- 1Bacteriology Division, United States Army Medical Research Institute of Infectious Diseases, Frederick, MD, United States
- 2Defence Science and Technology Laboratory, Porton Down, Salisbury, United Kingdom
- 3Respiratory Sciences, University of Leicester, Leicester, United Kingdom
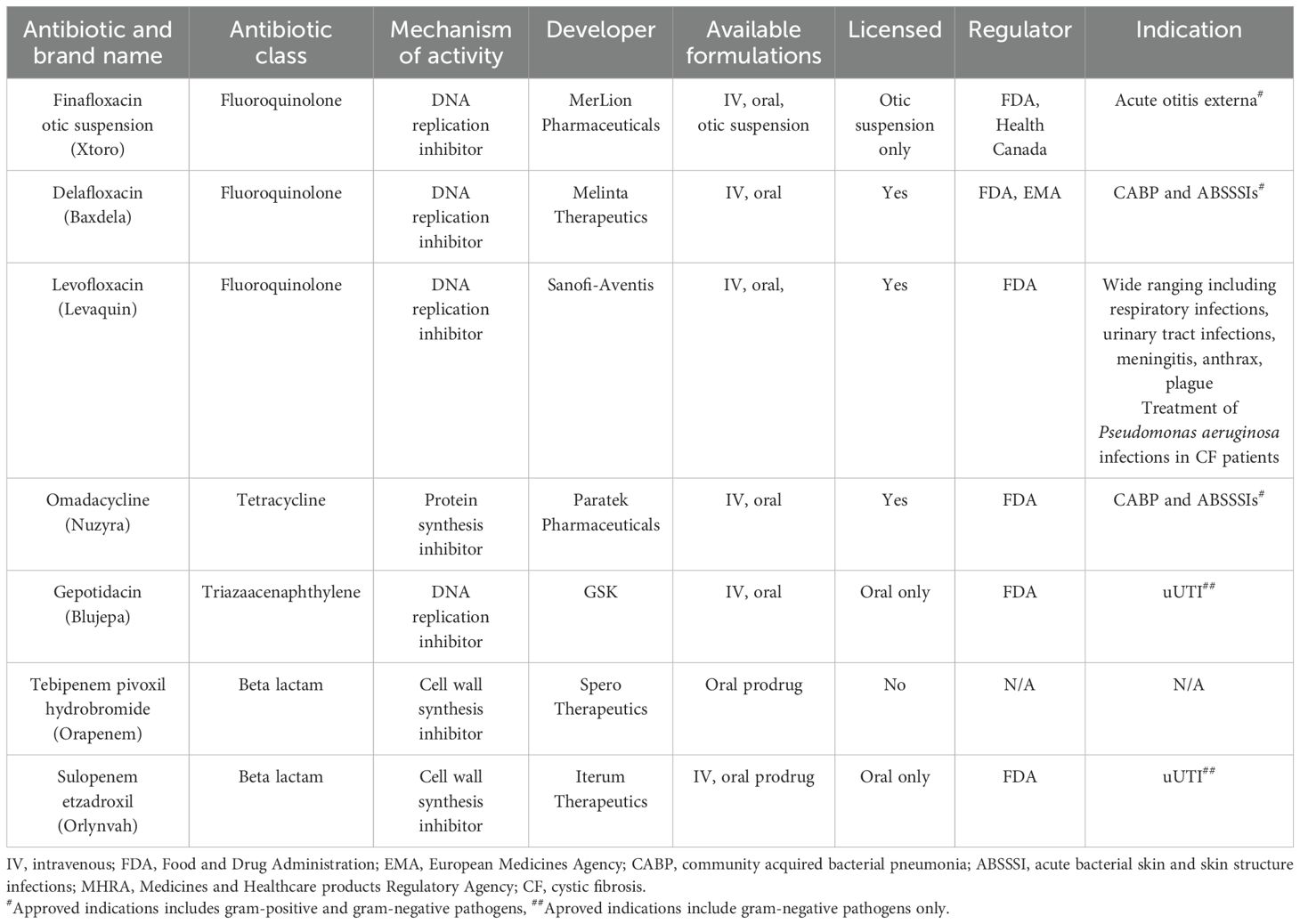
The development of medical countermeasures against pathogens of biodefense concern remains critical to protecting military and public health. This review compares data detailing antibacterial activity and efficacy for a selection of antibiotics evaluated against potential bacterial biothreat pathogens. The human safety and tolerability of these formulations were also considered. This review includes finafloxacin, levofloxacin, delafloxacin, omadacycline, gepotidacin, tebipenem and sulopenem. The selection criteria of these antibiotics were 1) the availability of an oral formulation, 2) the regulatory status (licensed by a regulatory authority or in an advanced stage of development) and 3) the availability of publicly available information on the biodefence pathogens of concern. We hope to highlight approved or advanced clinical candidates that have significant and unique potential in the biodefense space which may be deployed to protect both the public and warfighter against these bacterial infections.
Introduction
Effective and efficient biodefence strategies can be addressed, in part, through the use of broad spectrum antibiotics to provide an enhanced treatment capability against potential bacterial biothreat pathogens. These pathogens may include Yersinia pestis, Francisella tularensis, Burkholderia pseudomallei, Burkholderia mallei, Bacillus anthracis, and Coxiella burnetii, which cause the diseases plague, tularaemia, melioidosis, glanders, anthrax and Q fever, respectively1,2. They can be challenging to treat, particularly when patients have severe symptoms, and advanced disseminated disease, sepsis, or chronic infection, all of which require efficacious and lengthy courses of antibiotics. Current treatments for these infections include ciprofloxacin and levofloxacin (e.g., plague, tularaemia, anthrax), gentamicin (e.g., plague, tularaemia), doxycycline (e.g., plague, tularaemia, anthrax, Q fever) and ceftazidime/meropenem with co-trimoxazole/co-amoxiclav (e.g., melioidosis, glanders) (Van Zandt et al., 2013; Nelson et al., 2021; Bower et al., 2023; Currie et al., 2023; Nelson et al., 2024).
Seven antibiotics were selected for review based on the availability of an oral formulation and their licensure status by the Food and Drug Administration (FDA), the European Medicines Agency (EMA), or the Medicines and Healthcare Products Regulatory Agency (MHRA), either being licensed or close to being licensed for a non-biothreat clinical indication. Antibiotics with oral formulations were selected as they can be self-administered without the need for in-patient care. An open-source literature review was performed, identifying published in vitro antibacterial activity and in vivo efficacy data for the fluoroquinolones finafloxacin, delafloxacin and levofloxacin, the tetracycline omadacycline, the triazaacenaphthylene gepotidacin and the β-lactams tebipenem and sulopenem (Table 1). The literature reviewed included published manuscripts. We recognise other unpublished data may have been generated which is not accessible and is therefore excluded from this review.
Antimicrobial susceptibility tests (AST) including the broth microdilution assay are well characterized and are generally used to establish in vitro drug efficacy. The lowest concentration of an antibiotic at which bacterial growth is completely inhibited is termed the minimum inhibitory concentration (MIC). Using bacterial strain panels, the MIC50 (the MIC value where ≥ 50% of the strain panel is inhibited) and the MIC90 (the MIC value where ≥ 90% of the strain panel is inhibited) can be calculated (Schwarz et al., 2010). These values are useful benchmarks of therapeutic drug activity and where available are included herein. In vivo evaluation data that is publicly available was also included.
The evaluation of medical countermeasures in well-characterised animal models is fundamental, as clinical trials for these diseases may not be ethically justified. Typically, efficacy is determined in mouse models should the disease model be appropriate, and if warranted, be transitioned into higher order animal species. Parameters included in this review include survival (often the primary indicator of efficacy) and bacterial clearance in tissues (if determined). Although an attempt has been made to compare in vivo data sets, direct comparisons are challenging due to diverse experimental parameters (e.g., different aerobiology equipment, laboratory process differences, bacterial and animal species/strains, different challenge doses used and antibiotic dosing regimens (e.g., time of initiation, dose, and regularity of dosing).
Antibiotics
Finafloxacin
Finafloxacin (MerLion Pharmaceuticals) is a fifth-generation fluoroquinolone under development for the treatment of complicated urinary tract infections (cUTIs) and pyelonephritis (Table 1). There are three formulations available/in development, including a topical suspension which is licensed by the FDA and Health Canada for acute otitis externa. Additionally, intravenous (IV) and oral formulations have been evaluated in phase 1 and 2 clinical trials for cUTI. Finafloxacin binds to the bacterial DNA gyrase and topoisomerase IV preventing DNA replication. It is mainly differentiated from previous generations of the fluoroquinolones by its ability to retain antibacterial activity in acidic conditions, which is typical of infected body sites or in patients with acute sepsis (Higgins et al., 2010; Lemaire et al., 2011; Stubbings et al., 2011). Finafloxacin was shown to be superior to the second-generation fluoroquinolone ciprofloxacin in two cUTI/pyelonephritis clinical trials and retained potency against clinical strains shown to be resistant to ciprofloxacin (Vente et al., 2018; Wagenlehner et al., 2018).
Broad spectrum in vitro activity has been demonstrated for finafloxacin against Y. pestis, F. tularensis, B. pseudomallei, B. mallei, B. anthracis and C. burnetii at both neutral and acidic pH (Barnes et al., 2019; Peyrusson et al., 2021) (Table 2). The MIC90 values obtained for finafloxacin were low and comparable with standard-of-care antibiotics typically used as positive controls in these assays (fluoroquinolones and ceftazidime) with improved potency at acidic pH (Barnes et al., 2019). At pH 5 these were: ≤0.03 μg/mL (Y. pestis), 4 μg/mL (B. pseudomallei), 0.5 μg/mL (B. mallei) and ≤0.03 μg/mL (B. anthracis) and at pH 7: 0.06 μg/mL (Y. pestis), ≤0.03 μg/mL (F. tularensis), 4 μg/mL (B. pseudomallei), 0.5 μg/mL (B. mallei) and 0.12 μg/mL (B. anthracis). In addition, bactericidal activity was demonstrated in time kill assays against all of the bacterial agents, except for C. burnetii, where a cell culture model was used to demonstrate a 300-fold reduction in the intracellular bacterial load following finafloxacin treatment (Peyrusson et al., 2021) (Table 2). It has been suggested that this improved activity is due to the rapid influx of finafloxacin into cells, the accumulation of high levels within the cell and a slow efflux rate out (Chalhoub et al., 2020).
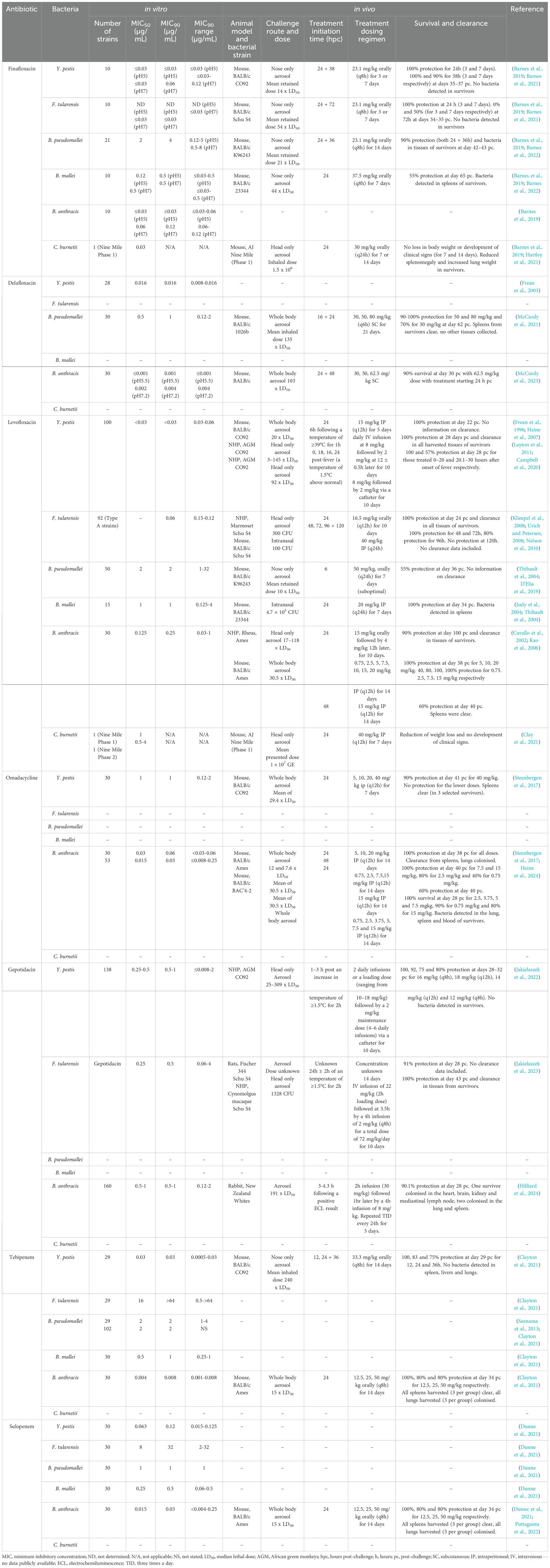
Table 2. A summary of the published in vitro and in vivo data for the biodefence pathogens and the antibiotics discussed in this review.
In vivo efficacy of finafloxacin has also been demonstrated using an orally delivered human equivalent dose in murine models of inhalational tularaemia, plague, Q fever, melioidosis and glanders (Table 2). Finafloxacin offered protection that was not statistically different to that afforded by ciprofloxacin and bacterial clearance when administered as treatment for plague. It was also comparable to co-trimoxazole as a treatment for glanders (Table 2) (Barnes et al., 2021; Barnes et al., 2022). Finafloxacin offered a significant improvement in survival compared to ciprofloxacin and doxycycline for the treatment of melioidosis and ciprofloxacin for the treatment of tularaemia (Barnes et al., 2021; Barnes et al., 2022). In a non-lethal mouse model of Q fever, finafloxacin reduced the clinical signs of infection and weight loss when compared to ciprofloxacin and doxycycline (Hartley et al., 2021).
Delafloxacin
Delafloxacin (Melinta Therapeutics) is a fourth-generation fluoroquinolone, approved by the FDA and the EMA for the treatment of community acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSIs) (Table 1) (Melinta Therapeutics, 2017). Both IV and oral formulations are available, allowing for administration in both the inpatient and outpatient settings (McCurdy et al., 2023). Like finafloxacin, delafloxacin inhibits bacterial DNA gyrase and topoisomerase IV, and also has enhanced MICs at low pH, demonstrating a bactericidal effect against gram-negative and gram-positive organisms (Kocsis et al., 2021).
The MIC90 values obtained for Y. pestis (0.016 μg/mL [pH 7.2]) and B. anthracis (≤0.001 μg/mL [pH 5.5] and 0.04 μg/mL [pH 7.2]), are low and comparable to those obtained for the fluoroquinolone class (0.016-0.06 μg/mL) (Table 2) (Frean et al., 2003; McCurdy et al., 2023). The MIC90 for B. pseudomallei (1 μg/mL) is comparable to standard-of-care antibiotics. Delafloxacin also demonstrates activity against B. pseudomallei overexpressing RND efflux pumps such as BpeEF-OprC (McCurdy et al., 2021; McCurdy et al., 2022).
Delafloxacin has been shown to be efficacious in murine models of inhalational melioidosis and anthrax (Table 2). Delafloxacin afforded protection not significantly different to ciprofloxacin in mice infected with B. anthracis (McCurdy et al., 2023). Bacterial clearance was observed in the spleens of survivors from the anthrax study; however, lungs were colonized, likely due to spore persistence. When evaluated against inhalational melioidosis, delafloxacin offered a significant improvement in survival compared to ceftazidime and cleared colonizing bacteria from spleens (McCurdy et al., 2021).
Levofloxacin
Levofloxacin is a third-generation fluoroquinolone licensed by the MHRA and FDA for indications including pneumonia, rhinosinusitis, chronic bronchitis, pyelonephritis, urinary tract infections and skin or skin structure infections (Table 1) (Podder et al., 2025). In addition, it is the only antibiotic discussed in this review which is licensed by the FDA for the treatment of Y. pestis and B. anthracis infections. Levofloxacin has the same mechanism of action as finafloxacin and delafloxacin and has broad spectrum activity against gram-negative and gram-positive organisms including methicillin resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis (Croom and Goa, 2003).
The MIC90 values obtained for Y. pestis, F. tularensis, and B. anthracis are low (< 0.03, 0.06, and 0.25 μg/mL respectively) and comparable to those for other fluoroquinolones (Table 2) (Frean et al., 1996; Cavallo et al., 2002; Urich and Petersen, 2008). Similarly, the MIC90s for B. pseudomallei and B. mallei (2 μg/mL and 1 μg/mL respectively) are comparable with comparator antibiotics (Thibault et al., 2004). There is limited in vitro data generated for C. burnetii, however an MIC of 1 μg/mL has been reported for strain Nine Mile (Phase I) with an intracellular MIC of 0.16 μg/mL (Clay et al., 2021; Hartley et al., 2021).
In vivo efficacy studies delivering the antibiotic by the IV and oral routes have been performed in murine and non-human primate (NHP) models (Table 2). Levofloxacin completely protected animals and cleared bacteria from tissues in an African Green Monkey (AGM) model of plague and a marmoset model of tularaemia (Nelson et al., 2010; Layton et al., 2011). Delaying treatment resulted in a reduction in survival in the AGM (Campbell et al., 2020). High levels of protection and clearance was also demonstrated in a rhesus macaque model of anthrax treated with levofloxacin (Kao et al., 2006).
Levofloxacin provided complete protection when delivered early in a murine model of plague (Heine et al., 2007). High levels of protection were demonstrated when levofloxacin was delivered following an intranasal challenge of F. tularensis and B. mallei (Judy et al., 2004; Klimpel et al., 2008). Limited information is available for the in vivo evaluation of B. pseudomallei infections with levofloxacin as fluoroquinolones are not clinically recommended for melioidosis; however, 55% survival was reported when a suboptimal course of levofloxacin was initiated at 6 hours post-challenge in a mouse model (D'Elia et al., 2019). Levofloxacin delivered by the intraperitoneal route reduced weight loss and the development of clinical signs of disease in a mouse model of Q fever (Clay et al., 2021).
Omadacycline
Omadacycline (Paratek Pharmaceuticals) is a first-in-class aminomethylcycline of the tetracycline family, approved by the FDA in 2018 for the treatment of CABP and ABSSSIs (Watkins and Deresinski, 2019) (Table 1). In addition, it is the first once-daily multi-indication oral antibiotic to be approved by the FDA in 10 years (Watkins and Deresinski, 2019). Both oral and IV formulations are available. Mechanistically, it binds the 30S ribosomal subunit, preventing the binding of aminoacyl-tRNA and inhibiting protein synthesis. Omadacycline is active against a wide range of pathogens including MRSA, vancomycin resistant Enterococcus and penicillin resistant S. pneumoniae (Tanaka et al., 2016).
The MIC90 obtained for Y. pestis (1 μg/mL), which, whilst higher than the previously discussed fluoroquinolones, is within the range of susceptible gram-negative pathogens for the class (Table 2) (Steenbergen et al., 2017). The MIC90 for B. anthracis has been reported as 0.06 μg/mL and 0.03 μg/mL which is comparable to previous generations of the fluoroquinolones (Steenbergen et al., 2017; Heine et al., 2024). Omadacycline also demonstrated high potency against the ciprofloxacin-resistant strain of Ames (BAC’4-2) (Heine et al., 2024).
In vivo efficacy delivering the antibiotic by the IP route has also been demonstrated in murine models of inhalational plague and anthrax (Table 2). Omadacycline was shown to offer an equivalent level of protection to ciprofloxacin when administered as treatment for infection with Y. pestis (Steenbergen et al., 2017). Bacterial clearance was observed in spleens. When evaluated against infection with B. anthracis, omadacycline also offered an equivalent level of protection to ciprofloxacin (Table 2) (Steenbergen et al., 2017). Spleens were clear from colonizing bacteria in survivors. In a separate study, omadacycline provided complete protection in an inhalational anthrax mouse model with strain BAC’4-2 (Heine et al., 2024).
Gepotidacin
Gepotidacin (GSK) is a bactericidal first-in-class triazaacenaphthylene that was recently approved by the FDA for the treatment of uncomplicated UTIs (uUTIs) (Wagenlehner et al., 2024) (Table 1). It is also in development for the treatment of gonorrhoea and both oral and IV formulations have been produced. Gepotidacin inhibits bacterial DNA gyrase and the type IIA topoisomerase at a site and mechanism distinct from the fluoroquinolones. As the first approved novel bacterial topoisomerase inhibitor (NBTI), gepotidacin is of interest as its potency is not impaired by the on-target mutations associated with fluoroquinolone resistance. Two phase 3 clinical trials evaluating gepotidacin as a therapeutic for uUTIs were stopped early due to the superiority of results obtained, leading to the FDA approving the use for the treatment of uUTIs in female adults and paediatric patients over 12 (GSK, 2022; GSK, 2025). Gepotidacin has demonstrated in vitro activity against gram-positive and gram-negative organisms, including MRSA, Shigella species, S. pneumoniae and Mycobacteria (Biedenbach et al., 2016; Ahmad et al., 2022).
Potency has been demonstrated in vitro for gepotidacin against Y. pestis, F. tularensis, and B. anthracis, all with MIC90 values between 0.5 and 1 μg/mL (Table 2) (Jakielaszek et al., 2022; Jakielaszek et al., 2023; Hilliard et al., 2024). It is worth noting that the in vitro MIC screening with gepotidacin utilised large panels of bacterial strains (120+), which is impressive. It also retained activity against aminoglycoside and doxycycline resistant mutants of Y. pestis and fluoroquinolone resistant mutants of B. anthracis (Jakielaszek et al., 2022; Hilliard et al., 2024).
Several studies utilizing large animal models have been published that demonstrate the efficacy of gepotidacin against Y. pestis, F. tularensis, and B. anthracis. This includes in vivo efficacy data in NHP models of plague and tularaemia where fever was used as a trigger-to-treat (Table 2). Gepotidacin provided a high level of protection (75-100%) and bacterial clearance in an AGM model of inhalational plague, irrespective of the antibiotic dose and dosing regimen (Jakielaszek et al., 2022). There were no differences between the level of protection offered in relation to the number of doses of antibiotic administered. This is similar to the data previously generated for ciprofloxacin and levofloxacin in this NHP model (Layton et al., 2011; Campbell et al., 2020). When administered to cynomolgus macaques following an inhalational F. tularensis exposure, gepotidacin provided complete protection and bacterial clearance (Jakielaszek et al., 2023). This is similar to data generated with levofloxacin in a marmoset model of tularaemia (Nelson et al., 2010). Gepotidacin was also shown to be 90% protective in a lethal, trigger-to-treat New Zealand white rabbit model of inhalational anthrax (Hilliard et al., 2024).
Tebipenem
Tebipenem pivoxil hydrobromide (Spero Therapeutics) is an oral carbapenem prodrug being developed for the treatment of cUTIs (Table 1). Carbapenems are bactericidal agents that enter the periplasm space and acylate penicillin-binding proteins (PBPs). This weakens the peptidoglycan of the cell wall which lyses the bacterial cell (Mahalingam and Shenoy, 2020). Traditionally, carbapenems have only been available for IV administration; therefore, the potential to leverage carbapenem activity in an orally-available drug would be significant. Tebipenem has been evaluated in a phase 3 clinical trial for the treatment of cUTIs and pyelonephiritis; however, the FDA has requested further data to be generated and submitted before considering licensure. Tebipenem is active against gram-negative and gram-positive organisms including extended spectrum β-lactamase (ESBL) and AmpC β-lactamase producing Klebsiella pneumoniae, Escherichia coli, Proteus spp, and MRSA (Cotroneo et al., 2020).
The MIC90 values obtained for Y. pestis, B. pseudomallei, B. mallei and B. anthracis are low (0.03, 2, 1 and 0.008 μg/mL, respectively (Table 2)) (Seenama et al., 2013; Clayton et al., 2021). The MIC for a ciprofloxacin resistant Ames strain of B. anthracis was similar (0.008 μg/mL). There was no measurable in vitro activity for F. tularensis (MIC90 of > 64 μg/mL), which is consistent with the activity of other carbapenems (Caspar and Maurin, 2017).
Oral tebipenem has been evaluated in murine models of pneumonic plague and inhalational anthrax (Table 2). It offered an equivalent level of protection to ciprofloxacin when administered as treatment for infection with Y. pestis (Clayton et al., 2021). Bacterial clearance was observed in lungs, livers and spleens. When evaluated against an infection with B. anthracis, tebipenem also offered an equivalent level of protection to ciprofloxacin (Clayton et al., 2021). Spleens were clear at the end of the study with lungs colonized.
Sulopenem
Sulopenem (Iterum Therapeutics) is a broad spectrum thiopenem β-lactam, being developed for the treatment of infections caused by multi-drug resistant bacteria (Table 1). Two formulations are currently being evaluated, an orally-available prodrug (sulopenem etzadroxil) or sulopenem for IV administration. Sulopenem retains many characteristics of the carbapenem family and shares the same mechanism of action (Zhanel et al., 2022). It has been evaluated in multiple phase clinical 3 trials for the treatment of uUTIs, cUTIs and pyelonephritis and is active against gram-negative and gram-positive organisms including penicillin resistant S. pneumoniae and H. influenzae and M. catarrhalis strains able to produce β-lactamases (Butler et al., 2023; Dunne et al., 2023). It was recently approved by the FDA to treat uUTIs in adult women with limited or no alternative oral antibacterial treatment options (delivered with the renal tubular transport inhibitor probenecid) (FDA, 2024).
The MIC90 values obtained for Y. pestis, B. pseudomallei, B. mallei and B. anthracis are low (0.12, 1, 0.5 and 0.03 μg/mL, respectively and similar to carbapenems (Dunne et al., 2021) (Table 2). Like tebipenem, there is limited in vitro activity for sulopenem against strains of F. tularensis (MIC90 of 32 μg/mL). Sulopenem has been evaluated for efficacy in a murine model of inhalational anthrax where it offered an equivalent level of protection to ciprofloxacin (Table 2) (Puttagunta et al., 2022). Spleens were clear of bacteria at the end of the study with lungs colonized.
Conclusions
The identification and evaluation of novel broad spectrum medical countermeasures antibiotics for the treatment of the diseases caused by the bacterial pathogens of biodefence interest remains a significant priority to both military and public health. This review discusses several antibiotics that are in advanced clinical development that, although not being developed for this purpose, have demonstrated efficacy against these pathogens, and offer potential alternatives or improvements to first-line therapies. Novel or newer generations of antibiotics such as those discussed here bring innovative tools to fight an increasingly variable biothreat landscape. Robust preclinical evaluation of candidates provides in vitro and in vivo efficacy data that can support regulatory approval or be leveraged in an emergency to rapidly identify alternative therapies. Continued work is needed to ensure the most appropriate and effective therapies are prepositioned to combat these virulent pathogens.
Author contributions
JM: Formal analysis, Investigation, Methodology, Writing – review & editing. MN: Conceptualization, Writing – review & editing. CC: Formal analysis, Supervision, Writing – review & editing. SE: Supervision, Writing – review & editing. SH: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was written with support from the UK Ministry of Defence and the Defense Threat Reduction Agency (project CB11395).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The opinions, interpretations, conclusions, and recommendations presented are those of the authors and are not necessarily endorsed by the U.S. Army or Department of Defense. The use of either trade or manufacturers’ names in this report does not constitute an official endorsement of any commercial products. This report may not be cited for purposes of advertisement.
Footnotes
References
Ahmad M. N., Garg T., Singh S., Shukla R., Malik P., Krishnamurthy R. V., et al. (2022). In vitro and in vivo activity of gepotidacin against drug-resistant mycobacterial infections. Antimicrob. Agents Chemother. 66, e0056422. doi: 10.1128/aac.00564-22
Barnes K. B., Bayliss M., Davies C., Richards M. I., Laws T. R., and Vente A. (2022). Harding SV Efficacy of finafloxacin in a murine model of inhalational glanders. Front. Microbiology Infect. Agents Disease 13, 1057202.
Barnes B., Richards M., Burgess G., Armstrong S. J., Bentley C., Maishman T., et al. (2022). Investigation of a combination therapy approach for the treatment of melioidosis. Front. Microbiology Infect. Agents Disease 13, 934312. doi: 10.3389/fmicb.2022.934312
Barnes K. B., Richards M. I., Laws T. R., Nunez A., Thwaite J. E., Bentley C., et al. (2021). Finafloxacin is an effective treatment for inhalational tularemia and plague in mouse models of infection. Antimicrob. Agents Chemother. 65, e02294–e02220. doi: 10.1128/AAC.02294-20
Barnes K. B., Zumbrun S. D., Halasohoris S. A., Desai P. D., Miller L. L., Richards M. I., et al. (2019). Demonstration of the broad spectrum in vitro activity of finafloxacin against pathogens of biodefence interest. Antimicrob. Agents Chemother. 63 (12), e01470–19. doi: 10.1128/AAC.01470-19
Biedenbach D. J., Bouchillon S. K., Hackel M., Miller L. A., Scangarella-Oman N. E., Jakielaszek C., et al. (2016). In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob. Agents Chemother. 60, 1918–1923. doi: 10.1128/AAC.02820-15
Bower W. A., Yu Y., Person M. K., Parker C. M., Kennedy J. L., Sue D., et al. (2023). CDC guidelines for the prevention and treatment of anthrax, 2023. MMWR Recomm Rep. 72, 1–47. doi: 10.15585/mmwr.rr7206a1
Butler M. S., Henderson I. R., Capon R. J., and Blaskovich M. A. T. (2023). Antibiotics in the clinical pipeline as of December 2022. J. Antibiot (Tokyo) 76, 431–473. doi: 10.1038/s41429-023-00629-8
Campbell J. L., Fay M. P., Lanning L. L., and Hewitt J. A. (2020). Effect of delaying treatment on efficacy of ciprofloxacin and levofloxacin in the african green monkey model of pneumonic plague. Clin. Infect. Dis. 70, S60–S65. doi: 10.1093/cid/ciz1234
Caspar Y. and Maurin M. (2017). Francisella tularensis Susceptibility to Antibiotics: A Comprehensive Review of the Data Obtained In vitro and in Animal Models. Front. Cell Infect. Microbiol. 7, 122. doi: 10.3389/fcimb.2017.00122
Cavallo J. D., Ramisse F., Girardet M., Vaissaire J., and Mock M. (2002). Hernandez E Antibiotic susceptibilities of 96 isolates of Bacillus anthracis isolated in France between 1994 and 2000. Antimicrob. Agents Chemother. 46, 2307–2309. doi: 10.1128/AAC.46.7.2307-2309.2002
Chalhoub H., Harding S. V., Tulkens P. M., and Van Bambeke F. (2020). Influence of pH on the activity of finafloxacin against extracellular and intracellular Burkholderia Thailandensis, Yersinia pseudotuberculosis and Francisella philomiragia and on its cellular pharmacokinetics in THP-1 monocytes. Clin. Microbiol. Infect. 26, e1–1254.e8. doi: 10.1016/j.cmi.2019.07.028
Clay K. A., Hartley M. G., Armstrong S., Bewley K. R., Godwin K., Rayner E., et al. (2021). Evaluation of the efficacy of doxycycline, ciprofloxacin, levofloxacin, and co-trimoxazole using in vitro and in vivo models of Q fever. Antimicrob. Agents Chemother. 65, e0067321. doi: 10.1128/AAC.00673-21
Clayton N. P., Jain A., Halasohoris S. A., Pysz L. M., Lembirik S., Zumbrun S. D., et al. (2021). In vitro and in vivo characterization of tebipenem (TBP), an orally active carbapenem, against biothreat pathogens. Antimicrob. Agents Chemother. 65, e02385–e02320. doi: 10.1128/AAC.02385-20
Cotroneo N., Rubio A., Critchley I. A., Pillar C., and Pucci M. J. (2020). In vitro and in vivo characterization of tebipenem, an oral carbapenem. Antimicrob. Agents Chemother. 64, e02240–e02219. doi: 10.1128/AAC.02240-19
Croom K. F. and Goa K. L. (2003). Levofloxacin. Drugs. 69, 2769–2802. doi: 10.2165/00003495-200363240-00008
Currie B. J., Janson S., Meumann E. M., Martin G. E., Ewin T., and Marshall C. S. (2023). The 2024 revised darwin melioidosis treatment guideline. Northern Territory Dis. Control Bull. 30, 3–12. Available at: https://health.nt.gov.au/professionals/centre-for-disease-control/northern-territory-disease-control-bulletin
D'Elia R. V., Woods S., Butcher W., McGahon J., Khadke S., Perrie Y., et al. (2019). Exploitation of the bilosome platform technology to formulate antibiotics and enhance efficacy of melioidosis treatments. J. Control Release 298, 202–212. doi: 10.1016/j.jconrel.2019.02.002
Dunne M. W., Aronin S. I., Das A. F., Akinapelli K., Breen J., Zelasky M. T., et al. (2023). Sulopenem for the treatment of complicated urinary tract infections including pyelonephritis: A phase 3, randomized trial. Clin. Infect. Dis. 76, 78–88. doi: 10.1093/cid/ciac704
Dunne M., Aronin S. I., Halasohoris S. A., Pysz L. M., Lembirik S., and Meinig J. M. (2021). In vitro antibacterial susceptibility testing of sulopenem against category A and B biothreat bacterial pathogens. Open Forum Infect. Diseases 8, S628. doi: 10.1093/ofid/ofab466.1266
FDA (2024). FDA approves new treatment for uncomplicated urinary tract infections in adult women who have limited or no alternative oral antibiotic treatment options. Available online at: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-treatment-uncomplicated-urinary-tract-infections-adult-women-who-have-limited-or-no (Accessed June 20, 2025).
Frean J. A., Arntzen L., Capper T., Bryskier A., and Klugman K. P. (1996). In vitro activities of 14 antibiotics against 100 human isolates of Yersinia pestis from a southern African plague focus. Antimicrob. Agents Chemother. 40, 2646–2647. doi: 10.1128/AAC.40.11.2646
Frean J., Klugman K. P., Arntzen L., and Bukofzer S. (2003). Susceptibility of Yersinia pestis to novel and conventional antimicrobial agents. J. Antimicrob. Chemother. 2, 294–296. doi: 10.1093/jac/dkg363
GSK (2022). EAGLE-2 and EAGLE-3 phase III trials for gepotidacin stopped early for efficacy following pre-planned interim analysis by Independent Data Monitoring Committee. Available online at: https://www.gsk.com/en-gb/media/press-releases/gsk-announces-phase-iii-trials-for-gepotidacin/ (Accessed June 20, 2025).
GSK (2025). Gepotidacin accepted for priority review by US FDA for treatment of uncomplicated urinary tract infections in female adults and adolescents. Available online at: https://www.gsk.com/en-gb/media/press-releases/gepotidacin-accepted-for-priority-review-by-us-fda-for-treatment-of-uncomplicated-urinary-tract-infections-in-female-adults-and-adolescents/ (Accessed June 20, 2025).
Hartley M. G., Norville I., Richards M., Barnes K., Bewley K., Vipond J., et al. (2021). Finafloxacin, a novel fluoroquinolone, reduces the clinical signs of infection and pathology in a mouse model of Q fever. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.760698
Heine H. S., Drusano G., Purcell B. K., Anastasiou D., Tanaka S. K., and Serio A. W. (2024). Omadacycline is active in vitro and in vivo against ciprofloxacin-resistant Bacillus anthracis. Antimicrob. Agents Chemother. 68, e0059524. doi: 10.1128/aac.00595-24
Heine H. S., Louie A., Sorgel F., Bassett J., Miller L., Sullivan L. J., et al. (2007). Comparison of 2 antibiotics that inhibit protein synthesis for the treatment of infection with Yersinia pestis delivered by aerosol in a mouse model of pneumonic plague. J. Infect. Dis. 196, 782–787. doi: 10.1086/520547
Higgins P. G., Stubbings W., Wisplinghoff H., and Seifert H. (2010). Activity of the investigational fluoroquinolone finafloxacin against ciprofloxacin-sensitive and resistant Acinetobacter baumannii isolates. Antimicrob. Agents Chemother. 54, 1613–1615. doi: 10.1128/AAC.01637-09
Hilliard J. J., Jakielaszek C., Mannino F., Hossain M., Qian L., Fishman C., et al. (2024). Efficacy of therapeutically administered gepotidacin in a rabbit model of inhalational anthrax. Antimicrob. Agents Chemother. 68, e0149723. doi: 10.1128/aac.01497-23
Jakielaszek C., Hilliard J. J., Mannino F., Hossain M., Qian L., Fishman C., et al. (2023). Efficacy of Intravenously Administered Gepotidacin in Cynomolgus Macaques following a Francisella tularensis Inhalational Challenge. Antimicrob. Agents Chemother. 67, e0138122. doi: 10.1128/aac.01381-22
Jakielaszek C., Hossain M., Qian L., Fishman C., Widdowson K., Hilliard J. J., et al. (2022). Gepotidacin is efficacious in a nonhuman primate model of pneumonic plague. Sci. Transl. Med. 14, eabg1787. doi: 10.1126/scitranslmed.abg1787
Judy B. M., Whitlock G. C., Torres A. G., and Estes D. M. (2004). Comparison of the in vitro and in vivo susceptibilities of Burkholderia mallei to Ceftazidime and Levofloxacin. BMC Microbiol. 9, 88. doi: 10.1186/1471-2180-9-88
Kao L. M., Bush K., Barnewall R., Estep J., Thalacker F. W., Olson P. H., et al. (2006). Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (post-exposure) rhesus monkey model. Antimicrob. Agents Chemother. 50, 3535–3542. doi: 10.1128/AAC.00090-06
Klimpel G. R., Eaves-Pyles T., Moen S. T., Taormina J., Peterson J. W., Chopra A. K., et al. (2008). Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine. 26, 6874–6882. doi: 10.1016/j.vaccine.2008.09.077
Kocsis B., Gulyás D., and Szabó D. (2021). Delafloxacin, finafloxacin, and zabofloxacin: novel fluoroquinolones in the antibiotic pipeline. Antibiotics. 10, 1506. doi: 10.3390/antibiotics10121506
Layton R. C., Mega W., McDonald J. D., Brasel T. L., Barr E. B., Gigliotti A. P., et al. (2011). Levofloxacin cures experimental pneumonic plague in African green monkeys. PloS Negl. Trop. Dis. 5, e959. doi: 10.1371/journal.pntd.0000959
Lemaire S., Van Bambeke F., and Tulkens P. M. (2011). Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int. J. Antimicrob. Agents 38, 52–59. doi: 10.1016/j.ijantimicag.2011.03.002
Mahalingam A. and Shenoy B. (2020). Tebipenem: A novel oral carbapenem. Pediatr. Infect. Disease 2, 25–28. doi: 10.5005/jp-journals-10081-1237
McCurdy S., Duffy E., Hickman M., Halasohoris S., and Zumbrun S. D. (2021). Efficacy of Delafloxacin against the Biothreat Pathogen Burkholderia pseudomallei. Antimicrob. Agents Chemother. 65, e0073621. doi: 10.1128/AAC.00736-21
McCurdy S., Halasohoris S. A., Babyak A. L., Lembirik S., Hoover R., Hickman M., et al. (2023). Efficacy of delafloxacin against the biothreat pathogen Bacillus anthracis. J. Antimicrob. Chemother. 78, 810–816. doi: 10.1093/jac/dkad015
McCurdy S. P., Somprasong N., and Schweizer H. P. (2022). Evaluation of Delafloxacin against a Burkholderia pseudomallei Efflux Mutant Panel. Microbiol. Spectr 10, e0090322. doi: 10.1128/spectrum.00903-22
Melinta Therapeutics (2017). Delafloxacin (Baxdela) UPSI. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf (Accessed June 20, 2025).
Nelson M., Lever M. S., Dean R. E., Pearce P. C., Stevens D. J., and Simpson A. J. (2010). Bioavailability and efficacy of levofloxacin against Francisella tularensis in the common marmoset (Callithrix jacchus). Antimicrob. Agents Chemother. 54, 3922–3926. doi: 10.1128/AAC.00390-10
Nelson C. A., Meaney-Delman D., Fleck-Derderian S., Cooley K. M., Yu P. A., Mead P. S., et al. (2021). Antimicrobial treatment and prophylaxis of plague: recommendations for naturally acquired infections and bioterrorism response. MMWR Recomm Rep. 70, 1–27. doi: 10.15585/mmwr.rr7003a1
Nelson C. A., Winberg J., Bostic T. D., Davis K. M., and Fleck-Derderian S. (2024). Systematic review: clinical features, antimicrobial treatment, and outcomes of human tularemia, 1993-2023. Clin. Infect. Dis. 78, S15–S28. doi: 10.1093/cid/ciad736
Peyrusson F., Whelan A. O., Hartley M. G., Norville I. H., Harding S. V., and Van Bambeke F. (2021). Intracellular activity of antibiotics against Coxiella burnetii in a model of activated human THP-1 cells. Antimicrob. Agents Chemother. 65, e0106121. doi: 10.1128/AAC.01061-21
Podder V., Patel P., and Sadiq N. M. (2025). “Levofloxacin,” in StatPearls (StatPearls Publishing, Treasure Island (FL).
Puttagunta S., Aronin S. I., Dunne M., Halasohoris S. A., Babyak A., Hourihan M. K., et al. (2022). Murine efficacy studies of sulopenem against bacillus anthracis. Open Forum Infect. Dis. 9, ofac492. doi: 10.1093/ofid/ofac492.1354
Schwarz S., Silley P., Simjee S., Woodford N., van Duijkeren E., Johnson A. P., et al. (2010). Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrobial Chemotherapy 65, 601–604. doi: 10.1093/jac/dkq037
Seenama C., Tiengrim S., and Thamlikitkul V. (2013). In vitro activity of tebipenem against Burkholderia pseudomallei. Int. J. Antimicrob. Agents 42, 375. doi: 10.1016/j.ijantimicag.2013.06.016
Steenbergen J., Tanaka S. K., Miller L. L., Halasohoris S. A., and Hershfield J. R. (2017). In Vitro and In Vivo Activity of Omadacycline against Two Biothreat Pathogens, Bacillus anthracis and Yersinia pestis. Antimicrob. Agents Chemother. 61, e02434–e02416. doi: 10.1128/AAC.02434-16
Stubbings W., Leow P., Yong G. C., Goh F., Körber-Irrgang B., Kresken M., et al. (2011). In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob. Agents Chemother. 9), 4394–4397. doi: 10.1128/AAC.00833-10
Tanaka S. K., Steenbergen J., and Villano S. (2016). Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med. Chem. 24, 6409–6419. doi: 10.1016/j.bmc.2016.07.029
Thibault F. M., Hernandez E., Vidal D. R., Girardet M., and Cavallo J. D. (2004). Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J. Antimicrob. Chemother. 54, 1134–1138. doi: 10.1093/jac/dkh471
Urich S. K. and Petersen J. M. (2008). In vitro susceptibility of isolates of Francisella tularensis types A and B from North America. Antimicrob. Agents Chemother. 52, 2276–2278. doi: 10.1128/AAC.01584-07
Van Zandt K. E., Greer M. T., and Gelhaus H. C. (2013). Glanders: an overview of infection in humans. Orphanet J. Rare Dis. 8, 131. doi: 10.1186/1750-1172-8-131
Vente A., Bentley C., Lückermann M., Tambyah P., and Dalhoff A. (2018). Early clinical assessment of the antimicrobial activity of finafloxacin compared to ciprofloxacin in subsets of microbiologically characterized isolates. Antimicrob. Agents Chemother. 62, e02325–e02317. doi: 10.1128/AAC.02325-17
Wagenlehner F., Nowicki M., Bentley C., Lückermann M., Wohlert S., Fischer C., et al. (2018). Explorative randomized phase II clinical study of the efficacy and safety of finafloxacin versus ciprofloxacin for treatment of complicated urinary tract infections. Antimicrob. Agents Chemother. 62, e02317–e02317. doi: 10.1128/AAC.02317-17
Wagenlehner F., Perry C. R., Hooton T. M., Scangarella-Oman N. E., Millns H., Powell M., et al. (2024). Oral gepotidacin versus nitrofurantoin in patients with uncomplicated urinary tract infection (EAGLE-2 and EAGLE-3): two randomised, controlled, double-blind, double-dummy, phase 3, non-inferiority trials. Lancet. 403, 741–755. doi: 10.1016/S0140-6736(23)02196-7
Watkins R. R. and Deresinski S. (2019). Omadacycline: A novel tetracycline derivative with oral and intravenous formulations. Clin. Infect. Dis. 69, 890–896. doi: 10.1093/cid/ciz242
Keywords: antibiotics, biodefence, medical countermeasures, antimicrobial susceptibility, biocontainment
Citation: Meinig JM, Nelson M, Cote CK, Emmett SR and Harding SV (2025) An evaluation of antibiotic options for the treatment of biothreat pathogens. Front. Antibiot. 4:1611588. doi: 10.3389/frabi.2025.1611588
Received: 14 April 2025; Accepted: 12 June 2025;
Published: 14 August 2025.
Edited by:
Abid Ali, Texas A and M University, United StatesReviewed by:
Jianhua Wang, Chinese Academy of Agricultural Sciences (CAAS), ChinaCrown Copyright © 2025 DSTL. Authors: Meinig, Nelson, Cote, Emmett and Harding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah V. Harding, c3ZoYXJkaW5nQGRzdGwuZ292LnVr; J. Matthew Meinig, amFtZXMubS5tZWluaWcuY2l2QGhlYWx0aC5taWw=
 J. Matthew Meinig
J. Matthew Meinig Michelle Nelson
Michelle Nelson Christopher K. Cote
Christopher K. Cote Stevan R. Emmett2
Stevan R. Emmett2 Sarah V. Harding
Sarah V. Harding