- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Neurology, Zigong Third People’s Hospital, Zigong, China
- 3Department of Cardiology, The General Hospital of Western Theater Command, Chengdu, China
- 4College of Medicine, Southwest Jiaotong University, Chengdu, Sichuan, China
Cardiovascular risk factors such as hypertension, hyperlipidemia, and hyperglycemia are closely associated with ocular diseases including glaucoma, diabetic retinopathy, and dry eye syndrome. These conditions are characterized by microvascular damage, hemodynamic alterations, and pathological neovascularization, ultimately leading to significant visual impairment. Traditional treatments often suffer from limitations, such as invasiveness and poor target specificity, highlighting the urgent need for innovative therapeutic approaches. Recent advancements in biomaterials have substantially improved therapeutic efficacy, particularly in the areas of targeted drug delivery, smart sensors, and tissue repair. Smart sensors like contact lenses enable continuous monitoring of intraocular pressure, enhancing glaucoma management. Nanotechnology and drug delivery systems improve drug targeting and bioavailability, enhancing anti-angiogenic therapies. Additionally, biocompatible materials and nanomaterials have shown promise in promoting retinal and optic nerve repair, facilitating neural regeneration and reducing aberrant neovascularization. Despite ongoing challenges, the rapid evolution of materials science holds transformative potential for developing more effective and personalized treatments for ocular diseases.
1 Introduction
Ocular diseases increasingly reflect the systemic burden of cardiovascular risk factors - including hypertension, hyperlipidemia, hyperglycemia, and hyperuricemia (Modjtahedi et al., 2016; Skrzypecki et al., 2019; Wang and Bao, 2019; Liu L. et al., 2020; Zhou et al., 2022)—which contribute to visual impairment through mechanisms such as microvascular dysfunction, chronic inflammation, and metabolic stress. These systemic conditions result in retinal damage via pathways involving oxidative stress and microvascular injury. For example, hypertension induces abnormal shear stress in retinal vessels, leading to endothelial dysfunction and neovascularization, while hyperglycemia disrupts the blood-retinal barrier through VEGF imbalance and inflammatory pathways (Liu L. et al., 2020; Jiang et al., 2024). Hyperlipidemia promotes lipid deposition, oxidative stress, and dysfunction of corneal endothelial pumps, ultimately reducing cell density, impairing intercellular junctions, and diminishing regenerative capacity—highlighting for the first time that the corneal endothelium is a previously unrecognized target tissue of hyperlipidemic injury (Bu et al., 2020). As the global prevalence of chronic systemic diseases continues to rise, ocular complications are becoming increasingly common, often coexisting in patients with complex cardiometabolic profiles.
Cardiovascular risk factors exert widespread effects on both the anterior and posterior ocular segments. These factors not only contribute to microvascular dysfunction in the retina but also impair anterior structures such as the corneal endothelium and uveal blood flow. Corneal endothelial dysfunction reduces transparency and disrupts fluid regulation, while uveal hypoperfusion compromises nutrient delivery to intraocular tissues (Aşıkgarip et al., 2022; Zeng et al., 2022). Importantly, systemic vascular conditions such as hypertension are strongly associated with retinal vascular occlusions, underscoring a unified pathophysiological mechanism in which cardiovascular dysregulation leads to vascular insufficiency and ischemic damage across the entire ocular system (Cheung et al., 2012).
Traditional ocular therapies often limited by factors such as invasive delivery, low bioavailability, and poor patient compliance. Standard treatments—including anti-VEGF agents and laser photocoagulation—face therapeutic ceilings and procedural burdens that restrict their long-term efficacy. In this context, materials science offers powerful interdisciplinary solutions by enabling precise drug delivery, real-time biosensing, and bioengineered tissue regeneration. Recent breakthroughs include microenvironment-responsive smart materials, on-demand drug release systems, and integrated physiological monitoring technologies. Smart contact lenses and injectable hydrogels exemplify these advances, providing improved drug retention, enhanced tissue integration, and personalized treatment modalities (Zhu et al., 2022; Wang L. et al., 2024). Emerging technologies such as smart contact lenses, sustained-release platform, and nanostructured hydrogels are expanding therapeutic possibilities for ocular diseases, particularly those linked to systemic dysfunction (Liu J. et al., 2020; Jiang et al., 2024).
This review presents a comprehensive analysis of six major ocular diseases from a dual perspective: the pathogenesis driven by cardiovascular risk factors and the therapeutic opportunities enabled by advanced biomaterials. For each condition, we discuss the underlying mechanisms, current treatment challenges, and how materials science offers targeted, functional, and clinically relevant interventions. Finally, we highlight existing challenges and propose future directions for translational application and interdisciplinary research (Figure 1).
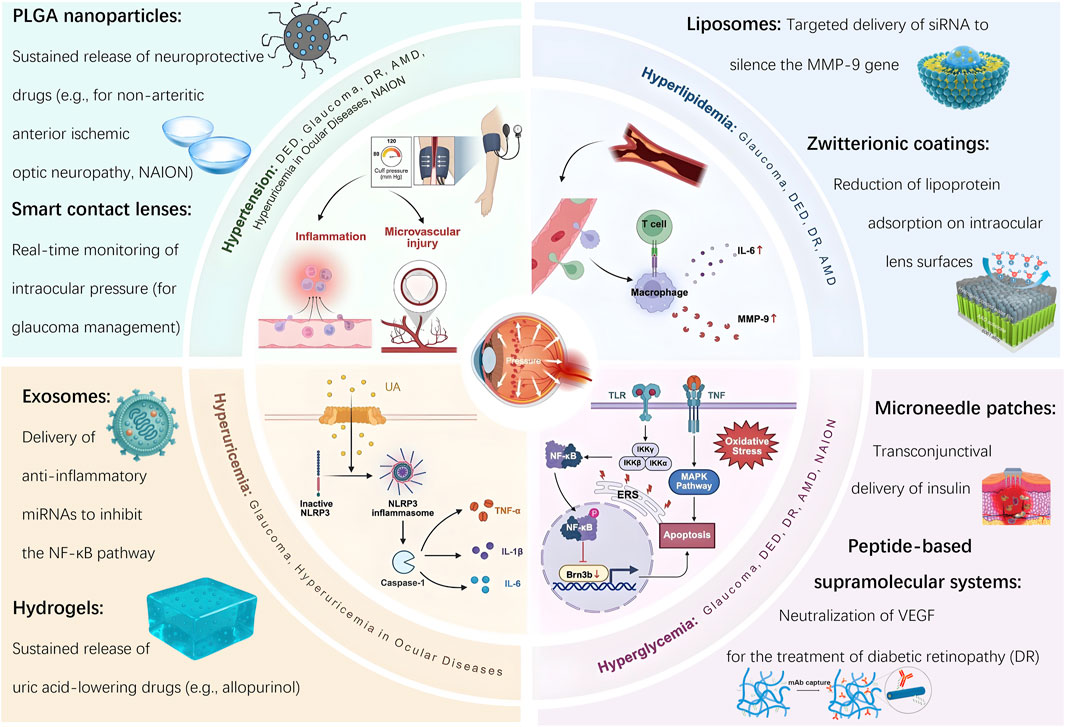
Figure 1. Bioaterial-based interventions for ocular diseases induced by cardiovascular risk factors.
2 Dry eye disease
The pathogenesis of Dry Eye Disease (DED) is influenced by systemic conditions such as hypertension, hyperlipidemia, and hyperglycemia, which exacerbate disease progression through mechanisms involving chronic inflammation and oxidative stress (Thang et al., 2024; Tran Tat et al., 2024; Park and Park, 2016; Su et al., 2022). Recent advances in materials science—particularly in targeted drug delivery systems and biosensing technologies—have introduced innovative diagnostic and therapeutic strategies that support the development of personalized treatment approaches.
2.1 Pathological mechanisms of dry eye disease induced by cardiovascular risk factors
Clinical evidence demonstrates a strong association between cardiovascular risk factors and the severity of DED. In patients with primary hypertension, the prevalence of DED reaches 41.7%, significantly higher than 18.8% observed in control groups (P < 0.001). Moreover, hypertension exhibits a stage-dependent relationship with DED prevalence, increasing from 27.1% in stage I to 57.6% in stage III hypertension (P < 0.001), with comorbid diabetes further elevating risk to 55.6% in T2DN patients (vs. 37.3% in general diabetics). Independent risk factors include advanced age, longer hypertension duration, concurrent diabetes, and elevated levels of plasma creatinine and high-sensitivity C-reactive protein (hs-CRP) (P < 0.001) (Thang et al., 2024; Tran Tat et al., 2024). Notably, antidiabetic medications choice may also influence DED risk—a recent study found that patients with type 2 diabetes initiating sodium-glucose cotransporter 2 inhibitors had a significantly lower incidence of DED compared to those receiving glucagon-like peptide-1 receptor agonists (9.0 vs. 11.5 cases per 1,000 person-years, HR = 0.78) (Su et al., 2022). The impact of cardiovascular conditions on DED is further exacerbated by comorbidities such as diabetes, which compromises ocular surface integrity through metabolic dysregulation. This indicates DED may serve as a manifestation of broader systemic dysfunction involving inflammation, vascular health and disturbances in the ocular microenvironment. Dyslipidemia also contributes significantly to the pathogenesis of DED and meibomian gland dysfunction (MGD). Elevated total cholesterol and triglycerides are associated with an increased risk of DED (OR = 1.6, 95% CI 1.2–2.1), with hypertriglyceridemia independently linked to DED symptoms in females (OR = 1.13) (Park and Park, 2016). While MGD is commonly related to lipid abnormalities, a cohort study suggests that dyslipidemia may be more closely tied to non-MGD forms of DED, possibly through mechanisms involving tear film instability or the upregulation of inflammatory markers such as interleukin-6 (IL-6) and matrix metalloproteinase-9 (MMP-9) (Mussi et al., 2021). This finding underscores the importance of a systems-based perspective on DED, recognizing the interconnection between cardiovascular risk factors and ocular surface health.
2.2 Materials science in dry eye disease
In response to the pathological mechanisms described above, materials science has introduced innovative strategies for the treatment of DED. These advancements have transformed DED management through two synergistic approaches: advanced diagnostic systems and targeted therapeutic platforms. Cutting-edge sensing technologies capable of multi-parameter detection and biomarker identification have greatly improved diagnostic accuracy. For example, smart contact lenses embeded with fluorescent corneal lenses enable quantitative analysis of tear film properties—such as pH and electrolyte concentration—through smartphone integration (Yetisen et al., 2020). This innovation is particularly relevant for patients with cardiovascular comorbidities, whose tear film dynamics and inflammatory responses may fluctuate due to systemic conditions like hypertension, diabetes, and dyslipidemia. Label-free biosensors based on localized surface plasmon resonance (LSPR), incorporating gold nanoshell-hydrogel composites (e.g., Al-OEGA-coated AuNSs, AuNS@PNM), detect tear proteins such as lysozyme and lactoferrin through covalent or electrostatic binding. These sensors generate linear LSPR wavelength shifts, enabling highly sensitive, portable detection of tear biomarkers. Such systems support early screening of chronic DED, facilitate grading of ocular surface inflammation, and promote personalized therapeutic strategies (Culver et al., 2018; Wechsler et al., 2021). Targeted drug delivery technologies address the limitations of conventional eye drops, which typically exhibit bioavailability below 5% and often lead to side effects. Advanced liposomal nanosystems target the ocular surface using electrostatic adhesion and lysosomal escape, co-delivering SS-31 peptides and insulin, demonstrating significant anti-inflammatory, antioxidant and mitochondrial repair effects that restore tear secretion, reduce pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and improve mitochondrial function in DED models (Xia et al., 2024). Contact lens-based sustained-release systems, utilizing silicone hydrogels or vitamin E-modified carriers, have been developed to extend the release duration of drugs such as cyclosporine A for up to 14 days. These platforms support long-term immunomodulation and ocular surface repair, while minimizing dosing frequency—an important factor in improving patient compliance in chronic disease management (Mondal et al., 2023). Furthermore, integrated theranostic systems that combine fluorescent sensing contact lenses with drug-loaded nanocarriers (e.g., lipid nanocapsules, micelles) offer real-time monitoring of tear film parameters alongside hydrophobic drug delivery. These systems extend ocular retention time and reduce administration frequency, enhancing both therapeutic efficacy and user convenience (Joshi et al., 2023). Despite these significant advancements, challenges related to sensitivity, comfort, and production cost continue to limit widespread clinical translation. Nevertheless, the integration of real-time diagnostics, targeted drug delivery, and sustained-release systems represents a promising paradigm shift in the management of ocular diseases influenced by cardiovascular risk factors.
3 Glaucoma
Glaucoma, a leading cause of irreversible blindness, is primarily associated with elevated intraocular pressure (IOP). Cardiovascular risk factors - such as hypertension, hyperlipidemia, and hyperuricemia - can impair ocular blood flow, contributing to abnormal increases in IOP and thereby elevating the risk of glaucoma development (Kuang et al., 2020; Wang L. et al., 2024; Zhou et al., 2022; Biggerstaff et al., 2021).
3.1 Pathological mechanisms of glaucoma induced by cardiovascular risk factors
Systemic hypertension exhibits a bidirectional relationship with IOP, with epidemiological studies demonstrating a 1.4-fold increased risk of primary open-angle glaucoma (POAG) (95% CI 1.2–1.7). This association is primarily mediated through impaired ocular hemodynamics and disrupted axonal transport in the optic nerve (Kuang et al., 2020; Wang L. et al., 2024). In addition to elevated blood pressure, dyslipidemia plays a significant role in the pathogenesis of glaucoma. Elevated triglyceride levels are associated with an increased risk of glaucoma (HR = 1.4, 95% CI: 1.2–1.7), and polymorphisms in the APOB gene further modulate susceptibility (Kang et al., 2024). Interestingly, statin use demonstrates population-specific effects, with increased glaucoma risk observed in individuals aged 60–69 or those with LDL-C levels ≥4.1 mmol/L (RR = 1.2, P = 0.04) (Lee et al., 2024). Disorders of glucose metabolism, particularly diabetes, also represent a major risk factor for glaucoma, though the underlying mechanisms are multifaceted. Diabetes significantly elevates glaucoma risk - as evidenced in the Blue Mountain Eye Study (OR = 2.12, 1997) - with hyperglycemia-induced retinal ganglion cell (RGC) apoptosis through endoplasmic reticulum stress (ERS). Notably, ERS inhibitors such as 4-phenylbutyric acid have been shown to reverse these effects (Zhou et al., 2022). Furthermore, hyperglycemia suppresses the expression of Brn3b via signaling pathways involving NO, NF-κB, and TNF-α, further accelerating RGC apoptosis (Tjandra et al., 2020). The risk is compounded by other metabolic syndrome components such as hypertension and prolonged hyperglycemia. Elevated fasting glucose levels and longer diabetes duration are strongly correlated with increased glaucoma risk (AlDarrab et al., 2023; Li et al., 2024). In postmenopausal women, the severity of diabetes is particularly impactful, with insulin use associated with a nearly twofold increased glaucoma risk (HR = 1.884) (Jung et al., 2021). Neovascular glaucoma, often secondary to diabetic retinopathy (DR) requires comprehensive management. However, genetic heterogeneity - such as GLIS3 mutations - and paradoxical findings, such as reduced open-angle glaucoma (OAG) risk in some diabetic populations, suggest that distinct molecular subtypes may underlie disease progression (Boddu et al., 2022; 2022; Virtanen et al., 2023). Overall, hyperglycemia promotes glaucoma development through mechanisms involving ERS, inflammation, and vascular injury, underscoring the importance of early screening and glycemic control (Yang et al., 2021). Among metabolic risk factors, uric acid (UA) plays a paradoxical and still controversial role. While gout has been associated with a 19% lower risk of POAG (HR = 0.81) (HR = 0.81) (Biggerstaff et al., 2021), lower serum UA levels have also been correlated with increased POAG risk (Serra et al., 2021). These findings suggest that uric acid may participate in glaucoma pathogenesis via complex inflammatory mediator networks, highlighting the potential role of anti-inflammatory strategies. Proinflammatory cytokines such as IL-6 and TNF-α serve as molecular bridges between cardiovascular and ocular diseases. IL-6 may enhance aqueous humor outflow in the short term but contributes to long-term trabecular meshwork damage, thereby impairing IOP regulation (Xiao et al., 2023). TNF-α facilitates RGC apoptosis and glaucomatous tissue injury by activating the NF-κB and MAPK signaling pathways (Li et al., 2021). Taken together, these findings reveal a complex, multifactorial interplay between cardiovascular risk factors and glaucoma development, mediated by cellular dysfunction, inflammatory responses, and vascular remodeling. Understanding these mechanisms underscores the importance of exploring molecular pathways and investigating innovative material-based strategies targeting these pathological processes. Ultimately, an integrated, systemic-ocular approach is essential to improve clinical outcomes in glaucoma management.
3.2 Application of materials science in glaucoma
Given the influence of cardiovascular comorbidities such as hypertension and diabetes on the pathogenesis of glaucoma, biosensing technologies that integrate intraocular pressure (IOP) monitoring with metabolic parameters (e.g., tear glucose and inflammatory cytokines) represent promising tools for early detection and intervention in patients at cardiometabolic risk. Sensing technologies have rapidly advanced in medical applications, especially for real-time disease monitoring and therapeutic guidance. Modern systems now combine biosensors with data acquisition platforms, enabling continuous, real-time tracking of IOP and other relevant parameters (Shean et al., 2024; Shao et al., 2025). Compared to traditional diagnostic approaches, these technologies support more precise, personalized, and multi-parametric health management (Shin et al., 2021). As mechanistic understanding of cardiovascular–IOP interactions deepens, intelligent sensors are becoming indispensable in translating this knowledge into individualized glaucoma care. Nonetheless, limitations remain, including suboptimal sensitivity, limited stability, constrained integration capacity, and dependence on single time-point measurements—as typified by Goldmann applanation tonometry (Moses, 1958). To address these challenges, smart contact lenses incorporating flexible sensors and wireless communication modules have been developed (Kim et al., 2022; Zhu et al., 2022). Innovative sensor designs using silver nanowires and hollow gold nanowires have demonstrated excellent sensitivity and biocompatibility for ocular applications (Kim et al., 2021; Kim et al., 2022). Most significantly, the hollow gold nanowire-based design achieves 11%–25% greater sensitivity compared to conventional thick Parylene C substrates when measuring equivalent IOP levels. Now providing 24-h IOP monitoring, facilitating early glaucoma detection and personalized treatment (Zhang J. et al., 2022). These platforms are evolving into multifunctional systems, where sensor feedback can trigger on-demand drug release (Kim et al., 2022), Hydrogel-based biosensors—such as those using Ti3C2Tx MXene—allow for simultaneous detection of tear glucose and IOP, supporting remote monitoring and real-time health display (Kim et al., 2017; Zhu et al., 2022; Duan et al., 2024). These multifunctional platforms enable long-term, non-invasive, real-time continuous IOP monitoring, marking a transformative shift in glaucoma diagnosis and the broader management of chronic diseases.
Drug delivery technologies are also crucial in addressing the limitations of conventional treatments, particularly for glaucoma and diabetic retinopathy. Ocular anatomical barriers—such as tear turnover and the blood–retinal barrier—severely restrict the bioavailability of topical agents, often reducing it to below 5% (Tomi and Hosoya, 2010). Therefore, innovative delivery platforms are essential for enhancing drug retention, decreasing dosing frequency, and minimizing systemic exposure (Akulo et al., 2022; Lin et al., 2023). Cardiovascular risk factors exacerbate glaucoma progression through multiple pathways (see Section 2.1), making precisely targeted delivery systems imperative: Nanocarrier platforms utilizing extracellular vesicles and exosomes can encapsulate RNA, proteins, and lipids, thereby enhancing drug targeting while minimizing side effects (Liu J. et al., 2020; Durmaz et al., 2024). Furthermore, Brugenera et al. developed a novel preservative-free liposomal delivery system (LAT-HA-LIP) that simultaneously achieves sustained IOP control and protects the ocular surface—addressing the limitations of conventional anti-glaucoma eyedrops, which may destabilize the tear film and cause DED due to preservative toxicity (Brugnera et al., 2025). Moreover, recent studies show that core-shell nanoparticles with PLGA carriers achieve 1.8-fold higher bioavailability in the choroid compared to PLA carriers when delivered via the conjunctival-scleral pathway (p = 0.003), offering new therapeutic strategies for posterior segment diseases (Mahaling and Katti, 2016). This underscores a shift toward more precise, controlled, and sustainable drug delivery, with nanotechnology enhancing both drug penetration and the safety profile of ocular therapies. Periocular routes—such as subconjunctival injections—also enable sustained drug delivery to the posterior segment, offering a minimally invasive yet effective approach (Rafiei et al., 2020). Sustained-release technologies, including drug-loaded contact lenses, wearable devices, and intraocular implants, offer precise control over drug release kinetics and improve patient adherence (Kompella et al., 2021; Al-Qaysi et al., 2023). Hydrogel-based systems and dendritic polymers—owing to their high water content, drug-protective capacity, tunable release profiles, and anti-inflammatory properties—optimize retention on the ocular surface (Akulo et al., 2022; Wang et al., 2023). Overall, these systems allow efficient penetration into ocular tissues and represent a significant improvement over traditional topical therapies.
Tissue repair materials are widely applied in ophthalmology, especially for managing postoperative scarring and neurodegeneration in glaucoma. Chronic inflammation induced by cardiovascular risk factors exacerbates fibrosis following glaucoma surgery. These biomaterials work by accelerating tissue healing, modulating inflammation, and guiding cell growth using physical scaffolds or bioactive agents. However, challenges remain regarding biodegradability, long-term efficacy, and target specificity. Advances in biomaterials, nanotechnology, and bioengineering have greatly expanded their clinical potential (Kompella et al., 2021; Sun et al., 2021). In glaucoma surgery, photo crosslinkable hydrogels such as GelDex-S58 inhibit postoperative fibrosis by controlling TGF-β signaling (Lin et al., 2023). Likewise, RGD (arginine–glycine–aspartate)-functionalized hydrogels target β1-integrin/FAK/Akt signaling pathways to suppress Tenon’s fibroblast activation, thereby reducing fibrotic scar formation (Chen B. et al., 2024). Beyond surgical applications, hydrogels can serve as reservoirs for sustained drug delivery. Mitomycin C (MMC)-loaded hydrogels, including those combined with RGD-modified carriers, provide prolonged anti-fibrotic effects with fewer side effects compared to conventional MMC treatments (Tu et al., 2023; Wu et al., 2023). Importantly, hypertension—a key cardiovascular risk factor—can directly elevate IOP through mechanical compression of the optic nerve or induce ischemic optic nerve damage via microvascular pathologies. Thus, in addition to structural repair, thermosensitive hydrogels loaded with neuroprotective agents have been developed. These hydrogels not only promote optic nerve regeneration but also help preserve visual function (Wang L. et al., 2024). With sensor integration, these materials provide a platform for real-time, feedback-controlled therapy—supporting both tissue recovery and functional vision preservation.
4 Ocular diseases related to hyperuricemia
Hyperuricemia, defined by elevated serum uric acid (UA) levels, has been implicated in a variety of systemic conditions, including gout, renal dysfunction, and cardiovascular diseases. Emerging evidence suggests that hyperuricemia is also linked to several ocular disorders, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and glaucoma. Elevated UA levels may contribute to the development and progression of these ocular pathologies by inducing oxidative stress, promoting inflammatory responses, and compromising the integrity of the blood-retinal barrier (Biggerstaff et al., 2021; Serra et al., 2021).
4.1 Pathological mechanisms of hyperuricemia in ocular diseases
Elevated serum uric acid (UA) contributes to ocular damage through multiple pathogenic pathways. High UA levels can promote endothelial cell dysfunction and increase vascular permeability, thereby accelerating the progression of DR and other retinal disorders (Ao et al., 2017; Li et al., 2019). Hyperuricemia exacerbates retinal microvascular and neuronal damage, with distinct mechanistic insights emerging from recent studies. Lu et al. (2022) reported an 18.3% reduction in superficial capillary plexus density among hyperuricemic women (p < 0.01), showing a significant linear correlation with serum urate levels (β = −0.24, p = 0.003). Complementarily, Yang et al. (2023) found that in males, each 1 mg/dL increase in serum UA was associated with a 13% increase in deep retinal capillary non-perfusion areas (OR = 1.13, 95% CI 1.05–1.22). Wei et al. (2023) further demonstrated that hypertensive patients with cerebral white matter lesions exhibited impaired macular microvascular architecture (FD-300, r = −0.41, p = 0.007), implicating disruption of the blood–retinal barrier.
Gout, a condition intrinsically linked to hyperuricemia, also presents with distinct ocular manifestations. Studies by Sharon and Schlesinger and Ao et al. (Sharon and Schlesinger, 2016; Ao et al., 2017) reported conjunctival urate crystal deposits in 33% of patients and neurotrophic dry eye in 22%. Karti et al. (2024) quantified retinal neurodegeneration in gout patients, showing sectoral thinning of the retinal nerve fiber layer (RNFL: −9.6 μm nasal) and ganglion cell complex (GCC: −12.3 µm inferior; p ≤ 0.005). These findings suggest that crystalline deposition within ocular tissues can provoke both inflammation and neurodegeneration. Meyer et al. (2024) provided pathological evidence of this process through a case of eyelid tophus, linking IL-1β–driven M1 macrophage polarization to chronic ocular inflammation. UA crystals activate the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. These mediators contribute to retinal neovascularization and macular edema in conditions such as DR and AMD (Thounaojam et al., 2019). In the pathogenesis of OAG, UA induces oxidative stress in the trabecular meshwork, impairing aqueous humor outflow and leading to elevated IOP. Additionally, crystal deposition contributes to RGC apoptosis (Biggerstaff et al., 2021; Serra et al., 2021). Moreover, hyperuricemia may exacerbate AMD by promoting choroidal inflammation. Elevated UA levels trigger the release of inflammatory mediators in the retina, damaging retinal pigment epithelial (RPE) cells and fostering the development of choroidal neovascularization (Pai et al., 2021; Pai et al., 2024). These findings underscore the dual role of UA in AMD - as both a systemic marker of oxidative stress and a direct mediator of ocular tissue injury - contributing to disease mechanisms across both anterior and posterior segment disorders.
4.2 Materials science in the treatment of hyperuricemia-related ocular diseases
Emerging biomaterial-based strategies are transforming the management of hyperuricemia-related ocular pathologies through precision-targeted interventions. Hyperuricemia, which is closely linked to gout and ocular diseases such as uveitis and retinal vascular occlusion, can be effectively addressed using advanced drug delivery systems.
Hydrogel microneedles loaded with colchicine have shown promise in localized treatment by effectively reducing inflammatory cytokine levels (Jiang et al., 2023). Macrophage-targeted liposomes encapsulating melatonin have demonstrated the ability to reprogram macrophage metabolism, presenting a novel therapeutic approach for ocular inflammation (Ma et al., 2025). A chitosan-based microneedle platform co-delivering colchicine and uricase enables sustained drug release, thereby improving patient adherence and supporting systemic uric acid management (Yang et al., 2023). Manganese-doped albumin nanogels (MAGNs) loaded with berberine have also shown efficient bioavailability and targeted delivery to inflamed tissues, which could be beneficial in treating hyperuricemia-associated ocular inflammation (Sun et al., 2023). Furthermore, red blood cell-encapsulated uricase formulations extend circulation time and enhance uric acid reduction, offering a promising strategy for enzyme replacement therapy (Ban et al., 2024). Microneedles provide a controlled and localized drug delivery system, with potential applications for ocular diseases linked to hyperuricemia (Yi et al., 2024). Febuxostat-loaded microneedles and nanogels enhance drug bioavailability and penetration, offering a solution for treating hyperuricemia-related eye conditions (Patel and Thakkar, 2023; Khan et al., 2024). In conclusion, these advances in materials science offer effective, non-invasive treatment options for hyperuricemia-related ocular diseases by enhancing drug targeting, minimizing systemic side effects, and improving patient compliance.
5 Diabetic retinopathy
Diabetic retinopathy is the leading cause of vision loss among individuals with diabetes. As the global prevalence of diabetes continues to rise, the incidence of DR is also increasing. The hallmark pathological features of DR include microvascular damage and pathological neovascularization.
5.1 Pathological mechanisms of diabetic retinopathy induced by cardiovascular risk factors
The pathological mechanisms by which cardiovascular risk factors contribute to DR underscore the disease’s complexity and the profound influence of systemic health on ocular outcomes. Hypertension (HTN) independently elevates the risk of DR, with each 1-unit increase in systolic blood pressure variability associated with a 2% higher risk (RR = 1.02), and even high-normal blood pressure (≥120/80 mmHg) showing a significant association with DR incidence (aOR = 1.114) (Zhang et al., 2023; Noroozi et al., 2024). These findings suggest that effective DR management must incorporate systemic parameters such as blood pressure, as they play a critical role in modulating retinal damage progression. Mendelian randomization studies further support a causal relationship between HTN and DR, with elevated intraocular pressure (IOP) also contributing to DR risk (OR = 1.090) (Wang X.-F. et al., 2024). Hypertension accelerates both retinal neurodegeneration and microvascular damage, leading to decreased peripapillary retinal nerve fiber layer (pRNFL) thickness, ganglion cell complex thinning, and reduced microvascular density compared to normotensive individuals with DR. It also promotes disease progression from early arteriolar thickening to advanced capillary occlusion (Huang and Fawzi, 2024; Sung et al., 2024; Yu et al., 2025). While achieving blood pressure control (target <130/80 mmHg) reduces the risk of DR onset (RR = 0.78), its effect on disease progression is modest (RR = 0.94). Notably, up to 19.7% of diabetic patients with a disease duration of ≥8 years remain undiagnosed with HTN (Woodward et al., 2020; Qureshi et al., 2025), underscoring the need for integrated retinal and blood pressure monitoring. The progression from arteriolar thickening to capillary occlusion illustrates how vascular injury leads to impaired retinal perfusion, exacerbating ischemia and contributing to retinal degeneration.
Hyperglycemia also plays a central role in DR pathogenesis by amplifying oxidative stress and inflammation, largely via the metabolic memory effect (Taurone et al., 2020; Huang et al., 2024). Elevated glucose levels trigger pericyte apoptosis, blood-retinal barrier (BRB) disruption, and vascular leakage through several mechanisms: mitochondrial reactive oxygen species (ROS) generation, advanced glycation end-product (AGE)–receptor for AGE (RAGE) signaling, VEGF upregulation, and microglial exosomal release of miR-155 (Lin et al., 2021; Tang et al., 2023; Wang X. et al., 2024). These pathways synergistically exacerbate retinal ischemia, as evidenced by increased central foveal thickness (∆ = +45 μm, P = 0.002) (Wang X. et al., 2024). Thus, comprehensive DR management must address not only local retinal pathology but also systemic metabolic and hemodynamic dysfunction.
Among ocular diseases associated with cardiovascular risk factors, DR is one of the most prevalent and severe. Sensing technologies are proving highly valuable in the monitoring, diagnosis, and treatment of such conditions (Keum et al., 2020). While current treatments primarily rely on intravitreal injection of anti-VEGF antibodies, long-term use of these agents can result in complications such as endogenous endophthalmitis (Li Y.-N. et al., 2023). Consequently, there is an urgent need for non-invasive diagnostic and therapeutic alternatives. To overcome the limitations of conventional injection-based therapies, nanoparticle drug delivery systems offer unique advantages, including targeted delivery and sustained release. For example, core-shell polycaprolactone/Pluronic® F68 nanoparticles loaded with triamcinolone acetonide alleviate both inflammation and vascular abnormalities (Mahaling et al., 2018) loaded with triamcinolone acetonide simultaneously alleviate inflammation and vascular abnormalities. IL-12-loaded polymeric nanoparticles (IL-12-PNPs) inhibit VEGF-A and MMP-9 expression, helping to restore retinal thickness (Zeng et al., 2019). Fenofibrate-loaded nanoparticles (Feno-NPs) maintain therapeutic efficacy for up to 60 days after a single injection, reducing vascular leakage and neovascularization (Qiu et al., 2019). Chitosan/PLGA-based hydrogels delivered via subconjunctival injection modulate the VEGF/Occludin balance and reduce retinal apoptosis (Rong et al., 2019). Magnetic nanoparticle-optical coherence tomography (OCT) conjugates improve drug activity by over 100-fold and achieve targeted distribution to the retina (Amato et al., 2020). These advanced delivery systems integrate anti-inflammatory, anti-angiogenic, and neuroprotective mechanisms, offering sustained drug release (ranging from weeks to months) with minimal invasiveness via intravitreal or subconjunctival routes. As a result, they significantly enhance bioavailability and reduce treatment-associated risks, pushing DR therapy toward a precision medicine model. Emerging solutions include quantum dot-based immunosensors for tear biomarker detection in diabetic retinopathy (LOD = 110 pg/mL) and SGLT2 inhibitors reduce 46% ROS accumulation and significantly attenuate retinal apoptosis independent of glucose lowering, through the ERK1/2–cPLA2–AA–ROS signaling cascade (Wang et al., 2017; Hu et al., 2022).
In parallel, non-invasive treatment strategies have made notable progress. Smart supramolecular peptide-based eye drops capable of selectively binding soluble Semaphorin 4D effectively reduce pathological retinal neovascularization and leakage in DR models (Li Y.-N. et al., 2023). These innovations complement nanoparticle-based therapies, with sensing technologies - such as near-infrared contact lenses and fingertip AGE detectors-enabling early-stage diagnosis, while drug delivery systems target mid- and late-stage pathology through multi-target interventions (anti-inflammatory, anti-angiogenic, neuroprotective). The integration of sensor arrays with machine learning has further enabled rapid, cost-effective, and reliable diagnostic tools for diabetes and DR, particularly via non-invasive biomarker detection (Faura et al., 2022). These material-science - driven advancements not only improve patient compliance but also establish a new paradigm for DR management by precisely modulating key pathological pathways, including VEGF, ICAM-1, and Occludin.
6 Age-related macular degeneration
Age-related macular degeneration is the leading cause of vision loss in the elderly population. In addition to increasing the risk of cardiovascular diseases, cardiovascular risk factors are closely linked to both the onset and progression of AMD (Chen et al., 2023; Nahavandipour et al., 2020; Yadav et al., 2024). The underlying mechanisms connecting these conditions involve metabolic dysregulation, chronic inflammation, and vascular damage, which interact in complex and synergistic ways.
6.1 Pathological mechanisms of AMD induced by cardiovascular risk factors
Hypertension significantly increases the risk of wet age-related macular degeneration (wAMD). It is also associated with a greater need for anti-VEGF treatments, largely due to choroidal endothelial dysfunction. The impact of pharmacological interventions varies: β-blockers such as propranolol have been shown to reduce late-stage AMD risk by 30%, likely through improved choroidal perfusion and suppression of interleukin-6 (IL-6). In contrast, thiazide diuretics are associated with a 45% increased risk of AMD in women—an effect that appears to be mitigated when co-administered with ACE inhibitors or angiotensin receptor blockers (Xu et al., 2020; Faura et al., 2022; Luo et al., 2023). Dyslipidemia also contributes to AMD risk in a nonlinear fashion, with both very high (≥77 mg/dL) and very low (<40 mg/dL) high-density lipoprotein cholesterol (HDL-C) levels linked to increased susceptibility. Genetic polymorphisms related to lipid metabolism - such as CETP rs173539 and COLEC12 rs1999930 - further influence this relationship (Chen et al., 2025). A Korean study reported a 52% increased risk of AMD in individuals with hyperlipidemia (aHR = 1.52), while long-term statin use (e.g., atorvastatin for ≥5 years) was associated with a dose-dependent risk reduction (aHR = 0.70), likely due to statins’ anti-inflammatory and antioxidant properties (Chen et al., 2023). These findings support the potential for statins to play a dual role in cardiovascular risk reduction and AMD progression, promoting an integrated treatment strategy that addresses both systemic and ocular health.
Data from Iran showed a 6.4% prevalence of AMD among patients with hyperlipidemia, with increased risk observed in those with concurrent hypertension and diabetes, emphasizing the broader impact of metabolic syndrome on ocular health (Panahi et al., 2023). The relationship between diabetes and AMD is complex and heterogeneous. For instance, newly diagnosed diabetic patients face a 30% increased risk of wAMD, while insulin-treated individuals show a 23% higher incidence (aHR = 1.23). Those with vision-threatening diabetic retinopathy (DR) have an even greater risk (aHR = 1.35), likely mediated by hyperglycemia-induced VEGF activation (Hwang et al., 2023; Lee et al., 2023). Meta-analyses affirm a significant association between diabetes and advanced AMD (OR = 1.38, 95% CI: 1.12–1.71), although cross-sectional studies have reported inconsistent findings regarding wAMD prevalence (Zhang Y. P. et al., 2022; Virtanen et al., 2023). IL-6 has emerged as a key molecular mediator in this process, with systemic levels significantly elevated in late-stage age-related macular degeneration (AMD), including geographic atrophy and neovascular subtypes, while showing only marginal association with early AMD (Nahavandipour et al., 2020; Yadav et al., 2024). Within the local ocular microenvironment, IL-6 levels are closely correlated with VEGF-A and ICAM-1 expression, implicating activation of the STAT3 signaling pathway in neovascularization and disruption of the blood-retina barrier (Li et al., 2022). Systemic inflammatory markers also correlate strongly with disease severity. For instance, each 1 mg/L increase in CRP is associated with an 8.2 μm reduction in choroidal thickness. Elevated E-selectin levels not only predict AMD progression but also indicate heightened cardiovascular risk (Chen et al., 2021; Nashine et al., 2022), underscoring the shared pathophysiology between endothelial dysfunction, vascular compromise, and retinal degeneration.
The intricate interplay among systemic inflammation, vascular health, and retinal pathology calls for an integrated, cross-disciplinary approach that bridges cardiovascular and ocular care. Targeting systemic inflammation may be critical in halting AMD progression and mitigating its association with other systemic diseases.
6.2 Materials science in age-related macular degeneration
Materials science has revolutionized the treatment of AMD by addressing the limitations of conventional anti-VEGF therapies. Implantable drug depot systems now enable sustained release of ranibizumab for up to 6 months, eliminating the need for additional injections in 98% of patients and reducing intraocular drug level fluctuations by 60% compared to monthly dosing (Khanani et al., 2021; Patel et al., 2021). Nanotechnology-based delivery platforms have significantly enhanced targeting efficiency. For example, pH-sensitive PLGA nanoparticles extend the vitreous half-life of bevacizumab from 9.8 to 34.5 days, while RGD-modified liposomes achieve fivefold higher drug accumulation at lesion sites (Chen X. et al., 2024; Xu et al., 2024). Synthetic HDL nanoparticles engineered to deliver rapamycin to retinal pigment epithelial (RPE) cells have demonstrated a 68% reduction in choroidal neovascularization (Mei et al., 2022). Hydrogels, as multifunctional carriers, also show great promise. Nanofiber hydrogels co-loaded with dexamethasone and ranibizumab prolonged anti-VEGF efficacy up to 12 weeks in rabbit models and reduced vitreous inflammation by 73% (Gao et al., 2023). Gene therapy represents another major advancement. Adeno-associated virus (AAV) vectors, such as RGX-314 delivered via subretinal injection, can suppress VEGF expression for over 2 years. In phase I/IIa clinical trials, 84% of patients required no additional treatment during the study period (Campochiaro et al., 2024). This approach offers the potential to replace years of repeated intravitreal injections with a single, long-lasting intervention, exemplifying the promise of regenerative medicine for chronic ocular conditions.
In the regenerative domain, collagen glue hydrogels that mimic the biomechanical properties of the native extracellular matrix have been used to support the differentiation of human embryonic stem cells into RPE-like cells. These constructs enhanced photoreceptor survival by 41% following transplantation (Moyo et al., 2024). Interdisciplinary innovations are shifting AMD therapy from passive treatment toward precision modulation. For example, the novel rGO/PBASE electrochemical biosensor enables rapid detection of complement C3 protein within 15 min (limit of detection: 0.43 ng/mL), offering a high-sensitivity tool for early AMD screening and laying the foundation for integrated diagnostic–therapeutic systems (Ghosh et al., 2024). In parallel, artificial intelligence is being increasingly applied to clinical decision-making. Deep learning models have been shown to improve AMD staging accuracy and treatment response prediction by 23% (Crincoli et al., 2024). These models, when integrated with biosensor-derived data, can guide personalized drug administration and optimize individualized care strategies for AMD patients. Despite these promising advances, challenges remain. Issues such as long-term stability (e.g., acidic microenvironments generated by PLGA degradation) and immune compatibility (e.g., immunogenicity of PEGylated liposomes) need to be resolved (Bonechi et al., 2023; Marquina et al., 2023). Nevertheless, these innovations collectively mark a paradigm shift in AMD management - from repetitive, reactive interventions to personalized, sustained, and precision-based therapeutic strategies.
7 Non-arteritic anterior ischemic optic neuropathy
Non-arteritic anterior ischemic optic neuropathy (NAION) is an acute optic neuropathy strongly associated with vascular dysfunction. Its pathogenesis involves the synergistic interaction of multiple cardiovascular and metabolic risk factors.
7.1 Pathological mechanisms of NAION induced by cardiovascular risk factors
Systematic reviews and meta-analyses have identified hypertension, diabetes, and hyperlipidemia as independent risk factors for NAION. In particular, malignant hypertension can directly induce optic disc edema, serving as a direct trigger for NAION (Liu et al., 2021; Chatziralli et al., 2022; Miralles Pechuan et al., 2024). Notably, hypertension poses a dual threat by increasing the incidence of NAION and elevating the risk of concomitant cerebral infarction (Li X. et al., 2023). Hyperlipidemia, especially elevated triglycerides (SMD = +0.58, 95% CI: +0.12 to +1.04) and lipoprotein(a) levels (OR = 2.88, 95% CI: 1.01–8.21)—worsens optic nerve ischemia by promoting atherosclerotic changes (Chatziralli et al., 2022). The association between diabetes mellitus and an increased risk of NAION is well documented (Chen et al., 2013; Sharma et al., 2017). While visual prognosis in diabetic patients may not differ significantly from that in non-diabetics, coexisting cardiovascular conditions such as ischemic heart disease may further exacerbate optic nerve injury (Sharma et al., 2017). Among individuals with metabolic syndrome, key contributors to NAION include hyperglycemia, elevated triglycerides, and low high-density lipoprotein (HDL) levels (Kohli et al., 2022). Recent attention has focused on a potential link between glucagon-like peptide-1 receptor agonist, especially semaglutide, and NAION. This association presents a clinical paradox: while semaglutide provides substantial benefits in glucose regulation and cardiovascular protection, studies have reported a significantly increased NAION risk in patients with obesity or diabetes (hazard ratio [HR] = 4.28–7.64; cumulative incidence = 8.9%). However, broader population studies show a more modest association (HR = 1.32), highlighting the influence of population heterogeneity (Shin et al., 2021; Grauslund et al., 2024; Cai et al., 2025; Chou et al., 2025). Given these findings, clinicians should weigh semaglutide’s benefits against its potential ocular risks, particularly in high-risk patients, and prioritize optic disc evaluation and regular ophthalmic follow-up (Malerbi and Bertoluci, 2025). Furthermore, obstructive sleep apnea syndrome significantly increases the risk of NAION (RR = 3.28, 95% CI: 2.08–5.17) and coronary heart disease (RR = 1.68, 95% CI: 1.24–2.27). Inherited thrombophilic conditions, such as Factor V Leiden mutation, have also been associated with NAION (RR = 2.21, 95% CI: 1.19–4.09) (Liu et al., 2021).
7.2 Application of materials science in NAION
Although the application of materials science in the treatment of non-arteritic anterior ischemic optic neuropathy (NAION) remains in the exploratory phase, early advances show promising potential. Notably, curcumin-polydopamine nanocomposite hydrogels (Cur@PDA@GelCA) have demonstrated significant neuroprotective effects in optic nerve injury models. These materials act by inhibiting reactive oxygen species (ROS)-mediated oxidative stress, indicating potential utility for acute-phase intervention in NAION.
In addition to their therapeutic efficacy, the hydrogel’s strong tissue adhesion and excellent biocompatibility highlight its suitability for localized drug delivery and targeted treatment (Liu et al., 2024). These findings underscore the broader feasibility of integrating materials science into NAION therapy.
Future research should aim to further elucidate the molecular mechanisms underlying NAION and leverage multi-omics technologies alongside biomaterials innovation to develop personalized treatment strategies that can improve patient outcomes.
8 Outlook and summary
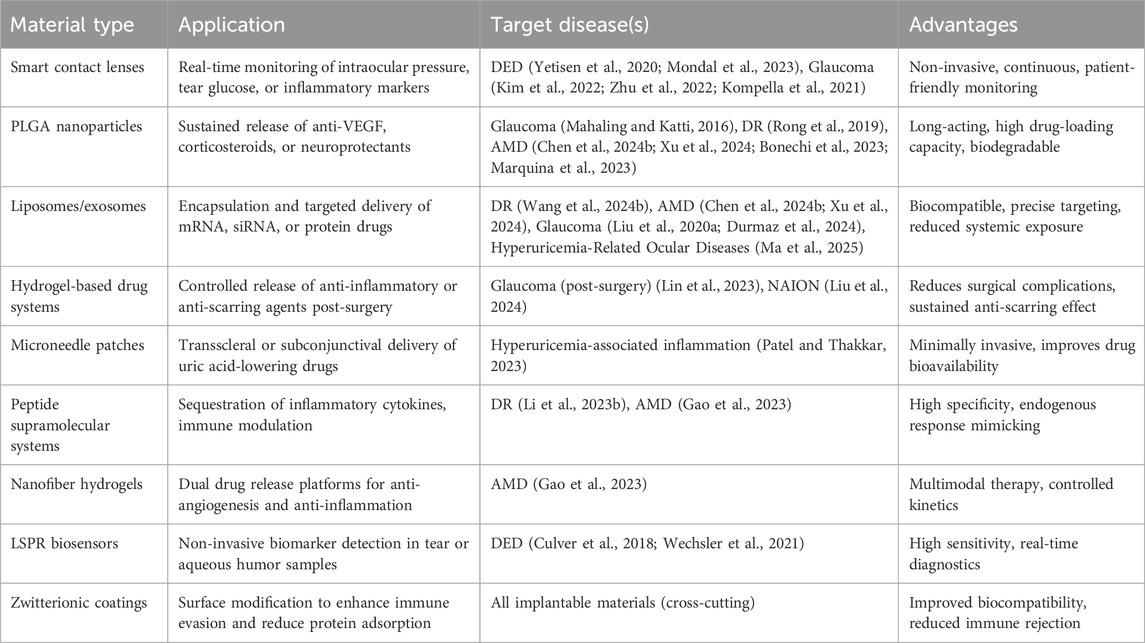
Materials science is increasingly recognized as a pivotal avenue for addressing ocular diseases associated with cardiovascular risk factors. Innovations such as biosensor-integrated smart contact lenses, PLGA-based nanocarriers, and neuroprotective hydrogels are driving therapeutic strategies toward greater precision, reduced invasiveness, and prolonged efficacy (Table 1). However, several critical challenges remain, such as the acidic microenvironment generated by PLGA degradation, the long-term biocompatibility of implants, and the limited efficiency of clinical translation.
Future research should advance along multiple fronts. In material design, pH-buffering coatings and environment-responsive modifications may help reduce local tissue irritation. For preclinical validation, organoid models and microfluidic “eye-on-a-chip” systems offer physiologically relevant platforms for evaluating safety and efficacy. To improve immune compatibility, approaches such as PEGylation and zwitterionic surface engineering can minimize inflammation and extend in vivo functionality.
Facilitating clinical translation will require early and proactive engagement with regulatory agencies to navigate approval pathways for nanomedicines and combination therapeutic devices. Additionally, stronger collaboration between academia and industry, paired with standardized, scalable manufacturing processes, will be essential for bridging the gap between laboratory innovation and clinical application.
In conclusion, the integration of advanced materials, biosensing technologies, and intelligent drug delivery systems offers a comprehensive and promising framework for the personalized management of ocular diseases in patients with cardiovascular comorbidities. A deeper alignment between engineering innovation and clinical practice will be vital to achieving long-lasting, widely applicable therapeutic outcomes.
Author contributions
PC: Writing – original draft. BZ: Writing – original draft. PW: Resources, Writing – original draft, Writing – review and editing. HL: Writing – review and editing. HP: Conceptualization, Funding acquisition, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project (2024ZD0526900), Tianfu Qingcheng Project-Tianfu Science and Technology Elite (No. 1358), Xining Joint Logistics Support Center-Technology Top Talent (to HP) and General Hospital of Western Theater Command-Head Goose Project Training Object (to HP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AGE, advanced glycation end; AMD, age-related macular degeneration; hs-CR, Phigh-sensitivity C-reactive protein; DED, dry eye disease; DR, diabetic retinopathy; ERS, endoplasmic reticulum stress; HDL, high-density lipoprotein; HTN, hypertension; IL-6, interleukin-6; IOP, intraocular pressure; LSPR, localized surface plasmon resonance; MAGNs, Manganese-doped albumin nanogels; MGD, meibomian gland dysfunction; MMC, mitomycin C; MMP-9, metalloproteinase-9; NAION, non-arteritic anterior ischemic optic neuropathy; OAG, open-angle glaucoma; POAG, primary open-angle glaucoma; RGC, retinal ganglion cell; RGD, arginine-glycine-aspartate; RNFL, retinal nerve fiber layer; ROS, reactive oxygen species; RPE, retinal pigment epithelium; UA, uric acid; wAMD, wet age-related macular degeneration.
References
Akulo, K. A., Adali, T., Moyo, M. T. G., and Bodamyali, T. (2022). Intravitreal injectable hydrogels for sustained drug delivery in glaucoma treatment and therapy. Polym. (Basel) 14, 2359. doi:10.3390/polym14122359
AlDarrab, A., Al Jarallah, O. J., and Al Balawi, H. B. (2023). Association of diabetes, fasting glucose, and the risk of glaucoma: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 27, 2419–2427. doi:10.26355/eurrev_202303_31776
Al-Qaysi, Z. K., Beadham, I. G., Schwikkard, S. L., Bear, J. C., Al-Kinani, A. A., and Alany, R. G. (2023). Sustained release ocular drug delivery systems for glaucoma therapy. Expert Opin. Drug Deliv. 20, 905–919. doi:10.1080/17425247.2023.2219053
Amato, R., Giannaccini, M., Dal Monte, M., Cammalleri, M., Pini, A., Raffa, V., et al. (2020). Association of the somatostatin analog octreotide with magnetic nanoparticles for intraocular delivery: a possible approach for the treatment of diabetic retinopathy. Front. Bioeng. Biotechnol. 8, 144. doi:10.3389/fbioe.2020.00144
Ao, J., Goldblatt, F., and Casson, R. J. (2017). Review of the ophthalmic manifestations of gout and uric acid crystal deposition. Clin. Exp. Ophthalmol. 45, 73–80. doi:10.1111/ceo.12749
Aşıkgarip, N., Temel, E., Kıvrak, A., and Örnek, K. (2022). Choroidal structural changes and choroidal vascularity index in patients with systemic hypertension. Eur. J. Ophthalmol. 32, 2427–2432. doi:10.1177/11206721211035615
Ban, Z., Sun, M., Ji, H., Ning, Q., Cheng, C., Shi, T., et al. (2024). Immunogenicity-masking delivery of uricase against hyperuricemia and gout. J. Control Release 372, 862–873. doi:10.1016/j.jconrel.2024.06.042
Biggerstaff, K. S., White, D. L., Frankfort, B. J., Richardson, P., Orengo-Nania, S., Garcia, J., et al. (2021). Gout and open-angle glaucoma risk in a veteran population. Graefes Arch. Clin. Exp. Ophthalmol. 259, 3371–3379. doi:10.1007/s00417-021-05273-2
Boddu, P. K., Velumula, P. K., Sharif, S., and Monika, B. (2022). A neonate with diabetes mellitus, congenital hypothyroidism, and congenital glaucoma. Cureus 14, e29488. doi:10.7759/cureus.29488
Bonechi, C., Mahdizadeh, F. F., Talarico, L., Pepi, S., Tamasi, G., Leone, G., et al. (2023). Liposomal encapsulation of citicoline for ocular drug delivery. Int. J. Mol. Sci. 24, 16864. doi:10.3390/ijms242316864
Brugnera, M., Vicario-de-la-Torre, M., González-Cela-Casamayor, M. A., González-Fernández, F. M., Ferraboschi, I., Andrés-Guerrero, V., et al. (2025). Disclosing long-term tolerance, efficacy and penetration properties of hyaluronic acid-coated latanoprost-loaded liposomes as chronic glaucoma therapy. J. Control Release 379, 730–742. doi:10.1016/j.jconrel.2025.01.041
Bu, J., Yu, J., Wu, Y., Cai, X., Li, K., Tang, L., et al. (2020). Hyperlipidemia affects tight junctions and pump function in the corneal endothelium. Am. J. Pathol. 190, 563–576. doi:10.1016/j.ajpath.2019.11.008
Cai, C. X., Hribar, M., Baxter, S., Goetz, K., Swaminathan, S. S., Flowers, A., et al. (2025). Semaglutide and nonarteritic anterior ischemic optic neuropathy. JAMA Ophthalmol. 143, 304. doi:10.1001/jamaophthalmol.2024.6555
Campochiaro, P. A., Avery, R., Brown, D. M., Heier, J. S., Ho, A. C., Huddleston, S. M., et al. (2024). Gene therapy for neovascular age-related macular degeneration by subretinal delivery of RGX-314: a phase 1/2a dose-escalation study. Lancet 403, 1563–1573. doi:10.1016/S0140-6736(24)00310-6
Chatziralli, I. P., Kazantzis, D., Chatzirallis, A. P., Machairoudia, G., Papageorgiou, E. G., Theodossiadis, G. P., et al. (2022). Cardiometabolic factors and risk of non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 260, 1445–1456. doi:10.1007/s00417-021-05522-4
Chen, B., Liang, L., Jia, D., Qin, M., He, L., Liu, S., et al. (2024a). Inhibitory effect of RGD peptide hydrogel on inflammation and angiogenesis in vitro. J. Biomater. Appl. 39, 723–733. doi:10.1177/08853282241296520
Chen, C.-H., Lin, H.-C., Lin, H.-L., Keller, J. J., and Wang, L.-H. (2023). Association between antihyperlipidemic agent use and age-related macular degeneration in patients with hyperlipidemia: a population-based retrospective cohort study. Biomedicines 11, 1508. doi:10.3390/biomedicines11061508
Chen, J. S., Esko, J. D., Walker, E., Gordts, P. L. S. M., Baxter, S. L., and Toomey, C. B. (2025). High-density lipoproteins associated with age-related macular degeneration in the all of us research program. Ophthalmology 132, 684–691. doi:10.1016/j.ophtha.2024.12.039
Chen, R. C., Palestine, A. G., Lynch, A. M., Patnaik, J. L., Wagner, B. D., Mathias, M. T., et al. (2021). Increased systemic C-reactive protein is associated with choroidal thinning in intermediate age-related macular degeneration. Transl. Vis. Sci. Technol. 10, 7. doi:10.1167/tvst.10.12.7
Chen, T., Song, D., Shan, G., Wang, K., Wang, Y., Ma, J., et al. (2013). The association between diabetes mellitus and nonarteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. PLoS One 8, e76653. doi:10.1371/journal.pone.0076653
Chen, X., Liu, S., Chen, M., Ni, N., Zhou, R., Wang, Y., et al. (2024b). Novel therapeutic perspectives for wet age-related macular degeneration: RGD-modified liposomes loaded with 2-deoxy-D-glucose as a promising nanomedicine. Biomed. Pharmacother. 175, 116776. doi:10.1016/j.biopha.2024.116776
Cheung, C. Y., Ikram, M. K., Sabanayagam, C., and Wong, T. Y. (2012). Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 60, 1094–1103. doi:10.1161/HYPERTENSIONAHA.111.189142
Chou, C.-C., Pan, S.-Y., Sheen, Y.-J., Lin, J.-F., Lin, C.-H., Lin, H.-J., et al. (2025). Association between semaglutide and nonarteritic anterior ischemic optic neuropathy: a multinational population-based study. Ophthalmology 132, 381–388. doi:10.1016/j.ophtha.2024.10.030
Crincoli, E., Sacconi, R., Querques, L., and Querques, G. (2024). Artificial intelligence in age-related macular degeneration: state of the art and recent updates. BMC Ophthalmol. 24, 121. doi:10.1186/s12886-024-03381-1
Culver, H. R., Wechsler, M. E., and Peppas, N. A. (2018). Label-free detection of tear biomarkers using hydrogel-coated gold nanoshells in a localized surface plasmon resonance-based biosensor. ACS Nano 12, 9342–9354. doi:10.1021/acsnano.8b04348
Duan, Z., Yuan, M., Liu, Z., Pei, W., Jiang, K., Li, L., et al. (2024). An ultrasensitive Ti3C2Tx MXene-based soft contact lens for continuous and nondestructive intraocular pressure monitoring. Small 20, e2309785. doi:10.1002/smll.202309785
Durmaz, E., Dribika, L., Kutnyanszky, M., and Mead, B. (2024). Utilizing extracellular vesicles as a drug delivery system in glaucoma and RGC degeneration. J. Control Release 372, 209–220. doi:10.1016/j.jconrel.2024.06.029
Faura, G., Boix-Lemonche, G., Holmeide, A. K., Verkauskiene, R., Volke, V., Sokolovska, J., et al. (2022). Colorimetric and electrochemical screening for early detection of diabetes mellitus and diabetic retinopathy-application of sensor arrays and machine learning. Sensors (Basel) 22, 718. doi:10.3390/s22030718
Gao, H., Chen, M., Liu, Y., Zhang, D., Shen, J., Ni, N., et al. (2023). Injectable anti-inflammatory supramolecular nanofiber hydrogel to promote anti-VEGF therapy in age-related macular degeneration treatment. Adv. Mater 35, e2204994. doi:10.1002/adma.202204994
Ghosh, T. N., Rotake, D. R., and Singh, S. G. (2024). Succinimide-functionalized reduced graphene oxide nanosheets: a high-throughput resistive sensing platform for age-related macular degeneration biomarker determination using human tears. ACS Appl. Bio Mater 7, 6014–6024. doi:10.1021/acsabm.4c00636
Grauslund, J., Taha, A. A., Molander, L. D., Kawasaki, R., Möller, S., Højlund, K., et al. (2024). Once-weekly semaglutide doubles the five-year risk of nonarteritic anterior ischemic optic neuropathy in a Danish cohort of 424,152 persons with type 2 diabetes. Int. J. Retina Vitr. 10, 97. doi:10.1186/s40942-024-00620-x
Hu, Y., Xu, Q., Li, H., Meng, Z., Hao, M., Ma, X., et al. (2022). Dapagliflozin reduces apoptosis of diabetic retina and human retinal microvascular endothelial cells through ERK1/2/cPLA2/AA/ROS pathway independent of hypoglycemic. Front. Pharmacol. 13, 827896. doi:10.3389/fphar.2022.827896
Huang, B. B., and Fawzi, A. A. (2024). Hypertension likely drives arteriolar wall thickening in preclinical diabetic retinopathy while diabetes drives wall thickness in clinical retinopathy. Transl. Vis. Sci. Technol. 13, 8. doi:10.1167/tvst.13.6.8
Huang, J., Liang, C., Huang, J., and Liu, L. (2024). Update on diabetic retinopathy during pregnancy. Eur. J. Ophthalmol. 34, 1695–1706. doi:10.1177/11206721241248868
Hwang, S., Kang, S. W., Kim, S. J., Lee, K. N., Han, K., and Lim, D. H. (2023). Diabetes-related risk factors for exudative age-related macular degeneration: a nationwide cohort study of a diabetic population. Invest Ophthalmol. Vis. Sci. 64, 10. doi:10.1167/iovs.64.10.10
Jiang, F., Lei, C., Chen, Y., Zhou, N., and Zhang, M. (2024). The complement system and diabetic retinopathy. Surv. Ophthalmol. 69, 575–584. doi:10.1016/j.survophthal.2024.02.004
Jiang, S., Wang, W., Ke, J., Huang, S., Wang, J., Luo, C., et al. (2023). A mechanically tough and ultra-swellable microneedle for acute gout arthritis. Biomater. Sci. 11, 1714–1724. doi:10.1039/d2bm01937j
Joshi, V. P., Singh, S., Thacker, M., Pati, F., Vemuganti, G. K., Basu, S., et al. (2023). Newer approaches to dry eye therapy: nanotechnology, regenerative medicine, and tissue engineering. Indian J. Ophthalmol. 71, 1292–1303. doi:10.4103/IJO.IJO_2806_22
Jung, Y., Han, K., Ohn, K., Kim, D. R., and Moon, J. I. (2021). Association between diabetes status and subsequent onset of glaucoma in postmenopausal women. Sci. Rep. 11, 18272. doi:10.1038/s41598-021-97740-3
Kang, T., Zhou, Y., Fan, C., Zhang, Y., Yang, Y., and Jiang, J. (2024). Genetic association of lipid traits and lipid-related drug targets with normal tension glaucoma: a Mendelian randomization study for predictive preventive and personalized medicine. EPMA J. 15, 511–524. doi:10.1007/s13167-024-00373-5
Karti, O., Kiyat, P., Sak, T., and Gercik, O. (2024). Evaluation of choroidal thickness and optic disc parameters in patients with chronic phase gout. Int. Ophthalmol. 44, 365. doi:10.1007/s10792-024-03294-4
Keum, D. H., Kim, S.-K., Koo, J., Lee, G.-H., Jeon, C., Mok, J. W., et al. (2020). Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 6, eaba3252. doi:10.1126/sciadv.aba3252
Khan, B. A., Ahmad, N., Alqahtani, A., Baloch, R., Rehman, A. U., and Khan, M. K. (2024). Formulation development of pharmaceutical nanoemulgel for transdermal delivery of feboxostat: physical characterization and in vivo evaluation. Eur. J. Pharm. Sci. 195, 106665. doi:10.1016/j.ejps.2023.106665
Khanani, A. M., Callanan, D., Dreyer, R., Chen, S., Howard, J. G., Hopkins, J. J., et al. (2021). End-of-Study results for the ladder phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmol. Retina 5, 775–787. doi:10.1016/j.oret.2020.11.004
Kim, J., Kim, M., Lee, M.-S., Kim, K., Ji, S., Kim, Y.-T., et al. (2017). Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997. doi:10.1038/ncomms14997
Kim, T. Y., Mok, J. W., Hong, S. H., Jeong, S. H., Choi, H., Shin, S., et al. (2022). Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma. Nat. Commun. 13, 6801. doi:10.1038/s41467-022-34597-8
Kim, T. Y., Shin, S., Choi, H., Jeong, S. H., Myung, D., and Hahn, S. K. (2021). Smart contact lenses with a transparent silver nanowire strain sensor for continuous intraocular pressure monitoring. ACS Appl. Bio Mater 4, 4532–4541. doi:10.1021/acsabm.1c00267
Kohli, D., Wu, K. Y., White, L. J., Hodge, D. O., Chen, J. J., and Roddy, G. W. (2022). Metabolic syndrome and its components are associated with non-arteritic anterior ischaemic optic neuropathy. BMJ Open Ophthalmol. 7, e001111. doi:10.1136/bmjophth-2022-001111
Kompella, U. B., Hartman, R. R., and Patil, M. A. (2021). Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog. Retin Eye Res. 82, 100901. doi:10.1016/j.preteyeres.2020.100901
Kuang, T.-M., Xirasagar, S., Kao, Y.-W., Shia, B.-C., and Lin, H.-C. (2020). Association of systemic hypertension with primary open-angle glaucoma: a population-based case-control study. Am. J. Ophthalmol. 218, 99–104. doi:10.1016/j.ajo.2020.04.020
Lee, H., Han, K.-D., and Shin, J. (2023). Association between glycemic status and age-related macular degeneration: a nationwide population-based cohort study. Diabetes Metab. 49, 101442. doi:10.1016/j.diabet.2023.101442
Lee, S. Y., Paul, M. E., Coleman, A. L., Kitayama, K., Yu, F., Pan, D., et al. (2024). Associations between statin use and glaucoma in the all of us research program. Ophthalmol. Glaucoma 7, 563–571. doi:10.1016/j.ogla.2024.07.008
Li, F., Luo, Y., Li, X., Dai, Y., and Xiang, Q. (2024). Association between metabolic syndrome and the risk of glaucoma: a meta-analysis of observational studies. Diabetol. Metab. Syndr. 16, 300. doi:10.1186/s13098-024-01532-4
Li, Q., Cheng, Y., Zhang, S., Sun, X., and Wu, J. (2021). TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. J. Neuroinflammation 18, 271. doi:10.1186/s12974-021-02315-8
Li, Q., Lin, F., Gao, Z., Huang, F., and Zhu, P. (2019). Sex-specific association between serum uric acid and retinal microvessels. Med. Sci. Monit. 25, 9973–9980. doi:10.12659/MSM.919972
Li, X., Cao, X., Ma, F., Jia, P., Wang, F., and Cao, X. (2023a). The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction. Transl. Neurosci. 14, 20220281. doi:10.1515/tnsci-2022-0281
Li, X., Cao, X., Zhao, M., and Bao, Y. (2022). The changes of irisin and inflammatory cytokines in the age-related macular degeneration and retinal vein occlusion. Front. Endocrinol. (Lausanne) 13, 861757. doi:10.3389/fendo.2022.861757
Li, Y.-N., Liang, H.-W., Zhang, C.-L., Qiu, Y.-M., Wang, D., Wang, H.-L., et al. (2023b). Ophthalmic solution of smart supramolecular peptides to capture Semaphorin 4D against diabetic retinopathy. Adv. Sci. (Weinh) 10, e2203351. doi:10.1002/advs.202203351
Lin, T., Gubitosi-Klug, R. A., Channa, R., and Wolf, R. M. (2021). Pediatric diabetic retinopathy: updates in prevalence, risk factors, screening, and management. Curr. Diab Rep. 21, 56. doi:10.1007/s11892-021-01436-x
Lin, Y., Luo, W., Jiang, B., Lin, Q., Tang, M., Li, X., et al. (2023). The effect of GelDex-S58 hydrogel on anti-conjunctival scarring after glaucoma filtration surgery. iScience 26, 107633. doi:10.1016/j.isci.2023.107633
Liu, B., Yu, Y., Liu, W., Deng, T., and Xiang, D. (2021). Risk factors for non-arteritic anterior ischemic optic neuropathy: a large scale meta-analysis. Front. Med. (Lausanne) 8, 618353. doi:10.3389/fmed.2021.618353
Liu, J., Jiang, F., Jiang, Y., Wang, Y., Li, Z., Shi, X., et al. (2020a). Roles of exosomes in ocular diseases. Int. J. Nanomedicine 15, 10519–10538. doi:10.2147/IJN.S277190
Liu, L., Quang, N. D., Banu, R., Kumar, H., Tham, Y.-C., Cheng, C.-Y., et al. (2020b). Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS One 15, e0229665. doi:10.1371/journal.pone.0229665
Liu, Y.-C., Lin, Y.-K., Lin, Y.-T., Lin, C.-W., Lan, G.-Y., Su, Y.-C., et al. (2024). Injectable, antioxidative, and tissue-adhesive nanocomposite hydrogel as a potential treatment for inner retina injuries. Adv. Sci. (Weinh) 11, e2308635. doi:10.1002/advs.202308635
Lu, Y., Yue, J., Chen, J., Li, X., Wang, L., Huang, W., et al. (2022). Retinal microvasculature and choriocapillaris flow deficit in relation to serum uric acid using swept-source optical coherence tomography angiography. Transl. Vis. Sci. Technol. 11, 9. doi:10.1167/tvst.11.8.9
Luo, Y., Liu, J., Feng, W., Lin, D., Song, G., Chen, M., et al. (2023). Use of β-blockers and risk of age-related macular degeneration among hypertensive patients: an insight from the National Health and Nutrition Examination Survey. Med. Int. (Lond) 3, 10. doi:10.3892/mi.2023.70
Ma, C., Jiang, Y., Xiang, Y., Li, C., Xie, X., Zhang, Y., et al. (2025). Metabolic reprogramming of macrophages by biomimetic melatonin-loaded liposomes effectively attenuates acute gouty arthritis in a mouse model. Adv. Sci. (Weinh) 12, e2410107. doi:10.1002/advs.202410107
Mahaling, B., and Katti, D. S. (2016). Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomedicine 12, 2149–2160. doi:10.1016/j.nano.2016.05.017
Mahaling, B., Srinivasarao, D. A., Raghu, G., Kasam, R. K., Bhanuprakash Reddy, G., and Katti, D. S. (2018). A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale 10, 16485–16498. doi:10.1039/c8nr00058a
Malerbi, F. K., and Bertoluci, M. C. (2025). Semaglutide, type 2 diabetes, and the risk of nonarteritic anterior ischemic optic neuropathy. Int. J. Retina Vitr. 11, 8. doi:10.1186/s40942-024-00622-9
Marquina, S., Ozgul, M., Robertson-Brown, K., and Kenney, M. C. (2023). A review on PLGA particles as a sustained drug-delivery system and its effect on the retina. Exp. Eye Res. 235, 109626. doi:10.1016/j.exer.2023.109626
Mei, L., Yu, M., Liu, Y., Weh, E., Pawar, M., Li, L., et al. (2022). Synthetic high-density lipoprotein nanoparticles delivering rapamycin for the treatment of age-related macular degeneration. Nanomedicine 44, 102571. doi:10.1016/j.nano.2022.102571
Meyer, A. M., Dai, H., and Sokol, J. A. (2024). Primary gouty tophi involving the eyelid. Ophthalmic Plast. Reconstr. Surg. 40, e198–e200. doi:10.1097/IOP.0000000000002708
Miralles Pechuan, V., González-Martín-Moro, J., Castro Rebollo, M., and Cobo Soriano, R. (2024). Non-arteritic anterior ischemic optic neuropathy as the only manifestation of a malignant hypertensive crisis: report of a case. Arch. Soc. Esp. Oftalmol. Engl. Ed. 99, 450–454. doi:10.1016/j.oftale.2024.06.007
Modjtahedi, B. S., Bose, N., Papakostas, T. D., Morse, L., Vavvas, D. G., and Kishan, A. U. (2016). Lipids and diabetic retinopathy. Semin. Ophthalmol. 31, 10–18. doi:10.3109/08820538.2015.1114869
Mondal, H., Kim, H.-J., Mohanto, N., and Jee, J.-P. (2023). A review on dry eye disease treatment: recent progress, diagnostics, and future perspectives. Pharmaceutics 15, 990. doi:10.3390/pharmaceutics15030990
Moses, R. A. (1958). The Goldmann applanation tonometer. Am. J. Ophthalmol. 46, 865–869. doi:10.1016/0002-9394(58)90998-x
Moyo, M. T. G., Adali, T., and Tulay, P. (2024). Exploring gellan gum-based hydrogels for regenerating human embryonic stem cells in age-related macular degeneration therapy: a literature review. Regen. Ther. 26, 235–250. doi:10.1016/j.reth.2024.05.018
Mussi, N., Haque, W., and Robertson, D. M. (2021). The association between risk factors for metabolic syndrome and meibomian gland disease in a dry eye cohort. Clin. Ophthalmol. 15, 3821–3832. doi:10.2147/OPTH.S322461
Nahavandipour, A., Krogh Nielsen, M., Sørensen, T. L., and Subhi, Y. (2020). Systemic levels of interleukin-6 in patients with age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 98, 434–444. doi:10.1111/aos.14402
Nashine, S., Cohen, P., Wan, J., and Kenney, M. C. (2022). Effect of Humanin G (HNG) on inflammation in age-related macular degeneration (AMD). Aging (Albany NY) 14, 4247–4269. doi:10.18632/aging.204074
Noroozi, M., Ghasemirad, H., Ghaedi, A., Kargar, M., Alipour, M., Mahmoudvand, G., et al. (2024). Visit-to-visit variability of blood pressure and risk of diabetic retinopathy: a systematic review and meta-analysis. Am. J. Cardiovasc Dis. 14, 281–294. doi:10.62347/DFSZ9202
Pai, H.-L., Chang, H.-H., and Lin, D. P.-C. (2021). The need to investigate hyperuricemia as a factor in the onset of age-related macular degeneration. Eye (Lond) 35, 1804–1807. doi:10.1038/s41433-021-01456-7
Pai, H.-L., Lin, D. P.-C., and Chang, H.-H. (2024). Current updates for hyperuricemia and gout in age-related macular degeneration. FASEB J. 38, e23676. doi:10.1096/fj.202400421R
Panahi, P., Kabir, A., and Falavarjani, K. G. (2023). Age-related macular degeneration prevalence and its risk factors in Iran: a systematic review and meta-analysis study. J. Curr. Ophthalmol. 35, 305–312. doi:10.4103/joco.joco_40_23
Park, H. W., and Park, J. W. (2016). The association between symptoms of dry eye syndrome and metabolic outcome in a general population in korea. J. Korean Med. Sci. 31, 1121–1126. doi:10.3346/jkms.2016.31.7.1121
Patel, A. J., Pieramici, D. J., and Bagheri, N. (2021). Evaluation of Port Delivery System with ranibizumab for the treatment of neovascular age-related macular degeneration. Ther. Deliv. 12, 191–200. doi:10.4155/tde-2020-0132
Patel, B., and Thakkar, H. (2023). Formulation development of fast dissolving microneedles loaded with cubosomes of febuxostat: in vitro and in vivo evaluation. Pharmaceutics 15, 224. doi:10.3390/pharmaceutics15010224
Qiu, F., Meng, T., Chen, Q., Zhou, K., Shao, Y., Matlock, G., et al. (2019). Fenofibrate-loaded biodegradable nanoparticles for the treatment of experimental diabetic retinopathy and neovascular age-related macular degeneration. Mol. Pharm. 16, 1958–1970. doi:10.1021/acs.molpharmaceut.8b01319
Qureshi, S. S., Amer, W., Mueez, M., Farok, M., and Shehzad, S. K. (2025). Frequency of undiagnosed hypertension among diabetic patients with micro vascular complications. Pak J. Med. Sci. 41, 210–213. doi:10.12669/pjms.41.1.9715
Rafiei, F., Tabesh, H., and Farzad, F. (2020). Sustained subconjunctival drug delivery systems: current trends and future perspectives. Int. Ophthalmol. 40, 2385–2401. doi:10.1007/s10792-020-01391-8
Rong, X., Ji, Y., Zhu, X., Yang, J., Qian, D., Mo, X., et al. (2019). Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomedicine 14, 45–55. doi:10.2147/IJN.S184574
Serra, R., Coscas, F., Pinna, A., Peri, M., Zucca, I., Sellam, A., et al. (2021). Detection of serum uric acid in primary open angle glaucoma: a pilot study. Eur. J. Ophthalmol. 31, 1857–1861. doi:10.1177/1120672120944012
Shao, Y., Hu, B., Liu, X., Ni, Z., Shu, Y., Zhang, X., et al. (2025). Multi-functional, conformal systems with ultrathin crystalline-silicon-based bioelectronics for characterization of intraocular pressure and ocular surface temperature. Biosens. Bioelectron. 267, 116786. doi:10.1016/j.bios.2024.116786
Sharma, S., Kwan, S., Fallano, K. A., Wang, J., Miller, N. R., and Subramanian, P. S. (2017). Comparison of visual outcomes of nonarteritic anterior ischemic optic neuropathy in patients with and without diabetes mellitus. Ophthalmology 124, 450–455. doi:10.1016/j.ophtha.2016.11.029
Sharon, Y., and Schlesinger, N. (2016). Beyond joints: a review of ocular abnormalities in gout and hyperuricemia. Curr. Rheumatol. Rep. 18, 37. doi:10.1007/s11926-016-0586-8
Shean, R., Yu, N., Guntipally, S., Nguyen, V., He, X., Duan, S., et al. (2024). Advances and challenges in wearable glaucoma diagnostics and therapeutics. Bioeng. (Basel) 11, 138. doi:10.3390/bioengineering11020138
Shin, H., Seo, H., Chung, W. G., Joo, B. J., Jang, J., and Park, J.-U. (2021). Recent progress on wearable point-of-care devices for ocular systems. Lab. Chip 21, 1269–1286. doi:10.1039/d0lc01317j
Skrzypecki, J., Ufnal, M., Szaflik, J. P., and Filipiak, K. J. (2019). Blood pressure and glaucoma: at the crossroads between cardiology and ophthalmology. Cardiol. J. 26, 8–12. doi:10.5603/CJ.2019.0008
Su, Y.-C., Hung, J.-H., Chang, K.-C., Sun, C.-C., Huang, Y.-H., Lee, C.-N., et al. (2022). Comparison of sodium-glucose cotransporter 2 inhibitors vs glucagonlike peptide-1 receptor agonists and incidence of dry eye disease in patients with type 2 diabetes in taiwan. JAMA Netw. Open 5, e2232584. doi:10.1001/jamanetworkopen.2022.32584
Sun, J., Liu, X., Du, J., An, J., Li, Y., Hu, Y., et al. (2023). Manganese-doped albumin-gelatin composite nanogel loaded with berberine applied to the treatment of gouty arthritis in rats via a SPARC-dependent mechanism. Int. J. Biol. Macromol. 253, 126999. doi:10.1016/j.ijbiomac.2023.126999
Sun, Y., Wei, X., Fang, F., Shen, Y., Wei, H., Li, J., et al. (2021). HPDL deficiency causes a neuromuscular disease by impairing the mitochondrial respiration. J. Genet. Genomics 48, 727–736. doi:10.1016/j.jgg.2021.01.009
Sung, J.-Y., Kim, J.-J., Hwang, J.-Y., and Lee, M.-W. (2024). Retinal neurodegeneration in diabetic retinopathy with systemic hypertension. Acta Diabetol. 61, 495–504. doi:10.1007/s00592-023-02226-5
Tang, Y., Shi, Y., and Fan, Z. (2023). The mechanism and therapeutic strategies for neovascular glaucoma secondary to diabetic retinopathy. Front. Endocrinol. (Lausanne) 14, 1102361. doi:10.3389/fendo.2023.1102361
Taurone, S., Ralli, M., Nebbioso, M., Greco, A., Artico, M., Attanasio, G., et al. (2020). The role of inflammation in diabetic retinopathy: a review. Eur. Rev. Med. Pharmacol. Sci. 24, 10319–10329. doi:10.26355/eurrev_202010_23379
Thang, T. T., Phuong, P. H., Huynh, N. S., Kien, N. T., Toan, N. D., Ha, N. T. T., et al. (2024). Dry eye rate and its relationship with disease stage in patients with primary hypertension: a cross-sectional study in Vietnam. Int. J. Ophthalmol. 17, 653–658. doi:10.18240/ijo.2024.04.07
Thounaojam, M. C., Montemari, A., Powell, F. L., Malla, P., Gutsaeva, D. R., Bachettoni, A., et al. (2019). Monosodium urate contributes to retinal inflammation and progression of diabetic retinopathy. Diabetes 68, 1014–1025. doi:10.2337/db18-0912
Tjandra, I., Soeharso, P., Artini, W., Siregar, N. C., and Victor, A. A. (2020). Ganglion cells apoptosis in diabetic rats as early prediction of glaucoma: a study of Brn3b gene expression and association with change of quantity of NO, caspase-3, NF-κB, and TNF-α. Int. J. Ophthalmol. 13, 1872–1879. doi:10.18240/ijo.2020.12.05
Tomi, M., and Hosoya, K. (2010). The role of blood-ocular barrier transporters in retinal drug disposition: an overview. Expert Opin. Drug Metab. Toxicol. 6, 1111–1124. doi:10.1517/17425255.2010.486401
Tran Tat, T., Ngo Duc, K., Pham Hong, P., Nguyen Sa, H., Nguyen Trung, K., Nguyen Thi Thu, H., et al. (2024). Dry eye and some related factors in patients with type 2 diabetic nephropathy: a cross-sectional study in vietnam. Clin. Ophthalmol. 18, 1217–1224. doi:10.2147/OPTH.S458633
Tu, S., Luo, Z., Yang, R., Hu, D., Xian, B., Zhao, F., et al. (2023). Mitomycin C-loaded PTMC15-F127-PTMC15 hydrogel maintained better bleb function after filtering glaucoma surgery in monkeys with intraocular hypertension. RSC Adv. 13, 13604–13615. doi:10.1039/d3ra01002c
Virtanen, A., Haukka, J., Loukovaara, S., and Harju, M. (2023). Diabetes mellitus and risk of open-angle glaucoma-A population-based follow-up study. Acta Ophthalmol. 101, 160–169. doi:10.1111/aos.15240
Wang, J., Li, B., Kompella, U. B., and Yang, H. (2023). Dendrimer and dendrimer gel-derived drug delivery systems: breaking bottlenecks of topical administration of glaucoma medications. MedComm Biomater. Appl. 2, e30. doi:10.1002/mba2.30
Wang, J.-C., Ku, H.-Y., Chen, T.-S., and Chuang, H.-S. (2017). Detection of low-abundance biomarker lipocalin 1 for diabetic retinopathy using optoelectrokinetic bead-based immunosensing. Biosens. Bioelectron. 89, 701–709. doi:10.1016/j.bios.2016.11.014
Wang, L., Zhang, S., Han, Y., Tang, S., Li, J., Bu, L., et al. (2024a). An effective pharmacological hydrogel induces optic nerve repair and improves visual function. Sci. China Life Sci. 67, 529–542. doi:10.1007/s11427-023-2394-3
Wang, S., and Bao, X. (2019). Hyperlipidemia, blood lipid level, and the risk of glaucoma: a meta-analysis. Invest Ophthalmol. Vis. Sci. 60, 1028–1043. doi:10.1167/iovs.18-25845
Wang, X., Xu, C., Bian, C., Ge, P., Lei, J., Wang, J., et al. (2024b). M2 microglia-derived exosomes promote vascular remodeling in diabetic retinopathy. J. Nanobiotechnology 22, 56. doi:10.1186/s12951-024-02330-w
Wang, X.-F., Zhang, X.-W., Liu, Y.-J., Zheng, X.-Y., Su, M.-R., Sun, X.-H., et al. (2024c). The causal effect of hypertension, intraocular pressure, and diabetic retinopathy: a Mendelian randomization study. Front. Endocrinol. (Lausanne) 15, 1304512. doi:10.3389/fendo.2024.1304512
Wechsler, M. E., Jocelyn Dang, H. K. H., Simmonds, S. P., Bahrami, K., Wyse, J. M., Dahlhauser, S. D., et al. (2021). Electrostatic and covalent assemblies of anionic hydrogel-coated gold nanoshells for detection of dry eye biomarkers in human tears. Nano Lett. 21, 8734–8740. doi:10.1021/acs.nanolett.1c02941
Wei, R., Xie, J., Meng, F., He, F., Liu, J., Zhao, Y., et al. (2023). Serum uric acid levels are associated with macula microvasculature changes in hypertensive white matter hyperintensity patients. Curr. Neurovasc Res. 20, 132–139. doi:10.2174/1567202620666221027095220
Woodward, R., Mgaya, E., Mwanansao, C., Peck, R. N., Wu, A., and Sun, G. (2020). Retinopathy in adults with hypertension and diabetes mellitus in Western Tanzania: a cross-sectional study. Trop. Med. Int. Health 25, 1214–1225. doi:10.1111/tmi.13463
Wu, P., Wang, Z., Liang, L., Chen, B., and Xu, N. (2023). Characteristics of mitomycin C-loaded peptide hydrogel in vitro and antiscarring effects in rat ocular injury model. J. Ocul. Pharmacol. Ther. 39, 139–147. doi:10.1089/jop.2022.0102
Xia, Y., Zhang, Y., Du, Y., Wang, Z., Cheng, L., and Du, Z. (2024). Comprehensive dry eye therapy: overcoming ocular surface barrier and combating inflammation, oxidation, and mitochondrial damage. J. Nanobiotechnology 22, 233. doi:10.1186/s12951-024-02503-7
Xiao, R., Lei, C., Zhang, Y., and Zhang, M. (2023). Interleukin-6 in retinal diseases: from pathogenesis to therapy. Exp. Eye Res. 233, 109556. doi:10.1016/j.exer.2023.109556
Xu, J.-F., Wang, Y.-P., and Liu, X.-H. (2024). Novel fabrication of anti-VEGF drug ranibizumab loaded PLGA/PLA co-polymeric nanomicelles for long-acting intraocular delivery in the treatment of age-related macular degeneration therapy. Regen. Ther. 26, 620–634. doi:10.1016/j.reth.2024.06.019
Xu, X., Ritz, B., Coleman, A., Liew, Z., Deapen, D., Lee, E., et al. (2020). Hypertension, antihypertensive medications use and risk of age-related macular degeneration in California Teachers Cohort. J. Hum. Hypertens. 34, 568–576. doi:10.1038/s41371-019-0269-9
Yadav, A., Phogat, J., Yadav, M., Bhardwaj, A., Yadav, R., Nada, M., et al. (2024). Analysis of interleukin-6 gene polymorphism and its serum levels in Indian age-related macular degeneration patients. Mol. Vis. 30, 434–446.
Yang, J., Qu, Y., Li, B., Zhong, Z., Shi, L., Tian, X., et al. (2021). Epidemiological characteristics of inpatients undergoing surgery for glaucoma at tianjin eye hospital from 2013 to 2017. J. Ophthalmol. 2021, 3628481–3628488. doi:10.1155/2021/3628481
Yang, Y., Li, Z., Huang, P., Lin, J., Li, J., Shi, K., et al. (2023). Rapidly separating dissolving microneedles with sustained-release colchicine and stabilized uricase for simplified long-term gout management. Acta Pharm. Sin. B 13, 3454–3470. doi:10.1016/j.apsb.2023.02.011
Yetisen, A. K., Jiang, N., Castaneda Gonzalez, C. M., Erenoglu, Z. I., Dong, J., Dong, X., et al. (2020). Scleral lens sensor for ocular electrolyte analysis. Adv. Mater 32, e1906762. doi:10.1002/adma.201906762
Yi, H., Yu, H., Wang, L., Wang, Y., Ouyang, C., and Keshta, B. E. (2024). Microneedle transdermal drug delivery as a candidate for the treatment of gouty arthritis: material structure, design strategies and prospects. Acta Biomater. 187, 20–50. doi:10.1016/j.actbio.2024.08.032
Yu, H.-Y., Kim, J.-J., Kim, J.-T., and Lee, M.-W. (2025). Impact of systemic hypertension on inner retinal layer thickness and macular microvasculature in patients with diabetic retinopathy. Acta Diabetol. 62, 271–279. doi:10.1007/s00592-024-02355-5
Zeng, L., Ma, W., Shi, L., Chen, X., Wu, R., Zhang, Y., et al. (2019). Poly(lactic-co-glycolic acid) nanoparticle-mediated interleukin-12 delivery for the treatment of diabetic retinopathy. Int. J. Nanomedicine 14, 6357–6369. doi:10.2147/IJN.S214727
Zeng, R., Garg, I., Bannai, D., Kasetty, M., Katz, R., Park, J. Y., et al. (2022). Retinal microvasculature and vasoreactivity changes in hypertension using optical coherence tomography-angiography. Graefes Arch. Clin. Exp. Ophthalmol. 260, 3505–3515. doi:10.1007/s00417-022-05706-6
Zhang, J., Kim, K., Kim, H. J., Meyer, D., Park, W., Lee, S. A., et al. (2022a). Smart soft contact lenses for continuous 24-hour monitoring of intraocular pressure in glaucoma care. Nat. Commun. 13, 5518. doi:10.1038/s41467-022-33254-4
Zhang, M., Wu, J., Wang, Y., Wu, J., Hu, W., Jia, H., et al. (2023). Associations between blood pressure levels and diabetic retinopathy in patients with diabetes mellitus: a population-based study. Heliyon 9, e16830. doi:10.1016/j.heliyon.2023.e16830
Zhang, Y. P., Wang, Y. X., Zhou, J. Q., Wang, Q., Yan, Y. N., Yang, X., et al. (2022b). The influence of diabetes, hypertension, and hyperlipidemia on the onset of age-related macular degeneration in north China: the kailuan eye study. Biomed. Environ. Sci. 35, 613–621. doi:10.3967/bes2022.081
Zhou, J., Chen, F., Yan, A., Jiang, J., and Xia, X. (2022). Hyperglycemia induces retinal ganglion cell endoplasmic reticulum stress to the involvement of glaucoma in diabetic mice. Transpl. Immunol. 73, 101636. doi:10.1016/j.trim.2022.101636
Keywords: cardiovascular risk factors, ocular diseases, materials science, smart sensors, drug delivery systems, tissue repair
Citation: Chen P, Zheng B, Wang P, Liu H and Pei H (2025) Advances in materials science for ocular diseases induced by cardiovascular risk factors. Front. Bioeng. Biotechnol. 13:1618232. doi: 10.3389/fbioe.2025.1618232
Received: 25 April 2025; Accepted: 18 June 2025;
Published: 27 June 2025.
Edited by:
Gaocan Li, Sichuan University, ChinaReviewed by:
Boxuan Ma, Zhejiang University, ChinaBinapani Mahaling, Harvard Medical School, United States
Fanjun Zhang, Sichuan University, China
Copyright © 2025 Chen, Zheng, Wang, Liu and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Pei, cGVpaGFpZmVuZ0Bzd2p0dS5lZHUuY24=; Hao Liu, bGl1aGFvZ3p6eUAxNjMuY29t
†These authors have contributed equally to this work
 Peiwen Chen
Peiwen Chen Bo Zheng2†
Bo Zheng2† Hao Liu
Hao Liu Haifeng Pei
Haifeng Pei