- Department of Industrial Engineering, University of Salerno, Fisciano, Italy
In this short review, drug delivery systems, formed by polysaccharide-based (i.e., agarose, alginate, and chitosan) aerogels, are analyzed. In particular, the main papers, published in the period 2011–2020 in this research field, have been investigated and critically discussed, in order to highlight strengths and weaknesses of the traditional production techniques (e.g., freeze-drying and air evaporation) of bio-aerogels with respect to supercritical CO2 assisted drying. Supercritical CO2 assisted drying demonstrated to be a promising technique to produce nanostructured bio-aerogels that maintain the starting gel volume and shape, when the solvent removal occurs at negligible surface tension. This characteristic, coupled with the possibility of removing also cross-linking agent residues from the aerogels, makes these advanced devices safe and suitable as carriers for controlled drug delivery applications.
Introduction
Pharmaceutical industry is evolving from traditional drug delivery systems, in which a biopolymeric matrix is used to provide weight, volume and flowability, toward new formulations, in which a biopolymer is adopted as drug performance enhancer in terms of release time and bioavailability (Agüero et al., 2017; Yuan et al., 2018; Shi et al., 2019; Wei et al., 2020; Liu et al., 2021).
In this field, the production of micro- and nanoparticles has been widely investigated, since they provide effective ways to address issues related to poorly water-soluble drugs and patient compliance (Agnihotri et al., 2004; Markman et al., 2013; Singh et al., 2019; Guastaferro et al., 2020).
Nowadays, also polymeric gels are becoming promising matrices for drug delivery, thanks to their nanostructured morphology that allows to reach larger drug loadings and an improved controlled release of the active compounds over time. In this regard, Cardea et al. (2018) realized poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) aerogels loaded with curcumin in order to obtain a prolonged drug release. Nanofibrous aerogels characterized by open interconnected pores and high porosity value (almost 94%) were produced by supercritical drying, and curcumin release was extended up to 44 h. Also Follmann et al. (2020) realized nanofibrous silica-based hybrid gels, with the aim to deliver camptothecin (CPT), a poorly water-soluble anticancer drug, in a sustained manner. In this case, aerogels ensured CPT release for more than 2 weeks. Giray et al. (2012) realized a composite gel consisting of a silica core coated by polyethyleneglycol (PEG). The results indicated that a slower release of ketoprofen was achieved increasing PEG diacrylate concentration, since it lowered aerogel permeability when it was immersed in an aqueous solution. Therefore, the main advantages of these systems over the traditional ones are: (i) tendency to deliver pharmaceutical compounds more selectively to a specific site, (ii) to maintain drug levels in the desired range, (iii) to increase patient compliance, and (iv) to prevent side effects (Van der Lubben et al., 2001; Guenther et al., 2008; Mehling et al., 2009; García-González et al., 2011; Marin et al., 2014; Lovskaya et al., 2015; Sosnik et al., 2021).
In this context, biocompatibility and biodegradability are essential features; therefore, polysaccharide-based polymers can be considered as “key formulation ingredients,” due to their natural properties (Zheng et al., 2015; Ferreira et al., 2016; Manivasagan and Oh, 2016; Wang et al., 2019). However, the final porous structure of these gels, required for drug release, depends on the kind of drying technique used. In particular, gels can be termed as “xerogel” when sample drying is carried out under ambient pressure and at room temperature, for some days (Conzatti et al., 2017; Takeshita et al., 2020b). Despite the energy-saving advantage of this technique, it leads to the formation of a condense structure that may have low porosity values and large shrinkage (Mirzaei et al., 2013; Buchtová and Budtova, 2016; Sukhodub et al., 2018; López-Iglesias et al., 2019). “Cryogels” are produced when the solvent inside the gel matrix is extracted by freeze drying. During this process, the liquid part in the wet-gel is frozen and, subsequently, under low pressure, the frozen wet-gel is dried by sublimation (Cheng et al., 2012; Gupta and Nayak, 2016; Mahmoud and Salama, 2016; You et al., 2017; Rubio-Elizalde et al., 2019; Yang et al., 2019).
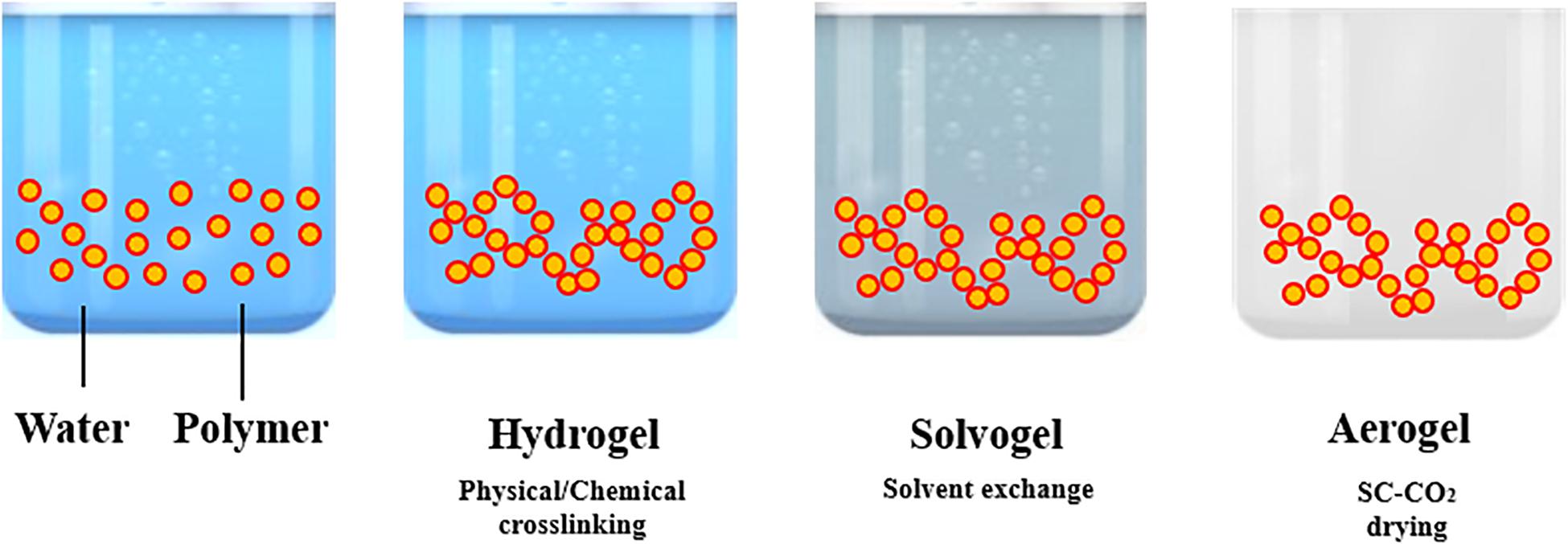
The processing steps involved in “aerogels” production are summarized in Figure 1. Aerogel production frequently starts from the formation of a gel in an aqueous solution, adding a chemical, physical or enzymatic cross-linker. Operating in this way, a hydrogel is obtained. The following step is the replacement of the water present in the 3-D network of the hydrogel by an organic solvent; the resulting gel is named as solvogel. Solvent exchange step is a critical and dynamic process that can strongly affect the final aerogel morphology. In particular, solvent composition, kind of solvent and exchange rate are the main parameters to be investigated (Takeshita et al., 2020a). According to Takeshita et al. (2020a), a low affinity between the polymer forming the gel and the substituting solvent induces the formation of an aerogel-like structure during the solvent exchange itself and a drastic shrinkage of the gel. Therefore, to minimize this phenomenon, some guidelines can be followed: (i) to select an organic solvent at high affinity with the biopolymer (Takeshita et al., 2020a); (ii) to perform a multi-step solvent exchange (García-González et al., 2011; Baldino et al., 2019), at increasing percentage by volume of the solvent substituting water. Operating in this way, the liquid-liquid extraction and substitution of water with the organic solvent selected, evolves gradually, reducing gel shrinkage and other undesired structure modifications. Then, the solvent is extracted from the solid network by supercritical CO2 (SC-CO2): a supercritical mixture with an almost zero surface tension is formed, at the opportune operative conditions of pressure and temperature, between CO2 and the organic solvent that avoids the collapse of the delicate gel nanostructure (Reverchon et al., 2008; Cardea et al., 2009; Baldino et al., 2016; Muñoz-Ruíz et al., 2019).
Gels dried under supercritical conditions show unique properties, such as large porosity, uniform pore sizes, and high surface area, in the range of 500–1,200 m2/g, due to mesoporous (<50 nm and >2 nm) and micropores (<2 nm) distribution inside the polymeric matrix (Soleimani Dorcheh and Abbasi, 2008; Baldino et al., 2015, 2019; García-González et al., 2015). Moreover, they are made up of about 95% of air or gas by volume and, consequently, are very light in weight (Del Gaudio et al., 2013; Della Porta et al., 2013; Lu et al., 2014; Mallepally et al., 2015; Quraishi et al., 2015; Baldino et al., 2019). Aerogels could overcome the problems associated with slow drug dissolution rate, unfavorable pharmacokinetics, poor bio-distribution and lack of selectivity for target tissues (Ulker and Erkey, 2014; Lovskaya et al., 2015; Mohammadian et al., 2018). Therefore, the combination of the outstanding structural properties of aerogels with the physiological compatibility of polysaccharides would result in high potential drug delivery systems (Huang et al., 2011; Matricardi et al., 2013; Shelke et al., 2014; Ren et al., 2018).
In this short review, the attention will be focused on the production of chitosan (CS), alginate (ALG), and agarose (AGR) aerogels for the pharmaceutical field. Indeed, among the other applications, these biopolymers have also been studied for drug delivery, and the respective percentages of investigation are: 11.92% agarose, 37.74% alginate and 50.34% chitosan. They were calculated using the database Science Direct, looking at the number of papers written in the period 2011–2020. The path used was the following one: “biopolymer (AGR/ALG/CS), drug delivery, aerogel/cryogel.” Strengths and weaknesses of the traditional production techniques (e.g., freeze-drying and air evaporation) of these bio-aerogels will be critically compared with supercritical CO2 assisted drying, to highlight possible indications to obtain advanced bio-carriers for controlled drug delivery applications.
Chitosan-Based Gels
Chitosan is derived from chitin that is the major component of the crustacean exoskeleton, and is naturally hydrophilic. CS exhibits biocompatible, biodegradable, and non-toxic properties (Pillai et al., 2009; Venkatesan and Kim, 2010).
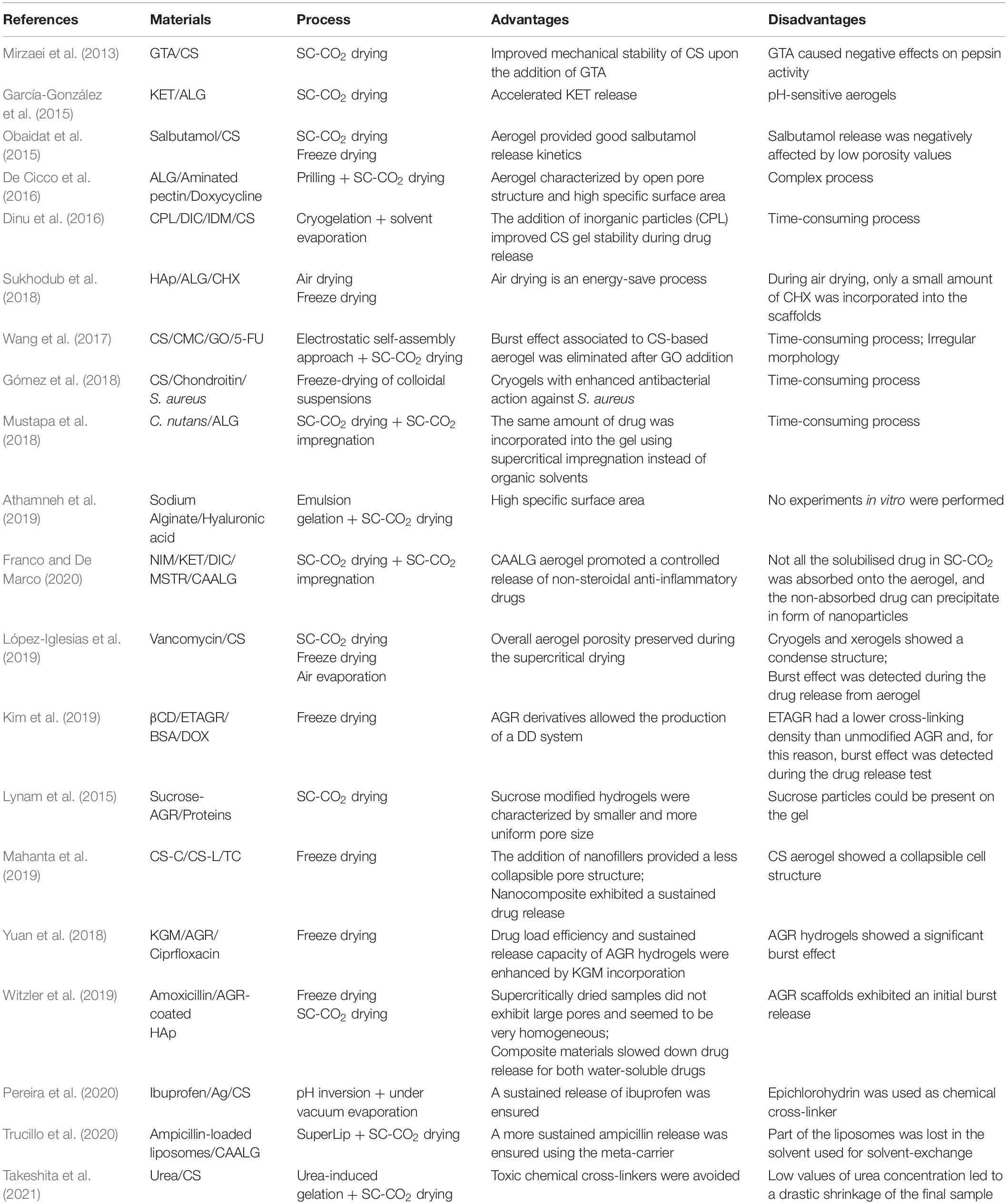
The first step toward CS-based aerogel production is represented by hydrogel formation (Shi et al., 2021). CS hydrogels can be prepared by non-covalent strategies that take advantage of ionic interactions, H-bonding and Van der Waals forces. In these cases, gel formation can be reversed (Cerchiara et al., 2002; Berger et al., 2004; Boucard et al., 2005). However, physically cross-linked gels could present some drawbacks; i.e., weak mechanical strength, uncontrolled gel pore size and fast dissolution kinetics (Dash et al., 2011). Improvements of mechanical properties can be obtained by permanent hydrogel networks, using covalent bonding between polymeric chains. In particular, the presence of –NH2 and –OH groups on CS chains offers the possibility to create different linkages, such as amide and ester bonding, as well as Schiff base formation (Croisier and Jérôme, 2013). The production of chemically cross-linked gels is achieved by mixing chitosan aqueous solutions with cross-linkers or charged polymers, under specific conditions of pH and temperature (López-León et al., 2005; Liang et al., 2009; Cui et al., 2014; Pellá et al., 2018). Glutaraldehyde (GTA), diglycidyl ether, diisocyanate, and diacrylate, are generally used for this purpose (Hoare and Kohane, 2008). However, their use in pharmaceutical and biomedical applications is restricted, since these cross-linking agents can deactivate or limit drug efficiency and can be cytotoxic for cells (Zeiger et al., 2005; Takigawa and Endo, 2006). In order to overcome these limitations, Takeshita et al. (2021) synthesized chitosan aerogels using a green technology that avoided the use of harmful chemicals, such as aldehyde cross-linkers. In particular, CS aerogels were produced by urea-induced gelation. Urea is an industrial reagent that is widely used due to its low cost and low toxicity. In this study, urea-induced gelation was followed by ethanol exchange and SC-CO2 drying. However, a drastic shrinkage was observed for CS samples at each value of urea concentration. Also genipin, that is a natural chemical compound ables to bind amino groups between amino molecules, can be used to produce a cross-linked CS at good mechanical and degradative properties (Dimida et al., 2015). CS hydrogels can be also prepared by increasing pH of the acidic polymer solutions. In this way, a sol-gel transition is promoted, due to hydrophobic interactions (Moura et al., 2007; Tabernero et al., 2020). Pereira et al. (2020) synthesized CS microspheres loaded with silver (Ag) nanoparticles, through pH inversion mechanism, to be used as bactericidal agent. The results revealed that these systems were effective against Gram-positive and Gram-negative microorganisms. The Ag-loaded CS microspheres were also tested as drug delivery systems, and ibuprofen was used as model drug. The addition of Ag nanoparticles into polymeric matrix promoted a significant delay of ibuprofen release that was extended up to 6 h, with respect to CS-only based microparticles. Gómez et al. (2018) fabricated colloidal suspensions of CS/chondroitin that were subsequently freeze dried to obtain lyophilized nanocomplexes. These cryogels were loaded with a garlic extract and tested against Staphylococcus aureus (S. aureus), a pathogenic microorganism in chronic skin lesions. The dried gels showed a non-homogeneous morphology consisting of fibers and sheets. The introduction of the extract led to an increase of pristine gel hardness and to an enhanced antibacterial action against S. aureus. Obaidat et al. (2015) prepared CS-based aerogel carriers using two different technologies, i.e., SC-CO2 assisted drying and freeze drying. Then, these carriers were loaded with salbutamol. SEM analysis revealed several cracks and voids in the freeze dried samples; whereas aerogels preserved a high open porosity and textural properties. The release profile of salbutamol from samples produced by supercritical drying could be considered suitable for pulmonary drug delivery systems; the release from cryogels was instead negatively affected by a low porosity and a low value of surface area. Terzić et al. (2018) prepared CS-based gels and investigated how the drying technique used could affect the drug release system behavior. With the aim of increasing CS hydrophilicity, it was blended with itaconic acid and methacrylic acid. Then, thymol, that represents the main constituent of oregano essential oil and has a strong antibacterial action, was supercritically impregnated into the polymeric matrix. Two different techniques were used for drying: air drying that led to xerogels formation, and SC-CO2 drying, for aerogel production. SEM analysis showed that xerogels structure was non-porous; indeed, stresses occurred on the pore walls during the extraction of the solvent, inducing the collapse of the gel native structure. On the other hand, during SC-CO2 drying, time resulted the key factor of the process to ensure the desired value of porosity: short processing time led to an incomplete removal of the solvent and, subsequently, a low value of porosity was detected. Due to the high specific surface area, the amount of thymol incorporated into CS aerogel was much higher than the amount loaded into CS xerogel. López-Iglesias et al. (2019) produced CS aerogels loaded with vancomycin to treat infections in chronic wounds. Hydrogel macroparticles were prepared via sol-gel processing and alcogels were subsequently dried. However, xerogels were not able to preserve the intrinsic gel nanoporous structure and high shrinkage values were detected; whereas CS aerogels preserved the overall porosity. A fast release of vancomycin from CS aerogel particles was measured during the first hour and it was followed by a slower release during the next hours.
The initial drug burst effect can be considered a relevant drawback when a sustained drug release is required (Spinks et al., 2006; Aryaei et al., 2014; Xie et al., 2018; Wahba, 2020). To address this issue, CS hydrogels can be covalently cross-linked using UV irradiation or GTA, to obtain an improved mechanical stability (Zeiger et al., 2005; Takigawa and Endo, 2006; Baldino et al., 2015). Baldino et al. (2015) produced CS aerogels by SC-CO2 drying. These samples were characterized by a nanofibrous structure, with an average pore size of 100 nm. Moreover, they demonstrated that, thanks to this process, it was possible to obtain a complete removal of GTA from CS gels: the supercritical mixture (CO2 + ethanol) showed a high affinity toward GTA, favoring its removal from the samples. For this reason, SC-CO2 drying can be considered a promising process to purify chemically cross-linked CS aerogels, to be used for pharmaceutical applications. Mirzaei et al. (2013) prepared CS aerogels cross-linked with GTA for drug delivery. SEM images demonstrated that these xerogels had an average pore size ranging from 100 to 500 μm. Moreover, the swelling trend of these CS xerogels decreased by increasing the amount of cross-linker.
Recently, nanohybrid gels have been used as drug delivery carriers. These pharmaceutical systems are composite materials; they are made up using organic polymers loaded with inorganic nanoparticles. Nanoparticles are supposed to suppress burst drug release behavior, leading to a slower and more continuous release of drugs. Wang et al. (2017) prepared hybrid cryogels of CS, carboxymethyl cellulose (CMC) and graphene oxide (GO), crosslinked by Ca+2. These gels were synthesized using an electrostatic self-assembly approach, followed by freeze drying. SEM images showed a cryogel morphology that was mainly characterized by irregular CS-CMC clusters located on GO sheets. These samples were used to investigate the release of 5-fluorouracil (5-FU), a chemotherapeutic agent adopted in the treatment of cancer. GO addition delayed the release of 5-FU and overcame burst release problems associated with CS-based aerogels. Dinu et al. (2016) synthesized CS/clinoptilolite (CPL) biocomposite cryogels by cryogelation. DIC and indomethacin (IDM) were loaded into these cryogels using the solvent evaporation technique. The release profiles of DIC and IDM from CS/CPL composites were pH-dependent, and drug release increased when pH varied from 1.2 to 7.4. Mahanta et al. (2019) realized CS nanohybrid cryogels using two kinds of disk-shaped nanofillers of opposite surface charges, 30B nanoclay (CS-C), negatively charged, and layer double hydroxide (CS-L), positively charged. The antibacterial drug, tetracycline hydrochloride (TC), was used as a model drug to investigate the release kinetics. Nanohybrids exhibited sustained release kinetics in both cases (hydrogel and dried gels). Scaffolds induced a 90, 69, and 56% of drug release in 15 h, from CS, CS-C, and CS-L, respectively; whereas a 58, 40, and 28% of drug release was measured using the respective hydrogels. A critical summary of these papers is reported in Table 1.
Alginate-Based Gels
Alginate is a naturally occurring anionic polymer, typically obtained from brown seaweed, and has been extensively used for pharmaceutical applications, thanks to its biocompatibility, low toxicity, low cost and easy gelation (García-González et al., 2015; Pantić et al., 2016; Athamneh et al., 2019; Lovskaya and Menshutina, 2020). ALG hydrogels formation can be induced by different cations, such as: H+, Ca+2, Ba+2, Cu+2, Sr+2, Zn+2, Mn+2, Fe+2, Al+3, and Fe+3 (Cao et al., 2020). Indeed, the presence of negatively charged ions in ALG molecules can lead to the formation of polyelectrolyte complexes, because they give the possibility to bind positively charged ions (Hu et al., 2021). Ca+2 is the most used divalent cation to induce alginate gelation, since it shows a high affinity toward the bio-polymer guluronate (G) blocks (Hu et al., 2021). Reynolds and Enquist (1973) proposed, for the first time, the theory of Ca+2 induced ALG gelation mechanism, defining the gel structure as an egg-box model. In this structure, Ca+2 coordinates with six oxygen atoms of two neighboring G units and one to three oxygen atoms of H2O to form a stable structure. However, depending on the final application, different kind of ions can be used: e.g., Ba-ALG gels have been widely used in nanomedicine and Sr-ALG gels show a great potential for tissue regeneration, since they can enhance cell proliferation (Hu et al., 2021). One critical drawback of ionically cross-linked ALG gels is the limited long-term stability in physiological conditions, because these gels can easily dissolve due to the release of divalent ions into the surrounding media, as a consequence of exchange reactions with monovalent cations (Waldman et al., 1998; Qin, 2004, 2005; Santos Miranda et al., 2006).
ALG hydrogels similarity to the extracellular matrices of living tissues allows wide applications in the delivery of small chemical drugs and proteins (Rowley et al., 1999; Augst et al., 2006; Bidarra et al., 2014). Athamneh et al. (2019) realized aerogel microspheres based on sodium alginate and hyaluronic acid for pulmonary drug delivery. Emulsion gelation was combined with SC-CO2 gel drying and, at the end, aerogels, at high specific surface area and good aerodynamic properties, were obtained. García-González et al. (2015) investigated the release kinetics of ketoprofen (KET) from ALG-based aerogels. These authors found that ALG aerogels accelerated KET release at simulated gastric pH conditions. Moreover, it was noted that KET release was mainly governed by a Fickian diffusion mechanism. Sukhodub et al. (2018) synthesized a hydroxyapatite-alginate (HAp)-ALG nanostructured composite for the controlled release of chlorexidine (CHX). The dried samples were obtained by hydrogel drying at 37°C in warm ambient and by freeze drying for 24 h. The densest morphology corresponded to the composite xerogel dried at 37°C; whereas the freeze dried sample had a porous morphology whose homogeneity was slightly improved after HAp addition. Increasing the amount of ALG in the composite gels led to an increase in the volume of adsorbed and released CHX, and the release time also increased from 24 to 72 h. Trucillo et al. (2020) produced a meta-carrier; namely, a carrier entrapped inside another carrier, formed by a liposome loaded aerogel. The antibiotic (ampicillin) was encapsulated into the liposomes, produced by a supercritical assisted liposomes formation (SuperLip) technique. SC-CO2 drying was selected to produce ALG aerogels loaded with ampicillin loaded liposomes. The structures obtained in the case of water exchange with ethanol and acetone were characterized by different morphologies; in particular, the structures obtained in the first case showed nanofibers and open pores, whereas the other ones were uniformly nanoporous. Drug release tests demonstrated that ampicillin release time from these meta-carriers was about twice than its release time from liposomes alone.
Franco and De Marco (2020) used SC-CO2 adsorption to incorporate three non-steroidal anti-inflammatory drugs (NSAIDs), nimesulide (NIM), (KET) and diclofenac sodium (DIC), into maize starch (MSTR) and calcium alginate (CAALG) aerogels. For each NSAID, it can be noted that the amount of drug adsorbed in CAALG was generally higher than the amount loaded in MSTR. Both aerogels were formed by a microporous structure, preserved after supercritical adsorption. The dissolution tests revealed that the adsorption into MSTR allowed a faster release of NSAIDs than pure crystalline drugs; whereas CAALG promoted a controlled release of NSAIDs. Gonçalves et al. (2016) realized ALG-based hybrid aerogels in form of particles to be used as mucosal drug delivery systems. Gel drying and KET loading were performed using SC-CO2. All ALG-based macroparticles showed high specific surface area and large pore volume. Moreover, these formulations were able to provide a slower release of KET in comparison with the pure one. De Cicco et al. (2016) realized aerogel formulations based on ALG and amidated pectin, in form of core-shell microparticles, that were dried using SC-CO2. At the end of the process, these samples were characterized by an open pore structure and high specific surface area. These polymeric aerogels were loaded with doxycycline and the results demonstrated that drug release was affected by pectin and ALG amount. Moreover, doxycycline release kinetics was mainly governed by swelling matrix and erosion phenomena. Mustapa et al. (2018) produced ALG hydrogels that were supercritically dried. Plant extracts of Clinacanthus nutans (C. nutans) were impregnated into ALG aerogels via SC-CO2 assisted impregnation. C. nutans-50 extract was released faster from ALG when was impregnated using supercritical conditions. A critical summary of these papers is reported in Table 1.
Agarose-Based Gels
Agarose is currently used in various research fields including food, DD, DNA electrophoresis, and tissue engineering, owing to its thermo-reversible gel forming ability (Sakai et al., 2007; Gu et al., 2017).
In order to create an AGR gel, heating and cooling processes are involved, and gelation occurs at high temperatures, making difficult to load heat-sensitive drugs. Therefore, researchers have developed AGR gels with low gelling temperatures by modification through acetylation (García-Ruiz et al., 2001), alkylation, alkenylation, acylation, and oxyalkylation (Zhang et al., 2018). The introduction of functional groups hinders the formation of the helicoidal structure at low temperatures, thereby lowering the gelling temperature of AGR (Forget et al., 2015). Moreover, for the development of more innovative AGR-based materials, AGR should comprise special functional groups, such as tosyl or amine moieties (Gericke and Heinze, 2015).
The diffusion characteristics of various substances, including drugs, from AGR gels, are related to the rheological properties of the gels (Normand et al., 2000; Kim et al., 2019). Therefore, AGR derivatives with low gelation temperatures can deliver the substance quickly because the double helicoidal structure during gelation is reduced and, subsequently, the storage modulus is lowered. For this reason, there is a limit in developing effective DD systems that can control the release rates using AGR derivatives with low gelation temperatures (Kim et al., 2019).
Kim et al. (2019) described the introduction of β-cyclodextrin (βCD) into an ethylenediamine-modified agarose (ETAGR) for the development of AGR at low gelling temperatures. The modified gels were prepared by freeze drying and used for both bovine serum albumin (BSA) and doxorubicin (DOX) release. The section of non-functionalized AGR gel was characterized by a non-uniform distribution of pore sizes. The release profiles showed that increasing the cross-linking density of the gel or increasing AGR concentration, the diffusion rate and, subsequently, the release kinetics of BSA, slowed down. Since CFAs had lower cross-linking density than AGR, due to the presence of ethylenediamine groups, BSA was released within a few hours with a non-negligible initial burst effect. Lynam et al. (2015) developed AGR scaffolds used for the controlled release of proteins, for nerve repair. Solvogels were supercritically dried after the replacement of water in AGR hydrogel with ethanol. Pores of 0.10 μm were identified; moreover, SEM images showed a homogeneous and nanoporous morphology. Witzler et al. (2019) investigated the release behavior from AGR-coated Hap, using two model drugs, i.e., adenosine 5′-triphosphate (ATP) and suramin. A prolonged release over 4 days was found for both samples (cryogel and aerogel). However, freeze dried samples exhibited large pores in the range of several hundred micrometers. This was due to the growth of ice crystals during the freezing process. In contrast to the freeze dried samples, the SC-CO2 dried ones were characterized by a higher specific surface area. Yuan et al. (2018) produced a polymeric blend with konjac glucomannan (KGM) and AGR, for ciprofloxacin release, using freeze drying. KGM is a natural polysaccharide found in the tuber of Amorphophallus konjac. The addition of KGM determined a clear effect on the internal morphology of AGR gels: the increase in KGM led to a more compact internal structure with smaller pores. The release results demonstrated that encapsulation, drug loading efficiencies, and sustained release capacity of AGR cryogels, were enhanced by the incorporation of KGM. However, more than 95% of ciprofloxacin was released in the first half hour, because most of the drug was localized on the surface of the polymeric matrix. A critical summary of these papers is reported in Table 1.
Conclusion
In this short review, the main production techniques of drug delivery systems, based on natural, biocompatible and biodegradable polymers, were analyzed. Bio-based aerogels are supposed to be promising candidates as drug carriers, thanks to the native overall nanoporosity and the high specific surface area. These features allow to reach a high drug loading and to obtain a sustained drug release over time.
Freeze drying and air evaporation are the most consolidate and frequently used drying techniques to produce these bio-polymeric systems. However, they can lead to dense and/or not homogeneous final gel structures; therefore, alternative drying techniques should be selected to preserve these relevant characteristics for drug release. Supercritical CO2 assisted drying can overcome these drawbacks, allowing to produce nanostructured bio-aerogels, that maintain the starting gel volume and shape. These aerogel properties are preserved when (i) water/solvent exchange step is carefully performed by selecting the opportune organic solvent and a slow exchange rate, and (ii) the process operative conditions guarantee the formation of a supercritical mixture (CO2 + organic solvent) at negligible surface tension. Moreover, SC-CO2 assisted drying can also remove cross-linking agent residues from the aerogels, making these advanced bio-carriers safe and suitable for controlled drug delivery applications.
Author Contributions
MG and LB: conceptualization. MG: writing—original draft preparation. LB: writing—review and editing. ER: supervision. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
Ag, silver; AGR, agarose; ALG, alginate; ATP, adenosine 5′-triphosphate; BSA, bovine serum albumin; CAALG, calcium alginate; CPT, camptothecin; β CD, β-cyclodextrin; CMC, carboxymethyl cellulose; CHX, chlorexidine; CPL, clinoptilolite; CS, chitosan; CS-C, nanoclay-loaded chitosan; CS-L, layer double hydroxide-loaded chitosan; DIC, diclofenac sodium; DOX, doxorubicin; ETAGR, ethylenediamine-modified agarose; 5-FU, 5-fluorouracil; GO, graphene oxide; GTA, gluteraldehyde; G, guluronate; HAp, hydroxyapatite; IDM, indomethacin; KET, ketoprofen; KGM, konjac glucomannan; MSTR, maize starch; NIM, nimesulide; NSAIDs, non-steroidal anti-inflammatory drugs; PEG, polyethyleneglycol; PVDF-HFP, poly(vinylidene fluoride-hexafluoropropylene); S. aureus, staphylococcus aureus; SC-CO2, supercritical CO2; SuperLip, supercritical assisted liposomes formation; TC, tetracycline hydrochloride.
References
Agnihotri, S. A., Mallikarjuna, N. N., and Aminabhavi, T. M. (2004). Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 100, 5–28. doi: 10.1016/j.jconrel.2004.08.010
Agüero, L., Zaldivar-Silva, D., Peña, L., and Dias, M. (2017). Alginate microparticles as oral colon drug delivery device: a review. Carbohydr. Polym. 168, 32–43. doi: 10.1016/j.carbpol.2017.03.033
Aryaei, A., Jayatissa, A. H., and Jayasuriya, A. C. (2014). Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. A 102, 2704–2712. doi: 10.1002/jbm.a.34942
Athamneh, T., Amin, A., Benke, E., Ambrus, R., Leopold, C. S., Gurikov, P., et al. (2019). Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 150, 49–55. doi: 10.1016/j.supflu.2019.04.013
Augst, A. D., Kong, H. J., and Mooney, D. J. (2006). Alginate hydrogels as biomaterials. Macromol. Biosci. 6, 623–633. doi: 10.1002/mabi.200600069
Baldino, L., Cardea, S., Scognamiglio, M., and Reverchon, E. (2019). A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 146, 152–158. doi: 10.1016/j.supflu.2019.01.016
Baldino, L., Concilio, S., Cardea, S., and Reverchon, E. (2016). Interpenetration of natural polymer aerogels by supercritical drying. Polymers 8:106. doi: 10.3390/polym8040106
Baldino, L., Concilio, S., Cardea, S., De Marco, I., and Reverchon, E. (2015). Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 103, 70–76. doi: 10.1016/j.supflu.2015.04.020
Berger, J., Reist, M., Mayer, J. M., Felt, O., and Gurny, R. (2004). Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 57, 35–52. doi: 10.1016/S0939-6411(03)00160-7
Bidarra, S. J., Barrias, C. C., and Granja, P. L. (2014). Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 10, 1646–1662. doi: 10.1016/j.actbio.2013.12.006
Boucard, N., Viton, C., and Domard, A. (2005). New aspects of the formation of physical hydrogels of chitosan in a hydroalcoholic medium. Biomacromolecules 6, 3227–3237. doi: 10.1021/bm050653d
Buchtová, N., and Budtova, T. (2016). Cellulose aero-, cryo- and xerogels: towards understanding of morphology control. Cellulose 23, 2585–2595. doi: 10.1007/s10570-016-0960-8
Cao, L., Lu, W., Mata, A., Nishinari, K., and Fang, Y. (2020). Egg-box model-based gelation of alginate and pectin: a review. Carbohydr. Polym. 242:116389. doi: 10.1016/j.carbpol.2020.116389
Cardea, S., Baldino, L., and Reverchon, E. (2018). Comparative study of PVDF-HFP-curcumin porous structures produced by supercritical assisted processes. J. Supercrit. Fluids 133, 270–277. doi: 10.1016/j.supflu.2017.10.026
Cardea, S., Gugliuzza, A., Sessa, M., Aceto, M. C., Drioli, E., and Reverchon, E. (2009). Supercritical gel drying: a powerful tool for tailoring symmetric porous PVDF-HFP membranes. ACS Appl. Mater. Interfaces 1:171–180. doi: 10.1021/am800101a
Cerchiara, T., Luppi, B., Bigucci, F., Orienti, I., and Zecchi, V. (2002). Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. J. Pharm. Pharmacol. 54, 1453–1459. doi: 10.1211/00223570281
Cheng, Y., Lu, L., Zhang, W., Shi, J., and Cao, Y. (2012). Reinforced low density alginate-based aerogels: preparation, hydrophobic modification and characterization. Carbohydr. Polym. 88, 1093–1099. doi: 10.1016/j.carbpol.2012.01.075
Conzatti, G., Faucon, D., Castel, M., Ayadi, F., Cavalie, S., and Tourrette, A. (2017). Alginate/chitosan polyelectrolyte complexes: a comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 172, 142–151. doi: 10.1016/j.carbpol.2017.05.023
Croisier, F., and Jérôme, C. (2013). Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 49, 780–792. doi: 10.1016/j.eurpolymj.2012.12.009
Cui, L., Jia, J., Guo, Y., Liu, Y., and Zhu, P. (2014). Preparation and characterization of IPN hydrogels composed of chitosan and gelatin cross-linked by genipin. Carbohydr. Polym. 99, 31–38. doi: 10.1016/j.carbpol.2013.08.048
Dash, M., Chiellini, F., Ottenbrite, R. M., and Chiellini, E. (2011). Chitosan - A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 36, 981–1014. doi: 10.1016/j.progpolymsci.2011.02.001
De Cicco, F., Russo, P., Reverchon, E., García-González, C. A., Aquino, R. P., and Del Gaudio, P. (2016). Prilling and supercritical drying: a successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 147, 482–489. doi: 10.1016/j.carbpol.2016.04.031
Del Gaudio, P., Auriemma, G., Mencherini, T., Porta, G., Della Reverchon, E., and Aquino, R. P. (2013). Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 102, 185–194. doi: 10.1002/jps.23361
Della Porta, G., Del Gaudio, P., De Cicco, F., Aquino, R. P., and Reverchon, E. (2013). Supercritical drying of alginate beads for the development of aerogel biomaterials: optimization of process parameters and exchange solvents. Ind. Eng. Chem. Res. 52, 12003–12009. doi: 10.1021/ie401335c
Dimida, S., Demitri, C., De Benedictis, V. M., Scalera, F., Gervaso, F., and Sannino, A. (2015). Genipin-cross-linked chitosan-based hydrogels: reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 132:42256. doi: 10.1002/app.42256
Dinu, M. V., Cocarta, A. I., and Dragan, E. S. (2016). Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels. Carbohydr. Polym. 153, 203–211. doi: 10.1016/j.carbpol.2016.07.111
Ferreira, A. R. V., Alves, V. D., and Coelhoso, I. M. (2016). Polysaccharide-based membranes in food packaging applications. Membranes 6:22. doi: 10.3390/membranes6020022
Follmann, H. D. M., Oliveira, O. N., Martins, A. C., Lazarin-Bidóia, D., Nakamura, C. V., Rubira, A. F., et al. (2020). Nanofibrous silica microparticles/polymer hybrid aerogels for sustained delivery of poorly water-soluble camptothecin. J. Colloid Interface Sci. 567, 92–102. doi: 10.1016/j.jcis.2020.01.110
Forget, A., Pique, R. A., Ahmadi, V., Lüdeke, S., and Shastri, V. P. (2015). Mechanically tailored agarose hydrogels through molecular alloying with β-sheet polysaccharides. Macromol. Rapid Commun. 36, 196–203. doi: 10.1002/marc.201400353
Franco, P., and De Marco, I. (2020). Supercritical CO2 adsorption of non-steroidal anti-inflammatory drugs into biopolymer aerogels. J. CO2 Util. 36, 40–53. doi: 10.1016/j.jcou.2019.11.001
García-González, C. A., Alnaief, M., and Smirnova, I. (2011). Polysaccharide-based aerogels - promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 86, 1425–1438. doi: 10.1016/j.carbpol.2011.06.066
García-González, C. A., Jin, M., Gerth, J., Alvarez-Lorenzo, C., and Smirnova, I. (2015). Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 117, 797–806. doi: 10.1016/j.carbpol.2014.10.045
García-Ruiz, J. M., Novella, M. L., Moreno, R., and Gavira, J. A. (2001). Agarose as crystallization media for proteins I: transport processes. J. Cryst. Growth 232, 165–172. doi: 10.1016/S0022-0248(01)01146-0
Gericke, M., and Heinze, T. (2015). Homogeneous tosylation of agarose as an approach toward novel functional polysaccharide materials. Carbohydr. Polym. 127, 236–245. doi: 10.1016/j.carbpol.2015.03.025
Giray, S., Bal, T., Kartal, A. M., Kizilel, S., and Erkey, C. (2012). Controlled drug delivery through a novel PEG hydrogel encapsulated silica aerogel system. J. Biomed. Mater. Res. A 100, 1307–1315. doi: 10.1002/jbm.a.34056
Gómez, M. A., Bonilla, J. M., Coronel, M. A., Martínez, J., Morán-Trujillo, L., Orellana, S. L., et al. (2018). Antibacterial activity against Staphylococcus aureus of chitosan/chondroitin sulfate nanocomplex aerogels alone and enriched with erythromycin and elephant garlic (Allium ampeloprasum L. var. ampeloprasum) extract. Pure Appl. Chem. 90, 885–900. doi: 10.1515/pac-2016-1112
Gonçalves, V. S. S., Gurikov, P., Poejo, J., Matias, A. A., Heinrich, S., Duarte, C. M. M., et al. (2016). Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 107, 160–170. doi: 10.1016/j.ejpb.2016.07.003
Gu, Y., Cheong, K. L., and Du, H. (2017). Modification and comparison of three Gracilaria spp. agarose with methylation for promotion of its gelling properties. Chem. Cent. J. 11:104. doi: 10.1186/s13065-017-0334-9
Guastaferro, M., Baldino, L., Cardea, S., and Reverchon, E. (2020). Supercritical assisted electrospray/spinning to produce PVP+quercetin microparticles and microfibers. J. Taiwan Inst. Chem. Eng. 117, 278–286. doi: 10.1016/j.jtice.2020.12.017
Guenther, U., Smirnova, I., and Neubert, R. H. H. (2008). Hydrophilic silica aerogels as dermal drug delivery systems - dithranol as a model drug. Eur. J. Pharm. Biopharm. 69, 935–942. doi: 10.1016/j.ejpb.2008.02.003
Gupta, P., and Nayak, K. K. (2016). Optimization of keratin/alginate scaffold using RSM and its characterization for tissue engineering. Int. J. Biol. Macromol. 85, 141–149. doi: 10.1016/j.ijbiomac.2015.12.010
Hoare, T. R., and Kohane, D. S. (2008). Hydrogels in drug delivery: progress and challenges. Polymer 49, 1993–2007. doi: 10.1016/j.polymer.2008.01.027
Hu, C., Lu, W., Mata, A., Nishinari, K., and Fang, Y. (2021). Ions-induced gelation of alginate: mechanisms and applications. Int. J. Biol. Macromol. 177, 578–588. doi: 10.1016/j.ijbiomac.2021.02.086
Huang, D., Zuo, Y., Zou, Q., Zhang, L., Li, J., Cheng, L., et al. (2011). Antibacterial chitosan coating on nano-hydroxyapatite/polyamide66 porous bone scaffold for drug delivery. J. Biomater. Sci. Polym. Ed. 22, 931–944. doi: 10.1163/092050610X496576
Kim, C., Jeong, D., Kim, S., Kim, Y., and Jung, S. (2019). Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 222:115011. doi: 10.1016/j.carbpol.2019.115011
Liang, S., Liu, L., Huang, Q., and Yam, K. L. (2009). Preparation of single or double-network chitosan/poly(vinyl alcohol) gel films through selectively cross-linking method. Carbohydr. Polym. 77, 718–724. doi: 10.1016/j.carbpol.2009.02.007
Liu, Z., Zhang, S., He, B., Wang, S., and Kong, F. (2021). Synthesis of cellulose aerogels as promising carriers for drug delivery: a review. Cellulose 28, 2697–2714. doi: 10.1007/s10570-021-03734-9
López-Iglesias, C., Barros, J., Ardao, I., Monteiro, F. J., Alvarez-Lorenzo, C., Gómez-Amoza, J. L., et al. (2019). Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 204, 223–231. doi: 10.1016/j.carbpol.2018.10.012
López-León, T., Carvalho, E. L. S., Seijo, B., Ortega-Vinuesa, J. L., and Bastos-González, D. (2005). Physicochemical characterization of chitosan nanoparticles: electrokinetic and stability behavior. J. Colloid Interface Sci. 283, 344–351. doi: 10.1016/j.jcis.2004.08.186
Lovskaya, D. D., Lebedev, A. E., and Menshutina, N. V. (2015). Aerogels as drug delivery systems: in vitro and in vivo evaluations. J. Supercrit. Fluids 106, 115–121. doi: 10.1016/j.supflu.2015.07.011
Lovskaya, D., and Menshutina, N. (2020). Alginate-based aerogel particles as drug delivery systems: investigation of the supercritical adsorption and in vitro evaluations. Materials 13:329. doi: 10.3390/ma13020329
Lu, T., Li, Q., Chen, W., and Yu, H. (2014). Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 94, 132–138. doi: 10.1016/j.compscitech.2014.01.020
Lynam, D. A., Shahriari, D., Wolf, K. J., Angart, P. A., Koffler, J., Tuszynski, M. H., et al. (2015). Brain derived neurotrophic factor release from layer-by-layer coated agarose nerve guidance scaffolds. Acta Biomater. 18, 128–131. doi: 10.1016/j.actbio.2015.02.014
Mahanta, A. K., Patel, D. K., and Maiti, P. (2019). Nanohybrid scaffold of chitosan and functionalized graphene oxide for controlled drug delivery and bone regeneration. ACS Biomater. Sci. Eng. 5, 5139–5149. doi: 10.1021/acsbiomaterials.9b00829
Mahmoud, A. A., and Salama, A. H. (2016). Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: preparation, evaluation and in-vivo wound healing assessment. Eur. J. Pharm. Sci. 83, 155–165. doi: 10.1016/j.ejps.2015.12.026
Mallepally, R. R., Marin, M. A., Surampudi, V., Subia, B., Rao, R. R., Kundu, S. C., et al. (2015). Silk fibroin aerogels: potential scaffolds for tissue engineering applications. Biomed. Mater. 10:035002. doi: 10.1088/1748-6041/10/3/035002
Manivasagan, P., and Oh, J. (2016). Marine polysaccharide-based nanomaterials as a novel source of nanobiotechnological applications. Int. J. Biol. Macromol. 82, 315–327. doi: 10.1016/j.ijbiomac.2015.10.081
Marin, M. A., Mallepally, R. R., and McHugh, M. A. (2014). Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 91, 84–89. doi: 10.1016/j.supflu.2014.04.014
Markman, J. L., Rekechenetskiy, A., Holler, E., and Ljubimova, J. Y. (2013). Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 65, 1866–1879. doi: 10.1016/j.addr.2013.09.019
Matricardi, P., Di Meo, C., Coviello, T., Hennink, W. E., and Alhaique, F. (2013). Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 65, 1172–1187. doi: 10.1016/j.addr.2013.04.002
Mehling, T., Smirnova, I., Guenther, U., and Neubert, R. H. H. (2009). Polysaccharide-based aerogels as drug carriers. J. Non. Cryst. Solids 355, 2472–2479. doi: 10.1016/j.jnoncrysol.2009.08.038
Mirzaei, B. E., Ramazani, S. A., Shafiee, M., and Danaei, M. (2013). Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 62, 605–611. doi: 10.1080/00914037.2013.769165
Mohammadian, M., Kashi, T. S. J., Erfan, M., and Soorbaghi, F. P. (2018). In-vitro study of ketoprofen release from synthesized silica aerogels (As drug carriers) and evaluation of mathematical kinetic release models. Iran. J. Pharm. Res. 17, 818–829. doi: 10.22037/ijpr.2018.2255
Moura, M. J., Figueiredo, M. M., and Gil, M. H. (2007). Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 8, 3823–3829. doi: 10.1021/bm700762w
Muñoz-Ruíz, A., Escobar-García, D. M., Quintana, M., Pozos-Guillén, A., and Flores, H. (2019). Synthesis and characterization of a new collagen-alginate aerogel for tissue engineering. J. Nanomater. 2019:2875375. doi: 10.1155/2019/2875375
Mustapa, A. N., and Martín, Á, and Cocero, M. J. (2018). Alginate aerogels dried by supercritical CO2 as herbal delivery carrier. Malays. J. Anal. Sci. 22, 522–531. doi: 10.17576/mjas-2018-2203-21
Normand, V., Lootens, D. L., Amici, E., Plucknett, K. P., and Aymard, P. (2000). New insight into agarose gel mechanical properties. Biomacromolecules 1, 730–738. doi: 10.1021/bm005583j
Obaidat, R. M., Tashtoush, B. M., Bayan, M. F., Al Bustami, R., and Alnaief, M. (2015). Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS PharmSciTech 16, 1235–1244. doi: 10.1208/s12249-015-0312-2
Pantić, M., and Knez, Ž, and Novak, Z. (2016). Supercritical impregnation as a feasible technique for entrapment of fat-soluble vitamins into alginate aerogels. J. Non. Cryst. Solids 432, 519–526. doi: 10.1016/j.jnoncrysol.2015.11.011
Pellá, M. C. G., Lima-Tenório, M. K., Tenório-Neto, E. T., Guilherme, M. R., Muniz, E. C., and Rubira, A. F. (2018). Chitosan-based hydrogels: from preparation to biomedical applications. Carbohydr. Polym. 196, 233–245. doi: 10.1016/j.carbpol.2018.05.033
Pereira, A. K., dos, S., Reis, D. T., Barbosa, K. M., Scheidt, G. N., da Costa, L. S., et al. (2020). Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohydr. Res. 488:107891. doi: 10.1016/j.carres.2019.107891
Pillai, C. K. S., Paul, W., and Sharma, C. P. (2009). Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci. 34, 641–678. doi: 10.1016/j.progpolymsci.2009.04.001
Qin, Y. (2004). Gel swelling properties of alginate fibers. J. Appl. Polym. Sci. 91, 1641–1645. doi: 10.1002/app.13317
Qin, Y. (2005). Ion-exchange properties of alginate fibers. Text. Res. J. 75, 165–168. doi: 10.1177/004051750507500214
Quraishi, S., Martins, M., Barros, A. A., Gurikov, P., Raman, S. P., Smirnova, I., et al. (2015). Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 105, 1–8. doi: 10.1016/j.supflu.2014.12.026
Ren, B., Chen, X., Du, S., Ma, Y., Chen, H., Yuan, G., et al. (2018). Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 118, 1257–1266. doi: 10.1016/j.ijbiomac.2018.06.200
Reverchon, E., Cardea, S., and Rapuano, C. (2008). A new supercritical fluid-based process to produce scaffolds for tissue replacement. J. Supercrit. Fluids 45, 365–373. doi: 10.1016/j.supflu.2008.01.005
Reynolds, A. E., and Enquist, L. W. (1973). Biological interactions between polysaccharides. Rev. Med. Virol. 16, 393–403.
Rowley, J. A., Madlambayan, G., and Mooney, D. J. (1999). Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53. doi: 10.1016/S0142-9612(98)00107-0
Rubio-Elizalde, I., Bernáldez-Sarabia, J., Moreno-Ulloa, A., Vilanova, C., Juárez, P., Licea-Navarro, A., et al. (2019). Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 206, 455–467. doi: 10.1016/j.carbpol.2018.11.027
Sakai, S., Hashimoto, I., and Kawakami, K. (2007). Synthesis of an agarose-gelatin conjugate for use as a tissue engineering scaffold. J. Biosci. Bioeng. 103, 22–26. doi: 10.1263/jbb.103.22
Santos Miranda, M. E., Marcolla, C., Rodriguez, C. A., Wilhelm, H. M., Sierakowski, M. R., BelleBresolin, T. M., et al. (2006). Chitosan and N-carboxymethylchitosan: I. the role of N-carboxymethylation of chitosan in the thermal stability and dynamic mechanical properties of its films. Polym. Int. 55, 961–969. doi: 10.1002/pi
Shelke, N. B., James, R., Laurencin, C. T., and Kumbar, S. G. (2014). Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 25, 448–460. doi: 10.1002/pat.3266
Shi, M., Zhang, H., Song, T., Liu, X., Gao, Y., Zhou, J., et al. (2019). Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl. Mater. Interfaces 11, 22730–22744. doi: 10.1021/acsami.9b04750
Shi, W., Ching, Y. C., and Chuah, C. H. (2021). Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: a review. Int. J. Biol. Macromol. 170, 751–767. doi: 10.1016/j.ijbiomac.2020.12.214
Singh, A. P., Biswas, A., Shukla, A., and Maiti, P. (2019). Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 4:33. doi: 10.1038/s41392-019-0068-3
Soleimani Dorcheh, A., and Abbasi, M. H. (2008). Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 199, 10–26. doi: 10.1016/j.jmatprotec.2007.10.060
Sosnik, A., García-gonz, C. A., Marco, I., De Erkey, C., Concheiro, A., and Alvarez-lorenzo, C. (2021). Aerogels in drug delivery: from design to application. J. Control. Release 332, 40–63. doi: 10.1016/j.jconrel.2021.02.012
Spinks, G. M., Shin, S. R., Wallace, G. G., Whitten, P. G., Kim, S. I., and Kim, S. J. (2006). Mechanical properties of chitosan/CNT microfibers obtained with improved dispersion. Sens. Actuators B Chem. 115, 678–684. doi: 10.1016/j.snb.2005.10.047
Sukhodub, L. F., Sukhodub, L. B., Litsis, O., and Prylutskyy, Y. (2018). Synthesis and characterization of hydroxyapatite-alginate nanostructured composites for the controlled drug release. Mater. Chem. Phys. 217, 228–234. doi: 10.1016/j.matchemphys.2018.06.071
Tabernero, A., Baldino, L., Misol, A., Cardea, S., and del Valle, E. M. M. (2020). Role of rheological properties on physical chitosan aerogels obtained by supercritical drying. Carbohydr. Polym. 233:115850. doi: 10.1016/j.carbpol.2020.115850
Takeshita, S., Sadeghpour, A., Sivaraman, D., Zhao, S., and Malfait, W. J. (2020a). Solvents, CO2 and biopolymers: structure formation in chitosan aerogel. Carbohydr. Polym. 247:116680. doi: 10.1016/j.carbpol.2020.116680
Takeshita, S., Zhao, S., and Malfait, W. J. (2021). Transparent, aldehyde-free chitosan aerogel. Carbohydr. Polym. 251:117089. doi: 10.1016/j.carbpol.2020.117089
Takeshita, S., Zhao, S., Malfait, W. J., and Koebel, M. M. (2020b). Chemistry of chitosan aerogels: three-dimensional pore control for tailored applications. Angew. Chemie Int. Ed. Engl. 60, 9828–9851. doi: 10.1002/anie.202003053
Takigawa, T., and Endo, Y. (2006). Effects of glutaraldehyde exposure on human health. J. Occup. Health 48, 75–87. doi: 10.1539/joh.48.75
Terzić, I., Ivanović, J., Žižović, I., Lučić Škorić, M., Milosavljević, N., Milašinović, N., et al. (2018). A novel chitosan gels: supercritical CO2 drying and impregnation with thymol. Polym. Eng. Sci. 58, 2192–2199. doi: 10.1002/pen.24834
Trucillo, P., Cardea, S., Baldino, L., and Reverchon, E. (2020). Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 39:101161. doi: 10.1016/j.jcou.2020.101161
Ulker, Z., and Erkey, C. (2014). An emerging platform for drug delivery: aerogel based systems. J. Control. Release 177, 51–63. doi: 10.1016/j.jconrel.2013.12.033
Van der Lubben, I. M., Verhoef, J. C., Borchard, G., and Junginger, H. E. (2001). Chitosan for mucosal vaccination. Adv. Drug Deliv. Rev. 52, 139–144. doi: 10.1016/S0169-409X(01)00197-1
Venkatesan, J., and Kim, S. K. (2010). Chitosan composites for bone tissue engineering - an overview. Mar. Drugs 8, 2252–2266. doi: 10.3390/md8082252
Wahba, M. I. (2020). Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 31, 350–375. doi: 10.1080/09205063.2019.1692641
Waldman, A. S., Schechinger, L., Govindarajoo, G., Nowick, J. S., Pignolet, L. H., and Labuza, T. (1998). The alginate demonstration: polymers, food science, and ion exchange. J. Chem. Educ. 75, 1430–1431. doi: 10.1021/ed075p1430
Wang, R., Shou, D., Lv, O., Kong, Y., Deng, L., and Shen, J. (2017). pH-Controlled drug delivery with hybrid aerogel of chitosan, carboxymethyl cellulose and graphene oxide as the carrier. Int. J. Biol. Macromol. 103, 248–253. doi: 10.1016/j.ijbiomac.2017.05.064
Wang, Y., Su, Y., Wang, W., Fang, Y., Riffat, S. B., and Jiang, F. (2019). The advances of polysaccharide-based aerogels: preparation and potential application. Carbohydr. Polym. 226:115242. doi: 10.1016/j.carbpol.2019.115242
Wei, S., Ching, Y. C., and Chuah, C. H. (2020). Synthesis of chitosan aerogels as promising carriers for drug delivery: a review. Carbohydr. Polym. 231:115744. doi: 10.1016/j.carbpol.2019.115744
Witzler, M., Ottensmeyer, P. F., Gericke, M., Heinze, T., Tobiasch, E., and Schulze, M. (2019). Non-Cytotoxic Agarose/Hydroxyapatite composite scaffolds for drug release. Int. J. Mol. Sci. 20:3565. doi: 10.3390/ijms20143565
Xie, Y., Liao, X., Zhang, J., Yang, F., and Fan, Z. (2018). Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 119, 402–412. doi: 10.1016/j.ijbiomac.2018.07.060
Yang, W., Xu, H., Lan, Y., Zhu, Q., Liu, Y., Huang, S., et al. (2019). Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 130, 58–67. doi: 10.1016/j.ijbiomac.2019.02.120
You, C., Li, Q., Wang, X., Wu, P., Ho, J. K., Jin, R., et al. (2017). Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 7:10489. doi: 10.1038/s41598-017-10481-0
Yuan, Y., Wang, L., Mu, R. J., Gong, J., Wang, Y., Li, Y., et al. (2018). Effects of konjac glucomannan on the structure, properties, and drug release characteristics of agarose hydrogels. Carbohydr. Polym. 190, 196–203. doi: 10.1016/j.carbpol.2018.02.049
Zeiger, E., Gollapudi, B., and Spencer, P. (2005). Genetic toxicity and carcinogenicity studies of glutaraldehyde - A review. Mutat. Res. Rev. Mutat. Res. 589, 136–151. doi: 10.1016/j.mrrev.2005.01.001
Zhang, Z., Wang, X., Wang, Y., and Hao, J. (2018). Rapid-forming and self-healing agarose-based hydrogels for tissue adhesives and potential wound dressings. Biomacromolecules 19, 980–988. doi: 10.1021/acs.biomac.7b01764
Keywords: agarose, alginate, chitosan, aerogel, supercritical CO2, drug delivery
Citation: Guastaferro M, Reverchon E and Baldino L (2021) Agarose, Alginate and Chitosan Nanostructured Aerogels for Pharmaceutical Applications: A Short Review. Front. Bioeng. Biotechnol. 9:688477. doi: 10.3389/fbioe.2021.688477
Received: 30 March 2021; Accepted: 20 April 2021;
Published: 12 May 2021.
Edited by:
Francesca Taraballi, Houston Methodist Research Institute, United StatesReviewed by:
Elena Vladimirovna Boldyreva, Novosibirsk State University, RussiaKirsi S. Mikkonen, University of Helsinki, Finland
Copyright © 2021 Guastaferro, Reverchon and Baldino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Baldino, bGJhbGRpbm9AdW5pc2EuaXQ=
 Mariangela Guastaferro
Mariangela Guastaferro Ernesto Reverchon
Ernesto Reverchon Lucia Baldino
Lucia Baldino