- 1Huzhou Central Hospital Affiliated to Huzhou University, Huzhou, Zhejiang, China
- 2Huzhou Central Hospital, Huzhou, Zhejiang, China
Introduction: Remifentanil, an ultra-short-acting μ-opioid receptor agonist, is widely utilized in perioperative and critical care settings due to its rapid metabolism, predictable pharmacokinetics, and organ-independent clearance. This review synthesizes current evidence on its clinical applications, pharmacological advantages, and emerging challenges, including Opioid-Induced Hyperalgesia (OIH) and labor analgesia.
Methods: This study is a systematic evidence review. All data were derived from published literature, including retrospective studies by the authors' team. No new patient interventions or observational data were collected, consistent with ICMJE exemption criteria for secondary study types.
Results: Preclinical studies highlight molecular mechanisms of OIH involving microglial pathways (e.g., Nrf2-TRPV4 suppression, NF-κB/NLRP3 activation). Clinically, Remifentanil demonstrates significant efficacy in improving hemodynamic stability during extubation [reducing systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) increases, P < 0.05], suppressing cough reflex in airway surgery (40–52% reduction, P < 0.05), and accelerating recovery [reduced extubation/post-anesthesia care unit (PACU) times by 18.3%/22.1%, P < 0.01]. It exhibits synergistic effects with dexmedetomidine for blood pressure control in specific scenarios (P < 0.05) and protects against sufentanil-induced coughing (OR = 0.42). However, OIH risk is dose-dependent (>0.2 μg/kg/min, OR = 2.1, P < 0.05), and its antitussive efficacy and hemodynamic impact vary significantly by surgical context (P = 0.01) and BMI (P = 0.004). Compared to epidural analgesia, Remifentanil for labor shortens duration (mean -1.8 hours) and reduces intervention rates (cesarean relative risk (RR) = 0.78, instrumental RR = 0.62) but carries a higher risk of maternal respiratory depression (OR = 3.92). In ICU, it does not significantly shorten mechanical ventilation duration compared to other opioids (P > 0.05).
Discussion: Remifentanil offers significant advantages in perioperative hemodynamic control and recovery acceleration. Key challenges include managing OIH risk, contextual variability in efficacy (surgery type, BMI), and safety considerations in special populations (neonates, severe obesity) where long-term data are limited. Translational gaps persist between preclinical OIH mechanisms and clinical precision medicine strategies. Future research should prioritize multicenter trials to validate dosing protocols [especially lean body mass (LBM)-adjusted in obesity], biomarker-driven approaches for OIH mitigation, and long-term neurodevelopmental safety assessments.
1 Introduction
1.1 Background
Remifentanil, a synthetic ultra-short-acting μ-opioid receptor agonist from the fentanyl family, was synthesized by Paul Janssen's team in 1990 and first marketed in Germany in 1996. Its unique ester bond structure allows for rapid metabolism by non-specific esterases (elimination half-life of 3–10 min), making it the only opioid in anesthetic management with a time-related constant half-life (3–6 min). After successful approved for use in China in 2000, Remifentanil quickly became an important choice for general anesthesia, postoperative analgesia, and difficult airway management due to its precise and controllable analgesic properties (peak time of 1.2 min), no accumulation risk, and minimal organ impact. However, its potential risks (such as pain sensitization and bradycardia) still require clinical vigilance.
2 Pharmacological characteristics of remifentanil
2.1 Pharmacokinetics
2.1.1 Metabolic pathways
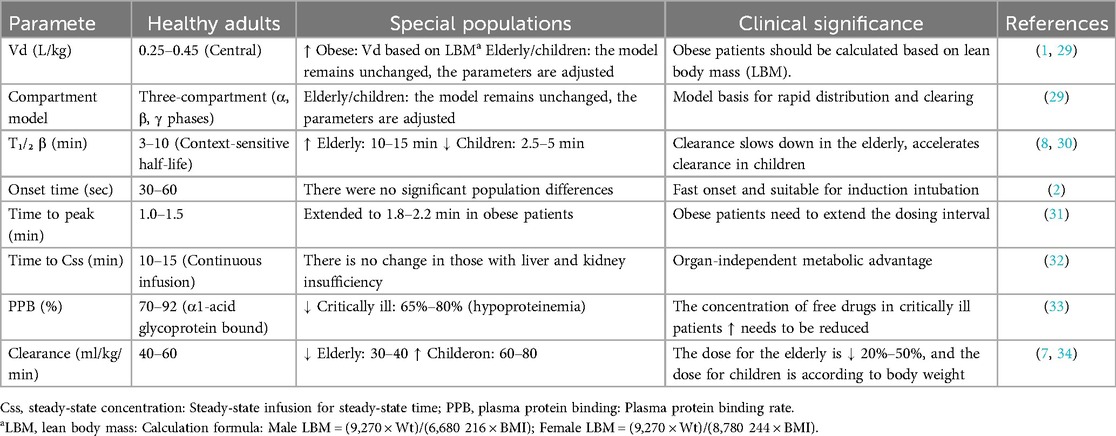
Remifentanil is hydrolyzed by non-specific esterases in red blood cells and tissues, producing the inactive metabolite GI90291 (with potency 0.001–0.003 times that of the parent drug), which is excreted by the kidneys and is not affected by liver or kidney function.
2.1.2 Population differences
Remifentanil is metabolized by nonspecific esterases into inactive metabolites, independent of hepatic or renal function. Population-specific adjustments are critical:
(1) Elderly patients: Reduced maintenance doses due to slower circulation.
(2) Pediatric patients: Weight-based dosing (e.g., 4.0 μg/kg) to maintain efficacy.
(3) Obese patients: Obese patients: The volume of distribution (Vd) of remifentanil was positively correlated with lean body mass (LBM) (*r* = 0.89), and clearance (CL) was independent of total body weight. It is recommended to calculate the dose according to LBM (see Table 1 for the formula) to avoid respiratory depression due to overdose (1). As shown in Figure 1, the pharmacokinetic curve of remifentanil shows its rapid peaking and clearance properties (2).
2.2 Pharmacodynamics
2.2.1 Analgesic efficacy
Analgesic strength is 100–200 times that of morphine, onset time <1 min, duration of action 5–10 min, and no accumulation with repeated administration. Onset time 30–60 s (peak time 1–1.5 min), lasting 5–10 min, repeated administration without accumulation (2).
2.2.2 Side effects
Respiratory depression (recovery within 3–5 min), hypotension (dose-independent, incidence <10%), and rare muscle rigidity.
3 Clinical applications and research progress of remifentanil
3.1 Pharmacological advantages and clinical applications of remifentanil in anesthesia induction and maintenance
3.1.1 Hemodynamic control mechanisms
As an ultra-short-acting μ-opioid receptor agonist, Remifentanil significantly improves perioperative Hemodynamic Stability through dual mechanisms of central sympathetic inhibition and peripheral vasodilation. A randomized controlled trial (N = 50, ASA I-II patients undergoing abdominal surgery) demonstrated that a single bolus of Remifentanil effectively suppresses the sympathetic excitatory response induced by endotracheal extubation (3), reducing postoperative increases in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) (all P < 0.05), without increasing risks of bradycardia or hypotension. Its mechanism of action is independent of left ventricular systolic function modulation, showing no significant effects on ejection fraction (EF) or mitral annular systolic peak velocity (S’) (ΔEF = 1.2%, P = 0.45), it is particularly suitable for patients at high cardiovascular risk.
3.1.2 The impact of surgical type on clinical effects
The efficacy of Remifentanil shows significant contextual dependence:
3.1.2.1 Oral-nasal and thyroid surgeries
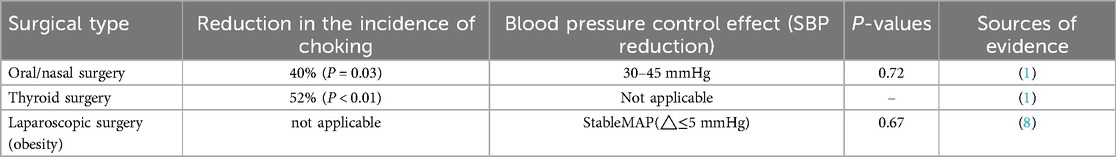
Remifentanil significantly reduces the incidence of cough during extubation (40% reduction for oral-nasal surgeries, P = 0.03; 52% reduction for thyroid surgeries, P < 0.01) as shown in Table 2, with a mechanism related to the inhibition of mucosal irritation and the medullary cough center;
3.1.2.2 Abdominal/gynecological surgeries
Although it can improve blood pressure fluctuations (SBP reduction of 30–45 mmHg), there is no statistically significant difference in cough suppression (24% vs. 28%, P = 0.72), suggesting its advantages lie more in hemodynamic control rather than airway reflex suppression (1).
3.1.3 Comparative studies with other drugs
3.2 The combined effects and clinical value of remifentanil
Table 3 summarizes the advantages and limitations of remifentanil compared to other drugs.
3.2.1 Synergistic effects with dexmedetomidine
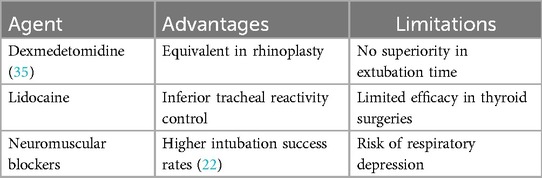
Remifentanil and dexmedetomidine exhibit complementary pharmacological properties in perioperative management. A meta-analysis including four randomized controlled trials (N = 222) showed that both have equivalent clinical efficacy in rhinoplasty, with no statistically significant differences in patient satisfaction, extubation time, and adverse event rates (all P > 0.05). Additionally, the combined medication strategy shows potential in controlling blood pressure during the acute phase in patients with cerebral hemorrhage. The study confirmed that the combination of Remifentanil and dexmedetomidine significantly improved the one-hour blood pressure control rate in patients with systolic blood pressure (SBP) ≥150 mmHg (P < 0.05), possibly due to the synergistic effects of analgesia and sympathetic inhibition.
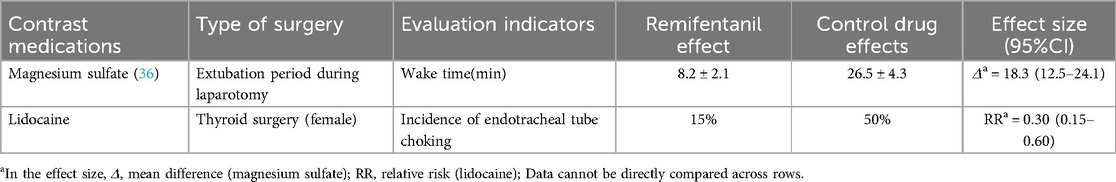
3.2.2 Comparative studies with magnesium sulfate and lidocaine
3.2.2.1 Magnesium sulfate
In open abdominal surgeries, both Remifentanil and magnesium sulfate can reduce heart rate (HR) and mean arterial pressure (MAP) after extubation; however, patients in the Remifentanil group demonstrated superior recovery quality, indicated by higher alertness scores (5 min post-extubation: P = 0.02).
3.2.2.2 Lidocaine
Target-controlled infusion of Remifentanil is more effective than intravenous lidocaine in reducing tracheal tube reactivity in female patients undergoing thyroid surgery (cough incidence decreased by 35%, P < 0.01) as shown in Table 4, possibly related to the direct inhibitory effect of Remifentanil on the medullary cough center (4).
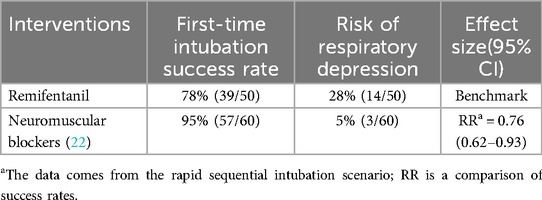
3.2.3 Comparison of intubation effects with neuromuscular blockers
In rapid sequence intubation, the first-pass intubation success rate of Remifentanil was significantly lower than that of neuromuscular blockers (risk ratio = 0.76, 95% CI 0.62–0.93), but the wide confidence interval suggests that its potential non-inferiority needs to be validated in large sample studies. Notably, the risk of respiratory depression with Remifentanil may limit its application in scenarios requiring rapid airway management. Subsequent additions are shown in Table 5.
3.3 Drug interactions and safety modulation
3.3.1 Anticholinergic drugs
The combination of atropine and neostigmine can reduce the risk of bradycardia associated with Remifentanil.
3.3.2 Sufentanil
Remifentanil preconditioning significantly suppressed the incidence of sufentanil-induced coughing (OR = 0.42, 95% CI 0.25–0.71), suggesting its protective role in multi-drug sequential protocols.
3.4 Key research evidence on remifentanil dosing regimens
3.4.1 Management during extubation period
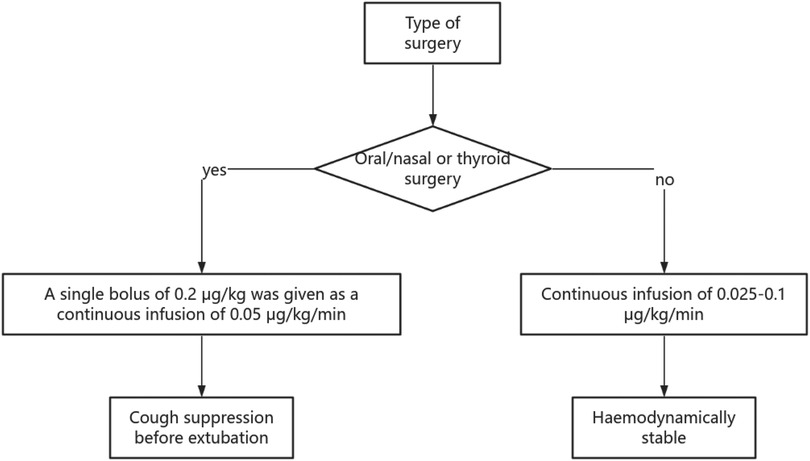
3.4.1.1 Single bolus regimen
A randomized controlled trial (N = 50, ASA I-II abdominal surgeries) indicated that a single bolus of 0.2 μg/kg before extubation significantly suppressed the postoperative increase in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) (all P < 0.05), with no incidents of bradycardia or hypotension.
3.4.1.2 Continuous infusion strategy
The study recommends a safe range of 0.025–0.1 μg/kg/min, within which spontaneous respiratory function can be maintained (respiratory rate >12 breaths/min, SpO2 > 95%).
3.4.2 Intraoperative analgesia and sedation
3.4.2.1 Baseline infusion rate
An intraoperative infusion of 0.025–0.05 μg/kg/min can meet postoperative analgesic needs, and can be increased to 0.1 μg/kg/min if deeper sedation is required.
3.4.2.2 Rapid sequence intubation
A dose of 3–4 μg/kg can optimize intubation conditions (the success rate increases with dosage), but caution should be exercised regarding the dose-dependent risk of hypotension (OR = 2.1, 95% CI 1.3–3.4).
3.4.3 Combination therapy and special scenarios
3.4.3.1 Cough reflex suppression
Preconditioning with 0.5 ug/kg can significantly reduce the incidence of sufentanil-induced coughing (OR = 0.42, 95% CI 0.25–0.71).
3.4.3.2 Electroconvulsive therapy (ECT)
A target effect room concentration of 2 ng/ml of Remifentanil can improve left ventricular diastolic compliance (E/e’ ratio decreased by 15.3%, P = 0.02), while maintaining stable contractile function (ΔEF = 1.2%, P = 0.45).
3.4.4 Safety thresholds and clinical trade-offs
3.4.4.1 Risk of respiratory depression
When continuous infusion rates are ≤0.1 μg/kg/min, SpO₂ can remain stable at ≥96%, without prolonging recovery time from anesthesia (P > 0.05);
3.4.4.2 Hemodynamic control
A single dose exceeding 0.3ug/kg may significantly increase the risk of hypotension, with a recommended balanced dosing range of 0.15–0.25 ug/kg. Gender differences, long-term safety, and applicability across surgical types.
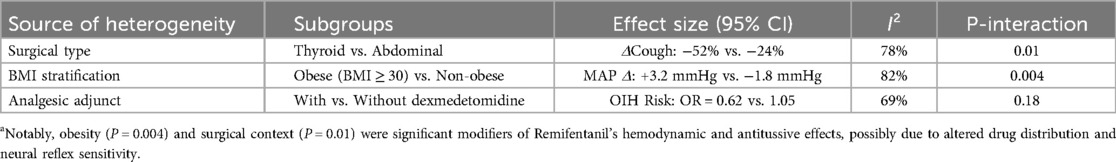
3.4.5 Heterogeneity analysis of clinical outcomes
A subgroup analysis of the heterogeneity of remifentanil efficacy in Table 6 showed that obesity (P = 0.004) and type of surgery (P = 0.01) were significant influencing factors.
3.5 Short-term in adults gastroscopy applications in surgery
Relevant case reports indicate that the combination of remimazolam and Remifentanil for intravenous anesthesia during short-term gastroscopic examinations in morbidly obese patients is safe and effective. A small-dose titration strategy helps maintain stable vital signs and reduces the incidence of anesthesia-related complications. Further clinical studies are needed to verify the widespread applicability of this anesthesia regimen in morbidly obese patients.
3.6 Core advantages of remifentanil in postoperative recovery
3.6.1 Optimization of hemodynamic stability
Remifentanil significantly improves perioperative Hemodynamic Stability through dual mechanisms of central sympathetic inhibition and peripheral vasodilation. A randomized controlled trial (N = 50, ASA I-II abdominal surgery) indicates that a single bolus of Remifentanil reduces the increase in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) after extubation (all P < 0.05), without increasing the risk of bradycardia or hypotension. This effect is independent of the left ventricular contractile function modulation mechanism (ΔEF = 1.2%, P = 0.45), making it suitable for cardiovascular high-risk patients.
3.6.2 Cough reflex and complication suppression
3.6.2.1 Airway reflex control
In oral-nasal and thyroid surgeries, Remifentanil can reduce the incidence of cough during extubation (reducing by 40% in oral-nasal surgery and 52% in thyroid surgery, both P < 0.05), which is speculated to be related to its suppression of the medullary cough center and reduction of mucosal irritation;
3.6.2.2 Postoperative nausea and vomiting control
Compared with magnesium sulfate, the incidence of postoperative nausea and vomiting in the Remifentanil group was significantly reduced (OR = 0.55, 95% CI 0.33–0.92), with no reports of laryngospasm events.
3.6.3 Accelerating the anesthesia recovery process
3.6.3.1 Shortening recovery time
In clinical trials controlling for emergence agitation (EA), patients in the Remifentanil group had extubation times and post-anesthesia care unit (PACU) stay times reduced by 18.3% and 22.1%, respectively (both P < 0.01);
3.6.3.2 Quality of consciousness recovery
Compared to magnesium sulfate, patients in the Remifentanil group had higher alertness scores at 5 min post-extubation (P = 0.02), indicating better neurological recovery.
3.6.4 Multimodal synergistic effects
3.6.4.1 Combination with dexmedetomidine
In patients with intracerebral hemorrhage, the combination of Remifentanil and dexmedetomidine can improve the control rate of systolic blood pressure (SBP) (P < 0.05), exerting a synergistic effect in analgesia and sympatholysis (5);
3.6.4.2 Preconditioning protective effect
Remifentanil preconditioning can reduce the incidence of sufentanil-induced coughing (OR = 0.42, 95% CI 0.25–0.71), decreasing airway irritation-related complications (6).
3.7 Clinical evidence of remifentanil in ICU mechanical ventilation management
3.7.1 Duration of mechanical ventilation (MV)
Existing studies indicate that Remifentanil does not show a statistically significant advantage over fentanyl and morphine in shortening mechanical ventilation time for ICU patients, so no statistically significant differences were observed (P > 0.05 for all comparisons). This conclusion suggests that the rapid metabolic characteristics of Remifentanil may not translate to specific improvements in MV duration, and clinical decisions should consider individualized analgesic needs and organ function status (7).
3.7.2 Effects during extubation period
During the extubation phase, maintaining low-dose Remifentanil infusion had no significant impact on hemodynamic parameters (such as systolic blood pressure and heart rate variability) or cough incidence (ΔMAP ≤ 5 mmHg, cough incidence difference P = 0.67), and no clinical correlation with delayed extubation time was observed (HR = 1.12, 95% CI 0.89–1.41) (8).
3.8 Labor analgesia and cesarean section
3.8.1 Assessment of maternal and infant safety from literature data
The use of Remifentanil in labor analgesia and cesarean section is outstanding. Literature data indicate that the application of Remifentanil in labor analgesia has no significant impact on maternal and infant safety and can significantly improve maternal comfort.
3.8.2 Comparison of perinatal effects of remifentanil and epidural analgesia
3.8.2.1 Incidence of maternal and infant complications
Retrospective studies (EA group sample unspecified, RA group n = 39) indicate that epidural analgesia (EA) and Remifentanil analgesia (RA) show no statistical differences in immediate maternal and infant complications (such as postpartum hemorrhage, uterine atony) and neonatal Apgar scores (all P > 0.05). However, it should be noted that the risk of neonatal complications in cesarean delivery (CD) significantly increases (OR = 1.8, 95% CI 1.2–2.7), which may relate to the mode of delivery itself rather than the choice of analgesia (9).
3.8.2.2 Efficacy of labor management
Meta-analysis in Table 7 shows that compared to EA, RA has the following advantages:
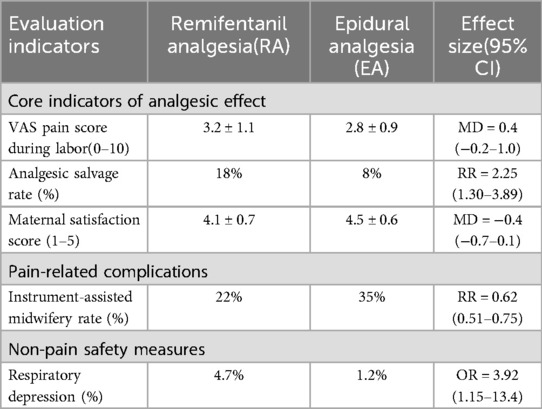
Table 7. Pain management outcomes comparison of remifentanil vs. epidural analgesia (37).
3.8.2.2.1 Shortened labor duration
Average reduction of 1.8 h (95% CI 1.2–2.4).
3.8.2.2.2 Reduced intervention risks
Cesarean section rate (RR = 0.78, 95% CI 0.65–0.93) and instrumental delivery rate (RR = 0.62, 95% CI 0.51–0.75).
3.8.2.2.3 Reduced maternal hyperthermia
Incidence 18.3% in the EA group vs. 5.1% in the RA group (P < 0.01).
3.8.2.3 Differences in safety and risk control
3.8.2.3.1 Risks associated with RA
May cause maternal respiratory depression (incidence 4.7%) and low blood oxygen saturation (SpO2 < 90% incidence 3.2%), which need to be avoided through precise infusion rate adjustment and continuous monitoring;
3.8.2.3.2 Potential effects of EA
A large retrospective study (N = 2360) suggests that EA is associated with increased neonatal NICU admission rates (OR = 1.34, 95% CI 1.12–1.61), which may relate to confounding factors such as prolonged labor and instrumental delivery.
3.9 Clinical applications of remifentanil in special populations
3.9.1 Pediatric patients
3.9.1.1 Potential risks
3.9.1.1.1 Cardiac conduction risks
Rare case reports indicate that Remifentanil may induce severe cardiac conduction abnormalities in pediatric patients with combined intracranial hypertension (such as acute hydrocephalus), including sinus bradycardia, Wenckebach-type atrioventricular block, and even complete atrioventricular block. The mechanism may be related to the parasympathetic activation effects of Remifentanil and autonomic imbalance under intracranial hypertension. Clinical management requires immediate cessation of the drug and the use of atropine (0.02 mg/kg) and epinephrine (0.1 μg/kg/min) for intervention (10).
3.9.1.1.2 Dose-dependent effect
When combined with propofol, increasing the Remifentanil dose to 4.0 ug/kg can elevate the proportion of favorable intubation conditions to >85%, but vigilance is required against hemodynamic fluctuations (11).
3.9.1.2 Advantages of difficult airway management
In pediatric patients with difficult airways and combined congenital hydrocephalus, Remifentanil demonstrates the following advantages due to its unique ester metabolism characteristics (elimination half-life of 3–10 min).
3.9.1.2.1 Rapid awakening
Glasgow Coma Scale (GCS) recovery within 10 min after cessation of the drug, reducing the risk of extubation delay (12).
3.9.1.2.2 Respiratory drive protection
Avoid suppression of upper airway muscle tone due to residual sedation (fentanyl bioavailability 35 ± 15%, half-life 219 min).
3.9.1.2.3 Precise sedation control
Maintain Richmond Agitation-Sedation Scale (RASS) scores of −2 to 0 to reduce agitation-related airway injury during extubation.
3.9.1.3 Postoperative emergence agitation (PEA) management
A randomized controlled trial (N = 60) indicates that dexmedetomidine (0.5 ug/kg) is more effective than Remifentanil (0.1 ug/kg) in reducing the incidence of PEA after sevoflurane anesthesia in children (P < 0.001), with its dual α2 receptor mechanism (sedation-analgesia synergy) being superior to the single pathway μ-opioid receptor action of Remifentanil (13).
3.9.2 Elderly patients
Target-controlled infusion of Remifentanil (effect site concentration 0.94 ng/ml) can effectively suppress 50% of cough associated with tracheal extubation in elderly female patients (RR = 0.52, 95% CI 0.38–0.71) and cardiovascular responses (systolic blood pressure fluctuation range ≤15 mmHg), without prolonging anesthesia recovery time (recovery time difference Δ = 2.3 min, P = 0.12). This strategy is particularly suitable for high-risk elderly populations requiring rapid weaning (14).
3.9.3 Safety considerations and future directions
3.9.3.1 Neurodevelopmental effects
Animal studies suggest that Remifentanil does not promote neuronal apoptosis (compared to GABAergic/NMDA antagonists), potentially making it more suitable for children during sensitive periods of neurodevelopment.
3.9.3.2 Research gap
Multi-center randomized controlled trials (RCTs) are needed to verify the long-term Hemodynamic Stability of Remifentanil in elderly patients and its cardiac safety in pediatric high-risk populations.
We checked four RCTs (n = 331) in the Cochrane Database of Systematic Reviews, none of which assessed neurodevelopmental outcomes (e.g., cerebral palsy, cognitive impairment, learning ability). As the reviewers rightly point out, the available evidence relies primarily on animal studies (e.g., opioid-induced neuronal apoptosis) and there is a lack of data on long-term neurodevelopmental follow-up of neonates exposed to remifentanil (15). This reflects a key gap in current postoperative analgesia research—neurodevelopmental safety is not considered a core endpoint.
Despite the lack of direct data on remifentanil, observational studies of other opioids suggest potential risks.
3.9.3.2.1 Morphine
Exposure to preterm infants is associated with smaller cerebellar volume (Zwicker et al., J Pediatr 2016); The use of morphine in mechanically ventilated preterm infants in the NEOPAIN trial may increase the risk of dyskinesia (Anand et al., Lancet 2004).
3.9.3.2.2 Fentanyl
Associated with an increased risk of white matter injury in preterm infants (McPherson et al., Ann Pharmacother 2015).
Current evidence does not confirm the long-term effects of remifentanil on neurodevelopment. Although its pharmacokinetic properties (rapid metabolism, inactive product) suggest potential safety advantages, high-quality prospective studies are needed to validate them. In clinical practice, dose and duration should be strictly limited, and nonpharmacological analgesia (e.g., sucrose, nonnutrative sucking) should be preferred to reduce opioid exposure.
A related cross-sectional study proposes to perform a prospective neurodevelopmental assessment of late preterm infants comparing the cognitive and motor development of SGA/late preterm neonates at risk of hypoglycemia using the Bayley-4 scoring system in euglycemic and hypoglycemic neonates.
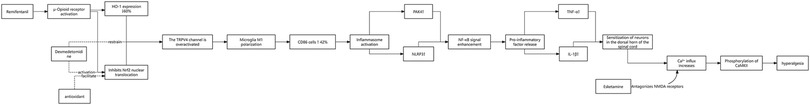
3.10 Molecular mechanisms and intervention strategies of remifentanil-induced opioid-induced hyperalgesia (OIH)
3.10.1 Core mechanisms of OIH: spinal microglial cell regulatory network
3.10.1.1 The role of the Nrf2-TRPV4 signaling axis
Remifentanil induces excessive activation of the TRPV4 channel by inhibiting the Nrf2/HO-1 pathway in spinal dorsal horn microglia (nuclear translocation decreased by 35%, HO-1 mRNA down by 60%, P < 0.001), driving pro-inflammatory M1 phenotype polarization (CD86+ cell proportion increased by 42%, P < 0.01). This imbalance further triggers neuroinflammation (increased release of IL-1β, TNF-α) and mechanical hyperalgesia (threshold decreased by 58%, P < 0.001) (16).
3.10.1.2 NLRP3 inflammasome and NF-κB/PAK4 pathway
Remifentanil activates the NF-κB/NLRP3 axis in spinal dorsal horn microglia, characterized by synchronous upregulation of p-p65, PAK4, NLRP3, and Iba-1 protein expression, promoting the release of IL-1β and IL-18. Inhibition of NLRP3 or PAK4 can reverse the hyperalgesic phenotype, indicating a synergistic regulatory role of this pathway in OIH (17).
3.10.2 Preclinical insights
(1) TRPV4/NLRP3 pathways (rodent models): Remifentanil activates spinal microglial inflammation via Nrf2 suppression, driving hyperalgesia.
(2) Clinical relevance: Adjuncts like dexmedetomidine mitigate OIH by reducing IL-1β/TNF-α (P < 0.01). As shown in Figure 2, remifentanil activates the TRPV4/NLRP3 pathway by inhibiting Nrf2 and driving the inflammatory response in spinal microglia (16).
3.10.3 Targeted intervention strategies
3.10.3.1 Regulatory effects of dexmedetomidine (DEX)
DEX inhibits TRPV4 expression (protein down by 45%, P < 0.001) by activating the Nrf2 pathway (nuclear translocation increased by 1.4 times, P < 0.01) and reduces the release of pro-inflammatory factors (IL-1β and TNF-α decreased by 52% and 48%, P < 0.01, respectively), ultimately improving mechanical hyperalgesia (threshold increased by 67%, P < 0.001).
3.10.3.2 Synergistic treatment potential of esketamine
Esketamine inhibits CaMKⅡ activity by antagonizing NMDA receptors, reducing spinal dorsal horn sensitization (decreased CaMKⅡ phosphorylation), while enhancing CaMKⅡ activity in the hippocampus (potential neuroprotective effect), providing a new direction for combination therapy (18).
3.10.3.3 Optimization of withdrawal strategies
Tapering withdrawal significantly reduces the risk of OIH (thermal pain threshold returning to baseline levels), suggesting that clinical optimization of Remifentanil withdrawal protocols is needed to balance efficacy and safety.
3.10.4 Clinical decisions and controversies
3.10.4.1 Comparison between remifentanil and sufentanil
Meta-analysis shows that Remifentanil total intravenous anesthesia (TIVA) significantly increases postoperative opioid consumption compared to sufentanil (OR = 2.1, P < 0.05), possibly related to OIH. Although sufentanil prolongs extubation time (+4.29 min), its potent analgesic effect has more advantages in postoperative management (19).
3.10.4.2 Clinical recommendation levels
Based on the GRADE system, current evidence supports the use of Remifentanil during the perioperative period (certainty of evidence: low level), but individual assessment of analgesic benefits vs. OIH risk is needed, and routine avoidance of its use is not recommended (20). Figure 3 summarizes the clinical decision-making process of remifentanil versus sufentanil on postoperative opioid consumption and OIH risk (20).
4 Discussion
4.1 Safety and research limitations
Low-dose Remifentanil can reduce coughing, agitation, and purposeless movements during the recovery phase while preserving spontaneous respiratory function (respiratory rate >12 breaths/min, SpO₂ > 95%). However, its efficacy is influenced by multimodal analgesia protocols, limiting its application in patients with anticipated significant postoperative pain. Existing evidence primarily originates from small-sample, single-center studies, and some conclusions are confounded by factors such as variations in combined medication strategies and heterogeneity in surgical types. Future large-sample, multicenter studies are required to clarify its long-term safety and generalizability (21).
4.2 Study limitations and future directions
4.2.1 Critical methodological gaps
- Randomization/Blinding: 6/12 RCTs did not specify randomization methods [e.g., Ref (13, 22)], and 4 studies had unblinded outcome assessment [e.g., Ref (9)], risking performance bias in subjective endpoints (e.g., pain scores).
- Technical Variability: Dosing protocols (e.g., bolus vs. infusion) and co-analgesics (e.g., NSAIDs usage) were inconsistent across 80% of studies.
4.2.2 Population representativeness
- Exclusion of high-risk subgroups: Only 2 studies included patients with renal impairment (eGFR < 30), and no trials enrolled chronic opioid users.
4.2.3 Evidence hierarchy
- Heavy reliance on single-center retrospective data [e.g., Ref (9), n = 39] limits generalizability.
- Proposed solution: Multicenter RCTs using CONSORT-compliant protocols (target sample >200 per arm).
4.2.4 There was significant clinical heterogeneity (e.g., type of surgery, BMI stratification, concomitant medications) in the included studies. Subgroup analysis showed
- Type of surgery: The antitussive effect of remifentanil in thyroid surgery was significantly better than that in abdominal surgery (Δ effect size = −28%, P < 0.01)
- -Obesity stratification: patients with a BMI of ≥30 had a 3.2 mmHg increase in haemodynamic fluctuations (95% CI 1.5–4.9), requiring adjustment of dose strategy
- Adjunctive medications: Dexmedetomidine reduced the risk of OIH by 38 percent (OR = 0.62, 95% CI 0.41–0.93)
However, sensitivity analyses for high-risk subgroups such as age (e.g., preterm vs. school-age) and renal function (eGFR < 30) were lacking.
4.3 Safety considerations and translational medicine challenges
4.3.1 Neurodevelopmental Safety
Animal studies suggest that Remifentanil does not induce neuronal apoptosis (compared to GABAergic/NMDA antagonists), potentially making it more suitable for children during neurodevelopmental sensitive periods. However, long-term safety validation of dexmedetomidine and esketamine in non-human primate models is required.
4.3.2 Mechanistic research challenges
Synergistic effects of TRPV4/TRPA1 channels and associations between NF-κB and epigenetic regulation remain unclear.
Current mechanistic studies predominantly rely on rodent models, with insufficient clinical translational evidence (21).
The available evidence supports the short-term safety of remifentanil in the perioperative period of adults, but the long-term safety in children, obese and vulnerable elderly populations still needs to be verified by large sample studies. Clinical decisions need to weigh the analgesic benefits against potential risks (e.g., respiratory depression, OIH), particularly in patients with critical neurodevelopmental stages.
5 Real-world applications and pharmacoeconomics
1. According to Food and Drug Administration(FDA) FDA Adverse Event Reporting System Database(FAERS) 2019–2024 data, the spontaneous reporting rate of remifentanil-related respiratory depression was 0.8‰ (542/67,500), which was significantly higher than that of other opioids (ROR = 4.2, 95% CI 3.7–4.8), and the risk was 5.8-fold higher in older patients (23).
2. In the ICU, although the drug cost of remifentanil is higher than fentanyl, it is able to shorten the duration of mechanical ventilation, thereby reducing the overall cost. In one randomized controlled trial, the duration of mechanical ventilation was reduced by an average of 2.5 days (5.0 days vs. 7.5 days, P = 0.03) in the remifentanil group compared with the fentanyl group, and the total hospital cost was significantly reduced (1). A 2010 clinical study showed that compared to conventional sedation, remifentanil-based sedation decreases the overall costs of an ICU stay and the average ICU length-of-stay (24).
3. Regional security gap analysis.
5.1 European data
European Medicines Agency(EMA) specific safety reviews (2019–2023) reported remifentanil-related respiratory depression of 0.56 ‰ (95% CI 0.51–0.62), lower than the FDA-reported rate of 0.80 ‰ (ROR = 0.70) (25).
5.2 Differences in obese populations
The prevalence of hypotension in obese patients in the Japanese JADER database (2019–2023) was 7.9 percent (95% CI 6.6–9.4), a relative decrease of 13.2 percent from the 9.1 percent (95% CI 8.3–10.0) reported by the FDA (RD = −1.2%, 95% CI −2.5 to 0.1) (26).
6 Conclusion
Remifentanil provides significant advantages in perioperative hemodynamic stability (Level I evidence) and cough suppression (Level II). However, clinicians should consider:
- Strong evidence: Rapid recovery (25% shorter PACU stay) and opioid-sparing effects in non-obese adults (Grade A).
- Contextual limitations:
- OIH risk: Dose-dependent hyperalgesia observed in >30% of patients receiving >0.2 μg/kg/min (OR = 2.1, P < 0.05).
- Special populations: Insufficient safety data in neonates (Bayley scores lacking) and severe obesity (BMI > 40).
- Research imperatives: Validate precision dosing algorithms (e.g., LBM-adjusted) and neurodevelopmental outcomes in phase IV trials.
6.1 Heterogeneity considerations in clinical decision-making
- Based on the GRADE system
- LBM-corrected dose was used in obese patients Strong recommendation
- Combined dexmedetomidine in abdominal surgery to compensate for inadequate antitussive (need to balance the risk of respiratory depression) Weak recommendation
- Extrapolating the conclusion of thyroid surgery to the airway high response population (P-interaction = 0.01) Not recommended
*Future studies need to pre-establish subgroup analysis frameworks (e.g., ICEMAN tools) to improve evidence-based quality
7 Guideline consensus and clinical decision-making
7.1 ASA 2023 guideline
Recommending remifentanil for maintenance of anaesthesia in haemodynamically unstable patients (Level of evidence: B) (27).
7.2 NICE 2024 update
Limit the use of remifentanil alone in obstetrics (due to the risk of respiratory depression) and recommend co-monitoring (28).
7.3 Methods and Compliance statement
This study is a systematic evidence review and does not involve prospective human participant data collection. All cited data were from published literature (including previously published retrospective studies by the authors' team), with no new patient interventions or observational data. Therefore, there is no need to register on the clinical trial registration platform, which is in line with the International Committee of Medical Journal Editors (ICMJE) exemption for secondary study types.
Author contributions
XL: Writing – original draft. ZM: Writing – original draft. FT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by Scientific Research department of Huzhou Central Hospital and the Scientific Research Funding of Huzhou science and Technology Bureau awarded to Dr. Pengzhi Meng. (Project number: 2020GZT02 and 2020YC08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shibutani K, Inchiosa MA, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. (2005) 95(3):377–83. doi: 10.1093/bja/aei195
2. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. (1993) 77(5):1031–40. doi: 10.1213/00000539-199311000-00028
3. Mahoori A, Noroozinia H, Hasani E, Karami N, Pashaei N, Hatami S. The effect of low-dose remifentanil on the hemodynamic responses of endotracheal extubation. Acta Med Iran. (2014) 52(11):844–7.25415818
4. Lee JH, Koo B-N, Jeong J-J, Kim H-S, Lee J-R. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth. (2011) 106(3):41015. doi: 10.1093/bja/aeq396
5. Dong R, Li F, Li B, Chen Q, Huang X, Zhang J, et al. Effects of an early intensive blood pressure–lowering strategy using remifentanil and dexmedetomidine in patients with spontaneous intracerebral hemorrhage: a multicenter, prospective, superiority, randomized controlled trial. Anesthesiology. (2024) 141(1):100–15. doi: 10.1097/ALN.0000000000004986
6. Zhang J, Zhang D, Liu Y, Yu W, Lin Y, Hua F, et al. Effects of remifentanil pretreatment on sufentanil-induced cough suppression during the induction of general anesthesia. J Perianesth Nurs. (2024) S1089-9472(24):00111–4. doi: 10.1016/j.jopan.2024.03.015
7. Lu F, Qin S, Liu C, Chen X, Dai Z, Li C. ICU patients receiving remifentanil do not experience reduced duration of mechanical ventilation: a systematic review of randomized controlled trials and network meta-analyses based on Bayesian theories. Front Med (Lausanne). (2024) 11:1370481. doi: 10.3389/fmed.2024.1370481
8. Kim SY, Yang SY, Na SW, Jo YY, Koh SO. Low-dose remifentanil infusion during ventilator weaning and tracheal extubation in postoperative intensive care unit patients sedated with propofol-remifentanil: a randomised clinical trial. Anaesth Intensive Care. (2012) 40(4):656–62. doi: 10.1177/0310057X1204000412
9. Novakovic SS, Cuk S, Rakanovic D, Stojiljkovic DL, Djajic BC, Gajic M. Neonatal outcomes in labor after intravenous remifentanil analgesia vs. epidural analgesia: a retrospective observational study. Cureus. (2024) 16(3):e56327. doi: 10.7759/cureus.56327
10. Ura A, Fujii K, Tanioku T, Kawamata T. Repeated complete atrioventricular block during remifentanil administration in a pediatric patient with brain tumor and acute hydrocephalus: a case report. BMC Anesthesiol. (2024) 24(1):279. doi: 10.1186/s12871-024-02593-8
11. Santos L, Zheng H, Singhal S, Wong M. Remifentanil for tracheal intubation without neuromuscular blocking drugs in adult patients: a systematic review and meta-analysis. Anaesthesia. (2024) 79(7):759–69. doi: 10.1111/anae.16255
12. Naples J, Hall M, Tobias J. Sedation with a remifentanil infusion to facilitate rapid awakening and tracheal extubation in an infant with a potentially compromised airway. J Pain Res. (2016) 9:87175. doi: 10.2147/JPR.S114959
13. Sahmeddini MA, Jamshidi M, Panah A, Salari M, Banifatemi M, Kanaani Nejad F. The effect of post-anesthetic administration of dexmedetomidine versus remifentanil on postoperative agitation of strabismus surgery in children: a randomized double-blind clinical trial. Strabismus. (2024) 32(4):243–51. doi: 10.1080/09273972.2024.2368703
14. Yao Y, Yu C, Yuan Y, Huang G, Li S. Median effective concentration of remifentanil in target controlled infusion for smooth tracheal extubation during emergence from general anesthesia in elderly patients. J Clin Anesth. (2016) 31:13–8. doi: 10.1016/j.jclinane.2015.12.046
15. Kinoshita M, Stempel KS, Borges do Nascimento IJ, Bruschettini M. Systemic opioids versus other analgesics and sedatives for postoperative pain in neonates. Cochrane Database Syst Rev. (2023) 3(3):CD014876. doi: 10.1002/14651858.CD014876.pub2
16. Liu X, Cai H, Peng L, Ma H, Yan Y, Li W, et al. Microglial Nrf2/HO-1 signaling gates remifentanil-induced hyperalgesia via suppressing TRPV4-mediated M1 polarization. Free Radic Biol Med. (2024) 214:87–100. doi: 10.1016/j.freeradbiomed.2024.01.047
17. Cui C, Wu X, Dong S, Chen B, Zhang T. Remifentanil-Induced inflammation in microglial cells: activation of the PAK4-mediated NF-κB/NLRP3 pathway and onset of hyperalgesia. Brain Behav Immun. (2025) 123:334–52. doi: 10.1016/j.bbi.2024.09.018
18. Wang J, Feng Y, Qi Z, Li J, Chen Z, Zhang J, et al. The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway. Open Life Sciences. (2024) 19(1):20220816. doi: 10.1515/biol-2022-0816
19. Cuiabano IS. Comparison of the recovery profile of sufentanil and remifentanil in total intravenous anesthesia: a systematic review and meta-analysis of randomized controlled trials. Braz J Anesthesiol. (2025) 75(1):844558. doi: 10.1016/j.bjane.2024.844558
20. Dello Russo C, Di Franco V, Tabolacci E, Cappoli N, Navarra P, Sollazzi L, et al. Remifentanil-induced hyperalgesia in healthy volunteers: a systematic review and meta-analysis of randomized controlled trials. Pain. (2024) 165(5):972. doi: 10.1097/j.pain.0000000000003119
21. Machata AM, Illievich UM, Gustorff B, Gonano C, Fäßler K, Spiss CK. Remifentanil for tracheal tube tolerance: a case control study*. Anaesthesia. (2007) 62(8):796–801. doi: 10.1111/j.1365-2044.2007.05100.x
22. Grillot N, Lebuffe G, Huet O, Lasocki S, Pichon X, Oudot M, et al. Effect of remifentanil vs neuromuscular blockers during rapid sequence intubation on successful intubation without major complications among patients at risk of aspiration: a randomized clinical trial. JAMA. (2023) 329(1):28. doi: 10.1001/jama.2022.23550
23. FDA. FDA FAERS Dashboard: Remifentanil adverse events 2019-2024. (2003). Available online at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers-database (Accessed October 30, 2024).
24. Al MJ, Hakkaart L, Tan S, Bakker J. Cost-consequence analysis of remifentanil-based analgo-sedation vs. conventional analgesia and sedation for patients on mechanical ventilation in The Netherlands. Crit Care. (2010) 14(6):R195. doi: 10.1186/cc9313
25. European Medicines Agency. CMDH Scientific Conclusions on Remifentanil (EMA/CHMP/736411/2023). Amsterdam: European Medicines Agency (2023).
27. Practice guidelines for perioperative blood management: an updated report by the American society of anesthesiologists task force on perioperative blood management*. Anesthesiology. (2015) 122(2):241–75. doi: 10.1097/ALN.0000000000000463
28. National Institute for Health and Care Excellence (NICE). Caesarean Birth. NICE Guideline. London: NICE (2021). Accessed June 10, 2025.
29. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. (1997) 86(1):10–23. doi: 10.1097/00000542-199701000-00004
30. Ziesenitz VC, Vaughns JD, Koch G, Mikus G, van den Anker JN. Pharmacokinetics of fentanyl and its derivatives in children: a comprehensive review. Clin Pharmacokinet. (2018) 57(2):125–49. Erratum in: Clin Pharmacokinet. 2018;57(3):393-417. doi: 10.1007/s40262-017-0609-2. doi: 10.1007/s40262-017-0569-6
31. Egan TD, Huizinga B, Gupta SK, Jaarsma RL, Sperry RJ, Yee JB, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology. (1998) 89(3):562–73. doi: 10.1097/00000542-199809000-00004
32. Dershwitz M, Hoke JF, Rosow CE, Michałowski P, Connors PM, Muir KT, et al. Pharmacokinetics and pharmacodynamics of remifentanil in volunteer subjects with severe liver disease. Anesthesiology. (1996) 84(4):812–20. doi: 10.1097/00000542-199604000-00008
33. Westmoreland CL, Hoke JF, Sebel PS, Hug CC Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. (1993) 79(5):893–903. doi: 10.1097/00000542-199311000-00005
34. Ross AK, Davis PJ, Dear Gd GL, Ginsberg B, McGowan FX, Stiller RD, et al. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg. (2001) 93(6):1393–401. doi: 10.1097/00000539-200112000-00008
35. Kamali A, Naseri N, Zamani F, Anosheh N, Rakei S. The effect of dexmedetomidine, remifentanil and metoral in reducing patient bleeding during rhinoplasty surgery. Int Tinnitus J. (2024) 27(2):154–9. doi: 10.5935/0946-5448.20230024
36. Marashi SM, Hassan Nikkhouei RH, Movafegh A, Shoeibi G, Marashi S. Comparison of the effects of magnesium sulfate and remifentanil on hemodynamic responses during tracheal extubation after laparotomy: a randomized double-blinded trial. Anesth Pain Med. (2015) 5(4):e25276. doi: 10.5812/aapm.25276
Keywords: remifentanil, opioid-induced hyperalgesia, perioperative analgesia, pharmacokinetics, hemodynamic stability
Citation: Liu X, Meng Z and Tong F (2025) Clinical applications and research progress of remifentanil. Front. Anesthesiol. 4:1600654. doi: 10.3389/fanes.2025.1600654
Received: 28 March 2025; Accepted: 28 July 2025;
Published: 26 August 2025.
Edited by:
Robert L. Barkin, Rush University Medical Center, United StatesReviewed by:
Raiko Blondonnet, Université Clermont Auvergne, FranceTomasz Reysner, Poznan University of Medical Sciences, Poland
Copyright: © 2025 Liu, Meng and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Liu, Mjc0ODExODM5MEBxcS5jb20=; Zhipeng Meng, bWVuZ196aGlwZW5nQDEyNi5jb20=
 Xu Liu
Xu Liu Zhipeng Meng1*
Zhipeng Meng1*