1
RIKEN Brain Science Institute (BSI), Japan
2
Institute for Genetics, University of Bonn, Germany
3
Institute of Cellular Neurosciences, University of Bonn, Germany
Connexin43 (Cx43), a major component of astrocytic gap junctions, is abundantly expressed in Bergmann glial cells (BGCs) in the cerebellum, but the function of Cx43 in BGCs is largely unknown. BGCs are specialized astrocytes closely associated with Purkinje cells. Here, we review our recent studies of the role of Cx43 in gap junctional coupling between BGCs and in cerebellar function. We generated Cx43 conditional knockout mice with an S100b-Cre transgenic line (Cx43fl/fl:S100b-Cre), in which there was a significant postnatal loss of Cx43 in BGCs and cerebellar astrocytes. Gap junctional coupling between BGCs measured by dye coupling was virtually abolished in Cx43fl/fl:S100b-Cre mice. Electrophysiologic and behavioral analyses suggested that Cx43-mediated gap junctions and Cx43 hemichannels in BGCs are not necessary for the neuron-glia interactions required for cerebellum-dependent motor coordination and motor learning. These findings raise questions regarding the regional differences in the impact of the loss of Cx43 in the brain.
Astrocytes, the most abundant cell type in the mammalian brain, are extensively coupled by gap junctions (Giaume and McCarthy, 1996
) through which ionic and metabolic homeostasis (e.g., spatial buffering of K+ and glutamate) is maintained, and electrical coupling and intercellular signaling (e.g., Ca2+ wave) occur (Ransom and Ye, 2005
). The gap junction channel is formed by two hemichannels, each of which comprises six protein subunits called connexins. Connexin43 (Cx43), the major constituent of astrocytic gap junctions
, is abundantly expressed in astrocytes throughout the brain (Dermietzel et al., 1989
; Giaume and McCarthy, 1996
). Cx43 knockout in mice causes early postnatal lethality due to heart malfunction (Reaume et al., 1995
), and therefore conditional knockout
(CKO) of Cx43 in mice with Cre/loxP system has been used to study the function of Cx43 in the brain.
The first Cx43 CKO mice, exhibiting the loss of Cx43 essentially in all astrocytes in the brain, were generated using the Cx43 floxed allele (Cx43fl) in which the Cx43 coding sequence is flanked by two loxP sites (Theis et al., 2001
), in combination with a Cre transgenic line under the control of the human glial fibrillary acidic protein promoter (hGFAP-Cre) (Theis et al., 2003
). Cx43fl/fl:hGFAP-Cre mice are viable and show no histologic abnormalities in the brain (Theis et al., 2003
), but exhibit several features in situ and in vivo that are related to brain physiology and/or function, as follows: [in situ] accelerated hippocampal spreading depression (Theis et al., 2003
) and impaired Ca2+ wave propagation in neocortex (Haas et al., 2006
); [in vivo] enhanced locomotor activity (Theis et al., 2003
); increased exploratory behavior, impaired motor capacity, and changes in brain acetylcoline level (Frisch et al., 2003
); and increased apoptosis and inflammation after cerebral ischemia (Nakase et al., 2004
). In addition, Cx43fl/fl:hGFAP-Cre mice in combination with a null mutation of Cx30, another astrocytic connexin, have impaired spatial K+ buffering and a reduced threshold for the generation of epileptiform events in the hippocampus in situ (Wallraff et al., 2006
). Figiel et al. (2007)
showed that deletion of Cx43 in cortical astrocytes causes a loss of glutamate transporter GLT-1. Recently, Lin et al. (2008
) reported that Cx43fl/fl:hGFAP-Cre mice are insensitive to hypoxic preconditioning, and in this case Cx43 functions as hemichannels that serve as a pathway for the efflux of ATP. Although these findings successfully demonstrated the involvement of astrocytic Cx43 in brain physiology and/or function, questions remain regarding the role of Cx43 in the cerebellum.
Bergmann glial cells (BGCs)
are unipolar astrocytes that extend long processes across the molecular layer of the cerebellum (Figures 1
A,B). BGC processes form intimate structural relationships with the dendrites of Purkinje cells (Figures 1
B,C) (Grosche et al., 2002
; Yamada and Watanabe, 2002
), which are inhibitory neurons that act as the sole source of output from the cerebellar cortex. The processes of mature BGCs surround the synapses on Purkinje cells that are formed with glutamatergic excitatory axon parallel fibers and climbing fibers (Palay and Chan-Palay, 1974
; Spacek, 1985
). Extensive gap junctional coupling is observed in mature BGC processes (Clark and Barbour, 1997
; Müller et al., 1996
) where Cx43 is abundantly expressed (Figure 1
D)(Nagy et al., 2001
), but the role of Cx43 is largely unknown.
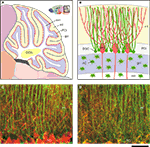
Figure 1. Structure of the cerebellum and cerebellar molecular layer in mice. (A,B) Schematic illustration of the structure of the cerebellum in mice. (A) Parasagittal view of mouse cerebellum. The boxed region (upper right) is magnified. wm, white matter; ml, molecular layer; PCl, Purkinje cell layer; gcl, granule cell layer; DCN, deep cerebellar nucleus. Figures are modified from Allen Mouse Brain Atlas [Internet]. Seattle (WA): Allen Institute for Brain Science. ©2008. Available from: http//www.brain-map.org.
(B) Cell bodies of Bergmann glial cells (BGC) are localized in the Purkinje cell layer (PCl), where somata of Purkinje cells (PC) align. In the molecular layer (ml), BGC processes associate closely with PC dendrites. Cerebellar astrocytes (AC) are present in the granule cell layer (gcl) and white matter (wm). (C,D) Double immunofluorescence analysis of parasagittal sections of the cerebellum from Cx43fl/+ mice (1.5-month-old) that served as controls in Tanaka et al. (2008)
. (C) GFAP (green)-positive BGC processes show intimate structural relationships with calbindin-D (red)-positive PC dendrites in the molecular layer. Modified and reproduced from Tanaka et al. (2008)
. Front. Behav. Neurosci. 2, 1. (D) GFAP (green)-positive BGC processes are positive for Cx43 (red). Scale bar, 50 μm.
To investigate the role of Cx43 in cerebellar function, we constructed a new Cx43 CKO model with temporal and regional specificity of a Cre-mediated recombination directed to the cerebellum (Tanaka et al., 2008
), as deficits in other brain areas, e.g., striatum, can also lead to impaired motor coordination (Blundell et al., 2008
). Furthermore, Cx43fl/fl:hGFAP-Cre mice generated on a different genetic background exhibit cellular disorganization of the cortex, hippocampus, and cerebellum, accompanied by ataxia and motor deficits (Wiencken-Barger et al., 2007
), suggesting the importance of controlling the onset and regional specificity of Cx43 CKO to study neuron-glia interactions in the adult cerebellum. Using a new Cx43 CKO model, we recently investigated the contribution of Cx43 to gap junctional coupling between BGCs, and examined whether Cx43 in BGCs, either as a gap junction channel or a hemichannel, plays an important role in cerebellar functions via Purkinje cell-BGC interactions.
Generation of a New Cx43 CKO Mice with Efficient Postnatal Recombination in BGCs and Cerebellar Astrocytes Mediated By S100b-Cre Transgene
First we generated a new Cre transgenic line that can be used to make Cx43 CKO mice suitable for studying cerebellar functions (Tanaka et al., 2008
). S100B is an EF-hand-type protein expressed primarily in astrocytes in the mammalian central nervous system (Boyes et al., 1986
; Haan et al., 1982
; Van Eldik et al., 1984
). In rodents, the pattern of S100b expression shows a rostral-caudal gradient during postnatal development with robust expression in the BGCs in the cerebellum (Landry et al., 1989
). The generation of a transgenic mouse line using a 5.4-kb genomic sequence of S100b to drive Cre recombinase (S100b-Cre) led to efficient deletion of Cx43fl in the cerebellum (Figure 2
A), which can be monitored by β-galactosidase expression with a nuclear localization signal under control of the Cx43 promoter upon Cre-mediated recombination of the floxed Cx43 coding region (Theis et al., 2003
). Since β-galactosidase is expressed instead of Cx43, reporter gene expression marks those cells which have lost Cx43 expression driven by the Cx43fl allele. The recombination pattern of Cx43fl/+: S100b-Cre mice with a rostral-caudal gradient as indicated by lacZ staining (Figure 2
A, upper left), was very similar to that of the endogenous S100b pattern during postnatal development (Landry et al., 1989
). Immunohistochemical analysis of the Cx43fl/+:S100b-Cre mice revealed that nuclear β-galactosidase immunoreactivity was located in BGCs in the Purkinje cell layer, and in cerebellar astrocytes in the granule cell layer and white matter, all of which were S100B-positive and NeuN negative (Figure 2
B).
Figure 2. S100b-Cre-mediated recombination in the brain. (A) Left panels: LacZ staining of 2-month-old Cx43fl/+:S100b-Cre mice showing the most extensive Cre-mediated recombination in the cerebellum as compared to positive control Cx43del/+ mice (Theis et al., 2001
). No lacZ-positive cells were detected in negative control Cx43fl/+ mice (data not shown). Scale bar, 1 mm. Right panels: Immunofluorescence analysis of parasagittal sections of the cerebellum from 2-month-old Cx43fl/+:S100b-Cre mice and positive control Cx43del/+ mice using antibodies directed to β-galactosidase. β-galactosidase immunoreactivity localized in the nucleus is observed in the Purkinje cell layer (arrowheads), granule cell layer (gcl) and white matter (wm). ml, molecular layer. Scale bar, 200 μm. (B) Double-immunofluorescence analysis of parasagittal sections of the cerebellum of Cx43fl/+:S100b-Cre mice. Nuclear lacZ immunoreactivity that colocalizes with S100B and not with NeuN corresponds to Bergmann glial cell bodies (magnified in inserts) in the Purkinje cell layer (arrowheads) and astrocytes in the granule cell layer. Scale bar, 100 μm. (C) Time course of S100b-Cre-mediated deletion of Cx43fl allele in the postnatal cerebellum. Scale bar, 1 mm. Modified and reproduced from Tanaka et al. (2008). Front. Behav. Neurosci. 2, 1.
S100b-Cre-mediated recombination of Cx43fl in the cerebellum began and progressed during postnatal development (Figure 2
C). Excision in most of the BGCs in the Cx43fl/+:S100b-Cre mice was completed by P28 (Figure 2
C). The results obtained using different reporter strains consistently indicated that S100b-Cre-mediated recombination in the cerebellum was restricted to the BGCs and cerebellar astrocytes and did not occur in Purkinje cells or granule cells (Tanaka et al., 2008
). It is therefore likely that the efficient and cell type-specific recombination in BGCs and cerebellar astrocytes in the adult cerebellum is due to the postnatal onset of S100b-Cre-mediated recombination in the precursors of those cells. The temporal and spatial Cre-mediated recombination pattern induced by S100b-Cre is markedly different from that of GFAP-Cre lines using either the human or mouse Gfap promoter, in which Cre-mediated recombination occurs in neural progenitors of prenatal embryos, resulting in widespread recombination in neurons and glial cells (Bajenaru et al., 2002
; Casper and McCarthy, 2006
; Garcia et al., 2004
; Kwon et al., 2001
; Zhuo et al., 2001
). Thus, our transgenic S100b-Cre line appears to be an ideal tool for studying glial function in the cerebellar molecular layer and/or granule cell layer in postnatal late developmental stages and adulthood in mice.
The S100b-Cre transgenic line was then used to generate Cx43 CKO (Cx43fl/fl:S100b-Cre) mice, to examine the role of Cx43 in the postnatal cerebellum, particularly in the Purkinje cell-BGC interactions in the cerebellar molecular layer. In Cx43fl/fl:S100b-Cre mice, a loss of Cx43 was evident in most of the cerebellar astrocytes and in virtually all of the BGCs in the cerebellar molecular layer in the adult (Figure 3
A). No compensatory changes were observed in the expression of Cx30, another connexin expressed in astrocytes and BGCs (Nagy et al., 1999
, 2001
), in the cerebellum of Cx43fl/fl:S100b-Cre mice (Tanaka et al., 2008
). The cerebellar architecture of the Cx43fl/fl:S100b-Cre mice was normal, including normal morphology of both the BGCs and Purkinje cells (Tanaka et al., 2008
), suggesting that Cx43 does not have a key role in the structural support of BGCs or cerebellar astrocytes in the late cerebellar developmental stages or adulthood.
Figure 3. Extent of S100b-Cre-mediated loss of Cx43 and Lucifer yellow coupling between BGC in Cx43fl/+, Cx43fl/fl, and Cx43fl/fl:S100b-Cre mice. (A) Immunofluorescence analysis of parasagittal sections of the cerebellum from Cx43fl/+, Cx43fl/fl, and Cx43fl/fl:S100b-Cre mice (2.5-month-old) using antibodies directed to Cx43. Arrowheads indicate Purkinje cell layer. gcl, granule cell layer; ml, molecular layer. Scale bar, 100 μm. (B) Intercellular Lucifer yellow transfer in BGC in parasagittal cerebellar slices from Cx43fl/+, Cx43fl/fl, and Cx43fl/fl:S100b-Cre mice (3.5-month-old) after Lucifer yellow injection into one BGC soma. The asterisk indicates a dye-injected BGC cell body. Arrows indicate representative dye-coupled BGC cell bodies. (C) Average numbers of dye-stained neighboring cells when processes of injected cells were stained as shown in (B) (n = 8 for Cx43fl/+, n = 5 for Cx43fl/fl, and n = 6 for Cx43fl/fl:S100b-Cre). Error bars represent SEM. *, p< 0.05; **, p< 0.01 in ANOVA. Modified and reproduced from Tanaka et al. (2008)
. Front. Behav. Neurosci. 2, 1.
We then investigated whether the loss of Cx43 in the BGCs affected gap junctional coupling in situ. Dye coupling experiments using Lucifer yellow demonstrated that Cx43 contributes significantly to gap junctional coupling between BGCs (Figures 3
B,C). The magnitude of Lucifer yellow dye coupling was closely related with the amount of Cx43 protein, which was reduced in Cx43fl/fl mice to approximately 30% that of wild-type (WT) mice, and in Cx43fl/fl:S100b-Cre mice to approximately 10% that of WT mice (Tanaka et al., 2008
).
The failure of glutamate uptake at Purkinje cell synapses by the glutamate transporters GLAST and GLT-1 expressed in BGCs results in motor discoordination in mice (Rothstein et al., 1996
; Watase et al., 1998
), which most likely is caused by the multiple innervation of Purkinje cell by climbing fibers (Watase et al., 1998
). Defects in Gfap KO mice (Shibuki et al., 1996
), such as impaired cerebellar long-term depression (LTD) at the parallel fiber (PF)-Purkinje cell (PC) synapses and impaired motor learning “eyeblink conditioning
”, may be caused by enhanced glutamate uptake through the upregulation of GLT-1 in the cerebellum (Hughes et al., 2004
). Importantly, the deletion of Cx43 in cortical astrocytes is associated with a loss in GLT 1 expression (Figiel et al., 2007
). In addition, gap junctions are permeable to glutamate (Goldberg et al., 1999
; Hansson et al., 2000
; Weber et al., 2004
). Based on these reports, we postulated that Cx43 in BGCs is involved in two distinct cerebellar functions, motor coordination and motor learning, by regulating glutamate uptake via maintaining the expression of glutamate transporters and/or controlling the spatial buffering of glutamate through gap junctions. Alternatively, Cx43 may be required for other regulatory systems as reported for other brain regions (e.g., K+ buffering through gap junctions and ATP release from hemichannels) in the cerebellum.
To determine whether the loss of Cx43 in BGCs affects Purkinje cell synaptic plasticity, which is substantially involved in cerebellum-dependent behaviors (Ito, 2001
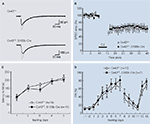
), we analyzed the kinetic properties of basal synaptic transmission and cerebellar LTD at PF-PC synapses in slices of adult Cx43fl/fl:S100b-Cre cerebellum. First, we stimulated parallel fibers in the middle molecular layer and recorded excitatory postsynaptic currents (EPSCs) from Purkinje cells. There were no significant differences in the basal kinetic properties of the PF-EPSCs (10–90% rising time, 1.6 ± 0.2 and 1.3 ± 0.2 ms; p> 0.2; decay time constant, 15.5 ± 2.0 and 15.6 ± 1.3 ms; p> 0.9) between control (n = 8) and Cx43fl/fl:S100b-Cre (n = 9) cells (Figure 4
A). To induce LTD of PF-EPSCs, we administered PF-stimulation with simultaneous somatic depolarization of Purkinje cells (140 ms, − 70 to + 10 mV) at 1 Hz for 5 min (Koekkoek et al., 2005
). The conjunctive stimuli induced a significant decrease in the amplitude of PF EPSCs in both control (Cx43fl/+) and Cx43fl/fl:S100b-Cre cells (Figure 4
B; p< 0.01 for each). The magnitude of LTD in the Cx43fl/fl:S100b-Cre cells measured during a 25- to 30-min period after stimulation was comparable with that in the control cells (Tanaka et al., 2008
).
Figure 4. Basal PF-PC EPSC, LTD induction, and behavioral analysis of control (Cx43fl/+) and Cx43fl/fl:S100b-Cre mice. (A) A representative example of PF-EPSC before (gray) and after (black) conjunctive stimulation in Cx43fl/+ and Cx43fl/fl:S100b-Cre mice. Six records are averaged. (B) PF-EPSC amplitude was plotted against time before and after conjunctive stimulation averaged for eight cells from 8 Cx43fl/+ mice or for nine cells from 8 Cx43fl/fl:S100b-Cre mice. Each point represents the average of three successive responses acquired for 1 min at 0.05 Hz. Bar indicates the period of conjunctive stimulation (CJ). (C) Accelerating rotarod test. The mean (±SEM) time an animal remained on an accelerating rod (6–40 rpm) during training with one trial per day for five consecutive days (n = 14 for Cx43fl/+, and n = 11 for Cx43fl/fl:S100b-Cre). (D) Delay eyeblink conditioning. The mean (±SEM) conditioned response (CR) percentage of Cx43fl/+ (n = 10) and Cx43fl/fl:S100b-Cre (n = 7) during acquisition (days 1–7), extinction (days 8–11), and relearning (days 12–13) sessions. Modified and reproduced from Tanaka et al. (2008).
Front. Behav. Neurosci. 2, 1.
To study the motor behavior of Cx43fl/fl:S100b-Cre mice, we first performed an open field test with 2.5-month-old control Cx43fl/+ (n = 13) and Cx43fl/fl:S100b-Cre (n = 10) mice. The genotypes did not significantly differ in either horizontally directed locomotor activity or in time spent in the center of the open field (data not shown). We then performed a rotarod test to determine whether these mice had normal motor coordination. We trained 2.5-month-old control Cx43fl/+ (n = 14) and Cx43fl/fl:S100b-Cre (n = 11) mice over 5 days to balance on an accelerating rotating rod (Figure 4
C). Although the latency of Cx43fl/fl:S100b-Cre mice to fall decreased slightly on the third day of training compared to that of control mice, their performance improved on the fourth and fifth days. There was no statistically significant difference in overall performance between the genotypes. There was no significant difference in the body weight of the mice used for the rotarod test (data not shown).
The impact of the loss of Cx43 in BGCs and the reduction of Cx43 in astrocytes in the deep cerebellar nuclei (data not shown) on delay eyeblink conditioning were then evaluated in 3- to 3.5-month-old control Cx43fl/+ (n = 10) and Cx43fl/fl:S100b-Cre (n = 7) mice. Eyeblink conditioning is a task in which an animal learns to associate a conditioned stimulus (CS) with a noxious unconditioned stimulus (US) that elicits an eyeblink (Christian and Thompson, 2003
). The memory trace for delay eyeblink conditioning, in which the preceding CS and the US terminate simultaneously, is considered to be formed in the cerebellar cortex and deep cerebellar nuclei (Attwell et al., 2002
; Christian and Thompson, 2003
; Mauk and Buonomano, 2004
), and cerebellar LTD appears to be a neural correlate of delay eyeblink conditioning (Christian and Thompson, 2003
). During eyeblink conditioning of control and Cx43fl/fl:S100b-Cre mice, a CS (tone 1 kH, 352 ms, 83–85 dB) was paired with the US, a periorbital shock (100 ms, 100 Hz pulses). Both genotypes exhibited an increased frequency of conditioned responses during the 7-day training period (Figure 4
D). There were no statistically significant differences in acquisition, extinction, or relearning kinetics of the conditioned responses, however, between control and Cx43fl/fl:S100b-Cre mice (Tanaka et al., 2008
).
A new model of Cx43 CKO (Cx43fl/fl:S100b-Cre) mice in which Cx43 ablation occurs preferentially in the postnatal cerebellum has been developed. In contrast to previous reports of Cx43fl/fl:hGFAP-Cre mice (Frisch et al., 2003
; Theis et al., 2003
), behavioral analyses of the Cx43fl/fl:S100b-Cre mice in the present study did not reveal enhanced locomotor activity nor increased exploratory behavior in the open field test (data not shown), further suggesting limited S100b-Cre-mediated recombination of the Cx43fl allele in the forebrain of Cx43fl/fl:S100b-Cre mice (Figure 2
A) (Tanaka et al., 2008
). Cerebellum dependent behaviors, such as motor coordination and eyeblink conditioning, were not significantly impaired in Cx43fl/fl:S100b-Cre mice (Figure 4
C,D), in contrast to the previously reported impairment in rotarod performance of Cx43fl/fl:hGFAP-Cre mice (Frisch et al., 2003
). The restricted nature of the S100b-Cre-mediated deletion might explain the lack of rotarod impairment in the Cx43fl/fl:S100b-Cre mice, because the functions of other brain regions can also affect motor coordination (Blundell et al., 2008
).
Our results suggest that Cx43 expressed in BGCs, either as a gap junction channel or hemichannel, is not required for glutamate uptake, K+ buffering, or other regulatory mechanisms that may be involved in the Purkinje cell synaptic plasticity related to cerebellum-dependent behaviors in adult mice. These results further suggest that Cx43 in BGCs or, more broadly, in cerebellar astrocytes including the BGCs, do not have an essential role in the eyeblink conditioning motor learning circuit. It remains unclear, however, whether other connexins expressed in BGCs, such as Cx30 and Cx29 (Altevogt and Paul, 2004
; Eiberger et al., 2006
; Nagy et al., 2001
), functionally compensate for the absence of Cx43. Cx30 forms functional gap junction channels that are not permeable to Lucifer yellow (Manthey et al., 2001
) and is expressed in the BGCs of Cx43fl/fl:S100b-Cre mice (Tanaka et al., 2008
). The role of these other connexins must be clarified by studying the consequences of their combined ablation in BGCs using [Cx30−/−, Cx43fl/fl:S100b-Cre] mice or [Cx29−/−, Cx30−/−, Cx43fl/fl:S100b-Cre] mice.
Although mutual interactions between Purkinje cells and BGCs have been described in several reviews (Bellamy, 2006
; Lopez-Bayghen et al., 2007
; Metea and Newman, 2006
), our results suggest that these Purkinje cell-BGC interactions are less dependent on gap junctional coupling between BGCs. The morphology of the BGCs, which forms a perpendicular array, may facilitate the spatial buffering of molecules as in the case of astrocytes in the stratum radiatum in the hippocampus (Wallraff et al., 2006
). In addition, the functional independence of Cx43 in BGCs compared to astrocytes in other brain regions where the loss of Cx43 results in physiologic and/or functional impairments (Frisch et al., 2003
; Haas et al., 2006
; Theis et al., 2003
) may reflect a functional heterogeneity of these astrocytic subsets located in different brain regions. For example, Ca2+ increases in BGCs restricted to microdomains (Kettenmann and Schipke, 2004
) are different from the intrinsic calcium oscillations observed in hippocampal astrocytes (Fiacco and McCarthy, 2006
).
Several lines of evidence indicate brain region-specific differences in response to the knockout of astrocyte-specific genes: (1) In Gfap KO mice (Shibuki et al., 1996
), glutamate uptake is reduced in association with a failure in glial transporter GLT-1 trafficking in the cortex and hippocampus, whereas glutamate uptake is enhanced in the cerebellum (Hughes et al., 2004
). (2) In S100b KO mice, microarray analysis revealed alterations of different groups of genes between the hippocampus and cerebellum (Ohshima, Kim, Konishi, and Itohara, unpublished data), which might be responsible for functional impairments detected in the hippocampus (Nishiyama et al., 2002
; Sakatani et al., 2007
, 2008
) and for the preserved cerebellar functions (Ohshima, Kim, Konishi and Itohara, unpublished data). Based on these observations, the impact of Cx43 knockout may be different in the cerebellum compared to other brain areas. It should be also noted that large-scale global transcriptomic alterations that compensate for the functional consequences of Cx43 deletion in the cerebellum may occur in the Cx43 CKO, as reported for Cx43−/− astrocytes and newborn mouse brains (Iacobas et al., 2007
, 2008
). Our results, together with the results of others, suggest that transcriptomic alterations in Cx43 CKO may differ between the cerebellum and other brain areas. Further studies of Cx43 CKO mice using different Cre lines with region-specificity will help to elucidate the heterogeneity of astrocytic subsets in the context of their functional connections to each brain area.
We thank C. Nishioka for her tireless help throughout the study, H. Nishiyama for his help in the early stage of the study, and T. Iwasato, J.S. Park, Y. Sano, and S. Nishimura-Akiyoshi for advice on the experiments; the BSI Research Resources Center for animal care and technical assistance; J. Miyazaki for the CAG-CAT-Z mice; and T. Iwasato for the CAG-Δ-Z mice. This work was supported, in part, by a Grant-in-Aid for Scientific Research on Priority Areas from the MEXT, Japan (to M. Tanaka). Work in the Bonn laboratory was supported by grants of the German Research Association (SFB 400, B3 and SFB 645, B2) to K. Willecke.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Conditional knockout (CKO) mouse: Tissue- or cell type-specific knock out of genes can be generated in mice by using Cre/loxP system, which requires genomic region flanked by two loxP sites (floxed allele) and tissue- or cell type-specific expression of Cre recombinase. Cre-mediated excision deletes the genomic region flanked by two loxP sites.
Frisch, C., Theis, M., De Souza Silva, M. A., Dere, E., Söhl, G., Teubner, B., Namestkova, K., Willecke, K., and Huston, J. P. (2003). Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 18, 2313–2318.
Hansson, E., Muyderman, H., Leonova, J., Allansson, L., Sinclair, J., Blomstrand, F., Thorlin, T., Nilsson, M., and Rönnbäck, L. (2000). Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem. Int. 37, 317–329.
Koekkoek, S. K., Yamaguchi, K., Milojkovic, B. A., Dortland, B. R., Ruigrok, T. J., Maex, R., De Graaf, W., Smit, A. E., VanderWerf, F., Bakker, C. E., Willemsen, R., Ikeda, T., Kakizawa, S., Onodera, K., Nelson, D. L., Mientjes, E., Joosten, M., De Schutter, E., Oostra, B. A., Ito, M., and De Zeeuw, C. I. (2005). Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron 47, 339–352.
Rothstein, J. D., Dykes-Hoberg, M., Pardo, C. A., Bristol, L. A., Jin, L., Kuncl, R. W., Kanai, Y., Hediger, M. A., Wang, Y., Schielke, J. P., and Welty, D. F. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686.
Shibuki, K., Gomi, H., Chen, L., Bao, S., Kim, J. J., Wakatsuki, H., Fujisaki, T., Fujimoto, K., Katoh, A., Ikeda, T., Chen, C., Thompson, R. F., and Itohara, S. (1996). Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron 16, 587–599.
Tanaka, M., Yamaguchi, K., Tatsukawa, T., Chieko, N., Nishiyama, H., Theis, M., Willecke, K., and Itohara, S. (2008). Lack of connexin43-mediated Bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Front. Behav. Neurosci. 2, 1.
Theis, M., Jauch, R., Zhuo, L., Speidel, D., Wallraff, A., Döring, B., Frisch, C., Söhl, G., Teubner, B., Euwens, C., Huston, J., Steinhäuser, C., Messing, A., Heinemann, U., and Willecke, K. (2003). Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 23, 766–776.
Watase, K., Hashimoto, K., Kano, M., Yamada, K., Watanabe, M., Inoue, Y., Okuyama, S., Sakagawa, T., Ogawa, S., Kawashima, N., Hori, S., Takimoto, M., Wada, K., and Tanaka, K. (1998). Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur. J. Neurosci. 10, 976–988.