Abstract
Introduction:
The hemodynamic management of septic patients involves initial fluid therapy, followed by the use of vasoconstrictors in case of treatment failure. The latest Surviving Sepsis Campaign guidelines suggest the synergistic use of argipressin in addition to norepinephrine when hemodynamic optimization is not achieved with norepinephrine alone.
Methods:
In our single-center retrospective observational study, the primary endpoint is the safety of initial norepinephrine-argipressin association treatment, assessed through a reduction in Resistance Index. Our secondary endpoint includes the efficacy of this combination, measured by an increase in Mean Arterial Pressure and a reduction in Resistance Index as an indicator of organ perfusion. The Resistance Index (RI) is evaluated through Power Doppler ultrasound. RI is crucial for assessing multi-district vascular tone and multiorgan perfusion. Patients were categorized into three groups based on their treatment. In Group 1, we analyzed patients treated with norepinephrine alone in incremental doses; in Group 2, we analyzed patients receiving the initial norepinephrine-argipressin association treatment (norepinephrine 0.05 mcg/kg/min-argipressin 0.03 IU/min); in the third group (Group 3), we analyzed patients given argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25≥ mcg/kg/min) to stabilize their hemodynamics (MAP > 65 mmHg). RI measurements were taken in the Renal Artery (ARE), Radial Artery (AR), Central Retinal Artery (CRA), and Superior Mesenteric Artery (AMS) at four different time points: T0 before vasopressor therapy, T1 at 1 h, T2 at 24 h, and T3 at 48 h after vasopressor infusion.
Results:
A total of 48 patients were divided into three groups: 17 patients in Group 1, 16 in Group 2, and 15 in Group 3. In Group 1, an increase in Mean Arterial Pressure (MAP) was observed, but there was an increase in RIs in the right CRI and left ARE. In Group 2, there was an improvement in MAP and a reduction in RIs in the right/left CRI, left ARE, AMS, and right AR. In Group 3, there was an increase in MAP and a reduction in RIs in the right/left CRI, left ARE, AMS, and right AR.
Conclusion:
Early norepinephrine-argipressin association treatment appears to be a valid strategy for hemodynamic optimization in this patient population.
1. Introduction
Septic shock is a condition characterized by an uncontrolled inflammatory response to an infectious agent, resulting in severe hypotension and leading to inadequate organ perfusion pressure and severe organ damage (1–4).
The Surviving Sepsis Campaign (SSC) guidelines recommend norepinephrine as the first-line vasopressor agent in patients with septic shock after volume replenishment (1). The SSC guidelines suggest adding argipressin (up to 0.03 IU/min) with norepinephrine to achieve a target mean arterial pressure (MAP) of 65 mmHg or adding argipressin (up to 0.03 IU/min) to reduce the norepinephrine dosages. In septic shock patients, a “catecholamine-sparing strategy” is suggested to help minimize the adverse events associated with the administration of catecholamines such as tachycardia, arrhythmias, clot formation, etc. (5, 6). Hence, several alternative vasopressors such as argipressin have been suggested to reduce the dose of norepinephrine. In septic shock and other states of shock, the proinflammatory state initially induces the release of high levels of argipressin, which rapidly drop below the normal range (7–12). This could be due to the depletion of stores in the pituitary gland caused by massive release in the early stages of septic shock, autonomic dysfunction, and the inhibition of argipressin release by nitric oxide (which is produced in large quantities by the vascular endothelium under these circumstances) (7). The rationale for the use of argipressin in septic shock lies primarily in its vasopressor and osmoregulatory activity. In fact, it has been hypothesized that stimulation of the V1 receptors induces anti-edematous activity, reduces vascular permeability, and increases non-responsiveness to fluid resuscitation. Further, it has been shown that under-expression of V2 receptors during sepsis, induced by the NF-kB signaling pathway, contributes to the onset of acute kidney injury. Therefore, it is possible that the renal V2 receptors stimulation by argipressin could reduce this phenomenon (7). Several models have been proposed to describe the interaction between argipressin and anti-inflammatory cytokine expression (13–19), which is suggestive of the immunomodulatory properties of argipressin (13). Patients with septic shock have reduced argipressin activity, and exogenous administration of argipressin can restore vascular tone and blood pressure, reducing the need for catecholamines (20–26).
Observational studies have shown that administering argipressin below 0.1 U per minute in patients with vasodilatory shock can improve their short-term blood pressure (7, 8). However, argipressin might decrease the blood flow to the heart, kidneys, and intestine (27–31).
Careful monitoring of peripheral circulation is crucial to the management of patients in intensive care units. Particularly, the hemodynamic assessment in patients with septic shock is critical.
Doppler ultrasound is a non-invasive method used widely for assessing cardiac functionality and peripheral resistances.
The Resistance Index (RI) is a power doppler ultrasound assessment of vascular compliance to detect organ perfusion in septic shock patients.
The RI is an important ultrasound parameter that evaluates the multi-district vascular tone and multiorgan perfusion.
The RI is calculated using the following formula:It not only indicates the absolute value of blood flow velocity but also reflects variations in the velocity of the Doppler waveform.
In our single-center retrospective observational study, the primary endpoint was to use the RI to determine the safety of an early administration of norepinephrine-argipressin. The secondary endpoint was to determine the efficacy of norepinephrine and argipressin by evaluating the mean arterial pressure and RI as markers of organ perfusion.
2. Materials and methods
This single-center, retrospective, observational study was conducted from September 2021 to November 2022. The study did not include any medical, pharmacological, or behavioural interventions in addition to hospital standards of care. This research was carried out in accordance with the principles of the Declaration of Helsinki.
The study was approved by a local ethics committee: reference number 63/2023 and registration number 20230038704.
2.1. Study population
Data of patients admitted to the Intensive Care Unit were collected. Patients over the age of 18 years who were treated with vasopressor drugs for septic shock [in accordance with the definition of the Surviving Sepsis Campaign (1)] and resistant to fluid infusion were considered.
The exclusion criteria were as follows: patients with unstable acute coronary syndrome, proven or suspected acute mesenteric ischemia, cancer, or other irreversible conditions with estimated 6-month mortality ≥50%, chronic cardiomyopathy (NYHA III or IV), history of eye disease or previous eye surgery, severe hyponatremia (serum Na+ <130 mmol/L), traumatic brain injury (GCS < 8 before sepsis onset), and those for whom more than 24 hours passed after the verification of the eligibility criteria.
2.2. Treatment
The patients were divided into three groups based on the treatments they had received.
In the first group (Group 1), we analyzed data from patients who were given norepinephrine in incremental doses. In the second group (Group 2), we analyzed patients who were given norepinephrine (0.05 mcg/kg/min) and argipressin association treatment (argipressin 0.03 IU/min). In the third group (Group 3), we analyzed patients given argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25≥ mcg/kg/min) to stabilize the hemodynamics (MAP > 65 mmHg).
Measurements were taken at the beginning of each treatment (T0), at 1 h of treatment (T1), at 24 h of treatment (T2), and at 48 h of treatment (T3).
2.3. Data collection and measurements
Demographic data were collected, and vital signs were analyzed at T0, T1, T2, and T3.
We evaluated heart rate (HR), MAP, Cardiac Index (CI), Stroke Volume Variation (SVV), Systemic Vascular Resistance Index (SVRI), SOFA(Sequential Organ Failure Assessment) score, and lactate levels.
The Resistance Indices we analyzed were from the right and left side of the central retinal artery (CRA), the radial artery at the anatomical snuffbox (AR), the renal interlobar arteries (ARE), and the superior mesenteric artery (AMS).
A portable ultrasound system equipped with a 3.5–5 MHz convex probe, a linear probe (7.5 MHz), and color Doppler/Pulsed Wave Doppler (PW) software was used for the measurements.
Measurements of Ris for the central retinal artery and the radial artery were taken with a linear probe, and the renal interlobar arteries and superior mesenteric artery measurements were taken with a convex probe. The vascular landmarks were sampled in line with existing literature (32–35).
The maximum systolic velocity (Vmax) and the minimum end-diastolic velocity (Vmin) of the pulsed Doppler wave were determined.
To locate the central renal artery location, a color Doppler ultrasound examination was conducted with the patients lying in a supine position with closed eyes. A measurement sample of 1.5 mm and Doppler angle correction were used. The vessels, identified in the region of interest were analyzed by Pulsed Wave Doppler (PW) and quantified using a velocimetric spectral analysis. Peak systolic velocity and end-diastolic velocity were measured after correcting the angle of insonation to less than 60°. Vmin was measured immediately before the next systolic peak. To reduce variability, an average of 3 measurements per side were taken.
The RI for the radial artery at the anatomical snuffbox was measured using a linear probe. The B-mode ultrasound when applied to the anatomical snuffbox measures the artery that connects the dorsal branch of the radial artery to the deep palmar arch. The Vmax and Vmin of the pulsed Doppler wave were determined, and the RI was calculated (36–38).
The renal intraparenchymal vascularization was determined by velocimetric analysis of the interlobar or arcuate arteries (identified using a convex probe). For correct sampling, measurements of at least three overlapping waves in three different areas of each kidney were taken. The RI was calculated as the mean of the measured waves (39–46).
The superior mesenteric artery was measured using a convex probe (3.5–5 MHz), with a Doppler frequency of 2.8 MHz and a Pulse Repetition Frequency (PRF) of 5 KHz. Longitudinal scans were performed 2–3 cm from the origin of the artery with a 50–60 degree Doppler angle (47, 48).
2.4. Endpoints
The primary endpoint was to evaluate the safety of the initial administration of norepinephrine-argipressin. The secondary endpoint was to evaluate the efficacy of this association in comparison to norepinephrine alone.
This hemodynamic optimization is evaluated by means of hemodynamic parameters (non-invasive and invasive) and the values of the peripheral Resistance Indices at three different time points (T0) before vasopressor therapy, (T1) at 1 h, T2 at 24 h, and T3 at 48 h. Other outcomes included the evaluation of the patient's clinical improvement in terms of SOFA SCORE, laboratory-blood gas data (Lactates), and the side effects of Catecholamines (Heart Rate, Systemic Vascular Resistance Index). We also evaluated rates of serious adverse events.
2.5. Statistical analyses
Baseline demographical and clinical patients' characteristics were reported as median and inter-quartile ranges for each group and compared using Kruskall–Wallis and Fisher exact tests for continuous and categorical variables, respectively. Correlations between continuous variables were assessed using Spearman's coefficient. Changes in variables over time (baseline, after 1, 24, and 48 h) were analyzed using longitudinal linear models with a spatial power covariance matrix to account for unequal space visits. All variables were logarithmically transformed before analyses. Results were reported as regression coefficients (slopes) along with standard errors and p-values for each arm. Pairwise slope comparisons were done within the longitudinal model using pre-specified statistical contrasts. All analyses are performed using R software. P-values <0.05 were considered statistically significant.
Because of the multiplicity of the endpoints at issue in this study, the power study was conducted while considering the potential detectable Cohen's effect size. A three-arm study, enrolling 15 patients in each arm, has 80% power, fixing a Type I Error (alpha) equal to 0.05, to detect a statistically significant pairwise difference (between arms pairwise comparisons) equal at least to 1.1 in terms of effect size. There were 17 patients in one arm, 16 in another, and 15 patients in the last arm, and the power slightly increased.
3. Results
Of the 48 analyzed patients, 17 were treated with only norepinephrine alone in incremental doses (Group 1); 16 used the initial norepinephrine-argipressin association treatment (Group 2); and there were 15 patients in Group 3. The baseline characteristics of the three Groups are shown in Table 1.
Table 1
| Group 1 | Group 2 | Group 3 | P-value | |
|---|---|---|---|---|
| N.Patients | 17 | 16 | 15 | |
| Age (median, interquartile range) | 62.00 (54.00, 78.00) | 61.00 (55.50, 71.00) | 61.00 (59.50, 67.00) | 0.075 |
| Male | 8 (47.1%) | 12 (75%) | 10 (66.7%) | 0.256 |
| Female | 9 (52.9%) | 4 (25.0%) | 5 (33.3%) | 0.256 |
| SOFA SCORE (median, interquartile range) | 12.00 (11.00, 13.00) | 12.00 (10.50, 14.00) | 13.00 (11.50, 14.00) | 0.384 |
Baseline characteristics of the study population.
The table shows the demographic and baseline clinical data of the study.
Table 2 summarizes the hemodynamic parameters of the patients. The heart rate measurements in Group 2 were significantly reduced at the different time points after treatment (Table 2). No significant differences in heart rates were seen in Groups 1 and 3 at different time points.
Table 2
| Baseline (median, interquartile range) | 1 h (median, interquartile range) | 24 h (median, interquartile range) | 48 h (median, interquartile range) | P-value | ||
|---|---|---|---|---|---|---|
| HR(bpm) | Group 1 | 88.00 [69.00, 01.00] | 88.00 [57.00, 110.00] | 97.00 [75.00, 103.00] | 87.00 [64.00, 109.00] | 0.9454 |
| HR (bpm) | Group 2 | 81.00 [61.00, 102.00] | 80.50 [57.00, 110.00] | 78.00 [58.00, 100.00] | 73.00 [64.00, 87.00] | 0 . 0235 |
| HR (bpm) | Group 3 | 82.00 [59.00, 102.00] | 83.00 [67.00, 97.00] | 77.00 [67.00, 91.00] | 75.00 [67.00, 87.00] | 0.1221 |
| MAP (mmHg) | Group 1 | 59.00 [55.00, 84.00] | 66.00 [61.00, 89.00] | 78.00 [59.00, 97.00] | 84.00 [71.00, 96.00] | <0 . 001 |
| MAP (mmHg) | Group 2 | 62.50 [56.00, 88.00] | 68.50 [61.00, 91.00] | 79.50 [70.00, 97.00] | 83.00 [72.00, 96.00] | <0 . 001 |
| MAP (mmHg) | Group 3 | 59.00 [51.00, 67.00] | 65.00 [55.00, 76.00] | 73.00 [59.00, 97.00] | 78.50 [71.00, 87.00] | <0 . 001 |
| Svri (dyn*s*cm-5*m2) | Group 1 | 1,443.00 [1,200.00, 1,877.00] | 1,455.00 [1,322.00, 1,988.00] | 1,899.00 [1,678.00, 2,988.00] | 2,134.00 [1,779.00, 2,888.00] | <0 . 001 |
| Svri (dyn*s*cm-5*m2) | Group 2 | 1,498.00 [1,124.00, 1,788.00] | 1,505.00 [1,232.00, 1,980.00] | 1,807.00 [1,653.00, 2,433.00] | 1,970.00 [1,700.00, 2,309.00] | <0 . 001 |
| Svri (dyn*s*cm-5*m2) | Group 3 | 1,543.00 [1,228.50, 1,566.50] | 2,112.00 [1,326.00, 2,766.00] | 1,788.00 [1,565.00, 1,988.00] | 1,912.00 [1,700.00, 1,998.00] | 0 . 0002 |
| Lactate (mmol/L) | Group 1 | 2.10 [2.00, 7.90] | 2.80 [0.90, 7.40] | 3.00 [1.70, 7.80] | 3.00 [1.40, 7.10] | 0.903 |
| Lactate (mmol/L) | Group 2 | 2.05 [2.00, 5.40] | 1.60 [0.80, 5.90] | 1.50 [0.70, 5.40] | 1.20 [0.80, 4.90] | 0.065 |
| Lactate (mmol/L) | Group 3 | 2.70 [2.50, 5.60] | 2.60 [1.00, 7.70] | 2.20 [0.90, 7.80] | 1.80 [0.90, 3.80] | 0.0212 |
Heart rate, mean arterial pressure, systemic vascular resistance index, and lactate values at T0, T1, T2, and T3.
HR, MAP, Svri, and Lactate values at T0, T1, T2, and T3: values are expressed in median and interquartile ranges and statistical significance (P-Value); The statistically significant P-Value are reported in bold; Group 1= Norepinephrine Group; Group 2= Initial Synergism Group (T0) Argipressin/Norepinephrine; Group 3= Argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25 ≥ mcg/kg/min) to stabilise the hemodynamics (MAP > 65 mmHg).
HR = Heart Rate; MAP = Mean Arterial Pressure; Svri = Systemic Vascular Resistance Index.
The MAP was similar in all three groups. A statistically significant increase in MAP was observed in each group at different time points.
In Group 3, the lactate levels were significantly reduced at different time points after treatment. (Table 2; Figure 1). No significant differences in lactate levels were seen s in Groups 1 and 2.
Figure 1

Variations in lactate levels in the three groups at different timings expressed as a median. Ne = Norepinephrine Group; NVtO = Initial Synergism Group (TO) Argipressin/Norepinephrine; NVtl = Argipressin/Norepinephrine synergism group due to failure to optimize Mean Arterial Pressure (>65mrnHg) for Norepinephrine dosages <0.25mcg/k:g/min.
Data on the RIs are summarized in Table 3.
Table 3
| Group 1 | Group 2 | Group 3 | P-value | |
|---|---|---|---|---|
| RI Radial Artery Left -anatomical snuffbox- | 0.99 (0.96, 1.20) | 1.06 (0.94, 1.29) | 1.15 (1.10, 1.22) | 0.698 |
| RI Radial Artery Right -anatomical snuffbox- | 0.96 (0.93, 1.00) | 0.97 (0.95, 1.25) | 1.10 (0.96, 1.20) | 0.46 |
| RI Central Retinal Artery Left | 0.94 (0.85, 1.01) | 0.91 (0.79,0.94) | 0.96 (0.85, 1.01) | 0.592 |
| RI Central Retinal Artery Right | 0.90 (0.71, 0.97) | 0.90 (0.80, 0.93) | 0.90 (0.83, 0.99) | 0.715 |
| RI Renal Left | 0.74 (0.72, 0.87) | 0.79 (0.72, 0,90) | 0.77 (0.74,0.90) | 0.77 |
| RI Renal Right | 0.78 (0.72, 0.88) | 0.85 (0.75, 0.89) | 0.82 (0.73, 0.86) | 0.755 |
| RI superior mesenteric Artery | 0.90 (0.78, 0.96) | 0.84 (0.78, 0.88) | 0.86 (0.78, 0.90) | 0.505 |
Resistance indices at T0.
Resistance indices at T0: values are expressed in median and interquartile ranges; Group 1 = Norepinephrine Group; Group 2= Initial Synergism Group (T0) Argipressin/Norepinephrine; Group 3= Argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25≥ mcg/kg/min) to stabilize the hemodynamics (MAP >65 mmHg).; MAP = Mean Arterial Pressure.
There are no significant differences in musculoskeletal circulation as assessed by the right and left radial artery at T0.
The right radial artery RI was reduced in Groups 2 and 3 at timepoint T3, which was statistically significant (Table 4). No significant differences were seen in Group 1.
Table 4
| 1 h (median, interquartile range) | 24 h (median, interquartile range) | 48 h (median, interquartile range) | P-value | ||
|---|---|---|---|---|---|
| RI superior mesenteric Artery | Group 1 | 0.85 [0.76, 0.97] | 0.90 [0.72, 0.99] | 0.89 [0.78, 0.99] | 0.3529 |
| RI superior mesenteric Artery | Group 2 | 0.82 [0.64, 0.93] | 0.77 [0.62, 0.89] | 0.75 [0.59, 0.81] | 0 . 0002 |
| RI superior mesenteric Artery | Group 3 | 0.83 [0.64, 0.93] | 0.74 [0.68, 0.89] | 0.68 [0.64, 0.81] | <0 . 001 |
| RI Central Retinal Artery Right | Group 1 | 0.96 [0.72, 1.11] | 0.92 [0.66, 1.06] | 0.97 [0.79, 1.14] | 0 . 0384 |
| RI Central Retinal Artery Right | Group 2 | 0.87 [0.71, 0.97] | 0.79 [0.59, 0.92] | 0.79 [0.58, 0.87] | 0 . 0081 |
| RI Central Retinal Artery Right | Group 3 | 0.86 [0.74, 1.11] | 0.86 [0.55, 0.95] | 0.78 [0.66, 0.88] | 0 . 003 |
| RI Central Retinal Artery Left | Group 1 | 0.92 [0.76, 1.20] | 0.97 [0.63, 1.00] | 0.95 [0.82, 1.09] | 0.3381 |
| RI Central Retinal Artery Left | Group 2 | 0.81 [0.60, 0.97] | 0.78 [0.44, 0.91] | 0.76 [0.58, 0.87] | 0 . 0285 |
| RI Central Retinal Artery Left | Group 3 | 0.90 [0.75, 1.20] | 0.87 [0.58, 0.98] | 0.79 [0.61, 0.88] | 0 . 0025 |
| RI Renal Right | Group 1 | 0.93 [0.76, 1.08] | 0.89 [0.69, 1.11] | 0.93 [0.72, 0.99] | 0.0918 |
| RI Renal Right | Group 2 | 0.78 [0.60, 0.95] | 0.69 [0.61, 1.11] | 0.73 [0.62, 0.86] | 0.1261 |
| RI Renal Right | Group 3 | 0.73 [0.60, 0.95] | 0.69 [0.61, 0.87] | 0.70 [0.62, 0.82] | 0 . 0074 |
| RI Renal Left | Group 1 | 0.89 [0.79, 1.11] | 0.89 [0.69, 1.17] | 0.96 [0.82, 1.17] | 0 . 0062 |
| RI Renal Left | Group 2 | 0.86 [0.63, 0.89] | 0.69 [0.61, 1.17] | 0.72 [0.59, 0.83] | 0 . 0211 |
| RI Renal Left | Group 3 | 0.78 [0.60, 1.00] | 0.72 [0.57, 0.85] | 0.67 [0.61, 0.85] | 0 . 0016 |
| RI Radial Artery Right | Group 1 | 1.09 [0.94, 1.20] | 1.09 [0.79, 1.19] | 1.10 [0.89, 1.21] | 0.4808 |
| RI Radial Artery Right | Group 2 | 1.05 [0.84, 1.29] | 1.02 [0.78, 1.23] | 1.01 [0.64, 1.22] | 0 . 0465 |
| RI Radial Artery Right | Group 3 | 1.10 [0.89, 1.27] | 0.84 [0.65, 1.00] | 0.87 [0.64, 1.22] | <0 . 001 |
| RI Radial Artery Left | Group 1 | 1.14 [0.87, 1.25] | 1.08 [0.89, 1.32] | 1.11 [0.87, 1.24] | 0.8975 |
| RI Radial Artery Left | Group 2 | 1.11 [0.81, 1.23] | 1.04 [0.62, 1.22] | 1.10 [0.87, 1.26] | 0.8011 |
| RI Radial Artery Left | Group 3 | 0.93 [0.69, 1.17] | 0.86 [0.61, 1.11] | 1.09 [0.87, 1.24] | 0.6983 |
Resistance indices variations at T1, T2, and T3.
Resistance indices variations at T1, T2, and T3: values are expressed in median and interquartile ranges and statistical significance (P-value). The statistically significant P-Value are reported in bold; group 1= norepinephrine group; group 2= initial synergism group (T0) argipressin/norepinephrine; group 3= argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25≥ mcg/kg/min) to stabilize the hemodynamics (MAP > 65 mmHg).
There were no significant differences in the intracranial circulation (as bilaterally assessed by the central retinal artery) in the three Groups at timepoint T0 (Table 3). In the measurements taken for both the right and left side CRAs, there were significant reductions in the RIs in Groups 2 and 3. In Group 1, there was a significant increase in the RI for the right side CRA (Table 4). No significant differences were seen in renal circulation (assessed bilaterally at the level of the interlobar renal artery) in the three Groups at timepoint T0 (Table 3). In Groups 2 and 3, the RIs for the left side ARE at time point T3 were significantly reduced. In Group 1, the RI for the left side ARE significantly increased. In Group 3, the RI for the right side ARE significantly reduced (Table 4). No significant differences were seen in the RI for the right side ARE in Groups 1 and 2.
No differences were seen in the splanchnic circulation (assessed by the superior mesenteric artery) in the three Groups at time point T0. In Groups 2 and 3, there was a statistically significant reduction of RI. No significant differences were seen in Group 1 (Table 4).
The norepinephrine drug dosage was significantly reduced over time in Groups 2 and 3 but significantly increased in Group 1 (Table 5).
Table 5
| 0 h (median, interquartile range) | 1 h | 24 h (median, interquartile range) | 48 h (median, interquartile range) | P-value | ||
|---|---|---|---|---|---|---|
| NORA DOSE | Group 1 | 0.18 [0.15, 0.25] | 0.28 [0.10, 0.45] | 0.30 [0.12, 0.50] | <0 . 001 | |
| NORA DOSE | Group 2 | 0.15 [0.10, 0.25] | 0.11 [0.03, 0.30] | 0.10 [0.03, 0.25] | <0 . 001 | |
| NORA DOSE | Group 3 | 0.18 [0.13, 0.25] | 0.20 [0.10, 0.25] | 0.15 [0.10, 0.25] | <0 . 001 |
Variations of norepinephrine dosage at T0, T1, T2, and T3.
Variations of Norepinephrine Dosage at T0, T1, T2, and T3: values are expressed in median and interquartile ranges and statistical significance (P-Value). The statistically significant P-Value are reported in bold. NORA DOSE = Norepinephrine Dosage; group 1 = Norepinephrine Group; group 2= Initial Synergism Group (T0) Argipressin/Norepinephrine; group 3= Argipressin (0.03 IU/min) after norepinephrine (<0.10–0.25≥ mcg/kg/min) to stabilize the hemodynamics (MAP > 65 mmHg).
A significant reduction in the SOFA SCORE was seen in Groups 2 and 3 compared to the Ne Group. No significant differences were seen in the SOFA SCORE in Group 1 (Figure 2).
Figure 2
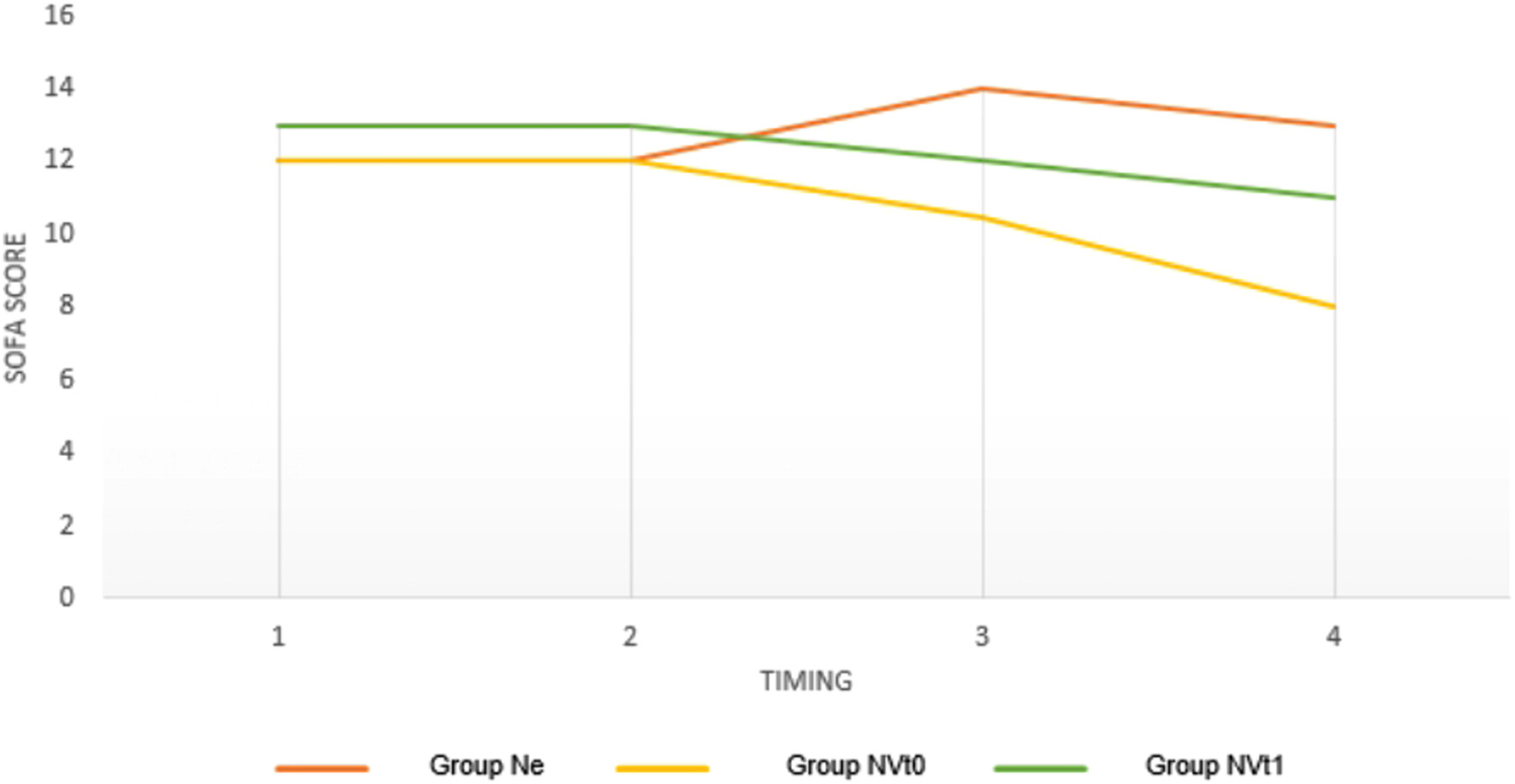
Variations in SOFA SCOREs in the three groups at different timings expressed as a median. Ne = Norepinepluine Group; NVtO = Initial Synergism Group (TO) Argipressin/Norepinephrine; NVtl = Argipressin/Norepineplu·ine synergism group due to failure to optimize Mean Alterial Pressure (>65mmHg) for Norepinephrine dosages <0.25mcg/kg/min.
3.1. Correlations between the parameters recorded in the study
The correlations between the dosage of norepinephrine and the polydistrectual multi-district RIs at different time points are presented in Figure 3.
Figure 3

The graph shows the coITelations between all the vatiables analyzed in the study at O hours (A), 24 hours (B) and 48 hours (C). The intensity of Red indicates Inverse Conelations, while the intensity of Blue indicates Direct CoITelations. It is also possible to highlight how the conelation turns out to be all the stronger the greater the diameter of the circumferences. What is highlighted in the Graph is a direct coITelation between the dose of norepinephrine and the Index of Resistance, i.e. for each increase in the dosage of Norepinephrine there is an increase in the Index of Resistance in the various districts. This coITelation is greater at 48 hours. The hypothesis that can be made to explain this is related to the mechanism of “Receptor Desensitization”. Receptor Desensitization means that the dosage ofnorepinephrine must be increased over time to produce the desired effect (Mean A1tetial Pressure >65mmHg). This increase therefore dete1mines, in addition to the desired effect, also an increase in side effects such as tachyphylaxis, vasoconsttiction and therefore the increase of the Resistance Indices. AMS RI= Resistance Index of the Superior Mesenteric Atte1y CRA RI right/left= Right and left Central Retinal A1tery Resistance Index; ARE RI right/left = Right and Left Renal Arte1y Resistance Index; AR RI right/left = Resistance Index Right and left radial arte1y to the anatomical snuff box; HR = Hea1t Rate; MAP = Mean A1terial Pressure; Svri = Systemic Vascular Resistance Index; SAP = Systemic A1terial Pressure; DAP = Diastolic a1te1ial pressure; SOFA (Sequential Organ failure Assessment) SCORE; PCT = Procalcitonin; C1t = Creatinine; NORA DOSE = norepinephrine dosage.
The correlation appears to be stronger at 48 h.
4. Discussion
This retrospective observational study evaluates the safety of early administration of argipressin drug association used in the treatment of septic shock.
The drug argipressin is not the first choice for treatment because it could cause an increase in splanchnic resistance.
Resistance Index is an important ultrasound parameter that evaluates the multi-district vascular tone and multiorgan Perfusion.
Studies comparing the Doppler ultrasound technique with standard techniques for measuring SVRI (such as transpulmonary thermodilution) have reported accurate measurements taken by Doppler ultrasound. Moreover, the Doppler ultrasound technique is low in cost and reproducible (36, 37). Previous studies have been conducted using a small number of samples and usually measured a single landmark or vascular district (53). It is known that the specific vasopressor receptors are distributed all along the vascular system. Hence, the hemodynamic disturbances after shock are not uniform along the whole body. Therefore, vascular RI measurement at a single site is not representative of systemic vascular resistance in patients with distributive shock. Hence, we measured the response to shock in several regions of the body including the intracranial circulation, the musculoskeletal circulation, the splanchnic circulation, and the renal circulation.
Our results showed an improvement in the pressure parameters (MAP) in all three groups. This increase was confirmed by hemodynamic monitoring using Systemic Vascular Resistances Indices over 48 h. However, the lactate levels in the three groups varied over 48 h. In particular, there was an increase in the lactate levels in the group given norepinephrine only, whereas the other two groups had a decrease in lactate levels (Table 2). Lactates are an important biomarker of tissue hypoxia and dysfunction but are not a direct measure of perfusion. A recent retrospective study by Sacha et al. (20) demonstrated a correlation between high doses of norepinephrine, lactate levels, and mortality in patients with septic shock.
In our study, the RI was used to evaluate other organ perfusion parameters. The RIs varied across the three groups in our study (Table 4).
We analyzed the perfusion in the splanchnic region, which has a higher incidence of mesenteric ischemia after infusion therapy with argipressin. Our study showed a significant reduction in RI in the two groups that used argipressin compared to the group that used norepinephrine alone. There were no differences between Groups 2 and 3.
In the musculoskeletal district, there was a significant reduction in the right radial artery RIs in the two groups that used argipressin compared to the norepinephrine group. This reduction was not significant when the two groups that used argipressin were compared.
In the renal district, there was a significant reduction in the RIs in the two groups that used argipressin compared to the norepinephrine group. There were no differences in Groups 2 and 3.
Finally, in the intracranial district, there was a significant reduction in the RIs in the two groups that used argipressin compared to the norepinephrine group. This reduction was not significant when the two groups that received argipressin were compared.
These findings suggested that the use of argipressin reduced resistance and improved multi-organ perfusion.
This better multi-organ perfusion could be explained by “decatecholaminization”, i.e., a catecholamine-sparing strategy associated with the early effect of norepinephrine-argipressin treatment.
Reducing norepinephrine dosages decreased the activation of α1 receptors. The α1-agonist action, in fact, increases pulmonary vascular resistance, increases cardiac work, and causes myocardial ischemia and severe hypertension (51). Systemic vasoconstriction can impair mesenteric perfusion resulting in organ dysfunction and metabolic acidosis.
It is essential to observe the trend of the norepinephrine dosage in the 48 h in the three groups. In the norepinephrine group, there is a highly significant increase in norepinephrine dosage (p-value < .0001) while in the two argipressin groups, there is a highly significant reduction (p-value < .0001) (Table 5).
The direct correlation between the dosages of norepinephrine and the multi-district RIs at 0 h, 24 h, and 48 h is presented in Figure 3.
This correlation appears to be greater at 48 h. This correlation can be explained by the mechanism of “receptor desensitization”.
It has been established that desensitization of norepinephrine receptors can occur through phosphorylation and internalization of α1-receptors (49–52). It is therefore essential to increase the dosage of norepinephrine over time to produce the desired effect (MAP > 65 mmHg). This increase can be associated with adverse effects such as tachyphylaxis and vasoconstriction with an increase in the RIs. In such scenarios, therapeutic agents with different pharmacodynamics should be used.
We can attribute the effect of reducing the RIs in the argipressin groups to the argipressin agonism on the V2 receptors of the kidney. V2 receptors act through the retention of free water and the release of von Willenbrand factor, factor VIII, and tissue plasminogen activator from endothelial cells (54–56). The selective V2 agonism exerted by argipressin causes vasodilation through the activation of endothelial NOS via c-AMP. Argipressin may therefore have vasodilatory effects in some vascular beds, including the pulmonary arterial system, probably through the activation of oxytocin receptors.
From the results of this study, it is not possible to say which of the two groups with argipressin is better at reducing the vasoconstrictive effects. There were no significant differences in the measurements in the groups that started the norepinephrine-argipressin combination treatment from the beginning and in the group in which the infusion was started for norepinephrine dosages less than 0.25 mcg/kg/min. The small sample size limits the results obtained, and further studies are needed.
Furthermore, the improvement of Multiorgan Perfusion can also be highlighted by the Correlation Graph (Figure 3) in terms of improvement of the SOFA SCORE. In particular, a direct correlation can be observed between dosages of norepinephrine, Ris, and SOFA SCORE.
It is likely that an early administration of argipressin is associated with a clinical prognostic improvement due to endocrine replacement. Patients with septic shock initially have high argipressin levels, which decrease over the subsequent 48 h (7–12). The administration of exogenous argipressin restores the physiological plasma concentrations, and this can improve the outcome and organ perfusion.
It is possible that a high SOFA SCORE value in the norepinephrine group is associated with the immunological effects of norepinephrine. Norepinephrine suppresses the synthesis of proinflammatory cytokines, such as TNFα and IL-1β, and increases the production of anti-inflammatory cytokines, such as IL-10, in a dose-dependent manner. This could explain the immunological impairment of the patient treated with high-dose norepinephrine and in general its worse outcome. Argipressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock (13;16;18) (Figures 4, 5).
Figure 4

Intracellular mechanisms behind the effect of a and ∼-AR activation on cytokine production. (A) Stimulation of the a-AR results in activation of PKC, which induces IKB phospho1ylation, and traslocation of NF-KB to the nucleus. NF-KB facilitates proinflammatory cytokine transcription. (B) Stimulation of the ∼-AR increases intracellular cAMP levels, which activate PKA. PKA prevents NF-KB from entering the nucleus, resulting in reduced proinflammatory cytokine transcription and production and increased production of antinflammatory IL-10. AR = adrenoceptor; IKB = inhibitor nuclear factor; NF-KB = nuclear factor K - lightchai-enhancer of activated B cell; PKA = Protein kinase A; PKC = Protein Kinase C; TNF = Tumor Necrosis factor.
Figure 5
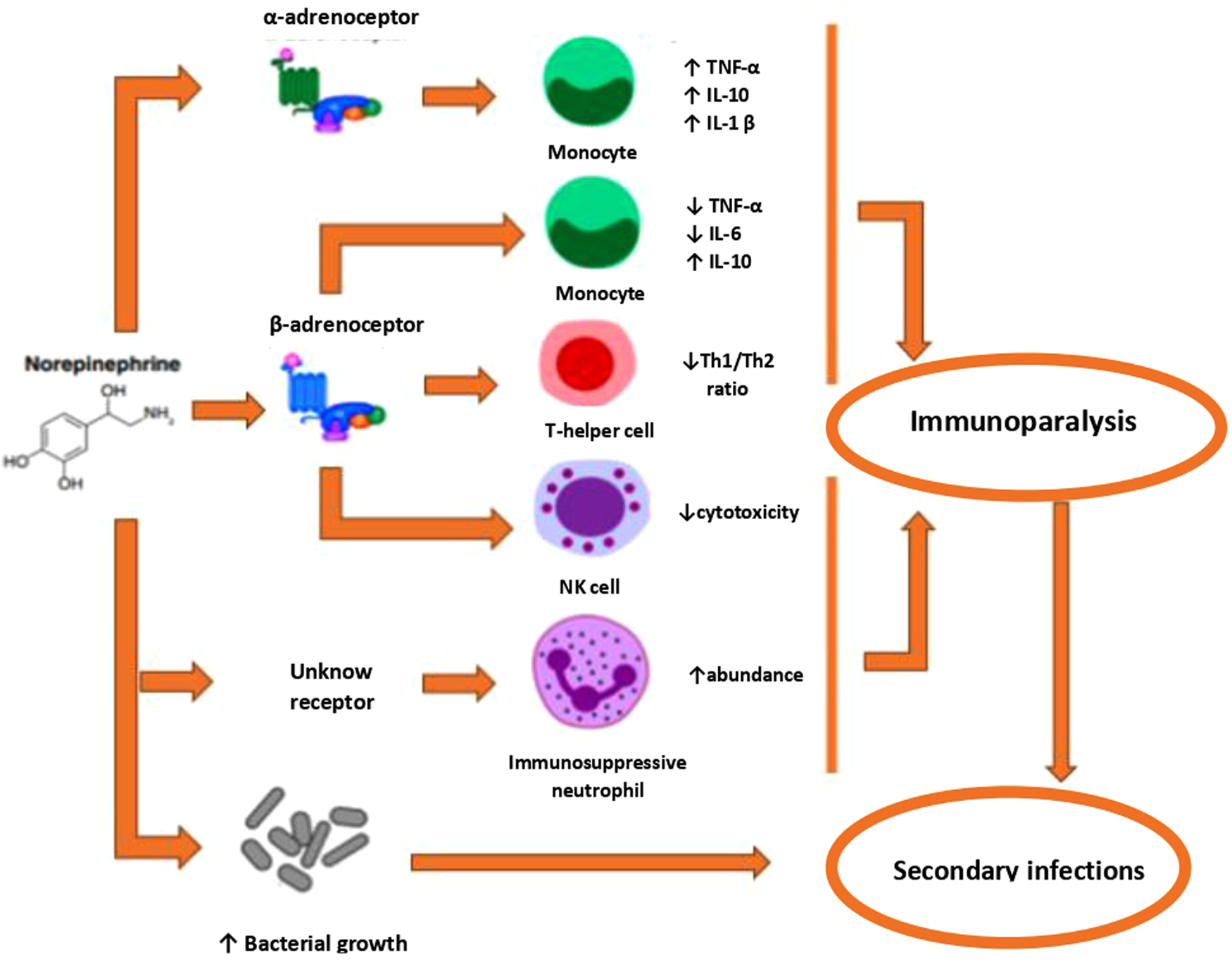
Mechanisms by which norepinephrine may contribute to sepsis-induced immunoparalysis. Activation of the a-AR has been associated with both pro- and anti-inflammatory effects. Activation of ∼-AR exe11s anti-inflammatory effects Fm1hermore, norep1ineph1ine induces the generation of immunosuppressive neutrophils and directly promotes bacterial growth. AR = adrenoreceptor; NK = Natmal Killer; Th = T-helper; TNF = tumor necrosis factor.
Our study also showed a statistically significant reduction in Heart Rate in Group 2. This could be due to the lower dosages of norepinephrine whose effects on arrhythmias are well known (57–59).
4.1. Limitations
The retrospective nature of the study represents a limitation, and this should be taken into consideration when interpreting our preliminary results.
As for the retrospective nature of the study, we had the chance to balance the number of patients in each group. The sample size uniformity among groups adds an important limitation.
Another limitation is the resistive index evaluations, as this requires a training period and is not a procedure that can always be replicated.
5. Conclusions
In light of the obtained results, we can hypothesize that the use of the early norepinephrine-argipressin association treatment could be a valid strategy for hemodynamic optimization in this patient setting. Our results suggest an efficacy and safety profile of the early argipressin-norephynephrine association. Considering the already discussed limitations of our study, further and more complete studies will serve to confirm (or not) the results of our observation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The San Carlo Hospital Ethics Committee, Potenza, Italy, (63/2023 num 20230038704). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because a retrospective observational study was conducted. The study did not involve medical, pharmacological, or behavioral interventions in addition to hospital standards of care.
Author contributions
AB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. BM: Data curation, Investigation, Supervision, Visualization, Writing – review & editing. AI: Visualization, Writing – review & editing. LM: Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GC: Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GM: Visualization, Writing – review & editing. AR: Visualization, Writing – review & editing. MT: Visualization, Writing – review & editing. AM: Visualization, Writing – review & editing. MC: Data curation, Formal Analysis, Investigation, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LR: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MD’A: Validation, Visualization, Writing – review & editing. AD: Supervision, Validation, Visualization, Writing – review & editing. PI: Validation, Visualization, Writing – review & editing. AG: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Rhodes A Evans LE Alhazzani W Levy MM Antonelli M Coopersmith C et al Gsurviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47(11):1181–247. 10.1007/s00134-021-06506-y
2.
Liu M Wang G Wang L Wang Y Bian Y Shi H . Immunoregulatory functions of mature CD10+ and immature CD10− neutrophils in sepsis patients. Front Med (Lausanne). (2023) 9:1100756. 10.3389/fmed.2022.1100756.eCollection 2022.
3.
Hu B Ji W Bo L Bian J . How to improve the care of septic patients following “surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021”. J Intensive Med. (2022) 3(2):144–6. 10.1016/j.jointm.2022.08.001
4.
Hiemstra B Eck RJ Keus F van der Horst IC . Clinical examination for diagnosing circulatory shock. Curr Opin Crit Care. (2017) 23(4):293. 10.1097/MCC.0000000000000420
5.
Andreis DT Singer M . Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. (2016) 42(9):1387–97. 10.1007/s00134-016-4249-z
6.
Landry DW Oliver JA . The pathogenesis of vasodilatory shock. N Engl J Med. (2001) 345:588–95. 10.1056/NEJMra002709
7.
Holmes CL Patel BM Russell JA Wal ley KR . Physiology of vasopressin relevant to management of septic shock. Chest. (2001) 120:989–1002. 10.1378/chest.120.3.989
8.
Reid IA . Role of vasopressin deficiency in the vasodilation of septic shock. Circulation. (1997) 95(5):1108–10. 10.1161/01.CIR.95.5.1108
9.
Sharshar T Carlier R Blanchard A Feydy A Gray F Paillard M et al Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med. (2002) 30(3):497–500. 10.1097/00003246-200203000-00001
10.
Barrett LK Singer M Clapp LH . Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. (2007) 35(1):33–40. 10.1097/01.CCM.0000251127.45385.CD
11.
Sharshar T Carlier R Blanchard A Feydy A Gray F Paillard M et al Circulating vasopressin levels in septic shock. Crit Care Med. (2003) 31(6):1752–8. 10.1097/01.CCM.0000063046.82359.4A
12.
Giusti-Paiva A Santiago MB . Neurohypophyseal dysfunction during septic shock. Endocr Metab Immune Disord Drug Targets. (2010) 10(3):247–51. 10.2174/187153010791936838
13.
Boyd JH Holmes CL Wang Y Roberts H Walley KR . Vasopressin decreases sepsis-induced pulmonary inflammation through the V2R. Resuscitation. (2008) 79(2):325–31. 10.1016/j.resuscitation.2008.07.006
14.
Russell JA Walley KR . Vasopressin and its immune effects in septic shock. J Innate Immun. (2010) 2(5):446–60. 10.1159/000318531
15.
Höcherl K Schmidt C Kurt B Bucher M . Inhibition of NF-κB ameliorates sepsis-induced downregulation of aquaporin-2/V 2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol. (2010) 298(1):F196–204. 10.1152/ajprenal.90607.2008
16.
Zhao L Brinton RD . Suppression of proinflammatory cytokines interleukin-1β and tumor necrosis factor-α in astrocytes by a V1 vasopressin receptor agonist: a cAMP response element-binding protein-dependent mechanism. J Neurosci. (2004) 24(9):2226–35. 10.1523/JNEUROSCI.4922-03.2004
17.
Stolk RF van der Pasch E Naumann F Schouwstra J Bressers S Van Herwaarden A et al Norepinephrine dysregulates the immune response and compromises host defense during sepsis. Am J Respir Crit Care Med. (2020) 202(6):830–42. 10.1164/rccm.202002-0339OC
18.
Stolk RF van der Poll T Angus DC van der Hoeven JG Pickkers P Kox M . Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med. (2016) 194(5):550–8. 10.1164/rccm.201604-0862CP
19.
Russell JA Fjell C Hsu JL Lee T Boyd J Thair S et al Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. Am J Respir Crit Care Med. (2013) 188(3):356–64. 10.1164/rccm.201302-0355OC
20.
Landry DW Levin HR Gallant EM Ashton RC Jr Seo S D'Alessandro D et al Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. (1997) 95(5):1122–5. 10.1161/01.cir.95.5.1122
21.
Sacha GL Lam SW Wang L Duggal A Reddy AJ Bauer SR . Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med. (2022) 50(4):614–23. 10.1097/CCM.0000000000005317
22.
Wieruszewski PM . KhannaAK. Early multimodal vasopressors-are we ready for it?Crit Care Med. (2022) 50(4):705–8. 10.1097/CCM.0000000000005344
23.
Bauer SR Sacha GL Siuba MT Lam SW Reddy AJ Duggal A . VachharajaniV. Association of arterial pH with hemodynamic response to vasopressin in patients with septic shock: an observational cohort study. Crit Care Explor. (2022) 4(2):e0634. 10.1097/CCE.0000000000000634.
24.
Bauer SR Sacha GL Siuba MT Wang L Wang X Scheraga RG et al Vasopressin response and clinical trajectory in septic shock patients. J Intensive Care Med. (2023) 38(3):273–9. 10.1177/08850666221118282
25.
Xu J Cai H Zheng X . Timing of vasopressin initiation and mortality in patients with septic shock: analysis of the MIMIC-III and MIMIC-IV databases. BMC Infect Dis. (2023) 23(1):199. 10.1186/s12879-023-08147-6
26.
Yerke JR Sacha GL Scheraga RG Culver DA Abraham S Torbic H et al Vasopressin plasma concentrations are not associated with hemodynamic response to exogenous vasopressin for septic shock. Pharmacotherapy. (2020) 40(1):33–9. 10.1002/phar.2346
27.
Patel BM Chittock DR Russell JA Walley KR . Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. (2002) 96:576–82. 10.1097/00000542-200203000-00011
28.
Landry DW Levin HR Gallant EM Seo S D'Alessandro D Oz MC et al Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. (1997) 25:1279–82. 10.1097/00003246-199708000-00012
29.
Malay MB Ashton RC Jr Landry DW Townsend RN . Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma. (1999) 47:699–70310.1097/00005373-199910000-00014
30.
Dunser MW Mayr AJ Ulmer H Knotzer H Sumann G Pajk W et al Arginine vasopressin in advanced vasodi latory shock: a prospective, randomized, controlled study. Circulation. (2003) 107:2313–9. 10.1161/01.CIR.0000066692.71008.BB
31.
Luckner G Dünser MW Jochberger S Mayr VD Wenzel V Ulmer H et al Arginine vasopressin in 316 patients with advanced vasodilatory shock. Crit Care Med. (2005) 33:2659–66. 10.1097/01.CCM.0000186749.34028.40
32.
Baxter GM Williamson TH . Color Doppler imaging of the eye: normal ranges, reproducibility, and observer variation. J Ultrasound Med. (1995) 14(2):91–6. 10.7863/jum.1995.14.2.91
33.
Orihashi K Mtsuura Y Sueda T . Clinical implication of orbital ultrasound monitoring during selective cerebral perfusion. Ann Thorac Surg. (2001) 71:673–7. 10.1016/S0003-4975(00)02162-7
34.
Arai T Numata K Tanaka K Kiba T Kawasaki S Saito T et al Ocular arterial flow hemodynamics in patients with diabetes mellitus. J Ultrasound Med. (1998) 17(11):675–81. 10.7863/jum.1998.17.11.675
35.
Querfurth HW Arms SW Lichy CM Irwin WT Steiner T . Prediction of intracranial pressure from noninvasive transocular venous and arterial hemodynamic measurements: a pilot study. Neurocrit Care. (2004) 1(2):183–94. 10.1385/NCC:1:2:183
36.
Lee EP Hsia SH Huang CC Kao K-C Chan O-W Lin C-Y et al Strong correlation between Doppler snuffbox resistive index and systemic vascular resistance in septic patients. J Crit Care. (2019) 49:45–9. 10.1016/j.jcrc.2018.10.010
37.
Ban K Kochi K Imai K Okada K Orihashi K Sueda T . Novel Doppler technique to assess systemic vascular resistance: the snuffbox technique. Circ J. (2005) 69(6):688–94. 10.1253/circj.69.688
38.
Kochi K Sueda T Orihashi K Matsuura Y . New noninvasive test alternative to Allen’s test: snuff box technique. J Thorac Cardiovasc Surg. (1999) 118(4):756–8. 10.1016/S00225223(99)70028-0
39.
Keogan MT Kliewer MA Hertzberg BS DeLong DM Tupler RH Carrollet BA . Renal resistive indexes: var ability in Doppler US measurement in a healthy population. Radiology. (1996) 199(1):165–9. 10.1148/radiology.199.1.8633141
40.
Norris CS Barnes RW . Renal artery flow velocity analysis: a sensitive measure of experimental and clinical renovascular resistance. J Surg Res. (1984) 36(3):230–6. 10.1016/0022-4804(84)90092-1
41.
Lerolle N Guérot E Faisy C Bornstain C Diehl JL Fagon JY . Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med. (2006) 32(10):1553–9. 10.1007/s00134-006-0360-x
42.
Darmon M Schortgen F Vargas F Liazydi A Schlemmer B Brun-Buissonet C et al Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. (2011) 37(1):68–76. 10.1007/s00134-010-2050-y
43.
Deruddre S Cheisson G Mazoit JX Vicaut E Benhamou D Duranteau J . Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. (2007) 33:1557–62. 10.1007/s00134-007-0665-4
44.
Schnell D Camous L Guyomarc’h S Duranteau J Canet E Gery P et al Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med. (2013) 41:1214–20. 29. 10.1097/CCM.0b013e31827c0a36
45.
Ninet S Schnell D Dewitte A Zeni F Meziani F Darmon M . Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and meta-analysis. J Crit Care. (2015) 30:629–35. 10.1016/j.jcrc.2015.02.008
46.
Moussa MD Scolletta S Fagnoul D Pasquier P Brasseur A Taccone FS et al Effects of fluid administration on renal perfusion in critically ill patients. Crit Care. (2015) 19:250. 10.1186/s13054-015-0963-0
47.
Giovagnorio F . L'eco Doppler dell'arteria mesenterica superiore nelle infiammazioni croniche intestinali [Doppler ultrasonography of the upper mesenteric artery in chronic intestinalinflammation]. Radiol Med. (1999) 98(1–2):43–7.
48.
Taourel P Perney P Dauzat M Gallix B Pradel J Blanc F et al Doppler study of fasting and postprandial resistance indices in the superior mesenteric artery in healthy subjects and patients with cirrhosis. J Clin Ultrasound. (1998) 26(3):131–6. 10.1002/(SICI)1097-0096(199803/04)26:3%3C131::AID-JCU4%3E3.0.CO;2-N
49.
García-Sáinz JA Vázquez-Prado J Del Carmen Medina L . Alpha 1-adrenoceptors: function and phosphorylation. Eur J Pharmacol. (2000) 389(1):1–12. 10.1016/s0014-2999(99)00896-1
50.
Rudner XL Berkowitz DE Booth JV Funk BL Cozart KL D'Amico EB et al Subtype specifc regulation of human vascular alpha (1)-adrenergic receptors by vessel bed and age. Circulation. (1999) 100:2336–43. 10.1161/01.CIR.100.23.2336
51.
Akinaga J Lima V Kiguti LR Hebeler-Barbosa F Alcántara-Hernández R García-Sáinz JA et al Differential phosphorylation, desensitization, and internalization of α1A-adrenoceptors activated by norepinephrine and oxymetazoline. Mol Pharmacol. (2013) 83:870–81. 10.1124/mol.112.082313
52.
Perez-Aso M Segura V Montó F Barettino D Noguera MA Milligan G et al The three α1-adrenoceptor subtypes show different spatio-temporal mechanisms of internalization and ERK1/2 phosphorylation. Biochim Biophys Acta. (2013) 1833(10):2322–33. 10.1016/j.bbamcr.2013.06.013
53.
Chow JH Abuelkasem E Sankova S Henderson RA Mazzeffi MA Tanaka KA . Reversal of vasodilatory shock: current perspectives on conventional, rescue, and emerging vasoactive agents for the treatment of shock. Anesth Analg. (2020) 130(1):15–30. 10.1213/ANE.0000000000004343
54.
Kaufmann JE Iezzi M Vischer UM . Desmopressin (DDAVP) induces NO production in human endothelial cells via V2 receptor- and cAMP-mediated signaling. J Thromb Haemost. (2003) 1:821–8. 10.1046/j.1538-7836.2003.00197.x
55.
Juul KV Bichet DG Nielsen S Nørgaard JP . The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol. (2014) 306(9):F931–40. 10.1152/ajprenal.00604.2013
56.
Hew-Butler T Hummel J Rider BC Verbalis JG . Characterization of the effects of the vasopressin V2 receptor on sweating, fluid balance, and performance during exercise. Am J Physiol Regul Integr Comp Physiol. (2014) 307(4):R366–75. 10.1152/ajpregu.00120.2014
57.
Lamontagne F Richards-Belle A Thomas K Harrison DA Mouncey PR ; 65 Trial Investigators. Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA. (2020) 323(10):938–49. 10.1001/jama.2020.0930
58.
Hylands M Moller MH Asfar P Lamontagne F Toma A Frenette AJ et al A systematic review of vasopressor blood pressure targets in critically ill adults with hypotension. Can J Anaesth. (2017) 64(7):703–15. 10.1007/s12630-017-0877-1
59.
Lamontagne F Meade MO Hébert PC Asfar P Heyland DR Seely AJ et al Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med. (2016) 42(4):542–50. 10.1007/s00134-016-4237-3
Summary
Keywords
septic shock, association, argipressin, decatecholaminization, resistance index, echodynamic monitoring
Citation
Barile A, Mazzotta B, Izzi A, Mirabella L, Cinnella G, Paternoster G, Mincolelli G, Recchia A, Tonti MP, Manuali A, Copetti M, Restivo L, D’Amora M, Di Fazio A, Innelli P and Del Gaudio A (2023) Argipressin-norepinephrine association in the treatment of septic shock: the use of the polydistrectual resistance index as an assessment of vascular compliance. Front. Anesthesiol. 2:1322825. doi: 10.3389/fanes.2023.1322825
Received
16 October 2023
Accepted
27 November 2023
Published
15 December 2023
Volume
2 - 2023
Edited by
Vincenzo Pota, University of Campania Luigi Vanvitelli, Italy
Reviewed by
Antonio Pisano, Hospital of the Hills, Italy
Giuliana Scarpati, University of Salerno, Italy
Updates
Copyright
© 2023 Barile, Mazzotta, Izzi, Mirabella, Cinnella, Paternoster, Mincolelli, Recchia, Tonti, Manuali, Copetti, Restivo, D'Amora, Di Fazio, Innelli and Del Gaudio.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Alessio Barile a.barile@operapadrepio.it Gianluca Paternoster gianluca.paternoster@ospedalesancarlo.it
Abbreviations RI, resistance index; ARE, renal artery; AR, radial artery; CRA, central retinal artery; AMS, superior mesenteric artery; SNUFF, snuffbox; HR, heart rate; MAP, mean arterial pressure; CI, cardiac index; SVV, stroke volume variation; SVRI, systemic vascular resistance index; Ne, norepinephrine group; NVt0, initial synergism group (T0) argipressin/norepinephrine; NVt1, argipressin/norepinephrine synergism group due to failure to optimize MAP(>65 mmHg) for Norepinephrine dosages.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.