1
Cognitive Neuroscience Sector, SISSA, Trieste, Italy
2
Institute of Cognitive Neuroscience, University College, London, UK
3
Rotman Research Institute, Baycrest, Toronto, ON, Canada
4
Departments of Psychology and Medicine, University of Toronto, Toronto, ON, Canada
5
Behavioral Neurology Unit, KS 253, Beth Israel Deaconess Medical Center, Boston, MA, USA
6
Harvard Medical School, Boston, MA, USA
This paper considers evidence provided by large neuropsychological group studies and meta-analyses of functional imaging experiments on the location in frontal cortex of the subprocesses involved in the carrying out of task-switching paradigms. The function of the individual subprocesses is also considered in the light of analyses of the performance of normal subjects.
Task switching is the area of research concerned with how a subject effects a change in the rules that govern how he or she responds to incoming stimuli. As an area of research, it is situated at the confluence of two intellectual traditions. Within human experimental psychology the study of cognitive control processes in normal subjects had seen important theoretical and experimental advances with the work of Atkinson and Shiffrin (1968) and Schneider and Shiffrin (1977)
. In complementary fashion, clinical neuropsychological investigations of lateral prefrontal functions had seen the development of tests for assessing executive functions including the Wisconsin Card-Sorting (Milner, 1963
) and the Extra-Dimensional Intra-Dimensional Shift test of the CANTAB battery (Owen et al., 1990
).
As an example of a relevant clinical test, in Wisconsin Card-Sorting, the patient is presented with four cards on each of which there is a set of coloured shapes, all the shapes on any one card being identical. These four ‘key’ cards differ in the form of their shapes, in their colour and in how many there are. The clinician has another set of cards each of which matches one of the key cards in colour, another in shape and so on. The patient is given one card at a time and has to place it with the key card which has the same value on one of the dimensions. The correct match is dictated by a rule in operation, which can be to match by colour or by shape or by number. Every so often, the rule changes and the patient must discover the new rule by trial and error. The cognitive component that is held to be impaired by lateral frontal lesions in such a task is set shifting. The prototypical lateral prefrontal patient continues to use the previously relevant rule after the rule changes, even though he or she is being told on each trial that the match is incorrect. What appears to be lost is the ability to shift from seeing one attribute of the stimulus as being relevant to seeing another.
Performance on such clinical tests, however, is very complex to analyse as there are many relevant cognitive components; Wisconsin Card-Sorting, for example, is affected in a qualitatively different fashion by lesions in different parts of prefrontal cortex (Stuss et al., 2000
). Moreover, such prefrontal clinical tests are poorly adapted to the isolation by purely behavioural means of critical subcomponents. So exploration of the performance of normal subjects in these paradigms in the fashion typical of human experimental psychology, virtually never occurred. Cognitive psychology and neuropsychology remained separate to their mutual disadvantage.
In the 1990s, developments in so-called ‘task-switching’ experimental paradigms provided an avenue to overcome this separation between the different research traditions. These experimental paradigms are also beginning to allow us to distinguish key components that contribute to performance in normal subjects, a key step in designing clinical tests which isolate different components of prefrontal functioning. The central cognitive component is again set-shifting. Typically on each trial of a task-switching experiment a stimulus is presented which can be conceived of in one of a number of ways, typically two (see Figure 1
). Two studies essentially initiated the modern use of this type of paradigm in normal subjects, those of Allport et al. (1994) and Rogers and Monsell (1995)
. In the second of these, subjects are presented with a letter and a digit. On some trials it is the letter which is relevant and the subject must respond by pressing one of two keys as quickly as possible as to whether the letter is a consonant or a vowel. On other trials the relevant part of the stimulus is the digit and the subject must make the corresponding decision between odd and even. In the Rogers and Monsell type of procedure it is possible for the subject to predict in advance which task is relevant. The letter-digit combination appears in one of four panes (quadrants) of a square on each trial, and the position of the pair moves consistently round the panes in turn; in the two upper positions one task is operative, in the two lower positions the other. Shortly thereafter, Meiran (1996)
introduced the procedure of the cueing of the task by a distinctive warning signal occurring before stimulus presentation; this enabled the relevant task to be varied randomly across trials. His paradigm also used four panes of a square (see Figure 1
). A circle appears in one of the four panes. The response key to be pressed depends on which dimension of the square is currently operative. If it is the vertical dimension, then the subject must make a top-bottom decision. If, instead, it is the horizontal dimension then the subject must make a left–right decision. Which of the two tasks is operative on a particular trial is indicated through a warning signal just before the stimulus. This means that the task that is relevant can be randomised across trials unlike on the Rogers and Monsell design.
Figure 1. The experimental paradigm used in Shallice et al. (2008b)
. It is derived from the paradigm developed by Meiran (1996)
.
This original group of experiments showed that if stimuli require different responses depending on which task is operative, then trials where the task switches from the previous trial are slower than those where the task repeats – the difference between the two reaction times is known as the switch effect. Moreover, the size of the switch effect generally decreases with an increase in the time available in which the subject can prepare for the specific task. This led Rogers and Monsell to propose that there is a top-down process of task reconfiguration which takes appreciable time and which is measurable by the decrease in switch effect. The paradigm appeared to provide a measure of the time to switch the dimension of the stimulus that should control behaviour. However, Allport et al. (1994)
found that the switch effect was less when switching is into a more difficult task than into an easier one, and argued that the switch effect was due instead to the need to inhibit a previously active task-set or action schema, namely the control process that determines which task is undertaken. These two types of explanation are not necessarily in conflict, since they can be represented in different levels of a relatively simple computational model of task-switching of the interactive activation type (Gilbert and Shallice, 2002
). On such a model, though, the switch cost as measured from reaction times, no longer corresponds quantitatively to the time that the internal process of task reconfiguration takes.
The incorporation of this type of experimental paradigm into the behavioural literature led to an explosion of studies. However, it became rapidly realised that the paradigm, apparently so simple, had many complexities and involved many different processes (e.g. Monsell, 2003
). For instance, Allport and Wylie (2000)
discovered that presentation of a stimulus associated with a previously active task-set would tend to reactivate it. At much the same time Meiran et al. (2000)
in somewhat conflicting fashion showed that task-set effects dissipate over time. Despite these complications the basic paradigms, together with the scores of subsequent variants (e.g. Brass and von Cramon, 2002
; Braver et al., 2003
), while complex, remain much simpler than the clinical tests from which they were originally derived. The complexity of the underlying processes, however, means that a return to brain-based studies – functional neuro-imaging and lesion studies – becomes critically necessary if the components are to be isolated. This is based on the assumption that the different cognitive resources involved are differently localised; if so the degree to which a particular cognitive resource is used in a given condition can be measured or the effect of its loss discovered.
Interpretation of the functional imaging studies, though, has not been straightforward. Many regions of cortex can be involved including parietal and even the anterior insula cortex (Wager et al., 2004
). Even restricting attention to prefrontal cortex, some studies (e.g. DiGirolamo et al., 2001
; Luks et al., 2002
) have obtained a large number of activation maxima (>10) for relevant contrasts. In a meta-analysis of task-switching, set-shifting and stimulus-response reversal studies, Derrfuss et al. (2005)
found many regions in frontal cortex that showed relatively consistent effects; these include the inferior frontal junction – a region in the vicinity of the junction of the inferior frontal sulcus and the inferior precentral sulcus – the inferior frontal sulcus itself, the inferior frontal gyrus, the medial superior frontal gyrus, the anterior cingulate and the pre-SMA, activations generally involving both hemispheres. However the consistency was only relative; for instance, of the seven pure task-switching studies, in only two was a maximum noted in the inferior frontal gyrus, which was the largest region and the second most significant in the meta-analysis. In a second meta-analysis by Buchsbaum et al. (2005)
, using many of the same source studies, the set of distinct regions found by Derrfuss et al. (2005)
essentially clustered into two prefrontal regions in each hemisphere. Buchsbaum et al. (2005)
related their findings to studies involving simple go/no-go tasks which require response inhibition, and so suggested that the right frontal regions involved in the switching task might mediate the inhibition of a previous response rule.
Given the potentially large number of prefrontal regions suggested to be relevant from the functional imaging meta-analyses, neuropsychological studies of patients with focal frontal lesions seem potentially important. In particular, they have the advantage that the impairment of a component can produce signature effects which help to narrow down hypotheses on the underlying functional processes. This is particularly the case if neuropsychological studies of task-switching are carried out in conjunction with parallel studies of related paradigms, which potentially involve different combinations of the same set of subsystems.
A number of studies of task switching have been carried out in neurological patients (see for instance, Mayr et al., 2006
; Mecklinger et al., 1999
), but they have mainly involved small numbers of patients and so are not suitable for differential localisation of subcomponents within prefrontal cortex. Our paper, that of Shallice et al. (2008b)
, is only the second study of task-switching with a sizeable number of patients with lesions in different parts of prefrontal cortex, the first being that of Aron et al. (2004)
. These two studies reported findings on 41 and 36 frontal patients respectively. Aron et al. (2004)
, using a version of the Rogers–Monsell paradigm, found that both patients with left frontal lesions and ones with right frontal lesions showed a significantly larger switch cost than controls. The right frontal group also showed a dramatically elevated error rate on switch trials. The Shallice et al. (2008b)
study which instead used the Meiran paradigm also found a left frontal effect, but this time on errors. The major reaction time effect we found was a striking slowing on both switch and repeat trials for patients with superior medial prefrontal lesions. An increase in errors in conditions where two tasks are potentially relevant on any given trial was also found for patients with inferior medial prefrontal lesions.
The results of the two studies, like the functional imaging studies, suggest that many parts of prefrontal cortex can potentially play distinct roles in task-switching. The divergence of results across the two studies highlights the importance of differences in both the specific behavioural paradigms and methodology.
Both studies followed an appropriate two-level method for localising patterns of deficits, a first-pass coarse-grain and then a second-pass fine-grain analysis. However, there was a difference between the two studies in both the coarse-grain and the fine-grain procedure employed. Thus, the coarse-grain analysis used by Aron et al. (2004)
involved comparing the effects of left frontal and right frontal lesions with control performance, but in the Shallice et al. (2008b)
case a contrast was drawn between each of four groups – left lateral, right lateral, superior medial and inferior medial – and the controls. In the fine-grain analysis, Aron et al. (2004)
took patients with lesions to one or other frontal lobe and correlated particular aspects of their performance with the proportion of each gyrus that was damaged. More simply, Shallice et al.
(2008b)
took each Petrides and Pandya (1999)
region of frontal cortex, and compared the performance of patients with damage to that region with that of all the other patients with frontal lesions. Both types of neuropsychological fine-grain analysis procedures make strong assumptions and both are subject to the possible problem of associated deficits from lesions extending into neighbouring regions. Therefore it is argued in our paper that it is critical that the fine-grain effects are consistent with the coarse-grain ones; if not, this increases the possibility that fine-grain effects could result from associated deficits.
At the coarse-grain level Aron et al. (2004)
compared left frontal and right frontal patients; the Shallice et al.(2008b)
study compared left lateral, right lateral and two medial groups, superior and inferior. On all reaction time measures in our study it is only the Superior Medial group that is slowed. Moreover, this slowing interacts with many variables such as switch trials versus repeat trials, incongruent trials (where the two tasks lead to different response outcomes given the same stimulus) versus congruent trials, harder versus easier tasks. This slowing with the Superior Medial lesions is closely analogous to effects found in a variety of other reaction time studies of frontal patients (e.g.. Alexander et al., 2005
, 2007
; Stuss et al., 2002
, 2005
). It fits with the idea that systems in the Superior Medial region, probably the anterior cingulate and/or the pre-SMA (see Rushworth et al., 2002
), are critical for the energisation (cognitive effort) necessary to activate operations not directly triggered in an overlearned fashion by perceptual and motivational inputs. Both functional imaging meta-analyses of task-switching produce activation of the anterior cingulate. Thus the four-group coarse-grain procedure is likely to be preferable to the two-group coarse grain procedure as far as localisation of prefrontal functional systems is concerned.
For the fine-grain analyses Aron et al. (2004)
used the percentage of particular gyri affected (superior, middle, and inferior frontal, orbital and medial) and Shallice et al.(2008b)
used difference between presence and absence of damage to particular frontal areas as defined by Petrides and Pandya (1999)
. Both studies obtained similar results on the coarse grain procedure as far as localisation is concerned in the left lateral frontal region. The fine-grain effects were consistent across the two studies, with the Aron et al. (2004)
study suggesting the middle frontal gyrus is the critical locus and the Shallice et al.(2008b)
one an area within it, 9/46v, but only as a trend, so there is no strong suggestion that either fine-grain procedure is inferior to the other. As far as the functional imaging meta-analyses are concerned, in the Derrfuss et al. (2005)
paper this region does not seem to be activated but in the Buchsbaum et al. (2005)
it is. There is thus a convergence in the pattern of results across all studies as far as the involvement of the left lateral prefrontal cortex is concerned, although there are possible differences in the specific region involved.
Even the apparently similar left lateral effects obtained in the two neuropsychological studies, though, differed in terms of the variable involved. In the Aron et al. (2004)
study, patients with left frontal lesions were slowed down more by a switch than were control subjects (a larger reaction time switch cost). In contrast, in our study the increased switch cost in patients with lateral frontal lesions occurred for the errors rather than reaction time. However, in addition, our paper points to the importance of a variable not considered in many task-switching studies, namely the degree of practice. The left lateral effect on errors concerned this variable; this group showed a significantly greater change in error rate from the first half to the second half of the experiment than the controls or the other patient groups. The left lateral patients had over double the error rate of the controls in the first half, but were virtually at the control rate in the second half. This is a similar effect to that shown by Alexander et al. (2005)
in a study of the acquisition of a serial reaction time paradigm. As in our study the left lateral group were as fast as any other group including the control group from the start. However, they made significantly more errors on the first 100 trials whereas on the next 400 trials their error rate was normal. This pair of studies point to the importance of the left lateral region in task-setting, the processes which allow task performance to move from a novel to a routinised state (see Stuss et al., 1995
). This is somewhat related to the argument presented by Aron et al. (2004)
for their left frontal effects, namely that there is weaker endogenous control of task-set in left frontal patients and to the argument of Derrfuss et al. (2005)
that the left inferior frontal junction is involved in the updating of task representations. Our position is that such updating is operating throughout the whole process of learning the task and not just on a single trial.
In addition to the difference concerning the Superior Medial findings, other findings also differed between the two studies. The Aron et al. (2004)
study found effects of right inferior frontal gyrus lesions and the Shallice et al.(2008b)
one of orbital frontal lesions, neither of which was found in the other study. This points to the importance of group composition, and to the specific behavioural characteristics of the task in addition to the analysis procedures. Thus as far as group composition is concerned, the absence of a Superior Medial effect in the Aron et al. (2004)
study may well be due to the fact that their patients had few sizeable lesions involving the medial region, especially in the critical left frontal group.
An effect which was found in our study but not in the Aron et al. (2004)
study was a highly significant increase in errors compared with controls in the Inferior Medial group. This effect was found both for repeat as well as switch trials and it was found for both preparation intervals, for the 1500 ms gap between cue and stimulus as well as for the 200 ms gap. The Inferior Medial region includes the orbital prefrontal cortex. Patients with orbital prefrontal lesions make much higher error rates on memory tasks where the chance of error is increased by the use of category cueing (Turner et al., 2007
). Such a lesion may well lower the subjective cost of an error (Rolls, 2004
); the subject is less careful. In a behavioural task switching study where there are competing task-sets such a tendency could well produce a higher error rate. The effects may also be similar to the inability of patients to evaluate risk in gambling paradigms (Floden et al., 2008
).
One contrasting effect that was found in the study of Aron et al. (2004)
but where no analogous effect was found in our study is an increased error rate on switch trials in their right frontal group. Aron et al. (2004)
link the error phenomenon they observed in their coarse-grain analysis with a reaction time effect they found only in their fine-grain analysis. This latter effect was that the larger the lesion in the region of the pars opercularis in the right inferior frontal gyrus the larger was the reaction time switch cost. They argue that this region contains a subsystem involved in inhibiting erroneous responses (see also Aron and Poldrack, 2006
). There was no analogous effect in our study. We have previously considered that regions of the right lateral frontal cortex might be involved in monitoring task performance – in reacting rapidly and accurately to multidimensional stimuli (Stuss et al., 2002
), in increasing preparation while a cued interval passes (Stuss et al., 2005
), in avoiding capture errors (Reverberi et al., 2005
), in judging the passage of time (Picton et al., 2006
) or in counting sounds (Shallice et al., 2008a
). Impairments in performance monitoring (e.g. not noticing that switching is required or had not been carried out) might also explain the findings in the Aron et al. (2004)
study.
The differences in patterns of performance between the two studies may derive from the composition of the two patient populations and from the group study analysis methodology used. However, as important a factor is likely to be the specific version of task-switching employed. Our study differed from that of Aron et al. (2004) and was unusual but far from unique in not finding a reduction in switch costs with an increased preparation interval. In our paradigm, preparation effects would be expected to be less as, unlike most other studies, we maintained the interval between stimuli in successive trials constant whatever the preparation interval, so as to avoid differences between conditions in task-set dissipation which Meiran et al. (2000)
had shown to be an important variable for the size of the switch cost. An increased preparation interval did, however, have a major effect. It led to an overall reduction in reaction time on both switch and repeat trials, of 287 ms in the control group and a dramatic 613 ms in the Superior Medial group. This suggests that the specific paradigm used led patients, and possibly also controls, to prepare when to respond rather than how to respond according to a specific task-set, a possibility that has been considered by Meiran et al. (2000) and Altmann (2005)
in other task-switching situations. It is relevant in this respect that the patient groups in the Aron et al. (2004)
study had if anything smaller preparation effects than the control group. This supports the possibility that there was a population difference between the two studies with medial patients, and in particular that Superior Medial patients were less well represented in the Aron et al. (2004)
study.
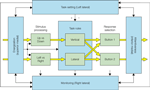
Overall our study shows the importance of a number of frontally located processes in task switching paradigms (see Figure 2
). These processes, which were analysed within the framework of a Supervisory system approach (see Shallice and Burgess, 1996
; Stuss et al. 1995
) are, however, held to have much wider relevance than task-switching per se. So-called task-setting may be conceived as operating over the short-term or the longer term; analogous effects to those obtained here in the left lateral prefrontal cortex have been found in a neuropsychological group study employing a rather different type reaction time task – that of Alexander et al. (2005)
(see also Stuss et al., 2002
). The energisation effects found in the Superior Medial group are consonant with those found in a range of studies using other paradigms, such as Stuss et al. (2002
, 2005)
, Alexander et al. (2005
, 2007)
, Picton et al. (2006
, 2007)
, Shallice et al. (2008a)
; they had the properties to be expected of an impairment to the energisation process. Finally, analogous error effects to those obtained in the Inferior Medial group attributable to impairments to an attentiveness process have not previously been found to our knowledge in reaction time paradigms with such patients. However, they can be related to analogous tendencies to produce errors in this patient group in other cognitive domains.
Figure 2. The conceptual framework of the paper based on the Supervisory System framework (Shallice and Burgess, 1996
; Stuss et al., 1995
). The inner green boxes and yellow arrows represent on-line processes which (following learning) would be realised in contention scheduling. The outer blue boxes and arrows represent supervisory control processes. These include (in the early stages of learning) task rules operating as explicit if-then contingencies. The four patient groups discussed – left lateral, right lateral, superior medial and inferior medial – are held to have impairments in different supervisory processes.
At the end of the 20th century the task switching type of paradigm was abstracted as an idealised simplification of set-switching processes known to be critical for effective performance on the most famous clinical test of prefrontal impairment, Wisconsin Card-Sorting. During the early years of the 21st century neuropsychological group studies have demonstrated how many processes are involved even in this simplified paradigm!
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
DiGirolamo, G. J., Kramer, A. F., Barad, V., Cepeda, N. J., Weissman, D. H., Milham, M. P., Wszalek, T. M., Cohen, N. J., Banich, M. T., Webb, A. Belopolsky, A. V., and McAuley, E. (2001). General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport 12, 2065–2071.
Stuss, D. T., Levine, B., Alexander, M. P., Hong, J., Palumbo, C., Hamer, L., Murphy, K. J., and Izukawa, D. (2000). Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38, 388–402.