- 1Research and Exploratory Development Department, Johns Hopkins University Applied Physics Laboratory, Laurel, MD, United States
- 2Center for Alternatives to Animal Testing (CAAT), Department of Environmental Health and Engineering, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 3CAAT-Europe, University of Konstanz, Konstanz, Germany
Service members and law enforcement personnel are frequently exposed to blast overpressure during training and combat due to the use of heavy weaponry such as large-caliber rifles, explosives, and ordnance. The cumulative effects of these repeated low-level (<4 psi) blast exposures can lead to physical and cognitive deficits that are poorly understood. Brain organoids—human stem cell-derived three-dimensional in vitro culture systems that self-organize to recapitulate the in vivo environment of the human brain—are a promising alternative biological model to traditional cellular cultures and animal models, offering a unique opportunity for studying the mechanisms of mild blast-induced traumatic brain injury (mbTBI) resulting from repeated exposure. In this article, we review the current state of brain organoid models and discuss future directions for advancing their physiological relevance for studying mbTBI. These will be presented within a framework for developing next-generation platforms that integrate relevant loading devices, as well as non-invasive technologies for assessing the brain organoid’s response while increasing throughput. These next-generation platforms aim to accelerate the development of new interventions for mbTBI.
1 Introduction
During training and combat, military and law enforcement personnel are frequently exposed to repetitive low-level blast (rLLB) from heavy weaponry, including artillery, mortars, shoulder-fired weapons, stun grenades, and breaching explosives (Kamimori et al., 2017; Skotak et al., 2019; Thangavelu et al., 2020; Belding et al., 2021; Boutté et al., 2021; Wiri et al., 2023; Gilmore et al., 2024). These blasts can generate overpressures which exceed 90 kPa during military training exercises (Wiri et al., 2023), surpassing the current safety standard of 28 kPa (or 4 psi) based on human tympanic membrane rupture (Hicks, 2024). Compounding this issue, personnel may experience over a hundred low-level blast overpressure events during a single training exercise (Wiri et al., 2023) and many more over the course of their career. While severe blast-induced traumatic brain injury (bTBI) from high-level blast explosions has been extensively investigated (Rosenfeld et al., 2013), growing evidence suggests that rLLB exposure can result in subconcussive or mild blast-induced traumatic brain injury (mbTBI) (Belding et al., 2021; Siedhoff et al., 2022). This form of bTBI is associated with chronic issues, including psychiatric disorders, motor and cognitive impairment, sleep disorders and pain (Siedhoff et al., 2022).
Preclinical models are essential for elucidating the underlying mechanisms of mbTBI, which remain poorly understood. This knowledge gap hinders the advancements in preventative measures (e.g., safe standoff distances, weapons modifications, personal protective equipment (PPE), and prophylactics), diagnostics (e.g., molecular biomarker assays and medical imaging), and treatments (e.g., pharmaceuticals). In the past decade, there has been an increasing number of animal studies that focus on rLLB (Ravula et al., 2022). These studies have provided important insights, revealing pathophysiological changes such as neuroinflammation, axonal damage, and glial activation, as well as behavioral deficits (Ravula et al., 2022). However, animal models face several challenges—such as low throughput, difficulties in generating rLLB exposure with appropriate mechanical boundary conditions, issues with reproducibility, and limited relevance to human neuroanatomy and neurophysiology—all of which are critical considerations when studying mbTBI.
In vitro cell culture models have also been extensively used to study bTBI. These models offer the advantage of isolating specific variables that would otherwise confound results. For example, in vitro models are easier to manipulate than in vivo models and offer the opportunity to independently study the effects of primary (i.e., from pressure wave), secondary (i.e., from tearing), or tertiary (i.e., from inertia or blunt forces) loading mechanisms, which are biomechanically distinct. Additionally, in vitro models are more accessible for a broader range of analytical tools, such as advanced imaging modalities, diverse assays for studying molecular pathways such as RNA sequencing and gene editing techniques to identify and manipulate specific genetic factors that influence injury responses and degenerative pathways (Chen et al., 2009; Beltrán et al., 2023; Lai et al., 2024). However, even with these advantages, these models do not capture the complexity of the in vivo human brain environment, even with more complex three-dimensional cellular cultures (Cullen et al., 2007; Bar-Kochba et al., 2016; Sawyer et al., 2017; 2018; Snapper et al., 2023; González-Cruz et al., 2024).
Recent advances in the generation of three-dimensional (3D) brain-like structures, called brain organoids, offer immense potential as a new in vitro model of the human brain (Smirnova and Hartung, 2024). These brain organoids are differentiated from human induced pluripotent stem cells (iPSCs) and form to resemble the cellular composition, diversity, and architecture of different anatomical regions of the human brain, e.g., midbrain, thalamus, and cerebral cortex (Susaimanickam et al., 2022). Brain organoids mimic key features of the human brain including myelination, synaptic connections, and patterns of gene expression (Vanvliet et al., 2007; Chesnut et al., 2021b; Modafferi, 2021). Functionally, brain organoids have shown spontaneous neural activity and the formation of neural circuits (Trujillo et al., 2019). Due to these unique properties, brain organoids have been used to study various neurodegenerative diseases and neurodevelopmental disorders (Pamies et al., 2017; 2022; Chesnut et al., 2021b; Modafferi, 2021; Eichmüller and Knoblich, 2022; Ravula et al., 2022; Susaimanickam et al., 2022; Smirnova and Hartung, 2024).
Studies have shown that brain organoids are able to recapitulate the key pathological changes associated with various TBI exposures (Zander et al., 2017; Ramirez et al., 2021; Silvosa et al., 2022; Beltrán et al., 2023; Lai et al., 2024). Zander et al. applied explosive blast overpressure waves to brain organoids and found increased formation of reactive oxygen species and membrane permeability (Zander et al., 2017). Silvosa et al. exposed cerebral organoids to pressure waves with varying frequencies and found that higher-frequency pressure resulted in increased apoptosis and network desynchronization (Silvosa et al., 2022). Ramirez et al. embedded cerebral organoids within a surrogate brain placed in a mouse skull and induced injury via controlled cortical impact. One week post impact, they found increased astrogliosis, neuronal damage, and apoptosis, which was similar to paired experiments with mice (Ramirez et al., 2021). In another controlled cortical impact study, Beltrán et al. used RNA sequencing to find genes that regulate inflammation, cell death, and immune dysregulation (Beltrán et al., 2023). Lastly, Lai et al. applied high-intensity focused ultrasound to cortical organoids, revealing tau phosphorylation and TDP-43, which was prominent in deep-layer neurons. Although brain organoids are still an emerging model for TBI research and require significant advancements to enhance their applicability to humans, these studies underscore their potential for investigating TBI (Jgamadze et al., 2020; LaPlaca and Brody, 2022).
In this article, we review the current state of brain organoids and present a framework (Figure 1) for developing next-generation platforms tailored to study mbTBI from rLLB. The framework focuses on three key technological areas, each discussed in context of current research:
• Loading devices capable of accurately replicating the biomechanical loading conditions in the human brain during rLLB exposure in vitro.
• Advancements in brain organoids to more effectively replicate the in vivo environment of the human brain.
• Non-invasive technologies for evaluating biological responses to loading while increasing throughput
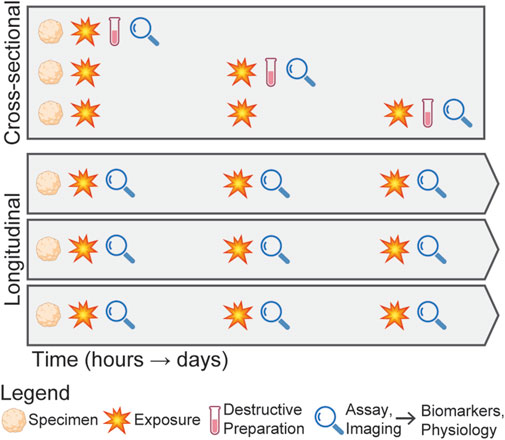
Figure 1. Framework for next-generation mbTBI research platforms, highlighting three core technological areas: (1) loading devices capable of accurately replicating the human brain’s biomechanical conditions, (2) advanced brain organoids that more effectively mimic the in vivo environment, and (3) technologies for non-invasively evaluating the biological responses to loading while increasing throughput. This framework aims to accelerate research into determining mbTBI mechanisms, enabling rapid assessment of new interventions.
The development of these new platforms will accelerate research to elucidate the mechanisms of mbTBI, including determining the dose-response relationship, molecular pathways involved, timelines of injury progression, recovery processes, and functional effects such as learning or memory. This understanding will enable researchers to rapidly evaluate the efficacy of interventions such as prophylactics and therapeutics.
2 Study design considerations
In studying mbTBI, it is critical to consider the overall experimental design as the injury response is not immediate (Hernandez et al., 2018), making such studies both time-intensive and costly. Further complicating these studies, both the pressure waveform characteristics (e.g., peak pressure and duration) and interval between exposures affect the biomechanical environment in the brain during rLLB exposure. Cross-sectional designs, where the specimen is loaded and then destructively processed and analyzed (Figure 2), for example, with bulk RNA sequencing or immunohistochemistry, provide a detailed snapshot of the injury state, but fail to capture the temporal dynamics of the injury progression. In contrast, longitudinal designs enable non-invasive evaluation of the biological responses, which reduces inter-subject variability and enables the assessment of injury progression within a single specimen, making it better suited for exploring a larger range of loading parameters. Despite these advantages, longitudinal designs face challenges due to the limited availability of techniques capable of non-invasive measurements. However, emerging technologies, including microfluidic systems (Zhao et al., 2024), label-free imaging approaches (Keshara et al., 2022), molecular assays (Abdollahi, 2021), and sensors (Kang et al., 2024), are beginning to address these limitations, making longitudinal designs increasingly feasible.
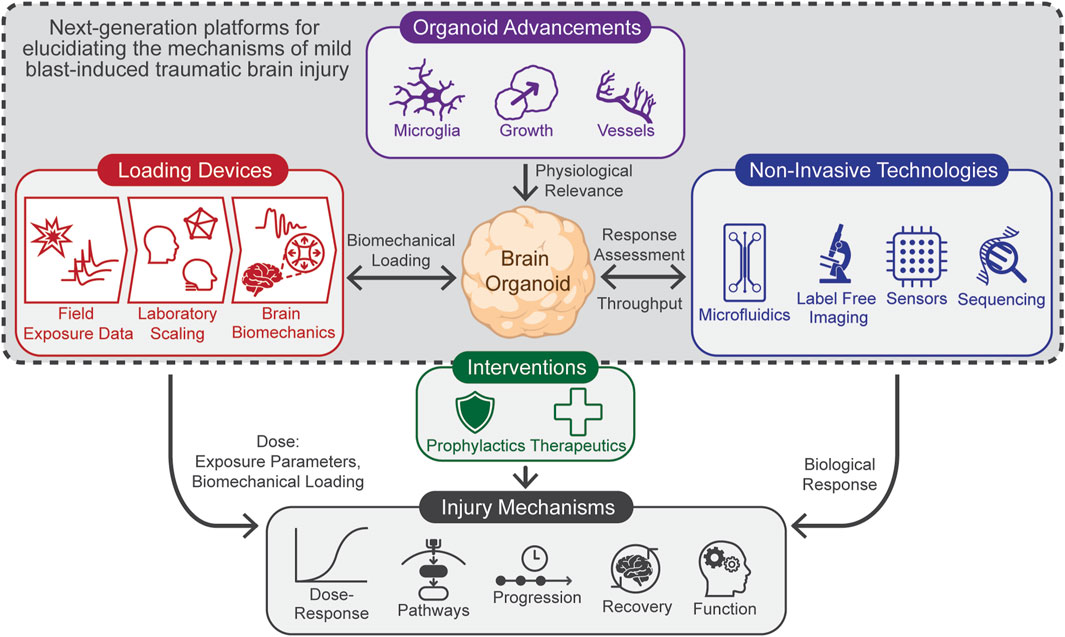
Figure 2. Schematic comparing cross-sectional and longitudinal experimental designs for studying mbTBI from repeated exposures. In the cross-sectional design, specimens are exposed to a blast condition, then destructively processed and analyzed at discrete time points, requiring three organoids to evaluate three post-exposure time points. In contrast, the longitudinal design involves exposing the same specimen to repeated blasts and continuously evaluating injury progression without destructive processing, resulting in more replicates for a single exposure condition.
3 Loading devices
A platform for studying mbTBI must effectively tease apart the complex relationship between exposure and injury response (LaPlaca and Brody, 2022). In vitro devices must methodically apply controlled loading to understand this complex relationship (LaPlaca and Brody, 2022); however the loading parameters need to accurately reflect the range of exposures experienced in real training and combat scenarios. Characterizing this range requires scaling externally measured field exposures (Kamimori et al., 2017; Skotak et al., 2019; Thangavelu et al., 2020; Wiri et al., 2023) to the biomechanical environment in the brain experimentally (Elster et al., 2023) or in silico (Gupta and Przekwas, 2013; Gupta et al., 2017), which are then applied as loading conditions to brain organoids. In this section, we review the biomechanics of blast loading to the head and approaches for scaling these loads to in vitro devices.
3.1 Characterizing the brain biomechanics
Primary bTBI occurs when a blast pressure wave propagates through the skull, loading the brain tissue directly. In contrast, secondary injuries from shrapnel, and tertiary injuries from rapid accelerations are typically associated with moderate to severe blast exposures (Rosenfeld et al., 2013). These secondary and tertiary effects are less relevant for LLB (Säljö et al., 2008; Rosenfeld et al., 2013), making the primary blast pressure response crucial to replicate experimentally when studying mbTBI.
The brain biomechanics underlying mbTBI are complex due to the interaction of the incident shockwave with the heterogeneous structures and mechanical properties of the human head (Liang et al., 2021). Across a variety of models, including post-mortem human subjects (Bir, 2011; Ganpule et al., 2013; Ott et al., 2013; Ouellet et al., 2014; Iwaskiw et al., 2018), in silico (Moore et al., 2009; Taylor and Ford, 2009; Nyein et al., 2010; Chafi et al., 2011; Panzer et al., 2012; Tan et al., 2017; Tan and Matic, 2020; Li et al., 2024), and anthropometric surrogates (Merkle and Carneal, 2012; Hua et al., 2014; Ouellet and Philippens, 2018), the skull acts as a filter, attenuating the high-frequency delta response of the incident shockwave. The transmitted pressure wave propagates through the brain tissue, undergoing multiple reflections within the intracranial cavity due to acoustic impedance mismatches between the cranium and brain (Gupta et al., 2021; Liang et al., 2021). These reflections result in intracranial pressure (ICP) wave interference and oscillatory characteristics with multiple peaks in the 0.5–10 kHz frequency range that persist for 1–10 m.
A full characterization of the tissue-level stress or strain state is challenging since deviatoric stresses in experimental studies are not measured due to sensor limitations. However, computational models have reported deviatoric stresses that are over 100 times lower than pressure (Taylor and Ford, 2009), which is attributable to the high bulk-to-shear modulus ratio of brain and it’s confinement within the intracranial cavity. Deviatoric stresses also persist milliseconds after the initial ICP wave (Taylor and Ford, 2009), suggesting they result from brain strains and displacements (Iwaskiw et al., 2018) caused by overall kinematic head motion, which is less relevant for LLB (Säljö et al., 2008; Rosenfeld et al., 2013). Elster et al. presents a comprehensive review of the experimental studies that measure the brain biomechanics during blast exposures (Elster et al., 2023). However, a comprehensive understanding of the brain biomechanics is challenging due to it being dependent upon many factors, including incident shockwave direction (Li et al., 2024), surface reflections (Tan et al., 2017), use of PPE (Moss et al., 2009; Nyein et al., 2010; Alphonse et al., 2020; Elster et al., 2023; Li et al., 2024), and anthropometric variations.
With recent field studies monitoring rLLB exposure (Kamimori et al., 2017; Skotak et al., 2019; Thangavelu et al., 2020; Wiri et al., 2023), there are opportunities to replicate these conditions experimentally or in silico to characterize the brain biomechanics. Critical to these studies is addressing the variations in exposures associated with large diversity of weaponry (Wiri et al., 2023), including the potential cumulative effects of automatic weapons with firing rates that approach the duration of ICP waves, potentially resulting in rapid ICP accumulation.
Additionally, several challenges related to spatiotemporal scales must be addressed when translating results between experimental and computational models, particularly when considering the differences between organoid models and the human brain. At the continuum level, the brain’s mechanical properties behaves as a nonlinear viscoelastic material that is highly rate-dependent (Procès et al., 2022). Therefore, characterizing and assigning these properties in computational models becomes difficult at the time scales of blast exposure. At the cellular scale, deformations are spatially heterogeneous due to the cell-extracellular matrix and cell-cell interactions, resulting in strain concentrations at micro-interfaces with impedance mismatches (Nakagawa et al., 2011), such as synapses (Gharahi et al., 2023). To accurately model these micro-interfaces, advancements in mechanobiology models that bridge continuum and molecular scales are essential (Montanino et al., 2020; Gharahi et al., 2023).
3.2 Load scaling to in vitro devices
Scaling the effective primary blast loading from an explosive event to an in vitro device is challenging. Various systems have been used to induce bTBI in cellular cultures, spanning a wide range of loading rates. Hydrostatic pressures have been applied to induce injury (Murphy and Horrocks, 1993; Salvador-Silva et al., 2004), but these systems do not capture the dynamics of the ICP. Systems utilizing shock tubes (Arun et al., 2011; Hue et al., 2013; Vogel et al., 2017; Campos-Pires et al., 2018) and pneumatic actuators (Ravin et al., 2012; 2016) expose cells to dynamic pressures. However, these systems are replicate the pressure from an incident shockwave—idealized as a Friedlander wave—instead of the tissue-level ICPs. Shockwaves generated by pulsed lasers (Selfridge et al., 2015; Gomez Godinez et al., 2021) and lithotripsy (Howard and Sturtevant, 1997) devices have also been utilized to induce cellular injury. However, the pressures generated are substantially more transient, in the microsecond time range, compared to the millisecond time range during blast loading.
In an interesting device design, Silvosa et al., used a piezo driven pressure chamber to control both pressure amplitude and frequency to induce primary bTBI in cerebral organoids (Silvosa et al., 2022). Approaches such as this are powerful to study mbTBI since they allow researchers to identify the relationship between specific loading parameters and injury response. Additionally, for rLLB exposures, the loading parameters require devices that are tunable to generate complex, low-magnitude pressure waveforms that are repeated for many hours (e.g., during training exercises) or at very high repetition rates to replicate firing rates of automatic weapons.
4 Advancing brain organoid models
Brain organoids have emerged as transformative tools for modeling human brain development and pathology, offering unprecedented opportunities to investigate complex neurobiological processes. Despite significant progress, several challenges remain for brain organoids to serve as a viable model for studying the effects of rLLB on cellular system. In this section, we review these challenges alongside recent developments aimed at addressing them.
4.1 Organoid growth and maturation
Due to the lack of an integrated vascular system, nutrient and waste exchange relies solely on diffusion (Nwokoye and Abilez, 2024). When organoids exceed a diameter of 0.4–0.5 mm, diffusion becomes increasingly inefficient, leading to hypoxia and necrosis of the inner core. These conditions reduce cellular viability and functional capacity in deeper regions. Additionally, brain organoids largely represent an immature state, akin to early fetal development, restricting their utility for modeling adult brain functions, such as advanced cognition or late-stage neurodegeneration. Addressing these limitations is essential for enhancing the physiological relevance and applicability of brain organoid models for mbTBI.
To overcome these challenges, various innovative techniques are being developed to support their growth and viability. One promising approach is organoid vascularization (Nwokoye and Abilez, 2024). By integrating endothelial cells into brain organoid cultures, either as a co-culture or during differentiation (Skylar-Scott et al., 2022), researchers promote vascularization, which enhances the survival of cells within the organoid’s core and promotes more complex tissue organization, closely resembling in vivo conditions. Vascularization is also an important component for studying neurovascular impairment, a common pathophysiology in bTBI (Siedhoff et al., 2022). Another significant advancement is the use of perfusion systems. Devices such as bioreactors and microfluidic systems enable dynamic medium flow, providing a constant supply of nutrients and oxygen while efficiently removing waste (Cai et al., 2021; Cho et al., 2021; Khan et al., 2021; Salmon et al., 2022). These systems create a more favorable microenvironment, supporting the prolonged growth and functional maintenance of larger organoids. Lastly, 3D bioprinting has emerged as a powerful tool for constructing organoids with precise spatial arrangement of cells and scaffolds, enabling the creation of vascular networks within organoids (Zhao et al., 2021; Salmon et al., 2022; Galpayage Dona et al., 2023). Together, these techniques are transforming the scalability and applicability of brain organoid models, paving the way for more advanced and realistic in vitro systems for studying mbTBI.
4.2 Cellular complexity and immune-response modeling with microglia
One of the critical limitations of current brain organoid models is their lack of cellular diversity, which restricts their ability to replicate key processes such as neuroinflammation and immune responses to injury or disease. While some brain organoids include oligodendrocytes and myelination (Pamies et al., 2017; Chesnut et al., 2021a; Chesnut et al., 2021b)—a particularly important feature to replicate—many still lack microglia. The absence of microglia represents a significant gap in these models since they are the brain’s resident immune cell and are essential in maintaining neural homeostasis, mediating synaptic pruning, and mounting immune responses to TBI (Loane and Byrnes, 2010; Huber et al., 2016; Shi et al., 2021; Ravula et al., 2022).
To incorporate microglia into organoid systems, researchers have employed various techniques (Zhang et al., 2023). Co-culture models involve the direct addition of microglia (Abreu et al., 2018; Song et al., 2019) or iPSCs into developing organoids (Wörsdörfer et al., 2019; Fagerlund et al., 2021; Sabate-Soler et al., 2022), facilitating their interaction with other brain cell types. Alternatively, endogenous development strategies use genetic engineering or cytokine treatments to encourage microglial differentiation within the organoid itself (Ormel et al., 2018), creating a more integrated and physiologically relevant model. Emerging dynamic immune-organoid systems, enabled by microfluidic systems, further enhance this integration by allowing the interaction of circulating immune cells with organoids, simulating systemic immune response (Ramadan et al., 2023).
5 Non-invasive technologies
Technologies to non-invasively evaluate brain organoid responses while reproducibly increasing throughput are essential for enabling longitudinal study designs encompassing a broad parameter space. In this section, we review a range of emerging technologies that can be integrated into next-generation mbTBI platforms.
5.1 Microfluidic systems
High-throughput systems that integrate brain organoids with microfluidics, known as organoid-on-a-chip systems, are revolutionizing their application in research and drug discovery (Anderson et al., 2021; Zhao et al., 2024). These systems provide several advantages that enhance the scalability and control of organoid-based experiments. These systems support parallelized experiments, allowing for the simultaneous testing of multiple loading parameters, assays, intervention strategies. As discussed previously, these systems also offer precise control over critical in vivo factors, such as fluid flow, temperature, pH, mechanical forces, nutrient gradients, and microglia circulation, thereby creating a physiological environment that more resemble the human brain. Achieving these environments typically involves precisely controlling incubation systems and tuning media exchange using low-flow pumps to minimize shear stress. However, a unique challenge in prolonged rLLB scenarios is ensuring a robust interface between the loading device and microfluidic system. Addressing this issue is essential for the development of future mbTBI platforms. Looking ahead, several innovations promise to further enhance the utility of organoid-on-a-chip systems. The development of automated systems for the production, maintenance, and testing of organoids will streamline workflows and increase reproducibility. Additionally, enhancing integration by combining multiple organoid types (e.g., brain, liver, and heart) on a single chip will facilitate multi-organ interactions (Zhao et al., 2024), particularly relevant for pharmacokinetics and polytrauma (Hubbard et al., 2017).
5.2 Sensors
New multimodal sensors enable real-time monitoring of brain organoid physiology, providing researchers with continuous feedback on dynamic parameters such as mechanical properties (Ryu et al., 2021), temperature, oxygen concentration, and neural activity without disrupting the organoid (Park et al., 2021). By utilizing MEAs alongside calcium imaging, studies have demonstrated that brain organoids form neural networks that generate oscillatory activity based on phase amplitude coupling (Trujillo et al., 2019), mutual information (Alam El Din et al., 2024a), network correlation or synchrony (Samarasinghe et al., 2021; Sharf et al., 2022), which has been shown to be disrupted by bTBI (Silvosa et al., 2022). Advancements in high-density (Schröter et al., 2022) and 3D MEAs (Li et al., 2019; Soscia et al., 2020; Huang et al., 2022; Martinelli et al., 2024) are expected to drastically enhance these electrophysiological measurements through unprecedented improvements in spatial resolution and access. An emerging area called organoid intelligence (Smirnova, 2023; Smirnova et al., 2023; Alam El Din et al., 2024a; Alam El Din et al., 2024b), combines these electrophysiological measurements with artificial intelligence, opening up the possibility to study cognition, learning, and memory, all of which are effected by mbTBI (Siedhoff et al., 2022).
5.3 Label-free imaging
Imaging provides unique insight into the 3D structure and function of brain organoids, enabling the researchers to characterize the injury progression and recovery processes as a result of mbTBI. Confocal, multiphoton, and light sheet fluorescent microscopy are the primary techniques for 3D imaging (Ettinger and Wittmann, 2014). However, these techniques typically rely on exogenous fluorophores that are diffusion-limited, cytotoxic, or require fixation, limiting their use for long-term time-lapse imaging of brain organoids (Ettinger and Wittmann, 2014; Fei et al., 2022). Genetically engineered brain organoids that express endogenous fluorophores (Artegiani et al., 2020; Romero et al., 2023), allowing for specific tagging of processes such as oligodendrogenesis and myelination (Romero et al., 2023) have begun to address the limitations with exogenous fluorophores. However, point scanning methods such as confocal and multiphoton microscopy can induce phototoxicity (Ettinger and Wittmann, 2014), which may confound the observed effects of mbTBI.
In recent years, there has been advancements in imaging techniques that overcome these limitations by taking advantage of untagged endogenous contrast agents (Fei et al., 2022; Keshara et al., 2022; Maharjan et al., 2024). Full-field optical coherence tomography (FF-OCT) is a full-field interferometry technique that resolves the temporal dynamics of intra-cellular structures (Scholler et al., 2020; Monfort et al., 2023). This technique has been used to image retinal organoids over the course of 17 days (Monfort et al., 2023) and has been shown to be correlated with cellular processes such as oxidative stress (Groux et al., 2022), differentiation, and cellular death (Monfort et al., 2023). Techniques such as fluorescence lifetime imaging microscopy (FLIM) and hyperspectral imaging (HSI) measure properties of endogenously fluorescing biomolecules, such as decay rates and spectral characteristics, respectively, that are involved in metabolic processes, as well as structural and molecular changes in organoids (Xue et al., 2021; Barroso et al., 2023).
One of the key challenges with FF-OCT, FLIM, and HSI is achieving imaging depths beyond a few hundred micrometers (Xue et al., 2021; Monfort et al., 2023). This capability is particularly important for visualizing the inner core of organoids, which in larger brain organoids can extend to depths of 1–2 mm and may respond differently to biomechanical loading compared to the surface. Recently, three-photon microscopy (3p.m.) has been used to image cerebral organoids at depths of up to 2 mm. (Yildirim et al., 2022). The endogenous contras detected by 3p.m. is based on third harmonic generation, which is sensitive to large refractive index changes, such as those occurring at the cell membrane. However, 3p.m. is limited by the working distance of high numerical aperture (>1) immersion objectives. Recent modifications to the collection pathway are beginning to address this limitation (Deng et al., 2024). The challenge of imaging at depth is expected to become more pronounced as researchers successfully grow larger brain organoids by mitigating inner core necrosis. Consequently, further advancements in these label-free imaging techniques are necessary.
5.4 Sequencing
bTBI initiates a cascade of key pathophysiological processes that disrupt brain homeostasis, including excitotoxicity, oxidative stress, inflammation, and apoptosis (Siedhoff et al., 2022). These processes exacerbate the initial damage caused by the primary injury, leading to widespread neuronal dysfunction and tissue loss. Omics technologies have been extensively employed to study bTBI (Tajik and Noseworthy, 2022), providing insights into global molecular changes but failing to capture the heterogeneity of the disease. The advent of single-cell omics has addressed this limitation by enabling the investigation of responses at the level of individual cells, uncovering cell-specific biomarkers and dynamic changes in cell population distributions. For example, single-cell RNA sequencing can identify distinct transcriptional states within neurons, glial cells, and infiltrating immune cells post-injury, offering a deeper understanding of the cellular signaling driving both damage and repair across different types of external injury models (Jha et al., 2024). Additionally, when combined with CRISPR, there is a path to towards identifying and modifying injury-induced degenerative processes (Lai et al., 2024).
Although single-cell RNA sequencing is a powerful tool for precisely assessing cellular signaling pathways triggered by bTBI, it is a destructive process. Extracellular vesicle (EV) based biomarkers represent a promising alternative since nearly all cell types release EVs, making them possible to characterize by processing the supernatant in brain organoid cultures. EVs containing lipids, proteins genetic material that are reflective of the cell-type specific complex biochemical environment, enabling dynamic assessment of neuroinflammation, gliosis, and neurodegeneration (Frühbeis et al., 2013). Additionally, EVs are able to cross the blood-brain barrier (Ramos-Zaldívar et al., 2022) and hold potential for inferring the brain’s state in vivo (Smirnova et al., 2024).
6 Conclusion
Brain organoids represent a transformative technology for studying the mechanisms of mbTBI by providing a physiologically accurate in vitro model with unprecedented control and throughput. However, to fully realize and harness the power of these next-generation platforms, the advancement of new loading devices, organoid models, and non-invasive technologies are essential. The presented framework aims to guide research to drive these innovations, establishing brain organoids as a cornerstone in trauma research.
Author contributions
EB-K: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review and editing. CC: Writing – original draft, Writing – review and editing. VA: Writing – original draft. AT: Writing – original draft, Writing – review and editing. AE: Writing – original draft, Writing – review and editing. CR: Writing – original draft, Writing – review and editing. IM: Writing – original draft. LS: Writing – original draft, Writing – review and editing. TH: Writing – original draft, Writing – review and editing. AM: Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the funding from the Johns Hopkins University Applied Physics Laboratory, Research and Exploratory Development Department. The authors gratefully acknowledge the financial support provided by the Independent Research and Development Program.
Acknowledgments
Figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, S. (2021). Extracellular vesicles from organoids and 3D culture systems. Biotech. and Bioeng. 118, 1029–1049. doi:10.1002/bit.27606
Abreu, C. M., Gama, L., Krasemann, S., Chesnut, M., Odwin-Dacosta, S., Hogberg, H. T., et al. (2018). Microglia increase inflammatory responses in iPSC-derived human BrainSpheres. Front. Microbiol. 9, 2766. doi:10.3389/fmicb.2018.02766
Alam El Din, D.-M., Moenkemoeller, L., Loeffler, A., Habibollahi, F., Schenkman, J., Mitra, A., et al. (2024a). Human neural organoid microphysiological systems show the building blocks necessary for basic learning and memory. BioRxiv., 2024.09.17.613333. doi:10.1101/2024.09.17.613333
Alam El Din, D.-M., Shin, J., Lysinger, A., Roos, M. J., Johnson, E. C., Shafer, T. J., et al. (2024b). Organoid intelligence for developmental neurotoxicity testing. Front. Cell. Neurosci. 18, 1480845. doi:10.3389/fncel.2024.1480845
Alphonse, V., Luong, Q., Tumperi, M., Schuman, C., Herman, S., Clark, J., et al. (2020). “Helmet blast attenuation performance,” in Personal armor systems symposium.
Anderson, W. A., Bosak, A., Hogberg, H. T., Hartung, T., and Moore, M. J. (2021). Advances in 3D neuronal microphysiological systems: towards a functional nervous system on a chip. Vitro Cell.Dev.Biol.-Animal 57, 191–206. doi:10.1007/s11626-020-00532-8
Artegiani, B., Hendriks, D., Beumer, J., Kok, R., Zheng, X., Joore, I., et al. (2020). Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. Nat. Cell Biol. 22, 321–331. doi:10.1038/s41556-020-0472-5
Arun, P., Spadaro, J., John, J., Gharavi, R. B., Bentley, T. B., and Nambiar, M. P. (2011). Studies on blast traumatic brain injury using in-vitro model with shock tube. NeuroReport 22, 379–384. doi:10.1097/WNR.0b013e328346b138
Bar-Kochba, E., Scimone, M., Estrada, J., and Franck, C. (2016). Strain and rate-dependent neuronal injury in a 3D in vitro compression model of traumatic brain injury. Sci. Rep. 6, 30550. doi:10.1038/srep30550
Barroso, M., Monaghan, M. G., Niesner, R., and Dmitriev, R. I. (2023). Probing organoid metabolism using fluorescence lifetime imaging microscopy (FLIM): the next frontier of drug discovery and disease understanding. Adv. Drug Deliv. Rev. 201, 115081. doi:10.1016/j.addr.2023.115081
Belding, J. N., Englert, R. M., Fitzmaurice, S., Jackson, J. R., Koenig, H. G., Hunter, M. A., et al. (2021). Potential health and performance effects of high-level and low-level blast: a scoping review of two decades of research. Front. Neurol. 12, 628782. doi:10.3389/fneur.2021.628782
Beltrán, S. M., Bobo, J., Habib, A., Kodavali, C. V., Edwards, L., Mamindla, P., et al. (2023). Characterization of neural mechanotransduction response in human traumatic brain injury organoid model. Sci. Rep. 13, 13536. doi:10.1038/s41598-023-40431-y
Bir, C. (2011). Measuring blast-related intracranial pressure within the human head. Fort Belvoir, VA: Defense Technical Information Center. doi:10.21236/ADA547306
Boutté, A. M., Thangavelu, B., Nemes, J., LaValle, C. R., Egnoto, M., Carr, W., et al. (2021). Neurotrauma biomarker levels and adverse symptoms among military and law enforcement personnel exposed to occupational overpressure without diagnosed traumatic brain injury. JAMA Netw. Open 4, e216445. doi:10.1001/jamanetworkopen.2021.6445
Cai, H., Ao, Z., Wu, Z., Song, S., Mackie, K., and Guo, F. (2021). Intelligent acoustofluidics enabled mini-bioreactors for human brain organoids. Lab. Chip 21, 2194–2205. doi:10.1039/D1LC00145K
Campos-Pires, R., Yonis, A., Macdonald, W., Harris, K., Edge, C. J., Mahoney, P. F., et al. (2018). A novel in vitro model of blast traumatic brain injury. JoVE, 58400. doi:10.3791/58400-v
Chafi, M. S., Ganpule, S., Gu, L., and Chandra, N. (2011). Dynamic response of brain subjected to blast loadings: influence of frequency ranges. Int. J. Appl. Mech. 03, 803–823. doi:10.1142/S175882511100124X
Chen, Y. C., Smith, D. H., and Meaney, D. F. (2009). In-Vitro approaches for studying blast-induced traumatic brain injury. J. Neurotrauma 26, 861–876. doi:10.1089/neu.2008.0645
Chesnut, M., Hartung, T., Hogberg, H., and Pamies, D. (2021a). Human oligodendrocytes and myelin in vitro to evaluate developmental neurotoxicity. IJMS 22, 7929. doi:10.3390/ijms22157929
Chesnut, M., Paschoud, H., Repond, C., Smirnova, L., Hartung, T., Zurich, M.-G., et al. (2021b). Human IPSC-derived model to study myelin disruption. IJMS 22, 9473. doi:10.3390/ijms22179473
Cho, A.-N., Jin, Y., An, Y., Kim, J., Choi, Y. S., Lee, J. S., et al. (2021). Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 12, 4730. doi:10.1038/s41467-021-24775-5
Cullen, D. K., Simon, C. M., and LaPlaca, M. C. (2007). Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal–astrocytic co-cultures. Brain Res. 1158, 103–115. doi:10.1016/j.brainres.2007.04.070
Deng, P., Liu, S., Zhao, Y., Zhang, X., Kong, Y., Liu, L., et al. (2024). Long-working-distance high-collection-efficiency three-photon microscopy for in vivo long-term imaging of zebrafish and organoids. iScience 27, 110554. doi:10.1016/j.isci.2024.110554
Eichmüller, O. L., and Knoblich, J. A. (2022). Human cerebral organoids — a new tool for clinical neurology research. Nat. Rev. Neurol. 18, 661–680. doi:10.1038/s41582-022-00723-9
Elster, N., Boutillier, J., Magnan, P., Naz, P., Willinger, R., and Deck, C. (2023). A critical review of experimental analyses performed on animals, post-mortem human subjects, and substitutes to explore primary blast-induced Traumatic Brain Injuries. Front. Mech. Eng. 9, 1185231. doi:10.3389/fmech.2023.1185231
Ettinger, A., and Wittmann, T. (2014). “Fluorescence live cell imaging,” in Methods in cell biology (Elsevier), 77–94. doi:10.1016/B978-0-12-420138-5.00005-7
Fagerlund, I., Dougalis, A., Shakirzyanova, A., Gómez-Budia, M., Pelkonen, A., Konttinen, H., et al. (2021). Microglia-like cells promote neuronal functions in cerebral organoids. Cells 11, 124. doi:10.3390/cells11010124
Fei, K., Zhang, J., Yuan, J., and Xiao, P. (2022). Present application and perspectives of organoid imaging technology. Bioengineering 9, 121. doi:10.3390/bioengineering9030121
Frühbeis, C., Fröhlich, D., Kuo, W. P., and Krämer-Albers, E.-M. (2013). Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 7, 182. doi:10.3389/fncel.2013.00182
Galpayage Dona, K. N. U., Ramirez, S. H., and Andrews, A. M. (2023). A next-generation 3D tissue-engineered model of the human brain microvasculature to study the blood-brain barrier. Bioengineering 10, 817. doi:10.3390/bioengineering10070817
Ganpule, S., Salzar, R., and Chandra, N. (2013). “Response of post-mortem human head under primary blast loading conditions: effect of blast overpressures,” in Volume 3A: biomedical and biotechnology engineering (San Diego, California, USA: American Society of Mechanical Engineers). doi:10.1115/IMECE2013-63910
Gharahi, H., Garimella, H. T., Chen, Z. J., Gupta, R. K., and Przekwas, A. (2023). Mathematical model of mechanobiology of acute and repeated synaptic injury and systemic biomarker kinetics. Front. Cell. Neurosci. 17, 1007062. doi:10.3389/fncel.2023.1007062
Gilmore, N., Tseng, C.-E. J., Maffei, C., Tromly, S. L., Deary, K. B., McKinney, I. R., et al. (2024). Impact of repeated blast exposure on active-duty United States Special Operations Forces. Proc. Natl. Acad. Sci. U.S.A. 121, e2313568121. doi:10.1073/pnas.2313568121
Gomez Godinez, V., Morar, V., Carmona, C., Gu, Y., Sung, K., Shi, L. Z., et al. (2021). Laser-induced shockwave (LIS) to study neuronal Ca2+ responses. Front. Bioeng. Biotechnol. 9, 598896. doi:10.3389/fbioe.2021.598896
González-Cruz, R. D., Wan, Y., Burgess, A., Calvao, D., Renken, W., Vecchio, F., et al. (2024). Cortical spheroids show strain-dependent cell viability loss and neurite disruption following sustained compression injury. PLoS ONE 19, e0295086. doi:10.1371/journal.pone.0295086
Groux, K., Verschueren, A., Nanteau, C., Clémençon, M., Fink, M., Sahel, J.-A., et al. (2022). Dynamic full-field optical coherence tomography allows live imaging of retinal pigment epithelium stress model. Commun. Biol. 5, 575. doi:10.1038/s42003-022-03479-6
Gupta, R. K., and Przekwas, A. (2013). Mathematical models of blast-induced TBI: current status, challenges, and prospects. Front. Neurol. 4, 59. doi:10.3389/fneur.2013.00059
Gupta, R. K., Tan, X. G., Somayaji, M. R., and Przekwas, A. J. (2017). Multiscale modelling of blast-induced TBI mechanobiology - from body to neuron to molecule. Def. Life Sci. J. 2, 3. doi:10.14429/dlsj.2.10369
Gupta, S., Haiat, G., Laporte, C., and Belanger, P. (2021). Effect of the acoustic impedance mismatch at the bone-soft tissue interface as a function of frequency in transcranial ultrasound: a simulation and in vitro experimental study. IEEE Trans. Ultrason. Ferroelect., Freq. Contr. 68, 1653–1663. doi:10.1109/TUFFC.2020.3043893
Hernandez, A., Tan, C., Plattner, F., Logsdon, A. F., Pozo, K., Yousuf, M. A., et al. (2018). Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes. Mol. Brain 11, 64. doi:10.1186/s13041-018-0408-1
Hicks, K. (2024). Department of defense requirements for managing brain health risks from blast overpressure. Available online at: https://media.defense.gov/2024/Aug/09/2003521276/-1/-1/1/DEPARTMENT-OF-DEFENSE-REQUIREMENTS-FOR-MANAGING-BRAIN-HEALTH-RISKS-FROM-BLAST-OVERPRESSURE-OSD005281-24-RES-FINAL.PDF (Accessed December 26, 2024).
Howard, D., and Sturtevant, B. (1997). In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound Med. and Biol. 23, 1107–1122. doi:10.1016/S0301-5629(97)00081-1
Hua, Y., Kumar Akula, P., Gu, L., Berg, J., and Nelson, C. A. (2014). Experimental and numerical investigation of the mechanism of blast wave transmission through a surrogate head. J. Comput. Nonlinear Dyn. 9, 031010. doi:10.1115/1.4026156
Huang, Q., Tang, B., Romero, J. C., Yang, Y., Elsayed, S. K., Pahapale, G., et al. (2022). Shell microelectrode arrays (MEAs) for brain organoids. Sci. Adv. 8, eabq5031. doi:10.1126/sciadv.abq5031
Hubbard, W. B., Greenberg, S., Norris, C., Eck, J., Lavik, E., and VandeVord, P. (2017). Distinguishing the unique neuropathological profile of blast polytrauma. Oxidative Med. Cell. Longev. 2017, 5175249. doi:10.1155/2017/5175249
Huber, B. R., Meabon, J. S., Hoffer, Z. S., Zhang, J., Hoekstra, J. G., Pagulayan, K. F., et al. (2016). Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 319, 206–220. doi:10.1016/j.neuroscience.2016.01.022
Hue, C. D., Cao, S., Haider, S. F., Vo, K. V., Effgen, G. B., Vogel, E., et al. (2013). Blood-brain barrier dysfunction after primary blast injury in vitro. J. Neurotrauma 30, 1652–1663. doi:10.1089/neu.2012.2773
Iwaskiw, A. S., Ott, K. A., Armiger, R. S., Wickwire, A. C., Alphonse, V. D., Voo, L. M., et al. (2018). The measurement of intracranial pressure and brain displacement due to short-duration dynamic overpressure loading. Shock Waves 28, 63–83. doi:10.1007/s00193-017-0759-z
Jgamadze, D., Johnson, V. E., Wolf, J. A., Kacy Cullen, D., Song, H., Ming, G., et al. (2020). Modeling traumatic brain injury with human brain organoids. Curr. Opin. Biomed. Eng. 14, 52–58. doi:10.1016/j.cobme.2020.05.004
Jha, R. M., Rajasundaram, D., Sneiderman, C., Schlegel, B. T., O’Brien, C., Xiong, Z., et al. (2024). A single-cell atlas deconstructs heterogeneity across multiple models in murine traumatic brain injury and identifies novel cell-specific targets. Neuron 112, 3069–3088.e4. doi:10.1016/j.neuron.2024.06.021
Kamimori, G. H., Reilly, L. A., LaValle, C. R., and Olaghere Da Silva, U. B. (2017). Occupational overpressure exposure of breachers and military personnel. Shock Waves 27, 837–847. doi:10.1007/s00193-017-0738-4
Kang, R., Park, S., Shin, S., Bak, G., and Park, J.-C. (2024). Electrophysiological insights with brain organoid models: a brief review. BMB Rep. 57, 311–317. doi:10.5483/BMBRep.2024-0077
Keshara, R., Kim, Y. H., and Grapin-Botton, A. (2022). Organoid imaging: seeing development and function. Annu. Rev. Cell Dev. Biol. 38, 447–466. doi:10.1146/annurev-cellbio-120320-035146
Khan, I., Prabhakar, A., Delepine, C., Tsang, H., Pham, V., and Sur, M. (2021). A low-cost 3D printed microfluidic bioreactor and imaging chamber for live-organoid imaging. Biomicrofluidics 15, 024105. doi:10.1063/5.0041027
Lai, J. D., Berlind, J. E., Fricklas, G., Lie, C., Urenda, J.-P., Lam, K., et al. (2024). KCNJ2 inhibition mitigates mechanical injury in a human brain organoid model of traumatic brain injury. Cell Stem Cell 31, 519–536.e8. doi:10.1016/j.stem.2024.03.004
LaPlaca, M. C., and Brody, D. L. (2022). Learning about blast injuries from brain organoids: a new frontier. J. Neurotrauma 39, 1453–1454. doi:10.1089/neu.2022.29132.editorial
Li, Q., Nan, K., Le Floch, P., Lin, Z., Sheng, H., Blum, T. S., et al. (2019). Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19, 5781–5789. doi:10.1021/acs.nanolett.9b02512
Li, Y., Lin, J., Liu, S., Zhu, H., Zhang, H., and Fan, H. (2024). Experimental and numerical study on the protective mechanism of the full helmet subjected to blast loadings. Thin-Walled Struct. 198, 111666. doi:10.1016/j.tws.2024.111666
Liang, B., Wang, S., Shen, F., Liu, Q. H., Gong, Y., and Yao, J. (2021). Acoustic impact of the human skull on transcranial photoacoustic imaging. Biomed. Opt. Express 12, 1512. doi:10.1364/BOE.420084
Loane, D. J., and Byrnes, K. R. (2010). Role of microglia in neurotrauma. Neurotherapeutics 7, 366–377. doi:10.1016/j.nurt.2010.07.002
Maharjan, S., Ma, C., Singh, B., Kang, H., Orive, G., Yao, J., et al. (2024). Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications. Adv. Drug Deliv. Rev. 208, 115237. doi:10.1016/j.addr.2024.115237
Martinelli, E., Akouissi, O., Liebi, L., Furfaro, I., Maulà, D., Savoia, N., et al. (2024). The e-Flower: a hydrogel-actuated 3D MEA for brain spheroid electrophysiology. Sci. Adv. 10, eadp8054. doi:10.1126/sciadv.adp8054
Merkle, A., and Carneal, I. W. C. (2012). “Effect of helmet systems on the two-phased brain response to blast loading,” in Personal armor systems symposium.
Modafferi, S., Zhong, X., Kleensang, A., Murata, Y., Fagiani, F., Pamies, D., et al. (2021). Gene–environment interactions in developmental neurotoxicity: a case study of synergy between chlorpyrifos and CHD8 knockout in human BrainSpheres. Environ. Health Perspect. 129, 77001. doi:10.1289/ehp8580
Monfort, T., Azzollini, S., Brogard, J., Clémençon, M., Slembrouck-Brec, A., Forster, V., et al. (2023). Dynamic full-field optical coherence tomography module adapted to commercial microscopes allows longitudinal in vitro cell culture study. Commun. Biol. 6, 992. doi:10.1038/s42003-023-05378-w
Montanino, A., Saeedimasine, M., Villa, A., and Kleiven, S. (2020). Localized axolemma deformations suggest mechanoporation as axonal injury trigger. Front. Neurol. 11, 25. doi:10.3389/fneur.2020.00025
Moore, D. F., Jérusalem, A., Nyein, M., Noels, L., Jaffee, M. S., and Radovitzky, R. A. (2009). Computational biology — modeling of primary blast effects on the central nervous system. NeuroImage 47, T10–T20. doi:10.1016/j.neuroimage.2009.02.019
Moss, W. C., King, M. J., and Blackman, E. G. (2009). Skull flexure from blast waves: a mechanism for brain injury with implications for helmet design. Phys. Rev. Lett. 103, 108702. doi:10.1103/PhysRevLett.103.108702
Murphy, E. J., and Horrocks, L. A. (1993). A model for compression trauma: pressure-induced injury in cell cultures. J. Neurotrauma 10, 431–444. doi:10.1089/neu.1993.10.431
Nakagawa, A., Manley, G. T., Gean, A. D., Ohtani, K., Armonda, R., Tsukamoto, A., et al. (2011). Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research. J. Neurotrauma 28, 1101–1119. doi:10.1089/neu.2010.1442
Nwokoye, P. N., and Abilez, O. J. (2024). Bioengineering methods for vascularizing organoids. Cell Rep. Methods 4, 100779. doi:10.1016/j.crmeth.2024.100779
Nyein, M. K., Jason, A. M., Yu, L., Pita, C. M., Joannopoulos, J. D., Moore, D. F., et al. (2010). In silico investigation of intracranial blast mitigation with relevance to military traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 107, 20703–20708. doi:10.1073/pnas.1014786107
Ormel, P. R., Vieira De Sá, R., Van Bodegraven, E. J., Karst, H., Harschnitz, O., Sneeboer, M. A. M., et al. (2018). Microglia innately develop within cerebral organoids. Nat. Commun. 9, 4167. doi:10.1038/s41467-018-06684-2
Ott, K., Voo, L., Merkle, A., Iwaskiw, A., Wickwire, A., Wester, B., et al. (2013). “Experimental determination of pressure wave transmission to the brain during head-neck blast tests,” in Summer bioengineering conference (American Society of Mechanical Engineers).
Ouellet, S., Bir, C., and Bouamoul, A. (2014). Direct comparison of the primary blast response of a physical head model with post-mortem human subjects. Def. Res. Dev. Canada-Valcartier Res. Cent. Quebec. Available online at: https://apps.dtic.mil/sti/citations/AD1004215.
Ouellet, S., and Philippens, M. (2018). The multi-modal responses of a physical head model subjected to various blast exposure conditions. Shock Waves 28, 19–36. doi:10.1007/s00193-017-0771-3
Pamies, D., Barreras, P., Block, K., Makri, G., Kumar, A., Wiersma, D., et al. (2017). A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity, 34, 362, 376. doi:10.14573/altex.1609122
Pamies, D., Wiersma, D., Katt, M. E., Zhao, L., Burtscher, J., Harris, G., et al. (2022). Human IPSC 3D brain model as a tool to study chemical-induced dopaminergic neuronal toxicity. Neurobiol. Dis. 169, 105719. doi:10.1016/j.nbd.2022.105719
Panzer, M. B., Myers, B. S., Capehart, B. P., and Bass, C. R. (2012). Development of a finite element model for blast brain injury and the effects of CSF cavitation. Ann. Biomed. Eng. 40, 1530–1544. doi:10.1007/s10439-012-0519-2
Park, Y., Franz, C. K., Ryu, H., Luan, H., Cotton, K. Y., Kim, J. U., et al. (2021). Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci. Adv. 7, eabf9153. doi:10.1126/sciadv.abf9153
Procès, A., Luciano, M., Kalukula, Y., Ris, L., and Gabriele, S. (2022). Multiscale mechanobiology in brain physiology and diseases. Front. Cell Dev. Biol. 10, 823857. doi:10.3389/fcell.2022.823857
Ramadan, Q., Hazaymeh, R., and Zourob, M. (2023). Immunity-on-a-Chip: integration of immune components into the scheme of organ-on-a-chip systems. Adv. Biol. 7, 2200312. doi:10.1002/adbi.202200312
Ramirez, S., Mukherjee, A., Sepulveda, S., Becerra-Calixto, A., Bravo-Vasquez, N., Gherardelli, C., et al. (2021). Modeling traumatic brain injury in human cerebral organoids. Cells 10, 2683. doi:10.3390/cells10102683
Ramos-Zaldívar, H. M., Polakovicova, I., Salas-Huenuleo, E., Corvalán, A. H., Kogan, M. J., Yefi, C. P., et al. (2022). Extracellular vesicles through the blood–brain barrier: a review. Fluids Barriers CNS 19, 60. doi:10.1186/s12987-022-00359-3
Ravin, R., Blank, P. S., Busse, B., Ravin, N., Vira, S., Bezrukov, L., et al. (2016). Blast shockwaves propagate Ca2+ activity via purinergic astrocyte networks in human central nervous system cells. Sci. Rep. 6, 25713. doi:10.1038/srep25713
Ravin, R., Blank, P. S., Steinkamp, A., Rappaport, S. M., Ravin, N., Bezrukov, L., et al. (2012). Shear forces during blast, not abrupt changes in pressure alone, generate calcium activity in human brain cells. PLoS ONE 7, e39421. doi:10.1371/journal.pone.0039421
Ravula, A. R., Das, T., Gosain, A., Dolalas, T., Padhi, S., Chandra, N., et al. (2022). An update on repeated blast traumatic brain injury. Curr. Opin. Biomed. Eng. 24, 100409. doi:10.1016/j.cobme.2022.100409
Romero, J. C., Berlinicke, C., Chow, S., Duan, Y., Wang, Y., Chamling, X., et al. (2023). Oligodendrogenesis and myelination tracing in a CRISPR/Cas9-engineered brain microphysiological system. Front. Cell. Neurosci. 16, 1094291. doi:10.3389/fncel.2022.1094291
Rosenfeld, J. V., McFarlane, A. C., Bragge, P., Armonda, R. A., Grimes, J. B., and Ling, G. S. (2013). Blast-related traumatic brain injury. Lancet Neurology 12, 882–893. doi:10.1016/S1474-4422(13)70161-3
Ryu, H., Park, Y., Luan, H., Dalgin, G., Jeffris, K., Yoon, H.-J., et al. (2021). Transparent, compliant 3D mesostructures for precise evaluation of mechanical characteristics of organoids. Adv. Mater. 33, 2100026. doi:10.1002/adma.202100026
Sabate-Soler, S., Nickels, S. L., Saraiva, C., Berger, E., Dubonyte, U., Barmpa, K., et al. (2022). Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 70, 1267–1288. doi:10.1002/glia.24167
Säljö, A., Arrhén, F., Bolouri, H., Mayorga, M., and Hamberger, A. (2008). Neuropathology and pressure in the pig brain resulting from low-impulse noise exposure. J. Neurotrauma 25, 1397–1406. doi:10.1089/neu.2008.0602
Salmon, I., Grebenyuk, S., Abdel Fattah, A. R., Rustandi, G., Pilkington, T., Verfaillie, C., et al. (2022). Engineering neurovascular organoids with 3D printed microfluidic chips. Lab. Chip 22, 1615–1629. doi:10.1039/D1LC00535A
Salvador-Silva, M., Aoi, S., Parker, A., Yang, P., Pecen, P., and Hernandez, M. R. (2004). Responses and signaling pathways in human optic nerve head astrocytes exposed to hydrostatic pressure in vitro. Glia 45, 364–377. doi:10.1002/glia.10342
Samarasinghe, R. A., Miranda, O. A., Buth, J. E., Mitchell, S., Ferando, I., Watanabe, M., et al. (2021). Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 24, 1488–1500. doi:10.1038/s41593-021-00906-5
Sawyer, T. W., Lee, J. J., Villanueva, M., Wang, Y., Nelson, P., Song, Y., et al. (2017). The effect of underwater blast on aggregating brain cell cultures. J. Neurotrauma 34, 517–528. doi:10.1089/neu.2016.4430
Sawyer, T. W., Ritzel, D. V., Wang, Y., Josey, T., Villanueva, M., Nelson, P., et al. (2018). Primary blast causes delayed effects without cell death in shell-encased brain cell aggregates. J. Neurotrauma 35, 174–186. doi:10.1089/neu.2016.4961
Scholler, J., Groux, K., Goureau, O., Sahel, J.-A., Fink, M., Reichman, S., et al. (2020). Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci. Appl. 9, 140. doi:10.1038/s41377-020-00375-8
Schröter, M., Wang, C., Terrigno, M., Hornauer, P., Huang, Z., Jagasia, R., et al. (2022). Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 47, 530–544. doi:10.1557/s43577-022-00282-w
Selfridge, A., Preece, D., Gomez, V., Shi, L. Z., and Berns, M. W. (2015). “A model for traumatic brain injury using laser induced shockwaves,”. Editors K. Dholakia, and G. C. Spalding (San Diego, California, United States), 95480P, 95480P. doi:10.1117/12.2189724
Sharf, T., Van Der Molen, T., Glasauer, S. M. K., Guzman, E., Buccino, A. P., Luna, G., et al. (2022). Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat. Commun. 13, 4403. doi:10.1038/s41467-022-32115-4
Shi, W., Dong, P., Kuss, M. A., Gu, L., Kievit, F., Kim, H. J., et al. (2021). Design and evaluation of an in vitro mild traumatic brain injury modeling system using 3D printed mini impact device on the 3D cultured human iPSC derived neural progenitor cells. Adv. Healthc. Mater. 10, 2100180. doi:10.1002/adhm.202100180
Siedhoff, H. R., Chen, S., Song, H., Cui, J., Cernak, I., Cifu, D. X., et al. (2022). Perspectives on primary blast injury of the brain: translational insights into non-inertial low-intensity blast injury. Front. Neurol. 12, 818169. doi:10.3389/fneur.2021.818169
Silvosa, M. J., Mercado, N. R., Merlock, N., Vidhate, S., Mejia-Alvarez, R., Yuan, T. T., et al. (2022). Understanding primary blast injury: high frequency pressure acutely disrupts neuronal network dynamics in cerebral organoids. J. Neurotrauma 39, 1575–1590. doi:10.1089/neu.2022.0044
Skotak, M., LaValle, C., Misistia, A., Egnoto, M. J., Chandra, N., and Kamimori, G. (2019). Occupational blast wave exposure during multiday 0.50 caliber rifle course. Front. Neurol. 10, 797. doi:10.3389/fneur.2019.00797
Skylar-Scott, M. A., Huang, J. Y., Lu, A., Ng, A. H. M., Duenki, T., Liu, S., et al. (2022). Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 6, 449–462. doi:10.1038/s41551-022-00856-8
Smirnova, L., and Hartung, T. (2024). The promise and potential of brain organoids. Adv. Healthc. Mater. 13, 2302745. doi:10.1002/adhm.202302745
Smirnova, L., Modafferi, S., Schlett, C., Osborne, L. M., Payne, J. L., and Sabunciyan, S. (2024). Blood extracellular vesicles carrying brain-specific mRNAs are potential biomarkers for detecting gene expression changes in the female brain. Mol. Psychiatry 29, 962–973. doi:10.1038/s41380-023-02384-6
Smirnova, L., Morales Pantoja, I. E., and Hartung, T. (2023). Organoid intelligence (OI) – the ultimate functionality of a brain microphysiological system. ALTEX 40, 191–203. doi:10.14573/altex.2303261
Snapper, D. M., Reginauld, B., Liaudanskaya, V., Fitzpatrick, V., Kim, Y., Georgakoudi, I., et al. (2023). Development of a novel bioengineered 3D brain-like tissue for studying primary blast-induced traumatic brain injury. J Neurosci. Res. 101, 3–19. doi:10.1002/jnr.25123
Song, L., Yuan, X., Jones, Z., Vied, C., Miao, Y., Marzano, M., et al. (2019). Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci. Rep. 9, 11055. doi:10.1038/s41598-019-47444-6
Soscia, D. A., Lam, D., Tooker, A. C., Enright, H. A., Triplett, M., Karande, P., et al. (2020). A flexible 3-dimensional microelectrode array for in vitro brain models. Lab. Chip 20, 901–911. doi:10.1039/C9LC01148J
Susaimanickam, P. J., Kiral, F. R., and Park, I.-H. (2022). Region specific brain organoids to study neurodevelopmental disorders. IJSC 15, 26–40. doi:10.15283/ijsc22006
Tajik, M., and Noseworthy, M. D. (2022). A review of molecular and genetic factors for determining mild traumatic brain injury severity and recovery. Brain Disord. 8, 100058. doi:10.1016/j.dscb.2022.100058
Tan, X. G., Przekwas, A. J., and Gupta, R. K. (2017). Computational modeling of blast wave interaction with a human body and assessment of traumatic brain injury. Shock Waves 27, 889–904. doi:10.1007/s00193-017-0740-x
Tan, X. G., and Matic, P. (2020). Simulation of cumulative exposure statistics for blast pressure transmission into the brain. Mil. Med. 185, 214–226. doi:10.1093/milmed/usz308
Taylor, P. A., and Ford, C. C. (2009). Simulation of blast-induced early-time intracranial wave Physics leading to traumatic brain injury. J. Biomechanical Eng. 131, 061007. doi:10.1115/1.3118765
Thangavelu, B., LaValle, C. R., Egnoto, M. J., Nemes, J., Boutté, A. M., and Kamimori, G. H. (2020). Overpressure exposure from .50-Caliber rifle training is associated with increased amyloid beta peptides in serum. Front. Neurol. 11, 620. doi:10.3389/fneur.2020.00620
Trujillo, C. A., Gao, R., Negraes, P. D., Gu, J., Buchanan, J., Preissl, S., et al. (2019). Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569.e7. doi:10.1016/j.stem.2019.08.002
Vanvliet, E., Stoppini, L., Balestrino, M., Eskes, C., Griesinger, C., Sobanski, T., et al. (2007). Electrophysiological recording of re-aggregating brain cell cultures on multi-electrode arrays to detect acute neurotoxic effects. NeuroToxicology 28, 1136–1146. doi:10.1016/j.neuro.2007.06.004
Vogel, E. W., Morales, F. N., Meaney, D. F., Bass, C. R., and Morrison, B. (2017). Phosphodiesterase-4 inhibition restored hippocampal long term potentiation after primary blast. Exp. Neurol. 293, 91–100. doi:10.1016/j.expneurol.2017.03.025
Wiri, S., Massow, T., Reid, J., Whitty, J., Dunbar, C., Graves, W., et al. (2023). Dynamic monitoring of service members to quantify blast exposure levels during combat training using BlackBox Biometrics Blast Gauges: explosive breaching, shoulder-fired weapons, artillery, mortars, and 0.50 caliber guns. Front. Neurol. 14, 1175671. doi:10.3389/fneur.2023.1175671
Wörsdörfer, P., Dalda, N., Kern, A., Krüger, S., Wagner, N., Kwok, C. K., et al. (2019). Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 9, 15663. doi:10.1038/s41598-019-52204-7
Xue, Y., Browne, A. W., Tang, W. C., Delgado, J., McLelland, B. T., Nistor, G., et al. (2021). Retinal organoids long-term functional characterization using two-photon fluorescence lifetime and hyperspectral microscopy. Front. Cell. Neurosci. 15, 796903. doi:10.3389/fncel.2021.796903
Yildirim, M., Delepine, C., Feldman, D., Pham, V. A., Chou, S., Ip, J., et al. (2022). Label-free three-photon imaging of intact human cerebral organoids for tracking early events in brain development and deficits in Rett syndrome. eLife 11, e78079. doi:10.7554/eLife.78079
Zander, N. E., Piehler, T., Hogberg, H., and Pamies, D. (2017). Explosive blast loading on human 3D aggregate minibrains. Cell Mol. Neurobiol. 37, 1331–1334. doi:10.1007/s10571-017-0463-7
Zhang, W., Jiang, J., Xu, Z., Yan, H., Tang, B., Liu, C., et al. (2023). Microglia-containing human brain organoids for the study of brain development and pathology. Mol. Psychiatry 28, 96–107. doi:10.1038/s41380-022-01892-1
Zhao, X., Xu, Z., Xiao, L., Shi, T., Xiao, H., Wang, Y., et al. (2021). Review on the vascularization of organoids and organoids-on-a-chip. Front. Bioeng. Biotechnol. 9, 637048. doi:10.3389/fbioe.2021.637048
Keywords: traumatic brain injury, brain organoids, repeated blast, low-level blast, primary blast, in vitro model
Citation: Bar-Kochba E, Carneal CM, Alphonse VD, Timm AC, Ernlund AW, Rodriguez CL, Morales Pantoja IE, Smirnova L, Hartung T and Merkle AC (2025) Advancing next-generation brain organoid platforms for investigating traumatic brain injury from repeated blast exposures. Front. Bioeng. Biotechnol. 13:1553609. doi: 10.3389/fbioe.2025.1553609
Received: 31 December 2024; Accepted: 05 June 2025;
Published: 18 June 2025.
Edited by:
Dong Zeng, Southern Medical University, ChinaReviewed by:
Reuben H. Kraft, The Pennsylvania State University (PSU), United StatesCopyright © 2025 Bar-Kochba, Carneal, Alphonse, Timm, Ernlund, Rodriguez, Morales Pantoja, Smirnova, Hartung and Merkle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eyal Bar-Kochba, ZXlhbC5iYXIta29jaGJhQGpodWFwbC5lZHU=
 Eyal Bar-Kochba
Eyal Bar-Kochba Catherine M. Carneal1
Catherine M. Carneal1 Andrea C. Timm
Andrea C. Timm Carissa L. Rodriguez
Carissa L. Rodriguez Itzy E. Morales Pantoja
Itzy E. Morales Pantoja Lena Smirnova
Lena Smirnova Thomas Hartung
Thomas Hartung Andrew C. Merkle
Andrew C. Merkle