- 1Department Bio-Adaptive production, Fraunhofer Institute for Production Technology (FHG), Aachen, Germany
- 2WZL RWTH Aachen, Chair of Intelligence in Quality Sensing, Laboratory for Machine Tools and Production Engineering, RWTH Aachen University, Aachen, Germany
Mesenchymal stem/stromal cells (MSCs) have been identified as a promising therapeutic option for osteoarthritis, graft vs. host disease and cardiovascular diseases, among others. For widespread application of these therapies, robust and scaled manufacturing processes are required that reliably yield high amounts of high quality MSCs. One of the primary challenges in MSC manufacturing is achieving robustness, due to the high donor-to-donor and batch-to-batch variability seen in MSC manufacturing. To achieve more consistent manufacturing, standardization of the manufacturing process and analytical methods to determine cell quality and control process parameters will be needed. Traditionally, MSCs are cultivated in two dimensional (2D) systems, such as flasks or plates. However, these systems are limited in their scalability. To enhance volumetric productivity, upscaling may be achieved using agitated bioreactors where the MSCs are grown on microcarriers or other types of scaffolds. In this article, we have reviewed existing publications on the manufacturing of MSCs in agitated bioreactor systems regarding the process conditions used and the quality parameters measured to define more clearly the most relevant cell quality and process parameters. Key cell quality parameters measured are cell number and viability, immunophenotype and differentiation potential, while key process parameters include the cultivation system (cell source, bioreactor type, media composition), physiochemical properties of the media such as pH and dissolved oxygen (DO), as well as nutrient supply. Defining these parameters more clearly will support the development of robust MSC manufacturing processes at scale using improved process control and facilitate the widespread clinical application of MSC-based cell therapies.
Introduction
The lack of curative treatments for widespread diseases such as osteoarthritis, cardiovascular diseases, and autoimmune deficiencies has led to the rise of Advanced Therapy Medicinal Products (ATMPs). Among these, mesenchymal stem/stromal cells (MSCs) are considered a promising treatment for many therapeutic applications, such as osteoarthritis, graft versus host disease (GvHD), and cardiovascular diseases (García-Gómez et al., 2010; Rodríguez-Fuentes et al., 2021). Globally, there are twelve approved MSC cell therapies with nine originating from Asia. In Europe, two products were approved by the European Medicines Agency (EMA): Holoclar, which facilitates corneal epithelium repair (European Medicines Agency, 2021), and Alofisel, used for treating complex fistulas in Crohn’s disease (European Medicines Agency (EMA)), whose authorisation was withdrawn by request of the marketing-authorisation holder. In the United States, one therapy has been approved by the Food and Drug Administration (FDA): Remestemcel-L for treating GvHD (RYONCIL, 2025). As more MSC therapies advance through clinical development, the demand for MSC manufacturing is expected to rise significantly. MSCs are multipotent, primary cells, which can be extracted from different tissue sources, such as bone marrow, umbilical cord, or adipose tissue, or differentiated from induced pluripotent stem cells (iPSCs) (Wruck et al., 2021; Pittenger et al., 1999).
For widespread application of MSCs as a therapy, the manufacturing must become more industrialized and overcome several critical challenges (de Almeida Fuzeta et al., 2020; García-Fernández et al., 2020). One limitation is the prevalent use of two-dimensional cultivation systems for MSC expansion, which restricts scalability. Transitioning to three-dimensional bioreactor systems can enhance productivity and allow for greater scalability (Tsai and Pacak, 2021). Maintaining quality during this scale-up process is essential to ensure therapeutic efficacy. Secondly, as MSCs are extracted from varying tissue sources, they are subject to substantial biological variability stemming from the tissue source and the donor (Costa et al., 2021; Győrgy et al., 2019; Kanawa et al., 2013; Mareschi et al., 2006; Pachón-Peña et al., 2016; Sammour et al., 2016). This has led to an effort to better characterize the extraction and cultivation process of MSCs. However, further standardization is needed for the cultivation process and the process metrology involved as MSC heterogeneity remains a challenge for clinical translation (Costa et al., 2021; Nikolits et al., 2021). Thirdly, this variability in MSC growth and phenotype necessitates a well characterized and standardized method for quality control (Silva Couto et al., 2020). The International Society for Cell and Gene Therapy (ISCT) defined minimal criteria for MSCs in 2009 as being plastic adherent, must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface markers and must differentiate to osteoblasts, adipocytes and chondroblasts in vitro (Dominici et al., 2006). This definition was recently updated as part of an expert survey (Renesme et al., 2025).
The regulatory pathway for the approval of an MSC product differs by region, Europe, US, Canada, Asia and regulatory authority (López-Beas et al., 2020). To engineer a pharmaceutical processes with a primary focus on product quality, the International Council for Harmonization (ICH) introduced the Quality-by-Design approach in 2009 (EMA, 2009). The guideline Q8 details a scientific and risk-based approach, where the desired product quality is detailed early in the product development and the focus of process development is set on meeting this defined quality efficiently. In a first step, a Quality Target Product Profile (QTPP) is defined, which is used as a basis to specify critical quality attributes (CQA). CQAs are key product parameters which must be met to ensure the drug product’s safety and efficacy. Thirdly, existing process understanding is utilized, and the production process is systematically evaluated to establish process controls ensuring CQAs are met throughout. QbD has been applied to the manufacturing of MSCs to identify the QTPP and define critical process parameters (CPP) (Sharma et al., 2014; Lipsitz et al., 2016; Maillot et al., 2021; Guadix et al., 2019). These studies suggest a QTPP of dosage (cell number and viability), Potency (Identity, differentiation potential and in vivo effect), and product quality (genetic stability, purity). The processes considered for these studies include the cell extraction and the two-dimensional expansion of MSCs, and give a list of CPPs for these unit operations (Maillot et al., 2021). As widespread application of MSCs will likely necessitate the expansion of MSCs in bioreactor systems, this review focuses on evaluating the expansion of MSCs in bioreactors in terms of the quality attributes and process parameters considered in current process development.
Systematic literature review and analysis
The literature search for this review was carried out according to the PRISMA methodology (PRISMA statement). The search string “(stirred OR agitated) AND (bioreactor OR expansion) AND mesenchymal AND (stem OR stromal) AND cell” was entered in the web of science search engine. The results were filtered for original research articles, reviews and meeting abstracts were excluded (I). Only articles using human MSCs were considered, animal based MSCs, co-cultivation with other cell types and tissue culture are not considered (II). Only articles conducting experimental work were considered; simulations and models using data published elsewhere were excluded to avoid redundancies (III). A list of the articles and exclusion reasoning is given in the Supplementary S1 along a PRISMA chart in S2. A total of 142 articles were evaluated, with 76 articles being included in the following analysis. The list of quality attributes (S3) and process parameters (S4), along with their frequency and weightings in terms of implementation of costs and criticality to quality is given in the supplementary. A detailed description of the Pareto Analysis performed is given as well (S5).
Quality attributes measured in bioreactor-based expansion of MSCs
Of the 79 quality attributes recorded, 27 refer to the immunophenotype of the MSCs, while others characterise cell growth or health of the MSCs. The quality attributes sorted by number of occurrences and weighted according to S5 are given in Figure 1A.
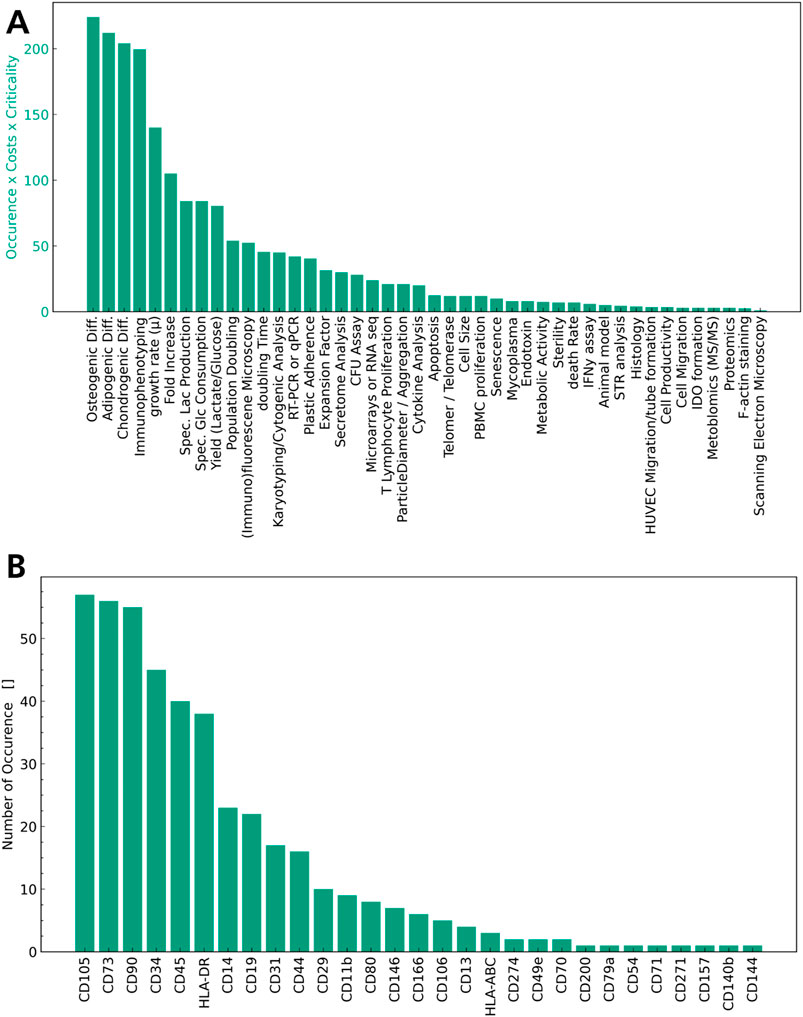
Figure 1. Quality attributes sorted by number of occurrences. In (A) All attributes characterising the immunophenotype are accumulated into one QA. (B) Surface markers measured as QA of MSCs by number of occurrences.
Cell Count and viability are measured ubiquitously as quality attributes in MSC expansion in bioreactors in these articles (28–103) as illustrated in Figure 1A. Cell count and viability characterise the dosage of the final product, which is a well-established part of the QTPP for MSCs and across all cell-based therapies. It is also highlighted as a central aspect in the EMA Guideline on quality, non-clinical and clinical requirements for investigational ATMPs (European Medicines A gency (EMA)). Figure 1A illustrates the immunophenotype of the MSCs (Carmelo et al., 2015; Chen et al., 2015; Cunha et al., 2017; de Da Sá Silva et al., 2020; Dos Santos et al., 2014; Eibes et al., 2010; Gadelorge et al., 2018; Gao et al., 2023; Gonzalez Gil et al., 2020; Jorgenson et al., 2018; Kehoe et al., 2012; Kurogi et al., 2021; Kurogi et al., 2022; Leber et al., 2017; López-Fernández et al., 2024a; López-Fernández et al., 2024b; Mizukami et al., 2016; Moreira et al., 2020; Nienow et al., 2016; Noronha et al., 2021; Osiecki et al., 2015; Petry et al., 2016; Rafiq et al., 2016; Rafiq et al., 2013; Santos et al., 2011; Schirmai et al., 2014; Sion et al., 2021; Sousa et al., 2015; Sunil et al., 2013; Timmins et al., 2012; Tozetti et al., 2017; Yan et al., 2020; Wang et al., 2023; Yu et al., 2009; Yuan et al., 2014; Zhang et al., 2022; Zhang et al., 2023) as well as the differentiation capability into one or all three mesodermal lines (Carmelo et al., 2015; Chen et al., 2015; Cunha et al., 2017; de Da Sá Silva et al., 2020; Dos Santos et al., 2014; Dosta et al., 2020; Eibes et al., 2010; Gadelorge et al., 2018; Gao et al., 2023; Gonzalez Gil et al., 2020; Jorgenson et al., 2018; Lawson et al., 2017; Kurogi et al., 2021; Lam et al., 2017; Leber et al., 2017; López-Fernández et al., 2024a; López-Fernández et al., 2024b; Mizukami et al., 2016; Moreira et al., 2020; Nienow et al., 2016; Noronha et al., 2021; Osiecki et al., 2015; Petry et al., 2016; Rafiq et al., 2016; Rafiq et al., 2013; Rotondi et al., 2021; Santos et al., 2011; Sart et al., 2013; Sart et al., 2009; Sart et al., 2010; Schirmai et al., 2014; Sion et al., 2020; Sousa et al., 2015; Sunil et al., 2013; Timmins et al., 2012; Tozetti et al., 2017; Yan et al., 2020; Wang et al., 2023; Yu et al., 2009; Yuan et al., 2014; Zhang et al., 2022; Zhou et al., 2013) are measured frequently, concurring with the minimal criteria defined by the ISCT. Apart from phenotyping and differentiation potential, indices of growth, cell age and metabolic activity, such as the growth rate, fold increase, metabolic consumption and production rates, and yields are also used as seen in Figure 1A (Carmelo et al., 2015; Chen et al., 2015; Cunha et al., 2017; de Da Sá Silva et al., 2020; Dos Santos et al., 2014; Dosta et al., 2020; Eibes et al., 2010; Gao et al., 2023; Gonzalez Gil et al., 2020; Grein et al., 2016; Jorgenson et al., 2018; Lawson et al., 2017; Kehoe et al., 2012; Lam et al., 2017; Leber et al., 2017; López-Fernández et al., 2024a; López-Fernández et al., 2024b; Mizukami et al., 2016; Moreira et al., 2020; Nienow et al., 2016; Noronha et al., 2021; Osiecki et al., 2015; Petry et al., 2016; Rafiq et al., 2016; Rafiq et al., 2013; Santos et al., 2011; Sart et al., 2009; Sart et al., 2010; Schirmai et al., 2014; Sion et al., 2020; Sion et al., 2021; Sousa et al., 2015; Sunil et al., 2013; Tozetti et al., 2017; Yan et al., 2020; Wang et al., 2023; Yuan et al., 2014; Zhang et al., 2022; Zhou et al., 2013). A wide range of in vitro assays targeting a specific function of MSCs, such as the T Lymphocyte proliferation assay (Dosta et al., 2020; Elseberg et al., 2012; Gadelorge et al., 2018; Lawson et al., 2017; Kurogi et al., 2021; Mizukami et al., 2016; Noronha et al., 2021; Tozetti et al., 2017) to assess modulation of the immune system or apoptosis (Cunha et al., 2017; Gonzalez Gil et al., 2020; Schirmai et al., 2014; Sion et al., 2020; Sousa et al., 2015; Timmins et al., 2012) to assess cell health are considered, but not used frequently overall. Interestingly, though plastic adherence is a characteristic of MSCs according to the ISCT criteria, it is not commonly mentioned as a quality attribute for MSCs in the articles surveyed (Cunha et al., 2017; López-Fernández et al., 2024b; Nienow et al., 2016; Noronha et al., 2021; Rafiq et al., 2013). As the MSCs in these studies are typically grown on microcarriers, many of them exhibit plastic adherence without it being expressly stated as an attribute of their identity.
When considering identity and potency in the QTPP, the most prevalent methods to determine quality attributes are identified based on the literature analysis in Figure 1A. In terms of identity, immunophenotyping is used widely to characterise the MSCs and the absence of any undesirable cells. In Figure 1B the different surface markers measured across the articles reviewed are sorted by number of occurrence. When comparing these to the minimal criteria established by the ISCT, the positive markers (CD105, CD90, CD73) are determined in every study using immunophenotyping, while HLA-DR, CD45 and CD34 are the most prevalent negative markers used, with a number of less frequently used markers also shown in Figure 1B. These findings illustrate the degree of standardisation in measurement of positive markers with a lack of standardisation for negative surface markers. This limitation was addressed by the ISCT recently, suggesting to amend the definition to focus on the positive MSC markers (CD105, CD90, CD73) and allow for variation in the negative markers based on the cell source and application of MSCs, though CD45 was highlighted as the negative MSC marker to be used ubiquitously (Renesme et al., 2025). Overall immunophenotyping is a frequently used and suitable method to determine identity of the cells in an MSC product, and some degree of standardisation is observed.
A key attribute in terms of potency of MSCs is their multipotency and ability to differentiate into osteoblasts, chondrocytes, and adipocytes. This mechanism of action is intended to be used in treating osteoarthritis and other tissue defects and is determined by performing trilinear differentiation of MSCs in vitro post manufacturing. While this quality attribute characterises the differentiation capabilities, its application as a potency assay is limited. The differentiation capacity of MSCs is not the sole or main mode of action in many therapeutic MSC applications, whereas the immunomodulatory properties, the secretion of bioactive factors or any combination of the three may be utilised. To this extent the analysis of other attributes, such as characterisation of the transcriptome, immunomodulatory assays, DNA methylation profiling or secretome profiling have been suggested to additionally characterise MSCs (Kadri et al., 2023; Krampera et al., 2021; Wong et al., 2021). This variability is evident in the literature analysis performed as seen in Figure 1A, as a number of assays are listed such as the T lymphocyte or PBMC proliferation assay to assess immunomodulatory properties, secretome analysis to assess secretion of bioactive factors or Metabolomics, Proteomics or gene expression profiling to characterise cell function in depth. To this date, the discussion around increased standardisation of potency assays in the therapeutic applications of MSCs has not been conclusive (Renesme et al., 2025) concurring with the study conducted in Figure 1A. The potency of an MSC product in contrast to identity and safety is inherently difficult to standardise across therapeutic applications, as it ideally should align with the main mode of action of the MSCs. MSC therapeutics are explored across a wide range of indications, and this should be reflected in their respective potency assays. While the literature review conducted suggests differentiation capacity of MSCs as a CQA, differentiation capacity of MSCs alone is insufficient to elucidate potency of the MSC product and additional assays are needed.
With regard to safety, only a limited number of studies report on quality attributes relating to the genetic stability, such as karyotyping or the telomerase activity of the MSCs, with even less studies reporting on the sterility, endotoxin or mycoplasma analysis in the MSC product. Considering the regulatory frameworks, these parameters are crucial to the QTPP of MSC products. The absence of microbial contaminations must be determined for any ATMP. Similarly, the genetic stability and tumorgenicity are important risk factors in the application of MSCs and must be considered as CQAs (López-Beas et al., 2020; Maillot et al., 2021; European Medicines A gency (EMA)). For the EMA, assays to verify the absence of contaminants as well as genetic stability and tumorigenicity are expressly stated as part of the guidelines (European Medicines A gency (EMA)). This discrepancy in the literature review conducted is likely due to the articles reviewed focusing on earlier product and process development, where safety quality attributes are not ubiquitously measured yet.
Process parameters measured in bioreactor based expansion of MSCs
In addition to quality parameters, process parameters must be evaluated to successfully establish a process robustly yielding high-quality MSCs for cell therapy. In the literature analysis, 41 process parameters were identified overall. Ten of those refer to the origin of the MSCs used for the experiment, with the most frequently used cells being bone marrow derived MSCs, umbilical cord derived MSCs, and adipose derived MSCs (S3). Five of the process parameters listed refer to the type of bioreactor used for the experimental work, with spinner flasks and stirred tank bioreactors used most (S3). In Figure 2A, these parameters are summarised as “Cell Type” and “Bioreactor Type” respectively to facilitate visualisation, and the process parameters are sorted by the number of occurrences in the articles. The parameter “media formulation” refers not only to the type of basal media used, but also to the presence or absence of supplements, such as FBS or platelet lysate. As media formulation has been extensively reviewed elsewhere (Gottipamula et al., 2013; Bui et al., 2021; Doron and Temenoff, 2021), this is not a focus of this review. Similarly, “Microcarrier” summarises all types of microcarriers used in the different articles, as these have been evaluated previously (Leber et al., 2017; Rafiq et al., 2016). The parameter “amino acid” refers to any amino acid measured other than glutamine or glutamate, as these were seen to be measured individually.
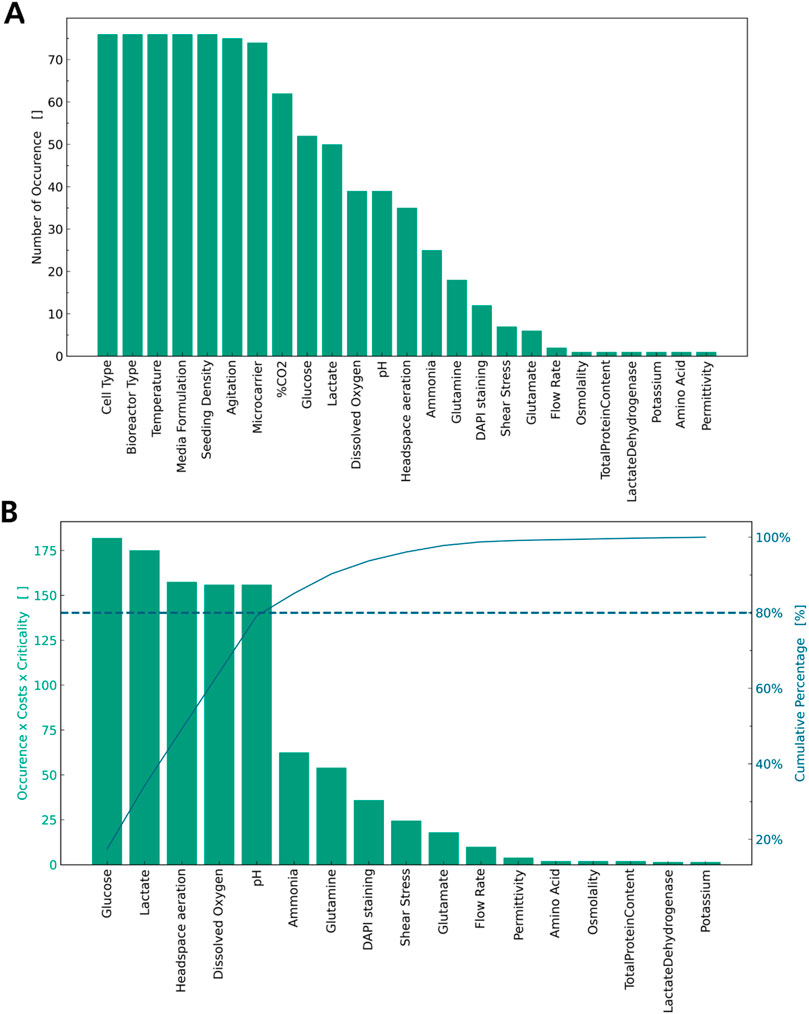
Figure 2. List of process parameters by number of occurrences. (A) Cell Type, Bioreactor Type, Media Formulation are accumulated. (B) Highly reoccurring parameters are dropped and a weighted Pareto Analysis is applied.
Figure 2A illustrates, which parameters are ubiquitously controlled for all experiments: agitation rate, bioreactor type, cell type, media formulation, microcarrier type, seeding density, and temperature are controlled in every article analysed and should thus be considered CPPs (28–78, 78, 79). Additionally, percentage of CO2 in the gaseous mixture is highly mentioned and should be considered a CPP. These parameters must be controlled for every cultivation or experiment to allow for a minimum of standardisation of the operation procedure and repeatability of the experiments. Thus, more consideration must be given to those parameters less frequently measured or controlled. Several process parameters determining the nutrient supply, such as glucose and amino acids, and the presence of inhibitory metabolites, such as lactate and ammonia, were identified in the literature review as seen in Figure 2A (Carmelo et al., 2015; Chen et al., 2015; Cunha et al., 2017; de Da Sá Silva et al., 2020; Dos Santos et al., 2014; Eibes et al., 2010; Elseberg et al., 2012; Gadelorge et al., 2018; Gao et al., 2023; Gonzalez Gil et al., 2020; Grein et al., 2016; Jorgenson et al., 2018; Lawson et al., 2017; Kehoe et al., 2012; Lam et al., 2017; Leber et al., 2017; López-Fernández et al., 2024a; López-Fernández et al., 2024b; Mizukami et al., 2016; Moreira et al., 2020; Noronha et al., 2021; Osiecki et al., 2015; Petry et al., 2016; Rafiq et al., 2016; Rafiq et al., 2013; Rotondi et al., 2021; Santos et al., 2011; Sart et al., 2010; Schirmai et al., 2014; Sion et al., 2020; Sousa et al., 2015; Sunil et al., 2013; Tozetti et al., 2017; Yu et al., 2009; Yuan et al., 2014; Zhang et al., 2022; Zhang et al., 2023; Zhou et al., 2013). Additionally, physicochemical properties of the media, such as the amount of dissolved oxygen (DO), pH, aeration, osmolality, and permittivity, were identified as seen in Figure 2A. Some of these parameters are interdependent, as most media are buffered using a carbonate system; the amount of CO2 in the gas supply is used to control pH, and headspace aeration is key to control DO in the media. Establishing metrologies for all process parameters given in Figure 2A is time-consuming and costly, so a prioritisation must be made. In Figure 2B the above-mentioned ubiquitous process parameters are excluded and a weighted Pareto analysis is applied to the remaining parameters considering criticality and cost of implementation for each process parameter. The weighted Pareto analysis identifies glucose and lactate concentration, headspace aeration, DO and pH as CPPs. The concentration of amino acids, glutamine, and glutamate, as well as the shear stress or osmolality in the bioreactor, are less often considered in comparison.
As glucose is the main substrate in most MSC cultivations, its levels are known to impact proliferation and viability of MSCs (Nosrati et al., 2024). Especially in combination with hypoxic conditions, it also influences differentiation capacity, mitochondrial activity and apoptosis (Lau et al., 2020; Almahasneh et al., 2024). Similarly, lactate levels have been shown to decrease proliferation, modulate gene expression and stemness of MSCs (Zieker et al., 2008; Schneider et al., 2012; Sun et al., 2020) stressing the criticality of glucose and lactate levels for MSC expansion. In addition, culturing MSCs from different cell sources in normoxic and hypoxic conditions has been shown to impact cell proliferation, modulate the differentiation capability and expression of pluripotency factors and influence the immunomodulatory capacity of the secretome of MSCs (Lavrentieva et al., 2020; Obradovic et al., 2019; Pezzi et al., 2017; Xu et al., 2022). Concerning the influence of pH, acidic cultivation conditions decrease expansion and viability of MSCs as well as modulate chemokine expression, while alkaline pH might improve differentiation of MSCs into osteoblasts (Wuertz et al., 2009; Bischoff et al., 2008; Fliefel et al., 2016). To ensure high-quality manufacturing of MSCs, these parameters should be considered as CPPs in addition to the ubiquitous measured parameters discussed above.
Discussion
The expansion of MSCs in bioreactor systems becomes increasingly relevant as more therapies transition through clinical trials. With increased throughput, QbD approaches are being applied to these therapies to ensure consistent, high-quality manufacturing of therapies. While some aspects of MSC manufacturing, such as the extraction of cells and cultivation in plate or flask-based cultures, have been reviewed in detail, the specifics of bioreactor-based MSC expansion were reviewed in this article. A survey of quality attributes and process parameters of MSCs expanded in bioreactor systems was conducted, and a list of quality attributes and process parameters compiled. For the quality parameters, findings of previous studies were confirmed with cell count and viability for dosage, immunophenotype for identity, and trilinear differentiation for potency being identified as CQAs. However, it has been discussed additional parameters relating to the potency of the MSCs depending on the application and additional parameters relating to safety, regarding regulatory guidelines, must be considered in addition. For process parameters, several parameters were identified as being ubiquitously controlled or monitored in bioreactor-based expansion of MSCs: agitation rate, bioreactor type, cell type, media formulation, microcarrier type, seeding density, and temperature are controlled in every article surveyed and percentage of CO2 in the gaseous mixture highly frequently. To improve consistency of quality, the process monitoring should be expanded to include more parameters that are not only monitored but also controlled. For prioritisation, a weighted Pareto Analysis was applied to the remaining process parameters and glucose and Lactate concentration, DO, headspace aeration and pH in the media were additionally classified as CPPs. A prerequisite for improved process not discussed so far is the availability of process analytical technology (PAT). As a direct process control is only feasible with in-line sensors for all relevant parameters integrated in the bioreactor system and connected to variables which can be manipulated. While DO and pH are often measured in-line, metabolite concentration is less frequently integrated in-line, but rather measured off-line or at-line. More sophisticated PAT based on enzymatic or spectroscopic principles is not yet frequently integrated in MSC manufacturing. This severely limits the degree of process control currently in use for these therapies and the achievable process consistency. More sophisticated PAT would allow for a detailed investigation of the influence that different levels of process parameters have on the quality of cells, which would be the basis for any process control established and improving consistency and quality of MSC therapy manufacturing.
Author contributions
LH: Conceptualization, Investigation, Visualization, Writing – original draft. BN: Supervision, Writing – review and editing. RS: Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The results published here were generated as a part of the AutoCRAT project. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 874671.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1608194/full#supplementary-material
References
Alimperti, S., Lei, P., Wen, Y., Tian, J., Campbell, A. M., and Andreadis, S. T. (2014). Serum-free spheroid suspension culture maintains mesenchymal stem cell proliferation and differentiation potential. Biotechnol. Prog. 30 (4), 974–983. doi:10.1002/btpr.1904
Almahasneh, F., Abu-El-Rub, E., Khasawneh, R. R., and Almazari, R. (2024). Effects of high glucose and severe hypoxia on the biological behavior of mesenchymal stem cells at various passages. World J. stem cells 16 (4), 434–443. doi:10.4252/wjsc.v16.i4.434
Bandarra-Tavares, H., Franchi-Mendes, T., Ulpiano, C., Morini, S., Kaur, N., Harris-Becker, A., et al. (2024). Dual production of human mesenchymal stromal cells and derived extracellular vesicles in a dissolvable microcarrier-based stirred culture system. Cytotherapy 26 (7), 749–756. doi:10.1016/j.jcyt.2024.03.001
Bischoff, D. S., Zhu, J.-H., Makhijani, N. S., and Yamaguchi, D. T. (2008). Acidic pH stimulates the production of the angiogenic CXC chemokine, CXCL8 (interleukin-8), in human adult mesenchymal stem cells via the extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and NF-κB pathways. J. Cell. Biochem. 104 (4), 1378–1392. doi:10.1002/jcb.21714
Bui, H. T. H., Nguyen, L. T., and Than, U. T. T. (2021). Influences of xeno-free media on mesenchymal stem cell expansion for clinical application. Tissue Eng. Regen. Med. 18 (1), 15–23. doi:10.1007/s13770-020-00306-z
Cao, Y., Li, D., Shang, C., Yang, S.-T., Wang, J., and Wang, X. (2010). Three-dimensional culture of human mesenchymal stem cells in a polyethylene terephthalate matrix. Biomed. Mater. Bristol, Engl. 5 (6), 065013. doi:10.1088/1748-6041/5/6/065013
Carmelo, J. G., Fernandes-Platzgummer, A., Diogo, M. M., da Silva, C. L., and Cabral, J. M. S. (2015). A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol. J. 10 (8), 1235–1247. doi:10.1002/biot.201400586
Chen, A. K.-L., Chew, Y. K., Tan, H. Y., Reuveny, S., and Weng Oh, S. K. (2015). Increasing efficiency of human mesenchymal stromal cell culture by optimization of microcarrier concentration and design of medium feed. Cytotherapy 17 (2), 163–173. doi:10.1016/j.jcyt.2014.08.011
Chen, Y., Xu, H., Zhang, Y., Guo, L., Lan, M., Yang, Y., et al. (2023). Large-scale cell production based on GMP-grade dissolvable porous microcarriers. J. Vis. Exp. JoVE 197. doi:10.3791/65469
Chung, H. J., Jung, J. S., and Park, T. G. (2011). Fabrication of adipose-derived mesenchymal stem cell aggregates using biodegradable porous microspheres for injectable adipose tissue regeneration. JPolymer Ed. 22 (1-3), 107–122. doi:10.1163/092050609X12580983495681
Costa, L. A., Eiro, N., Fraile, M., Gonzalez, L. O., Saá, J., Garcia-Portabella, P., et al. (2021). Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell. Mol. Life Sci. 78 (2), 447–467. doi:10.1007/s00018-020-03600-0
Costa, M. H. G., Costa, M. S., Painho, B., Sousa, C. D., Carrondo, I., Oltra, E., et al. (2023). Enhanced bioprocess control to advance the manufacture of mesenchymal stromal cell-derived extracellular vesicles in stirred-tank bioreactors. Biotechnol. Bioeng. 120 (9), 2725–2741. doi:10.1002/bit.28378
Cunha, B., Aguiar, T., Carvalho, S. B., Silva, M. M., Gomes, R. A., Carrondo, M. J., et al. (2017). Bioprocess integration for human mesenchymal stem cells: from up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 248, 87–98. doi:10.1016/j.jbiotec.2017.01.014
de Almeida Fuzeta, M., de Matos Branco, A. D., Fernandes-Platzgummer, A., da Silva, C. L., and Cabral, J. M. S. (2020). Addressing the manufacturing challenges of cell-based therapies. Adv. Biochem. engineering/biotechnology 171, 225–278. doi:10.1007/10_2019_118
de Da Sá Silva, J., Severino, P., Wodewotzky, T. I., Covas, D. T., Swiech, K., Cavalheiro Marti, L., et al. (2020). Mesenchymal stromal cells maintain the major quality attributes when expanded in different bioreactor systems. Biochem. Eng. J. 161, 107693. doi:10.1016/j.bej.2020.107693
de Soure, A. M., Fernandes-Platzgummer, A., Moreira, F., Lilaia, C., Liu, S. H., Ku, C. P., et al. (2017). Integrated culture platform based on a human platelet lysate supplement for the isolation and scalable manufacturing of umbilical cord matrix-derived mesenchymal stem/stromal cells. J. tissue Eng. Regen. Med. 11 (5), 1630–1640. doi:10.1002/term.2200
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 (4), 315–317. doi:10.1080/14653240600855905
Doron, G., and Temenoff, J. S. (2021). Culture substrates for improved manufacture of mesenchymal stromal cell therapies. Adv. Healthc. Mater. 10 (15), e2100016. doi:10.1002/adhm.202100016
Dos Santos, F., Campbell, A., Fernandes-Platzgummer, A., Andrade, P. Z., Gimble, J. M., Wen, Y., et al. (2014). A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol. Bioeng. 111 (6), 1116–1127. doi:10.1002/bit.25187
Dos Santos, N. C. D., Bruzadelle-Vieira, P., de Cássia Noronha, N., Mizukami-Martins, A., Orellana, M. D., Bentley, M. V. L. B., et al. (2024). Transitioning from static to suspension culture system for large-scale production of xeno-free extracellular vesicles derived from mesenchymal stromal cells. Biotechnol. Prog. 40 (3), e3419. doi:10.1002/btpr.3419
Dosta, P., Ferber, S., Zhang, Y., Wang, K., Ros, A., Uth, N., et al. (2020). Scale-up manufacturing of gelatin-based microcarriers for cell therapy. J. Biomed. Mater. Res. Part B, Appl. biomaterials 108 (7), 2937–2949. doi:10.1002/jbm.b.34624
Eibes, G., dos Santos, F., Andrade, P. Z., Boura, J. S., Abecasis, M. M., da Silva, C. L., et al. (2010). Maximizing the ex vivo expansion of human mesenchymal stem cells using a microcarrier-based stirred culture system. J. Biotechnol. 146 (4), 194–197. doi:10.1016/j.jbiotec.2010.02.015
Elseberg, C. L., Leber, J., Salzig, D., Wallrapp, C., Kassem, M., Kraume, M., et al. (2012). Microcarrier-based expansion process for hMSCs with high vitality and undifferentiated characteristics. Int. J. Artif. Organs 35 (2), 93–107. doi:10.5301/ijao.5000077
EMA (2009). International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use considerations (ICH) guideline Q8 (R2) on pharmaceutical development: ICH Q8 (R2), ema/chmp/ICH/167068/2004. London, United Kingdom: European Medicines Agency. Available online at: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline.
European Medicines Agency (EMA) Guideline on quality, non-clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials: EMA/CAT/852602/2018. Available online at: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-non-clinical-clinical-requirements-investigational-advanced-therapy-medicinal-products-clinical-trials-first-version_en.pdf.
European Medicines Agency (2021). European public assessment report - Holoclar: ex vivo expanded autologous human corneal epithelial cells containing stem cells. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/holoclar.
European Medicines Agency (EMA) Alofisel | European Medicines agency (EMA). Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel.
Fernandes-Platzgummer, A., Cunha, R., Morini, S., Carvalho, M., Moreno-Cid, J., García, C., et al. (2023). Optimized operation of a controlled stirred tank reactor system for the production of mesenchymal stromal cells and their extracellular vesicles. Biotechnol. Bioeng. 120 (9), 2742–2755. doi:10.1002/bit.28449
Fliefel, R., Popov, C., Tröltzsch, M., Kühnisch, J., Ehrenfeld, M., and Otto, S. (2016). Mesenchymal stem cell proliferation and mineralization but not osteogenic differentiation are strongly affected by extracellular pH. J. Cranio-Maxillofacial Surg. 44 (6), 715–724. doi:10.1016/j.jcms.2016.03.003
Gadelorge, M., Bourdens, M., Espagnolle, N., Bardiaux, C., Murrell, J., Savary, L., et al. (2018). Clinical-scale expansion of adipose-derived stromal cells starting from stromal vascular fraction in a single-use bioreactor: proof of concept for autologous applications. J. tissue Eng. Regen. Med. 12 (1), 129–141. doi:10.1002/term.2377
Gao, T., Zhao, X., Hao, J., Tian, Y., Ma, H., Liu, W., et al. (2023). A scalable culture system incorporating microcarrier for specialised mesenchymal stem cells from human embryonic stem cells. Mater. Today Bio. 20, 100662. doi:10.1016/j.mtbio.2023.100662
García-Fernández, C., López-Fernández, A., Borrós, S., Lecina, M., and Vives, J. (2020). Strategies for large-scale expansion of clinical-grade human multipotent mesenchymal stromal cells. Biochem. Eng. J. 159, 107601. doi:10.1016/j.bej.2020.107601
García-Gómez, I., Elvira, G., Zapata, A. G., Lamana, M. L., Ramírez, M., García Castro, J., et al. (2010). Mesenchymal stem cells: biological properties and clinical applications. Expert Opin. Biol. Ther. 10 (10), 1453–1468. doi:10.1517/14712598.2010.519333
Gonzalez Gil, L. V., Singh, H., da Silva, J. d. S., dos Santos, D. P., Covas, D. T., Swiech, K., et al. (2020). Feasibility of the taylor vortex flow bioreactor for mesenchymal stromal cell expansion on microcarriers. Biochem. Eng. J. 162, 107710. doi:10.1016/j.bej.2020.107710
Gottipamula, S., Muttigi, M. S., Kolkundkar, U., and Seetharam, R. N. (2013). Serum-free media for the production of human mesenchymal stromal cells: a review. Cell Prolif. 46 (6), 608–627. doi:10.1111/cpr.12063
Grein, T. A., Leber, J., Blumenstock, M., Petry, F., Weidner, T., Salzig, D., et al. (2016). Multiphase mixing characteristics in a microcarrier-based stirred tank bioreactor suitable for human mesenchymal stem cell expansion. Process Biochem. 51 (9), 1109–1119. doi:10.1016/j.procbio.2016.05.010
Guadix, J. A., López-Beas, J., Clares, B., Soriano-Ruiz, J. L., Zugaza, J. L., and Gálvez-Martín, P. (2019). Principal criteria for evaluating the quality, safety and efficacy of hMSC-based products in clinical practice: current approaches and challenges. Pharmaceutics 11 (11), 552. doi:10.3390/pharmaceutics11110552
Győrgy, R., Klontzas, M. E., Kostoglou, M., Panoskaltsis, N., Mantalaris, A., and Georgiadis, M. C. (2019). Capturing mesenchymal stem cell heterogeneity during osteogenic differentiation: an experimental–modeling approach. Ind. Eng. Chem. Res. 58 (31), 13900–13909. doi:10.1021/acs.iecr.9b01988
Heathman, T. R. J., Glyn, V. A. M., Picken, A., Rafiq, Q. A., Coopman, K., Nienow, A. W., et al. (2015). Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotechnol. Bioeng. 112 (8), 1696–1707. doi:10.1002/bit.25582
Heathman, T. R., Nienow, A. W., Rafiq, Q. A., Coopman, K., Kara, B., and Hewitt, C. J. (2018). Agitation and aeration of stirred-bioreactors for the microcarrier culture of human mesenchymal stem cells and potential implications for large-scale bioprocess development. Biochem. Eng. J. 136, 9–17. doi:10.1016/j.bej.2018.04.011
Jorgenson, K. D., Hart, D. A., Krawetz, R., and Sen, A. (2018). Production of adult human synovial fluid-derived mesenchymal stem cells in stirred-suspension culture. Stem cells Int. 2018, 1–16. doi:10.1155/2018/8431053
Jossen, V., Schirmer, C., Mostafa Sindi, D., Eibl, R., Kraume, M., Pörtner, R., et al. (2016). Theoretical and practical issues that are relevant when scaling up hMSC microcarrier production processes. Stem cells Int. 2016 (1), 4760414. doi:10.1155/2016/4760414
Kadri, N., Amu, S., Iacobaeus, E., Boberg, E., and Le Blanc, K. (2023). Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell. and Mol. Immunol. 20 (6), 613–625. doi:10.1038/s41423-023-01022-z
Kaiser, S., Jossen, V., Schirmaier, C., Eibl, D., Brill, S., van den Bos, C., et al. (2013). Fluid flow and cell proliferation of mesenchymal adipose-derived stem cells in small-scale, stirred, single-use bioreactors. Chem. Ing. Tech. 85 (1-2), 95–102. doi:10.1002/cite.201200180
Kanawa, M., Igarashi, A., Ronald, V. S., Higashi, Y., Kurihara, H., Sugiyama, M., et al. (2013). Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy 15 (9), 1062–1072. doi:10.1016/j.jcyt.2013.03.015
Kaneko, M., Sato, A., Ayano, S., Fujita, A., Kobayashi, G., and Ito, A. (2023). Expansion of human mesenchymal stem cells on poly(vinyl alcohol) microcarriers. J. Biosci. Bioeng. 136 (5), 407–414. doi:10.1016/j.jbiosc.2023.08.003
Kehoe, D., DiLeo, A., Simler, J., Ball, A., and Schnitzler, A. C. (2012). Scale-up of human mesenchymal stem cells on microcarriers in suspension in a single-use bioreactor. Biopharm. Int. 25 (3). Available online at: https://www.biopharminternational.com/view/scale-human-mesenchymal-stem-cells-microcarriers-suspension-single-use-bioreactor.
Krampera, M., and Le Blanc, K. (2021). Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell 28 (10), 1708–1725. doi:10.1016/j.stem.2021.09.006
Kurogi, H., Takahashi, A., Isogai, M., Sakumoto, M., Takijiri, T., Hori, A., et al. (2021). Umbilical cord derived mesenchymal stromal cells in microcarrier based industrial scale culture sustain the immune regulatory functions. Biotechnol. J. 16 (6), e2000558. doi:10.1002/biot.202000558
Kurogi, H., Takijiri, T., Sakumoto, M., Isogai, M., Takahashi, A., Okubo, T., et al. (2022). Study on the umbilical cord-mesenchymal stem cell manufacturing using clinical-grade culture medium. Tissue Eng. Part C. Methods 28 (1), 23–33. doi:10.1089/ten.TEC.2021.0207
Lam, A. T.-L., Li, J., Toh, J. P. W., Sim, E. J. H., Chen, A. K. L., Chan, J. K. Y., et al. (2017). Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy 19 (3), 419–432. doi:10.1016/j.jcyt.2016.11.009
Lam, A. T.-L., Sim, E. J. H., Shekaran, A., Li, J., Teo, K. L., Goggi, J. L., et al. (2019). Sub-confluent culture of human mesenchymal stromal cells on biodegradable polycaprolactone microcarriers enhances bone healing of rat calvarial defect. Cytotherapy 21 (6), 631–642. doi:10.1016/j.jcyt.2019.03.004
Lam, A. T.-L., Lee, A. P., Jayaraman, P., Tan, K. Y., Raghothaman, D., Lim, H. L., et al. (2021). Multiomics analyses of cytokines, genes, miRNA, and regulatory networks in human mesenchymal stem cells expanded in stirred microcarrier-spinner cultures. Stem Cell Res. 53, 102272. doi:10.1016/j.scr.2021.102272
Lau, F., Dalisson, B., Zhang, Y. L., Zhao, J., Eliopoulos, N., and Barralet, J. E. (2020). Effects of oxygen and glucose on bone marrow mesenchymal stem cell culture. Adv. Biosyst. 4 (11), 2000094. doi:10.1002/adbi.202000094
Lavrentieva, A., Hoffmann, A., and Lee-Thedieck, C. (2020). Limited potential or unfavorable manipulations? Strategies toward efficient mesenchymal stem/stromal cell applications. Front. cell Dev. Biol. 8, 316. doi:10.3389/fcell.2020.00316
Lawson, T., Kehoe, D. E., Schnitzler, A. C., Rapiejko, P. J., Der, K. A., Philbrick, K., et al. (2017). Process development for expansion of human mesenchymal stromal cells in a 50L single-use stirred tank bioreactor. Biochem. Eng. J. 120, 49–62. doi:10.1016/j.bej.2016.11.020
Leber, J., Barekzai, J., Blumenstock, M., Pospisil, B., Salzig, D., and Czermak, P. (2017). Microcarrier choice and bead-to-bead transfer for human mesenchymal stem cells in serum-containing and chemically defined media. Process Biochem. 59, 255–265. doi:10.1016/j.procbio.2017.03.017
Lin, Y. M., Lim, J. F. Y., Lee, J., Choolani, M., Chan, J. K. Y., Reuveny, S., et al. (2016). Expansion in microcarrier-spinner cultures improves the chondrogenic potential of human early mesenchymal stromal cells. Cytotherapy 18 (6), 740–753. doi:10.1016/j.jcyt.2016.03.293
Lipsitz, Y. Y., Timmins, N. E., and Zandstra, P. W. (2016). Quality cell therapy manufacturing by design. Nat. Biotechnol. 34 (4), 393–400. doi:10.1038/nbt.3525
López-Beas, J., Guadix, J. A., Clares, B., Soriano-Ruiz, J. L., Zugaza, J. L., and Gálvez-Martín, P. (2020). An overview of international regulatory frameworks for mesenchymal stromal cell-based medicinal products: from laboratory to patient. Med. Res. Rev. 40 (4), 1315–1334. doi:10.1002/med.21659
López-Fernández, A., Codinach, M., Coca, M. I., Prat-Vidal, C., Castaño, J., Torrents, S., et al. (2024a). Comparability exercise of critical quality attributes of clinical-grade human mesenchymal stromal cells from the Wharton's jelly: single-use stirred tank bioreactors versus planar culture systems. Cytotherapy 26 (5), 418–426. doi:10.1016/j.jcyt.2023.08.008
López-Fernández, A., Garcia-Gragera, V., Lecina, M., and Vives, J. (2024b). Identification of critical process parameters for expansion of clinical grade human Wharton's jelly-derived mesenchymal stromal cells in stirred-tank bioreactors. Biotechnol. J. 19 (2), e2300381. doi:10.1002/biot.202300381
Maillot, C., Sion, C., de Isla, N., Toye, D., and Olmos, E. (2021). Quality by design to define critical process parameters for mesenchymal stem cell expansion. Biotechnol. Adv. 50, 107765. doi:10.1016/j.biotechadv.2021.107765
Mareschi, K., Ferrero, I., Rustichelli, D., Aschero, S., Gammaitoni, L., Aglietta, M., et al. (2006). Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J. Cell. Biochem. 97 (4), 744–754. doi:10.1002/jcb.20681
Mizukami, A., Fernandes-Platzgummer, A., Carmelo, J. G., Swiech, K., Covas, D. T., Cabral, J. M. S., et al. (2016). Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnol. J. 11 (8), 1048–1059. doi:10.1002/biot.201500532
Mizukami, A., Thomé, C. H., Ferreira, G. A., Lanfredi, G. P., Covas, D. T., Pitteri, S. J., et al. (2019). Proteomic identification and time-course monitoring of secreted proteins during expansion of human mesenchymal stem/stromal in stirred-tank bioreactor. Front. Bioeng. Biotechnol. 7, 154. doi:10.3389/fbioe.2019.00154
Moreira, F., Mizukami, A., de Souza, L. E. B., Cabral, J. M. S., da Silva, C. L., Covas, D. T., et al. (2020). Successful use of human AB serum to support the expansion of adipose tissue-derived mesenchymal stem/stromal cell in a microcarrier-based platform. Front. Bioeng. Biotechnol. 8, 307. doi:10.3389/fbioe.2020.00307
Nienow, A. W., Rafiq, Q. A., Heathman, T. R. J., Coopman, K., and Hewitt, C. J. (2016). Mixing theory for culture and harvest in bioreactors of human mesenchymal stem cells on microcarriers. Theor. Found. Chem. Eng. 50 (6), 895–900. doi:10.1134/S0040579516060117
Nikolits, I., Nebel, S., Egger, D., Kreß, S., and Kasper, C. (2021). Towards physiologic culture approaches to improve standard cultivation of mesenchymal stem cells. Cells 10 (4), 886. doi:10.3390/cells10040886
Noronha, N. C., Mizukami, A., Orellana, M. D., Oliveira, M. C., Covas, D. T., Swiech, K., et al. (2021). Hypoxia priming improves in vitro angiogenic properties of umbilical cord derived-mesenchymal stromal cells expanded in stirred-tank bioreactor. Biochem. Eng. J. 168, 107949. doi:10.1016/j.bej.2021.107949
Nosrati, S., Gheisari, M., Zare, S., Dara, M., Zolghadri, S., and Razeghian-Jahromi, I. (2024). The impact of diabetic glucose concentration on viability and cardiac differentiation of mesenchymal stem cells. Tissue and cell 88, 102361. doi:10.1016/j.tice.2024.102361
Obradovic, H., Krstic, J., Trivanovic, D., Mojsilovic, S., Okic, I., Kukolj, T., et al. (2019). Improving stemness and functional features of mesenchymal stem cells from Wharton's jelly of a human umbilical cord by mimicking the native, low oxygen stem cell niche. Placenta 82, 25–34. doi:10.1016/j.placenta.2019.05.005
Ochs, J., Hanga, M. P., Shaw, G., Duffy, N., Kulik, M., Tissin, N., et al. (2022). Needle to needle robot-assisted manufacture of cell therapy products. Bioeng. and Transl. Med. 7 (3), e10387. doi:10.1002/btm2.10387
Osiecki, M. J., Michl, T. D., Kul Babur, B., Kabiri, M., Atkinson, K., Lott, W. B., et al. (2015). Packed bed bioreactor for the isolation and expansion of placental-derived mesenchymal stromal cells. PLOS ONE 10 (12), e0144941. doi:10.1371/journal.pone.0144941
Pachón-Peña, G., Serena, C., Ejarque, M., Petriz, J., Duran, X., Oliva-Olivera, W., et al. (2016). Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl. Med. 5 (4), 464–475. doi:10.5966/sctm.2015-0161
Padhiar, C., Aruni, A. W., Abhaya, M., Muthuchamy, M., Dhanraj, A. K., Ganesan, V., et al. (2022). GMP compliant clinical grade and xenofree manufacturing of human Wharton’s jelly derived mesenchymal stem cell from pooled donors. Biochem. Eng. J. 184, 108470. doi:10.1016/j.bej.2022.108470
Petry, F., Smith, J. R., Leber, J., Salzig, D., Czermak, P., and Weiss, M. L. (2016). Manufacturing of human umbilical cord mesenchymal stromal cells on microcarriers in a dynamic system for clinical use. Stem cells Int. 2016, 4834616. doi:10.1155/2016/4834616
Pezzi, A., Amorin, B., Laureano, Á., Valim, V., Dahmer, A., Zambonato, B., et al. (2017). Effects of hypoxia in long-term in vitro expansion of human bone marrow derived mesenchymal stem cells. J. Cell. Biochem. 118 (10), 3072–3079. doi:10.1002/jcb.25953
Phelps, J., Leonard, C., Shah, S., Krawetz, R., Hart, D. A., Duncan, N. A., et al. (2022). Production of mesenchymal progenitor cell-derived extracellular vesicles in suspension bioreactors for use in articular cartilage repair. Stem cells Transl. Med. 11 (1), 73–87. doi:10.1093/stcltm/szab008
Phelps, J., Hart, D. A., Mitha, A. P., Duncan, N. A., and Sen, A. (2024). Extracellular vesicles generated by mesenchymal stem cells in stirred suspension bioreactors promote angiogenesis in human-brain-derived endothelial cells. Int. J. Mol. Sci. 25 (10), 5219. doi:10.3390/ijms25105219
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Sci. (New York, N.Y.) 284 (5411), 143–147. doi:10.1126/science.284.5411.143
PRISMA statement PRISMA 2020 flow diagram — PRISMA statement. Available online at: https://www.prisma-statement.org/prisma-2020-flow-diagram.
Rafiq, Q. A., Brosnan, K. M., Coopman, K., Nienow, A. W., and Hewitt, C. J. (2013). Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol. Lett. 35 (8), 1233–1245. doi:10.1007/s10529-013-1211-9
Rafiq, Q. A., Coopman, K., Nienow, A. W., and Hewitt, C. J. (2016). Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol. J. 11 (4), 473–486. doi:10.1002/biot.201400862
Renesme, L., Cobey, K. D., Lalu, M. M., Bubela, T., Chinnadurai, R., De Vos, J., et al. (2025). Delphi-driven consensus definition for mesenchymal stromal cells and clinical reporting guidelines for mesenchymal stromal cell–based therapeutics. Cytotherapy 27 (2), 146–168. doi:10.1016/j.jcyt.2024.10.008
Rodríguez-Fuentes, D. E., Fernández-Garza, L. E., Samia-Meza, J. A., Barrera-Barrera, S. A., Caplan, A. I., and Barrera-Saldaña, H. A. (2021). Mesenchymal stem cells current clinical applications: a systematic review. Archives Med. Res. 52 (1), 93–101. doi:10.1016/j.arcmed.2020.08.006
Rotondi, M., Grace, N., Betts, J., Bargh, N., Costariol, E., Zoro, B., et al. (2021). Design and development of a new ambr250® bioreactor vessel for improved cell and gene therapy applications. Biotechnol. Lett. 43 (5), 1103–1116. doi:10.1007/s10529-021-03076-3
RYONCIL (2025). FDA. Available online at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/ryoncil.
Sammour, I., Somashekar, S., Huang, J., Batlahally, S., Breton, M., Valasaki, K., et al. (2016). The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia-induced lung injury. PLOS ONE 11 (10), e0164269. doi:10.1371/journal.pone.0164269
Santhagunam, A., Dos Santos, F., Madeira, C., Salgueiro, J. B., and Cabral, J. M. S. (2014). Isolation and ex vivo expansion of synovial mesenchymal stromal cells for cartilage repair. Cytotherapy 16 (4), 440–453. doi:10.1016/j.jcyt.2013.10.010
Santos, F. d., Andrade, P. Z., Abecasis, M. M., Gimble, J. M., Chase, L. G., Campbell, A. M., et al. (2011). Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng. Part C. Methods 17 (12), 1201–1210. doi:10.1089/ten.tec.2011.0255
Sart, S., Schneider, Y.-J., and Agathos, S. N. (2009). Ear mesenchymal stem cells: an efficient adult multipotent cell population fit for rapid and scalable expansion. J. Biotechnol. 139 (4), 291–299. doi:10.1016/j.jbiotec.2008.12.011
Sart, S., Schneider, Y.-J., and Agathos, S. N. (2010). Influence of culture parameters on ear mesenchymal stem cells expanded on microcarriers. J. Biotechnol. 150 (1), 149–160. doi:10.1016/j.jbiotec.2010.08.003
Sart, S., Errachid, A., Schneider, Y.-J., and Agathos, S. N. (2013). Modulation of mesenchymal stem cell actin organization on conventional microcarriers for proliferation and differentiation in stirred bioreactors. J. tissue Eng. Regen. Med. 7 (7), 537–551. doi:10.1002/term.545
Schirmaier, C., Jossen, V., Kaiser, S. C., Jüngerkes, F., Brill, S., Safavi-Nab, A., et al. (2014). Scale-up of adipose tissue-derived mesenchymal stem cell production in stirred single-use bioreactors under low-serum conditions. Eng. Life Sci. 14 (3), 292–303. doi:10.1002/elsc.201300134
Schneider, C.-C., Ateschrang, A., Königsrainer, I., Glatzle, J., Bühler, S., Schaefer, R., et al. (2012). Lactate influences the gene expression profile of human mesenchymal stem cells (hMSC) in a dose dependant manner. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol. 30 (6), 1547–1556. doi:10.1159/000343342
Sharma, R. R., Pollock, K., Hubel, A., and McKenna, D. (2014). Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54 (5), 1418–1437. doi:10.1111/trf.12421
Shekaran, A., Sim, E., Tan, K. Y., Chan, J. K. Y., Choolani, M., Reuveny, S., et al. (2015). Enhanced in vitro osteogenic differentiation of human fetal MSCs attached to 3D microcarriers versus harvested from 2D monolayers. BMC Biotechnol. 15 (1), 102. doi:10.1186/s12896-015-0219-8
Shekaran, A., Lam, A., Sim, E., Jialing, L., Jian, L., Wen, J. T. P., et al. (2016). Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 18 (10), 1332–1344. doi:10.1016/j.jcyt.2016.06.016
Silva Couto, P., Rotondi, M., Bersenev, A., Hewitt, C., Nienow, A., Verter, F., et al. (2020). Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds. Biotechnol. Adv. 45, 107636. doi:10.1016/j.biotechadv.2020.107636
Simão, V. A., Brand, H., da Silveira-Antunes, R. N., Fukasawa, J. T., Leme, J., Tonso, A., et al. (2023). Adipose-derived stem cells (ASCs) culture in spinner flask: improving the parameters of culture in a microcarrier-based system. Biotechnol. Lett. 45 (7), 823–846. doi:10.1007/s10529-023-03367-x
Sion, C., Loubière, C., Wlodarczyk-Biegun, M., Davoudi, N., Müller-Renno, C., Guedon, E., et al. (2020). Effects of microcarriers addition and mixing on WJ-MSC culture in bioreactors. Biochem. Eng. J. 157, 107521. doi:10.1016/j.bej.2020.107521
Sion, C., Ghannoum, D., Ebel, B., Gallo, F., de Isla, N., Guedon, E., et al. (2021). A new perfusion mode of culture for WJ-MSCs expansion in a stirred and online monitored bioreactor. Biotechnol. Bioeng. 118 (11), 4453–4464. doi:10.1002/bit.27914
Soder, R. P., Dudley, N. R., and Dawn, B. (2024). Microcarrier-based clinical-grade manufacturing of therapeutic Wharton's jelly mesenchymal stromal cells. Cytotherapy 26 (12), 1556–1565. doi:10.1016/j.jcyt.2024.07.003
Sousa, M. F. Q., Silva, M. M., Giroux, D., Hashimura, Y., Wesselschmidt, R., Lee, B., et al. (2015). Production of oncolytic adenovirus and human mesenchymal stem cells in a single-use, Vertical-Wheel bioreactor system: impact of bioreactor design on performance of microcarrier-based cell culture processes. Biotechnol. Prog. 31 (6), 1600–1612. doi:10.1002/btpr.2158
Sun, H., Zhang, X., Dai, J., Pan, Z., Wu, Y., Yu, D., et al. (2020). Sodium lactate promotes stemness of human mesenchymal stem cells through KDM6B mediated glycolytic metabolism. Biochem. Biophysical Res. Commun. 532 (3), 433–439. doi:10.1016/j.bbrc.2020.08.061
Sunil, N., Kehoe, D., Niss, K., Aysola, M., Murrel, J., Punreddy, S., et al. (2013). Growth kinetics of human mesenchymal stem cells in a 3-L single-use, stirred-tank bioreactor. Biopharm. Int. 26 (4), 40–45-40–45. Available online at: https://www.biopharminternational.com/view/growth-kinetics-human-mesenchymal-stem-cells-3-l-single-use-stirred-tank-bioreactor.
Tan, K. Y., Teo, K. L., Lim, J. F., Chen, A. K., Reuveny, S., and Oh, S. K. (2015). Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy 17 (8), 1152–1165. doi:10.1016/j.jcyt.2015.05.001
Timmins, N. E., Kiel, M., Günther, M., Heazlewood, C., Doran, M., Brooke, G., et al. (2012). Closed system isolation and scalable expansion of human placental mesenchymal stem cells. Biotechnol. Bioeng. 109 (7), 1817–1826. doi:10.1002/bit.24425
Tozetti, P. A., Caruso, S. R., Mizukami, A., Fernandes, T. R., da Silva, F. B., Traina, F., et al. (2017). Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions. Biotechnol. Prog. 33 (5), 1358–1367. doi:10.1002/btpr.2494
Tsai, A.-C., and Pacak, C. A. (2021). Bioprocessing of human mesenchymal stem cells: from planar culture to microcarrier-based bioreactors. Bioeng. Basel, Switz. 8 (7), 96. doi:10.3390/bioengineering8070096
Wang, X., Ouyang, L., Chen, W., Cao, Y., and Zhang, L. (2023). Efficient expansion and delayed senescence of hUC-MSCs by microcarrier-bioreactor system. Stem Cell Res. Ther. 14 (1), 284. doi:10.1186/s13287-023-03514-1
Wong, S. W., Lenzini, S., Giovanni, R., Knowles, K., and Shin, J.-W. (2021). Matrix biophysical cues direct mesenchymal stromal cell functions in immunity. Acta Biomater. 133, 126–138. doi:10.1016/j.actbio.2021.07.075
Wruck, W., Graffmann, N., Spitzhorn, L.-S., and Adjaye, J. (2021). Human induced pluripotent stem cell-derived mesenchymal stem cells acquire rejuvenation and reduced heterogeneity. Front. cell Dev. Biol. 9, 717772. doi:10.3389/fcell.2021.717772
Wuertz, K., Godburn, K., and Iatridis, J. C. (2009). MSC response to pH levels found in degenerating intervertebral discs. Biochem. biophysical Res. Commun. 379 (4), 824–829. doi:10.1016/j.bbrc.2008.12.145
Xu, Z., Lin, L., Fan, Y., Huselstein, C., De Isla, N., He, X., et al. (2022). Secretome of mesenchymal stem cells from consecutive hypoxic cultures promotes resolution of lung inflammation by reprogramming anti-inflammatory macrophages. Int. J. Mol. Sci. 23 (8), 4333. doi:10.3390/ijms23084333
Yan, X., Zhang, K., Yang, Y., Deng, D., Lyu, C., Xu, H., et al. (2020). Dispersible and dissolvable porous microcarrier tablets enable efficient large-scale human mesenchymal stem cell expansion. Part C. Methods 26 (5), 263–275. doi:10.1089/ten.TEC.2020.0039
Yu, Y., Li, K., Bao, C., Liu, T., Jin, Y., Ren, H., et al. (2009). Ex Vitro expansion of human placenta-derived mesenchymal stem cells in stirred bioreactor. Appl. Biochem. Biotechnol. 159 (1), 110–118. doi:10.1007/s12010-009-8556-5
Yuan, Y., Kallos, M. S., Hunter, C., and Sen, A. (2014). Improved expansion of human bone marrow-derived mesenchymal stem cells in microcarrier-based suspension culture. J. tissue Eng. Regen. Med. 8 (3), 210–225. doi:10.1002/term.1515
Zhang, J., Peng, Y., Guo, M., and Li, C. (2022). Large-scale expansion of human umbilical cord-derived mesenchymal stem cells in a stirred suspension bioreactor enabled by computational fluid dynamics modeling. Bioeng. Basel, Switz. 9 (7), 274. doi:10.3390/bioengineering9070274
Zhang, B., Lu, Q., Dai, G., Zhou, Y., Ye, Q., Zhou, Y., et al. (2023). Enhancing mesenchymal stem cells cultivated on microcarriers in spinner flasks via impeller design optimization for aggregated suspensions. Bioresour. Bioprocess. 10 (1), 89. doi:10.1186/s40643-023-00707-7
Zhou, L., Kong, J., Zhuang, Y., Chu, J., Zhang, S., and Guo, M. (2013). Ex vivo expansion of bone marrow mesenchymal stem cells using microcarrier beads in a stirred bioreactor. Biotechnol. Bioprocess Eng. 18 (1), 173–184. doi:10.1007/s12257-012-0512-5
Keywords: quality-by-design, critical process parameter, critical quality attributes, mesenchymal stem cells, standardization, bioreactor
Citation: Herbst L, Nießing B and Schmitt RH (2025) Identification of critical process parameters and quality attributes for bioreactor-based expansion of human MSCs. Front. Bioeng. Biotechnol. 13:1608194. doi: 10.3389/fbioe.2025.1608194
Received: 08 April 2025; Accepted: 30 July 2025;
Published: 21 August 2025.
Edited by:
Volker Huppert, Glycostem Therapeutics B.V., NetherlandsReviewed by:
Urban Švajger, Blood Transfusion Centre of Slovenia, SloveniaCopyright © 2025 Herbst, Nießing and Schmitt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Herbst, bGF1cmEuaGVyYnN0QGlwdC5mcmF1bmhvZmVyLmRl
 Laura Herbst
Laura Herbst Bastian Nießing
Bastian Nießing Robert H. Schmitt
Robert H. Schmitt