Meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. (2019) 104:3835–50. doi: 10.1210/jc.2018-02496
30. Chiou, KL, Montague, MJ, Goldman, EA, Watowich, MM, Sams, SN, Song, J, et al. Rhesus macaques as a tractable physiological model of human ageing. Philos Trans R Soc Lond Ser B Biol Sci. (1811) 375:20190612. doi: 10.1098/rstb.2019.0612
31. Rippy, MK, Lee, DR, Pearson, SL, Bernal, JC, and Kuehl, TJ. Identification of rhesus macaques with spontaneous endometriosis. J Med Primatol. (1996) 25:346–55.
32. Zondervan, KT, Weeks, DE, and Colman, R. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod. (2004) 19:448–55. doi: 10.1093/humrep/deh052
33. Assaf, BT, and Miller, AD. Pleural endometriosis in an aged rhesus macaque (Macaca mulatta): a histopathologic and immunohistochemical study. Vet Pathol. (2012) 49:636–41. doi: 10.1177/0300985811406890
34. Zondervan, K, Cardon, L, and Desrosiers, R. The genetic epidemiology of spontaneous endometriosis in the rhesus monkey. Ann N Y Acad Sci. (2002) 955:233–8. doi: 10.1111/j.1749-6632.2002.tb02784.x
35. Gurule, S, Sustaita-Monroe, J, Padmanabhan, V, and Cardoso, R. Developmental programming of the neuroendocrine axis by steroid hormones: insights from the sheep model of PCOS. Front Endocrinol (Lausanne). (2023) 14:1096187. doi: 10.3389/fendo.2023.1096187
36. Mansour, S, Hamed, S, and Kamal, R. Spectrum of ovarian Incidentalomas: diagnosis and management. Br J Radiol. (2023) 96:20211325. doi: 10.1259/bjr.20211325
37. Fortune, JE, Rivera, GM, Evans, AC, and Turzillo, AM. Differentiation of dominant versus subordinate follicles in cattle. Biol Reprod. (2001) 65:648–54. doi: 10.1095/biolreprod65.3.648
38. García-Guerra, A, Motta, JCL, Melo, LF, Kirkpatrick, BW, and Wiltbank, MC. Ovulation rate, antral follicle count, and circulating anti-Müllerian hormone in trio allele carriers, a novel high fecundity bovine genotype. Theriogenology. (2017) 101:81–90. doi: 10.1016/j.theriogenology.2017.05.026
39. Wall, KM, Kilembe, W, Vwalika, B, Haddad, LB, Hunter, E, Lakhi, S, et al. Risk of heterosexual HIV transmission attributable to sexually transmitted infections and non-specific genital inflammation in Zambian discordant couples, 1994-2012. Int J Epidemiol. (2017) 46:1593–606. doi: 10.1093/ije/dyx045
40. Greydanus, DE, Cabral, MD, and Patel, DR. Pelvic inflammatory disease in the adolescent and young adult: an update. Dis Mon. (2022) 68:101287. doi: 10.1016/j.disamonth.2021.101287
41. Yu, J, Zhou, Y, Luo, H, Su, X, Gan, T, Wang, J, et al. Mycoplasma genitalium infection in the female reproductive system: diseases and treatment. Front Microbiol. (2023) 14:1098276. doi: 10.3389/fmicb.2023.1098276
42. Langner, CA, Ortiz, AM, Flynn, JK, Kendall, H, Lagenaur, LA, and Brenchley, JM. The vaginal microbiome of nonhuman Primates can be only transiently altered to become Lactobacillus dominant without reducing inflammation. Microbiol Spectr. (2021) 9:e0107421. doi: 10.1128/Spectrum.01074-21
43. Rhoades, NS, Hendrickson, SM, Gerken, DR, Martinez, K, Slayden, OD, Slifka, MK, et al. Longitudinal profiling of the macaque vaginal microbiome reveals similarities to diverse human vaginal communities. mSystems. (2021) 6. doi: 10.1128/mSystems.01322-20
44. Huang, Y, Zhu, B, Ji, X, Wen, Y, Wang, Y, Hu, X, et al. Forty years of development of salpingitis animal modeling. Arch Gynecol Obstet. (2023) 308:1093–112. doi: 10.1007/s00404-023-06966-1
45. Gabriel, GC, Devine, WA, Redel, BK, Whitworth, KM, Samuel, M, Spate, LD, et al. Profiling development of abdominal organs in the pig. Sci Rep. (2022) 12:16245. doi: 10.1038/s41598-022-19960-5
46. Jana, B, Całka, J, Bulc, M, and Witek, K. Role of noradrenaline and Adrenoreceptors in regulating prostaglandin E2 synthesis Cascade in inflamed endometrium of pigs. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24065856
47. Packard, GC. Allometric growth in mass by the brain of mammals. Anat Rec (Hoboken). (2021) 304:1551–61. doi: 10.1002/ar.24555
48. Gómez-Segura, L, Boix-Montañes, A, Mallandrich, M, Parra-Coca, A, Soriano-Ruiz, JL, Calpena, AC, et al. Swine as the animal model for testing new formulations of anti-inflammatory drugs: Carprofen pharmacokinetics and bioavailability of the intramuscular route. Pharmaceutics. (2022) 14. doi: 10.3390/pharmaceutics14051045
49. Bošnjak, M, Kržan, M, Tratar, UL, Dolenc, J, Čemažar, M, and Seliškar, A. Pharmacokinetics of carprofen in anaesthetized pigs: a preliminary study. Vet Anaesth Analg. (2021) 48:35–41. doi: 10.1016/j.vaa.2020.09.006
50. De Clercq, E, Van Gils, M, and Schautteet, K. Chlamydia trachomatis L2c infection in a porcine model produced urogenital pathology and failed to induce protective immune responses against re-infection. Front Immunol. (2020) 11:555305. doi: 10.3389/fimmu.2020.555305
51. Ombelet, W. WHO fact sheet on infertility gives hope to millions of infertile couples worldwide. Facts Views Vis Obgyn. (2020) 12:249–51.
52. Farsimadan, M, Riahi, SM, Muhammad, HM, Emamvirdizadeh, A, Tabasi, M, Motamedifar, M, et al. The effects of hepatitis B virus infection on natural and IVF pregnancy: a meta-analysis study. J Viral Hepat. (2021) 28:1234–45. doi: 10.1111/jvh.13565
53. Abedal-Majed, MA, and Cupp, AS. Livestock animals to study infertility in women. Anim Front. (2019) 9:28–33. doi: 10.1093/af/vfz017
54. Schliep, KC, Mumford, SL, Hammoud, AO, Stanford, JB, Kissell, KA, Sjaarda, LA, et al. Luteal phase deficiency in regularly menstruating women: prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. J Clin Endocrinol Metab. (2014) 99:E1007–14. doi: 10.1210/jc.2013-3534
55. Lee, GH, Kim, HJ, Joo, YS, Kim, SY, Reed, B, Hart, LA, et al. Development of two Korean IACUC guidance documents to Foster implementation of the three Rs. Altern Lab Anim. (2023) 51:335–49. doi: 10.1177/02611929231194309
56. Cardoso-Moreira, M, Sarropoulos, I, Velten, B, Mort, M, Cooper, DN, Huber, W, et al. Developmental gene expression differences between humans and mammalian models. Cell Rep. (2020) 33:108308. doi: 10.1016/j.celrep.2020.108308
57. Carson, SA, and Kallen, AN. Diagnosis and Management of Infertility: a review. JAMA. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
58. Hansen, S, Fischer, K, Krabben, L, Rinke Carrapeiro, A, Klinger, B, Schnieke, A, et al. Detection of porcine cytomegalovirus, a roseolovirus, in pig ovaries and follicular fluid: implications for somatic cells nuclear transfer, cloning and xenotransplantation. Virol J. (2023) 20:15. doi: 10.1186/s12985-023-01975-7
59. Li, C, Zhou, M, He, X, di, R, Zhang, Z, Ren, C, et al. Comparative proteomics of ovaries elucidated the potential targets related to ovine prolificacy. Front Vet Sci. (2023) 10:1096762. doi: 10.3389/fvets.2023.1096762
60. Kong, C, Su, J, Wang, Q, Liu, K, Fu, R, and Sui, S. Signaling pathways of Periplaneta americana peptide resist H(2)O(2)-induced apoptosis in pig-ovary granulosa cells through FoxO1. Theriogenology. (2022) 183:108–19. doi: 10.1016/j.theriogenology.2022.02.004
61. Brännström, M, Racowsky, C, Richards, EG, Flyckt, R, Stillman, RJ, O’Brien, JE, et al. Absolute uterine infertility a cornelian dilemma: uterine transplantation or surrogacy? Fertil Steril. (2023) 119:918–29. doi: 10.1016/j.fertnstert.2023.04.005
62. Johannesson, L, Enskog, A, and Dahm-Kähler, P. Uterus transplantation in a non-human primate: long-term follow-up after autologous transplantation. Hum Reprod. (2012) 27:1640–8. doi: 10.1093/humrep/des093
63. Grigsby, PL. Animal models to study placental development and function throughout Normal and dysfunctional human pregnancy. Semin Reprod Med. (2016) 34:011–6. doi: 10.1055/s-0035-1570031
64. Rowell, EE, Corkum, KS, Even, KA, and Laronda, MM. Ovarian tissue health after laparoscopic unilateral oophorectomy: a porcine model for establishing optimized fertility preservation techniques in children. J Pediatr Surg. (2020) 55:1631–8. doi: 10.1016/j.jpedsurg.2019.12.014
65. Wei, L, Xue, T, Tao, KS, Zhang, G, Zhao, GY, Yu, SQ, et al. Modified human uterus transplantation using ovarian veins for venous drainage: the first report of surgically successful robotic-assisted uterus procurement and follow-up for 12 months. Fertil Steril. (2017) 108:346–356.e1. doi: 10.1016/j.fertnstert.2017.05.039
66. Maraschio, MA, Larcher, JMS, Alcaraz, A, Giordano, E, Reimondez, S, Luján, O, et al. Uterus transplantation in a sheep model: novel surgical technique with long-term survival and uterus vitality. First case series in Argentina. JBRA Assist Reprod. (2021) 25:557–62. doi: 10.5935/1518-0557.20210035
67. Carter, AM. Evolution of placentation in cattle and antelopes. Anim Reprod. (2020) 16:3–17. doi: 10.21451/1984-3143-ar2018-00145
68. Ginther, OJ. Intraovarian spatial and vascular harmony between follicles and corpus luteum in monovulatory heifers, mares, and women. Theriogenology. (2019) 128:31–9. doi: 10.1016/j.theriogenology.2019.01.019
69. Souza, SS, Aguiar, FLN, Alves, BG, Alves, KA, Brandão, FAS, Brito, DCC, et al. Equine ovarian tissue xenografting: impacts of cooling, vitrification, and VEGF. Reprod Fertil. (2021) 2:251–66. doi: 10.1530/raf-21-0008
70. Yatsenko, SA, and Rajkovic, A. Genetics of human female infertility†. Biol Reprod. (2019) 101:549–66. doi: 10.1093/biolre/ioz084
71. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
72. Wang, M, Zhao, J, Zhang, L, Wei, F, Lian, Y, Wu, Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. (2017) 8:761–73. doi: 10.7150/jca.17648
73. Serkova, NJ, Glunde, K, Haney, CR, Farhoud, M, de Lille, A, Redente, EF, et al. Preclinical applications of multi-platform imaging in animal models of Cancer. Cancer Res. (2021) 81:1189–200. doi: 10.1158/0008-5472.Can-20-0373
74. Deycmar, S, Gomes, B, Charo, J, Ceppi, M, and Cline, JM. Spontaneous, naturally occurring cancers in non-human primates as a translational model for cancer immunotherapy. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2022-005514
75. Córdoba, KM, Jericó, D, Jiang, L, Collantes, M, Alegre, M, García-Ruiz, L, et al. Systemic messenger RNA replacement therapy is effective in a novel clinically relevant model of acute intermittent porphyria developed in non-human primates. Gut. (2025) 74:270–83. doi: 10.1136/gutjnl-2024-332619
76. Morita, D, Nishio, N, Saito, S, Tanaka, M, Kawashima, N, Okuno, Y, et al. Enhanced expression of anti-CD19 chimeric antigen receptor in piggyBac transposon-engineered T cells. Mol Ther Methods Clin Dev. (2018) 8:131–40. doi: 10.1016/j.omtm.2017.12.003
77. Guo, L, Overholser, J, Good, AJ, Ede, NJ, and Kaumaya, PTP. Preclinical studies of a novel human PD-1 B-cell peptide Cancer vaccine PD1-Vaxx from BALB/c mice to beagle dogs and to non-human Primates (Cynomolgus monkeys). Front Oncol. (2022) 12:826566. doi: 10.3389/fonc.2022.826566
78. Herzog, TJ, Pignata, S, Ghamande, SA, Rubio, MJ, Fujiwara, K, Vulsteke, C, et al. Randomized phase II trial of farletuzumab plus chemotherapy versus placebo plus chemotherapy in low CA-125 platinum-sensitive ovarian cancer. Gynecol Oncol. (2023) 170:300–8. doi: 10.1016/j.ygyno.2023.01.003
79. Spannuth, WA, Sood, AK, and Coleman, RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin Biol Ther. (2010) 10:431–7. doi: 10.1517/14712591003592069
80. Ebel, W, Routhier, EL, Foley, B, Jacob, S, McDonough, J, Patel, RK, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. (2007) 7:6.
81. Hosono, Y, Niknafs, YS, Prensner, JR, Iyer, MK, Dhanasekaran, SM, Mehra, R, et al. Oncogenic role of THOR, a conserved Cancer/testis long non-coding RNA. Cell. (2023) 186:4254–5. doi: 10.1016/j.cell.2023.08.025
82. Han, X, Li, B, and Zhang, S. MIR503HG: a potential diagnostic and therapeutic target in human diseases. Biomed Pharmacother. (2023) 160:114314. doi: 10.1016/j.biopha.2023.114314
83. Choudhari, R, Yang, B, Rotwein, P, and Gadad, SS. Structure and expression of the long noncoding RNA gene MIR503 in humans and non-human primates. Mol Cell Endocrinol. (2020) 510:110819. doi: 10.1016/j.mce.2020.110819
84. Maltaris, T, Dittrich, R, Widjaja, W, Sindhuwinata, C, Hoffmann, I, Beckmann, MW, et al. The role of oestradiol in the uterine peristalsis in the perfused swine uterus. Reprod Domest Anim. (2006) 41:522–6. doi: 10.1111/j.1439-0531.2006.00707.x
85. Yang, X, Wang, C, Sun, X, Fan, Q, Yuan, J, Li, Y, et al. Cryoablation used in fertility-sparing treatment for early endometrial cancer: a pig model experiment using a new designed balloon cryoprobe. Cryobiology. (2020) 94:89–94. doi: 10.1016/j.cryobiol.2020.04.004
86. Mege, D, Mezouar, S, Dignat-George, F, Panicot-Dubois, L, and Dubois, C. Microparticles and cancer thrombosis in animal models. Thromb Res. (2016) 140:S21–6. doi: 10.1016/s0049-3848(16)30094-9
87. Li, Z, Zheng, W, Wang, H, Cheng, Y, Fang, Y, Wu, F, et al. Application of animal models in Cancer research: recent Progress and future prospects. Cancer Manag Res. (2021) 13:2455–75. doi: 10.2147/cmar.S302565
88. Palanki, R, Peranteau, WH, and Mitchell, MJ. Delivery technologies for in utero gene therapy. Adv Drug Deliv Rev. (2021) 169:51–62. doi: 10.1016/j.addr.2020.11.002
89. Chaffin, CL, and Vandevoort, CA. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp Biol Med (Maywood). (2013) 238:539–48. doi: 10.1177/1535370213489437
90. Simerly, CR, Castro, CA, Jacoby, E, Grund, K, Turpin, J, McFarland, D, et al. Assisted reproductive technologies (ART) with baboons generate live offspring: a nonhuman primate model for ART and reproductive sciences. Reprod Sci. (2010) 17:917–30. doi: 10.1177/1933719110374114
91. Stouffer, RL, and Woodruff, TK. Nonhuman Primates: a vital model for basic and applied research on female reproduction, prenatal development, and Women's health. ILAR J. (2017) 58:281–94. doi: 10.1093/ilar/ilx027
92. Ai, X, Wang, BJ, Wu, Z, Zhang, GX, Ju, ZH, Shi, TP, et al. New porcine model for training for laparoscopic ureteral reimplantation with horn of uterus to mimic enlarged ureter. J Endourol. (2010) 24:103–7. doi: 10.1089/end.2009.0148
93. Wang, Y, Nicholes, K, and Shih, IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol. (2020) 15:71–95. doi: 10.1146/annurev-pathmechdis-012419-032654
94. Jalali, BM, Lukasik, K, Witek, K, Baclawska, A, and Skarzynski, DJ. Changes in the expression and distribution of junction and polarity proteins in the porcine endometrium during early pregnancy period. Theriogenology. (2020) 142:196–206. doi: 10.1016/j.theriogenology.2019.09.041
95. Dubuisson, J, Vilmin, F, Boulvain, M, Combescure, C, Petignat, P, and Brossard, P. Do laparoscopic pelvic trainer exercises improve residents' surgical skills? A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. (2016) 206:177–80. doi: 10.1016/j.ejogrb.2016.09.026
96. Kemp, MW, Jobe, AH, Usuda, H, Nathanielsz, PW, Li, C, Kuo, A, et al. Efficacy and safety of antenatal steroids. Am J Phys Regul Integr Comp Phys. (2018) 315:R825–r839. doi: 10.1152/ajpregu.00193.2017
97. Bessette, NW, and Rurak, DW. Chronic fetal and maternal instrumentation in pregnant sheep: effect on gestation length and birthweight. Reprod Fertil Dev. (2010) 22:459–67. doi: 10.1071/rd09156
98. Turner, ZB, Lima, FS, Conley, AJ, McNabb, BR, Rowe, JD, Garzon, A, et al. Cystic ovarian disease in dairy cattle: diagnostic accuracy when using B-mode and color Doppler ultrasound. J Dairy Sci. (2023) 106:3411–20. doi: 10.3168/jds.2022-22498
99. Notaro, US, Huber, E, Stassi, AF, Ormaechea, NE, Chiaraviglio, JA, Baravalle, ME, et al. Estrogens receptors, nuclear coactivator 1 and ligand-dependent corepressor expression are altered early during induced ovarian follicular persistence in dairy cattle. Theriogenology. (2023) 210:17–27. doi: 10.1016/j.theriogenology.2023.07.004
100. Stensen, MH, Tanbo, T, Storeng, R, Byholm, T, and Fèdorcsak, P. Routine morphological scoring systems in assisted reproduction treatment fail to reflect age-related impairment of oocyte and embryo quality. Reprod Biomed Online. (2010) 21:118–25. doi: 10.1016/j.rbmo.2010.03.018
101. Broekmans, FJ, Soules, MR, and Fauser, BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. (2009) 30:465–93. doi: 10.1210/er.2009-0006
102. Styer, AK, and Toth, TL. Antral follicle count in clinical practice: building the bridge from ovarian reserve to in vitro fertilization outcome. Fertil Steril. (2011) 95:480–1. doi: 10.1016/j.fertnstert.2010.11.054
103. Garcia Barros, R, Lodde, V, Franciosi, F, and Luciano, AM. A refined culture system of oocytes from early antral follicles promotes oocyte maturation and embryo development in cattle. Reproduction. (2023) 165:221–33. doi: 10.1530/rep-22-0277
104. Pinnapureddy, AR, Stayner, C, McEwan, J, Baddeley, O, Forman, J, and Eccles, MR. Large animal models of rare genetic disorders: sheep as phenotypically relevant models of human genetic disease. Orphanet J Rare Dis. (2015) 10:107. doi: 10.1186/s13023-015-0327-5
105. Ham, GX, Lim, KE, Augustine, GJ, and Leong, V. Synchrony in parent-offspring social interactions across development: a cross-species review of rodents and humans. J Neuroendocrinol. (2023) 35:e13241. doi: 10.1111/jne.13241
106. Siragher, E, and Sferruzzi-Perri, AN. Placental hypoxia: what have we learnt from small animal models? Placenta. (2021) 113:29–47. doi: 10.1016/j.placenta.2021.03.018
107. Malik, M, Roh, M, and England, SK. Uterine contractions in rodent models and humans. Acta Physiol (Oxford). (2021) 231:e13607. doi: 10.1111/apha.13607
108. Machado, DA, Ontiveros, AE, and Behringer, RR. Mammalian uterine morphogenesis and variations. Curr Top Dev Biol. (2022) 148:51–77. doi: 10.1016/bs.ctdb.2021.12.004
109. Wang, Q, Xu, X, He, B, Li, Y, Chen, X, and Wang, J. A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Hum Reprod. (2013) 28:1670–8. doi: 10.1093/humrep/det052
110. Bellofiore, N, Rana, S, Dickinson, H, Temple-Smith, P, and Evans, J. Characterization of human-like menstruation in the spiny mouse: comparative studies with the human and induced mouse model. Hum Reprod. (2018) 33:1715–26. doi: 10.1093/humrep/dey247
111. Bai, P, Fan, T, Sun, G, Wang, X, Zhao, L, and Zhong, R. The dual role of DNA repair protein MGMT in cancer prevention and treatment. DNA Repair (Amst). (2023) 123:103449. doi: 10.1016/j.dnarep.2023.103449
112. Lampreht Tratar, U, Horvat, S, and Cemazar, M. Transgenic mouse models in Cancer research. Front Oncol. (2018) 8:268. doi: 10.3389/fonc.2018.00268
113. Lv, W, Zheng, J, Luan, M, Shi, M, Zhu, H, Zhang, M, et al. Comparing the evolutionary conservation between human essential genes, human orthologs of mouse essential genes and human housekeeping genes. Brief Bioinform. (2015) 16:922–31. doi: 10.1093/bib/bbv025
114. Rayatdoost, F, and Grottke, O. The use of large animal models in trauma and bleeding studies. Hamostaseologie. (2023) 43:360–73. doi: 10.1055/a-2118-1431
115. Nardone, R, Florea, C, Höller, Y, Brigo, F, Versace, V, Lochner, P, et al. Rodent, large animal and non-human primate models of spinal cord injury. Zoology (Jena). (2017) 123:101–14. doi: 10.1016/j.zool.2017.06.004
116. Han, W, Li, Z, Guo, Y, He, K, Li, W, Xu, C, et al. Efficient precise integration of large DNA sequences with 3′-overhang dsDNA donors using CRISPR/Cas9. Proc Natl Acad Sci USA. (2023) 120:e2221127120. doi: 10.1073/pnas.2221127120
117. Yoshimatsu, S, Okahara, J, Yoshie, J, Igarashi, Y, Nakajima, R, Sanosaka, T, et al. Generation of a tyrosine hydroxylase-2A-Cre knockin non-human primate model by homology-directed-repair-biased CRISPR genome editing. Cell Rep Methods. (2023) 3:100590. doi: 10.1016/j.crmeth.2023.100590
118. Vajta, G, Chen, WB, and Machaty, Z. Production of cloned pigs by handmade cloning. Methods Mol Biol. (2023) 2647:183–95. doi: 10.1007/978-1-0716-3064-8_9
119. Zhang, Y, Yang, L, Zhang, Y, Liang, Y, Zhao, H, Li, Y, et al. Identification of important factors causing developmental arrest in cloned pig embryos by embryo biopsy combined with microproteomics. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms232415975
120. Chen, L, Qu, J, and Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. (2019) 10:1. doi: 10.1186/s13287-018-1105-9
121. Nakamura, T, Okamoto, I, Sasaki, K, Yabuta, Y, Iwatani, C, Tsuchiya, H, et al. A developmental coordinate of pluripotency among mice, monkeys and humans. Nature. (2016) 537:57–62. doi: 10.1038/nature19096
122. Radtke, S, and Kiem, HP. Identification of nonhuman primate hematopoietic stem and progenitor cells. Methods Mol Biol. (2023) 2567:87–98. doi: 10.1007/978-1-0716-2679-5_6
123. Ribitsch, I, Baptista, PM, Lange-Consiglio, A, Melotti, L, Patruno, M, Jenner, F, et al. Large animal models in regenerative medicine and tissue engineering: to do or not to do. Front Bioeng Biotechnol. (2020) 8:972. doi: 10.3389/fbioe.2020.00972
124. Rogers, CS. Engineering large animal species to model human diseases. Curr Protoc Hum Genet. (2016) 90. doi: 10.1002/cphg.18
125. Ricci, C, Baumgartner, J, Malan, L, and Smuts, CM. Determining sample size adequacy for animal model studies in nutrition research: limits and ethical challenges of ordinary power calculation procedures. Int J Food Sci Nutr. (2020) 71:256–64. doi: 10.1080/09637486.2019.1646714
126. Crudeli, C, Kooragayala, K, Lou, J, Zilberman, B, Williams, J, DeLong, A, et al. Utilization large animal research as an adjunct for surgical education increases perceived resident confidence. Am Surg. (2023) 89:4496–500. doi: 10.1177/00031348221121538
127. Padrell, M, Llorente, M, and Amici, F. Invasive research on non-human Primates-time to turn the page. Animals (Basel). (2021) 11. doi: 10.3390/ani11102999
128. Kumar, S, Laurence, H, Owston, MA, Sharp, RM, Williams, P, Lanford, RE, et al. Natural pathology of the captive chimpanzee (Pan troglodytes): a 35-year review. J Med Primatol. (2017) 46:271–90. doi: 10.1111/jmp.12277
129. Bourgeois-Gironde, S, Addessi, E, and Boraud, T. Economic behaviours among non-human primates. Philos Trans R Soc Lond Ser B Biol Sci. (1819) 376:20190676. doi: 10.1098/rstb.2019.0676
130. BA, TH, Bogers, WM, Haanstra, KG, Verreck, FA, and Kocken, CH. The translational value of non-human primates in preclinical research on infection and immunopathology. Eur J Pharmacol. (2015) 759:69–83. doi: 10.1016/j.ejphar.2015.03.023
131. Andersen, ML, and Winter, LMF. Animal models in biological and biomedical research - experimental and ethical concerns. An Acad Bras Cienc. (2019) 91:e20170238. doi: 10.1590/0001-3765201720170238
132. Guo, J, He, S, Tu, Y, Zhang, Y, Wang, Z, Wu, S, et al. A stable large animal model for Dural defect repair with biomaterials and regenerative medicine. Tissue Eng Part C Methods. (2019) 25:315–23. doi: 10.1089/ten.TEC.2019.0014
133. Wang, Y, Kankala, RK, Ou, C, Chen, A, and Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact Mater. (2022) 9:198–220. doi: 10.1016/j.bioactmat.2021.07.005
134. Jogdand, A, Landolina, M, and Chen, Y. Organs in orbit: how tissue chip technology benefits from microgravity, a perspective. Front Lab Chip Technol. (2024) 3. doi: 10.3389/frlct.2024.1356688
135. Mahajan, G, Doherty, E, and To, T. Vaginal microbiome-host interactions modeled in a human vagina-on-a-chip. Microbiome. (2022) 10:201. doi: 10.1186/s40168-022-01400-1
136. Ingber, DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. (2022) 23:467–91. doi: 10.1038/s41576-022-00466-9
137. Picollet-D'hahan, N, Zuchowska, A, Lemeunier, I, and Le Gac, S. Multiorgan-on-a-Chip: A systemic approach to model and decipher inter-organ communication. Trends Biotechnol. (2021) 39:788–810. doi: 10.1016/j.tibtech.2020.11.014
138. Manickam, P, Mariappan, SA, Murugesan, SM, Hansda, S, Kaushik, A, Shinde, R, et al. Artificial intelligence (AI) and internet of medical things (IoMT) assisted biomedical Systems for Intelligent Healthcare. Biosensors (Basel). (2022) 12. doi: 10.3390/bios12080562
139. Kulkarni, PA, and Singh, H. Artificial intelligence in clinical diagnosis: opportunities, challenges, and hype. JAMA. (2023) 330:317–8. doi: 10.1001/jama.2023.11440
140. Walilko, TJ, Sewsankar, K, Wagner, CD, Podolski, A, Smith, K, Zai, L, et al. The application of machine learning to identify large animal blast exposure thresholds. Mil Med. (2023) 188:e600–6. doi: 10.1093/milmed/usab410
141. Shen, J, Sharifzadeh-Kermani, A, and Tayebi, M. Atlas-free automatic segmentation of sheep brain MRI. Annu Int Conf IEEE Eng Med Biol Soc. (2023) 2023:1–4. doi: 10.1109/embc40787.2023.10340739
142. Kritikos, M. The European Union as guardian of ethical research and integrity. Dev Med Child Neurol. (2023) 65:150. doi: 10.1111/dmcn.15449
143. De Simone, FI, and Serratosa, J. Biotechnology, animal health and animal welfare within the framework of European Union legislation. Rev Sci Tech. (2005) 24:89–99.
144. Vitale, A, and Ricceri, L. The principle of the 3Rs between aspiration and reality. Front Physiol. (2022) 13:914939. doi: 10.3389/fphys.2022.914939
145. Bailey, J. It's time to review the three Rs, to make them more fit for purpose in the 21st century. Altern Lab Anim. (2024) 52:155–65. doi: 10.1177/02611929241241187
146. Kim, SH, Lee, SJ, and Yu, SM. Study of lipid proton difference evaluation via 9.4T MRI analysis of fatty liver induced by exposure to methionine and choline-deficient (MCD) diet and high-fat diet (HFD) in an animal model. Chem Phys Lipids. (2022) 242:105164. doi: 10.1016/j.chemphyslip.2021.105164
147. Kisu, I, Banno, K, Mihara, M, Suganuma, N, and Aoki, D. Current status of uterus transplantation in primates and issues for clinical application. Fertil Steril. (2013) 100:280–94. doi: 10.1016/j.fertnstert.2013.03.004
148. Abbott, DH, Rogers, J, Dumesic, DA, and Levine, JE. Naturally occurring and experimentally induced Rhesus macaque models for polycystic ovary syndrome: translational gateways to clinical application. Med Sci (Basel). (2019) 7. doi: 10.3390/medsci7120107
149. Hayashi, K, Carpenter, KD, and Spencer, TE. Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology. (2004) 145:3247–57. doi: 10.1210/en.2004-0178
150. Moini Jazani, A, Nasimi Doost Azgomi, H, Nasimi Doost Azgomi, A, and Azgomi R, ND. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru. (2019) 27:863–77. doi: 10.1007/s40199-019-00312-0
151. Padmanabhan, V, and Veiga-Lopez, A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. (2013) 373:8–20. doi: 10.1016/j.mce.2012.10.005
152. Favre-Inhofer, A, Carbonnel, M, Revaux, A, Sandra, O, Mougenot, V, Bosc, R, et al. Critical steps for initiating an animal uterine transplantation model in sheep: experience from a case series. Int J Surg. (2018) 60:245–51. doi: 10.1016/j.ijsu.2018.11.017
153. Banstola, A, and Reynolds, JNJ. The sheep as a large animal model for the investigation and treatment of human disorders. Biology (Basel). (2022) 11. doi: 10.3390/biology11091251
154. Riddle, A, Dean, J, Buser, JR, Gong, X, Maire, J, Chen, K, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. (2011) 70:493–507. doi: 10.1002/ana.22501
155. Zhang, Q, Chen, Y, Lai, M, Li, Y, Li, Q, Fu, C, et al. Magnetic resonance imaging-guided focused ultrasound surgery in a swine Adenomyosis model. Acad Radiol. (2023) 30:S220–s226. doi: 10.1016/j.acra.2022.11.034
156. Dion, L, Le Lous, M, and Nyangoh Timoh, K. Single bilateral ovarian venous return in uterine transplant: validation in an orthotopic auto-transplant model in the Yucatan minipig. J Gynecol Obstet Hum Reprod. (2021) 50:102059. doi: 10.1016/j.jogoh.2021.102059
157. Clauss, S, Bleyer, C, Schüttler, D, Tomsits, P, Renner, S, Klymiuk, N, et al. Animal models of arrhythmia: classic electrophysiology to genetically modified large animals. Nat Rev Cardiol. (2019) 16:457–75. doi: 10.1038/s41569-019-0179-0
158. Adams, GP, Singh, J, and Baerwald, AR. Large animal models for the study of ovarian follicular dynamics in women. Theriogenology. (2012) 78:1733–48. doi: 10.1016/j.theriogenology.2012.04.010
159. Amonkar, DDB, Genovese, V, and De Gregorio, V. Impact of prepubertal bovine ovarian tissue pre-freeze holding duration on follicle quality. Reprod Biol. (2023) 23:100794. doi: 10.1016/j.repbio.2023.100794
160. Baerwald, AR, Adams, GP, and Pierson, RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. (2003) 69:1023–31. doi: 10.1095/biolreprod.103.017772
161. Scoggin, CF. Endometritis: nontraditional therapies. Vet Clin North Am Equine Pract. (2016) 32:499–511. doi: 10.1016/j.cveq.2016.08.002
162. Lu, H, Ma, L, Zhang, Y, Feng, Y, Zhang, J, and Wang, S. Current animal model Systems for Ovarian Aging Research. Aging Dis. (2022) 13:1183–95. doi: 10.14336/ad.2021.1209
163. Bernstein, LR, Mackenzie, ACL, Durkin, K, Kraemer, DC, Chaffin, CL, and Merchenthaler, I. Maternal age and gonadotrophin elevation cooperatively decrease viable ovulated oocytes and increase ootoxicity, chromosome-, and spindle-misalignments: '2-Hit' and 'FSH-OoToxicity' mechanisms as new reproductive aging hypotheses. Mol Hum Reprod. (2023) 29. doi: 10.1093/molehr/gaad030
164. Bauer, C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception. (2015) 92:120–3. doi: 10.1016/j.contraception.2015.06.007
165. Yun, JW, Kim, YY, Ahn, JH, Kang, BC, and Ku, SY. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng Regen Med. (2016) 13:323–34. doi: 10.1007/s13770-016-9091-4
166. Onyango, PO, Gesquiere, LR, Altmann, J, and Alberts, SC. Puberty and dispersal in a wild primate population. Horm Behav. (2013) 64:240–9. doi: 10.1016/j.yhbeh.2013.02.014
167. Sato, J, Nasu, M, and Tsuchitani, M. Comparative histopathology of the estrous or menstrual cycle in laboratory animals. J Toxicol Pathol. (2016) 29:155–62. doi: 10.1293/tox.2016-0021
168. MacLean, JA 2nd, and Hayashi, K. Progesterone actions and resistance in gynecological disorders. Cells. (2022) 11. doi: 10.3390/cells11040647
169. Weinbauer, GF, Niehoff, M, Niehaus, M, Srivastav, S, Fuchs, A, van Esch, E, et al. Physiology and endocrinology of the ovarian cycle in macaques. Toxicol Pathol. (2008) 36:7s–23s. doi: 10.1177/0192623308327412
170. Gong, JG, Campbell, BK, and Webb, R. Defining the gonadotrophin requirement for the selection of a single dominant follicle in cattle. Reprod Fertil Dev. (2020) 32:322–34. doi: 10.1071/rd19060
Keywords: large animal models, gynecological diseases, non-human primates, pigs, sheep
Citation: Zhao D, Li X, Zheng X, Xie X, Zhao Y and Liu Y (2025) Large animal models in gynecology: status and future perspectives. Front. Vet. Sci. 12:1588098. doi: 10.3389/fvets.2025.1588098
Edited by:
Isaac Karimi, Razi University, IranReviewed by:
Murat Findik, Ondokuz Mayis University, TürkiyeKomang Trisna Sumadewi, Universitas Warmadewa, Indonesia
Copyright © 2025 Zhao, Li, Zheng, Xie, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanan Zhao, enluamlheW91NDI1QDE2My5jb20=; Yang Liu, bGl1LXlhbmc2NkAxMjYuY29t
 Dan Zhao1
Dan Zhao1 Xu Zheng
Xu Zheng Xiangrui Xie
Xiangrui Xie Yanan Zhao
Yanan Zhao Yang Liu
Yang Liu