- 1Kansas City University, Kansas City, MO, United States
- 2Department of Anesthesiology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Background: Opioid-free anesthesia (OFA) is a multimodal strategy to avoid intraoperative opioids and minimize associated complications, though evidence remains variable.
Methods: A systematic search of PubMed and Google Scholar (2010–2025), supplemented by AI tools (Google Gemini) for earlier publications, summarized eligible studies (RCTs, cohorts, systematic reviews, and meta-analyses) comparing OFA to opioid-based anesthesia (OBA). Data were summarized following PRISMA-ScR guidelines.
Results: Across 23 randomized controlled trials and one cohort study, OFA consistently reduced PONV, while demonstrating analgesia and recovery outcomes comparable to OBA. Hemodynamic stability was variable, with dexmedetomidine-based OFA regimens sometimes associated with increased bradycardia and hypotension. PACU stay varied, ranging from 9 min shorter to 15–35 min longer with OFA. Long-term outcome data are limited.
Conclusion: OFA is a feasible approach that significantly reduces PONV while maintaining comparable analgesia and recovery. However, heterogeneous protocols, small sample sizes, and scarce long-term data limit external validity. Large, multicenter trials are needed to standardize OFA protocols and clarify long-term outcomes.
1 Introduction
The perioperative period has become a critical juncture leading to long-term opioid use and dependence (1, 2). While intraoperative opioid administration is a cornerstone of general anesthesia due to its potent analgesia, sympatholytic properties, and synergistic effect with anesthetic agents, its widespread use is linked to both acute and chronic complications (3, 4).
Acute complications, known as Opioid-Related Adverse Drug Events (ORADEs), include postoperative nausea and vomiting (PONV), constipation, urinary retention, dry mouth, dizziness, drowsiness, sedation, pruritus, and, more severely, respiratory depression. Affecting 10%–14% of surgical patients (5). Another serious acute risk is opioid-induced hyperalgesia (OIH), a paradoxical state where opioid administration increases pain sensitivity (6–8). ORADEs can prolong hospitalization and increase healthcare costs (5).
Beyond the acute setting, perioperative opioid exposure can also lead to Persistent Postoperative Opioid Use (PPOU) and Chronic Postsurgical Pain (CPSP) or persistent pain lasting over three months (2). The transition to CPSP is linked to central nervous system sensitization, which can be caused by poorly managed acute pain (9). The incidence of PPOU varies widely in different studies, from as low as 0.119% after caesarian delivery (10), 3% major elective surgery (11), 5%–54.4% after bariatric surgery (12–14), to 6% in some cohorts of adults undergoing both minor and major surgery (15). This highlights how perioperative opioid use could unintentionally lead to long-term dependence. In response to these risks, anesthesiologists are increasingly exploring opioid-free anesthesia (OFA) and opioid-sparing techniques. Given the diversity of OFA regimens and study designs, a scoping review was selected to synthesize its current evidence on the efficacy and safety and explore the practical challenges of its implementation.
2 Methods
A comprehensive literature review was conducted across PubMed and Google Scholar to identify relevant articles in patients undergoing abdominal, breast, gynecological, or orthopedic surgical procedures between January 2010 and August 2025. The search strategy included combinations of keywords such as “opioid-free anesthesia” OR “opioid-free anaesthesia”, “opioid-sparing”, “multimodal analgesia”, “multimodal anesthesia”, “non-opioid anesthesia”, “dexmedetomidine”, “ketamine”, “lidocaine”, “esmolol”, “acetaminophen”, “NSAID”, “magnesium sulfate”, “gabapentinoid”, “enhanced recovery after surgery”, “perioperative opioid”, “postoperative opioid use”, and “postsurgical pain.” AI-powered tools such as Google Gemini were used to uncover interconnected and relevant publications, including studies performed prior to 2010. Searches were restricted to human studies.
Eligibility criteria included randomized controlled trials, cohort studies, meta-analyses, or systematic reviews that compared OFA with opioid-based anesthesia (OBA) and reported acute perioperative outcomes or long-term outcomes. OFA included protocols that excluded opioid medications intraoperatively. OBA included any regimens that included intraoperative opioid use. Exclusion criteria included case reports, studies with a small sample size (total sample size <20 patients), conference abstracts, and opinion pieces.
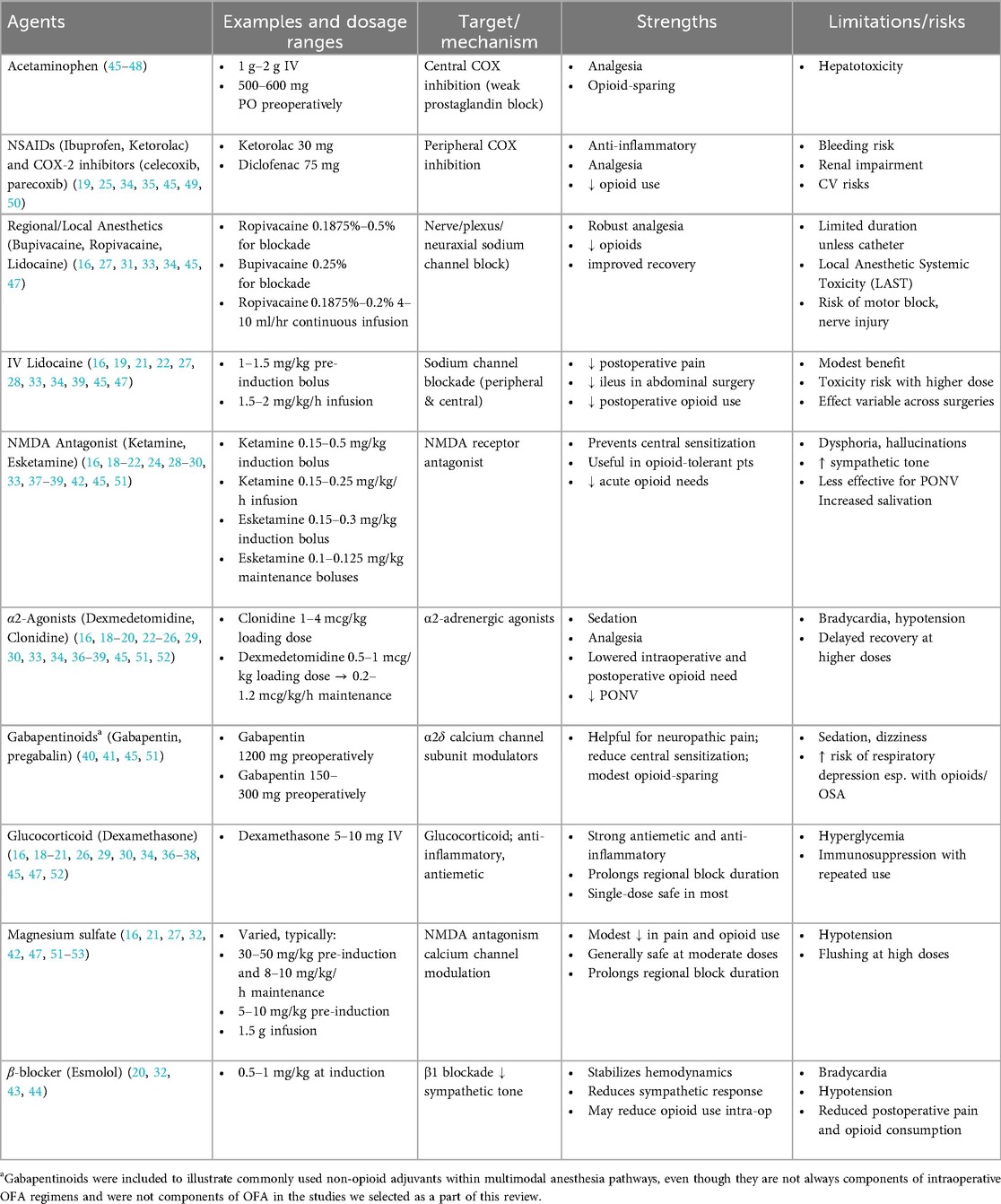
This scoping review was conducted and reported in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines. A total of 23 randomized controlled trials and 1 retrospective cohort study were included. Screening and data extraction were performed independently by the first author and verified for consistency. From each study, we extracted sample size, anesthetic regimens, medication dosages, ORADEs, chronic complications, and postoperative pain. Table 1 summarizes the mechanisms and roles of specific pharmacological agents in anesthesia. Trial characteristics are presented in Table 2. No formal review protocol was preregistered.
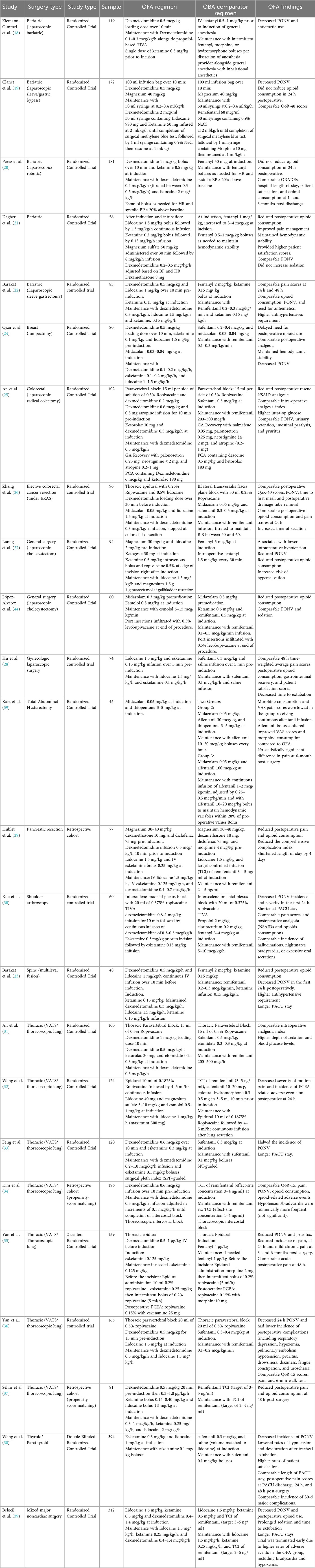
Table 2. Study characteristics and key findings of recent trials comparing opioid-free (OFA) and opioid-based anesthesia (OBA) regimens.
3 Non-opioid targets and mechanisms in opioid-free anesthesia
OFA is a multimodal anesthesia approach that targets multiple points along the nociceptive (pain) pathway to provide analgesia and manage the surgical stress response. Instead of opioids, OFA uses a combination of non-opioid medications, including α2-adrenergic agonists (e.g., dexmedetomidine), NMDA receptor antagonists (e.g., ketamine), local anesthetics (e.g., IV lidocaine), non-steroidal anti-inflammatory drugs, magnesium, acetaminophen, glucocorticoids (dexamethasone), local infiltration analgesia, regional, and neuraxial blocks, and others (14–39). These agents and drug classes are described in Table 2 below.
Through synergistic interactions, these agents can prevent central sensitization, maintain hemodynamic stability, and provide effective pain control. This combined approach may reduce ORADEs and the risk of long-term opioid misuse. For example, perioperative use of lidocaine, ketamine, and gabapentinoids has been shown to reduce the risk of CPSP for up to 6 months (40), and perioperative gabapentin decreased the time to opioid cessation post-surgery (41). Additionally, individually ketamine and magnesium can maintain stability of blood pressure and heart rate, respectively (42). Esmolol was found to reduce pain and postoperative opioid consumption (43) and has shown to pose an opioid-sparing effect intraoperatively (44). The effectiveness and safety of OFA can differ based on the type of surgery (Table 1).
4 Acute clinical outcomes
4.1 Postoperative nausea and vomiting (PONV)
The most consistent benefit of OFA compared to opioid-based anesthesia (OBA) is a significant reduction in PONV. Numerous randomized-controlled trials across various surgical specialties, including bariatric (18), thoracic (33, 36), thyroid (38), and orthopedic surgery (30) have demonstrated lower PONV incidence rates with OFA. For instance, OFA offered a clinically and statistically significant reduction in PONV rates from 30%–32% to 14%–15% in video-assisted thoracic surgery (36) and from 40% to 13% in shoulder arthroscopy (30). Meta-analyses have also consistently shown a clinically meaningful reduction in PONV with OFA (54–57). While a few studies in patients undergoing gynecologic laparoscopy (28) and thoracic surgery (34) have found no clinically or statistically significant difference, the overall evidence overwhelmingly supports OFA as a highly effective strategy for PONV prevention.
4.2 Pain control
The impact of OFA on immediate postoperative pain is variable. Some studies in breast surgery, laparoscopic cholecystectomy, laparoscopic colectomy, pancreatic resection, and spine surgery have reported improved early pain scores and reduced postoperative analgesic use (23–25, 27, 29, 44). A meta-analysis by Cheng et al. (56) supported these findings, reporting a reduced need for rescue analgesia in OFA groups undergoing laparoscopic surgery.
In contrast, other studies in bariatric surgery, gynecologic laparoscopy, and shoulder arthroscopy found no significant reduction in 24 h opioid consumption with OFA (19, 20, 22, 28, 30). Studies in thoracic surgery have also reported similar postoperative pain scores and opioid use between OFA and OBA groups (31, 34). Meta-analyses have also concluded that OFA provides little to no consistent improvement in postoperative pain requirements (54, 55). The effectiveness of OFA in managing pain appears highly dependent on the specific protocol and its meticulous execution. Nevertheless, the consensus is that OFA is not inferior to OBA in terms of postoperative pain control.
4.3 Postoperative recovery
Quality of Recovery (QoR), a composite score that assesses physical comfort, emotional state, pain, and other factors, has been used to evaluate the overall benefits of OFA (58). Some studies have demonstrated comparable QoR outcomes between OFA and OBA. Clanet et al. (19) reported similar QoR-40 scores at both 24 h and 30 days postoperatively in bariatric surgery patients. Kim et al. (34) and Yan et al. (36) found nearly identical QoR-15 scores between OFA and OBA groups in patients undergoing video-assisted thoracic surgery. However, a meta-analysis by Liu et al. (49) reported a clinically meaningful improvement in QoR-40 scores among OFA patients, primarily driven by enhanced pain control and physical comfort. This improvement was not reflected in QoR-15 scores, highlighting the sensitivity of different QoR instruments. The evidence suggests that, at a minimum, OFA is comparable to OBA in terms of overall postoperative recovery.
4.4 Intraoperative hemodynamic stability
Maintaining hemodynamic stability with OFA is a potential challenge due to the variety of regimens used and their pharmacodynamics, contributing to notable discrepancies in the literature. Two systematic reviews noted a higher incidence of bradycardia (14, 55), and some studies noted hypotension requiring increased use of vasopressors (52) or hypertension needing more antihypertensive agents (23), particularly with dexmedetomidine-based regimens. A large multicenter trial by Beloeil et al. (39) was even halted prematurely due to a higher incidence of severe bradycardia and hypoxemia in the dexmedetomidine-based OFA group. This study was criticized, however, by Mieszczański et al. (14) due to the high average doses of dexmedetomidine (1.2 mcg/kg/h) and long average anesthetic time of 268 min. Regardless, the possibility of increased intraoperative hemodynamic instability is clinically meaningful when comparing OFA and OBA.
Conversely, other research suggests OFA can lead to comparable hemodynamic stability (21, 29, 30). In a trial of patients undergoing lumpectomy, the OFA group experienced statistically and clinically significant lower rates of hypotension (5% vs. 38%) and bradycardia (8% vs. 32%) (24). Lower rates of intraoperative hypotension were also seen in laparoscopic cholecystectomy (1% vs. 8%) (27) and thyroid surgery (1% vs. 5%) (38). However, it remains unclear whether these differences translate into meaningful clinical consequences.
The discrepancy in outcomes highlights the critical need for developing robust, standardized protocols and providing comprehensive education to enhance clinician understanding and effective management of the unique pharmacodynamics of non-opioid agents.
4.5 Length of post-anesthesia care unit (PACU) stay
OFA may prolong PACU stays, a trade-off worth considering against reduced opioid-related complications. Studies in major spine (23), thoracic surgery (33), and mixed major non-cardiac surgery (39) have reported a longer PACU duration (15.5–35 min) for OFA patients, often attributed to the sedative effects of dexmedetomidine even in the absence of pain or nausea.
In contrast, other studies have found either no significant difference or even a shorter PACU stay with OFA protocols. A study on shoulder arthroscopy found a statistically significant reduction in PACU stay by 9.3 min (30). Similarly, a trial in thyroid and parathyroid surgery and a meta-analysis on laparoscopic surgery demonstrated comparable PACU stays between OFA and OBA (38, 56).
The differences in length of PACU stay are likely influenced by surgical complexity and the sedative profile of dexmedetomidine. An absolute reduction of 9.3 min with OFA for shoulder arthroscopy may not be clinically meaningful. However, an increased length of stay by 35 min with an opioid-free approach may negatively impact efficiency and resource use. This underscores the need to balance depth of sedation with the possible benefits of OFA.
5 A critical knowledge gap: the long-term impact of OFA
A critical knowledge gap in the OFA literature is the lack of robust, high-level evidence on long-term patient outcomes, particularly regarding PPOU and CPSP. The central hypothesis that OFA reduces these risks remains largely unproven. Only a handful of studies have assessed long-term outcomes, with mixed results. While one thoracic trial found a reduction in CPSP with a non-opioid epidural pathway (35), other studies found no difference in chronic pain incidence at six months (58, 59).
Crucially, no randomized controlled trials were found to have reported PPOU as a primary or secondary outcome, despite this being a key public health objective of OFA. The absence of data on opioid prescription fulfillment beyond the immediate postoperative period is a major limitation. Interestingly, some retrospective data have paradoxically suggested that higher intraoperative fentanyl doses may be associated with a lower incidence of PPOU, potentially by preventing inadequate pain control and subsequent central sensitization (60). This paradox underscores the complexity of the pain-anesthesia-dependence relationship and the urgent need for targeted, long-term investigation. Taken together, the scarcity of long-term data and the inconsistency of existing findings mirror the broader methodological issues in OFA literature, as discussed below.
6 Limitations of the evidence base and future directions
As summarized in Table 1, most available studies on OFA consist of small, single-center randomized controlled trials with heterogeneous anesthetic protocols and patient populations. Many trials included fewer than 100 participants and were powered to detect short-term outcomes such as PONV, postoperative pain, and quality-of-recovery scores, rather than long-term outcomes. It is possible that several trials that noted comparable findings for pain, hemodynamic stability, postoperative opioid use, or other ORADEs may be reflecting Type II error rather than true equivalence. Guo et al. (61) and Gricourt et al. (16) agree that there is a critical need for large, multicenter trials to improve the generalizability and external validity of findings across diverse surgical settings and patient demographics.
The marked heterogeneity among OFA regimens further complicates comparison across trials. Protocols varied in drug combinations, dosing, and timing. Several studies do not clearly outline titration or monitoring strategies. The inconsistency between blinding strategies across studies also introduces a source of bias and increases the difficulty of comparing across trials. Shanthanna and Joshi (62) emphasize that future studies should develop procedure- and patient-specific combinations with standardized dosing and administration.
As noted earlier, only a few studies assessed CPSP after discharge. PPOU was not outlined as an endpoint in any of the studies that we reviewed. Most studies that we reviewed had short observation periods, making it challenging to evaluate long-term opioid-related complications as they relate to OFA. This is compounded by the fact that inappropriate postoperative prescribing and a lack of discharge stewardship programs may lead to persistent opioid use, potentially offsetting the intraoperative benefits of OFA (2, 63). More studies are needed to clarify the long-term impact of OFA on CPSP and PPOU, which is a core rationale for its adoption.
The cost-effectiveness of OFA remains largely unexplored. While OFA may reduce complications and hospital stays, its higher upfront costs, driven by multi-agent regimens and increased monitoring, pose a barrier to widespread adoption (21). Further research is needed to evaluate the economic impact of OFA to guide its broader implementation.
7 Conclusion
Opioid-free anesthesia (OFA) offers a valuable strategy to reduce perioperative opioid exposure. The most consistent and immediate benefit reported is a significant reduction in PONV. While short-term pain control and recovery outcomes appear comparable to opioid-based approaches, substantial limitations remain. However, the long-term impact of OFA on PPOU and CPSP remains largely unknown. To advance the clinical utility of this technique, future research must prioritize robust, multicenter, well-powered trials with standardized protocols, established safety metrics, and sufficient longitudinal follow-up to definitively assess PPOU and CPSP. Furthermore, cost-effectiveness analyses are crucial for determining the broader economic and clinical implications of OFA and its appropriate role in mitigating the opioid crisis.
Author contributions
AP: Conceptualization, Investigation, Writing – original draft. OE: Conceptualization, Writing – review & editing, Project administration, Supervision. RW: Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Egan TD. Are opioids indispensable for general anaesthesia? Br J Anaesth. (2019) 122:e127–35. doi: 10.1016/j.bja.2019.02.018
2. Shanthanna H, Ladha KS, Kehlet H, Joshi GP. Perioperative opioid administration. Anesthesiology. (2021) 134:645–59. doi: 10.1097/ALN.0000000000003572
3. Ferry N, Hancock LE, Hendrix JM, Dhanjal ST. Opioid Anesthesia. Treasure Island, FL: StatPearls Publishing (2025).
4. Glass PSA, Gan TJ, Howell S, Ginsberg B. Drug interactions: volatile anesthetics and opioids. J Clin Anesth. (1997) 9:18S–22. doi: 10.1016/S0952-8180(97)00122-0
5. Yiu CH, Gnjidic D, Patanwala A, Fong I, Begley D, Khor KE, et al. Opioid-related adverse drug events in surgical patients: risk factors and association with clinical outcomes. Expert Opin Drug Saf. (2022) 21:1211–23. doi: 10.1080/14740338.2022.2049230
6. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. (2011) 14:145–61. doi: 10.36076/ppj.2011/14/145
7. Yu EHY, Tran DHD, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. (2016) 71:1347–62. doi: 10.1111/anae.13602
8. Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. (2014) 112:991–1004. doi: 10.1093/bja/aeu137
9. Moka E, Aguirre JA, Sauter AR, Lavand’homme P. Chronic postsurgical pain and transitional pain services: a narrative review highlighting European perspectives. Reg Anesth Pain Med. (2025) 50:205–12. doi: 10.1136/rapm-2024-105614
10. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. (2016) 176:1286–93. doi: 10.1001/jamainternmed.2016.3298
11. Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Br Med J. (2014) 348:g1251. doi: 10.1136/bmj.g1251
12. Gribsholt SB, Pedersen AM, Svensson E, Thomsen RW, Richelsen B. Prevalence of self-reported symptoms after gastric bypass surgery for obesity. JAMA Surg. (2016) 151:504–11. doi: 10.1001/jamasurg.2015.5110
13. Pierik AS, Coblijn UK, de Raaff CAL, van Veen RN, van Tets WF, van Wagensveld BA. Unexplained abdominal pain in morbidly obese patients after bariatric surgery. Surg Obes Relat Dis. (2017) 13:1743–51. doi: 10.1016/j.soard.2017.05.027
14. Mieszczański P, Kołacz M, Trzebicki J. Opioid-Free anesthesia in bariatric surgery: is it the one and only? A comprehensive review of the current literature. Healthcare. (2024) 12:1094. doi: 10.3390/healthcare12111094
15. Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. (2017) 152:e170504. doi: 10.1001/jamasurg.2017.0504
16. Gricourt Y, Cuvillon P, Forget P. Opioid-free anaesthesia as a valuable alternative to opioid-based practices: evidence and future challenges. Pain Manag. (2025) 15:721–31. doi: 10.1080/17581869.2025.2542719
17. Forget P. Opioid-free anaesthesia. Why and how? A contextual analysis. Anaesth Crit Care Pain Med. (2019) 38:169–72. doi: 10.1016/j.accpm.2018.05.002
18. Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. (2014) 112:906–11. doi: 10.1093/bja/aet551
19. Clanet M, Touihri K, El Haddad C, Goldsztejn N, Himpens J, Fils JF, et al. Effect of opioid-free versus opioid-based strategies during multimodal anaesthesia on postoperative morphine consumption after bariatric surgery: a randomised double-blind clinical trial. BJA Open. (2024) 9:100263. doi: 10.1016/j.bjao.2024.100263
20. Perez JJ, Strunk JD, Preciado OM, DeFaccio RJ, Chang LC, Mallipeddi MK, et al. Effect of an opioid-free anesthetic on postoperative opioid consumption after laparoscopic bariatric surgery: a prospective, single-blinded, randomized controlled trial. Reg Anesth Pain Med. (2024) 50:699–705. doi: 10.1136/rapm-2024-105632
21. Dagher C, Mattar R, Aoun M, Tohme J, Naccache N, Jabbour H. Opioid-free anesthesia in bariatric surgery: a prospective randomized controlled trial. Eur J Med Res. (2025) 30:320. doi: 10.1186/s40001-025-02565-9
22. Barakat H, Gholmieh L, Nader JA, Karam VY, Albaini O, Helou ME, et al. Opioid-free versus opioid-based anesthesia in laparoscopic sleeve gastrectomy: a single-center, randomized, controlled trial. Perioper Med. (2025) 14:16. doi: 10.1186/s13741-024-00486-5
23. Barakat H, Al Nawwar R, Abou Nader J, Aouad M, Yazbeck Karam V, Gholmieh L. Opioid-free versus opioid-based anesthesia in major spine surgery: a prospective, randomized, controlled clinical trial. Minerva Anestesiol. (2024) 90:482–90. doi: 10.23736/S0375-9393.24.17962-X
24. Qian XL, Li P, Chen YJ, Xu SQ, Wang X, Feng SW. Opioid free total intravenous anesthesia with dexmedetomidine-esketamine-lidocaine for patients undergoing lumpectomy. J Clin Med Res. (2023) 15:415–22. doi: 10.14740/jocmr5000
25. An G, Wang G, Zhao B, Zhang X, Li Z, Fu J, et al. Opioid-free anesthesia compared to opioid anesthesia for laparoscopic radical colectomy with pain threshold index monitoring: a randomized controlled study. BMC Anesthesiol. (2022) 22:241. doi: 10.1186/s12871-022-01747-w
26. Zhang L, Yu X-H, Zhang H-M, Wang S, Chen J-L, Li X-S, et al. Efficacy of opioid-free anesthesia in short-term recovery following laparoscopic-assisted colorectal tumor resection: a randomized trial. Front Oncol. (2025) 15:1588623. doi: 10.3389/fonc.2025.1588623
27. Luong NV. Evaluation of efficacy of free opioid anesthesia for laparoscopic cholecystectomy: a prospective, randomized double-blinded study. Open Anesth J. (2000) 14:73–9. doi: 10.2174/2589645802014010073
28. Hu Y, Zhang QY, Qin GC, Zhu GH, Long X, Xu JF, et al. Balanced opioid-free anesthesia with lidocaine and esketamine versus balanced anesthesia with sufentanil for gynecological endoscopic surgery: a randomized controlled trial. Sci Rep. (2024) 14:11759. doi: 10.1038/s41598-024-62824-3
29. Hublet S, Galland M, Navez J, Loi P, Closset J, Forget P, et al. Opioid-free versus opioid-based anesthesia in pancreatic surgery. BMC Anesthesiol. (2022) 22:9. doi: 10.1186/s12871-021-01551-y
30. Xue Z, Yan C, Liu Y, Yang N, Zhang G, Qian W, et al. Opioid-free anesthesia with esketamine-dexmedetomidine versus opioid-based anesthesia with propofol-remifentanil in shoulder arthroscopy: a randomized controlled trial. BMC Surg. (2024) 24:228. doi: 10.1186/s12893-024-02518-9
31. An G, Zhang Y, Chen N, Fu J, Zhao B, Zhao X. Opioid-free anesthesia compared to opioid anesthesia for lung cancer patients undergoing video-assisted thoracoscopic surgery: a randomized controlled study. PLoS One. (2021) 16:e0257279. doi: 10.1371/journal.pone.0257279
32. Wang S, Li Y, Liang C, Han X, Wang J, Miao C. Opioid-free anesthesia reduces the severity of acute postoperative motion-induced pain and patient-controlled epidural analgesia-related adverse events in lung surgery: randomized clinical trial. Front Med. (2023) 10:1243311. doi: 10.3389/fmed.2023.1243311
33. Feng C, Xu Y, Chen S, Song N, Meng X, Liu H, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. (2024) 132:267–76. doi: 10.1016/j.bja.2023.11.008
34. Kim M, Huh J, Choi H, Hwang W. No difference in postoperative recovery outcomes between opioid-free and opioid-sparing anesthesia under multimodal analgesic protocol for video-assisted thoracoscopic surgery: a propensity score matching cohort study. J Clin Med. (2024) 13:6581. doi: 10.3390/jcm13216581
35. Yan H, Chen W, Chen Y, Gao H, Fan Y, Feng M, et al. Opioid-Free versus opioid-based anesthesia on postoperative pain after thoracoscopic surgery: the use of intravenous and epidural esketamine. Anesth Analg. (2023) 137:399–408. doi: 10.1213/ANE.0000000000006547
36. Yan X, Liang C, Jiang J, Ji Y, Wu A-S, Wei C-W. Effects of balanced opioid-free anesthesia on post-operative nausea and vomiting in patients undergoing video-assisted thoracic surgery: a randomized trial. BMC Anesthesiol. (2025) 25:62. doi: 10.1186/s12871-025-02938-x
37. Selim J, Jarlier X, Clavier T, Boujibar F, Dusséaux M-M, Thill J, et al. Impact of opioid-free anesthesia after video-assisted thoracic surgery: a propensity score study. Ann Thorac Surg. (2022) 114:218–24. doi: 10.1016/j.athoracsur.2021.09.014
38. Wang D, Sun Y, Zhu Y-J, Shan X-S, Liu H, Ji F-H, et al. Comparison of opioid-free and opioid-inclusive propofol anaesthesia for thyroid and parathyroid surgery: a randomised controlled trial. Anaesthesia. (2024) 79:1072–80. doi: 10.1111/anae.16382
39. Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. (2021) 134:541–51. doi: 10.1097/ALN.0000000000003725
40. Doleman B, Mathiesen O, Sutton AJ, Cooper NJ, Lund JN, Williams JP. Non-opioid analgesics for the prevention of chronic postsurgical pain: a systematic review and network meta-analysis. Br J Anaesth. (2023) 130:719–28. doi: 10.1016/j.bja.2023.02.041
41. Hah J, Mackey SC, Schmidt P, McCue R, Humphreys K, Trafton J, et al. Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort: a randomized clinical trial. JAMA Surg. (2018) 153:303–12. doi: 10.1001/jamasurg.2017.4915
42. Forget P, Cata J. Stable anesthesia with alternative to opioids: are ketamine and magnesium helpful in stabilizing hemodynamics during surgery? A systematic review and meta-analyses of randomized controlled trials. Best Pract Res Clin Anaesthesiol. (2017) 31:523–31. doi: 10.1016/j.bpa.2017.07.001
43. Ozturk T, Kaya H, Aran G, Aksun M, Savaci S. Postoperative beneficial effects of esmolol in treated hypertensive patients undergoing laparoscopic cholecystectomy. Br J Anaesth. (2008) 100:211–4. doi: 10.1093/bja/aem333
44. López-Álvarez S, Mayo-Moldes M, Zaballos M, Iglesias BG, Blanco-Dávila R. Esmolol versus ketamine-remifentanil combination for early postoperative analgesia after laparoscopic cholecystectomy: a randomized controlled trial. Can J Anesth Can Anesth. (2012) 59:442–8. doi: 10.1007/s12630-012-9684-x
45. Kianian S, Bansal J, Lee C, Zhang K, Bergese SD. Perioperative multimodal analgesia: a review of efficacy and safety of the treatment options. Anesthesiol Perioper Sci. (2024) 2:9. doi: 10.1007/s44254-023-00043-1
46. Gerriets V, Anderson J, Patel P, Nappe TM. Acetaminophen. Treasure Island, FL: StatPearls Publishing (2025).
47. Wu CL, King AB, Geiger TM, Grant MC, Grocott MPW, Gupta R, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative opioid minimization in opioid-naive patients. Anesth Analg. (2019) 129:567–77. doi: 10.1213/ANE.0000000000004194
48. Simpson JC, Bao X, Agarwala A. Pain management in enhanced recovery after surgery (ERAS) protocols. Clin Colon Rectal Surg. (2019) 32:121–8. doi: 10.1055/s-0038-1676477
49. Liu Y, Ma W, Zuo Y, Li Q. Opioid-free anaesthesia and postoperative quality of recovery: a systematic review and meta-analysis with trial sequential analysis. Anaesth Crit Care Pain Med. (2025) 44:101453. doi: 10.1016/j.accpm.2024.101453
50. Ghlichloo I, Gerriets V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). Treasure Island, FL: StatPearls Publishing (2025).
51. Kaye AD, Urman RD, Rappaport Y, Siddaiah H, Cornett EM, Belani K, et al. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J Anaesthesiol Clin Pharmacol. (2019) 35:S40–5. doi: 10.4103/joacp.JOACP_51_18
52. Mieszczański P, Górniewski G, Ziemiański P, Cylke R, Lisik W, Trzebicki J. Comparison between multimodal and intraoperative opioid free anesthesia for laparoscopic sleeve gastrectomy: a prospective, randomized study. Sci Rep. (2023) 13:12677. doi: 10.1038/s41598-023-39856-2
54. Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. (2019) 74:651–62. doi: 10.1111/anae.14582
55. Feenstra ML, Jansen S, Eshuis WJ, van Berge Henegouwen MI, Hollmann MW, Hermanides J. Opioid-free anesthesia: a systematic review and meta-analysis. J Clin Anesth. (2023) 90:111215. doi: 10.1016/j.jclinane.2023.111215
56. Cheng L, Liu J, Qin S, Geng X, Jing L, Fang S. Safety and effectiveness of multimodal opioid-free anaesthesia for pain and recovery after laparoscopic surgery: a systematic review and meta-analysis. BMJ Open. (2025) 15:e085988. doi: 10.1136/bmjopen-2024-085988
57. Zhang Z, Li C, Xu L, Sun X, Lin X, Wei P, et al. Effect of opioid-free anesthesia on postoperative nausea and vomiting after gynecological surgery: a systematic review and meta-analysis. Front Pharmacol. (2024) 14:1330250. doi: 10.3389/fphar.2023.1330250
58. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. (2013) 118:1332. doi: 10.1097/ALN.0b013e318289b84b
59. Katz J, Clairoux M, Redahan C, Kavanagh BP, Carroll S, Nierenberg H, et al. High dose alfentanil pre-empts pain after abdominal hysterectomy. Pain. (1996) 68:109–18. doi: 10.1016/S0304-3959(96)03172-7
60. Santa Cruz Mercado LA, Liu R, Bharadwaj KM, Johnson JJ, Gutierrez R, Das P, et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. (2023) 158:854–64. doi: 10.1001/jamasurg.2023.2009
61. Guo Z, Shan Z, Wang F. Research trends and knowledge mapping of opioid-free anesthesia: a global bibliometric analysis. J Multidiscip Healthcare. (2025) 18:4145–57. doi: 10.2147/JMDH.S533687
62. Shanthanna H, Joshi GP. Opioid-free general anesthesia: considerations, techniques, and limitations. Curr Opin Anesthesiol. (2024) 37:384. doi: 10.1097/ACO.0000000000001385
Keywords: opioid-free anesthesia (OFA), multimodal analgesia, enhanced recovery after surgery (ERAS), postoperative pain, non-opioid analgesics, opioid crisis
Citation: Pershad A, Elvir Lazo OL and Wong R (2025) Opioid-free anesthesia: a scoping review of efficacy, safety, and implementation challenges. Front. Anesthesiol. 4:1714040. doi: 10.3389/fanes.2025.1714040
Received: 26 September 2025; Accepted: 16 October 2025;
Published: 4 November 2025.
Edited by:
Lucas Ferreira Gomes Pereira, Universidade de Sao Paulo Anestesiologia, BrazilReviewed by:
Carlos Darcy Alves Bersot, Federal University of São Paulo, BrazilJosé Eduardo Guimarães Pereira, Hospital Central do Exercito, Brazil
Vitor Felippe, National Cancer Institute (INCA), Brazil
Copyright: © 2025 Pershad, Elvir Lazo and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ofelia Loani Elvir Lazo, bG9hbmlkb2NAeWFob28uY29t
 Amogh Pershad
Amogh Pershad Ofelia Loani Elvir Lazo
Ofelia Loani Elvir Lazo Robert Wong2
Robert Wong2