Champalimaud Neuroscience Programme, Instituto Gulbenkian de Ciência, Rua da Quinta Grande, Oeiras, Portugal
A commentary on
Associative and non-associative plasticity in Kenyon cells of the honeybee mushroom body
by Paul Szyszka, Alexander Galkin and Randolf Menzel
Learning and memory lead to functional and structural changes in the brain, ultimately providing a basis for adaptive behavior. The honeybee is an elegant model for the study of learning and memory formation as it permits both the visualization of neural activity related to the events occurring in olfactory learning and the behavioral assessment of olfactory learning (Galizia and Menzel, 2000
). The formation of odor memories in the honeybee is thought to involve the two primary processing centers of the olfactory system, the antennal lobe (AL) and the mushroom body (MB). The intrinsic neurons of the MB – the Kenyon cells (KCs), located within the lip region of the MB calyx – are the site of convergence of the neural pathways that transmit odor information from the projection neurons (PNs) of the AL and reward information from the VUMmx1 neuron (Hammer, 1997
). In recent years, imaging studies performed in the honeybee AL and MB lip have indicated that pairing odor and reward induces changes in neural activity (Faber and Menzel, 2001
; Faber et al., 1999
), reinforcing the anatomical suggestion that KCs are likely to undergo associative plasticity during learning.
This study, Szyszka et al. (2008)
provides new evidence that odor-evoked activity of KCs can be modified by sensory experience, and the changes can be associative or non-associative. Szyszka et al. (2008)
visualized odor representations in the MB of the honeybee by imaging the calcium responses of a subpopulation of KCs – the clawed KCs, by retrogradely labeling the cell population using dye injections into the ventral alpha/gamma lobe. This allowed the authors to image, in a relatively selective way, the bulk dendritic activity of KCs over neuropil. Szyszka et al. (2008)
first examined KC activity in response to repeated stimulation with the same odor and found that it led to a decrease in KC responses, a non-associative form of plasticity resembling a phenomenon previously described in locust PNs (Stopfer and Laurent, 1999
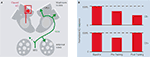
). Separate experiments found that honeybee PNs did not show repetition-induced depression, indicating that the effect is not a generalized run-down. The authors then compared KC activity before and after pairing one odor with sucrose reward and tested how the associative pairing procedure affects KC responses to the rewarded odor (CS+) and to the unrewarded odor (CS−). During pre-training, KC responses to both CS+ and CS− decreased with repeated stimulation. After pairing CS+ with sucrose CS+ responses recovered and CS− responses decreased further (Figure 1
). Intriguingly, examination of spatiotemporal patterns within the imaged region before and after conditioning suggested additional changes in both CS+ and CS− evoked KC activity following conditioning.
Figure 1. KC responses are subject to associative and non-associative plasticity.(A) Scheme of honeybee antennal lobes and mushroom bodies (MB). Calcium responses in the somata and dendrites of clawed Kenyon cells (clawed KCs, red) were recorded in the MB calyx (the square indicates the imaged area). The VUMmx1 neuron (green), which connects the subesophageal ganglion (SEG) with the antennal lobes and the MB calyces, mediates the reinforcing function of the reward. (B) Amplitude of KC responses to the rewarded odor (CS+) and to the unrewarded odor (CS-) before training, after repeated odor stimulation with both odors (pre-training), and after pairing CS+ with sucrose (post-training).
The results of this study support the suggestion that KC odor responses can be modified by sensory experience and that these changes can be associative, extending the earlier work of the Menzel group (Faber and Menzel, 2001
; Faber et al., 1999
). Although a causal link between this activity and learning and memory remains to be established, the strengthening of support for a leading candidate cellular substrate is an encouraging observation that can be followed up in several ways. Firstly, it should be possible to examine the same cell population at the molecular and cellular levels in order to elucidate the processes underlying these modifications. Secondly, the changes in spatiotemporal patterns hint at restructuring of KC odor representations that might reflect a correlate of information storage. It may now be feasible, using similar imaging approaches but at single cell resolution, to resolve the details of the changes. Finally, it should be possible, albeit technically challenging, to take advantage of the possibility of behavioral measurements in combination with imaging to try to directly link KC changes to memory storage itself.