- 1Department of Anesthesiology and Perioperative Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 2University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 3Surgery Service Line Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
- 4Office of Research and Development Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
- 5Medicine Service Line Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
Background: For major abdominal surgery, we explored “usual” opioid-avoiding effects of spinal magnesium 25 mg added to intrathecal morphine (250+ µg in women, 300+ µg in men; ITM + Mg+). We evaluated associated benefits of several integrated care “bundles”: 5-drug antiemetic prophylaxis, multiple-day postoperative antiemetic boosters (perphenazine and aprepitant), boosters for non-opioid transitional analgesia, and strategically-avoided usual opioids intraoperatively (fentanyl, hydromorphone, etc.). We also explored antiemetic outcomes, and pruritus, on postoperative days 0–2. We hypothesized these bundles would independently and interactively influence associated outcomes.
Methods: We used a mixed-method framework to demonstrate whether these bundles, integrated with ITM + Mg+, were all associated with one or more milestones en route to improving described outcomes [prevented postoperative nausea/vomiting (PONV), avoided postoperative opioids, etc.]. We did so via retrospective, case-matched quality improvement methodology for a single-hospital population of ITM-receiving Veterans, applying multiple regression to determine (i) PONV prevention success on days 0–1 separately from day 2, (ii) success of avoiding usual opioids (by withholding discretionary fentanyl/hydromorphone intraoperatively en route to avoiding the need for patient-requested hydromorphone/oxycodone postoperatively), and (iii) predictors/signals of itching, including related to the ITM-upgrade to ITM + Mg+.
Results: ITM + Mg+, at the described doses, supported by 5-antiemetic prophylaxis and three-drug non-opioid transitional analgesics, was associated with significant opioid-avoiding improvements. Postoperative avoidance of usual opioids was associated with both ITM + Mg+ use and intraoperative and immediate postoperative avoidance of “usual opioids” (fentanyl, hydromorphone, etc.). PONV on days 0–1 (vs. day 2) appears to have differing predictor patterns, warranting both 5-MMAEPPx preoperatively, and future antiemetic upgrade from 2-drug booster prophylaxis (perphenazine/aprepitant) to also include palonosetron every 40 h. ITM historical control major abdominal surgery cases before ITM + Mg+ had a 14% “usual opioid avoidance rate” (35/246), which showed significant associated improvements with ITM + Mg+ use, usual opioid avoidance, and integration with the other described care bundles (34/60, 57%, P < 0.001).
Conclusion: Multiple “bundles” appear to address both sustained antiemetic success and “usual opioid avoidance.” ITM-related pruritus requires further study regarding prophylaxis and treatment, in order to allow ITM + Mg+ to achieve its full enhanced recovery potential, when trying to avoid postoperative exposure to usual opioids.
1 Introduction
This manuscript (a) addresses minimizing postoperative exposure by combining preservative-free magnesium sulfate (MgSO4, or simply Mg+; at a dose of 25 mg) to intrathecal morphine (ITM), and (b) follows our 2023 (1) and 2025 (2, 3) publications addressing postoperative nausea and vomiting (PONV) prevention with vs. without ITM, alongside an off-patent 5-drug Multimodal AntiEmetic ProPhylaxis (5-MMAEPPx) combination.
5-MMAEPPx has shown an associated ∼95% PONV prevention success on Postoperative Day (POD) zero, and ∼90% success on POD#1, without booster antiemetic dosing (1–3), but POD#2 PONV effects thereafter are unknown. 5-MMAEPPx was first (1–3) developed for our institution's intrathecal morphine (ITM) enhanced recovery protocol for major abdominal/truncal surgery under general anesthesia (GA) in early 2021. Our objectives now include extending further observations addressing whether our MMAEPPx/ITM successes could be further leveraged into both meaningful opioid-sparing and continued antiemetic benefits (e.g., POD#2).
Specifically, we presume that any “usual” opioid exposure intraoperatively may trigger new persistent opioid use (NPOU); we ask the necessary question of “how successful can we be in avoiding downstream opioids by primary prevention (of exposure) via strategic avoidance in the operating theater?” As a frame of reference, we previously reported (2) that ITM use with day-of-surgery (DOS) 5-MMAEPPx was associated with 17% success (10/58 cases) of averted need for any intravenous (IV) or per os (PO) opioids during/after bariatric surgery (on POD #0–1) in-hospital. Importantly, this 17% success in bariatrics (2) was observed when in-hospital postoperative follow-up (i.e., non-opioid analgesic and antiemetic boosters) was fully systematized in a comprehensive enhanced recovery protocol (2). We now aim to evaluate observations across other types of surgery (including bariatrics) regarding enhanced analgesic and antiemetic quality, via several interacting “bundles” in an enhanced recovery integrated care plan. First, (i) ITM would be supplemented by preservative-free magnesium sulfate (ITM + Mg+) in the same spinal (4–6) without local anesthetic, while (ii) strategically withholding all “usual” intraoperative opioids (fentanyl/hydromorphone, etc.). Next (iii), 5-MMAEPPx routine preoperative dosing would be continued, while adding (iv) MMAEPPx scheduled booster dosing after surgery through at least POD#2 (we tested two drugs, but recommend three for future work). The next care bundle, (v), provides 3-drug, non-opioid, pre- and postoperative analgesic boosters to create necessary transitional analgesia once the ITM + Mg+ analgesic dose effects dissipate. We consider these concepts (i–v) as separate but interacting care “bundles” informed by a “sixth bundle” care concept: (vi) that POD#2 AEPPx should incorporate any different risk factors that emerge for POD#2 separately from expected PONV risk factors on POD#0–1, and that pruritus should be tracked and addressed.
The aims are to quantify how (a) ITM + Mg+ (and the described enhanced recovery multimodal booster dosing, i.e., bundles [i] and [ii–v]) may yield better associated antiemetic and analgesic outcomes, than does plain ITM and single-dose 5-MMAEPPx, (b) the extent to which POD#0–1 vs. POD#2 have differing PONV-predictor patterns and pruritus issues when ITM + Mg+ is used (i.e., bundle [vi]), (c) ITM + Mg+ in tandem with pre-and post-operative non-opioid multimodals (both analgesics and antiemetics, bundles [iv, v]) may be associated with a meaningful, associated significant downstream opioid avoidance, higher than our reported 17% (2) success rate above with ITM-only, particularly when usual IV opioids (fentanyl, remifentanil, hydromorphone—bundle [ii]) are deliberately avoided in the operating room, and (d) avoiding after-OR IV opioids being achievable via better analgesia from ITM + Mg+, and associated avoidance of opioid-induced itching (bundle [vi], as opposed to considering pruritus simply as a “fact of life” side effect from ITM ± Mg+).
2 Materials and methods
2.1 Ethics approval
As in our prior (2) report, this was an IRB-approved quality improvement (QI) initiative, exempt from patient research consent above and beyond clinical surgery and anesthesia consent. Institutional approval (approval/exemption 1670098, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA, most recently on 19 February 2025) allowed for tracking and external reporting of QI outcome data. For this report, we focused specifically on GA patients undergoing ITM + Mg+, and ITM case-matched controls.
2.2 External reporting precedents and guidance; patient identification/selection, and data collation
As previously (2) reported, following STROBE guidelines, we disclosed 5-MMAEPPx as institutional standard of care for ITM recipients, soon after our group's earliest case series (7) was accepted for publication [addressing joint replacement surgery (7) under bupivacaine spinal including ITM]. The institutional standard of care status was further reinforced after a subsequent publication (1). Patients having received both ITM (±Mg+) and GA were then case-matched as follows. We retrospectively reviewed QI data from our single United States (US) Veterans Administration hospital. As with our previous publications (1, 2), case matching was by the lead author (BAW), described further below (Section 2.5). Consecutive ITM + Mg+ cases from 1 February 2024 through 9 October 2024 (n = 60) were matched with ITM (without Mg+) cases (n = 246) occurring no earlier than 9 March 2021. Of the n = 246, n = 154 were previously case-matched with GA-sans-ITM cases in a previous publication (1). The remaining 92 ITM cases (without Mg+) were (a) not previously matched to non-ITM cases (n = 71), or (b) specifically matched to ITM + Mg+ cases since February 2024, or previously reported (1, 2) ITM or non-ITM GA cases previously published (n = 21). Of the n = 60 ITM + Mg+ cases, n = 45 cases were matched with the n = 154 previously-reported (1) ITM cases (in ratios most commonly ranging from 1:1 to 1:4). The remaining 15 ITM + Mg+ cases were matched (in similar ratios) with previously unpublished n = 71 ITM cases. By the time that ITM + Mg+ was initiated in February 2024, the following enhanced recovery processes were already co-established as ITM-related local standards: (a) 2-drug antiemetic booster dosing (bundle [iv]), and (b) oral non-opioid analgesics entailing acetaminophen, a COX-2 inhibitor (usually celecoxib), and dextromethorphan (8) both pre- and post-operatively (bundle [v]).
2.3 Anesthesia and related enhanced-recovery procedures
2.3.1 Spinal/intrathecal analgesia: technical details
Our spinal needles were primarily pencil-point, 24–25G, selected for directionality of injectate flow, described by Urmey et al. (9), along with goals of avoiding post-dural puncture headache and/or diplopia (10). Preservative-free MgSO4 (25–50 mg) was assumed to render injectates hyperbaric. Sterile water was the diluent, rendering a likely hypobaric effect (without Mg+) when the usual 2–3 ml morphine-water volume was injected, so we anticipated ITM + Mg+ in 3 ml to be hyperbaric, possibly imparting inadequate cephalad spread to achieve desired dermatome-focused analgesic effect. Future study will be needed to ultimately determine actual baricity with varying water diluent volumes. No local anesthetic was included in these injectates. Lead author BAW, based on decades of clinical experience with spinals, selected ∼8 ml as the volume start-point for water + ITM + Mg+, with volume adjustments considered up or down if needed; no volume changes were deemed necessary after n = 60 recorded ITM + Mg+ injections (or since then). “High spinal” was of relatively little concern (i.e., cardio-accelerator fiber anesthesia at T1-T4, or respiratory center anesthesia at C2–4), in the absence of bupivacaine/local anesthetics.
2.3.2 Enhanced recovery: established process details
In the process roll-out for ITM + Mg+ (bundle [i]), we had already (contemporaneously) enhanced our preoperative (5-drug, bundle [iii]) and around-the-clock postoperative (2-drug, aprepitant and perphenazine “booster prophylaxis,” bundle [iv]) MMAEPPx strategy, in tandem with the described non-opioid analgesics (and their daily [or more frequent] booster doses, bundle [v]). Our 5-MMAEPPx plan was “supersized” from palonosetron 75–150 µg IV, after early, ITM-induced pruritus tendencies (and anecdotal cumbersomeness) were observed with 50 mg MgSO4. By this point, dexamethasone was already commonly “supersized” from 4 to 8 mg (IV), based on analgesic success with antiemetic doses approaching 0.1 mg/kg, reported elsewhere (11). We also directed focus to strategically avoiding “usual” intraoperative IV opioids (bundle [ii]) during and after surgery, in an effort to also avoid the need for “usual” PO opioids postoperatively, via IV opioid-induced acute hyperalgesia, particularly since ITM + Mg+ would have already been in place. In the process, we opted for not only pan-prophylaxis against PONV, but also deliberate avoidance of any exposure to “usual” opioids during (bundle [ii]) or after surgery, specifically fentanyl, remifentanil, hydromorphone, and oxycodone/hydrocodone/tramadol, all of which we consider high abuse-liability opioids (HALO). We do not consider ITM ± Mg+ as “HALO”, given no documented euphoria, tolerance, or hyperalgesia descriptions of such with single-injection ITM exposures (OVID Medline, last accessed 22 November 2024). All described interventions, regardless of a priori PONV risk factors, had already been actively implemented for consecutive cases in advance of February 2024, at the time of initiating ITM + Mg+ (instead of plain ITM). Spinal procedures occurred in a separate location outside the operating room, as previously described (12), and as was the case in our most recent report in this journal (2).
2.4 Setting and participants, study size, and bias
As with our previous reports (1, 2), patients whose data were reviewed were already-hospitalized Veteran inpatients presenting for surgery, or were known in advance of surgery to be admitted to the hospital after surgery. ITM + Mg+ cases (n = 60, bundle [i]) occurred after the February 2024 addition of MgSO4 to our previous ITM program (n = 246). The total case number was a convenience sample, with no a priori power calculations. Regarding sample size and statistical power, our statistician co-author (MYBK) was consulted, and informed us regarding post hoc power analysis in such observational inquiries as being of questionable value (13–18). For this study, we could only (a) “case match” cases that were performed, and (b) assess similar historical control cases performed at the same institution as comparators. Based upon literature (13–18) focused on power analyses to assess reliability of research results, and to inform future research conducted, the application of post hoc power analysis seemed questionable due to concerns about the conceptual basis for such tests. We acknowledge post hoc power analyses as often being requested during peer review with the presence of non-significant results, to be able to assess if the same effect size would be statistically significant given a larger sample size, or to determine what effect size would have been required to be able to detect a difference between the same sample size groups. However, the purpose of these analyses for this specific query is unclear, and may be conceptually flawed, as described in the literature (13–18). No further efforts were made to address potential sources of bias, other than those listed above.
2.5 Quantitative variables, and statistical methods
2.5.1 Surgical setting
As reported (2), the setting was a single US Veterans hospital, this time with ITM + Mg+ cases matched with prior (1, 2) ITM-only cases. The ITM ± Mg+ cases represent a retrospective review of quality improvement (QI) data. Institutional approval (approval/exemption 1670098) within this report entailed tracking and external reporting of GA + ITM (or GA + ITM + Mg+) data, while GA (without ITM) case-matched controls were previously (1) reported.
2.5.2 Bariatric-specific
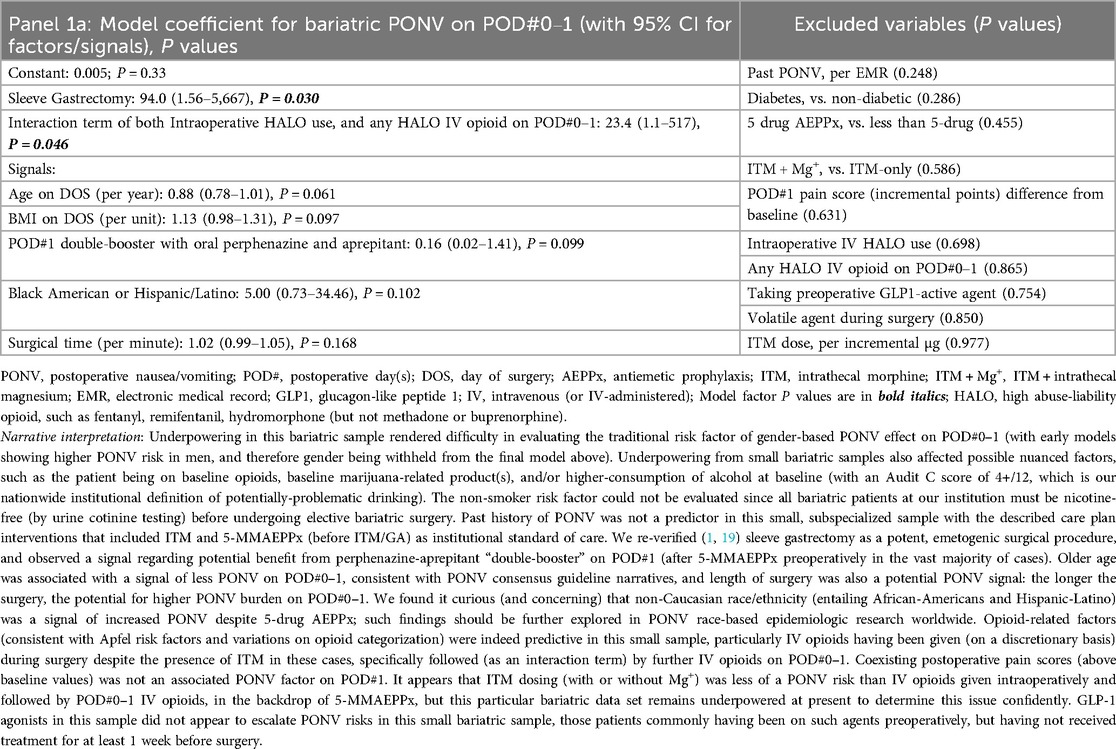
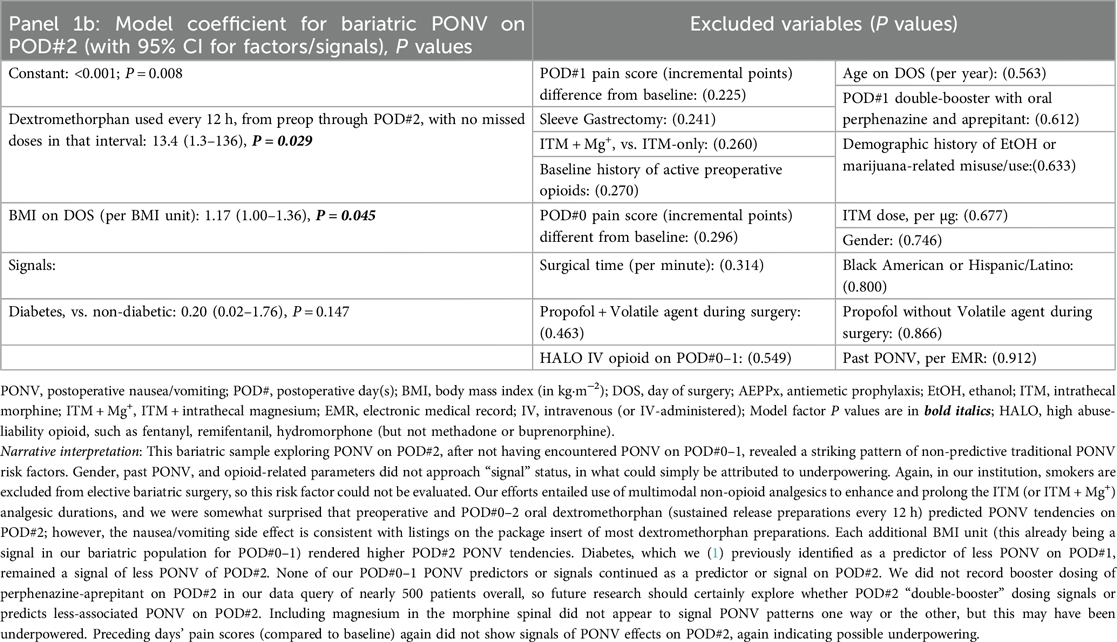
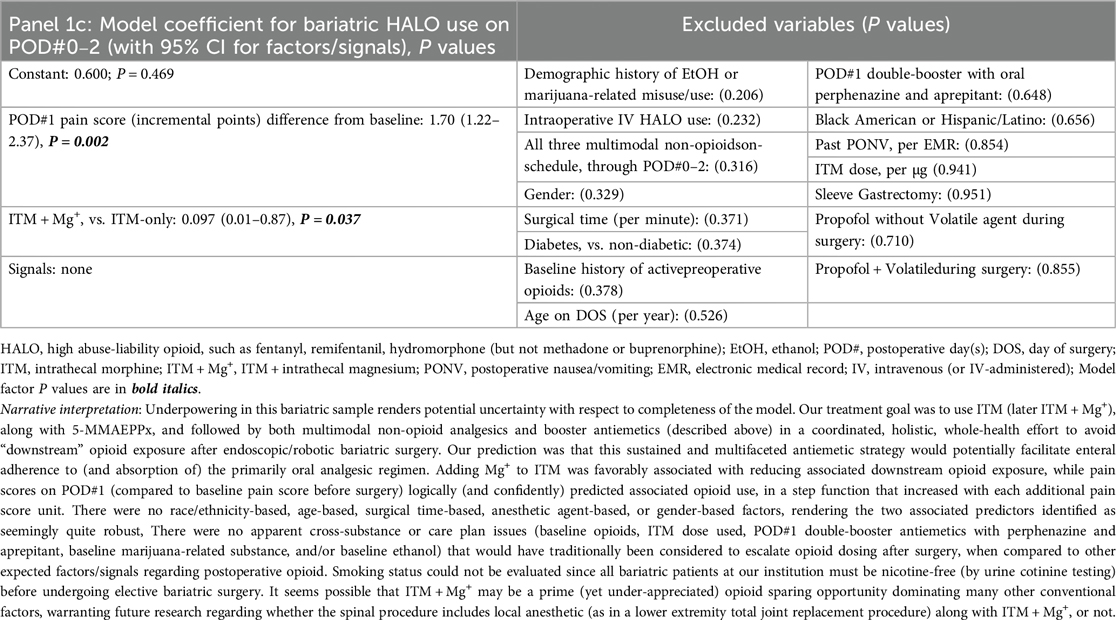
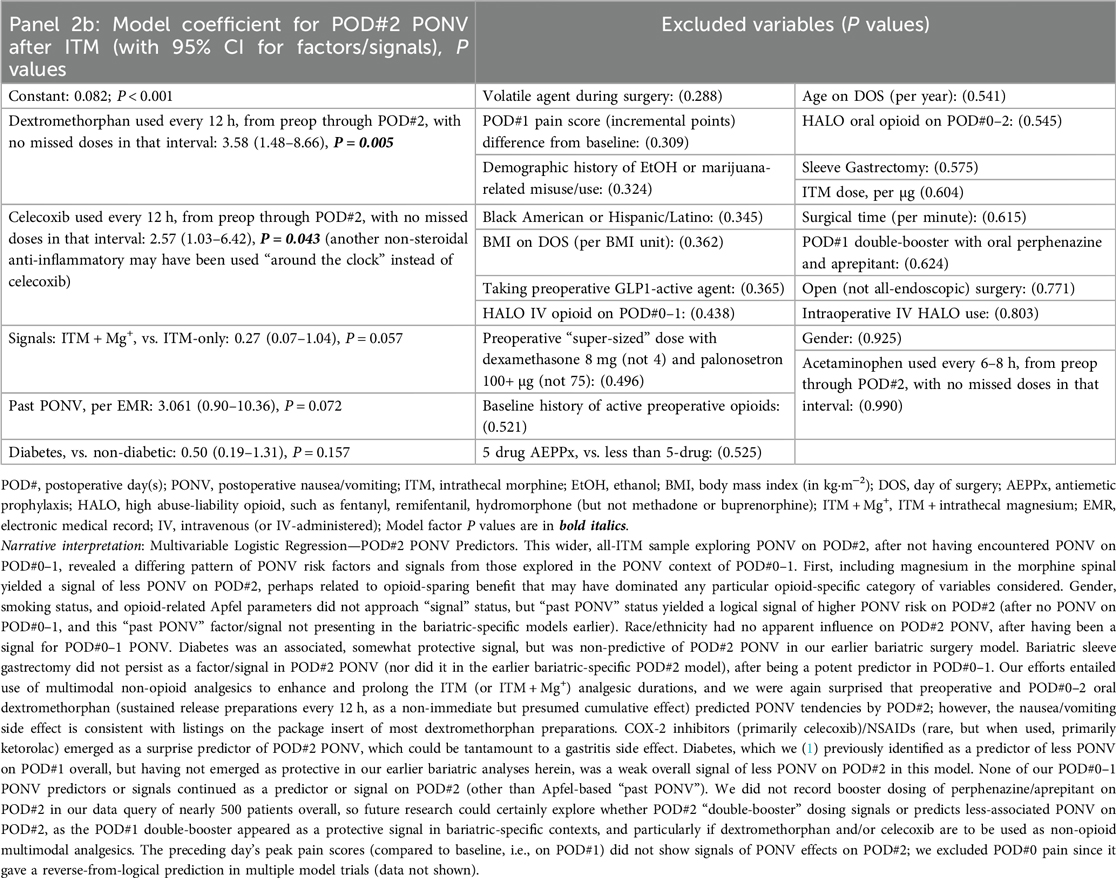
Our queries first include a consecutive case cohort of Veterans undergoing ITM-only (n = 60) or ITM + Mg+ (n = 13, bundle [i]) administration for bariatric surgery under GA. Sleeve gastrectomy and Roux-en-Y gastric bypass were the only “all-endoscopic” and/or “all-robotic” procedure types with necessary case volume for meaningful exploratory analysis. Descriptive statistics for these cases are presented in Supplementary Table S1. No PONV differences were seen based on ITM + Mg+ in this limited sample, so we constructed logistic regression models for PONV occurrence(s), identifying bundles of potentially novel factors (P ≤ 0.05) or signals (P > 0.05 but ≤0.175), as depicted in Table 1, Panels 1a,b, for PONV on POD#0–1 and POD#2, respectively. These factors and signals would then inform similar modeling across our entire ITM population (Table 2, Panels 2a,b, see below Section 2.5.3), i.e., not just restricted to bariatrics. Table 1, Panel 1c, addressed bundles of factors (or signals) associated with any downstream HALO opioid use (IV or PO) after bariatric operating room exit. After this, we addressed factors/signals associated with itching (Table 1, Panel 1d) requiring a nursing intervention.
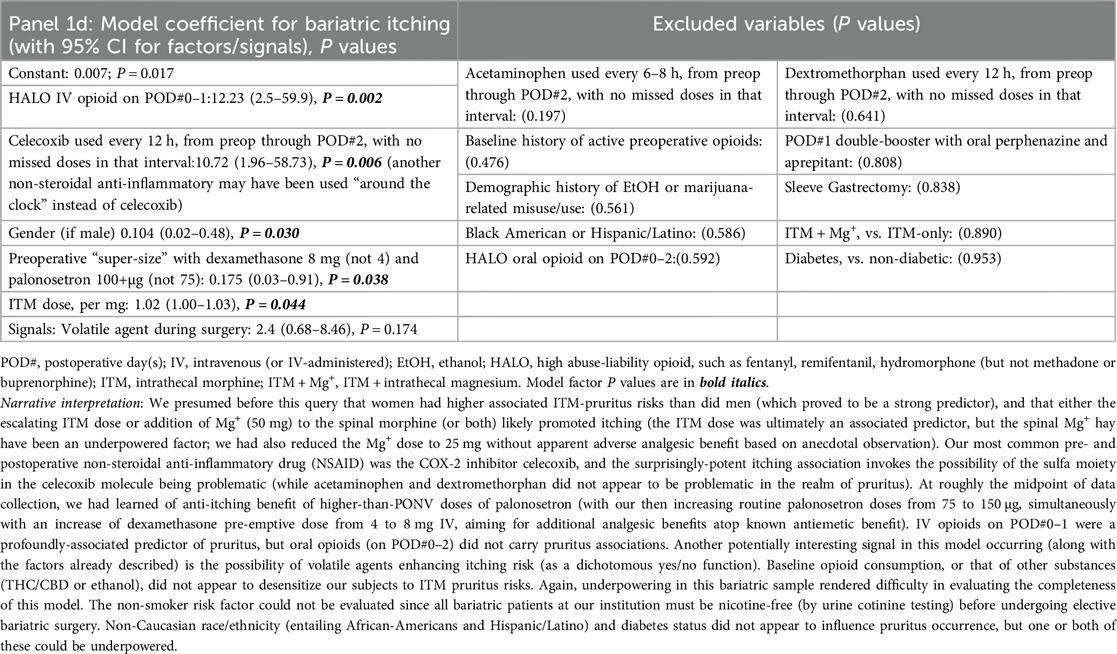
Table 1. Panels 1a–d; multivariable logistic regression models after bariatric surgery (with ITM or ITM + Mg+), outlining (1a) predictors of PONV on POD#0–1; (1b) predictors of PONV on POD#2 (after no PONV on POD#0–1); (1c) predictors of HALO Use on POD#0–2; and (1d) predictors of itching.
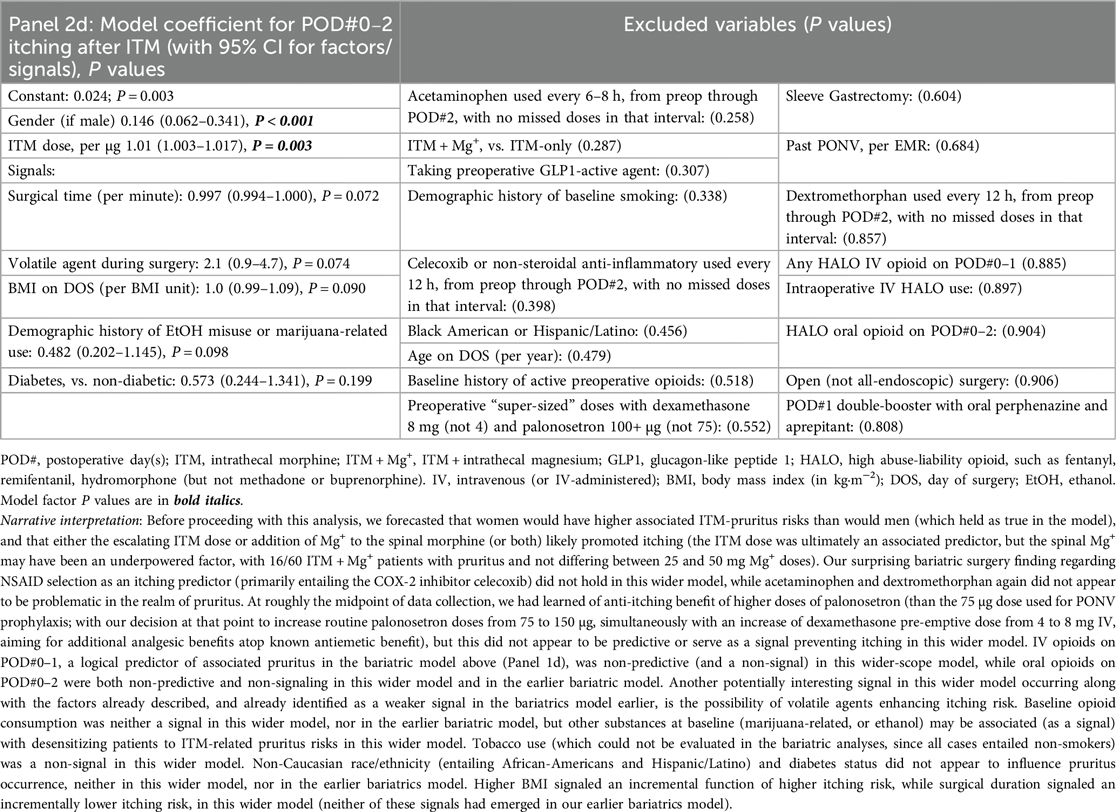
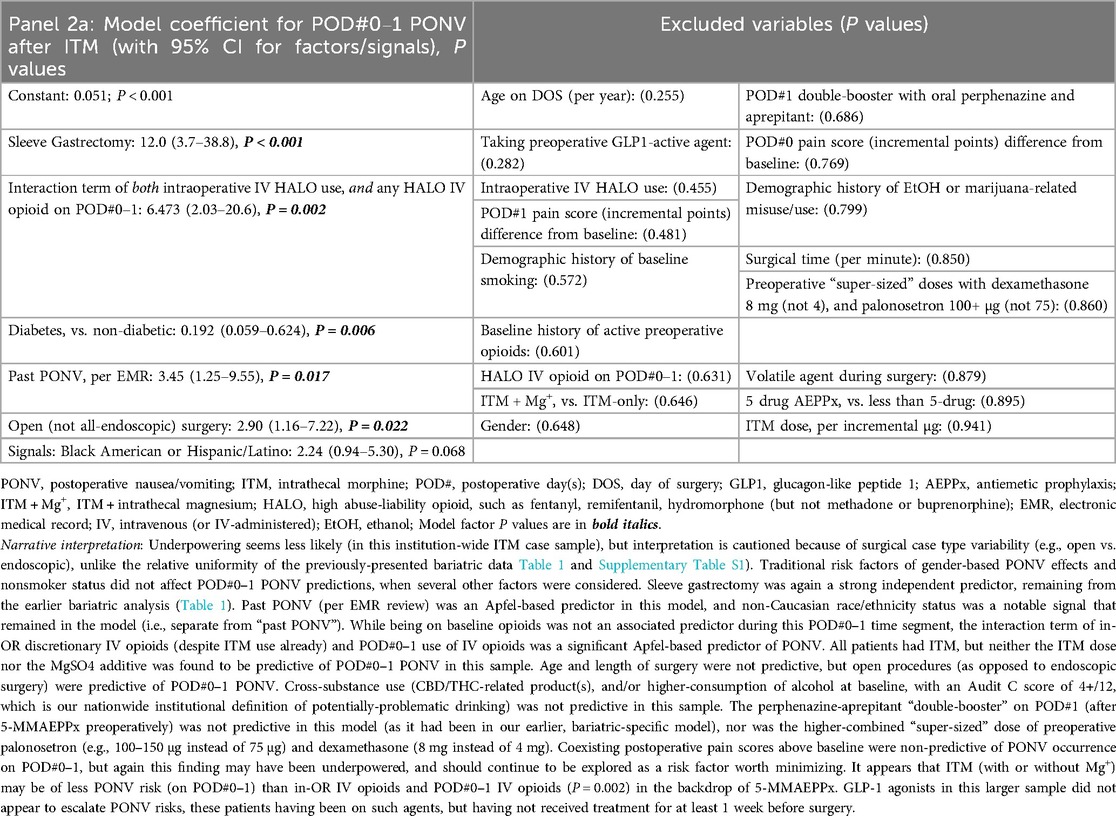
Table 2. Panels 2a–d; multivariable logistic regression models after all surgery types in our institution (with ITM or ITM + Mg+) outlining (2a) predictors of PONV on POD#0–1; (2b) predictors of PONV on POD#2 (after no PONV on POD#0–1); (2c) predictors of HALO Use on POD#0–2; and (2d) predictors of itching.
2.5.3 Entire single-center ITM ± Mg+ population
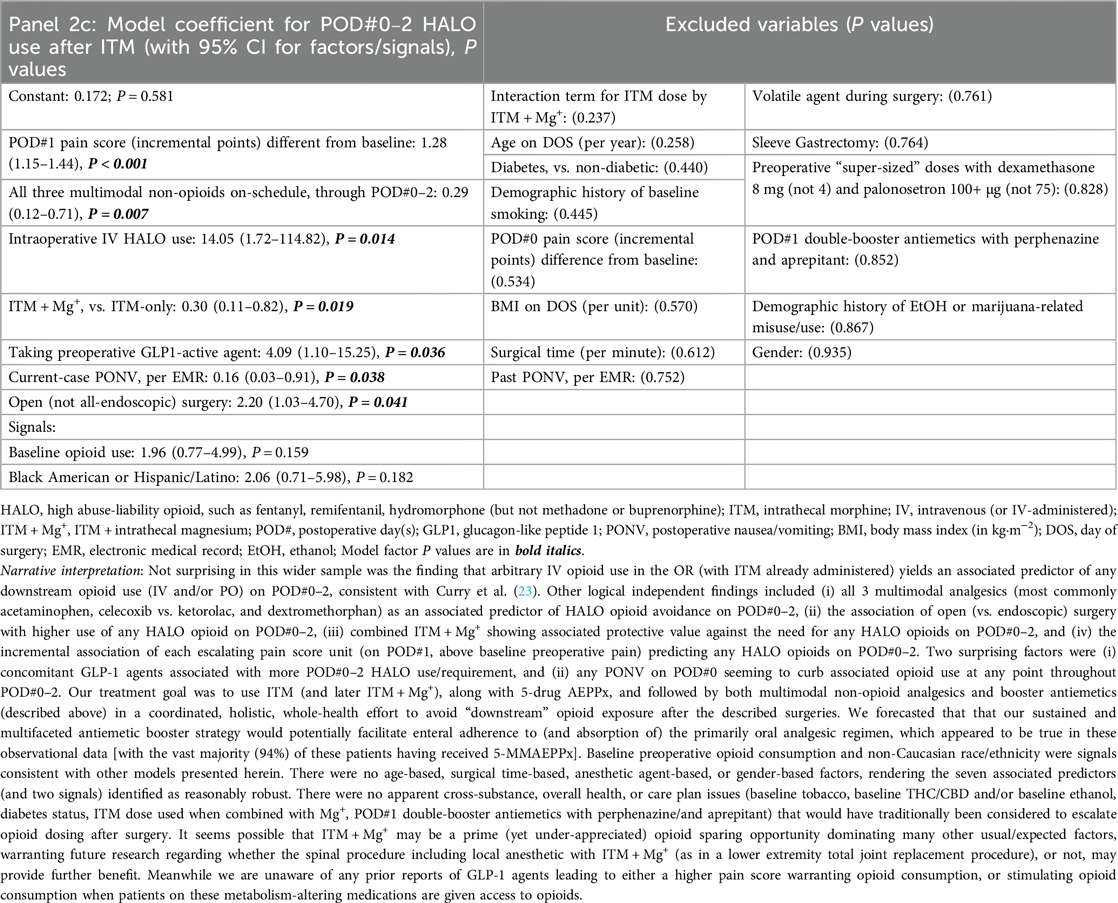
For the next analyses (after bariatrics-only, above), all ITM cases with GA (within or outside of bariatrics, including general/colorectal, and genitourinary major cancer) were queried to evaluate ITM without Mg+ (n = 246) or with ITM + Mg+ (n = 60, bundles [i–vi], all of which having been case-matched with at least one ITM-without-Mg+ case). There were no non-ITM historical controls in this analysis (similar to the bariatrics analysis above in Section 2.5.2). Again, we constructed logistic regression models to identify novel factors (P ≤ 0.05) or to signal potential predictors. These (n = 306) updated PONV outcomes are described below, along with HALO avoidance success and itching incidence. Descriptive statistics for these are presented in Supplementary Table S2. As with the Table 1 (bariatric only) panels described above, Table 2, Panels 2a,b address POD#0–1 and POD#2 PONV, respectively, while Table 2, Panel 2c, addressed downstream HALO opioid use (IV or PO) after operating room exit, and Table 2, Panel 2d addressed postoperative itching across all ITM case types.
2.5.4 Case-matching details
Similar to previous (1, 2) reports, for both bariatric-specific and expanded case analyses as described, case matching by lead author BAW utilized common procedural terminology codes, American Society of Anesthesiologists Physical Status designation, age, gender, surgery duration, and body mass index. Sleeve gastrectomy was coded separately from other bariatric (Roux-en-Y procedure) and general/genitourinary (usually colorectal or other cancer-related) surgery procedures. The sleeve gastrectomy nuance was incorporated based on statements from the International Society for Perioperative Care of the Patient with Obesity (ISPCPO) and the American Society for Metabolic and Bariatric Surgery (ASMBS), describing higher PONV risks for such cases (19). Medical records of all described cases were audited for rescue antiemetic administration on POD#0–2, and/or health professional electronic medical record (EMR) narrative notes indicating nausea and/or vomiting (e.g., nurses, physicians, other advanced care providers) on POD#0–2. POD#0–1 vs. POD#2 data were analyzed separately using multivariable logistic regression (of PONV predictors including ITM ± Mg+ use), to provide a preliminary assessment for factors' (and bundles') robustness and “believability” of 5-MMAEPPx (bundle [iii]) success, co-administered with ITM + Mg+ (bundle [i]), daily antiemetic booster doses (perphenazine and aprepitant, bundle [iv]), and scheduled postoperative multimodal analgesic/anti-hyperalgesic booster doses of acetaminophen, celecoxib, and dextromethorphan (bundle [v]). Prophylactic booster doses of ondansetron (after preoperative palonosetron) were not utilized, out of fear of creating “rebound PONV,” previously described (20, 21); rescue ondansetron doses were permitted only 40+ h following the initial palonosetron dose before surgery.
In determining factors/signals in the regression analyses (Tables 1, 2), or when evaluating clusters of 2–3 variables (e.g., two individual variables and a third interaction term), a factor with P < 0.2 was either retained in the multivariable model as a viable signal, or such variables/clusters were removed if the outcome of the multivariable model was opposite of expectation. For example, “increases in IV opioid use yielding less PONV” would be excluded from a subsequent iterative model, as an unexpected result. To re-run the model with a logical array (or bundle) of predictive factors and signals, the final models excluded variables (as potential signals) with P ≥ 0.2. In addition, if a variable was found to be a significant associated predictor on the multivariable PONV models (bundle [vi]) of POD#0–1 vs. POD#2, the factor/signal variable was similarly run in the other multivariable time-based model (and vice-versa), for completeness.
All data collation and statistical analysis utilized SPSS version 28 (IBM SPSS Statistics, Armonk, NY, USA), and all reported P-values are two-sided. In this report, other sociodemographic determinants of health were included in these analyses, such as race/ethnicity (Caucasian/not Latino vs. not, taking baseline opioids immediately before surgery, regularly using marijuana-related substance[s] and/or unhealthy alcohol consumption at baseline before surgery, etc.).
3 Results
3.1 Participants and descriptive data
As detailed and itemized in Supplementary Tables S1, S2, the number of patients per analyzed observational condition are summarized as follows. There were 60 bariatric ITM cases (without Mg+) and 13 bariatric ITM + Mg+ cases (bundle [i]) in Supplementary Table S1. Supplementary Table S2 shows 246 ITM cases overall (without Mg+), and 60 ITM + Mg+ cases (bundle [i]) overall (with Supplementary Table S2 including the aforementioned bariatric cases from Supplementary Table S1). One relevant raw data outcome (from Supplementary Table S2) that we will not discuss further below is the perceived longer analgesic duration of ITM + Mg+ (42 h, 95% CI 36–49 h) over plain ITM (31 h, 95% CI 28–33 h, P < 0.001 per t-test), likely explainable by local enhancement [e.g., N-methyl-D-aspartate (NMDA)-antagonism] of ITM by Mg+ (bundle [i]), particularly at the spinal cord level.
3.2 Raw data analysis of avoided downstream opioid when intrathecal Mg+ is added to ITM
In Section 1, we cited our recent observation (2) that ITM-only use, with day-of-surgery 5-MMAEPPx followed by 2-drug AEPPx boosters (bundles [iii, iv]), was associated with 17% success (10/58 cases) of averted need for any IV or PO HALO opioids during and after bariatric surgery (on POD#0–1). This was observed when in-hospital postoperative follow-up (i.e., non-opioids [bundle [v]] and antiemetic boosters [bundle [iv]]) was fully systematized in a comprehensive bariatric enhanced recovery protocol (2). In the current data set incorporating ITM + Mg+ and all described bundles, 9/13 (69%) of our bariatric cases with ITM + Mg+ did not need or receive downstream opioids after the initial ITM + Mg+, compared to 11/60 (18%, Supplementary Table S1) in bariatric cases receiving ITM only [i.e., one numerator and two denominator bariatric cases having been added to the previously-published (2) cases that entailed 10 of 58 cases at the time].
When considering the entire ITM + Mg+ vs. ITM-only cohorts at our center (Supplementary Table S2), ITM + Mg+ (34/60, 57%) was significantly more successful than was ITM-only (35/246, 14%, P < 0.001 by Chi-square test) on the “avoided HALO” parameter.
3.3 Models addressing clustered PONV occurrences across POD#0–1 (as a no/yes dichotomous variable)
When considering the bariatric (Table 1) and overall ITM cohort (Table 2) models incorporating comparisons of ITM against ITM + Mg+, as an aggregate exploration to inform potentially-predictive PONV bundles on POD#0–1 (Panels 1a, 2a, respectively), we observed logical prediction and signaling patterns. First (a), sleeve gastrectomy was profoundly emetogenic (19); (b) outside of sleeve gastrectomy, daily postoperative booster doses of perphenazine/aprepitant were of relatively little value [i.e., at least until booster doses of palonosetron (every 40 h) are added in future enhanced recovery QI work]. This latter point is based on Apfel's statements (20, 21) regarding ondansetron and rebound nausea risk. Continuing, (c) there may be a separate, race-based PONV risk factor distinct from the classic Apfel factor of “past PONV”; (d) operating room IV HALO, followed by ongoing IV HALO, appears to be profoundly associated with PONV risk on POD#0–1; and (e) younger age and higher BMI may be PONV-predictive factors, along with open (not endoscopic) surgery. Readers are directed to the fully descriptive captions and footnotes of Tables 1, 2, Panels 1a, 2a.
3.4 Models and predictive bundles addressing any PONV occurrence on POD#2 (dichotomous variable), specifically after no PONV on POD#0–1
When considering PONV predictors on POD#2, and addressing (among other comparisons) ITM vs. ITM + Mg+, we observed: (a) oral, slow-release dextromethorphan contributing cumulative analgesic effects as a non-opioid (Panel 2c), but also appearing to emerge as a POD#2 PONV factor (Panels 1b, 2b). This PONV side effect after associated analgesic benefit renders as reasonable the daily incorporation of not only oral booster antiemetics (perphenazine and aprepitant), but also palonosetron booster doses every 40 h, since incorporating 3 boosters (bundle [iv]) into the 5-MMAEPPx plan (bundle[iii]) should help avoid hospital cost escalations associated with usual rescue/work-up PONV care, and extended length of stay (22). Continuing, (b) ITM + Mg+ (bundle [i]) signals POD#2 PONV reduction and/or protection (Panel 2b), presumably via strategic avoidance of other/HALO opioids (bundle [ii] extended postoperatively). Next, (c), diabetes appears to be a PONV-protective factor [POD#0–1 Panel 2a, which has been previously reported (1)], along with diabetes signaling (Panels 1b, 2b) lower PONV risks into POD#2. Further, (d), Apfel factor “Past PONV” remains as a logical signal of PONV prediction on POD#2 (Panel 2b). Readers are directed to the fully descriptive captions and footnotes of Tables 1, 2, Panels 1b, 2b.
3.5 Models addressing any HALO use on POD#0–2 (dichotomous variable)
When considering both models (Tables 1, 2) and comparing ITM and ITM + Mg+ (among other comparisons), for identifying HALO use predictors on POD#0–2 (Panels 1c, 2c), we observed the following. First (a), ITM + Mg+ (bundle [i]) appearing to be a confident predictor of opioid sparing, in that the co-administered intrathecal MgSO4 may attenuate “rebound pain” possibilities (or pain severity) as initial-dose ITM effects dissipate. Next, (b), intraoperative HALO (Panel 2c) appears to induce hyperalgesia/tolerance (opposite of the objectives of bundle [ii]), as described elsewhere (23), including in rodent models (24), even with only brief exposure. Further, (c), any non-opioid analgesic (bundle [v]) reducing POD#1 pain scores seem likely to provide associated HALO avoidance; while (d), baseline opioid use preoperatively being a possibly overrated signal with ITM + Mg+ use. Next, (e), Current-case PONV (POD#0, Panel 2c) appears to curb opioid dosing thereafter, which may reflect (1) the care team withholding opioids with known new PONV, or (2) a patient not wanting opioids based on current PONV. Further, (f), all three (and not less than three) of our oral multimodal non-opioids on-schedule, started preoperatively (Panel 2c), appear to be predictive of less frequent future HALO use/need. Finally, (g), Agents such as semaglutide (Panel 2c) may warrant evaluation for possibly worsening postoperative pain perception and/or opioid requirement/utilization. Readers are directed to the fully descriptive captions and footnotes of Tables 1, 2, Panels 1c, 2c.
3.6 Models addressing pruritus on POD#0–2 (dichotomous variable)
When considering bundles of predictors (Panels 1d, 2d) evaluating ITM ± Mg+ and pruritus, we observed the following. First, (a), female gender and ITM dose, not surprisingly, were predictive of pruritus, but higher-dose preoperative palonosetron (e.g., 150 µg instead of 75 µg, Panel 1d) may be protective, based on bariatric observations. Next, (b), any IV HALO on POD#0–1 (in bariatrics) appeared to increase the occurrence of associated pruritus. Further, (c), volatile agents may signal more postoperative itching, perhaps via sensitization, while (d), celecoxib (in bariatrics) may enhance itching, along with marijuana and/or ethanol consumption (in all ITM cases) having signals of desensitization associated with fewer itching occurrences. Readers are directed to the fully descriptive captions and footnotes of Tables 1, 2, Panels 1c, 2c.
4 Discussion
4.1 Specific aims, and future recommended bundles for hypothesis testing and compassionate care
The specific aims, using multivariable logistic regression analyses, were to present bundles of integrated potential predictors related to (a) POD#0–1 PONV (Panels 1a, 2a) when evaluating an intrathecal analgesia plan entailing ITM ± Mg+; (b) POD#2 PONV (Panels 1b, 2b) when there had been no PONV on POD#0–1; (c) greater associated avoidance of downstream HALO after ITM ± Mg+ use (Panels 1c, 2c); and (d) POD#0–2 pruritus (Panels 1d, 2d). More compelling than the bundles of predictors and signals, however, was the raw data observation of 69% downstream opioid avoidance after bariatric surgery with ITM + Mg+ (bundle [i], compared with 18% after ITM-only and bundles [ii–v]), and 57% downstream opioid avoidance in our entire ITM + Mg+ cohort (bundles [i–v], vs. 14% after ITM-only). All of these outcomes entail routine use of off-patent products not carrying excessive expense or side effect burden. Also compelling (from raw data in Supplementary Table S2) were confirmations in follow-up to previously published (1) PONV and related outcome data: 5% POD#0 PONV after ITM with 5-MMAEPPx [vs. 14% in discretionary PONVCG-guided practice in pre-ITM Veteran historical controls (1)], 11% POD#1 PONV after ITM with 5-MMAEPPx [vs. 24% (1) in the same Veteran historical controls], and 15% PONV across POD#0–1 after ITM with 5-MMAEPPx [vs. 27% (1) in the same previously-reported Veteran historical controls].
4.1.1 The 5-MMAEPPx preoperative “bundle (iii)”, and its postoperative prophylactic boosters (“bundle [iv]”)
To restate and clarify next steps in hypothesis testing, from herein and from previous publications, the 5-MMAEPPx bundle had first entailed palonosetron (now recommending IV 250 µg, vs. data herein using 150 µg). Next in 5-MMAEPPx was perphenazine [4–8 mg per os (PO), with dose selection based on age and body mass index (BMI), assuming no Parkinson's disease and/or past extrapyramidal reactions to drugs of a similar class]. After palonosetron-perphenazine, aprepitant 40 mg PO, diphenhydramine 20 mg IV, and dexamethasone 8 mg IV, are incorporated, with all 5-MMAEPPx medications administered before operating room entry (1). We also confidently propose booster dosing postoperatively for future hypothesis testing (and compassionate care, within or outside the ITM ± Mg+ context), entailing daily perphenazine PO, 4–8 mg (based on age and BMI), daily aprepitant 40 mg PO, and every 40 h IV palonosetron, dose to be determined, likely 150–250 µg, with the latter already accepted and approved as a chemotherapy dose.
4.1.2 The non-opioid analgesic “bundle (v),” and the NMDA-based “bundles (i and v),” and their boosters
To restate herein the recommended non-opioid analgesic bundle for hypothesis testing and/or compassionate care, we suggest (a) pre-, intra- and post-operative acetaminophen, (b) pre- and daily post-operative celecoxib (400 then 200–400 mg) or instead meloxicam (15 then 7.5–15 mg), assuming no renal dysfunction, and (c) 60 mg PO sustained-release dextromethorphan, before surgery and every 12 h thereafter; all of these within or outside the ITM ± Mg+ context). The NMDA focus entails the described dextromethorphan (bundle [v]), and the 25 mg dose of IT Mg+ (bundle [i]) to be routinely used in conjunction with ITM. Intraoperative ketamine would be another likely beneficial NMDA-based strategy.
4.1.3 The HALO-avoiding bundle (ii)
To restate herein the recommended HALO-avoiding bundle for confident hypothesis testing and/or compassionate care, we suggest (a) IT Mg+ enhancing ITM (bundle [i]), (b) ITM (bundle [i]) starting at doses of 250 µg for women and 300 µg for men, with further mathematical adjustments, previously reported (2), and (c) avoided pre-/intra-operative fentanyl/remifentanil/hydromorphone (bundle [ii]). Future hypothesis testing (not based on our data) could further entail postoperative buprenorphine (25) (and the aforementioned non-opioids in Section 4.1.2 above and bundle [v]) replacing usual postoperative hydromorphone/oxycodone/hydrocodone sequences (and reflexive discharge prescriptions thereof).
4.2 Raw data regarding downstream opioid avoidance with ITM + Mg+; forecasting the value of methadone and/or buprenorphine intra-/peri-operatively based on ITM + Mg+ outcomes herein
4.2.1 Phase out the HALO?
The following data-informed opinion likely serves as a centerpiece to downstream opioid avoidance after surgery (in patients not taking opioids at baseline). Strategic avoidance of intraoperative usual HALO (fentanyl/remifentanil/hydromorphone, Panel 2c, bundle [ii]), and avoidance of usual HALO immediately postoperatively (hydromorphone/oxycodone/hydrocodone) appears to be a logical starting point. This can then logically incorporate bundles (i, ii, and v), with likely further facilitation by bundles (iii, iv, and vi), to promote associated postoperative HALO downstream avoidance, based on recommended enhanced recovery QI tracking/research, with the ITM + Mg+ being the “opioid of choice” instead of HALO. Our confidence in these statements is further based on (a) similarities to clinical observational findings of Curry et al. (23), and (b) similar basic science mechanistic findings in rodent models (24) by Chen et al. In the first of these, Curry et al. (23) reported that during joint replacement, increasing doses of intraoperative hydromorphone in an enhanced recovery pathway was associated with paradoxically higher pain scores and opioid requirements, which these authors attributed to intraoperative acute tolerance and/or opioid-induced hyperalgesia. Rephrased, any brief exposure to HALO opioids, be it intraoperatively (23), or in a rodent model (2022) (24), produces hyperalgesia and early tolerance. Hyperalgesia and early tolerance from fentanyl/remifentanil/hydromorphone/oxycodone, likely hydrocodone, and perhaps tramadol (26), etc., should be generally expected and accepted as likely to occur.
The scourge of oxycodone on society at large goes without saying (27, 28). Any HALO drug given to patients that are awake/conscious should be generally expected, and accepted, to be euphoria-producing (29), adding to baseline psychological and other behavioral factors that may contribute ultimately to conditions up to and including opioid use disorder (OUD), and colloquially stigmatized as “addiction.”
4.2.2 Is it more important to avoid all opioids, or instead to specifically avoid HALO?
Extending these principles from above leads to necessary re-interpretation of opioid-avoidance literature (30) (Santa Cruz Mercado, et al., 2023) in recent years. Specifically, their reported outcomes were converse to those in the above joint replacement case series (23) (upon superficial evaluation). This context now, in retrospect, may have since generated worrisome misinterpretations (and likely HALO use exacerbation). Specifically, they (30) reported less chronic persistent surgical pain (CPSP) and less new persistent opioid use (NPOU) with intraoperative fentanyl (as opposed to non-opioid techniques), but they excluded from their analyses cases that involved regional anesthesia, methadone use, and/or buprenorphine use. If academic dialogue entails “meeting in the middle” for important public health considerations, we opine that (i) fentanyl/hydromorphone/oxycodone/hydrocodone/tramadol avoidance of initial exposure is likely more important than the mitigation of chronic persistent surgical pain from non-opioid anesthesia techniques, and (ii) replacement of HALO with ITM + Mg+, methadone, and/or buprenorphine may better mitigate outcomes related to CPSP and NPOU than do the described HALO agents. These (30) authors considered only two opioids that we define as HALO (fentanyl and hydromorphone), this research having occurred during an earlier era (2016–2020, i.e., just after declaration of the opioid epidemic) where anesthesia QI/research was considering (briefly) “all-opioid avoidance,” or opioid-free anesthesia. Specifically, data (30) analyzed over 11 months concluding in October 2022 not only may have missed the Curry et al. institutional case series (23), but also likely pre-dated the basic science (24) demonstration of short-duration exposure to opioids mechanistically creating both tolerance and hyperalgesia in rodents. To their credit, these (30) authors used elegant institutional data harvesting of over 61,000 patients, and propensity-based data modeling, that unfortunately included only HALO while specifically excluding (a) non-HALO, and (b) interventions such as regional anesthesia use. Then these (30) authors, in good faith, reported that absence of fentanyl/hydromorphone had worse hospital outcomes and worse 3-month chronic pain reports, than did patients receiving fentanyl and/or hydromorphone. The methodological difference between our work (1–3) including herein, and the good-faith methodological advance (30) (“big data” and propensity scoring, but exclusively HALO use vs. no opioids) is that our protocol herein was not “opioid free.” Rather, our protocol was HALO-free, and showed important, logical, interacting associated bundles, factors, and trends that can confidently allow us to safely and reasonably remove fentanyl, hydromorphone, and remifentanil HALO opioids from ITM + Mg+-applicable cases. Further, HALO avoidance can urgently compel new enhanced recovery QI/research necessity for similar non-HALO characteristics, such as perineural (31–34) or other routes of buprenorphine delivery, and/or methadone via front-loaded IV bolus (35–46) or before surgery oral-enterally (47) (as recently shown in contexts of IV methadone drug shortage). In other words, we are confident that perioperative HALO avoidance is feasible in the opioid-naïve, when replaced by (i) non-HALO opioids, (ii) appropriate MMAEPPx “bundles” as outlined herein and elsewhere (1–3), and (iii) non-opioid analgesic bundles encompassing acetaminophen, nonsteroidals or COX-2 inhibitors, and N-methyl-D-aspartate agents (IV ketamine intraoperatively, and oral sustained-release dextromethorphan both pre- and post-operatively). The authors herein are not claiming an “easy button” process, but, rather, we are outlining a complex but feasible process, entailing all integrated facets, after needed re-education distinguishing HALO from the proposed non-HALO [ITM + Mg+, likely methadone, and likely buprenorphine, as described further below (Sections 4.2.3 through 4.2.6)], strengthened observationally by the multivariable logistic regression model and outcomes described herein.
4.2.3 Are there any non-HALO down-sides?
We acknowledge room for improvement on ITM-induced opioid complications, particularly itching, and perhaps urinary retention (pending necessary study), understanding that any route-of-delivery of methadone and/or buprenorphine may also cause, perhaps less-frequently, similar itching or urinary concerns as ITM ± Mg+. Logic may dictate that nuisance itching in ∼20% of patients (until further discovery of better prevention) might be an acceptable interim “trade-off” alternative if downstream HALO avoidance approaches or surpasses a 50% opioid-sparing avoidance outcome, as reported in our observational data.
4.2.4 Is postoperative oxycodone (as prescribed by our surgical colleagues) the real problem? Or is the problem truly any exposure to any HALO, sufficiently before any oxycodone exposure?
Taking the position above further (from Sections 4.2.2 and 4.2.3), health care professionals not appreciating these nuances (23, 24) may be unknowingly steering their patients via initial or any HALO exposure (23, 30) to both “part of the numerator and denominator” of the opioid epidemic, even with brief (24) exposure, involving now-documented mechanisms of action. It is generally accepted that 6% of surgical patients go on to develop new persistent opioid use (NPOU) (48). Regarding opioid overdose events within 1 year after surgery, Veterans are ascribed a 0.68%–0.8% risk, vs. propensity-matched non-surgical controls (0.1%) (49). Much of the work from these authors (48, 49) has entailed a well-intended effort, targeting surgeons’ postoperative prescriptions of oxycodone as the basis of analysis (i.e., number of pills), for what seems to be a secondary prevention focus. Neither of these citations (48, 49) seems to have considered the specific type of opioid exposure intraoperatively, as a “gateway” to subsequent oxycodone homegoing prescription “craving”, presumably related to such datasets not disclosing or analyzing intraoperative opioid used. We recommend reconsidering the definitions of primary vs. secondary prevention, by assuming that even brief intraoperative HALO exposure (23, 24, 30) may be just as “guilty of a culprit” as postoperative oxycodone prescriptions by our surgical colleagues, and therefore may be a more logical (and simultaneous) target of “primary prevention.”
Regarding primary prevention, we now have literature basis (35–46) to supplant all intraoperative HALO with either ITM + Mg+ (herein), or intraoperative methadone (while patients are on continuous telemetry, and esmolol/magnesium therapy is available), and we have early literature basis to replace HALO/oxycodone default with an option such as transdermal (25) or buccal buprenorphine, as recently reported, for patients that present to surgery not on baseline full-agonist HALO opioids.
4.2.5 Can we trust every consensus guidance or government statement every time?
Continuing the theme of agency guidance regarding “opioid safety,” we are grateful for the 2022 United States Veterans Administration and Department of Defense Clinical Practice Guidelines (USVADODCPG) (50) incorporating buprenorphine for the treatment of chronic pain. It seems that similar refinement of the guidance for the acute pain realm, also incorporating preoperative ITM + Mg+ and/or pre-/intra-operative methadone, would be similarly central to both adequate pain relief and primary prevention of NPOU. As background, since the release of the original US Centers for Disease Control (CDC) Guidelines for Prescribing Opioids for Chronic Pain (2016) (51), before USVADODCPG), medical providers, including in surgery, have better acknowledged abuse liability after any HALO exposure, and some have steered patients to incrementally shorter courses of HALO for acute, post-surgical pain (51–53). This change in practice has had some impact on preventing NPOU, given that the baseline NPOU rate is estimated to be 6% after at least 1 day of HALO therapy, 13.5% NPOU after a first extended episode of exposure entailing ≥8 days, and ∼30% NPOU when the first extended episode of exposure use was for ≥31 days (54) [this (54) citation not having addressed NPOU risk of methadone-as-first-opioid, or of buprenorphine-as-first-opioid]. Indeed, patients with even one day of HALO exposure can develop NPOU; the mandate for care process advances is evident. In other words, standard perioperative opioid regimens incorporating HALO agents (before any exposure to surgeons' oxycodone prescriptions) may be inadvertently leading patients to NPOU, and so the anesthesia process “gateway” can now serve as a logical portal for primary prevention. As a result, ITM + Mg+ (in conjunction with other proposed changes such as an “NMDA bundle” [i and v], a non-opioid analgesic bundle [v], and the described 5- and 3-MMAEPPx bundles [iii and iv]) may ultimately provide “net-less-addictive” alternatives for pain management (directly, with bundles [i and v], and indirectly, with bundles [iii, iv, and vi]). Furthering this HALO avoidance concept, Morrissey et al. (25) recently advocated for a paradigm shift toward buprenorphine for treating acute, post-surgical pain for orthopedic surgery; we support this change in practice, and offer the additional hypothesis that buprenorphine seems likely to further reduce NPOU post-surgery by facilitating downstream avoidance of HALO analgesics. Together, these integrated innovative bundles may lower the otherwise unchanged potential for NPOU post-surgery. The available bundles can hopefully compel refinement of current clinical practice guidelines grounded in the aftermath of Oxycontin®, which assume (50) that all long-acting opioids (i.e., methadone and buprenorphine) resemble Oxycontin® in its hyperalgesic neuroplasticity (an assumption possibly warranting a re-phrase by the CDC and in subsequent USVADODCPG).
4.2.6 Moving forward by defining “HALO” vs. “non-HALO”, and incorporating enhanced recovery bundles
We previously described our OVID Medline search failing to identify single-injection ITM as either euphoric, hyperalgesic, and/or creating worrisome acute tolerance effects. Our position is that methadone seems more likely to resemble the pharmacologic behavior of ITM + Mg+ than of HALO (fentanyl, hydromorphone, etc.). Our position continues to extend favorable non-HALO attributes to buprenorphine, with lead author BAW having fairly extensive experience with perineural buprenorphine being both duration-extending (31–34) and opioid-sparing (33, 34). There may be value in abandoning HALO opioids for routine use in perioperative contexts in favor of a combination or series of ITM + Mg+ (when anatomically appropriate), buprenorphine by any route, and/or methadone (IV and/or PO), along with esmolol infusions (55) as a “therapeutic substitution” for fentanyl/hydromorphone boluses and/or remifentanil infusions during surgery. Supplementation of all three non-HALO opioids by an NMDA antagonist bundle (e.g., IT Mg+ before surgery, IV ketamine during surgery, PO dextromethorphan both before and after surgery) appears to be rational. The work herein again (1–3) supports (a) the 5- and 3-MMAEPPx (bundle concepts [iii, iv], and (b) associated postoperative multimodal analgesic booster (bundle [v]) dosing daily, described above (Sections 4.1.1–4.1.3). After our having observed only a modest effect from just using booster perphenazine and aprepitant as non-sedating antiemetics, producing an associated antiemetic benefit on POD#1 in bariatric sleeve gastrectomy (2), but not for all ITM cases previously (2) evaluated, we have suggested (for bundle [iv] moving forward) booster palonosetron every 40 h for hypothesis testing and compassionate care, in addition to perphenazine/aprepitant boosters. Regarding other non-HALO options when ITM + Mg+ is anatomically inappropriate for the planned surgery, buprenorphine (inserted into bundles [ii and v] via the transdermal route was recently described by Morrissey et al. (25), steered in part by palliative care medicine expertise (see Section 4.1.3 above, while also incorporating Section 4.1.2 above). Perioperative methadone use has had a recent plethora of successful research, and a longstanding record of success for over 3 decades (35–46), with much of this perioperative methadone research unfortunately “contaminated,” at least partially, by a routinely-used perioperative HALO opioid; methadone could be inserted into: (a) bundle (i) as both a non-HALO opioid, and an IT Mg+-analogous agent addressing NMDA, (b) bundle (ii) as differing from the enlisted HALO opioids, and (c) bundle (v) with its co-analgesic effects again addressing NMDA.
4.3 Dosing guidance for ITM + Mg+, methadone, and/or buprenorphine intra-/peri-operatively
The recommended ITM dose has been reported by our group recently in this journal (2), and anticipated analgesic durations with ITM dosing (but without Mg+) were also presented as a potentially useful, statistically-driven formula/calculation (2). Anecdotally, our efforts to reduce ITM doses (below 250 µg in women and 300 µg in men) in the presence of IT Mg+ did not manifest as successful, after a limited number of cases required IV opioid in the post-anesthesia care unit; this could be an avenue for further study. As for other non-HALO, we (i) re-cite recommended IV methadone dosing as covered by Kharasch et al. both in outpatient same-day (45) and next-day (37) discharge, and (ii) reference perineural buprenorphine dosing from our center's work (31, 32), and associated precautions (56).
4.4 Central role of the 5-MMAEPPx bundle
Our described 5-MMAEPPx strategy (1–3) (bundle [iii]) appears to be a useful and necessary start point for the likely increase in nausea created with preferential use of ITM + Mg+, methadone, and/or buprenorphine. It is unlikely sufficient, however. Our models herein supported “double-booster” antiemetic dosing on POD#1 as a signal for our bariatric cases (Panel 1a), including the highly-emetogenic sleeve gastrectomy procedure, but “antiemetic double booster” postoperatively was neither a predictor nor a signal in our overall ITM cohort (Panel 2a). Our guidance for enhanced recovery protocols is to now embrace every-40-hour palonosetron boosters in hypothesis testing and in compassionate care, in addition to perphenazine/aprepitant, to re-evaluate whether the third MMAEPPx booster dose (bundle [iv], Section 4.4) is associated with an improved longer-term antiemetic outcome that is more generalizable than just in bariatrics/sleeve gastrectomy. In such a situation, IV diphenhydramine could preferentially serve as a PONV rescue agent (as it is in our center) due to its sedation risk (as opposed to being used as a booster).
4.5 PONV predictors (bundle [vi]) on POD#0–1 having a differing profile than PONV predictor bundles on POD#2
Our bundles of predictors and signals for PONV differed on POD#2 vs. POD#0–1. We are not aware of such having been previously reported. The Mg+ adjunct to ITM seemed to create opioid-sparing and antiemetic benefits downstream, even into POD#2. POD#0–2 multimodal analgesics dextromethorphan (Panels 1b, 2b) and celecoxib (Panel 2b) contributing (unfavorably) toward POD#2 PONV risk reinforces the probable usefulness of enhancing the antiemetic booster regimen (bundle [iv], Section 4.4), as described above. Apfel et al. (57) are to be congratulated and thanked for their original predictors, for which “past PONV” was a predictor in our POD#0–1 model (Panel 2a) and a signal in our POD#2 model (Panel 2b). Two other 1999 (57) predictors (gender, smoking status) from Apfel et al. did not enter any of our POD#0–2 PONV models, perhaps because of ITM (with or without Mg+) being a dominant predictor in these equations. It is logical to consider inflammatory response dynamics, likely cumulative, as also contributing to POD#2 PONV predictors differing from those of POD#0–1.
Intraoperative and POD#0–1 postoperative IV opioids (Panel 2a) were another astute predictor of POD#0–1 PONV that originated from Apfel et al. (57), although in our data, no particular opioids or routes of delivery (including ITM dose-response) were predictive of POD#2 PONV (after encountering no PONV on POD#0–1).
4.6 Predictors of downstream HALO need/consumption
No opioid specifics were factors or signals in our limited-sample, all-endoscopic, bariatric surgery ITM cases (Panel 1c). However, for the entire ITM ± Mg+ cohort (Panel 2c), baseline opioid was a signal (P = 0.159) for later opioid need/consumption, while in-OR IV HALO (opposite of bundle [ii]) was a potent associated predictor of downstream opioid need/consumption. In short, these two variations of Apfel et al. (57) opioid-related PONV predictors were associated in our data with postoperative HALO requirement/consumption. Enhanced recovery QI/research priorities should, hence, entail total HALO avoidance in the OR [reiterating esmolol infusion (55) as a therapeutic substitution], along with further testing of methadone and/or buprenorphine intra-/peri-operatively, to specifically detect patterns of downstream HALO avoidance, and associated satisfactory analgesia (conferred by the described multimodal non-opioid analgesics, and if insufficient, preferential rescue doses of methadone or of buprenorphine (25), as opposed to hydromorphone (58) or oxycodone (27, 28).
4.7 The pruritus dilemma
In our sample, women had more frequent ITM-associated pruritus (Table 2 Panel 2d) than did men. The ITM dose was an associated predictor across all ITM cases (Panel 2d), but we do not rule out the possibility that the spinal Mg+ effect may have been underpowered, with 16/60 ITM + Mg+ patients (27%) encountering pruritus, and not differing (in incidence) between 25 and 50 mg Mg+ doses. Anecdotally, the 50 mg-associated pruritus may have been more intense and bothersome than was itching after 25 mg Mg+. Future research may best start with 25 mg Mg+ (not 50–100 mg) as the intrathecal dose, and consider a visual analog scale for itching to better quantify this otherwise subjective response related to ITM ± Mg+. Also surprising, in bariatric surgery, the primary nonsteroidal class of drug used was celecoxib (technically, a type-2 cyclo-oxygenase inhibitor), and the “nonsteroidal drug class” was an itching predictor (Panel 1d). We later learned of anti-itching benefits of higher doses of palonosetron [than the 75 µg dose recommended for consensus-guided (59) PONV prophylaxis]; we then increased routine palonosetron doses from 75 to 150 µg, only to learn even later that 250 µg was the ED50-associated dose (60) of palonosetron required to meaningfully address itching. It may make sense to hereafter “supersize” palonosetron ITM-MMAEPPx dosing to 250 µg (an already-approved dose for nausea from chemotherapy) for both anti-itching and antiemetic purposes, particularly since Apfel et al. (20) have declared palonosetron as non-concerning for QT-interval prolongation on the electrocardiogram.
As far as other interventions with which better anti-itching may be achievable, IV opioids on POD#0–1 was a logical predictor of associated pruritus in bariatrics (Panel 1d). Another pruritus signal in both bariatrics (Panel 1d) and across the entire ITM sample (Panel 2d) entailed volatile agents enhancing itching risk, while baseline consumption of other substances [tetrahydrocannabinol/cannabidiol (THC/CBD), or ethanol] may have desensitizing effects with respect to ITM-related pruritus (Panel 2d). Higher BMI signaled an incremental (step) function of higher itching risk, while surgical duration signaled an incrementally lower itching risk (both Panel 2d) across the entire ITM sample, with or without Mg+-specific effects statistically apparent, in an acknowledged small sample.
4.8 Limitations
As previously reported recently in this journal (2), Veterans may be a lower-risk population for PONV than others, so future validation in diverse cohorts is indicated. As a result, forecasts of 5-MMAEPPx success in ITM cases (with or without Mg+) may differ in the non-Veteran population. To offset this, we again allowed for signals (with P ≥ 0.05 but <0.2) in our bundles for the final regression equations (Panels 1a–d, 2a–d), representing potentially interacting factors manifested, perhaps, by the further inclusion of Mg+ with ITM, but heretofore remaining possibly underpowered. Further, there may have been uncontrolled confounders such as surgical technique variations and patient adherence to the recommended regimen (such as not following the orders as written, and/or opting instead to skip a step in the recommended sequence of booster doses, and related).
For the next limitation, postoperative bladder catheters were near-ubiquitous in this ITM/major abdominal surgery patient population; issues regarding postoperative urinary retention will need to be separately addressed, related to Mg+ specifically. Importantly, our data did not entail co-administered local anesthetics such as bupivacaine, and their independent effects on recovery of bladder function.
Further, we acknowledge [as we did previously (2)] our inability to derive sample sizes and power analyses: our observations remain limited to a single-center Veterans population, which may not generalize to broader, diverse patient populations. Again, we could only “case match” the cases that were performed with ITM (with or without Mg+), from a smaller pool of historical control cases performed at the same institution, as was previously described (2). Future work by research teams internationally can certainly expand the cohort and include non-Veteran populations, to enhance generalizability. Accordingly, we cannot rule out the problematic potential of selection bias.
Next, we acknowledge the 27% pruritus incidence, regarding which our mitigation strategies were to first pre-empt with diphenhydramine (which was already part of the 5-MMAEPPx strategy, bundle [iii]), and next increase our 5-MMAEPPx palonosetron dose to a standardized 150 µg dose, with rescue diphenhydramine followed by transnasal butorphanol (based on repeated shortages of IV butorphanol/nalbuphine). We observed a possible reticence of healthcare professional support staff to use the ordered butorphanol for reasons unclear to the authors, opting instead for “easy button” repeated diphenhydramine doses and verbal reassurance. This may be related to requirements with butorphanol for two-person unused opioid discard practice, with pruritus often occurring during overnight shifts with possibly less nurse staffing, having been a potential contributor to this local cultural factor. Future antipruritic prophylaxis [beyond increasing palonosetron from 150 to 250 µg per dose, an approved dose for chemotherapy, and not (20) affecting QTc interval matters] would seem to be on the immediate forefront of pruritus prevention research, along with any other pruritus-related interventions. Future antipruritic research should also include clinical data collection of pruritus severity scales (for which a visual analog score should be sufficient), that would be easily incorporated into routine care for QI analysis when also ensuring comparability of groups including factors such as age and type of surgery, evaluating administered ITM with vs. without intrathecal Mg+.
Finally, incorporating all described multimodal processes and bundles (i–vi) into an overarching enhanced recovery protocol [including 5-MMAEPPx, and otherwise complete opioid avoidance (other than ITM + Mg+) until after extubation with the patient being physically stationed in PACU] may be a suitable start-point for prospective evaluations, for example, evaluating another type-2 cyclo-oxygenase inhibitor (e.g., meloxicam instead of celecoxib), or evaluating palonosetron-perphenazine-aprepitant boosters (instead of only perphenazine/aprepitant, to date).
5 Conclusions
We can confidently hypothesize for future enhanced recovery “bundle” research addressing HALO avoidance pre- and intraoperatively. First (a), ITM + Mg+ (supported by 5-MMAEPPx and the recommended 3-drug booster dosing enhancements, while limiting Mg+ to a 25 mg dose) will likely yield significant outcome improvements, including a potential “quantum leap” in downstream HALO opioid avoidance after surgery. Next (b), intraoperative and immediate postoperative avoidance of all described HALO opioids seems likely to facilitate downstream HALO avoidance success, with such intraoperative HALO opioids being easily substituted out and replaced by intraoperative esmolol infusions (55), knowing that analgesia would be confidently covered by preoperative intrathecal Mg+ added to a sufficient, previously reported (2), formula-based ITM dose. Further (c), PONV on POD#0–1 vs. POD#2 appears to have differing predictor bundle patterns, warranting maintained 5-MMAEPPx preoperatively, while further upgrading daily booster prophylaxis (perphenazine-aprepitant) to include every 40-hour palonosetron as the logical next step in booster PONV prophylaxis. Finally (d), ITM-related pruritus requires further study regarding prophylaxis and treatment, for ITM + Mg+ to seemingly achieve its full enhanced recovery potential in trying to avoid HALO exposure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional approval (approval/exemption 1670098, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Quality Improvement query of clinical routine is not deemed to be research.
Author contributions
BW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. RC: Conceptualization, Data curation, Methodology, Resources, Software, Writing – review & editing. CS: Conceptualization, Data curation, Methodology, Resources, Software, Writing – review & editing. KG: Conceptualization, Data curation, Methodology, Resources, Writing – review & editing. CE: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. MB-K: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – review & editing. LL: Conceptualization, Investigation, Resources, Writing – review & editing. JL: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to our Veterans for their service and that their understanding of the described maneuvers as a means to offset the risks of the opioid epidemic sufficiently resonated to receive the described procedures as part of our local recommended standard of care. We are also grateful for the clinical support of our chief anesthesiologist, James W. Ibinson MD, PhD, and schedule-coordinating anesthesiologist colleagues Drs. Todd M. Oravitz and Patrick J. Kennedy. We are grateful for the support of recently-added staff general surgeon Dr. Genia Dubrovsky, along with previously acknowledged (2) local surgeons, Drs. Ramesh Ramanathan (bariatric surgery), Daniel E. Hall, Gregory Watson, Robert Tessler, Robert Handzel, Christine Leeper, Brian Zuckerbraun, and Kelly McCoy (general surgery), and Amir Toussi (urology). This manuscript is dedicated to the career of Federal Service of the contemporaneous Executive Director of the United States Veterans Administration National Surgery Office, Mark A. Wilson, MD, PhD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2025.1592643/full#supplementary-material
References
1. Williams BA, Holder-Murray JM, Nettrour JF, Ibinson JW, DeRenzo JS, Dalessandro C, et al. Aim for zero: prevention of postoperative nausea and vomiting using an off-patent five-drug multimodal approach. Br J Anaesth. (2023) 131:e1–4. doi: 10.1016/j.bja.2023.01.005
2. Williams BA, Hall DE, Dalessandro C, Garbelotti KE, Ludden JM. Patient-centered intrathecal morphine dose-response in major abdominal surgeries when augmented by innovative five-drug antiemetic prophylaxis. Front Anesthesiol. (2025) 4:1521409. doi: 10.3389/fanes.2025.1521409
3. Williams BA, Schumacher CA, Choragudi R, Garbelotti KE, Ludden JM, Hall DE. Historical perspectives supporting the ambitious anesthetist aiming for zero nausea/vomiting: should one trust every consensus statement every time? Front Anesthesiol. (2025) 3:1525030. doi: 10.3389/fanes.2024.1525030
4. Staikou C, Paraskeva A. The effects of intrathecal and systemic adjuvants on subarachnoid block. Minerva Anestesiol. (2014) 80:96–112.23839318
5. Pascual-Ramírez J, Gil-Trujillo S, Alcantarilla C. Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trials. Minerva Anestesiol. (2013) 79:667–78. https://www.minervamedica.it/en/journals/minerva-anestesiologica/article.php?cod=R02Y2013N06A0667
6. Morrison AP, Hunter JM, Halpern SH, Banerjee A. Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: a systematic review and meta-analysis. Br J Anaesth. (2013) 110:702–12. doi: 10.1093/bja/aet064
7. Williams BA, Ibinson JW, Cellurale M, Nalepka T, Becker DB. Same-day and next-day pain and nausea parameters after intrathecal morphine for abdominal panniculectomy and mastectomy post-bariatric surgery. Pain Med. (2021) 22:3114–6. doi: 10.1093/pm/pnab171
8. King MR, Ladha KS, Gelineau AM, Anderson TA. Perioperative dextromethorphan as an adjunct for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. (2016) 124:696–705. doi: 10.1097/ALN.0000000000000950
9. Urmey WF, Stanton J, Bassin P, Sharrock NE. The direction of the Whitacre needle aperture affects the extent and duration of isobaric spinal anesthesia. Anesth Analg. (1997) 84:337–41. doi: 10.1097/00000539-199702000-00017
10. Nishio I, Williams BA, Williams JP. Diplopia: a complication of dural puncture. Anesthesiology. (2004) 100:158–64. doi: 10.1097/00000542-200401000-00025
11. Corcoran TB, Myles PS, Forbes AB, Cheng AC, Bach LA, O'Loughlin E, et al. Dexamethasone and surgical-site infection. N Engl J Med. (2021) 384:1731–41. doi: 10.1056/NEJMoa2028982
12. Williams BA, Kentor ML, Williams JP, Figallo CM, Sigl JC, Anders JW, et al. Process analysis in outpatient knee surgery: effects of regional and general anesthesia on anesthesia-controlled time. Anesthesiology. (2000) 93:529–38. doi: 10.1097/00000542-200008000-00033
13. Levine M, Ensom MH. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy. (2001) 21:405–9. doi: 10.1592/phco.21.5.405.34503
14. Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. (2006) 63:484–9. doi: 10.1001/archpsyc.63.5.484
15. Heonig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat. (2001) 55:19–24. doi: 10.1198/000313001300339897
16. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Mahwah, NJ: LEA (1988).
17. Quach NE, Yang K, Chen R, Tu J, Xu M, Tu XM, et al. Post-hoc power analysis: a conceptually valid approach for power based on observed study data. Gen Psychiatr. (2022) 35:e100764. doi: 10.1136/gpsych-2022-100764
18. Zhang Y, Hedo R, Rivera A, Rull R, Richardson S, Tu XM. Post hoc power analysis: is it an informative and meaningful analysis? Gen Psychiatr. (2019) 32:e100069. doi: 10.1136/gpsych-2019-100069
19. Schumann R, Ziemann-Gimmel P, Sultana A, Eldawlatly AA, Kothari SN, Shah S, et al. Postoperative nausea and vomiting in bariatric surgery: a position statement endorsed by the ASMBS and the ISPCOP. Surg Obes Relat Dis. (2021) 17:1829–33. doi: 10.1016/j.soard.2021.08.005
20. Apfel CC, Jukar-Rao S. Is palonosetron also effective for opioid-induced and post-discharge nausea and vomiting? Br J Anaesth. (2012) 108:371–3. doi: 10.1093/bja/aer516
21. Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi YY, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. (2012) 117:475–86. doi: 10.1097/ALN.0b013e318267ef31
22. Oderda GM, Senagore AJ, Morland K, Iqbal SU, Kugel M, Liu S, et al. Opioid-related respiratory and gastrointestinal adverse events in patients with acute postoperative pain: prevalence, predictors, and burden. J Pain Pall Care Pharmacother. (2019) 33:82–97. doi: 10.1080/15360288.2019.1668902
23. Curry CS, Craig WY, Richard JM, Ward DS. Increasing intraoperative hydromorphone does not decrease postoperative pain: a retrospective observational study. Br J Anaesth. (2021) 126:e95–7. doi: 10.1016/j.bja.2020.11.026
24. Chen SR, Chen H, Jin D, Pan HL. Brief opioid exposure paradoxically augments primary afferent input to spinal excitatory neurons via alpha-2-delta-1-dependent presynaptic NMDA receptors. J Neurosci. (2022) 42:9315–29. doi: 10.1523/JNEUROSCI.1704-22.2022
25. Morrissey PJ, Quinn M, Mikolasko B, Fadale PD. Optimizing safe opioid prescribing: a paradigm shift in buprenorphine management for orthopaedic surgery. J Arthroplasty. (2025) 40:8–12. doi: 10.1016/j.arth.2024.06.052
26. Das M, Jain R, Dhawan A, Kaur A. Assessment of abuse liability of tramadol among experienced drug users: double-blind crossover randomized controlled trial. J Opioid Manag. (2016) 12:421–30. doi: 10.5055/jom.2016.0361
27. Kibaly C, Alderete JA, Liu SH, Nasef HS, Law PY, Evans CJ, et al. Oxycodone in the opioid epidemic: high ‘liking’, ‘wanting’, and abuse liability. Cell Mol Neurobiol. (2021) 41:899–926. doi: 10.1007/s10571-020-01013-y
28. Barrett JE, Shekarabi A, Inan S. Oxycodone: a current perspective on its pharmacology, abuse, and pharmacotherapeutic developments. Pharmacol Rev. (2023) 75:1062–118. doi: 10.1124/pharmrev.121.000506
29. Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. (2008) 33:1179–91. doi: 10.1038/sj.npp.1301479
30. Santa Cruz Mercado LA, Liu R, Bharadwaj KM, Johnson JJ, Gutierrez R, Das P, et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. (2023) 158:854–64. doi: 10.1001/jamasurg.2023.2009
31. Williams BA, Ibinson JW, Mangione MP, Modrak RT, Tonarelli EJ, Rakesh H, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011-2014). Pain Med. (2015) 16:7–12. doi: 10.1111/pme.12609.
32. Williams BA, Ibinson JW, Mangione MP, Scanlan RL, Cohen PZ. Clinical benchmarks regarding multimodal peripheral nerve blocks for postoperative analgesia: observations regarding combined perineural midazolam-clonidine-buprenorphine-dexamethasone. Pain Med. (2015) 161:1–6. doi: 10.1111/pme.12599
33. Williams BA, Ibinson JW, Mikolic JM, Boudreaux-Kelly MY, Paiste HJ, Gilbert KL, et al. Day-one pain reductions after hip and knee replacement when buprenorphine-clonidine-dexamethasone is added to bupivacaine nerve/plexus blocks: a randomized clinical trial. Pain Med. (2022) 23:57–66. doi: 10.1093/pm/pnab325
34. Williams BA, Ibinson JW, Ritter ME, Ezaru CS, Rakesh HR, Paiste HJ, et al. Extended perineural analgesia after hip and knee replacement when buprenorphine-clonidine-dexamethasone is added to bupivacaine: preliminary report from a randomized clinical trial. Pain Med. (2020) 21:2893–902. doi: 10.1093/pm/pnaa229
35. Kharasch ED. Intraoperative methadone and postoperative anesthesia care unit outcomes: a retrospective cohort analysis. Anesthesiology. (2024) 141:408–10. doi: 10.1097/aln.0000000000005034
36. Nunn KP, Velazquez AA, Bebawy JF, Ma K, Sinedino BE, Goel A, et al. Perioperative methadone for spine surgery: a scoping review. J Neurosurg Anesthesiol. (2025) 37:31–9. doi: 10.1097/ana.0000000000000966
37. Kharasch ED, Brunt LM, Blood J, Komen H. Intraoperative methadone in next-day discharge outpatient surgery: a randomized, double-blinded, dose-finding pilot study. Anesthesiology. (2023) 139:405–19. doi: 10.1097/aln.0000000000004663
38. Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Deshur MA, et al. Clinical effectiveness and safety of intraoperative methadone in patients undergoing posterior spinal fusion surgery: a randomized, double-blinded, controlled trial. Anesthesiology. (2017) 126(5):822–33. doi: 10.1097/aln.0000000000001609
39. Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Deshur MA, et al. Intraoperative methadone for the prevention of postoperative pain: a randomized, double-blinded clinical trial in cardiac surgical patients. Anesthesiology. (2015) 122:1112–22. doi: 10.1097/aln.0000000000000633
40. Murphy GS, Avram MJ, Greenberg SB, Benson J, Bilimoria S, Maher CE, et al. Perioperative methadone and ketamine for postoperative pain control in spinal surgical patients: a randomized, double-blind, placebo-controlled trial. Anesthesiology. (2021) 134:697–708. doi: 10.1097/aln.0000000000003743
41. Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. (2011) 112:218–23. doi: 10.1213/ANE.0b013e3181d8a095
42. Murphy GS, Avram MJ, Greenberg SB, Shear TD, Deshur MA, Dickerson D, et al. Postoperative pain and analgesic requirements in the first year after intraoperative methadone for complex spine and cardiac surgery. Anesthesiology. (2020) 132:330–42. doi: 10.1097/ALN.0000000000003025
43. Kharasch ED. Intraoperative methadone: rediscovery, reappraisal, and reinvigoration? Anesth Analg. (2011) 112:13–6. doi: 10.1213/ANE.0b013e3181fec9a3
44. Kharasch ED. Opioid half-lives and hemlines: the long and short of fashion. Anesthesiology. (2015) 122:969–70. doi: 10.1097/aln.0000000000000634
45. Komen H, Brunt LM, Deych E, Blood J, Kharasch ED. Intraoperative methadone in same-day ambulatory surgery: a randomized, double-blinded, dose-finding pilot study. Anesth Analg. (2019) 128:802–10. doi: 10.1213/ANE.0000000000003464
46. Dunn LK, Yerra S, Fang S, Hanak MF, Leibowitz MK, Alpert SB, et al. Safety profile of intraoperative methadone for analgesia after major spine surgery: an observational study of 1,478 patients. J Opioid Manag. (2018) 14:83–7. doi: 10.5055/jom.2018.0435
47. Esfahani K, Tennant W, Tsang S, Naik BI, Dunn LK. Comparison of oral versus intravenous methadone on postoperative pain and opioid use after adult spinal deformity surgery: a retrospective, non-inferiority analysis. PLoS One. (2023) 18:e0288988. doi: 10.1371/journal.pone.0288988
48. Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. (2017) 152(6):e170504. doi: 10.1001/jamasurg.2017.0504
49. Larach DB, Waljee JF, Bicket MC, Brummett CM, Bruehl S. Perioperative opioid prescribing and iatrogenic opioid use disorder and overdose: a state-of-the-art narrative review. Reg Anesth Pain Med. (2024) 49:602–8. doi: 10.1136/rapm-2023-104944
50. VA/DoD Clinical Practice Guideline. Use of opioids in the management of chronic pain. Washington, DC: U.S. Government Printing Office (2022).
51. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. J Am Med Assoc. (2016) 315:1624–45. doi: 10.1001/jama.2016.1464
52. Michigan-Open.org. OPEN prescribing recommendations-adult. Available at: https://doi.org/10.56137/OPEN.000054 and/or https://michigan-open.org/wp-content/uploads/2024/08/All-Adult-Prescribing-Recs.pdf.pdf (Accessed May 28, 2025).
53. Overton HN, Hanna MN, Bruhn WE, Hutfless S, Bicket MC, Makary MA. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. (2018) 227:411–8. doi: 10.1016/j.jamcollsurg.2018.07.659
54. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. Morb Mortal Wkly Rep. (2017) 66:265–9.
55. Bahr MP, Williams BA. Esmolol, antinociception, and its potential opioid-sparing role in routine anesthesia care. Reg Anesth Pain Med. (2018) 43:815–8. doi: 10.1097/aap.0000000000000873
56. Ritter ME, Williams BA. Rare respiratory depression after adjuvant perineural buprenorphine dual lower extremity peripheral nerve blocks. Pain Med. (2022) 23:1194–7. doi: 10.1093/pm/pnac012
57. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. (1999) 91:693–700. doi: 10.1097/00000542-199909000-00022
58. Dunn KE, Brands B, Marsh DC, Bigelow GE. Characterizing the subjective, observer-rated, and physiological effects of hydromorphone relative to heroin in a human laboratory study. Psychopharmacology. (2018) 235:971–81. doi: 10.1007/s00213-017-4814-3
59. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2020) 131:411–48. doi: 10.1213/ane.0000000000004833
60. Sun L, Jin L, Jiao C, Wang LY, Xu Q, Wu H, et al. Comparison of the ED50 of prophylactic butorphanol in preventing morphine-induced pruritus with or without palonosetron: a prospective, double-blinded, randomized dose-response trial using an up-down sequential allocation method. Ann Med. (2023) 55:2304671. doi: 10.1080/07853890.2024.2304671
Keywords: spinal morphine, spinal magnesium, PONV (postoperative nausea and vomiting), palonosetron, perphenazine, aprepitant, diphenhydramine, dexamethasone
Citation: Williams BA, Choragudi R, Schumacher CA, Garbelotti KE, Ezaru CS, Boudreaux-Kelly MY, La Colla L and Ludden JM (2025) Upgrading intrathecal morphine for postoperative pain mitigation in abdominal surgery: an exploratory multiple regression analysis of observational data addressing co-administered spinal magnesium sulfate, en route to both enhanced systemic opioid sparing and opioid avoidance. Front. Anesthesiol. 4:1592643. doi: 10.3389/fanes.2025.1592643
Received: 12 March 2025; Accepted: 21 May 2025;
Published: 13 June 2025.
Edited by:
Alparslan Turan, Cleveland Clinic, United StatesReviewed by:
Chong Lei, Fourth Military Medical University, ChinaFiroozeh Madadi, Shahid Beheshti University of Medical Sciences, Iran
Raghuraman M. Sethuraman, Sree Balaji Medical College and Hospital, India
Copyright: © 2025 Williams, Choragudi, Schumacher, Garbelotti, Ezaru, Boudreaux-Kelly, La Colla and Ludden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian A. Williams, d2lsbGlhbXNiYUBhbmVzLnVwbWMuZWR1;, YnJpYW4ud2lsbGlhbXM2QHZhLmdvdg==;, d2lsbGlhbXNiYUBnbWFpbC5jb20=
 Brian A. Williams
Brian A. Williams Ridhi Choragudi
Ridhi Choragudi Christopher A. Schumacher2
Christopher A. Schumacher2 Kelly E. Garbelotti
Kelly E. Garbelotti