- 1Department of Pharmacology and Toxicology, School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2Department of Clinical Pharmacy, School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 3Department of Surgery, School of Medicine, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 4School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 5Department of Critical Care and Emergency Nursing, School of Nursing, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 6Department of Clinical Laboratory Sciences, School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 7Department of Epidemiology and Biostatistics, School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Background: Patients who suffer from surgical site infections (SSIs) bear a heavy clinical burden. Longer hospital stays are more likely for patients with SSIs because they are more likely to require treatment in an intensive care unit or to be readmitted to the hospital. Drug-resistant bacterial infections are becoming more common, which is one of the main challenges to effectively treating SSIs in hospitals.
Objective: The study aimed to determine the incidence, bacterial isolates, and susceptibility patterns among patients who had undergone major surgical procedures in eastern Ethiopia.
Methods: Hospital-based prospective multicenter cohort study design was used to determine the incidence of SSIs at public hospitals in eastern Ethiopia. A systematic sampling technique was used to determine the number of patients to participate from each hospital. Data were collected using pre-tested structured questionnaires, standard microbiological operating procedures were used, and the collected wound swab samples were transported to the Hiwot Fana Specialized Comprehensive University Hospital microbiology laboratory unit for identification of bacterial pathogens and antibiotics susceptibility test. The collected data was cleaned, entered and analyzed using SPSS version 22. A P-value <0.05 is considered as statistically significant.
Results: Out of 801 patients who had undergone surgery for different indications, 137(17.1%) (95% CI: 14.6–19.9) of them developed SSIs. Patients with contaminated wound type (AOR: 14.725, 95%CI: 8.210-26.410), who stayed more than 7 days in the hospitals (AOR: 2.45, 95%CI:1.113-5.402), those surgical procedures took more than 60 minutes (AOR:2.598, 95%CI: 1.217-5.546), who did not receive antibiotics prophylaxis (AOR: 5.506, 95%CI: 3.006-10.084), and the presence of comorbidity(AOR: 4.088, 95%CI: 2.266-7.375) were associated with a high risk of developing SSIs. A majority of bacterial isolates from SSIs were gram-negative (66.1%). Of the specific bacterial isolates, S. aureus was the predominant (30.5%) followed by E. coli (18.6%). S. aureus showed a multi-drug resistance against commonly used antimicrobials with some degree of susceptibility to vancomycin. Klebsiella pneumonia and E.coli were multi-drug resistant isolates, but they had a better susceptibility to meropenam, gentamycin, and piperacillin with tazobactam.
Conclusion: The presence of contaminated wound type, prolonged hospital stay, longer procedures, absence of antibiotic prophylaxis, and comorbidity increase the incidence of SSIs. S. aureus, Klebsiella pneumonia, and E.coli showed high resistance to multiple antibiotics commonly used in the management of SSIs. Therefore determining their antimicrobial susceptibility and providing defined antimicrobial therapy could lower morbidity, mortality, and healthcare costs, and could improve in-hospital outcomes is recommended to use vancomycin, meropenam, gentamycin, and piperacillin with tazobactam because of the observed susceptibility of common isolates for these drugs in the study area.
1 Background
Surgical site infection (SSI) is defined as an infection that occurs in surgical patients at the incision site within 30 days after surgery if there is no implant or within one year if there is an implant (Kim et al., 2016; Björnsson et al., 2013; Chong and Bartolo, 2008). It is a potential complication associated with any surgical procedure (Shin et al., 2013). Epidemiological evidence suggests that approximately 2–5% of surgical patients worldwide have developed SSIs (Chen et al., 2015). However, the incidence differs among developed and developing countries; more patients from developing countries are affected than those from developed countries. The incidence of SSI was 2.6% in the United States of America (USA), 1.6% in Germany, and 2.9% in different settings of European countries. However, in developing countries, it accounts for two times more than in developed countries (Ostapowicz et al., 2002). The average incidence of SSI in China was 4.5% among patients who underwent abdominal surgery (Lewis, 2006). In Sub-Saharan Africa, various study results showed that the SSI rate ranges from 11 to 18% (Lewis, 2006; Fernández-Castañer et al., 2008). More specifically, systematic reviews and meta-analyses in Ethiopia also showed that the rate of SSI ranged from 12.3% to 25.22% (Ibáñez et al., 2002; Larrey, 1995). SSIs also varied according to the type of procedures, where the highest risk was observed for orthopedic followed by cardiac and intra-abdominal surgery (Kaplowitz, 2004; Sun and Migaly, 2016).
Microorganisms from the patient’s skin flora or the environment surrounding the patient were the causes of SSIs (Brown, 2017; Pfenninger and Zainea, 2001; Yeo and Tan, 2014). In both cases, microorganisms can adhere to surgical instruments and consequently contaminate the incision wound, particularly during contaminated surgical procedures. Most of these infections are caused by multidrug-resistant microorganisms (Riss et al., 2012).
In most developing countries like Ethiopia, it is a common practice that antibiotics can be purchased without a prescription. This leads to the misuse of antibiotics by the public thus contributing to the emergence and spread of antimicrobial resistance (Dejenie et al., 2021; Abate et al., 2021; Fiseha et al., 2021). However, studies assessing the etiological agents of SSIs in Ethiopia are scarce. Thus, it is necessary to identify bacterial agents and determine their antibiotic susceptibility pattern from wounds for empirical treatment in the situation of inadequate culture and sensitivity test service in Ethiopia (Fiseha et al., 2021). Thus, this study aimed to determine the magnitude of SSIs, patterns of bacterial pathogens, and susceptibility in the surgical and GYN/OBS wards of hospitals in eastern Ethiopia.
2 Materials and methods
2.1 Study setting and period
The study was conducted in three public Hospitals in Eastern Ethiopia where different and multidimensional aspects of health care services are provided to the patients: Hiwot Fana Comprehensive Specialized University Hospital (HFCSUH) from Harari Regional State, DRH from Dire Dawa administrative council, and Sheik Hassen Yabare Specialized University hospital (SHYSUH) from Somali regional state. The study was conducted from March 2023 to April 2024.
2.2 Study designs
A hospital-based prospective multicenter cohort study was employed to determine the incidence of SSI, to identify bacterial pathogens responsible for SSI and susceptibility patterns of isolate against commonly used antimicrobials among patients who underwent major surgical procedures at public hospitals in eastern Ethiopia.
2.3 Source population
All patients underwent major surgical procedures and all patients admitted with SSIs at public hospitals in eastern Ethiopia were the source population to measure the incidence, predictors, bacterial pathogens, antibiotic susceptibility pattern of isolate, and treatment outcomes of SSIs.
2.4 Study populations
All systematically selected patients who had undergone major surgical procedures and patients admitted with SSIs at the selected public hospitals in eastern Ethiopia during the study period were the study population to measure the incidence, predictors, bacterial pathogens, and antibiotic susceptibility pattern of isolates.
2.5 Inclusion and exclusion criteria
2.5.1 Inclusion criteria
Patients of all age groups and genders underwent major surgical procedures with visible incision (laparotomy, operations in the intra-abdominal alimentary tract such as procedures for gastro-duodenal, small intestinal, and colorectal diseases or appendicitis, thyroidectomy, herniotomy, mastectomy, amputations, open prostatectomy, cholecystectomy, thoracotomy, or splenectomy in surgical wards or abdominal hysterectomy or Caesarian Section in Gynecology/Obstetrics wards) were included into the study.
2.5.2 Exclusion criteria
Patients who had an operation a month developed SSI before the study period or died within 48 hours of the operation and patients.
2.6 Sample size determination
The sample size required for this study was determined by using Epi-Info version 7.1 (Paltiel et al., 2020) statistical software. For objective one (incidence rate of SSI), the required sample size was determined by using a single population proportion formula by taking the following assumptions: 21.1 incidence rate of SSI taken from a study done in JMC (Misha et al., 2021a), 95% confidence level and 4% margin of error. After adding a non-response rate of 10% and multiplying by a design effect of 2, since a multistage sampling technique will be employed, the calculated final sample size was 888.
2.7 Sampling techniques
A multi-stage sampling technique was used to select a representative sample of the study subjects. First, four study sites based on the administrative unit (Harari regional state, Somali regional state, and Dire-Dawa administrative council) were selected purposely. Second, three public hospitals one from each study site: HFCSUH from Harari Regional State, Sheikh Hassen Yabbere Specialized University Hospital from Somali regional state, and Dil Chora Referral Hospital from the Dire-Dawa Administration Council were selected purposely considering the level of the hospital and provision of major surgical services in the selected study area. The sample size was proportionally allocated to the selected hospitals based on the previous year’s annual client flow.
2.8 Data collection methods
2.8.1 Data collection tools
The data were collected using a pre-tested structured questionnaire prepared after reviewing relevant literature on each topic. The questionnaires used to measure the incidence and predictors of SSI include preoperative questions on patient demographics, comorbidities, laboratory values, intraoperative questions, availability of antibiotic prophylaxis, sample collection for microbiological analysis and antimicrobial susceptibility test of bacterial isolates of surgical site infections.
2.8.2 Data collectors
Twelve (12) trained BSc nurses collected data for 24 months, and six Master of Public Health professionals supervised the data collectors and collection process. Twelve BSc nurses for data collection (four nurses per hospital) and six masters of public health for supervision (two individuals per hospital) were recruited for the data collection process during the study period. Both the data collectors and the supervisors were not selected from the same hospitals as it may have compromised the quality of data. At each hospital, the aim of the study was clearly explained to the study participants before they filled out the questionnaire.
2.8.3 Data collection procedures
The selected patients were followed throughout their hospital stay. The data collectors collected the data using a range of methods including reviewing operation room logbooks medical charts, and specimens collected from patients suspected of SSI. Accordingly, the operation room logbook was reviewed daily to identify patients meeting the inclusion criteria. Patients’ names, hospital numbers, and wards were identified via OR records. Medical records, operative notes, anesthetics records, diagnostic imaging reports, microbiological and biochemical data, data on the operative procedure, and data on the use of antibiotic prophylaxis were extracted.
Physical examinations of the patients were made by a surgeon, gynecologist or attending physician, and confirmed developing SSIs based on the presence of at least one of the following signs or symptoms of infection within 30 days of the operation (Young and Khadaroo, 2014), pain, tenderness, localized swelling, redness, heat or purulent discharge (Björnsson et al., 2013), evidence of abscess or fever of >38°C in infections of the deep incision (Allegranzi et al., 2016), localized pain or tenderness with an organism isolated from an organ/space infection were considered for bacteriological analysis.
2.8.3.1 Swab sampling and laboratory methods
For patients who showed signs of SSIs, wound swabs were taken, put in Ames transport medium and sent to the laboratory for culture and antibiotic sensitivity tests. Swab samples were inoculated on blood agar and MacConkey agar medium and incubated at 370c for 24-48Hr. After incubation, colony growth, colour, and size of colonies were examined. Then a gram stain on a sample from the colony was done to determine the gram reaction (positive or negative) and morphology (cocci, bacilli). Additional biochemical tests (e.g., catalase, oxidase, fermentation tests) were done to identify the organism. Finally antimicrobial susceptibility test disk diffusion (Kirby-Bauer), broth microdilution (Metchock et al., 1999). The Kirby-Bauer disc diffusion method on Mueller-Hinton agar plates was used for antibiotic susceptibility testing. Disc strengths were recommended by the latest updated Clinical and Laboratory Standards Institute(CLSI) during microbial analysis,interpretative criteria for susceptibility and resistance testing were used. The test was performed by applying a bacterial inoculum of approximately 1–2×108CFU/mL to the surface of a large (150 mm diameter) Mueller-Hinton agar plate. Up to 12 commercially-prepared, fixed concentrations, paper antibiotic disks were placed on the inoculated agar surface. Plates were incubated for 16–24 h at 35°C before the determination of results. The zones of growth inhibition around each of the antibiotic disks were measured to the nearest millimeter. The diameter of the zone is related to the susceptibility of the isolate and the diffusion rate of the drug through the agar medium. The results of the disk diffusion test are “qualitative,” in that, a category of susceptibility (i.e., susceptible, intermediate, or resistant) was derived from the test (Nijs et al., 2003).
2.9 Study variables
2.9.1 Dependent variables
The outcome variables of this study were the incidence of SSI within 30 days of postoperative, bacterial pathogens responsible for SSIs, and susceptibility pattern of SSIs isolated against commonly used antimicrobials.
2.9.2 Independent variables
In this study, the explanatory variables of SSIs were categorized as Socio-demographic factors, patient and clinical-related factors, healthcare facility-related factors, ventilation of operation room, procedure-related factors, wound class (clean or clean-contaminated), type of incision, American Society of Anesthesiologists (ASA) score, duration of operation and amount of blood lost during operation, antimicrobial related factors, and comorbid disease-related factors.
2.10 Operational definitions
Surgical site infection: a wound infection that will occur within 30 days of operative procedures.
Clean wound: a wound that is not infected has no signs of inflammation and does not contain any foreign material or debris.
Clean-contaminated wound: a surgical wound that has a low level of contamination and involves entry into the respiratory, alimentary, genital, or urinary tracts
Contaminated wound: a wound that has been compromised by an outside object, spillage from the gastrointestinal tract, or a break in sterile technique during an operation.
Dirty or infected wound: an incision undertaken during an operation in which the viscera was perforated or when acute inflammation with pus was encountered during the operation (for example, emergency surgery for fecal peritonitis), and for traumatic wounds where treatment was delayed, and there was fecal contamination or devitalized tissue present”
American Society of Anesthesiologists (ASA) score classification: ASA-I: a normal healthy patient, ASA-II: a patient with a mild systemic disease with no emergency, ASA-III: a patient with severe systemic disease with no emergency, ASA-IV: a patient with severe systemic disease that is a constant threat to life with no emergency, ASA-V: a moribund patient not expected to survive without the operation, ASA-VI: declared brain dead patient whose organs being removed for donor purposes no emergency (Knuf et al., 2018).
Antibiotic susceptibility test: a test that determines how effective antibiotics are against microorganisms that cause infection
Antibiotic resistance: where bacteria that cause illness change and become resistant to the antibiotics used to treat them. This means that the bacteria are not killed and can continue to grow, making infections difficult or impossible to treat.
2.11 Data quality control
Data quality was ensured by using a structured questionnaire that was developed after thoroughly reviewing the pertinent literature. The English language questionnaire was first translated into the local languages (Amharic and Afan Oromo) by experts in the language and then back-translated to English to maintain its consistency. To enhance the reliability of the instrument, a pre-test was done on 5% of the planned sample size drawn from outside of the study area in nearby healthcare facilities (Jogula General Hospital and Bisidimo Hospital) on patients with similar characteristics to those in the study. The tools were amended based on the feedback obtained before data collection. Training was provided for data collectors and supervisors for three days on the purpose of the study, sampling and interview techniques, data collection tools, and data collection and handling techniques. Wound swab/pus aspirate sample collection, how to clean and sterilize reusable laboratory materials for laboratory attendants. A manufacturer’s instruction was followed during culture media preparation and sterilization. Besides, supportive supervision was done throughout the data collection period to solve immediately any problem that might encountered on the spot in the data collection process. Before data entry, data cleaning was done by eyeballing for completeness, inconsistencies, numerical errors, and missing data. During data entry, double data entry was done to validate the data and if discrepancies were observed, the entered data was verified with the primary data sources. Before analysis, data were cleaned by using simple frequencies and cross tabulation, and edited by re-categorization of categorical variables and categorization of continuous variables done to suit for analyses.
2.12 Data processing and analysis
The data were coded, cleaned, and entered into Epi-Data version 3.2 and exported to SPSS version 22 for analyses. Descriptive statistics were computed; categorical variables were expressed as frequency counts and percentages; and continuous variables, as mean (standard deviation). A logistic regression model was utilized to identify factors associated with the outcomes variable (incidence rate of SSI among patients undergoing major surgical procedures). The basic assumptions of the binary logistic regression were maintained by checking the appropriateness of the outcome variables structure as binary (SSI present or absent), observations independence, and linearity of independent variables and log odds. In multivariate analysis, confounding factors were controlled through various ways like inclusion of confounding variables in the model to ensure that the relationship between the independent variables and the dependent variable is not distorted by the presence of other influencing factors. Potential multicollinearity among the independent variables was checked using variance inflation factor (VIF); a VIF of 10 or more was considered for multicollinearity. Bivariable and multivariable logistic regression analyses were computed to identify the association between the independent and outcome variables. Variables identified prior as clinically relevant variables and additional variables with a P-value less than 0.25 in bivariate analysis were entered into the multivariable analysis to determine the independent predictors of the outcome variables by controlling for possible confounding factors. An adjusted odds ratio (AOR) with 95% CIs was computed to assess the strength of the relationship between predictor and outcome variables. Variables with a P-value less than 0.05 in the final multivariable model were considered statistically significant predictors of the outcome variables.
Quality control (QC) methods were implemented throughout the laboratory work process to ensure the trustworthiness of the study findings. Every technique was carried out aseptically, and all supplies, tools, and methods were properly regulated. The performance and sterility of culture mediums were evaluated. In accordance with the CLSI, international control bacterial strains E. coli ATCC 25922, P. aeruginosa ATCC 27853, and S. aureus ATCC 25923 were used to control the experiments conducted in this investigation. For the susceptibility test, a barium sulfate turbidity standard, which is equivalent to a 0.5 McFarland standard, was utilized to standardize the inoculum’s density of bacterial suspension (Misha et al., 2021a).
2.13 Ethical consideration
This study was approved by the Institutional Health and Research Ethics Review Committee (IHRERC) of the College of Health and Medical Science, Haramaya University with a reference Number of IHRERC/007/2022. An official letter was submitted to concerned health bureaus and the administrative body of the hospitals to secure permission. Letters of cooperation were written to all study sites. Informed, voluntary, written, and signed consent was sought from the study participants (age greater than 18 years) after an adequate explanation of the study’s purpose, procedure, potential risk, and benefit was provided. Similarly, informed, voluntary, written, and signed consent was also obtained from the participants’ guardians and parents under 18 years old. Confidentiality of the patient information was maintained and the data were used only for research.
3 Result
3.1 Socio-demographic and behavioral characteristics of study participants
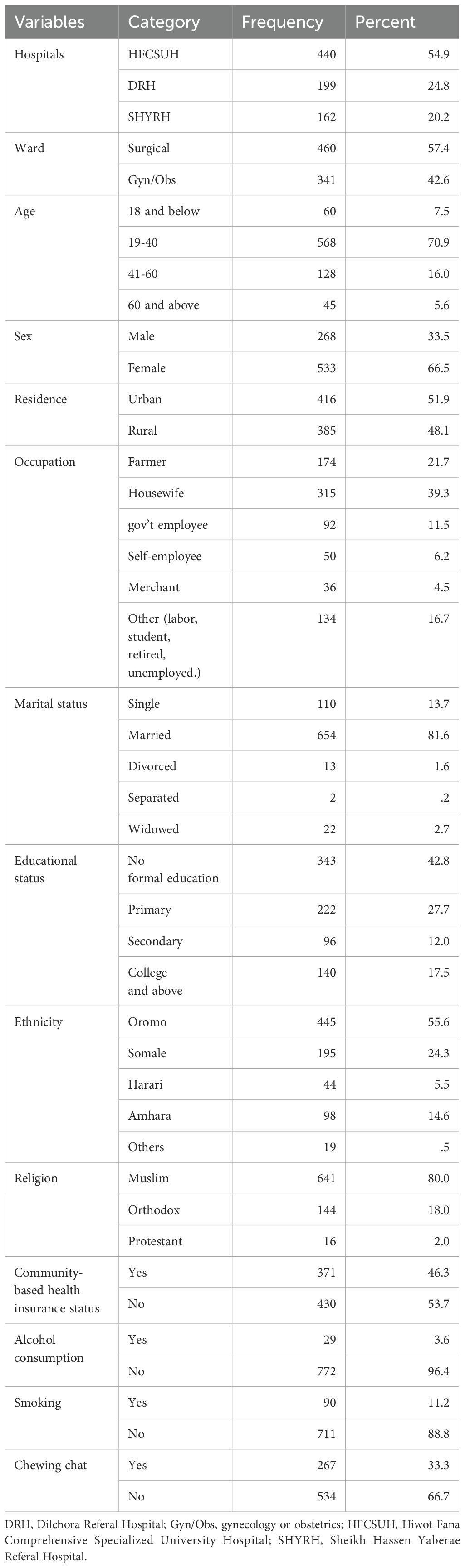
A total of 801 patients were included in the study with a response rate of 90.2%. More than half of the patients were from HFCSUH (440, 54.9%) followed by DRH (199, 24.8). Out of 801 participants, 460 (57.4%) were from the surgical ward and 341 (42.6%) were from Gyn/OBS ward. The majority of patients were in the age group of 19-40 (568, 70.9%) and females accounted for the highest proportion of patients (533, 66.5%). In terms of marital status, a large proportion of patients were married (654, 81.6%). About 343 (42.8%) of participating patients had no formal education. More than half of the participants had no community-based health insurance. Regarding lifestyle conditions, most of the participants did not drink alcohol or smoke cigarettes. However, one-third of the patients were chew-chat (Table 1).
3.2 Clinical characteristics of the study participants
The majority of the study participants had no previous surgical history (631, 78.8%). Near half of the patients, (374, 46.7%), surgical procedures took more than one hour. The duration of hospital stay for most of the patients was less than seven days. About 413(51.6%) surgical cases were done as an emergency case. Most of the procedures were conducted in the daytime with general anesthesia. About 87(12.2%) patients who had undergone surgery received blood transfusions in the pre-, intra-, or post-operation period. Most of the wound types were clean 377(92%) whereas clean-contaminated wounds were 64(8%). About 80%of patients received antimicrobial prophylaxis of which 579(72.3%) were received during the pre-operative period. The majority of study participants had no immunosuppression or comorbidities (Table 2).
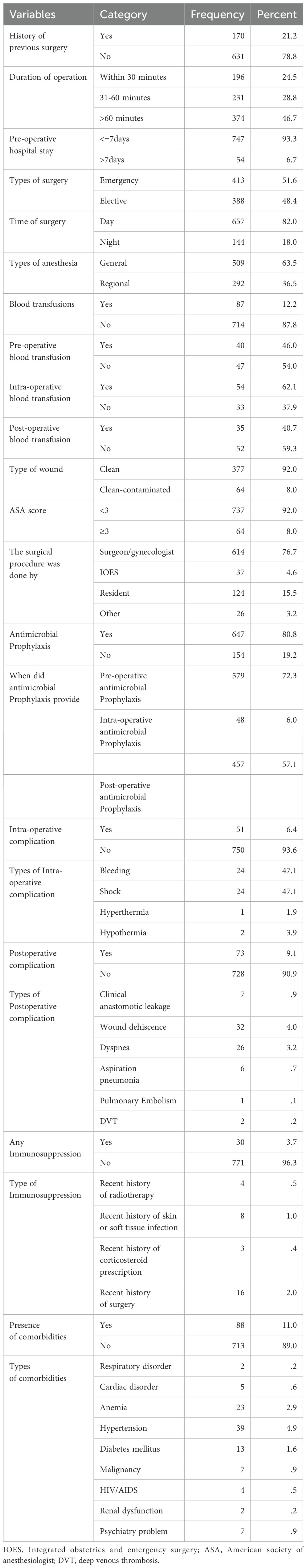
Table 2. Baseline clinical characteristics, surgical procedures type, and antimicrobial usage patterns of study participants.
3.3 Incidence of SSIs
In this study, out of 801 patients who underwent major surgery for different indications 137(17.1%) of them developed SSIs. Most of them 113(82.5%) were identified before discharge, while the remaining 24 were tracked down through follow-up and readmission (Figure 1).
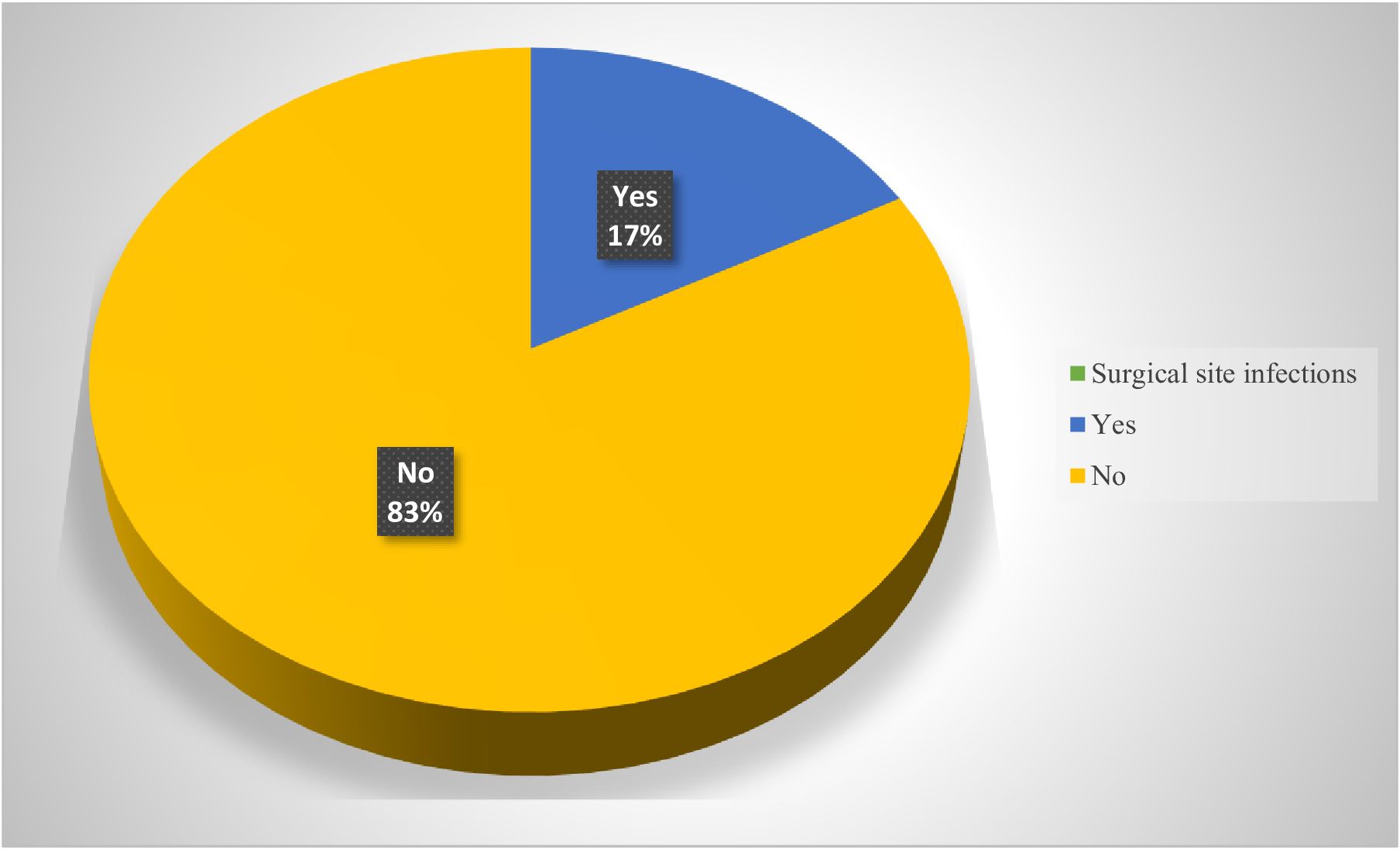
Figure 1. Incidence of SSIs among patients who underwent major surgical procedures in eastern Ethiopia.
3.4 Bivariate analysis of factors associated with SSIs among patients who have undergone major surgical procedures
In bivariate analysis being male, contaminated wound types, seven days and above pre-operative hospital stay, duration of surgical procedures lasting more than 60 minutes, having not received antibiotic prophylaxis, presence of comorbidities, substance use (Khat chewing and cigarette smoking), and being underweight were significantly associated with SSIs. In addition, patients who attained secondary education had less chance of developing SSIs. However, patients’ ages were not significantly associated with SSIs (Table 3).
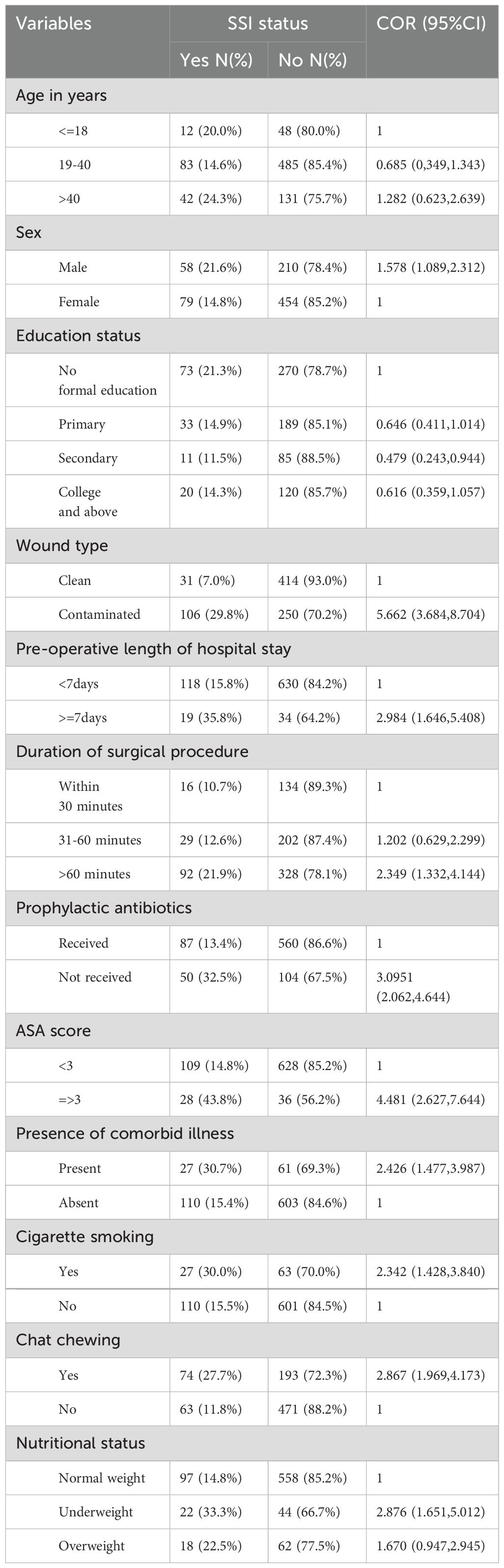
Table 3. Bivariate analysis of factors associated with SSIs among patients undergone major surgical procedures in public Hospitals in Eastern Ethiopia, 2024 (N = 801).
3.5 Multivariate analysis of factors associated with SSIs among patients undergone major surgical procedures
In multivariate analysis, factors like wound type, pre-operative hospital stay, duration of surgical procedure, prophylactic antibiotics use, ASA score, presence of comorbidity, and khat chewing remain statistically significantly associated with SSI development. Patients with contaminated wound type were nearly fifteen times more likely to develop SSIs compared to those patients with clean wound type (AOR: 14.725, 95%CI: 8.210-26.410). Those patients who stayed 7 days or more in the hospitals were more than two times at risk of developing SSIs compared to those who stayed less than 7 days ahead of surgical procedures (AOR: 2.45, 95%CI:1.113-5.402). The odds of developing SSIs among those patients whose surgical procedures took more than 60 minutes were 2.598 times more likely to develop SSIs relative to those patients whose procedures were completed within 30 minutes (AOR:2.598, 95%CI: 1.217-5.546). Compared to those patients who received antibiotics, patients who did not receive antibiotics prophylactic were more than five times at risk of developing SSIs (AOR: 5.506, 95%CI: 3.006-10.084). Patients with ASA scores three and above were 3.5 times more likely to develop SSIs compared to their counterparts (AOR:3.497, 95%CI:1.713-7.141). The presence of comorbidity increased the odds of contracting SSIs by about fourfold (AOR: 4.088, 95%CI: 2.266-7.375). Those patients chewing khat were 2.88 times more likely to acquire SSIs compared to those who did not chew khat (AOR: 2.884, 95%CI: 1.322-3.613) (Table 4).
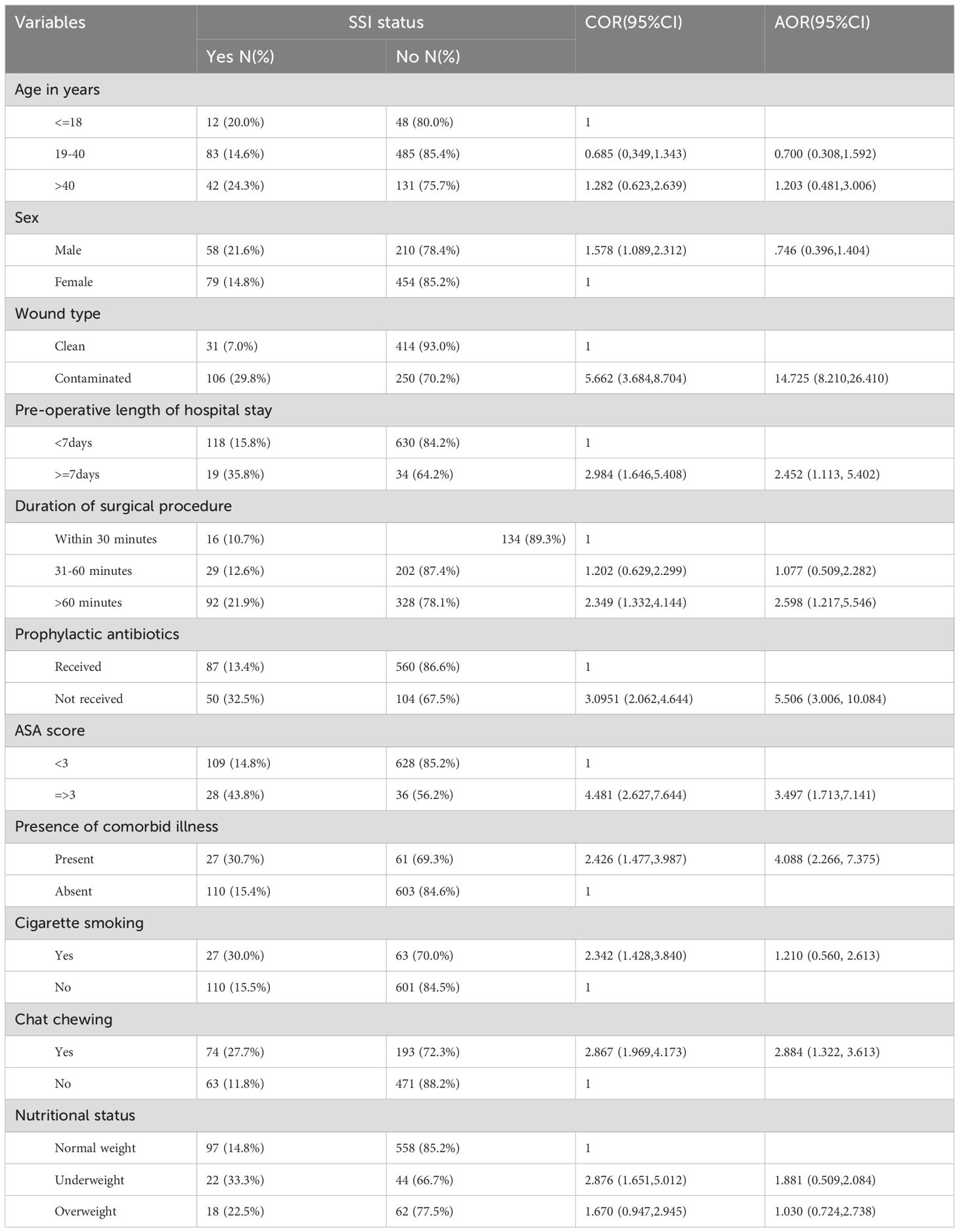
Table 4. Multivariate analysis of factors associated with SSIs among patients undergone major surgical procedures in public hospital in Eastern Ethiopia, 2024.
3.6 Patterns of bacterial isolate in the study area
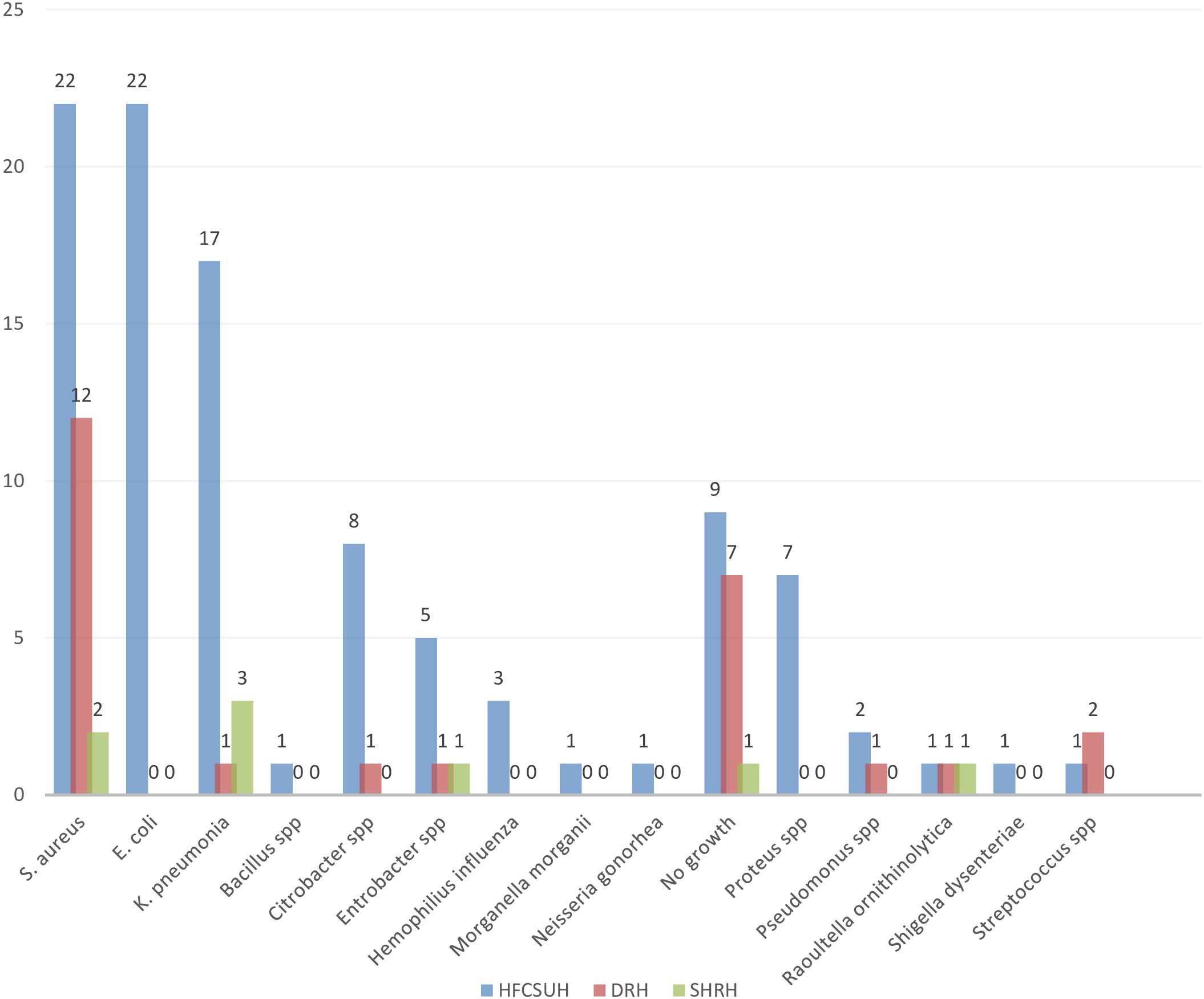
Wound swab samples were collected from 137 patients who developed SSI. Out of these, 112 (81.75%) were culture positive whereas 25(18.25%) were culture negative. Of the positive culture, 78% were with a single isolate and 4% were with a mixed isolate (Figure 2). Of the bacterial isolates, Staphylococcus species were the predominant 36 (30.5%) followed by E. coli 22(18.6%) whereas a single isolate of Bacillus, Morganella, Neisseria, and Shigella species was identified in the study area (Figure 3). Regarding the distribution of each isolate in the specific Hospital, E. coli, S. aureus and klebseilla species were the highest isolates in the HFCSUH. Staphylococcus species were the highest isolates in DRH. Overall, the number of bacterial isolates was low in SHYRH as compared to the other two Hospitals (Figure 4).
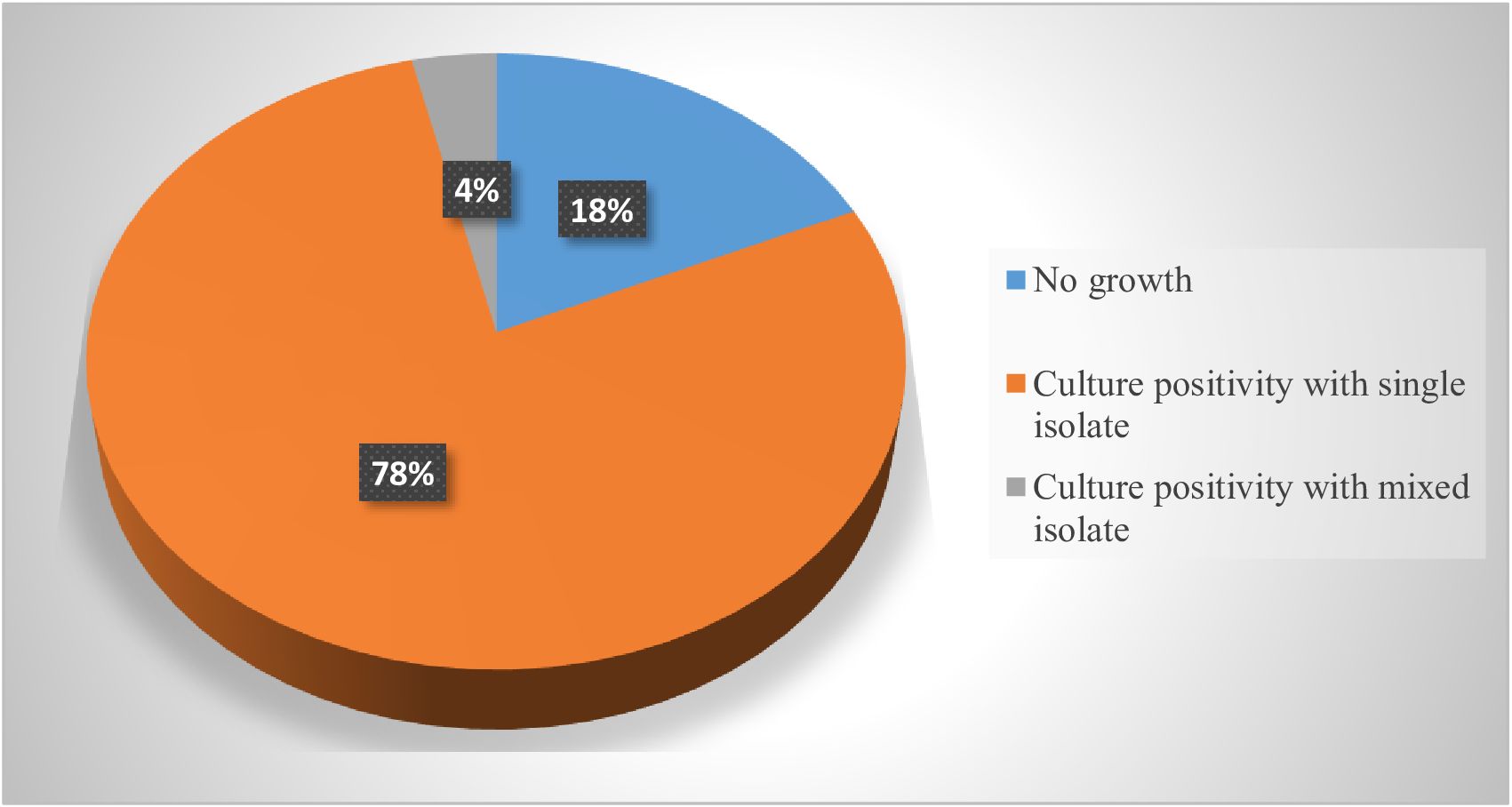
Figure 2. Growth rates of bacterial isolates from samples collected from SSI patients in eastern Ethiopian referral hospitals.

Figure 3. Prevalence of bacterial isolates among SSI patients in eastern Ethiopian referral hospitals.
3.7 Antimicrobial susceptibility patterns of isolated bacteria
In terms of antimicrobial susceptibility patterns, staphylococcus aureus showed the highest resistance to azithromycin (18/36), gentamycin (16/36), cotrimoxazole(15/36), erythromycin(11/36), tetracycline (10/36) and penicillin G(10/36). However, few isolates of Staphylococcus spp were susceptible to vancomycin. Out of 21 isolates of Klebsiella pneumonia, a higher resistance was observed for ceftriaxone (15/21), Ciprofloxacillin (10/21), and amoxicillin with clavulanic acid (8/21). Klebsiella pneumonia showed better susceptibility to meropenam(15/21), gentamycin (10/21), and piperacillin with tazobactam (8/21). E. coli demonstrated high resistance to ceftriaxone (13/22) and cotrimoxazole (8/22). However, it showed higher susceptibility to meropenam (16/22) followed by gentamycin and piperacillin with tazobactam in a similar pattern (10/22). In terms of gram reaction most bacterial isolates were gram-negative (66.1%) whereas gram-positive accounted for 33.9% (Table 5).
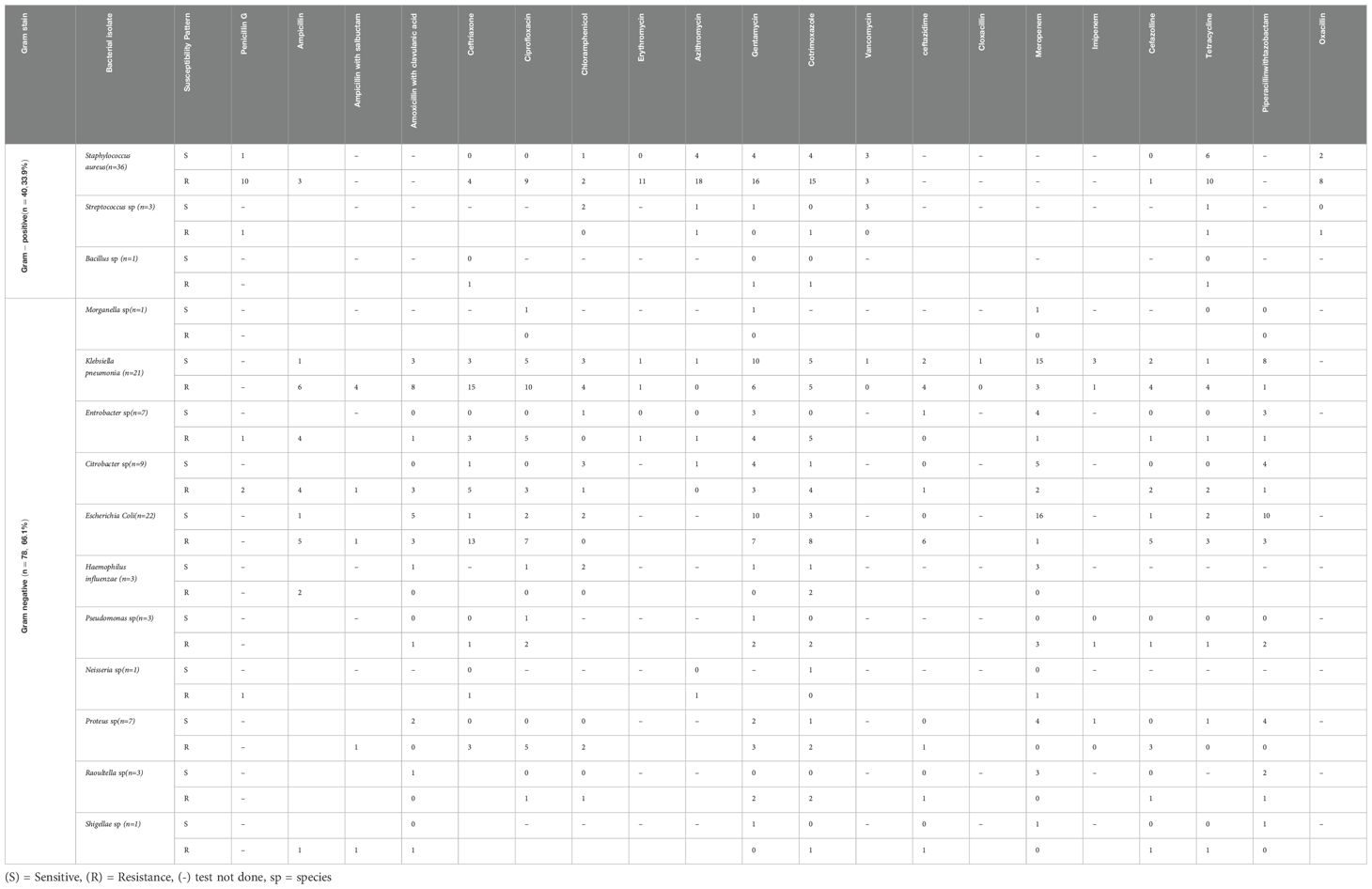
Table 5. Antimicrobial susceptibility patterns of isolated bacteria in three public tertiary hospitals in eastern Ethiopia.
3.8 Treatment outcomes of SSI patients
A thirty-day outcome of study participants revealed that overall 137 patients developed surgical site infections. Most (89%) were discharged improved, 6% developed complications and 5% of deaths were registered among the patients with SSIs during the study period (Figure 5).
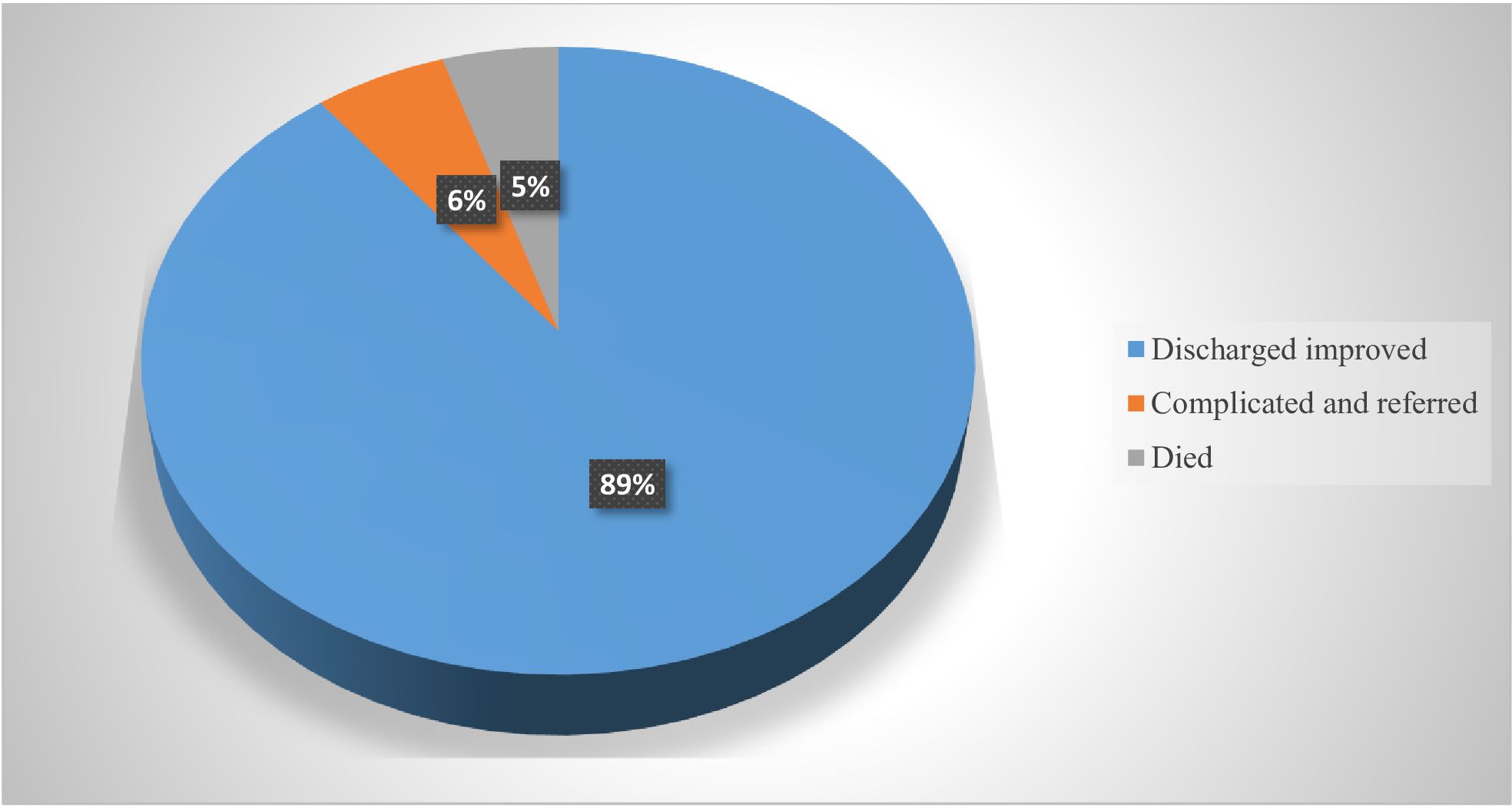
Figure 5. Treatment outcome among patients who developed SSI in three public hospitals of Eastern Ethiopia.
4 Discussion
In low- and middle-income nations, SSIs continue to be a leading cause of morbidity and mortality among patients who have had major surgical procedures. Longer hospital stays, more nursing care, more wound care, readmissions to the hospital, and further surgeries raise the cost of health care (Costabella et al., 2023). The identification of bacterial pathogens and the selection of an appropriate antibiotic to battle the organism are essential for the effective management of this infection (Evans, 2009).
This study assessed the incidence of SSIs, bacterial etiologies, associated factors, and antimicrobial susceptibility patterns of the bacterial isolates among patients who underwent major surgical procedures in eastern Ethiopia. Factors significantly associated with the incidence of SSIs were the type of wound, ASA score, duration of surgery, length of hospital stay, prophylactic antibiotic prescription, presence of comorbidity and Khat chewing.
The present study identified an SSI incidence of 17.1%. This finding was closely related to other studies conducted at Wachemo University Nigist Eleni hospital (16.5%) (Billoro et al., 2019) and Dilla University referral hospital (19.3%) (Birhanu et al., 2022). However, the current finding is higher than the estimated pooled prevalence of SSI reported from a systematic review and meta-analysis (12.3%) (Shiferaw et al., 2020) and other previous studies done at different settings of Ethiopian hospitals that reported SSI rates ranging from 9.9% to 13% (Alamrew et al., 2019; Awoke et al., 2019; Fisha et al., 2019; Kalayu et al., 2019; Shakir et al., 2021). It is also higher than the results of studies conducted in Morocco (6.3%) (Flouchi et al., 2022), Italy (5.3%), Nepal (2.6%) (Shrestha et al., 2016), Brazil (6.4%) (Gomes et al., 2014), Turkey (4.09%) (Isik et al., 2015), India (5%) (Pathak et al., 2014), Algeria (5.4%) (Rawabdeh et al., 2016b) and Saudi Arabia (11.4%) (Rawabdeh et al., 2016a). The observed variation in low-income countries like Ethiopia could be attributed to limited resources such as inadequate access to essential equipment and supplies necessary to maintain strict aseptic conditions that can hinder effective infection prevention and control. Likewise, infrastructure constraints like poor sanitation, lack of proper waste management, and overcrowding in healthcare facilities can create environments conducive to the spread of infections. It might also related to insufficient training of healthcare workers on infection prevention practices, which could lead to errors in technique and increased risk of contamination.
However, the incidence of SSIs in the current study is lower than the findings of other studies done in the hospitals in the Gojjam zone 25.5% (Afenigus et al., 2019), Dessie Referral Hospital 23.4% (Misganaw et al., 2020), Borumeda Hospital 46.7% (Moges et al., 2020), and Jimma Medical Center 21.1% (Misha et al., 2021b), Tertiary healthcare facility in Nigeria (27.6%) (Olowo-Okere et al., 2018) and Tanzania (26%) (Mawalla et al., 2011), Turkey 24% (Bilgiç et al., 2020), USA 24% (Elliott et al., 2017), USA 34% (McCracken et al., 2019), China 26.7% (Zhang et al., 2018). These disparities may be due to the differences in sample size, study design, duration, study population, diagnosis and reporting practices of SSIs. It could also be explained by variations in the distribution of pathogens and infectious agents, the type of surgery as complex procedures involving multiple organ systems or those that penetrate the abdomen or chest are often associated with higher SSI rates.
In the present study, factors significantly associated with the occurrence of SSIs were the type of wound, ASA scores, duration of surgery, length of hospital stay, prophylactic antibiotic prescription, presence of comorbidity and Khat chewing. Accordingly, wound class was independently associated with the occurrence of SSIs in patients undergoing major surgical procedures in this study. Patients with contaminated wounds were nearly 15 times more likely to develop SSIs as compared to those with clean wounds. This corroborates several previous studies (Lakoh et al., 2022; Alamrew et al., 2019; Fisha et al., 2019; Misganaw et al., 2020; Misha et al., 2021b; Moges et al., 2020; Shakir et al., 2021; Shrestha et al., 2016). This might be because dirty wounds have a higher probability of developing infections than clean wounds in similar care practices. In addition, surgeries with an increased microbial load in the operative field are associated with a higher risk of SSI. Moreover, evidence supports that treating infections on the operating site, if possible, or postponing the surgery until the infection has cleared or considering the use of wound protector devices in clean contaminated abdominal surgical procedures will reduce the incidence of SSIs (Gheorghe et al., 2012).
The odds of developing SSIs were about threefold higher in patients with an ASA score of three or more compared to those with an ASA score of less than three. This supports the findings of several other studies (Misha et al., 2021b; Shiferaw et al., 2020; Flouchi et al., 2022; Shrestha et al., 2016). The study indicated that a higher ASA score likely leads to worsening the general clinical status of the patient, prolonging the duration of surgery and making it more susceptible to infections (Misha et al., 2021b).
In addition, the study revealed that a lengthy operation period that exceeds 60 minutes was significantly associated with the development of SSIs. This finding is supported by several other studies conducted across the globe (Lakoh et al., 2022; Mawalla et al., 2011; Worku et al., 2023; Flouchi et al., 2022; Fisha et al., 2019; Misganaw et al., 2020; Misha et al., 2021b; Shakir et al., 2021) that identified a direct association between longer operation times and SSIs. It is also supported by guidelines recommendation limiting the length of the surgery; the longer the incision remains open, the higher the risk of introduction of microorganisms into the surgical incision (Ban et al., 2017). This might be due to a longer duration of surgery that resulted in prolonged exposure of tissue to the environment, prolonged hypothermia and declining levels of antibiotics or a greater chance of breach of the aseptic technique in the procedure.
In this study, patients who had 7 days or more of preoperative hospital stay were 2.45 times more likely to develop SSIs compared to those who had less stay. This finding was in line with results reported from several other studies (Awoke et al., 2019; Birhanu et al., 2022; Fisha et al., 2019; Misha et al., 2021b; Petrosillo et al., 2008; Shakir et al., 2021; Shiferaw et al., 2020; Worku et al., 2023) and also supported by a systematic review conducted on prolonged operative duration increases risk of SSIs (Cheng et al., 2017). The possible explanation might be due to patients with a long stay in hospital before surgery expose to contamination or colonization by pathogens which will contribute to the occurrence of SSIs.
In this study, patients who did not receive preoperative antibiotic prophylaxis were about 5 times more likely to develop SSIs compared to those who received it. This could reinforce the importance of providing preoperative antibiotic prophylaxis to prevent SSIs. This finding is in agreement with a study done at St. Paul’s Hospital Millennium Medical College, Addis Ababa among patients who underwent general surgery (Alamrew et al., 2019).
Moreover, the study indicated that comorbid conditions were an important predictive factor for SSIs. In our study, patients with comorbidity had around 4 fold higher risk of developing SSIs compared to their counterparts. This is consistent with other studies (Flouchi et al., 2022; Misganaw et al., 2020; Misha et al., 2021b). A study suggested that patient comorbidity is the primary driver of infection and poor wound healing (Lakoh et al., 2022). This is possible because HIV/AIDS and other immunosuppression-related conditions such as diabetes mellitus can lead to increased SSIs as they weaken the patient’s immunity.
Furthermore, substance use like Khat chewing was found to be one of the independent predictive factors of SSIs in the present study. The odds of developing SSIs were around three times higher among khat chewer patients compared to non-chewers. This can be explained by the fact that khat like other substances impacts nutrition and impairs the immune status of an individual which could increase the risk of acquiring SSIs.
The current results showed that 81.75% of bacterial isolates from SSI were culture-positive. This outcome is similar to a study carried out in Nigeria (82%) (Mohammed et al., 2013). However, it is higher than the reports from Jimma Medical Center (71.7%) (Misha et al., 2021a), Ayder teaching referral hospital (75%) (Mengesha et al., 2014), India (68%) (Vasundhara Devi et al., 2017) and lower than a report from a university of Gondar hospital (88.1%) (Gelaw et al., 2014). This variation might be associated with differences in sample size, study setting and bacterial isolates.
In the present study, 66.1% of bacterial isolates were gram-negative whereas 33.9% were gram-positive. This finding was inconsistent with the study conducted at Jimma Medical Centre where gram-negative was 78% and gram-positive was 11.5% (Misha et al., 2021a), and a multicenter study reported from different hospitals in Ethiopia (Worku et al., 2023). This could be caused by differences in how wounds are treated and how well preventative medications work in various contexts. Besides, the normal flora of the patient’s body and the hospital environment, various medical equipment, might harbor germs, potentially causing post-procedural contamination of surgical incisions (Abayneh et al., 2022).
Of the bacterial isolates, Staphylococcus aureus (30.5%) was predominant, followed by E. coli (18.6%) in the study area. This finding is comparable with a result reported from a multicenter study conducted at northern, central, southern, and southwest hospitals in Ethiopia (Worku et al., 2023), in public hospitals in the Harari region (Shakir et al., 2021), with a pooled systematic review and meta-analysis conducted in Ethiopia (Birhanu and Endalamaw, 2020) and a study conducted in Vietnam (An et al., 2024). However, it is lower than the study conducted at Tikur Anbesa Hospital, Dessie Hospital (Ali et al., 2023), and higher than the study from Behar Dar Felegehiwot Hospital (Mulu et al., 2013). It is also inconsistent with studies done in Sudan Teaching Hospital (Ahmed, 2012), and India (Verma et al., 2012). This could be due to the presence of bacterial pathogens such as S. aureus commonly in the skin and nasal flora, as well as its ability to spread through contact with surgical tools, the environment, and healthcare personnel’s hands (Anguzu and Olila, 2007). The prevalence of gram-negative isolates was lower in the present study as compared to the report from Mizan Tepi University Teaching Hospital (Abayneh et al., 2022). The discrepancies in these results may be due to differences in the pathogen epidemiology, the quality of surgical technique used and operating room standards.
Staphylococcus aureus resulted in a high resistance to azithromycin, gentamycin, cotrimoxazole erythromycin, tetracycline, and penicillin G from gram-positive isolates of SSI in our investigation. This study is concordant with other studies done in Ethiopia that demonstrated high resistance of S. aureus to amoxicillin-clavulanic acid, doxycycline, cotrimoxazole, clindamycin, erythromycin, cefoxitin, gentamicin, and chloramphenicol (Abayneh et al., 2022), and a report from India with high a resistance to cotrimoxazole (66.67%) (Verma et al., 2012). This high resistance may be attributed to empirical broad-spectrum antibiotic prescription in the treatment of various infections, which then disrupt normal flora and pose the risk of bacterial resistance. From gram-negative isolates of the present study, Klebsiella pneumonia and E.coli showed high resistance to different antibiotics. Klebsiella pneumonia demonstrated higher resistance to ceftriaxone, Ciprofloxacillin and amoxicillin with clavulanic acid. Similarly, the E. coli had shown high resistance to ceftriaxone and cotrimoxazole. However, both pathogens were found to be susceptible to meropenam, gentamycin and piperacillin with tazobactam in a similar pattern. These findings are comparable with the study conducted in India (Verma et al., 2012) and Southwestern Uganda (Hope et al., 2019) where gram-negative bacteria showed a high resistance to multiple antibiotics. This high resistance might be due to the indiscriminate and sub-therapeutic doses of antibiotics used for the treatment of different infections that could result in multidrug resistance in our health facilities and others. Furthermore, the widespread use of antibiotics, often without proper guidelines, can contribute to the development of antibiotic-resistant bacteria, and make SSIs more difficult to prevent and treat.
Overall, the study indicated multidrug resistance to the commonly used antibiotics with many SSI isolates, which ranged from 5-71%. This finding is lower than report from Ayder referral hospital Mekelle (Mengesha et al., 2014), hospital-based cross-sectional study in Ethiopia (Dessalegn et al., 2014), study from Northwest Ethiopia (Abosse et al., 2021), previous study done in Harar (78.8%) (Shakir et al., 2021), from Hawasa, Ethiopia (93.2%) (Dessalegn et al., 2014) and Nigeria (Mohammed et al., 2013). However, the current finding is higher than a report from Tikur Anbesa, Addis Ababa (65%) Ethiopia (Asres et al., 2017), and Egypt (37.2%) (Zein-Eldeen et al., 2018). This variation could be ascribed to regional antibiotic use policies and local resistance profiles of bacterial isolates. In addition, the prevalence of specific resistance mechanisms or the dissemination of resistant strains within different healthcare settings may contribute to the observed discrepancy (An et al., 2024).
5 Conclusion
The incidence of SSIs in the study areas was 17.1%, highlighting the need for targeted interventions to reduce this burden. Several factors, including wound type, ASA score, duration of surgery, length of hospital stay, prophylactic antibiotic prescription, comorbidity, and Khat chewing, were identified as significant predictors of SSI incidences. By addressing these modifiable factors, healthcare facilities can significantly reduce the incidence of SSIs and improve patient outcomes. Among 137 patients who developed SSIs, culture positivity was found to be 81.75%. About 78% of patients with SSIs were infected by a single isolate. Gram-negative bacteria were the dominant isolates, of which E.coli and Klebseila accounted for a larger proportion. Staphylococcus Spps contributed to a higher proportion of gram-positive isolates. Results of susceptibility patterns indicated that staphylococcus Spps were the most common multidrug-resistant isolates from gram-positive. Similarly, from gram-negative bacterial isolates Klebsiella pneumonia and E. coli were observed to be multidrug resistant. However, Klebsiella pneumonia and E. coli showed a better susceptibility to meropenam, gentamycin and piperacillin with tazobactam. In general, a multi-faceted approach that involves collaboration among healthcare providers, policymakers, and researchers is essential to achieve sustainable improvements in SSI prevention.
Author’s note
Additional data can be obtained from the corresponding author on reasonable request.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Institutional Health and Research Ethics Review Committee (IHRERC) of the College of Health and Medical Science, Haramaya University with a reference Number of IHRERC/007/2022. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MY: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation. FA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, Project administration, Supervision. AAm: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing, Project administration, Supervision. AAb: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Software. HM: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors received financial support from Haramaya University to conduct the study, regular annual grant with code HURG-2021-02-01-62.
Acknowledgments
First of all, we would like to express our deepest gratitude to Haramaya University for funding this project. Finally, we extend our special thanks to data collectors at all hospitals and HSCUH, medical microbiology staff for their cooperation in microbial analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abate K. H., Abdulahi M., Abdulhay F., Arage G., Mecha M., Yenuss M., et al. (2021). Consequences of exposure to prenatal famine on estimated glomerular filtration rate and risk of chronic kidney disease among survivors of the great Ethiopian famine, (1983-85): a historical cohort study. Nutr. J. 20, 19. doi: 10.1186/s12937-021-00675-8
Abayneh M., Asnake M., Muleta D., Simieneh A. (2022). Assessment of bacterial profiles and antimicrobial susceptibility pattern of isolates among patients diagnosed with surgical site infections at Mizan-Tepi University Teaching Hospital, Southwest Ethiopia: a prospective observational cohort study. Infect. Drug Resistance, 1807–1819. doi: 10.2147/IDR.S357704
Abosse S., Genet C., Derbie A. (2021). Antimicrobial resistance profile of bacterial isolates identified from surgical site infections at a referral hospital, northwest Ethiopia. Ethiop. J. Health Sci. 31, 635–644. doi: 10.4314/ejhs.v31i3.21
Afenigus A. D., Shbabawu A. T., Emrie H. C. (2019). Surgical site infection and associated factors among adult patients admitted in west and east Gojjam zone hospitals, Amhara region, Ethiopia. Nurs. Care Open Access J. 6, 107–112. doi: 10.15406/ncoaj.2019.06.00192
Ahmed M. I. (2012). Prevalence of nosocomial wound infection among postoperative patients and antibiotics patterns at a teaching hospital in Sudan. North Am. J. Med. Sci. 4, 29. doi: 10.4103/1947-2714.92900
Alamrew K., Tadesse T. A., Abiye A. A., Shibeshi W. (2019). Surgical antimicrobial prophylaxis and incidence of surgical site infections at Ethiopian tertiary-care teaching hospital. Infect. Dis. (Auckl) 12, 1178633719892267. doi: 10.1177/1178633719892267
Ali A., Gebretsadik D., Desta K. (2023). Incidence of surgical site infection, bacterial isolate, and antimicrobial susceptibility pattern among patients who underwent surgery at Dessie Comprehensive Specialized Hospital, Northeast Ethiopia. SAGE Open Med. 11, 20503121231172345. doi: 10.1177/20503121231172345
Allegranzi B., Bischoff P., De Jonge S., Zeynep N., Zayed B., Gomes S. (2016). Surgical site infections 1. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect. Dis. 16, e276–e287. doi: 10.1016/S1473-3099(16)30398-X
An N. V., Hai L. H. L., Luong V. H., Vinh N. T. H., Hoa P. Q., Hung L. V., et al. (2024). Antimicrobial resistance patterns of Staphylococcus aureus isolated at a General Hospital in Vietnam between 2014 and 2021. Infect. Drug Resistance, 259–273. doi: 10.2147/IDR.S437920
Anguzu J., Olila D. (2007). Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr. Health Sci. 7. doi: 10.5555/afhs.2007.7.3.148
Asres G., Legese M., Woldearegay G. (2017). Prevalence of multidrug-resistant Bacteria in postoperative wound infections at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Arch. Med. 9, 12. doi: 10.21767/1989-5216.1000233
Awoke N., Arba A., Girma A. (2019). The magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PloS One 14, e0226140. doi: 10.1371/journal.pone.0226140
Ban K. A., Minei J. P., Laronga C., Harbrecht B. G., Jensen E. H., Fry D. E., et al. (2017). American College of Surgeons and Surgical Infection Society: surgical site infection guidelines 2016 update. J. Am. Coll. Surgeons 224, 59–74. doi: 10.1016/j.jamcollsurg.2016.10.029
Bilgiç Ç., Keske Ş., Sobutay E., Can U., Zenger S., Gürbüz B., et al. (2020). Surgical site infections after pancreaticoduodenectomy: Preoperative biliary system interventions and antimicrobial prophylaxis. Int. J. Infect. Dis. 95, 148–152. doi: 10.1016/j.ijid.2020.04.005
Billoro B. B., Nunemo M. H., Gelan S. E. (2019). Evaluation of antimicrobial prophylaxis use and rate of surgical site infection in the surgical ward of Wachemo University Nigist Eleni Mohammed Memorial Hospital, Southern Ethiopia: prospective cohort study. BMC Infect. Dis. 19, 1–8. doi: 10.1186/s12879-019-3895-5
Birhanu A., Amare H. H., M G. M., Girma T., Tadesse M., Assefa D. G. (2022). The magnitude of surgical site infection and determinant factors among postoperative patients, A cross-sectional study. Ann. Med. Surg. (Lond) 83, 104324. doi: 10.1016/j.amsu.2022.104324
Birhanu Y., Endalamaw A. (2020). Surgical site infection and pathogens in Ethiopia: a systematic review and meta-analysis. Patient Saf. Surg. 14, 1–8. doi: 10.1186/s13037-020-00232-y
Björnsson E. S., Bergmann O. M., Björnsson H. K., Kvaran R. B., Olafsson S. (2013). Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144, 1419–1425. doi: 10.1053/j.gastro.2013.02.006
Brown S. R. (2017). Haemorrhoids: an update on management. Ther. Adv. Chronic Dis. 8, 141–147. doi: 10.1177/2040622317713957
Chen M., Suzuki A., Borlak J., Andrade R. J., Lucena M. I. (2015). Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 63, 503–514. doi: 10.1016/j.jhep.2015.04.016
Cheng H., Chen B. P., Soleas I. M., Ferko N. C., Cameron C. G., Hinoul P. (2017). Prolonged operative duration increases risk of surgical site infections: A systematic review. Surg. Infect. (Larchmt) 18, 722–735. doi: 10.1089/sur.2017.089
Chong P. S., Bartolo D. C. (2008). Hemorrhoids and fissure in ano. Gastroenterol. Clinics North America 37, 627–644. doi: 10.1016/j.gtc.2008.07.001
Costabella F., Patel K. B., Adepoju A. V., Singh P., Mahmoud H. A. H., Zafar A., et al. (2023). Healthcare cost and outcomes associated with surgical site infection and patient outcomes in low-and middle-income countries. Cureus 15. doi: 10.7759/cureus.42493
Dejenie M., Kerie S., Reba K. (2021). Undiagnosed hypertension and associated factors among bank workers in Bahir Dar City, Northwest, Ethiopia 2020. A cross-sectional study. PloS One [Electronic Resource] 16, e0252298. doi: 10.1371/journal.pone.0252298
Dessalegn L., Shimelis T., Tadesse E., Gebre-Selassie S. (2014). Aerobic bacterial isolates from the post-surgical wound and their antimicrobial susceptibility pattern: a hospital-based cross-sectional study. J. Med. Res. 3, 18–23.
Elliott I. A., Chan C., Russell T. A., Dann A. M., Williams J. L., Damato L., et al. (2017). Distinction of risk factors for superficial vs organ-space surgical site infections after pancreatic surgery. JAMA Surg. 152, 1023–1029. doi: 10.1001/jamasurg.2017.2155
Evans R. P. (2009). Surgical site infection prevention and control: an emerging paradigm. JBJS 91, 2–9. doi: 10.2106/JBJS.I.00549
Fernández-Castañer A., García-Cortés M., Lucena M., Borraz Y., Peláez G., Costa J., et al. (2008). An analysis of the causes, characteristics, and consequences of reexposure to a drug or compound responsible for a hepatotoxicity event. Rev. Española Enfermedades Digestivas 100, 278. doi: 10.4321/s1130-01082008000500006
Fiseha T., Ahmed E., Chalie S., Gebreweld A. (2021). Prevalence and associated factors of impaired renal function and albuminuria among adult patients admitted to a hospital in Northeast Ethiopia. PloS One [Electronic Resource] 16, e0246509. doi: 10.1371/journal.pone.0246509
Fisha K., Azage M., Mulat G., Tamirat K. S. (2019). The prevalence and root causes of surgical site infections in public versus private hospitals in Ethiopia: a retrospective observational cohort study. Patient Saf. Surg. 13, 26. doi: 10.1186/s13037-019-0206-4
Flouchi R., El Far M., Hibatallah A., Elmniai A., Rhbibou I., Touzani I., et al. (2022). Incidence of surgical site infections and prediction of risk factors in a hospital center in Morocco. J. Infect. Dev. Ctries 16, 1191–1198. doi: 10.3855/jidc.15289
Gelaw A., Gebre-Selassie S., Tiruneh M., Mathios E., Yifru S. (2014). Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at the University of Gondar Hospital, Northwest Ethiopia. J. Environ. Occup. Sci. 3, 103–108. doi: 10.5455/jeos.20140512124135
Gheorghe A., Calvert M., Pinkney T. D., Fletcher B. R., Bartlett D. C., Hawkins W. J., et al. (2012). A systematic review of the clinical effectiveness of wound-edge protection devices in reducing surgical site infection in patients undergoing open abdominal surgery. Ann. Surg. 255, 1017–1029. doi: 10.1097/SLA.0b013e31823e7411
Gomes A. E. B., Cavalcante R. D. S., Pavan É. C. P., Freitas E. D. S., Fortaleza C. M. C. B. (2014). Predictive factors of post-discharge surgical site infections among patients from a teaching hospital. Rev. da Sociedade Bras. Medicina Trop. 47, 235–238. doi: 10.1590/0037-8682-0069-2013
Hope D., Ampaire L., Oyet C., Muwanguzi E., Twizerimana H., Apecu R. O. (2019). Antimicrobial resistance in pathogenic aerobic bacteria causing surgical site infections in Mbarara regional referral hospital, Southwestern Uganda. Sci. Rep. 9, 17299. doi: 10.1038/s41598-019-53712-2
Ibáñez L., Pérez E., Vidal X., Laporte J.-R. (2002). Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J. Hepatol. 37, 592–600. doi: 10.1016/S0168-8278(02)00231-3
Isik O., Kaya E., Dundar H., Sarkut P. (2015). Surgical site infection: re-assessment of the risk factors. Chirurgia (Bucur) 110, 457–461.
Kalayu A. A., Diriba K., Girma C., Abdella E. (2019). Incidence and bacterial etiologies of surgical site infections in a public hospital, Addis Ababa, Ethiopia. Open Microbiol. J. 13, 301–307. doi: 10.2174/1874285801913010301
Kim W. S., Lee S. S., Lee C. M., Kim H. J., Ha C. Y., Kim H. J., et al. (2016). Hepatitis C and not Hepatitis B virus is a risk factor for anti-tuberculosis drug-induced liver injury. BMC Infect. Dis. 16, 1–7. doi: 10.1186/s12879-016-1344-2
Knuf K. M., Maani C. V., Cummings A. K. (2018). Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioperative Med. 7, 1–6. doi: 10.1186/s13741-018-0094-7
Lakoh S., Yi L., Russell J. B. W., Zhang J., Sevalie S., Zhao Y., et al. (2022). The burden of surgical site infections and related antibiotic resistance in two geographic regions of Sierra Leone: a prospective study. Ther. Adv. Infect. Dis. 9, 20499361221135128. doi: 10.1177/20499361221135128
Larrey D. (1995). Drug-induced hepatitis: epidemiologic, clinical, diagnostic and physiopathologic aspects in 1995. La Rev. Medecine Interne 16, 752–758. doi: 10.1016/0248-8663(96)80784-3
Lewis J. H. (2006). [amp]]lsquo;Hy’s law,’ the ‘Rezulin Rule,’ and other predictors of severe drug-induced hepatotoxicity: putting risk-benefit into perspective. Pharmacoepidemiol. Drug Saf. 15, 221–229. doi: 10.1002/pds.v15:4
Mawalla B., Mshana S. E., Chalya P. L., Imirzalioglu C., Mahalu W. (2011). Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC Surg. 11, 1–7. doi: 10.1186/1471-2482-11-21
McCracken E. K. E., Mureebe L., Blazer D. G. III (2019). Minimally invasive surgical site infection in procedure-targeted ACS NSQIP pancreaticoduodenectomies. J. Surg. Res. 233, 183–191. doi: 10.1016/j.jss.2018.07.041
Mengesha R. E., Kasa B. G.-S., Saravanan M., Berhe D. F., Wasihun A. G. (2014). Aerobic bacteria in post surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res. Notes 7, 1–6. doi: 10.1186/1756-0500-7-575
Metchock B., Nolte F., Wallace R., Murray P., Baron E., Pfaller M. (1999). Manual of clinical microbiology (Washington, DC: American Society for Microbiology).
Misganaw D., Linger B., Abesha A. (2020). Surgical antibiotic prophylaxis use and surgical site infection pattern in dessie referral hospital, Dessie, northeast of Ethiopia. BioMed. Res. Int. 2020, 1695683. doi: 10.1155/2020/1695683
Misha G., Chelkeba L., Melaku T. (2021a). Bacterial profile and antimicrobial susceptibility patterns of isolates among patients diagnosed with surgical site infection at a tertiary teaching hospital in Ethiopia: a prospective cohort study. Ann. Clin. Microbiol. Antimicrobials 20, 1–10. doi: 10.1186/s12941-021-00440-z
Misha G., Chelkeba L., Melaku T. (2021b). Incidence, risk factors and outcomes of surgical site infections among patients admitted to Jimma Medical Center, South West Ethiopia: Prospective cohort study. Ann. Med. Surg. (Lond) 65, 102247. doi: 10.1016/j.amsu.2021.102247
Moges G., Belete L., Mengesha Y., Ahmed S. (2020). Evaluation of surgical antimicrobial prophylaxis and incidence of surgical site infection at Borumeda hospital, northeast Ethiopia: retrospective cross-sectional study. Drug Healthc. Patient Saf. 12, 257–268. doi: 10.2147/DHPS.S280442
Mohammed A., Adeshina G. O., Ibrahim Y. K. (2013). Incidence and antibiotic susceptibility pattern of bacterial isolates from wound infections in a tertiary hospital in Nigeria. Trop. J. Pharm. Res. 12, 617–621. doi: 10.4314/tjpr.v12i4.26
Mulu W., Kibru G., Beyene G., Damtie H. (2013). Associated risk factors for postoperative nosocomial infections among patients admitted at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Clin. Med. Res. 2, 140–147. doi: 10.11648/j.cmr.20130206.15
Nijs A., Cartuyvels R., Mewis A., Peeters V., Rummens J., Magerman K. (2003). Comparison and evaluation of Osiris and Sirscan 2000 antimicrobial susceptibility systems in the clinical microbiology laboratory. J. Clin. Microbiol. 41, 3627–3630. doi: 10.1128/JCM.41.8.3627-3630.2003
Olowo-Okere A., Ibrahim Y. K. E., Sani A. S., Olayinka B. O. (2018). Occurrence of surgical site infections at a tertiary healthcare facility in Abuja, Nigeria: a prospective observational study. Med. Sci. 6, 60. doi: 10.3390/medsci6030060
Ostapowicz G., Fontana R. J., Schiødt F. V., Larson A., Davern T. J., Han S. H., et al. (2002). Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Internal Med. 137, 947–954. doi: 10.7326/0003-4819-137-12-200212170-00007
Paltiel O., Berhe E., Aberha A. H., Tequare M. H., Balabanova D. (2020). A public-private partnership for dialysis provision in Ethiopia: a model for high-cost care in low-resource settings. Health Policy Plann. 35, 1262–1267. doi: 10.1093/heapol/czaa085
Pathak A., Saliba E. A., Sharma S., Mahadik V. K., Shah H., Lundborg C. S. (2014). Incidence and factors associated with surgical site infections in a teaching hospital in Ujjain, India. Am. J. Infect. Control 42, e11–e15. doi: 10.1016/j.ajic.2013.06.013
Petrosillo N., Drapeau C. M., Nicastri E., Martini L., Ippolito G., Moro M. L., et al. (2008). Surgical site infections in Italian Hospitals: a prospective multicenter study. BMC Infect. Dis. 8, 34. doi: 10.1186/1471-2334-8-34
Pfenninger J. L., Zainea G. G. (2001). Common anorectal conditions: Part I. Symptoms and complaints. Am. Family Physician 63, 2391.
Rawabdeh A., Al Mulhim A. R. S., Khan Z. (2016a). Surgical site infections incidence, their predictors and causative organisms in a teaching hospital. Int. J. Community Fam Med. 1, 104. doi: 10.15344/2456-3498/2016/104
Rawabdeh A. A. A., Al Mulhim A. R. S., Khan Z. U. (2016b). Community & Family medicine. Hospital 1, 3498. doi: 10.15344/2456-3498/2016/104
Riss S., Weiser F. A., Schwameis K., Riss T., Mittlböck M., Steiner G., et al. (2012). The prevalence of hemorrhoids in adults. Int. J. Colorectal Dis. 27, 215–220. doi: 10.1007/s00384-011-1316-3
Shakir A., Abate D., Tebeje F., Weledegebreal F. (2021). Magnitude of surgical site infections, bacterial etiologies, associated factors and antimicrobial susceptibility patterns of isolates among post-operative patients in Harari region public hospitals, Harar, eastern Ethiopia. Infect. Drug Resist. 14, 4629–4639. doi: 10.2147/IDR.S329721
Shiferaw W. S., Aynalem Y. A., Akalu T. Y., Petrucka P. M. (2020). Surgical site infection and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Surg. 20, 107. doi: 10.1186/s12893-020-00764-1
Shin J., Hunt C. M., Suzuki A., Papay J. I., Beach K. J., Cheetham T. C. (2013). Characterizing phenotypes and outcomes of drug-associated liver injury using electronic medical record data. Pharmacoepidemiol. Drug Saf. 22, 190–198. doi: 10.1002/pds.v22.2
Shrestha S., Wenju P., Shrestha R., Karmacharya R. (2016). Incidence and risk factors of surgical site infections in Kathmandu University Hospital, Kavre, Nepal. Kathmandu Univ. Med. J. 14, 107–111.
Sun Z., Migaly J. (2016). Review of hemorrhoid disease: presentation and management. Clinics Colon Rectal Surg. 29, 22. doi: 10.1055/s-0035-1568144
Vasundhara Devi P., Sreenivasulu Reddy P., Shabnum M. (2017). Microbial profile and antibiotic susceptibility pattern of orthopedic infections in a tertiary care hospital: a study from South India. Int. J. Med. Sci. Public Health 6, 838–841. doi: 10.5455/ijmsph.2017.1165105122016
Verma A. K., Kapoor A., Bhargava A. (2012). Antimicrobial susceptibility pattern of bacterial isolates from surgical wound infections in tertiary care hospital in Allahabad, India. Internet J. Med. Update-EJOURNAL 7.
Worku S., Abebe T., Alemu A., Seyoum B., Swedberg G., Abdissa A., et al. (2023). Bacterial profile of surgical site infection and antimicrobial resistance patterns in Ethiopia: a multicentre prospective cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 22, 96. doi: 10.1186/s12941-023-00643-6
Yeo D., Tan K.-Y. (2014). Hemorrhoidectomy-making sense of the surgical options. World J. Gastroenterol: WJG 20, 16976. doi: 10.3748/wjg.v20.i45.16976
Young P. Y., Khadaroo R. G. (2014). Surgical site infections. Surg. Clinics 94, 1245–1264. doi: 10.1016/j.suc.2014.08.008
Zein-Eldeen A. A., Elsayed Sabal M. S., Hamam S. S., Zahran W. A. (2018). Surgical site infections: problem of multidrug-resistant bacteria. Menoufia Med. J. 30, 1005–1013. doi: 10.4103/mmj.mmj_119_17
Keywords: incidence, surgical site infection, bacterial isolates, susceptibility patterns, wound, antibiotic discs
Citation: Abdela J, Yusuf M, Adem F, Amin A, Mohammedsani B, Abdurke M, Mehadi A, Abdu A and Mohammed H (2025) Incidence, predictors, bacterial isolate, antibiotic susceptibility pattern and treatment outcomes among patients who underwent major surgery in public hospitals in Eastern Ethiopia: a prospective multicenter cohort study. Front. Bacteriol. 4:1552671. doi: 10.3389/fbrio.2025.1552671
Received: 28 December 2024; Accepted: 10 March 2025;
Published: 01 April 2025.
Edited by:
Akhilesh K. Chaurasia, Sungkyunkwan University, Republic of KoreaReviewed by:
Santosh Dulal, Management Sciences for Health, United StatesYulia Rosa Saharman, University of Indonesia, Indonesia
Copyright © 2025 Abdela, Yusuf, Adem, Amin, Mohammedsani, Abdurke, Mehadi, Abdu and Mohammed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmedmenewer Abdu, bWVuZXdlcjU5QGdtYWlsLmNvbQ==
 Jemal Abdela
Jemal Abdela Mohammed Yusuf2
Mohammed Yusuf2 Fuad Adem
Fuad Adem Abdi Amin
Abdi Amin Mohammed Abdurke
Mohammed Abdurke Ame Mehadi
Ame Mehadi Ahmedmenewer Abdu
Ahmedmenewer Abdu