- 1Virus and Prion Research Unit, National Animal Disease Center, Agricultural Research Service, United States Department of Agriculture, Ames, IA, United States
- 2Department of Biomedical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 3Oak Ridge Institute for Science and Education, Oak Ridge, TN, United States
- 4Department of Veterinary Pathology, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
Background: Chronic wasting disease (CWD) is a fatal prion disease that affects the cervid species, including white-tailed deer (WTD) (Odocoileus virginianus) and mule deer (MD) (Odocoileus hemionus). Interspecies transmission of CWD is highly variable and dependent upon multiple factors. CWD of MD is transmissible to sheep after intracranial inoculation, with clinical signs and incubation periods similar to scrapie.
Purpose: This study used sheep and transgenic mice to investigate the susceptibility of sheep to the CWD agent from WTD (WTD sheep CWD) when intracranially inoculated and to characterize the agent in subsequent passages.
Methods: Fifteen Suffolk sheep with PRNP genotypes VRQ/ARQ, ARQ/ARQ, or ARQ/ARR were inoculated intracranially with the CWD agent from WTD. Western blots and enzyme immunoassays (EIA) were performed on brain and lymphoid tissues to analyze misfolded prion protein (PrPSc) accumulation.
Results: PrPSc was detected in 2 of 15 sheep (both ARQ/ARQ sheep) in the brainstem at the level of the obex, with a mean incubation period (MIP) of 39 months. In affected sheep, the distribution of PrPSc was limited to the central nervous system (CNS). Brain material from one positive sheep (ARQ/ARQ) was used to inoculate mice expressing the cervid (Tg12) and ovine (Tg338) prion protein gene. Passage of the WTD sheep CWD agent into cervidized mice resulted in an attack rate of 83% for PrPSc detection, with a mean incubation period of 377 days for all mice, while passage into ovinized mice resulted in no clinical signs or demonstration of PrPSc. These results were compared to those of passage of MD CWD agent from sheep (MD sheep CWD) into cervidized and ovinized mice. There was an 86% attack rate in cervidized mice with a mean incubation period of 646 days for all mice and an attack rate of 100% in ovinized mice with a mean incubation period of 282 days.
Conclusions: This data suggests that WTD CWD is unlikely to present a major risk to sheep but could be transmissible back to the cervid population. However, MD sheep CWD could present a risk to both the cervid and sheep populations.
Introduction
Prion diseases or transmissible spongiform encephalopathies (TSEs) are the result of an accumulation of the misfolded prion protein (PrPSc), ultimately leading to fatal neurodegeneration (1). CWD and scrapie are TSEs occurring naturally in the cervid and sheep host species (2). Scrapie was first reported in the United States in 1947 from an imported sheep (3), while CWD was first confirmed in 1980 from an MD in Colorado (4).
Sheep’s susceptibility to the scrapie agent can be determined by the host prion protein gene (PRNP). Codons associated with susceptibility include 136 V (valine), 154 R (arginine), and 171 Q (glutamine), a genotype of VRQ/VRQ. Codons associated with resistance to the classical scrapie agent include the genotype of ARR/ARR (5).
The origins of CWD are unclear, though one theory suggests that CWD could have originated from a cervid being exposed to the sheep scrapie agent (6–8). Both diseases exhibit the presence of PrPSc in the central nervous system (CNS) and lymphoid tissues (9, 10), raising the possibility of horizontal transmission between the two species (11). As of January 2025, 36 U.S. states have reported confirmed cases of CWD (Figure 1), with additional cases detected in countries such as Canada, Norway, Finland, Sweden, and South Korea (12). As CWD continues to become increasingly distributed among the cervid population, it is also increasingly contaminating the environment. Notably, the geographic distribution of CWD in both captive and free-ranging cervids overlaps with areas where sheep populations are present and scrapie has been detected (Figures 1–3). This overlap raises an important question of whether sheep could be assisting in the continued spread of CWD.
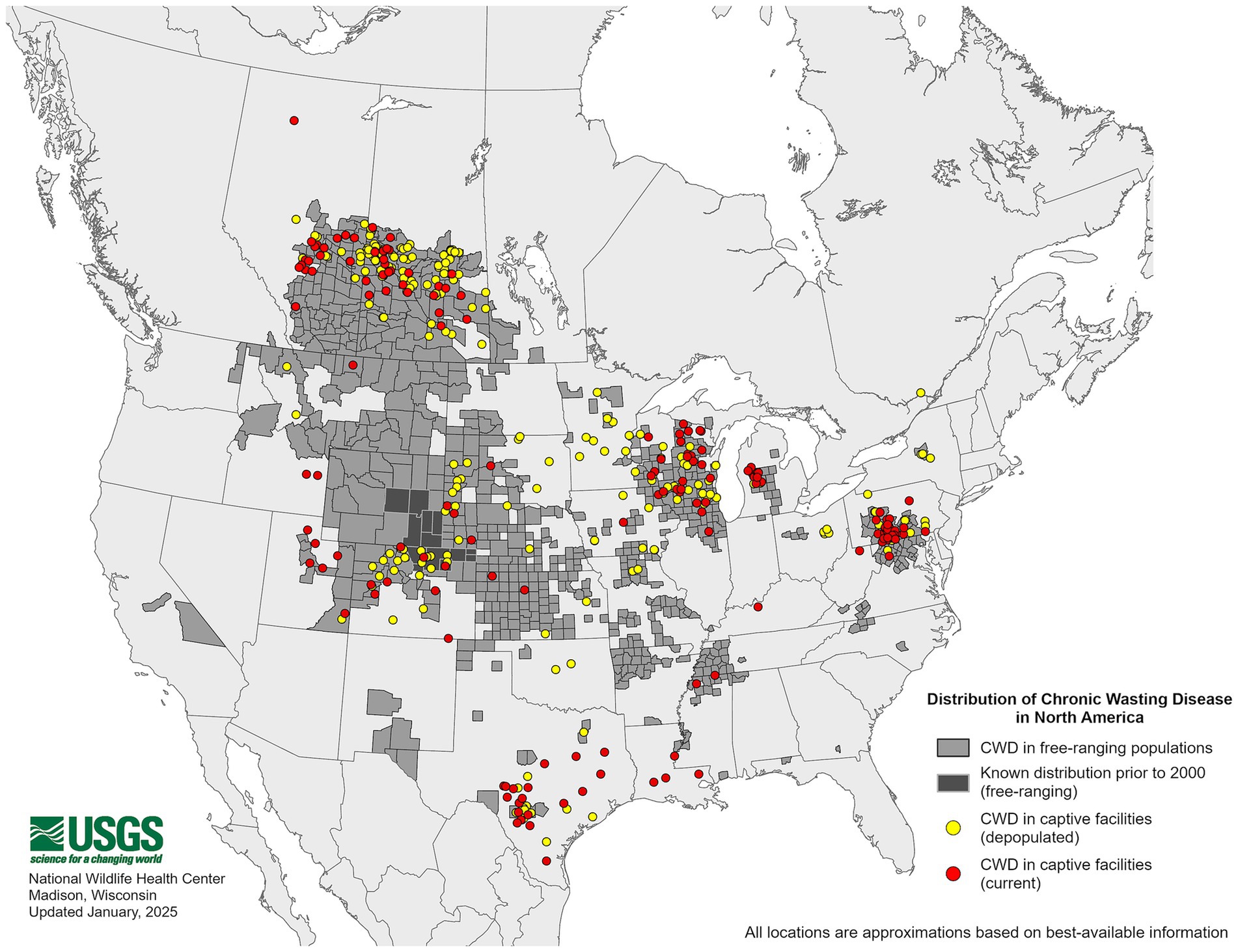
Figure 1. Distribution of chronic wasting disease in the United States. The map above shows free-ranging and captive cervids that tested positive for the chronic wasting disease agent. This map is courtesy of the United States Geological Survey (USGS).
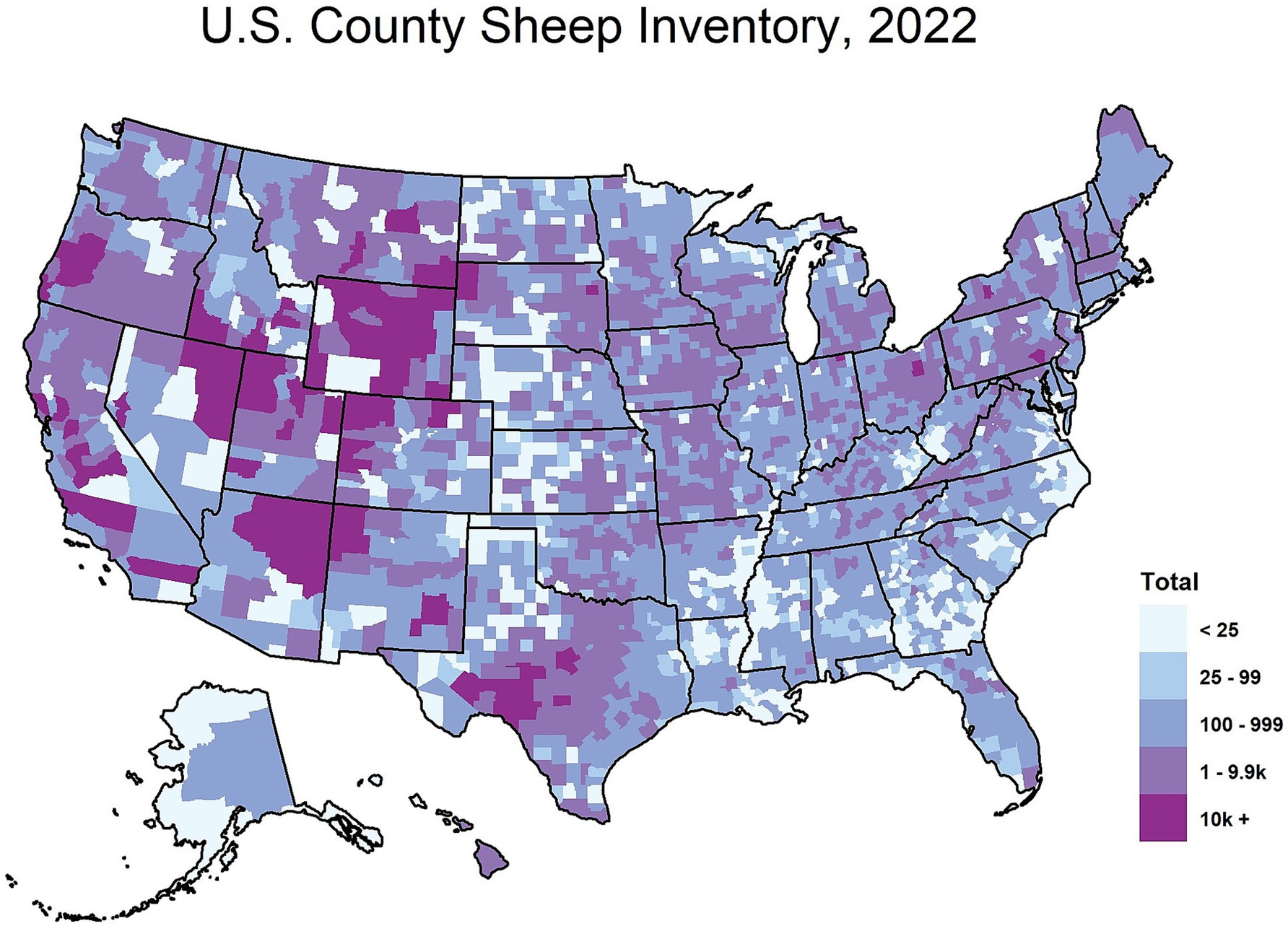
Figure 2. Total sheep inventory by county in the United States, 2022. The map above depicts the 2022 sheep inventory in the United States by county. This map is courtesy of the National Agricultural Statistics Service based on the 2022 United States Agricultural Census data.
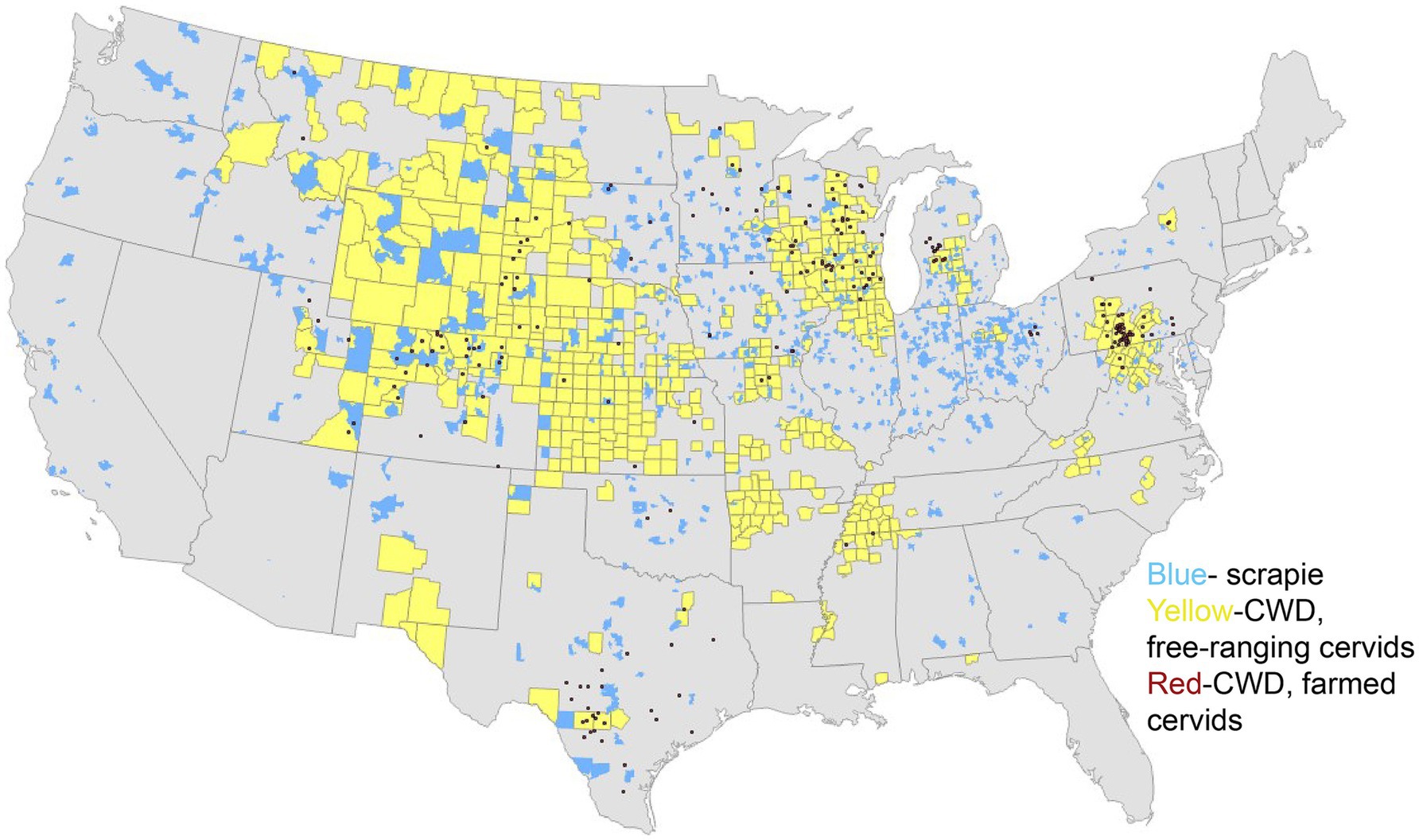
Figure 3. Distribution of scrapie and chronic wasting disease. The map above depicts scrapie events by zip code between 1988 and 2016 (blue) and positive cases of the chronic wasting disease agent in 2024, in free-ranging cervids (yellow) and captive cervids (red). This map is courtesy of Bryan Richards at the United States Geological Survey (USGS).
Previous work has shown that sheep are susceptible to both the WTD CWD agent (13) and the MD CWD agent (14, 15). This study demonstrates that sheep are oronasally susceptible to WTD CWD with a 14% attack rate, with PrPSc accumulating only in lymphoid tissues. Sheep are susceptible to the MD CWD agent intracranially with a 25% attack rate upon first passage and a 100% attack rate upon second passage. In the first passage of MD sheep CWD, PrPSc accumulation was present in both the CNS and lymphoid tissues of clinically affected sheep. Similarly, the second passage of MD sheep CWD also displayed PrPSc in the CNS and lymphoid tissues.
Oronasal inoculation most closely mimics natural routes of prion transmission; however, this study evaluates transmission following intracranial inoculation in sheep. Intracranial inoculation is commonly used in laboratory settings because it ensures efficient delivery of the agent to the central nervous system and bypasses barriers that may limit peripheral infection.
Building on previous findings, the current study focuses on CWD transmission involving WTD and MD, the two most widespread cervid species in the United States. These species are of significant focus due to their high population density in the United States and their potential role in the continual transmission of the CWD agent.
Materials and methods
Ethics statement
This study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC) and the Guide for the Care and Use of Agriculture Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). The protocol was approved by the Institutional Animal Care and Use Committee at the National Animal Disease Center (Protocol Number: 3985).
Animal and inoculum procedures
A total of 15 four-month-old male lambs (Cheviot and Suffolk) of different PRNP genotypes (at codons 136, 154, 171) were obtained from a scrapie-free flock at the National Animal Disease Center (NADC) to carry out this study. The sheep were divided into three groups corresponding to different genotypes. Inoculations included six lambs with VRQ/ARQ genotypes, four with ARQ/ARQ genotypes, and five with ARQ/ARR genotypes. All sheep were inoculated with pooled CWD that was prepared from two WTD brains (genotypes 96GG) (16) combined with phosphate buffer saline (PBS) and gentamycin (100 μg/mL) to make a 10% w/v homogenate. To perform intracranial inoculations, the sheep were anesthetized with xylazine (up to 0.2 mg/kg) and ketamine (2 mg/kg) intravenously before 1 mL of inoculum was injected into their brain as previously described (14). All sheep were monitored daily for clinical signs of disease and euthanized with an intravenous overdose of pentobarbital sodium (85 mg/kg) once the disease presented or at the end of the study [55 months post-inoculation (MPI)].
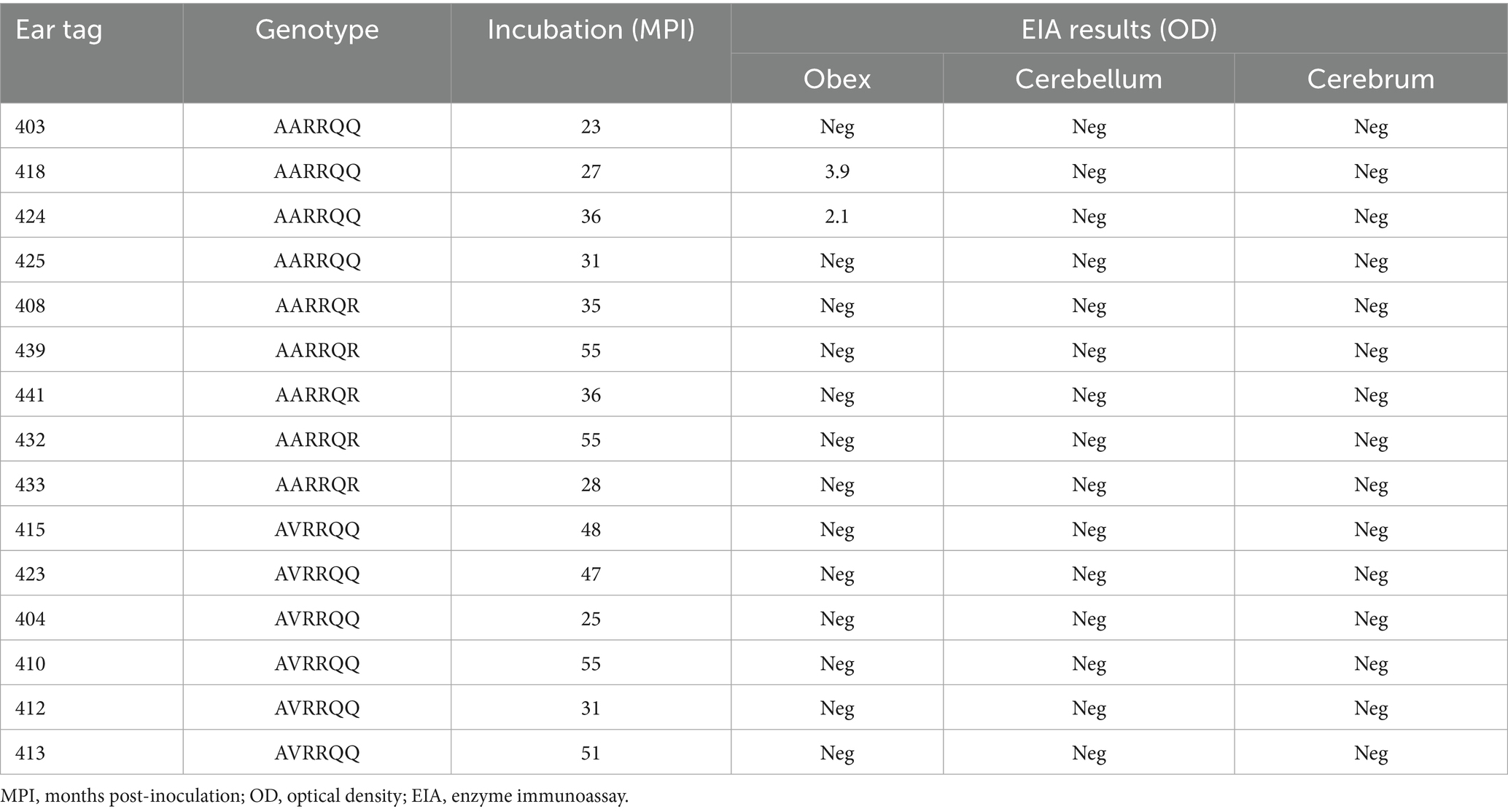
Brain material from a CWD-positive sheep (418, Table 1) (ARQ/ARQ) intracranially inoculated with the CWD agent from WTD was used to intracranially inoculate mice expressing the cervid PRNP at approximately 2-fold (Tg12; 20 μL of 10% homogenate) or ovine VRQ PRNP at approximately 8-fold (Tg338; 20 μL of 1% homogenate) (17). Intracranial inoculations of mice were performed as previously described (18). Brain material from sheep positive for PrPSc after inoculation with the MD CWD agent was also inoculated into mice expressing the cervid PRNP (Tg12; 20 μL of 10% homogenate) and ovine VRQ/VRQ (Tg338; 20 μL of 1% homogenate) PRNP. All mice were monitored daily and euthanized when neurological clinical signs (ataxia, tremors, moribund, loss of body condition, or head tilt) were displayed or at the end of the study (approximately 700 days post-inoculation). All animals were confirmed positive or non-detectable by enzyme immunoassay (EIA) (IDEXX HerdChek BSE-Scrapie Antigen EIA test kit, Westbrook, ME), upon euthanasia. Sheep samples ran included the brainstem at the level of the obex, cerebrum, cerebellum, and retropharyngeal tonsils, while all mice brains were tested. All tissues collected were made as a 20% w/v homogenate.
Western blot
Samples included in the western blots were all brainstem tissue at the level of the obex (sheep) and whole brain homogenates (transgenic mice). Tissues were homogenized to 20% (w/v) in phosphate buffer saline (PBS). First, a bicinchoninic acid assay (BCA) was performed on all samples to determine 50 μg of total protein. Tissue samples of 50–150 μL of were combined with 2.5 μL 20% sarkosyl/PBS, 2.5 μL proteinase K (PK) (1 mg/mL), and PBS to equal 25 μL. The samples were then incubated for 1 h at 37°C. Pefabloc was added to all samples to stop proteinase K (PK) digestion at room temperature for 20 min. Then, samples were combined with 2-ME and loading buffer and heated at 100°C for 5 min before gel electrophoresis.
Once the gel was transferred overnight, the membrane was blocked for 30 min with 3% BSA in 0.05% TBST. Signal detection was achieved using anti-PrP monoclonal antibodies, namely C-terminal binding SHA31, recognizing amino acids 145–152 on the human PRNP (Bertin Technologies, Montigny-le-bretonneux, France), and N-terminal binding 12B2, recognizing amino acids on the bovine PRNP at codons 101–105 (6) (Wageningen University and Research, The Netherlands), at a dilution of 1:10,000 as primary antibodies. The primary antibodies were incubated at room temperature for 1 h. The secondary antibody used was a biotinylated sheep anti-mouse IgG (1:400 dilution; GE Healthcare UK Limited, Amersham™, Buckinghamshire, United Kingdom) followed by streptavidin-HRP (1:10,000 dilution; GE Healthcare UK Limited, Amersham™, Buckinghamshire, United Kingdom). Both antibodies were incubated at room temperature for 1 h. ECL plus detection (1:10 dilution; Thermo Fisher Scientific, Carlsbad, CA) was used in conjunction with the iBright visualization system (Thermo Fisher, Invitrogen, Waltham, MA) for imaging.
Enzyme immunoassay
Frozen tissues tested by EIA (IDEXX HerdChek BSE-Scrapie Antigen EIA test kit, Westbrook, ME) included the cerebrum, cerebellum, and brainstem at the level of the obex, palatine tonsil, and retropharyngeal lymph node. Tissues were homogenized to a 20% (w/v) concentration with PBS and then loaded onto a 96-well plate. The samples were run according to kit instructions, and a BioTek 800TS microplate reader was used to measure the optical density (OD) of each sample, differentiating positive and non-detectable samples. The negative cutoff value for mice samples was 0.18 plus the optical density of the negative control.
Conformational stability assay
Brain homogenates (30 μg/μL) were denatured with three times the amount of guanidine hydrochloride (GdnHCl; 0–5.5 M) (Millipore-Sigma, G7294, Burlington, MA) for 1 h at room temperature. Utilizing a Bio-Dot Microfiltration apparatus (Bio-Rad, Hercules, CA), samples were filtered through Amersham Protran nitrocellulose membranes (Cytiva, Marlborough, MA) before washing twice with PBS. The membranes were air-dried for 1 h, then incubated at 37°C for 1 h with 5 μg/mL proteinase K (PK) in cell lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, and 0.5% Igepal CA-630). To inactivate the PK, 2 mM phenylmethylsulfonyl fluoride was put on the membranes, and 3 M guanidine thiocyanate in Tris-HCl (pH 7.8) was added for 10 min at room temperature. The membranes were washed with PBS four times, blocked with 5% non-fat milk in TBST for 1 hour, and probed with the primary antibody of SHA31 at a dilution of 1:5,000 (Bertin Technologies, Cat. A03213, France) overnight at 4°C. Primary antibody was followed by an HRP-conjugated goat anti-mouse IgG secondary antibody (1:5,000 dilution; Southern Biotech, Birmingham, AL). ECL Plus was used to develop membranes (1:10 dilution; Thermo Fisher, Scientific, Carlsbad, CA), and the iBright (Thermo Fisher, Invitrogen, Waltham, MA) was used for imaging. The AzureSpot Pro analysis software (Azure Biosystems) was used to analyze the signals of three biological replicates. The undenatured PrPSc (Fapp) was then plotted on the denaturation curve as a function of GdnHCl concentration using a non-linear least-squares four-parameter sigmoidal dose–response regression. The GraphPad Prism software (San Diego, CA) was used to calculate the half-maximal denaturation concentration, [GdnHCl]1/2, followed by a Student’s t-test to assess the statistical significance of [GdnHCl]1/2.
Results
Sheep results
For sheep intracranially inoculated with the WTD CWD agent, the mean incubation period was 39 months post-inoculation (MPI) for all sheep before death or euthanasia (Table 1). EIA was used to detect the amount of misfolded protein in CNS and lymphoid tissues after necropsy. No sheep showed unequivocal clinical signs of neurological disease; however, two sheep (418 and 424) tested positive for PrPSc by EIA on the brainstem at the level of the obex tissues (Table 1). All sheep displayed no detection of PrPSc in lymphoid tissues by EIA. All sheep cerebellum and cerebrum tissues were non-detectable when tested (Table 1).
Sheep western blot
In order to examine the molecular profile of brain tissue from WTD sheep CWD, a western blot was used to compare the brainstem at the level of the obex with brain samples from WTD inoculated with the CWD agent, and sheep inoculated with the scrapie agent. The results showed that the WTD sheep CWD agent (sheep 418) had a molecular profile similar to sheep scrapie, which was different from WTD with the CWD agent (Figure 4). The WTD CWD agent migrated a smaller distance, as the unglycosylated band is heavier at 20 kDa, compared to the WTD sheep CWD and sheep scrapie agent, which traveled a longer distance.
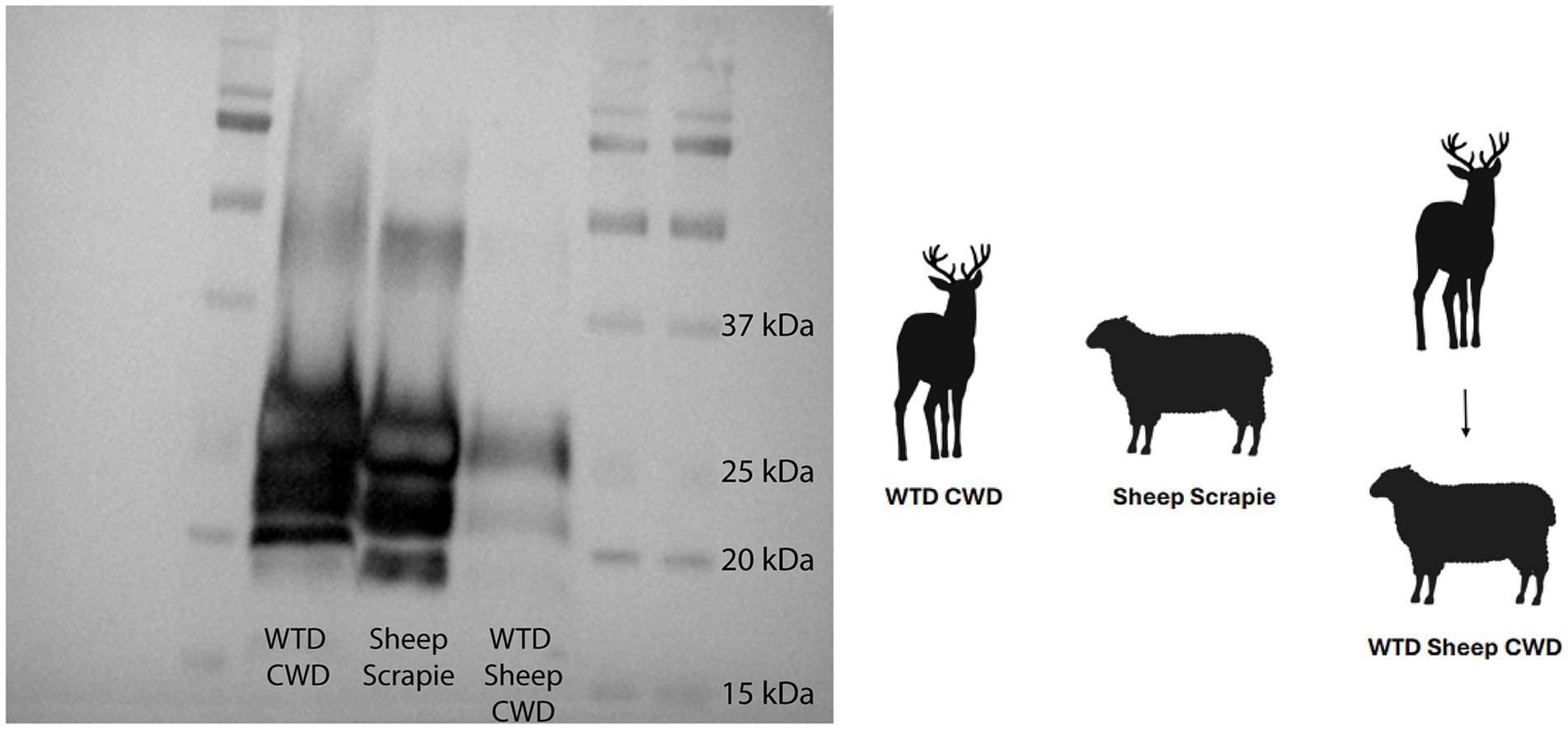
Figure 4. White-tailed deer sheep chronic wasting disease western blot. Western blot done on white-tailed deer with the chronic wasting disease agent, sheep with scrapie, and sheep with the white-tailed deer chronic wasting disease agent. All tissues on the blot are the brainstem at the level of the obex. Monoclonal antibody SHA31 was used to probe for PrPSc.
Cervidized mice results
Passage of the WTD sheep CWD agent into cervidized mice resulted in an attack rate of 83% with a mean incubation period of 377 days (Figure 5), while passage into ovinized mice resulted in 0% attack rate. These results were compared with the passage of the MD sheep CWD agent into cervidized and ovinized mice. There was positive detection in ovinized mice with a 100% attack rate and an incubation period of 282 days, whereas cervidized mice showed an 86% attack rate with an incubation period of 646 days (Figure 5).
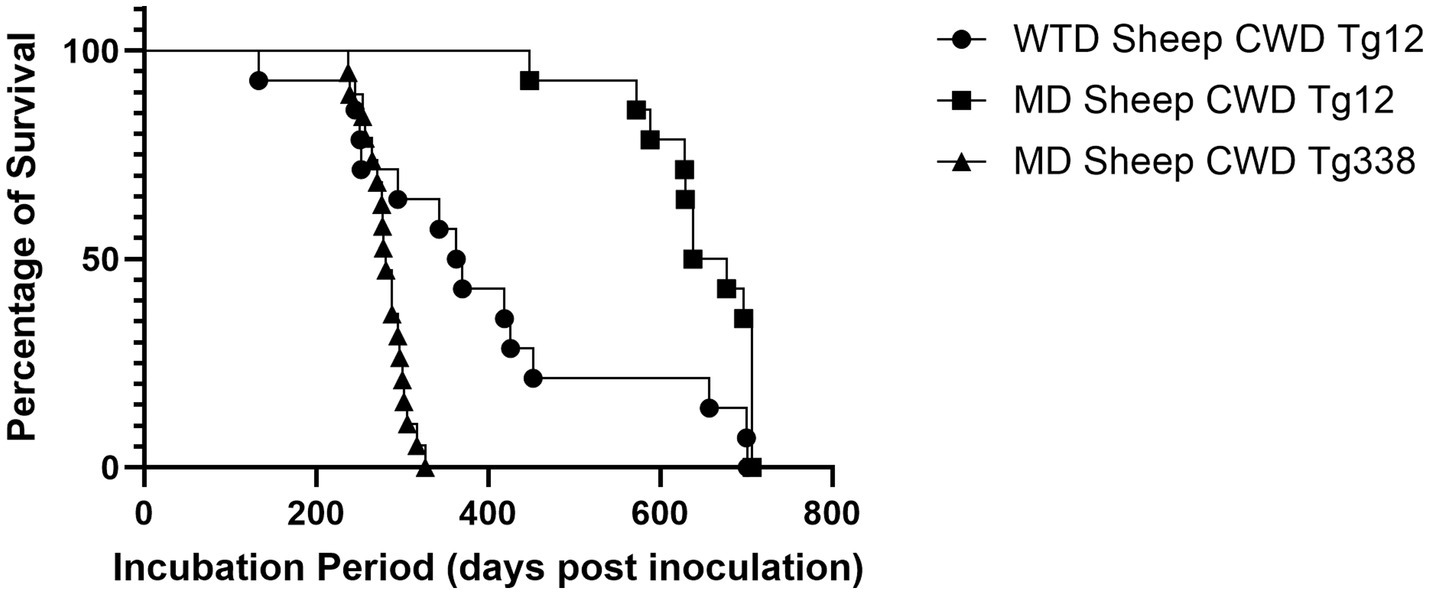
Figure 5. Incubation periods in transgenic mice. The graph above depicts incubation periods for cervidized (Tg12) and ovinized (Tg338) mice. Passage of the WTD sheep CWD agent into Tg12 mice (circle) resulted in a mean incubation period of 377 days. Passage of the MD sheep CWD agent resulted in a mean incubation period of 646 days in Tg12 mice (square) and 282 days in Tg338 mice (triangle).
Cervidized mice western blots
Cervidized mice were used to compare the WTD sheep CWD agent to the CWD agent from other cervids. Five groups of cervidized mice were inoculated with different prion agents. Group one was the WTD sheep CWD agent, group two was the MD sheep CWD agent, group three was the MD WTD CWD agent, group four was the MD CWD agent, and group five was the WTD CWD agent. The results showed that the MD sheep CWD agent (Figure 6, lane 2) displayed a different molecular profile with antibody SHA31 compared to the other groups. The MD sheep CWD agent appeared to have four bands, the unglycosylated band being lower than the other group’s unglycosylated band with antibody SHA31. To determine if there was a difference in PK cleavage point, N-terminal antibody 12B2 was used, and all five groups appeared to have similar molecular profiles (Figure 6).
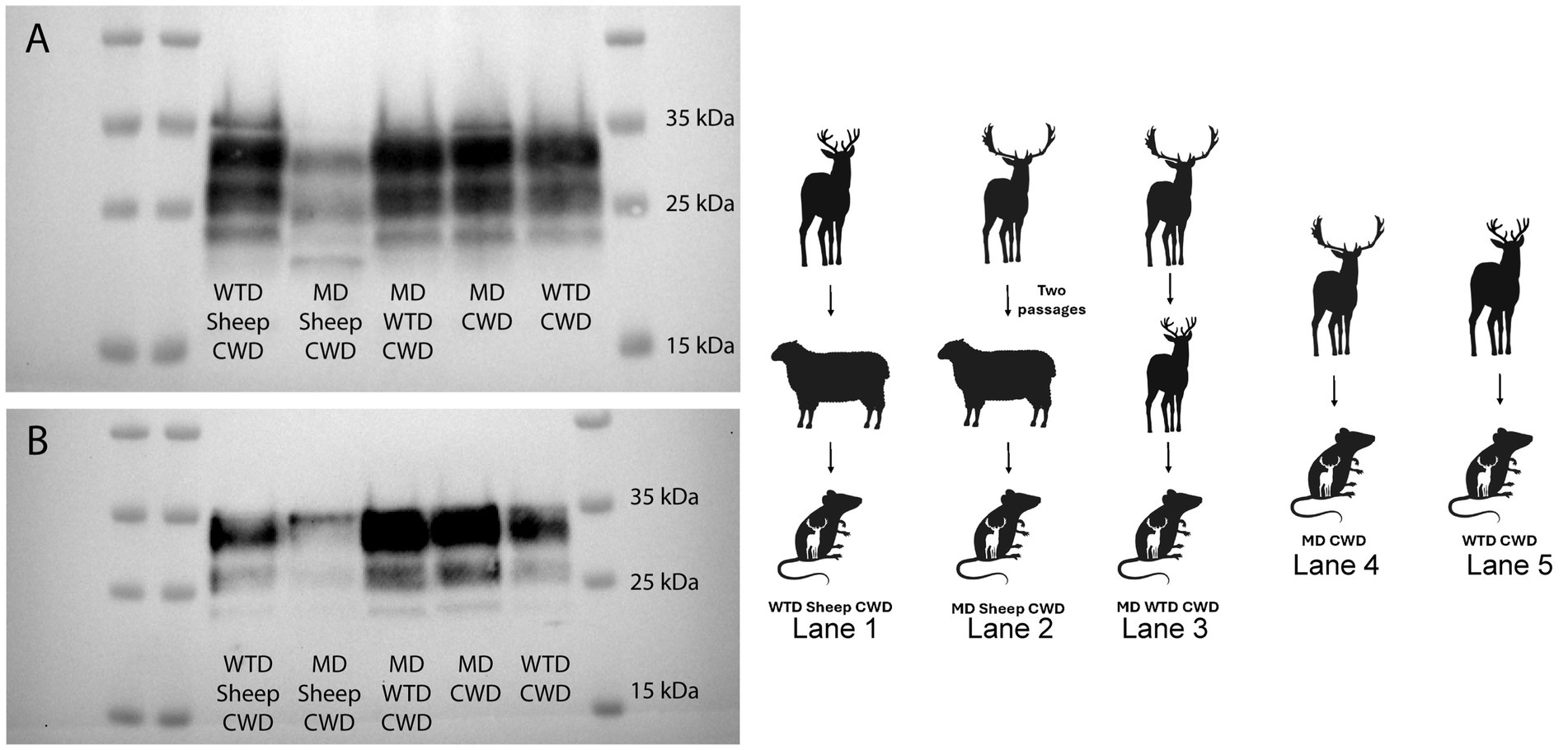
Figure 6. Cervidized mice western blots. Western blots were performed with monoclonal antibodies SHA31 (A) and 12B2 (B), utilizing brain tissues of cervidized mice (Tg12) inoculated with the chronic wasting disease agent from various sources. The western blot performed with SHA31 (A) shows that the mule deer sheep chronic wasting disease agent has a different molecular profile with four bands compared to other lanes with only three bands. Samples probed with antibody 12B2 are difficult to differentiate in the western blot performed.
Ovinized mice western blot
In order to determine the transmission potential of the WTD sheep CWD agent and the MD sheep CWD agent to sheep, ovinized mice were inoculated. The WTD sheep CWD agent did not transmit to ovinized mice; however, the MD sheep CWD agent did transmit to ovinized mice. A western blot was performed on the MD sheep CWD agent to compare the molecular profile to the scrapie agent in ovinized mice. The results showed that the MD sheep CWD agent had a molecular profile similar to two strains of classical scrapie, including No. 13-7 scrapie and x124 scrapie (Figure 7).
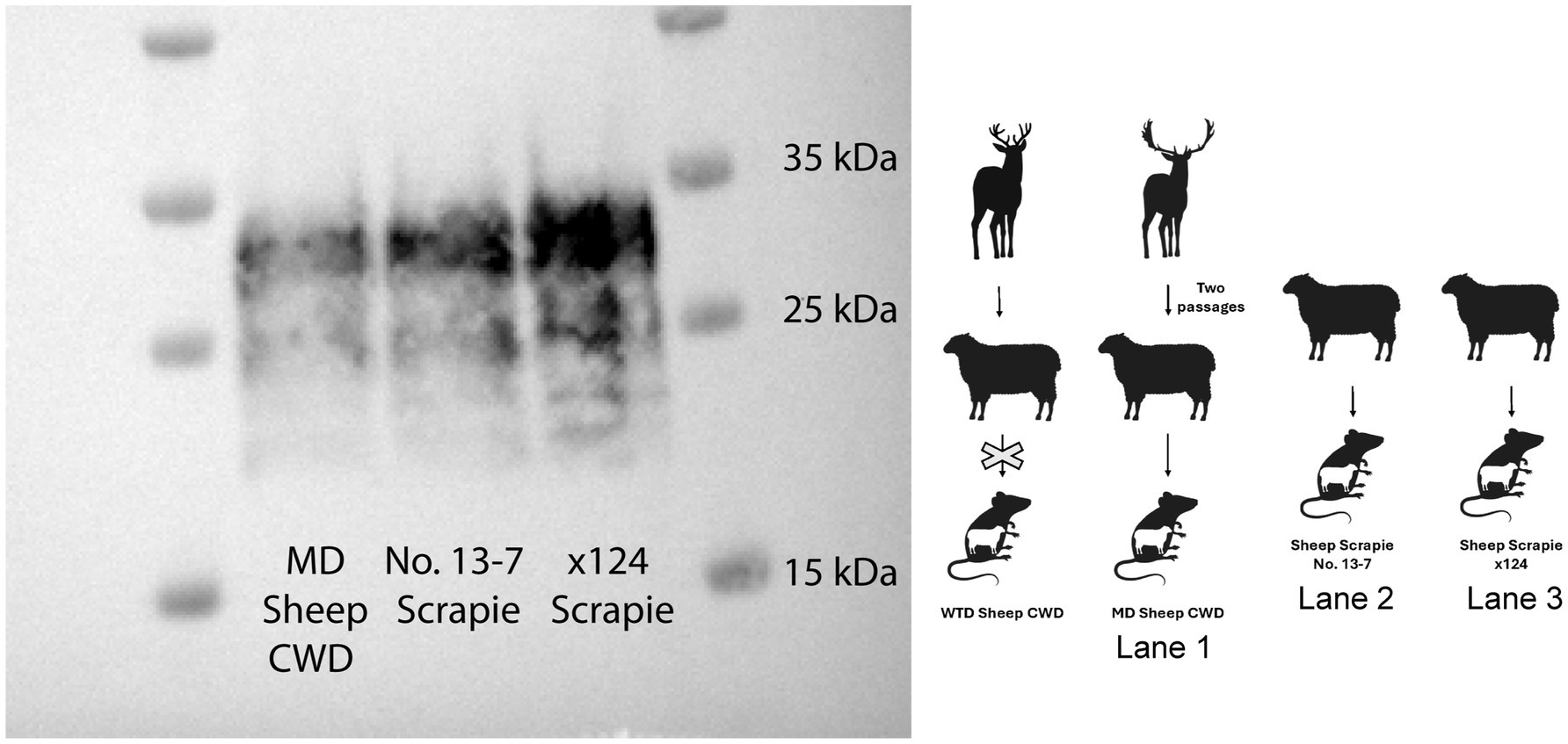
Figure 7. Ovinized mice western blot. The animal graphic aside the western blot displays the layout with the passage of white-tailed deer sheep chronic wasting disease agent into ovinized mice (western blot not performed as no infection occurred), the mule deer sheep chronic wasting disease agent into ovinized mice (lane 1), and two strains of classical scrapie, including No. 13-7 scrapie (lane 2) and x124 scrapie agent, into ovinized mice (lane 3). All samples on this western blot are ovinized mice (Tg338) probed with monoclonal antibody SHA31.
Conformational stability assay
To investigate the possibility of differences in strain stability between the CWD agents from various cervids transmitted to sheep, conformational stability was evaluated on all cervidized mouse brains. Half-maximal denaturation of PrPSc with GdnHCl occurred in group one at 1.74 M, group two at 1.91 M, group three at 1.85 M, group four at 1.93 M, and group five at 1.87 M. Results showed no significant stability differences between groups, regardless of inoculum source (Figure 8).
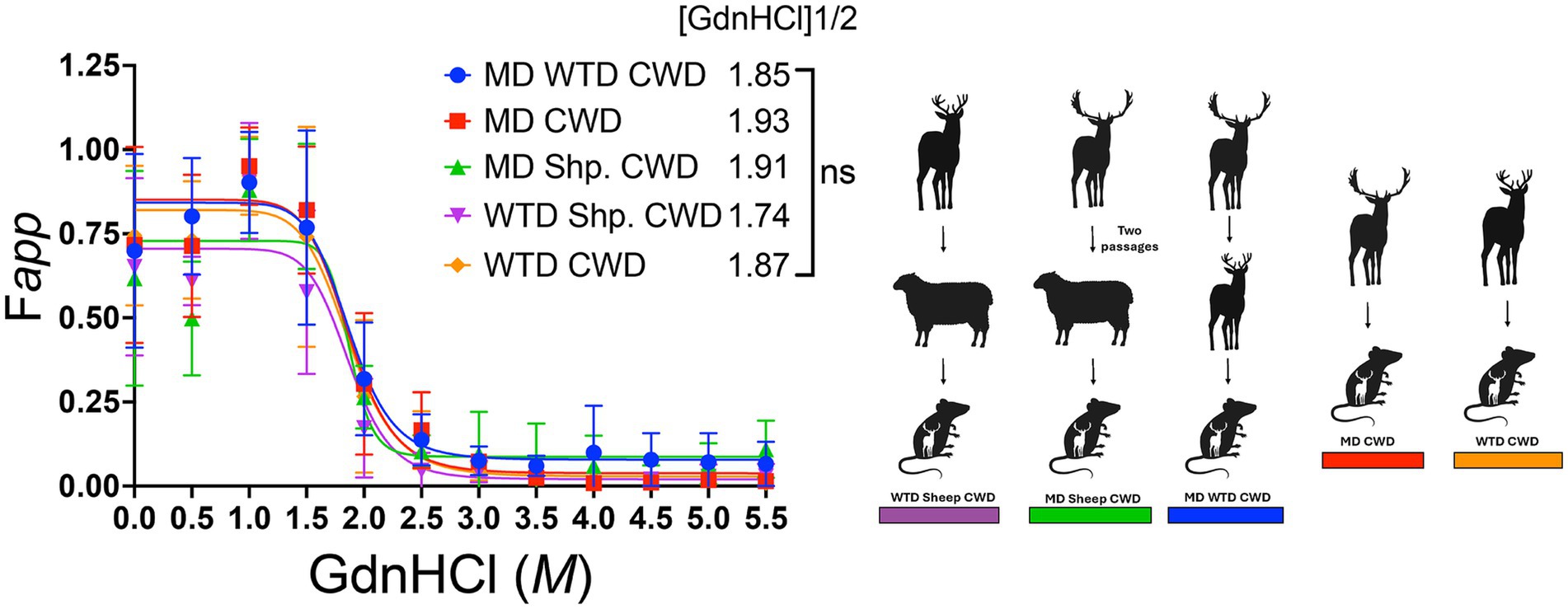
Figure 8. Conformational stability assay in cervidized mice. The conformational stability graph displays all brain samples from cervidized mice inoculated with the chronic wasting disease agent from various sources. The denaturation curve follows progressive amounts of guanidine hydrochloride (GdnHCl) on the x-axis while measuring the apparent fractional change of PrPSc at half-maximal amount of GdnHCl1/2 on the y-axis.
Discussion
Donor source of the CWD agent affects transmission to sheep
The results demonstrated that sheep are susceptible to the CWD agent from WTD when intracranially inoculated, but only the CNS tissues show positive PrPSc accumulation, which is different from sheep intracranially inoculated with the agent of CWD from MD where there is PrPSc accumulation in both the brain and lymphoid tissues (15). Additionally, we have shown that this same WTD sheep CWD agent can be transmitted to cervidized mice, but not ovinized mice. In contrast, the MD sheep CWD agent positively transmits to cervidized and ovinized mice. When a western blot was performed on all of the cervidized mice with various CWD sources, the MD sheep CWD agent displayed a profile different from the other groups. When the monoclonal antibody SHA31 was utilized, the MD sheep CWD agent displayed four bands, different from the other cervidized mice, which displayed only three bands on the blot.
The differing transmission patterns of these two sheep CWD agents (WTD and MD) align with previous research (13–15), which indicates that sheep are more susceptible to the MD CWD agent than to the WTD CWD agent. One possibility for these agents to show different transmission results could be a difference in host PRNP genotypes. The MD CWD agent caused disease in sheep expressing the AVRQ genotype (15), while the WTD CWD agent affected sheep with the ARQ genotype (Table 1). These results suggest that the host genotype could play a critical role in the successful transmission of the CWD agent.
Comparison to other research in sheep CWD transmission
Transgenic mice expressing the cervid and ovine prion protein genes are robust models for the transmission of both the CWD and scrapie agents. Previous studies have successfully demonstrated positive transmission of the CWD agent to cervidized mice and the sheep scrapie agent to ovinized mice (19). Notably, research comparing the transmission of the elk sheep CWD agent showed efficient transmission to both cervidized and ovinized mice (19), which highlights the potential for interspecies transmission of the CWD agent.
Risk of CWD infection in sheep population varies depending on the original source of CWD
Currently, no known cases exist of sheep being naturally infected with the CWD agent. Interspecies transmission remains a slight possibility due to the geographic overlap between the CWD agent and sheep populations. These experimental studies suggest that the WTD CWD agent can be transmitted to sheep intracranially and then subsequently to cervidized mice. While intracranial inoculation is a valuable method for assessing disease progression and host susceptibility in experimental settings, results may not fully reflect natural transmission dynamics. Notably, the study reveals differences in transmission to ovinized mice when inoculated with the WTD sheep CWD agent compared to the MD sheep CWD agent. Overall, the data suggest that the WTD CWD agent is unlikely to pose a significant risk to sheep, but it could potentially be transmissible back to the cervid population. In contrast, the MD sheep CWD agent could pose a greater risk to both the cervid and sheep populations. This study is significant as it will benefit the cervid and sheep industry, as well as researchers in the field. Future work will focus on further characterizing the sheep CWD agent from WTD and MD when passed back into both large animal and mouse models.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by National Animal Disease Center Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AF: Investigation, Conceptualization, Writing – review & editing, Writing – original draft, Formal analysis, Visualization, Methodology. EC: Writing – review & editing, Investigation. JB: Formal analysis, Supervision, Investigation, Methodology, Writing – review & editing. LM: Writing – review & editing, Investigation. SS: Investigation, Writing – review & editing. MG: Writing – review & editing, Writing – original draft, Supervision. JG: Project administration, Writing – original draft, Methodology, Supervision, Funding acquisition, Writing – review & editing, Resources, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded in its entirety by congressionally appropriated funds to the United States Department of Agriculture, Agricultural Research Service.
Acknowledgments
We thank Kevin Hassall, Ami Frank, Jessica Slight, and Samantha Lei for their technical contributions to this study. Our heartfelt thanks to Dr. Qingzhong Kong for the cervidized mice and Dr. Hubert Laude for the ovinized mice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Caughey, B, and Raymond, GJ. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. (1991) 266:18217–23. doi: 10.1016/S0021-9258(18)55257-1
2. Schneider, K, Fangerau, H, Michaelsen, B, and Raab, WH. The early history of the transmissible spongiform encephalopathies exemplified by scrapie. Brain Res Bull. (2008) 77:343–55. doi: 10.1016/j.brainresbull.2008.09.012
3. Detwiler, LA, and Baylis, M. The epidemiology of scrapie. Rev Sci Tech. (2003) 22:121–43. doi: 10.20506/rst.22.1.1386
4. Otero, A, Duque Velasquez, C, McKenzie, D, and Aiken, J. Emergence of CWD strains. Cell Tissue Res. (2023) 392:135–48. doi: 10.1007/s00441-022-03688-9
5. Frese, AJ, Greenlee, MHW, Bian, J, and Greenlee, JJ. Transmission of classical scrapie using lymph node inoculum. Res Vet Sci. (2024) 176:105348. doi: 10.1016/j.rvsc.2024.105348
6. Greenlee, JJ, Moore, SJ, Cassmann, ED, Lambert, ZJ, Kokemuller, RD, Smith, JD, et al. Characterization of classical sheep scrapie in white-tailed deer after experimental oronasal exposure. J Infect Dis. (2023) 227:1386–95. doi: 10.1093/infdis/jiac443
7. LaFauci, G, Carp, RI, Meeker, HC, Ye, X, Kim, JI, Natelli, M, et al. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elaphus nelsoni) PrPC. J Gen Virol. (2006) 87:3773–80. doi: 10.1099/vir.0.82137-0
8. Meyerett-Reid, C, Wyckoff, AC, Spraker, T, Pulford, B, Bender, H, and Zabel, MD. Generation of a unique cervid prion strain using protein misfolding cyclic amplification. mSphere. (2017) 2:e00372-16. doi: 10.1128/mSphere.00372-16
9. Haley, NJ, and Hoover, EA. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu Rev Anim Biosci. (2015) 3:305–25. doi: 10.1146/annurev-animal-022114-111001
10. Sigurdson, CJ, Williams, ES, Miller, MW, Spraker, TR, O’Rourke, KI, and Hoover, EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol. (1999) 80:2757–64. doi: 10.1099/0022-1317-80-10-2757
12. Richards, BJ. Chronic wasting disease distribution in the United States by state and county (April 2025) [Data set]. U.S. Geological Survey. (2025).
13. Cassmann, ED, Moore, SJ, and Greenlee, JJ. Experimental oronasal transmission of chronic wasting disease agent from white-tailed deer to Suffolk sheep. Emerg Infect Dis. (2021) 27:3156–8. doi: 10.3201/eid2712.204978
14. Cassmann, ED, Frese, RD, and Greenlee, JJ. Second passage of chronic wasting disease of mule deer to sheep by intracranial inoculation compared to classical scrapie. J Vet Diagn Invest. (2021) 33:711–20. doi: 10.1177/10406387211017615
15. Hamir, AN, Kunkle, RA, Cutlip, RC, Miller, JM, Williams, ES, and Richt, JA. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J Vet Diagn Invest. (2006) 18:558–65. doi: 10.1177/104063870601800606
16. Mammadova, N, Cassmann, E, and Greenlee, JJ. Successful transmission of the chronic wasting disease (CWD) agent to white-tailed deer by intravenous blood transfusion. Res Vet Sci. (2020) 133:304–306.
17. Marín-Moreno, A, Espinosa, JC, and Torres, JM. Transgenic mouse models for the study of prion diseases. Prog Mol Biol Transl Sci. (2020) 175:147–77. doi: 10.1016/bs.pmbts.2020.08.007
18. Smith, JD, Nicholson, EM, and Greenlee, JJ. Evaluation of a combinatorial approach to prion inactivation using an oxidizing agent, SDS, and proteinase K. BMC Vet Res. (2013) 9:151. doi: 10.1186/1746-6148-9-151
19. Madsen-Bouterse, SA, Schneider, DA, Zhuang, D, Dassanayake, RP, Balachandran, A, Mitchell, GB, et al. Primary transmission of chronic wasting disease versus scrapie prions from small ruminants to transgenic mice expressing ovine or cervid prion protein. J Gen Virol. (2016) 97:2451–60. doi: 10.1099/jgv.0.000539
Keywords: prions, chronic wasting disease, sheep, white-tailed deer, mule deer
Citation: Frese AJ, Cassmann ED, Bian J, Mandell LZ, Smadi S, Greenlee MHW and Greenlee JJ (2025) Differences between the white-tailed and mule deer chronic wasting disease agents after passage through sheep. Front. Vet. Sci. 12:1632936. doi: 10.3389/fvets.2025.1632936
Edited by:
Kohtaro Miyazawa, National Agriculture and Food Research Organization, JapanReviewed by:
Andrea Grindeland, McLaughlin Research Institute, United StatesJameson Mori, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Frese, Cassmann, Bian, Mandell, Smadi, Greenlee and Greenlee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin J. Greenlee, ampncmVlbmxlZTE5NzJAZ21haWwuY29t
 Alexis J. Frese
Alexis J. Frese Eric D. Cassmann
Eric D. Cassmann Jifeng Bian
Jifeng Bian Leisa Z. Mandell1
Leisa Z. Mandell1 M. Heather West Greenlee
M. Heather West Greenlee Justin J. Greenlee
Justin J. Greenlee