Abstract
The scientific literature referring to tumors in African pygmy hedgehog (Atelerix albiventris) is expanding, enhanced by an increased lifespan under human care. This study compiles the clinical records and pathologic findings in a 2-year and 7-month-old male African pygmy hedgehog (Atelerix albiventris) with a metastatic giant-cell renal sarcoma. Metastases to the lungs, retroperitoneum, lymph nodes, pericardium, and mediastinal adipose tissue were observed. Immunohistochemical assessment with a panel of 11 antibodies (cytokeratins [AE1/AE3, CK19, and CK20], vimentin, thyroid transcription factor-1, thyroglobulin, Ki67, Iba-1, S100, synaptophysin, and CD31) was performed, yielding negative immunolabeling in the neoplastic cells. To the author’s knowledge, neoplasms arising from the kidney in African pygmy hedgehogs are rarely reported, and a metastatic giant-cell renal sarcoma has not been previously described. This case report aims to contribute to the growing body of knowledge regarding neoplasm development and help veterinary specialists with the characterization of the pathologic conditions affecting this species.
Introduction
The African pygmy hedgehog (Atelerix albiventris; Family Erinaceidae), or four-toed hedgehog, is an insectivorous and nocturnal small mammal gaining popularity as an exotic pet species in Western countries (1). Increasing literature has revealed a high incidence of malignant neoplastic events in captive individuals as they reach adulthood and geriatric ages (2, 3). The majority of the reported neoplastic events have malignant features and are reserved for poor prognosis (2, 4, 5). In decreasing order, the most common tumor origins and affected organ systems are mesenchymal, epithelial, and round cell, involving integumentary, hemolymphatic, digestive, endocrine, and reproductive systems, respectively (2, 4, 5).
The diagnosis of “anaplastic” giant-cell sarcoma (GCS) represents a histologic and immunohistochemical diagnostic challenge in human and veterinary medicine (6). The literature attempting a detailed immunohistochemical characterization of GCS is scarce in domestic animal species (7–9). Herein, this study reports the clinical, macroscopic, and histologic features, together with an immunohistochemical assessment, of a renal GCS in an African pygmy hedgehog with metastasis contributing to the tumor characterization and the growing knowledge of neoplasms affecting this species.
Materials and methods
A 2-year and 7-month-old, male intact, white and black, domestic African hedgehog was presented to a private veterinary practice with a history of dry and itchy skin, ongoing lethargy, reduced fecal and urine output, anorexia, and weight loss (705–540 grams). Dermatophyte culture on skin was negative. The animal was found dead and submitted for full post-mortem examination. A standardized necropsy was performed, and representative samples from major organ systems, including skin, skeletal muscle, liver, kidney, spleen, lung, heart, adrenal gland, small intestine, large intestine, and brain, were collected and processed routinely in the histologic laboratory of the Veterinary Pathology Centre (University of Surrey). Ancillary histochemical stains, including Periodic-acid Schiff (PAS), Giemsa, Perls’, Masson Trichrome, and Von Kossa, were performed on the primary neoplasm and metastatic sites. Immunohistochemistry (IHC) was conducted on both the primary tumor and metastases for vimentin, thyroid transcription factor-1 [TTF1], Ki 67, thyroglobulin, cytokeratin (AE1/AE3, CK19, and CK20), Iba-1, S100, synaptophysin, and CD31 (Table 1) following standardized protocols (10). Internal positive controls included vascular endothelium for CD31, stromal and endothelial cells for vimentin, resident macrophages for Iba-1, Schwann cells and dendritic cells for S100, neuroendocrine cells for synaptophysin, bronchial epithelium for cytokeratins (AE1/AE3, CK19, and CK20), proliferating cells (mitotic figures) for Ki-67, respiratory epithelium for thyroid transcription factor-1 (TTF-1), and, if present, thyroid follicular cells for thyroglobulin. External positive tissue controls included canine normal-haired skin, lung, lymph node, and adrenal gland. Negative controls were produced using Primary Antibody Diluent (Leica Biosystems) instead of the primary antibody. The Bond Polymer Refine Detection Kit (Leica Biosystems) was used for visualization with hematoxylin counterstain.
Table 1
| Antibody | Source | Host | Type | Clone | Antigen retrieval | Dilution |
|---|---|---|---|---|---|---|
| AE1/AE3 | Dako | Mouse | Monoclonal | AE1 y AE3 | Citrate buffer | 1:100 |
| Vimentin | Dako | Mouse | Monoclonal | V9 | Citrate buffer | 1:100 |
| TTF1 | Abcam | Rabbit | Monoclonal | SP141 | Tris-EDTA buffer | 1:100 |
| Thyroglobulin | Dako | Rabbit | Polyclonal | 1D4 | Citrate buffer | 1:1500 |
| CK20 | Leica | Mouse | Monoclonal | B170 | Citrate buffer | 1:100 |
| CK19 | Leica | Mouse | Monoclonal | B170 | Citrate buffer | 1:100 |
| Ki67 | Dako | Mouse | Monoclonal | MIB-1 | Citrate buffer | 1:200 |
| S100 | Novacastra | Rabbit | Polyclonal | NCL-L-S100p | Citrate buffer | 1:2000 |
| IBA-1 | Wako | Rabbit | Polyclonal | IBA-1 | Citrate buffer | 1:1000 |
| Synaptophysin | Leica | Mouse | Monoclonal | 27G12 | Citrate buffer | 1:100 |
| CD31 | Dako | Mouse | Monoclonal | JC70A | Citrate buffer | 1:40 |
Primary antibodies and immunostaining protocols used in the current study.
Results
At necropsy, the right kidney was markedly expanded by a 2.5 × 2 × 1.5 cm, firm, multinodular, dark red to pale brown mass. The left kidney was less affected but exhibited similar findings (Figure 1A). The core of the mass was composed of multiple and coalescing blood-filled cavities intermixed with multifocal and extensive white and hard areas (dystrophic mineralization) (Figure 1B). The interrenal fat and lymph nodes, in close association with the renal artery and vein, were effaced by a focal 0.5 × 0.2 × 0.2 cm multinodular mass exhibiting identical gross features as described in the kidneys. Similar multinodular structures were present at the base of the heart and attached to the pericardial sac. The trachea was filled with moderate amounts of foam. Lung lobes were congested and edematous with occasional miliary dark red and firm areas randomly distributed throughout the pulmonary parenchyma on cut section.
Figure 1
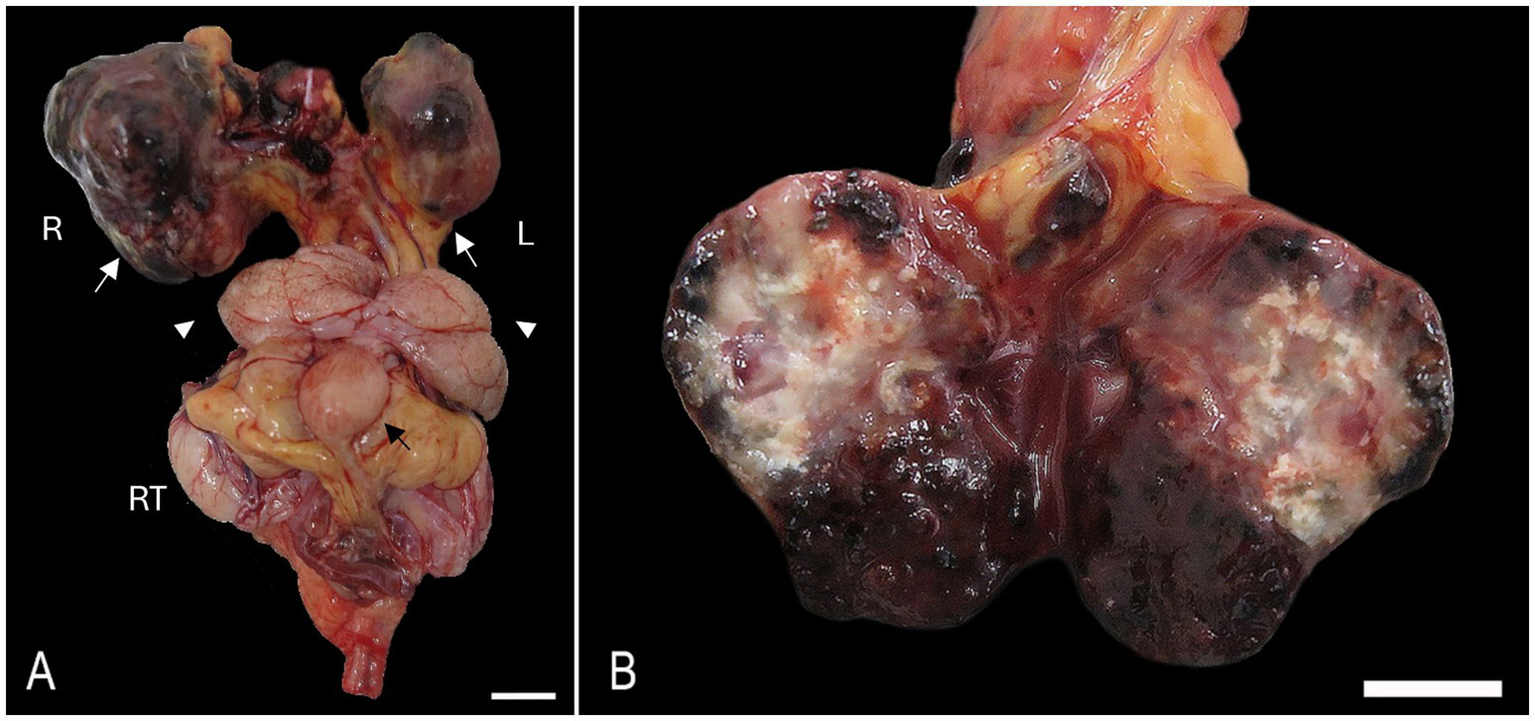
Bilateral anaplastic (giant cell) renal sarcoma in a hedgehog. (A) Both kidneys [white arrows, right (R) and left (L)] are expanded by poorly demarcated, irregularly round, raised, tan to dark-red nodules. The vesicular gland (white arrow heads), urinary bladder (black arrow), and right testis (RT) are visible. Bar = 1 cm. (B) The cut surface of the left kidney exhibits multiple colors with areas of necrosis, mineralization, and hemorrhage. Bar = 1 cm.
Histologically, the cortex and medulla of the right kidney were markedly disrupted by a multilobular, expansile, moderately cellular, poorly demarcated, non-encapsulated, and infiltrative neoplasm composed of a pleomorphic mesenchymal cell population. Neoplastic cells are disposed in interwoven streams and supported by moderate fibrovascular stroma often forming large blood-filled cavities. Cells are large (60–120 μm), round to polygonal with moderately distinct cell borders, a mild to moderate amount of finely granular to vacuolated cytoplasm, a large (5–30 μm) central to paracentral nucleus with finely stippled chromatin, with an often prominent single, occasionally two, magenta nucleolus. Anisocytosis, anisokaryosis, and karyomegaly are marked. Binucleated and multinucleated giant cells were common. The mitotic count was 9 in 10 high-power fields (2.37mm2). Multifocal areas within the neoplasm exhibit loss of any recognizable architecture and cellular integrity with karyorrhexis and karyolysis (necrosis), numerous acicular cholesterol clefts with xanthogranulomatous reaction, and multifocal well-differentiated, eosinophilic, mineralized, and anastomosis trabeculae containing a moderate number of osteocytes (osseous metaplasia) (Figures 2A–D). The left kidney exhibited identical but less prominent histologic findings. Other microscopic findings in the kidney included moderate and multifocal lymphoplasmacytic interstitial nephritis with moderate fibrosis, frequent loss of “back-to-back” tubular arrangement, and frequent thickening of Bowman’s capsule.
Figure 2
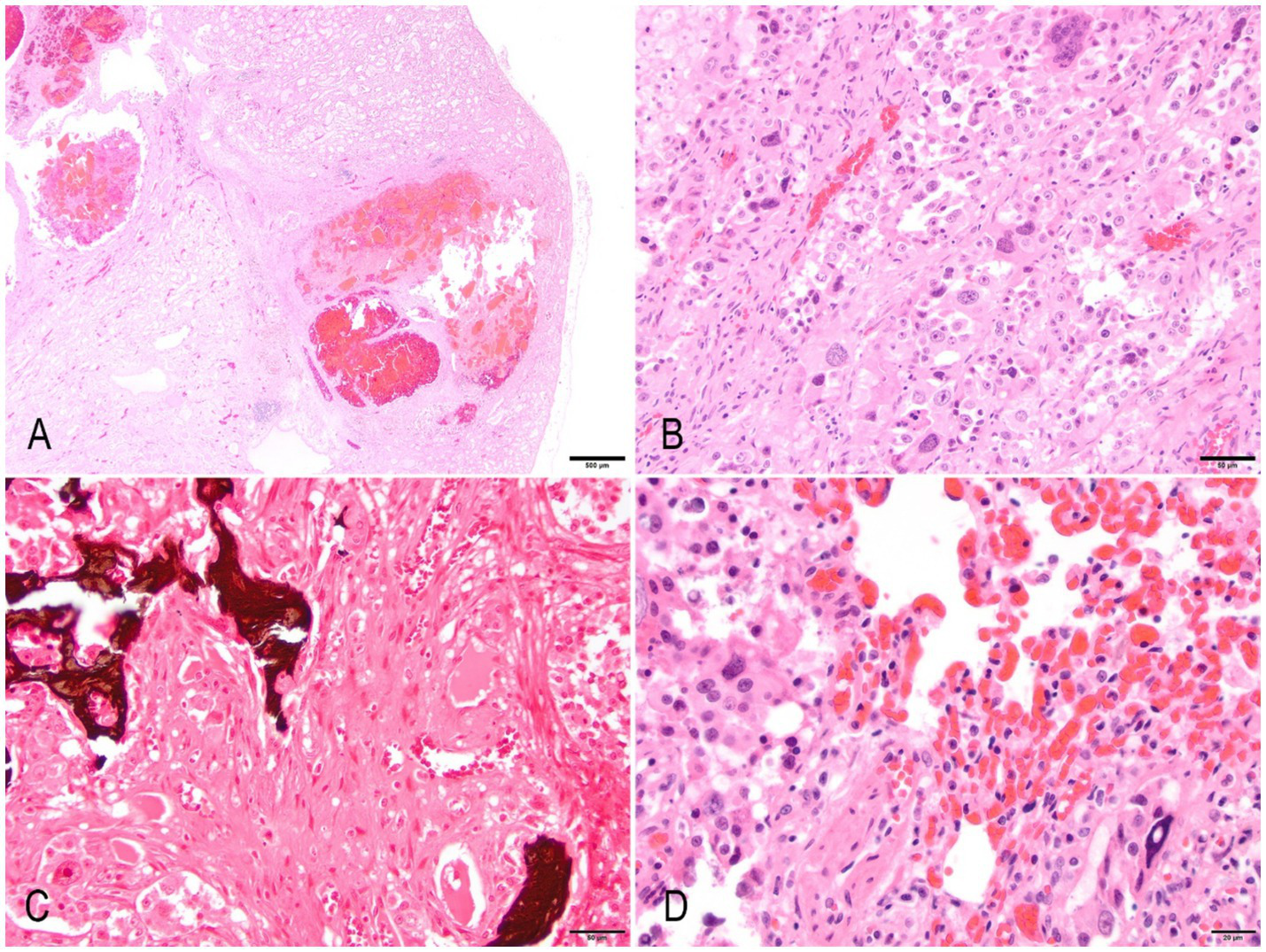
Anaplastic (giant cell) renal sarcoma in a hedgehog. (A) A low-power magnification image of the left kidney exhibits multifocal areas of neoplasia that efface and expand the parenchyma. The neoplasm exhibits multifocal cystic areas and hemorrhages. Hematoxylin & Eosin (HE). Bar = 500 μm. (B) The neoplasm is composed of spindle, pleomorphic cells that exhibit marked anisokaryosis, anisocytosis, cytomegaly, and karyomegaly. There are frequent multinucleated giant cells. Bar = 50 μm. (C) Multifocal areas exhibit osteoid that blends with neoplastic cells. Von Kossa. Bar = 50 μm. (D) Lung metastasis. Spindloid neoplastic cells infiltrate the lung parenchyma. There is diffuse marked congestion of alveolar capillary beds (HE). Bar = 20 μm.
The lung parenchyma was multifocally expanded and disrupted by coalescing nodules of neoplastic cells displaying similar features as previously described in the kidneys, often invading vascular structures. Multiple areas of necrosis, discrete dystrophic mineralization with occasional corpora amilacea, hemorrhages, and moderate alveolar histiocytosis were present within the neoplasm (Figure 2). The perirenal and mediastinal lymph nodes were multifocally effaced by neoplastic cells, which spread to the perinodal adipose tissue.
Secondary findings included moderate multifocal and centrilobular hepatic necrosis with marked midzonal congestion; marked splenic lymphoid depletion with abundant megakaryocytes and extramedullary hematopoiesis; mild, multifocal, chronic lymphoplasmacytic balanoposthitis with acanthosis; and mild, multifocal, perivascular lymphoplasmacytic superficial dermatitis with lymphangiectasia, oedema, reduced numbers of pilosebaceous units, and occasional superficial serocellular crusts. The presumed newly formed osteoid stained positive f von Kossa. Masson’s trichrome highlighted moderate intra-tumor proliferation of connective tissue supporting the neoplastic cells. Perls’ stain identified numerous hemosiderin-laden macrophages and multinucleated giant cells within the alveoli. Giemsa and PAS were unremarkable.
Neoplastic cells exhibited negative nuclear or cytoplasmic immunolabelling for cytokeratin (AE1/AE3, 19, 20), thyroglobulin, thyroid transcription factor 1 (TTF1), S100, synaptophysin, CD31, and Iba-1. External and internal positive controls stained appropriately in the expected different cell populations (epithelial cells, pneumocytes, nerve cells, endothelial cells, and macrophages). Vimentin and Ki-67 did not cross-react with hedgehog tissues, as evidenced by the absence of immunoreactivity in tissue elements and mitotic figures, respectively.
Discussion
The macroscopic, histologic, and overall immunohistochemical evaluation of targeted organs in this hedgehog supported the diagnosis of renal anaplastic giant-cell sarcoma (GCS) with invasion and metastasis of the lung, mediastinal, and renal lymph nodes. The morphology and immunoprofile of the neoplastic cells warranted differential diagnoses of osteosarcoma, due to an osteoid-like matrix, and rhabdomyosarcoma, based on pleomorphism and giant cell formation. Rapidly expanding literature reveals a high prevalence of neoplastic events in captive African pygmy hedgehogs (2, 11). As expected, age increases the incidence of neoplastic events in hedgehogs, although reports in young individuals have been described (12).
In a recent retrospective study, neoplastic lesions were present in 60% of the evaluated individuals, with 76% of those being malignant (3). Commonly reported cellular origins, in decreasing order, are: mesenchymal, epithelial, and round cell. Most commonly affected systems with examples of reported neoplasms include: integumentary and soft tissue (fibrosarcoma, hemangiosarcoma, histiocytic sarcomas, squamous cell carcinoma, lymphoma, mast cell tumor, liposarcoma, sebaceous gland carcinoma, schwannoma, neurofibroma, and multicentric), hemolymphatic (lymphoma, eosinophilic, and myelogenous leukemia), reproductive (uterine adenocarcinoma, adenosarcoma, leiomyosarcoma, spindle cell sarcoma, and endometrial stromal sarcoma), mammary gland (mammary adenocarcinoma), digestive (salivary gland adenocarcinoma, colonic mucinous adenocarcinoma, gastric adenocarcinoma, hepatocellular carcinoma, and oral squamous cell carcinoma), among others (3, 11, 13, 14). Occasionally, retroviral particles have been identified within multicentric soft tissue sarcomas, and there has been speculation about the role of viruses in the development of these neoplasms (4).
The kidney was identified as the primary site based on the presence of a large, well-demarcated, expansile, and infiltrative mass replacing a substantial portion of the renal parenchyma, with disruption of normal architecture. No comparable primary lesion of similar extent or organization was observed in other organs. The distribution and progression of lesions were consistent with secondary metastatic spread rather than synchronous tumor development, supporting the exclusion of a multicentric sarcoma (13). Descriptions of primary neoplasms arising from the urinary system in the African pygmy hedgehog are scarce, encompassing adenocarcinoma, hemangiosarcoma, stromal-type nephroblastoma, and a poorly differentiated renal neoplasia (12, 15, 16).
In the former, renal tumor cells displayed negative immunolabelling against all applied antibodies. An epithelial origin (carcinoma) is discarded following negative labeling for AE1/AE3 cytokeratin. In addition, there was no immunolabelling for CK19 (urothelium) and CK20 (variably immunolabelling for urothelium/oncocytomas) (17, 18). The absence of any nuclear labelling against TTF1, together with a negative assessment against thyroglobulin, disregards a thyroid origin of the neoplasm (19). Negative immunolabelling against synaptophysin, S100, Iba-1, may exclude potential neuroendocrine, peripheral nerve sheath, and histiocytic origin of the tumor, respectively (19). Ki67 was not identified within the neoplastic cells despite a moderate mitotic count, suggesting that the Ki67 antibody may not have cross-reacted with the target antigen in this species, likely due to species-specific antigenic differences (20). Similarly, vimentin did not cross-react with hedgehog tissues, likely due to species-specific antigenic differences. The histological and immunohistochemical results support a diagnosis of renal giant-cell sarcoma.
To the author’s knowledge, this case represents the first description of an anaplastic GCS with metastasis to lungs and lymph nodes, compiling macroscopic, histologic, and extensive immunohistochemical assessment in an African pygmy hedgehog.
Interestingly, the poor body condition with marked weight loss, together with the prominent splenic lymphoid depletion and abundant extramedullary hematopoiesis, could suggest a paraneoplastic syndrome secondary to the renal neoplasm (i.e., anemia) (6). Individuals with cancer-related cachexia could present anemia, weakness, weight loss, diminished immune function, and an altered metabolism of carbohydrates, lipids, and proteins. Paraneoplastic dermatoses associated with underlying neoplasms have been described in both humans and animals, often attributed to cytokine release or metabolic disturbances (21, 22). All the criteria needed to confirm the diagnosis of a paraneoplastic syndrome could not be proved, and this is a speculative association.
This study aims to contribute to the rapidly growing literature referring to neoplasms in the African pygmy hedgehog (Atelerix albiventris) with the first description of a GCS arising from the urinary tract. The high prevalence of neoplasms in this species could promote further investigations to characterize and understand the behavior of tumoral cells in non-domestic species.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The specific approval from the animal’s owner was not required for this study. The pathological materials and diagnostic data used in the report are the sole property of the Veterinary Pathology Centre at the University of Surrey, as indicated in our submission form prior to case acceptance. Per institutional policies and ethical standards, materials submitted to and archived by the Veterinary Pathology Centre at Surrey University become its property and may be used for research, education, and publication, provided no identifying information is disclosed. This case report does not include any personal data or identifiable owner information. The clinical material was handled in compliance with established ethical practices, and its use for publication aligns with the internal governance of the Veterinary Pathology Centre at University of Surrey. Here we share the website link for our post mortem submission form: https://www.surrey.ac.uk/sites/default/files/2023-11/private-pm-submission-form-2023.pdf. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
PD-S: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JD-M: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. CG: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. AS-B: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors recognize and are grateful for the contributions of the histology and post-mortem technicians at the University of Surrey Veterinary Pathology Centre, Royal Veterinary College, Francis Crick Institute of London, and Moorfields Eye Hospital. Without their contributions, this study would not be possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Priestnall S Suárez-Bonnet A . Additional stains and immunohistochemistry: what else can the pathologist tell us?In Pract. (2022) 44:385–93. doi: 10.1002/inpr.90
2.
Bertram CA Garner MM Reavill D Klopfleisch R Kiupel M . Giant cell sarcomas in domestic rabbits (Oryctolagus cuniculus). Vet Pathol. (2020) 57:490–6. doi: 10.1177/0300985820921814
3.
Silva GF Rêma A Teixeira S Pires MDA Taulescu M Amorim I . Pathological findings in African pygmy hedgehogs admitted into a Portuguese rehabilitation center. Animals. (2022) 12:1361. doi: 10.3390/ani12111361
4.
Johnson DH . Geriatric hedgehogs. Vet. Clin North America Exotic Animal Practice. (2020) 23:615–37. doi: 10.1016/j.cvex.2020.05.005
5.
Ueda K Imada T Ueda A Imada M Ozaki K . Stromal-type Nephroblastoma with or without anaplasia in two hedgehogs (Atelerix albiventris). J Comp Pathol. (2019) 172:48–52. doi: 10.1016/j.jcpa.2019.09.002
6.
Ramos-Vara JA Beissenherz ME . Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed, paraffin-embedded tissues: Experience with 63 markers. J. Vet. Diag. Inv., 12 (2000). 307–311 p.
7.
Wissink-Argilaga N . African pygmy hedgehogs In: KubiakM, editor. Handbook of exotic pet medicine. Amsterdam: Wiley (2020). 13–26.
8.
Heatley J Mauldin G Cho D . A review of neoplasia in the captive African hedgehog (). Semin Avian Exot Pet Med. (2005) 14:182–92. doi: 10.1053/j.saep.2005.07.002
9.
Raymond J Garner M . Spontaneous tumors in hedgehogs: a retrospective study of fifty cases. In: Proceedings of the Joint Conference of the American Association of Zoo Veterinarians, American Association of Wildlife Veterinarians, Association of Reptilian and Amphibian Veterinarians, and the National Association of Zoo and Wildlife Veterinarians. Orlando, FL (2001). p. 326–327.
10.
Peauroi JR Lowenstine LJ Wilson DW . Multicentric skeletal sarcomas associated with probable retrovirus particles in two African hedgehogs (Atelerix albiventris). Vet Pathol. (1994) 31:403–96.
11.
Harrison TM Kitchell BE . Principles and applications of medical oncology in exotic animals. Vet. Clin. North America Exotic Animal Practice. (2017) 20:209–34. doi: 10.1016/j.cvex.2016.07.007
12.
de Cecco BS Argenta FF Bianchi RM De Lorenzo C Wronski JG Bandinelli MB et al . Feline giant-cell pleomorphic sarcoma: cytologic, histologic and immunohistochemical characterization. J Feline Med Surg. (2021) 23:738–44. doi: 10.1177/1098612X20972667
13.
Okada K Kondo H Sumi A Kagawa Y . A retrospective study of disease incidence in African pygmy hedgehogs (Atelerix albiventris). J Vet Med Sci. (2018) 80:1504–10. doi: 10.1292/jvms.18-0238
14.
Díaz-Delgado J Pool R Hoppes S Cerezo A Quesada-Canales Ó Stoica G . Spontaneous multicentric soft tissue sarcoma in a captive african pygmy hedgehog (Atelerix albiventris): case report and literature review. J Vet Med Sci. (2017) 79:889–95. doi: 10.1292/jvms.17-0003
15.
Meuten D . Tumors in domestic animals. New York, NY: Wiley Blackwell (2017).
16.
Kim M-K Kim S . Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol. (2002) 10:332–8. doi: 10.1097/00129039-200212000-00008
17.
Ghibaudo G Bettini G Abramo F . Anaplastic and aggressive subcutaneous sarcoma in a seven-month-old dog. J Small Anim Pract. (2008) 49:310–3. doi: 10.1111/j.1748-5827.2007.00533.x
18.
Stopyra GA Warhol MJ Multhaupt HAB . Cytokeratin 20 immunoreactivity in renal Oncocytomas. J Histochem Cytochemistry. (2001) 49:919–20. doi: 10.1177/002215540104900712
19.
Pei-Chi H Jane-Fang Y Lih-Chiann W . A retrospective study of the medical status on 63 African hedgehogs (Atelerix albiventris) at the Taipei zoo from 2003 to 2011. J Exot Pet Med. (2015) 24:105–11. doi: 10.1053/j.jepm.2014.11.003
20.
Remnant L Kochanova NY Reid C Cisneros-Soberanis F Earnshaw WC . The intrinsically disorderly story of Ki-67. Open Biol. (2021) 11:210120. doi: 10.1098/rsob.210120
21.
Didona D Fania L Didona B Eming R Hertl M Di Zenzo G . Paraneoplastic dermatoses: a brief general review and an extensive analysis of paraneoplastic pemphigus and paraneoplastic dermatomyositis. Int J Mol Sci. (2020) 21:2178. doi: 10.3390/ijms21062178
22.
Turek MM . Cutaneous paraneoplastic syndromes in dogs and cats: a review of the literature. Vet Dermatol. (2003) 14:279–96. doi: 10.1111/j.1365-3164.2003.00346.x
Summary
Keywords
immunohistochemistry, hedgehog, kidney, metastatic, sarcoma
Citation
Díaz-Santana P, Déniz-Marrero J, Gola C and Suárez-Bonnet A (2025) Case Report: Metastatic renal giant-cell sarcoma in an African pygmy hedgehog (Atelerix albiventris). Front. Vet. Sci. 12:1609488. doi: 10.3389/fvets.2025.1609488
Received
10 April 2025
Accepted
30 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Davis Seelig, University of Minnesota Twin Cities, United States
Reviewed by
Javier Asin, University of California, Davis, United States
Victor Vasconcelos, Federal University of Acre, Brazil
Updates
Copyright
© 2025 Díaz-Santana, Déniz-Marrero, Gola and Suárez-Bonnet.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Díaz-Santana, Pablo.santana@surrey.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.