- 1Bioprocessing Technology Institute (BTI), Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
- 2Institute of Molecular and Cell Biology (IMCB), Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
Introduction: Cultivated meat, produced by in vitro cell culture in bioreactors, offers a sustainable alternative to traditional meat sources. A significant challenge in its production is the high cost of mitogenic growth factors, which are essential supplements in serum-free media for cultivating meat cells. One strategy to reduce cost involves minimizing purification cost by using a food-grade host to secrete growth factors. In this study, we investigate the production of recombinant FGF2 (Fibroblast Growth Factor 2) through secretion in Lactococcus lactis, a Generally Recognized As Safe (GRAS) organism.
Method: To enhance the secretion in L. lactis, we employed the USP45 secretory peptide and secretion propeptide (PP1) in the design of our recombinant FGF2-G3. Optimization was performed on various culture parameters that influence protein expression, including media formulation, nisin concentration, induction timing, temperature, and culture duration. Secreted FGF2-G3 produced under optimized conditions was purified and tested for bioactivity on Anguilla japonica pre-adipocytic cells, Aj1C-2x.
Results and Discussion: We have generated a recombinant L. lactis strain and an optimal expression strategy to enable the production of secreted bioactive growth factors. Our results demonstrate that this system can produce FGF2 which were able to promote the proliferation of fish Anguilla japonica pre-adipocytic cells. Despite minimal purification beyond affinity purification and buffer exchange, we were able to obtain comparable specific activity to commercial FGF2. The final yields can be derived at 1.97 mg/L and through simple protein purification and buffer exchange. Finally, this study highlights the potential use of L. lactis secretion as an endotoxin-free alternative, compared to E. coli, for production of growth factors for use in cultivated meat production.
Introduction
Cultivated meat, also known as cultured meat, has gained significant interest in recent years due to global movement towards achieving sustainability development goals. It provides a prospective alternative meat source to support demand from increasing population, reduce environmental impact from animal agriculture and avoids animal-borne diseases (Campbell et al., 2017; Godfray et al., 2018; Lynch and Pierrehumbert, 2019; Stephens et al., 2018). Cultivated meat is produced through in vitro culturing of animal cells in cell culture media. However, foetal bovine serum (FBS), a typical ingredient found in culture media, is derived from extracting blood serum of bovine foetuses from animal slaughter houses (Jochems et al., 2002; Kadim et al., 2015; Lee et al., 2022; Post et al., 2020; Reiss et al., 2021). This conflicts with the concept of producing meat via an animal-free approach. Thus, serum-free media formulations that are capable of sustaining cell culture were developed (Badenes et al., 2016; Das et al., 2009; Messmer et al., 2022; Skrivergaard et al., 2023; Stout et al., 2022). Subsequently, supplementation of growth factors, such as fibroblast growth factors or insulin-like growth factors, into serum-free media formulations are essential to mimic proliferative and developmental effects of FBS (Park et al., 2013; Santos et al., 2023; Venkatesan et al., 2022; Yu et al., 2023).
One such growth factor of interest is the basic fibroblast growth factor, also known as fibroblast growth factor 2 (FGF2), which is a member of the cytokine family. It acts by binding to cell surface receptors (FGFR), activating mitogenic pathways such as PI3k/Akt pathway, MAPK/ERK pathway and JNK pathway. Activation of these pathways regulates cellular responses such as growth, proliferation, migration, maintenance and differentiation (Ahmad et al., 2023; Bikfalvi et al., 1997; Yun et al., 2010). Production of recombinant growth factors, including FGF2, for supplementation into serum-free media are most frequently done in prokaryotic expression system using Escherichia coli. However, the recombinant proteins are produced intracellularly and has high tendency for inclusion bodies formation, which subsequently involve expensive and tedious downstream protein refolding and purification processes. Furthermore, host-cell derived impurities, in particular endotoxins, poses health risks to humans (Baneyx and Mujacic, 2004; Kaur et al., 2018; Petsch and Anspach, 2000; Sahdev et al., 2008; Thomas and Baneyx, 1996). This calls for an alternative endotoxin free expression system that is also economical for production of recombinant proteins.
Lactococcus lactis, a Gram-positive lactic acid bacterium that is widely used in food and therapeutic applications (Bahey-El-Din et al., 2010; Kumari et al., 2011; Song et al., 2017), presents a good alternative host for recombinant protein expression. The key feature of using Lactococcus lactis expression system is its ability to secrete recombinant proteins into culture medium, minimising the need for cell lysis, complex protein purification and refolding. Moreover, L. lactis does not produce lipopolysaccharides and has few extracellular proteases, that causes endotoxin toxicity and proteolytic degradation respectively (Frelet-Barrand, 2022; Garcia-Fruitos, 2012; Morello et al., 2008). As L. lactis are microaerophilic, they only require a simple static fermentation process without aeration, this makes possible for a simple and direct scale-up to industrial scale. Among the various L. lactis expression systems developed, the most widely used is the nisin-controlled gene expression (NICE) system, consisting of a nisRK regulatory gene integrated into bacterial host chromosome and an expression vector with nisA promoter to tightly regulate gene expression (de Ruyter et al., 1996; Mierau and Kleerebezem, 2005; Mierau et al., 2005a; Zhou et al., 2006). L. lactis expression system has been applied for production of several growth factor proteins (Cao et al., 2020; Gao et al., 2012; Huynh and Li, 2015; Zhou et al., 2021). In our lab, we have recently reported on valorisation of mammalian spent culture media waste to support intracellular FGF2 production in bioreactors (Rizal et al., 2024). However, comprehensive research regarding the production and secretion of functional FGF2 from L. lactis is not available. Hence, we set forth herein to investigate the possibility of employing L. lactis NICE expression system to produce and secrete biologically active FGF2. To enhance the secretion efficiency, we fused USP45 secretory peptide and secretion propeptide 1 (PP1) (Lim et al., 2017) to a thermostable FGF2 variant, FGF2-G3 (Dvorak et al., 2018). Together with optimisation of media formulation and culture conditions, we were able to obtain ∼2 mg/L of secreted FGF2-G3. Furthermore, FGF2-G3 purified from the medium was able to stimulate proliferation of the Japanese eel Anguilla japonica pre-adipocytic cells, comparable to commercial FGF2. Together, these results signal the potential application of L. lactis protein secretion system as an alternative strategy for recombinant FGF2 and potentially other growth factor production to circumvent issues faced with E. coli for cultured meat development.
Materials and methods
Bacterial strain, plasmid and cloning of FGF2-G3 gene
L. lactis NZ9000 and pNZ8148 plasmid (BoCa Scientific, United States) were used for cloning and expression studies. Sequence of the thermostable human FGF2-G3 was obtained from Dvorak et al. (2018). To enhance expression and secretion into medium, USP45 secretion peptide (Accession ABY84357) and propeptide 1 (PP1) (Lim et al., 2017) were fused at the N-terminus of FGF2-G3 sequence. For ease of purification with Ni-NTA affinity chromatography and Western blot detection, we have also included His6 sequence at the N-terminus. The nucleotide sequences corresponding to the amino acids were codon optimized and synthesized by IDT (Singapore) for expression in L. lactis. The coding sequence was cloned into the multiple cloning site (MCS) of pNZ8148 vector using NeBuilder HiFi Assembly (New England Biolabs, United States) (Fusion protein sequence available in Supplementary Figure S1), transformed into L. lactis NZ9000 and plated onto M17 agar plate containing 0.5% (w/v) glucose and 10 μg/mL chloramphenicol to screen for positive recombinant clones. The positive recombinant clones were further sequenced to ensure no mutations prior to protein expression with L. lactis NZ9000.
Culture optimization for FGF2-G3 expression and secretion in L. lactis
Productivity of FGF2-G3 production in L. lactis was assessed with varying M17 media and glucose concentration. They were expressed in either M17 (supplemented with 0.5% (w/v) glucose), 2xM17 (supplemented with 0.5% (w/v) glucose) or 2xM17 (supplemented with 2% (w/v) glucose). All cultures were also supplemented with 10 μg/mL chloramphenicol for selection and maintenance of cells containing pNZ8148-FGF2-G3 plasmids. To increase production and secretion level, FGF2-G3 expression was further optimized with different nisin inducer concentrations (10, 25, 50 ng/mL), induction time points (OD600nm 0.5, 1.0, 2.0), incubation temperatures (20, 25, 30, 35°C) and expression duration (4, 20 h post-induction). These parameters were investigated individually in 10 mL culture volume. The cultures were harvested by centrifugation at 3,845 g, 4°C for 20 min with Sorvall ST 40 centrifuge (Thermo Fisher Scientific, United States). The media fraction was aspirated and concentrated with a 10 kDa MWCO centrifugal filter (Merck Millipore, Ireland) to obtain secreted protein fraction, while the cell pellet was resuspended in PBS, incubated at 37°C for 1 h with 1 mg/mL lysozyme and 0.05 U/µL mutanolysin and then lysed via sonication. The cell lysate was centrifuged, supernatant collected as intracellular soluble fraction, and the lysed cell pellet resuspended in 6M urea to obtain intracellular insoluble fraction. All 3 fractions (secreted, soluble, insoluble) were analysed on SDS-PAGE to determine productivity level.
Optimized production of secreted FGF2-G3
A larger production volume of FGF2-G3 was performed under optimized conditions. 250 mL of 2xM17 (supplemented with 2% (w/v) glucose and chloramphenicol 10 μg/mL) was inoculated with pre-culture and incubated at 30°C until it reaches OD600nm 1.0. Nisin was then added to final concentration of 25 ng/mL to induce expression of FGF2-G3, and induction was done at 35°C for 20 h. The culture was centrifuged and the media fraction containing secreted FGF2-G3 was collected and purified via affinity chromatography.
Purification of FGF2-G3
Media fraction containing secreted FGF2-G3 was concentrated, and buffer exchanged to buffer A (50 mM NaH2PO4, 300 mM NaCl, 10 mM Imidazole, pH 8) using crossflow filtration system. The concentrated sample was loaded onto Ni-NTA Agarose (Qiagen, United States) column and incubated for 60 min. The column was first washed with 10 mM imidazole, next with 20 mM imidazole, and finally eluted with 250 mM imidazole. The eluted fraction was buffer exchanged to PBS for protein evaluation with SDS-PAGE and bioactivity assay. The purified FGF2-G3 was quantified using Bradford method, with BSA as standard.
Western blot and SDS-PAGE
An equal amount of protein from each fraction were run on NuPage 4%–12% Bis-Tris SDS-PAGE gel (Thermo Fisher Scientific, United States) and transferred onto nitrocellulose membrane using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, United States). The membrane was subsequently probed with His-Tag Antibody HRP Conjugate (Merck Millipore, United States) and detected with Clarity Western ECL Blotting Substrate (Bio-Rad, United States). Expression level of FGF2-G3 was determined by densitometric analysis of digital images using ImageJ software (National Institute of Health, United States).
To analyse purity of the large-scale purified media fraction eluted from Ni-NTA agarose column, 2 ug of protein was run on NuPage 4%–12% Bis-Tris SDS-PAGE gel (Thermo Fisher Scientific, United States) and stained with InstantBlue Coomassie Protein stain (Abcam, UK).
Bioactivity assay of FGF2-G3
Biological activity of the purified FGF2-G3 was assessed using Anguilla japonica (Japanese eel) pre-adipocytic cells, Aj1C-2x (Sugii et al., 2011). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 media (Thermo Fisher Scientific, United States) supplemented with reduced fetal bovine serum (2.5%) and 10 ng/mL of FGF2 at 27°C with 5% CO2 in a humidified incubator. Cell density and viability were determined using Vi-CELL XR Cell Viability Analyzer (Beckman Coulter, United States), according to manufacturer’s instructions. Each well contains fresh DMEM/F12 medium supplemented with 2.5% FBS and varying concentrations of purified recombinant FGF2-G3 or positive control. Commercial heat stable FGF2 (PHG0368, Thermo Fisher Scientific) was used as positive control. Aj1C-2x cells were seeded into 96-well plates at seeding density of 2 x 104 cells/well. After culturing for 3 days, cell viability was determined with CyQuant XTT cell viability assay (X12223, Thermo Fischer Scientific), according to manufacturer’s instructions. The absorbance reading of the media control was subtracted from the absorbance reading of each sample to determine the specific absorbance reading for each sample. Subsequently, the specific absorbance reading of each sample were normalized against the specific absorbance reading of the cells cultivated in basal media with 2.5% FBS (0 ng/mL FGF2) for Aj1C-2x cells to calculate relative absorbance fold changes. Cellular health was examined through inverted microscope under ×4 magnification (Nikon eclipse Ti with NIS-Elements AR 4.30.02 software, Nikon).
Biological activity of the purified FGF2-G3 was also further evaluated in a 6-well plate format. Aj1C-2x cells were seeded at a seeding density of 3 × 105 cells/well in a 6-well plate using DMEM/F12 medium (Thermo Fisher Scientific, United States) supplemented with 2.5% FBS and varying concentrations of purified recombinant FGF2-G3 or commercial heat-stable FGF2 (PHG0368, Thermo Fisher Scientific). After 4 days of culture, cellular health was examined through inverted microscope (Nikon eclipse Ti with NIS-Elements AR 4.30.02 software, Nikon). Subsequently, cells were dissociated using TrypLE™ Express Enzyme (Thermo Fisher Scientific, United States) and viable cell density were measured using a Vi-CELL XR Cell Viability Analyzer (Beckman Coulter, United States), following the manufacturer’s protocol.
Results and discussion
An enhancer propeptide to enhance secretion
To determine whether FGF-2 can be expressed and secreted out of L. lactis, we constructed an expression plasmid containing FGF2-G3, a modified and stable version that has nine amino acid mutations (Dvorak et al., 2018), with N-terminal fused to USP45 signal peptide. Our initial attempts to express the fusion protein resulted in low intracellular soluble yield and no protein was secreted. In previous study conducted by Lim et al. (2017), it was reported that addition of a short secretion propeptide 1 (PP1) to a USP45 fusion protein could significantly enhance protein secretion efficiency. Hence, we added PP1 between USP45 and FGF2-G3 as shown in Figure 1A.
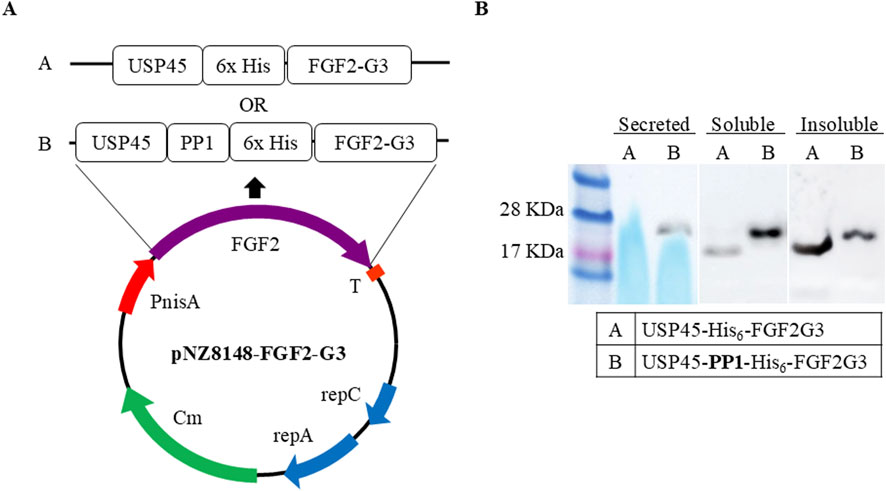
Figure 1. Expression vector constructs. (A) Schematic representation of expression vectors. FGF2-G3 with N-terminal fusion of signalling-secretion peptides (USP45 with and without PP1) and His6-tag in pNZ8148 vector. Protein sequence and insertion site available in Supplementary Figure S1 (B) Western blot of FGF2-G3 expression and secretion with and without propeptide (PP1) sequence in fusion plasmid.
L. lactis cells containing the two different recombinant plasmids (with and without PP1) were cultured in M17 media, supplemented with 0.5% glucose, and expression was induced at OD600nm 0.5 with nisin at final concentration of 10 ng/mL. FGF2-G3 was allowed to be expressed for 4 h at 30°C. The expression and secretion were assessed by Western blot. Appearance of bands corresponding to two different fusion FGF2-G3 constructs (with and without PP1) were observed. It was noted that addition of PP1 not only facilitates secretion of the fusion protein, but also increases the soluble yield (Figure 1B).
Optimisation of cultivation parameters
Next, optimization of culture and expression conditions were examined to increase protein secretion yield. Optimization of culture conditions began with expressing FGF2-G3 in varying M17 and glucose concentrations (M17 + 0.5% (w/v) glucose, 2xM17 + 0.5% (w/v) glucose, 2xM17 + 2% (w/v) glucose). 2xM17 + 2% (w/v) glucose medium proved to be the best for secretion of FGF2-G3 (Figure 2A).
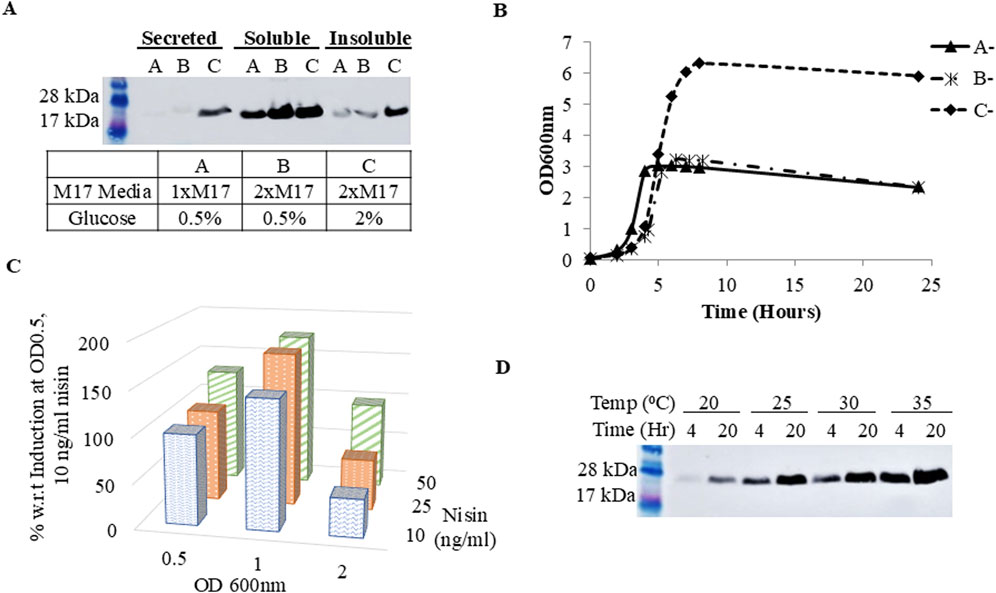
Figure 2. Expression of FGF2-G3 under different culture parameters. (A) Western blot of secreted, soluble and insoluble fraction of cell lysate. Cells were grown and induced in different media concentrations (A: M17 + 0.5% (w/v) glucose, (B) 2xM17 + 0.5% (w/v) glucose, (C) 2xM17 + 2% (w/v) glucose); (B) Growth curve of un-induced cells grown in different media formulations (A-: M17 + 0.5% (w/v) glucose, B-: 2xM17 + 0.5% (w/v) glucose, C-: 2xM17 + 2% (w/v) glucose) (C) Densitometry analysis of protein secretion yield under different induction OD (OD600nm 0.5, 1.0, 2.0) and nisin concentration (10, 25 or 50 ng/mL); (D) Western blot of secreted protein with post-induction temperature and expression duration at 20, 25, 30 or 35°C and 4 or 20 h, respectively.
Doubling the amount of M17 increased the soluble protein fractions. Looking at the growth curve of cells grown in different medias (Figure 2B), we hypothesize that doubling M17 not only provides additional nitrogen-based nutrients to support cell metabolism for a higher cell density culture, but also increases the buffering capacity against lactic acid produced during fermentation, due to increased amount of Disodium-β-glycerophosphate, a buffering agent found in M17 medium composition (Hayek et al., 2019; Terzaghi and Sandine, 1975; Zhang et al., 2009). These then worked in concert with increased glucose concentration, permitting L. lactis to extend its growth phase for higher FGF2-G3 production and, in particular, secretion titer. Further culture optimizations were performed using 2xM17 + 2% (w/v) glucose medium.
FGF2-G3 production also improved when the culture was induced at a higher cell density of OD600nm 1.0 (Figure 2C). This is anticipated since higher cell density would also mean more plasmids available for induction, which in-turn raises protein expression titer. With the increase in cell density, more nisin may be needed for complete induction and thus we proceeded to determine new optimal nisin concentration. Culture induction was performed when cells reached OD600nm 1.0 with nisin at final concentrations of 10, 25 or 50 ng/mL. The expression level of FGF2-G3 increased when nisin concentration was up from 10 to 25 ng/mL (Figure 2C), indicating correlation between cell density and nisin concentration needed for maximal induction. There was no further increase in expression level with 50 ng/mL nisin. Induction was also tested at OD600nm 2.0, but it did not lead to higher FGF2-G3 yield.
As the FGF2-G3 used in this study has been engineered for stability, we predicted that translation and folding within L. lactis is not limiting, rather the rate of translation/transcription can be further improved via fermentation optimisation. Temperature and expression duration are two important post-induction conditions for optimization as they balance between bacterial growth, functional protein yield and protein degradation. It has been suggested that lower temperature improves proper protein folding and solubility (Mierau et al., 2005; Sahdev et al., 2008; Yu et al., 2021), and prolonged expression should also be avoided as it leads to higher tendency for protein degradation caused by protein instability, lactate accumulation that disrupts energy metabolism for protein expression and/or triggering of cell stress response (Zhou et al., 2006). Interestingly, results for the present study showed an increase in secreted protein yield along with increasing temperature and expression duration, with 35°C and 20 h being the optimal temperature and harvest time-point (Figure 2D).
The highest expression and secretion of FGF2-G3 was achieved when cells were cultured in 2xM17 medium supplemented with 2% (w/v) glucose and induced at OD600nm 1.0 with 25 ng/mL nisin for 20 h at 35°C. The culture was scaled up from 10 mL to 250 mL for subsequent purification and bioactivity testing. The secreted fraction of the overexpressed FGF2-G3 was purified using immobilized metal affinity chromatography, which utilized Ni-NTA resin matrix, and the identity and purity were determined with Western blot and Coomassie staining (Figures 3A,B). The purified yield achieved herein is about 1.97 mg for 1 L of fermentation media, which is comparable to previous study by Rizal et al. (2024) where 2.6 mg/L of intracellular FGF2-G3 was produced from bioreactor fermentation. Based on an estimated requirement of 50 ng/mL recombinant FGF2-G3 in cultivated meat culture, secreted growth factors produced from 1 L fermentation provides enough growth factors for 39.4 L of cultivated meat culture. Secretion titers could be further raised with i) plasmid modifications, such as replacing promoters, ii) increasing membrane porosity by addition of chemicals, such as peptides and detergents, into culture media and iii) using bioreactor fermentation with controlled environment.
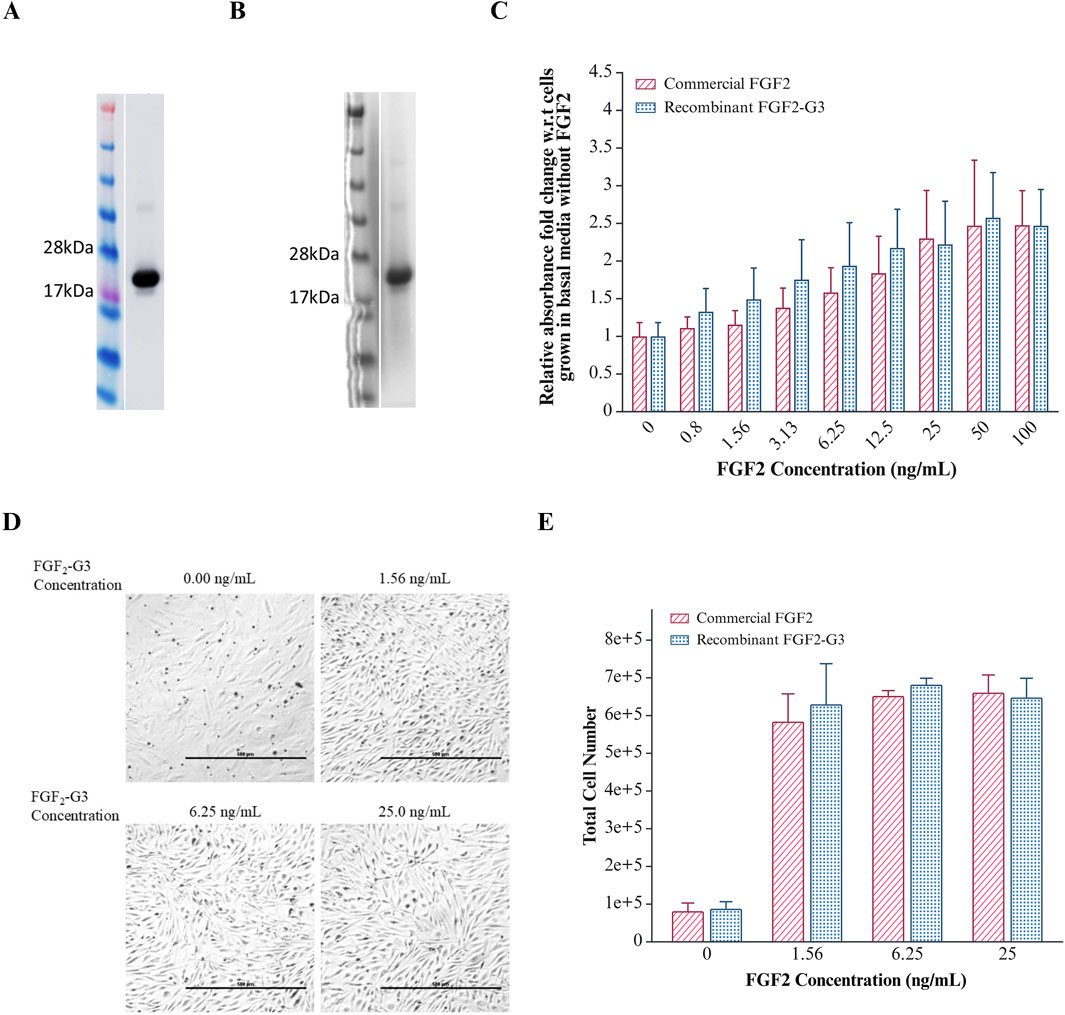
Figure 3. Purification and Effect of FGF2-G3 on proliferation of Anguilla japonica cells, Aj1C-2x. (A) Western blot analysis of purified FGF2-G3; (B) Coomassie stain of purified FGF2-G3; (C) XTT assay to determine cell proliferation effect of varying concentrations of commercial FGF2 and purified recombinant FGF2-G3 on Aj1C-2x cells. Absorbance readings were normalized to cells grown in medium without FGF2. Data plotted as average absorbance with error bars representing SD calculated for biological and technical triplicates. T-test analysis indicates no significant differences between commercial and recombinant FGF2 (P > 0.05); (D) Representative images of Anguilla japonica cells, Aj1C-2x, observed under ×4 magnification, grown in fresh DMEM/F12 medium supplemented with 2.5% FBS with varying concentrations of purified recombinant FGF2-G3; (E) Total cell number measured after 4 days of culture using Vi-CELL XR Cell Viability Analyzer. Data are presented as total cell counts, with error bars representing the standard deviation from biological duplicates.
Biological activity assessment of the purified FGF2
To assess the biological activity of the purified FGF2-G3, growth stimulation on Anguilla japonica (Japanese eel) pre-adipocytic cells, Aj1C-2x, was measured using XTT assay and compared against a commercial heat stable FGF2 (positive control). As shown in Figure 3C, an increase in metabolic activity was detected when cells were cultured with the purified FGF2-G3, indicating its ability to promote cell proliferation and exhibited comparable bioactivity profile to the commercial FGF2. This is further supported by an increase in cell density observed through microscopy and cell count using a cell viability analyzer (Figures 3D,E). The positive result suggests that our secreted FGF2-G3 can be used to stimulate fish stem cells for cultivated fish meat and cultivating adipocytes (fats) for enhancement of meat texture and flavour. Future bioactivity testing can be performed on mammalian cell lines, such as bovine, porcine and chicken muscle cells, to widen its application range in cultivated meat.
Conclusion
In summary, this study demonstrated that functional FGF2 can be expressed and secreted using the L. lactis expression system. We employed a multi-modal optimization strategy that included a secretion-enhancing propeptide and further cultivation optimizations. Specifically, we utilized a nutrient-rich 2xM17 medium containing 2% (w/v) glucose. The ability of the L. lactis-produced FGF2-G3 to stimulate growth of Japanese eel cells suggests that L. lactis could be used as a safer alternative for production of growth factors for cultured meat development. The secretion of recombinant proteins into culture medium by L. lactis simplifies the production process and presents opportunities to lower production cost for cultured meat. Future efforts could be directed at expressing other growth factors, such as EGF, IGF and TGFβ1, in L. lactis and scaling-up in bioreactors for precision fermentation of recombinant growth factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
PH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – Original draft, Project administration. YC: Formal Analysis, Investigation, Methodology, Validation, Writing – Review and editing. JQ: Formal Analysis, Validation, Writing – Review and editing. SN: Writing – Review and editing. FW: Writing – Review and editing. DO: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – Review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Agency for Science, Technology and Research (A∗STAR), Singapore, and was funded by Singapore Food Story (SFS) R&D Programme (H20H8a0003, W23W2D0009 and NRF-SFSRND2FF-0003).
Acknowledgments
We would like to thank Shigeki Sugii and Lamony Chew for providing us with the Anguilla japonica pre-adipocytic cell lines and Zarra Iwana Huang for her assistance in culturing work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1560426/full#supplementary-material
Abbreviations
EGF, Epidermal Growth Factor; FBS, Foetal Bovine Serum; FGF2, Fibroblast Growth Factor 2; GRAS, Generally Recognized As Safe; IGF, Insulin-like Growth Factor; NICE, Nisin Controlled Gene Expression System; TGF-β1, Transforming Growth Factor β1.
References
Ahmad, S. S., Chun, H. J., Ahmad, K., Shaikh, S., Lim, J. H., Ali, S., et al. (2023). The roles of growth factors and hormones in the regulation of muscle satellite cells for cultured meat production. J. Anim. Sci. Technol. 65 (1), 16–31. doi:10.5187/jast.2022.e114
Badenes, S. M., Fernandes, T. G., Cordeiro, C. S., Boucher, S., Kuninger, D., Vemuri, M. C., et al. (2016). Defined essential 8 medium and vitronectin efficiently support scalable xeno-free expansion of human induced pluripotent stem cells in stirred microcarrier culture systems. PLoS One 11 (3), e0151264. doi:10.1371/journal.pone.0151264
Bahey-El-Din, M., Gahan, C. G., and Griffin, B. T. (2010). Lactococcus lactis as a cell factory for delivery of therapeutic proteins. Curr. Gene Ther. 10 (1), 34–45. doi:10.2174/156652310790945557
Baneyx, F., and Mujacic, M. (2004). Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22 (11), 1399–1408. doi:10.1038/nbt1029
Bikfalvi, A., Klein, S., Pintucci, G., and Rifkin, D. B. (1997). Biological roles of fibroblast growth factor-2. Endocr. Rev. 18 (1), 26–45. doi:10.1210/edrv.18.1.0292
Campbell, B. M., Beare, D. J., Bennett, E. M., Hall-Spencer, J. M., Ingram, J. S. I., Jaramillo, F., et al. (2017). Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 22 (4), art8. doi:10.5751/es-09595-220408
Cao, W. Y., Dong, M., Hu, Z. Y., Wu, J., Li, Y. C., and Xu, H. D. (2020). Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21 reduced body weight of Db/Db mice through the activity of brown adipose tissue. Benef. Microbes 11 (1), 67–78. doi:10.3920/BM2019.0093
Das, M., Rumsey, J. W., Bhargava, N., Gregory, C., Reidel, L., Kang, J. F., et al. (2009). Developing a novel serum-free cell culture model of skeletal muscle differentiation by systematically studying the role of different growth factors in myotube formation. Vitro Cell Dev. Biol. Anim. 45 (7), 378–387. doi:10.1007/s11626-009-9192-7
de Ruyter, P. G., Kuipers, O. P., and de Vos, W. M. (1996). Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62 (10), 3662–3667. doi:10.1128/aem.62.10.3662-3667.1996
Dvorak, P., Bednar, D., Vanacek, P., Balek, L., Eiselleova, L., Stepankova, V., et al. (2018). Computer-assisted engineering of hyperstable fibroblast growth factor 2. Biotechnol. Bioeng. 115 (4), 850–862. doi:10.1002/bit.26531
Frelet-Barrand, A. (2022). Lactococcus lactis, an attractive cell factory for the expression of functional membrane proteins. Biomolecules 12 (2), 180. doi:10.3390/biom12020180
Gao, G., Qiao, J. J., Yang, C. H., Jiang, D. Z., Li, R. Q., Su, J. J., et al. (2012). Functional expression of mouse insulin-like growth factor-I with food-grade vector in Lactococcus lactis NZ9000. Lett. Appl. Microbiol. 54 (5), 404–409. doi:10.1111/j.1472-765X.2012.03222.x
Garcia-Fruitos, E. (2012). Lactic acid bacteria: a promising alternative for recombinant protein production. Microb. Cell Fact. 11, 157. doi:10.1186/1475-2859-11-157
Godfray, H. C. J., Aveyard, P., Garnett, T., Hall, J. W., Key, T. J., Lorimer, J., et al. (2018). Meat consumption, health, and the environment. Science 361 (6399), eaam5324. doi:10.1126/science.aam5324
Hayek, S. A., Gyawali, R., Aljaloud, S. O., Krastanov, A., and Ibrahim, S. A. (2019). Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 86 (4), 490–502. doi:10.1017/S002202991900075X
Huynh, E., and Li, J. (2015). Generation of Lactococcus lactis capable of coexpressing epidermal growth factor and trefoil factor to enhance in vitro wound healing. Appl. Microbiol. Biotechnol. 99 (11), 4667–4677. doi:10.1007/s00253-015-6542-0
Jochems, C. E., van der Valk, J. B., Stafleu, F. R., and Baumans, V. (2002). The use of fetal bovine serum: ethical or scientific problem? Altern. Lab. Anim. 30 (2), 219–227. doi:10.1177/026119290203000208
Kadim, I. T., Mahgoub, O., Baqir, S., Faye, B., and Purchas, R. (2015). Cultured meat from muscle stem cells: a review of challenges and prospects. J. Integr. Agric. 14 (2), 222–233. doi:10.1016/S2095-3119(14)60881-9
Kaur, J., Kumar, A., and Kaur, J. (2018). Strategies for optimization of heterologous protein expression in E. coli: roadblocks and reinforcements. Int. J. Biol. Macromol. 106, 803–822. doi:10.1016/j.ijbiomac.2017.08.080
Kumari, A., Catanzaro, R., and Marotta, F. (2011). Clinical importance of lactic acid bacteria: a short review. Acta Biomed. 82 (3), 177–180. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/22783712.
Lee, D. Y., Lee, S. Y., Yun, S. H., Jeong, J. W., Kim, J. H., Kim, H. W., et al. (2022). Review of the current research on fetal bovine serum and the development of cultured meat. Food Sci. Anim. Resour. 42 (5), 775–799. doi:10.5851/kosfa.2022.e46
Lim, P. Y., Tan, L. L., Ow, D. S., and Wong, F. T. (2017). A propeptide toolbox for secretion optimization of flavobacterium meningosepticum endopeptidase in Lactococcus lactis. Microb. Cell Fact. 16 (1), 221. doi:10.1186/s12934-017-0836-0
Lynch, J., and Pierrehumbert, R. (2019). Climate impacts of cultured meat and beef cattle. Front. Sustain Food Syst. 3, 5. doi:10.3389/fsufs.2019.00005
Messmer, T., Klevernic, I., Furquim, C., Ovchinnikova, E., Dogan, A., Cruz, H., et al. (2022). A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food 3 (1), 74–85. doi:10.1038/s43016-021-00419-1
Mierau, I., and Kleerebezem, M. (2005). 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68 (6), 705–717. doi:10.1007/s00253-005-0107-6
Mierau, I., Leij, P., van Swam, I., Blommestein, B., Floris, E., Mond, J., et al. (2005a). Industrial-scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: the case of lysostaphin. Microb. Cell Fact. 4, 15. doi:10.1186/1475-2859-4-15
Mierau, I., Olieman, K., Mond, J., and Smid, E. J. (2005b). Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb. Cell Fact. 4, 16. doi:10.1186/1475-2859-4-16
Morello, E., Bermudez-Humaran, L. G., Llull, D., Sole, V., Miraglio, N., Langella, P., et al. (2008). Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J. Mol. Microbiol. Biotechnol. 14 (1-3), 48–58. doi:10.1159/000106082
Park, Y. H., Gong, S. P., Kim, H. Y., Kim, G. A., Choi, J. H., Ahn, J. Y., et al. (2013). Development of a serum-free defined system employing growth factors for preantral follicle culture. Mol. Reprod. Dev. 80 (9), 725–733. doi:10.1002/mrd.22204
Petsch, D., and Anspach, F. B. (2000). Endotoxin removal from protein solutions. J. Biotechnol. 76 (2-3), 97–119. doi:10.1016/s0168-1656(99)00185-6
Post, M. J., Levenberg, S., Kaplan, D. L., Genovese, N., Fu, J., Bryant, C. J., et al. (2020). Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 1 (7), 403–415. doi:10.1038/s43016-020-0112-z
Reiss, J., Robertson, S., and Suzuki, M. (2021). Cell sources for cultivated meat: applications and considerations throughout the production workflow. Int. J. Mol. Sci. 22 (14), 7513. doi:10.3390/ijms22147513
Rizal, J., Mainali, P., Quek, J. P., Lee Ling, T., Bi, J., Chan, A. J., et al. (2024). Valorisation of spent cultivated meat media for recombinant FGF2 production in GRAS Lactococcus lactis. bioRxiv 2024. doi:10.1101/2024.08.01.606190
Sahdev, S., Khattar, S. K., and Saini, K. S. (2008). Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Mol. Cell Biochem. 307 (1-2), 249–264. doi:10.1007/s11010-007-9603-6
Santos, A. C. A., Camarena, D. E. M., Roncoli Reigado, G., Chambergo, F. S., Nunes, V. A., Trindade, M. A., et al. (2023). Tissue engineering challenges for cultivated meat to meet the real demand of a global market. Int. J. Mol. Sci. 24 (7), 6033. doi:10.3390/ijms24076033
Skrivergaard, S., Young, J. F., Sahebekhtiari, N., Semper, C., Venkatesan, M., Savchenko, A., et al. (2023). A simple and robust serum-free media for the proliferation of muscle cells. Food Res. Int. 172, 113194. doi:10.1016/j.foodres.2023.113194
Song, A. A., In, L. L. A., Lim, S. H. E., and Rahim, R. A. (2017). A review on lactococcus lactis: from food to factory. Microb. Cell Fact. 16 (1), 55. doi:10.1186/s12934-017-0669-x
Stephens, N., Di Silvio, L., Dunsford, I., Ellis, M., Glencross, A., and Sexton, A. (2018). Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. and Technol. 78, 155–166. doi:10.1016/j.tifs.2018.04.010
Stout, A. J., Mirliani, A. B., Rittenberg, M. L., Shub, M., White, E. C., Yuen, J. S. K., et al. (2022). Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 5 (1), 466. doi:10.1038/s42003-022-03423-8
Sugii, S., Kida, Y., Berggren, W. T., and Evans, R. M. (2011). Feeder-dependent and feeder-independent iPS cell derivation from human and mouse adipose stem cells. Nat. Protoc. 6 (3), 346–358. doi:10.1038/nprot.2010.199
Terzaghi, B. E., and Sandine, W. E. (1975). Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29 (6), 807–813. doi:10.1128/am.29.6.807-813.1975
Thomas, J. G., and Baneyx, F. (1996). Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing Heat-shock proteins. J. Biol. Chem. 271 (19), 11141–11147. doi:10.1074/jbc.271.19.11141
Venkatesan, M., Semper, C., Skrivergaard, S., Di Leo, R., Mesa, N., Rasmussen, M. K., et al. (2022). Recombinant production of growth factors for application in cell culture. iScience 25 (10), 105054. doi:10.1016/j.isci.2022.105054
Yu, B., Sun, W., Huang, Z., Sun, G., Li, L., Gu, J., et al. (2021). Large-scale preparation of highly stable recombinant human acidic fibroblast growth factor in Escherichia coli BL21(DE3) plysS strain. Front. Bioeng. Biotechnol. 9, 641505. doi:10.3389/fbioe.2021.641505
Yu, I. S., Choi, J., Kim, M. K., and Kim, M. J. (2023). The comparison of commercial serum-free media for hanwoo satellite cell proliferation and the role of fibroblast growth factor 2. Food Sci. Anim. Resour. 43 (6), 1017–1030. doi:10.5851/kosfa.2023.e68
Yun, Y. R., Won, J. E., Jeon, E., Lee, S., Kang, W., Jo, H., et al. (2010). Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 218142. doi:10.4061/2010/218142
Zhang, G., Mills, D. A., and Block, D. E. (2009). Development of chemically defined media supporting high-cell-density growth of lactococci, enterococci, and streptococci. Appl. Environ. Microbiol. 75 (4), 1080–1087. doi:10.1128/AEM.01416-08
Zhou, X. X., Li, W. F., Ma, G. X., and Pan, Y. J. (2006). The nisin-controlled gene expression system: construction, application and improvements. Biotechnol. Adv. 24 (3), 285–295. doi:10.1016/j.biotechadv.2005.11.001
Keywords: cultivated meat, serum-free media, fibroblast growth factor 2, FGF2, Lactococcus lactis, recombinant protein expression, precision fermentation
Citation: Ho PL, Chua YF, Quek JP, Ng SK, Wong FT and Ow DS-W (2025) Functional expression and secretion of basic fibroblast growth factor in Lactococcus lactis. Front. Bioeng. Biotechnol. 13:1560426. doi: 10.3389/fbioe.2025.1560426
Received: 14 January 2025; Accepted: 11 July 2025;
Published: 24 July 2025.
Edited by:
Artur Ribeiro, University of Minho, PortugalCopyright © 2025 Ho, Chua, Quek, Ng, Wong and Ow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dave Siak-Wei Ow, ZGF2ZV9vd0BhLXN0YXIuZWR1LnNnZGF2ZV9vd0BidGkuYS1zdGFyLmVkdS5zZw==; Fong Tian Wong, d29uZ2Z0QGltY2IuYS1zdGFyLmVkdS5zZw==
 Pooi Leng Ho
Pooi Leng Ho Yu Feng Chua
Yu Feng Chua Jun Ping Quek
Jun Ping Quek Say Kong Ng
Say Kong Ng Fong Tian Wong
Fong Tian Wong Dave Siak-Wei Ow
Dave Siak-Wei Ow