- Allergy Immunology Unit, Department of Pediatrics, Advanced Pediatrics Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India
Rheumatic heart disease (RHD), the principal long-term sequel of acute rheumatic fever (ARF), has been a major contributor to cardiac-related mortality in general population, especially in developing countries. With improvement in health and sanitation facilities across the globe, there has been almost a 50% reduction in mortality rate due to RHD over the last 25 years. However, recent estimates suggest that RHD still results in more than 300,000 deaths annually. In India alone, more than 100,000 deaths occur due to RHD every year (Watkins DA et al., N Engl J Med, 2017). Children and adolescents (aged below 15 years) constitute at least one-fourth of the total population in India. Besides, ARF is, for the most part, a pediatric disorder. The pediatric population, therefore, requires special consideration in developing countries to reduce the burden of RHD. In the developed world, Kawasaki disease (KD) has emerged as the most important cause of acquired heart disease in children. Mirroring global trends over the past two decades, India also has witnessed a surge in the number of cases of KD. Similarly, many regions across the globe classified as “high-risk” for ARF have witnessed an increasing trend in the incidence of KD. This translates to a double challenge faced by pediatric health care providers in improving cardiac outcomes of children affected with ARF or KD. We highlight this predicament by reviewing the incidence trends of ARF and KD over the last 50 years in ARF “high-risk” regions.
Introduction
Globally, acute rheumatic fever (ARF) remained the most important cause of acquired heart disease in children until the later part of the 20th century. Improvements in general standard of living, hygiene, sanitation, health care facilities, better understanding of the disease, appropriate use of antimicrobials, and directed public health policies resulted in a significant decrease in incidence of ARF and prevalence of rheumatic heart disease (RHD) in developed countries (1, 2). On the other hand, Kawasaki disease (KD) is increasingly being recognized in many developed and developing countries. KD is now the commonest cause of acquired heart disease in children in developed countries and the incidence of KD in developing countries also seems to be rising (3).
However, in many under-developed and developing regions, a significant burden of ARF/RHD still exists due to poor standard of living, suboptimal hygiene and sanitation facilities (4). Also, KD has clinical features overlapping with many infectious diseases and no specific diagnostic tests for KD are available. It is possible that children with KD in developing countries may end up being empirically treated with antimicrobials given the tremendous burden of infectious diseases in these countries (5). Additionally, timely intravenous immunoglobulin therapy remains a challenge due to concerns of limited availability and high costs involved in its procurement. Consequently, a higher proportion of children with KD in developing countries may suffer cardiac complications (as compared to the developed world) (6–10).
In this review, we note the trends in the incidence of ARF and KD [before the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic] in regions that have been traditionally described to have “high-risk”/“high-burden” for ARF/RHD. We highlight that the incidence of KD is increasing in these areas while a significant burden of RHD remains. So there is a dual challenge (of KD and ARF) in a majority of regions in the developing world that needs to be tackled to reduce the burden of cardiovascular morbidity in children.
History (Including Diagnostic Criteria)
ARF
The first descriptions indicative of acute rheumatic fever (ARF) and its cardiac sequel date back to the first century A.D (11, 12). Sydenham in late 1600s provided a detailed description of ARF and, for the first time in history, he differentiated this illness from gout (13). The first probable description of ARF in modern English language was published around 1700 (14). Around the turn of the 20th century, the complete spectrum of ARF in the pediatric population was described (15, 16). The year 1944 was a landmark in history of rheumatism when Dr. T. Duckett Jones, for the first time, proposed diagnostic criteria for ARF (17, 18). The Jones criteria have been revised on numerous instances with the latest version having been published in 2015 (19, 20). The latest version incorporates monoarthralgia as a minor criterion, and monoarthritis and polyarthralgia as major manifestations in high-risk settings. Additionally, lower cutoffs for fever and erythrocyte sedimentation rate have been promulgated for such populations (Supplementary Table 1). These substantial revisions will enhance the recognition of ARF in high-risk populations across the globe (20).
KD
The first descriptions of Kawasaki disease (KD) were published by Dr. Tomisaku Kawasaki in 1967 and 1974 in Japanese and English language, respectively (21–23). In 1975, Kato et al. published the first English language description of coronary artery aneurysms in KD detected by coronary angiography (24, 25). Two sets of diagnostic criteria are currently employed for KD – the American Heart Association (AHA) and the Japanese criteria. These diagnostic criteria are essentially based on the seminal observations made by Dr. Kawasaki in the 1960s. The latest version of the AHA diagnostic criteria has been published in 2017 (3) and the latest version of Japanese criteria has been published in the English Language in 2020 (26). Essentially, both these sets of criteria incorporate fever, rash, bilateral non-exudative conjunctival injection, oral mucosal changes, cervical lymphadenopathy, and edema or periungual skin peeling of hands or feet as major manifestations of the disease (Supplementary Tables 2, 3). However, clinical judgment is imperative as KD may present only with fever and coronary artery abnormalities, especially in young infants (3). The recent Japanese criteria have been updated to augment the diagnosis of KD – for example, reactivation of Bacillus Calmette–Guérin vaccination site, most commonly seen infants and young children, has been added as a major manifestation (26). Similar modifications to the AHA criteria may increase the sensitivity of KD diagnosis. Analogously, revisions have already been made by the AHA to the modified Jones criteria (in 2015).
Trend of Incidence
India
ARF
In a school-based study from rural South India in 1970s, the average incidence of rheumatic fever and newly diagnosed rheumatic heart disease was estimated to be 110/100,000 schoolchildren per year (27). In a population-based study from rural North India in late 1980s, the average incidence of first episode of ARF in children 5–15 years of age was estimated to be 54/100,000 per year (overall ~79/100,000) (28). Surveys conducted in 1980s and early 1990s estimated the annual incidence of ARF in pediatric age group to be 38–70/100,000 (29). In early 1990s, a study from North India estimated the annual incidence of first episode of ARF to be 19/100,000 school children (overall 32/100,000) (30). In a North Indian survey of ~16,000 children between 5–15 years of age, no cases of ARF were reported during the observation period of 2007 and 2008 (29). Multicentric studies have noted a declining trend in the prevalence and burden of RHD in India; however, studies on the incidence of ARF are sparse (Figure 1) (31).
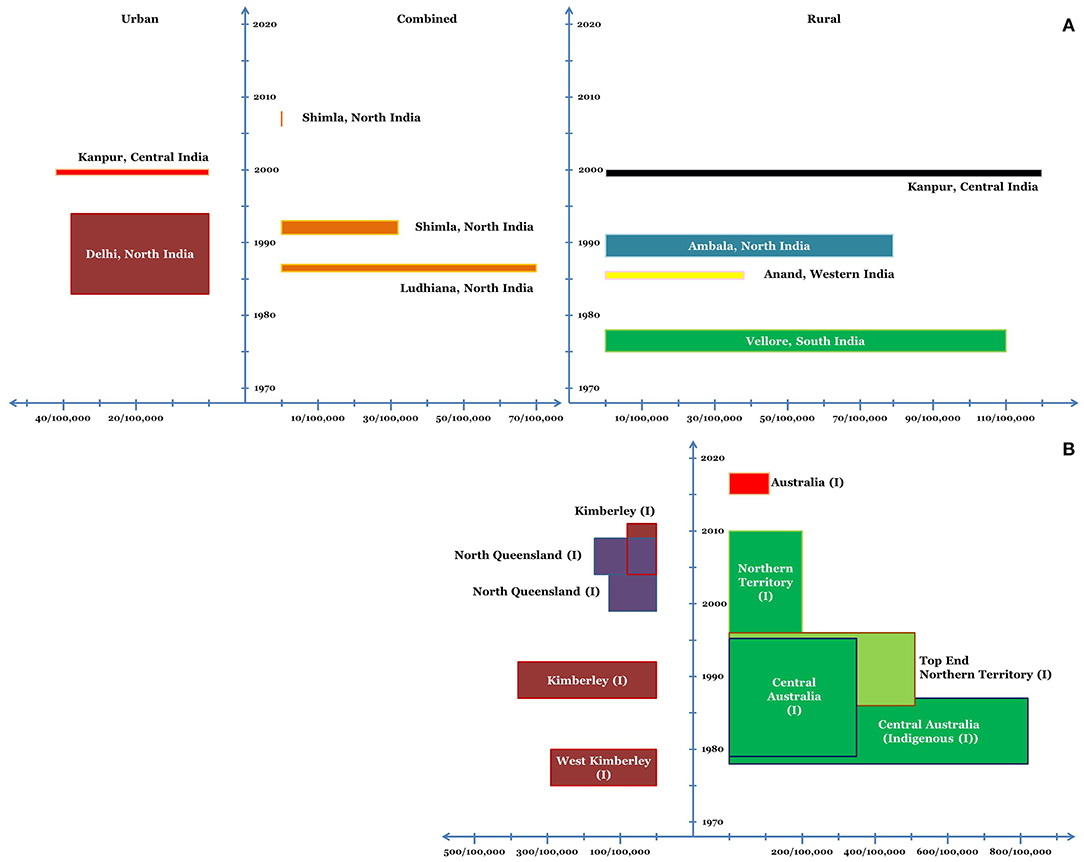
Figure 1. Incidence of ARF in various regions of India (A) and Australia (B) in school-age children. (I) refers to the indigenous population. The x-axis depicts the incidence and the y-axis depicts the timeline. The dimension of the bars along the x-axis (length of the bars) depicts the average annual incidence of ARF. The dimension of the bars along the y-axis (width of the bars) corresponds to the duration for which the said average annual incidence was noted.
KD
Prior to 1990, there were only three reports of KD from India. Subsequently, hospital-based incidence studies were undertaken predominantly from Chandigarh, North India (32). In children below 15 years, the incidence of KD was estimated to be 0.51/100,000 in 1994. The incidence gradually increased in the subsequent years and was estimated to be 4.54/100,000 in 2007 (33). In children <5, the average annual incidence of KD during 2009–14 was 5.35/100,000 (34). The incidence of KD in children <5 years at Chandigarh has been estimated to be 5.64/100,000 and 10.6/100,000 during 2015 and 2019, respectively (unpublished observations) (Figure 2). Other centers in India have also witnessed a similar increase in the number of cases with KD in the last decade, although nationwide estimates for incidence are lacking (35–37).
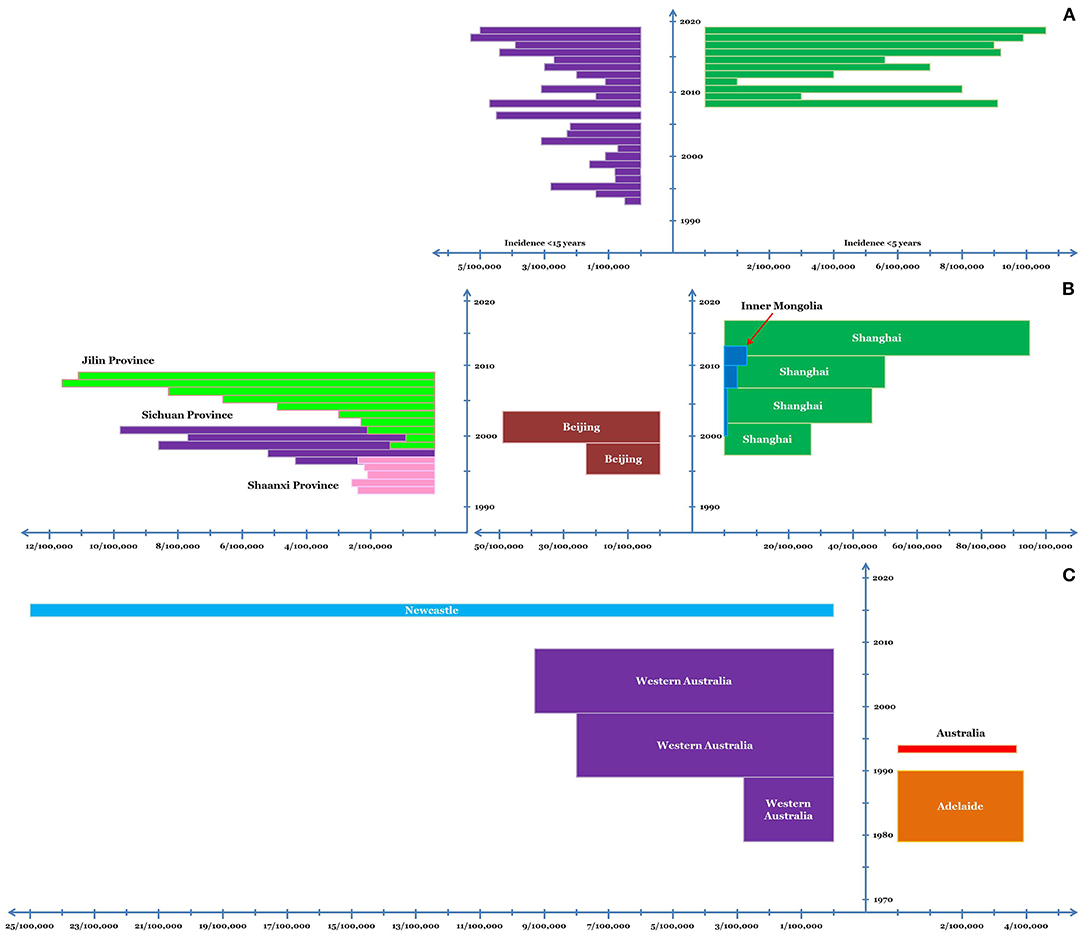
Figure 2. Incidence of KD in Chandigarh, India, (A) and various regions of China (B) and Australia (C). In case of Chandigarh, India, the incidence in children <5 and <15 years of age is shown; while for China and Australia, incidence in young children is depicted. The x-axis depicts the incidence and the y-axis depicts the timeline. The dimension of the bars along the x-axis (length of the bars) depicts the average annual incidence of KD. The dimension of the bars along the y-axis (width of the bars) corresponds to the duration for which the said average annual incidence was noted.
China
ARF
English language reports on incidence of ARF in China are scarce. In early 1990s, the annual incidence of ARF in the pediatric population in South Western China was estimated to be 12.87/100,000 (38). In a large survey across five provinces in mainland China conducted between 1992–1995, the average annual incidence of ARF in children 5–18 years of age was reported to be 20.05/100,000 (39, 40). Although population-based data reflecting the trend of ARF incidence in China are sparse, hospital-based data have noted a decreasing trend similar to other regions of the world (39, 41).
KD
Shanghai and Beijing
A number of studies have been conducted in China to assess the epidemiology of KD. By the turn of the 21st century, KD had already overtaken ARF in the annual incidence, at least in Shanghai and Beijing. In Shanghai, the average annual incidence of KD in children below five has gradually increased over the last two decades which was 27.32/100,000 during 1998–2002, 46.32/100,000 during 2003–07, 50.5/100,000 during 2008–12, and 94.7/100,000 during 2013–17 (42–44). In Beijing, the average annual incidence of KD has been similar to Shanghai; for example, during 1995–99 it was 22.9/100,000 (45), while during 2000–04 it was 49.4/100,000 (46). However, reports suggest significant variability in the incidence of KD among different regions in China.
Other Regions
The average annual incidence of KD in Jilin Province during 1999–2008 was 5.04/100,000 children under five (47). Similarly, in Inner Mongolia, the mean annual incidence of KD during 2001–13 was 3.55/100,000 children under 5 (48). Nonetheless, these surveys did note an increasing trend in the disease incidence during these periods. In Shaanxi province, the annual incidence of KD remained fairly constant at ~2.34/100,000 children <5 years of age during mid-1990s (49). However, an increasing trend in the incidence of KD was noted in Sichuan Province where the incidence of KD in children <5 years was 4.26/100,000 and 9.81/100,000 in 1997 and 2001 respectively (50) (Figure 2).
Australia
ARF
Western Australia
In the late 1970s, hospital-based studies from West Kimberley (Western Australia) estimated the annual incidence of ARF to be 230–350/100,000 in Indigenous school children (51). During 1988–92, the annual incidence of ARF in Indigenous school-children (5–14 years) in the Kimberley region was 375/100,000 (52). The annual incidence in Indigenous population in the age group of 15–29 years was also high at 258/100,000 (52). Hospital-based incidence data, however, suggested a decrease in incidence of ARF in the region. During 1988–92, the annual incidence as per 100,000 hospitalized school-children was 278 (52). The annual age-standardized hospitalization rates for ARF during 2005–11 ranged between 50–100/100,000 Indigenous population in Kimberley (except for 2008, when it was <50/100,000) (53). In the same study, a significant yearly decrease of ~9% in the age-standardized hospitalizations of ARF/RHD was noted during 2003–11 (continued decrease noted for both ARF and RHD) (53, 54).
Queensland
The annual incidence of ARF in Indigenous children (5–14 years) in north Queensland during 1999–2004 was 133/100,000. The highest incidence was noted in Northern Peninsula Area and Torres Strait Health Service District (349/100,000 children), whereas, no ARF case was detected in Innisfail District (55). A significant increase in the incidence of ARF was noted over the next 5 years (2004–09) with the annual incidence in children 5–14 years of age being 155/100,000. This finding also reflected that the transition from intensified to usual surveillance in the year 2004 had no adverse bearing on the notification of ARF (56).
Northern Territory
During 1978–87, the incidence of ARF in Central Australian Indigenous children (5–14 years) was estimated to be 815/100,000 (57). From 1987–96, the incidence in Indigenous children in Top End Northern Territory was estimated to be 224/100,000. However, the incidence in Indigenous communities where complete data was available was higher at 508/100,000 and in Non-Indigenous children (5–14 years) the incidence was 1.3/100,000 (58). In a combined study assessing the epidemiology of ARF in Northern Territory during 1979–96, the incidence of ARF in 5–14 year old indigenous children in Top End and Central Australia was estimated to be 245/100,000 and 351/100,000 respectively (59). During 1997–2010, the annual incidence of the first episode of ARF in the Indigenous children (5–14 years) of the Northern Territory was 194/100,000 (60). Notably, in this large and well-designed study, a decreasing trend in the annual incidence of ARF was not noted on multivariate analysis (60).
The annual incidence of the first episode of ARF across 5 Australian Jurisdictions during 2015–2017 was 107.6/100,000 for Indigenous children (5–14 years) and 1/100,000 for non-Indigenous children (61). More than 80% of the ARF episodes were reported from North Australia (61) (Figure 1).
KD
During 1979–90, the average annual incidence of KD in Adelaide was 3.9/100,000 children aged 0–5 years, with highest incidence being noted in the year 1986 (7.7/100,000) (62). In 1994, the incidence of KD in Australian children <5 years was noted to be 3.7/100,000 (63). A large study analyzing the 30-year epidemiology of KD in Western Australia from 1979–2009 noted a gradual increase in the incidence of KD in children <5 years of age. The average annual incidence rate for the said population during the consecutive decades of the study was 2.82, 7.96, and 9.34 per 100,000 respectively (64). A hospital-based study from Newcastle estimated the average annual incidence of KD (in children <5 years) to be ~25/100,000 during 2015–16 (65) (Figure 2). In a nationwide study, an increase in the hospitalizations due to KD (0–19 years) was noted during the 25-year period from 5.2/100,000 in early 1990s to 12.4/100,000 in 2017–18 (66). Notably, very few Indigenous children have been diagnosed to have KD in these large studies (64, 66).
Africa
ARF
Most of the studies have focused on the prevalence of RHD from Africa; however, epidemiological studies assessing the incidence of ARF are quite scarce. It has been estimated that half of the global RHD population under the age of 15 years lives in Africa (67). In the year 1990, the annual incidence of ARF in school-going children was estimated to be 30/100,000 (68). In Algerian children and adolescents (aged 4–19 years), the annual incidence of ARF was estimated to be 11.1/100,000 in 1997 and 6.2/100,000 in 2000 (69). A systematic review on the global burden of group A streptococcal disease by Carapetis et al. has noted a lesser incidence of ARF in sub-Saharan and North Africa in comparison to other “high-risk” regions (70). Poor case ascertainment and documentation seem to be the likely reasons for the lesser incidence of ARF in Africa (70).
KD
Similar to ARF, the actual incidence of KD in many African countries is not known. Variable incidence rates amongst children below 5 years of age have been documented from different African countries, for example, 3.15/100,000 from Algeria, 0.95/100,000 from Tunisia, and 4.52/100,000 from Morocco (71). Although incidence studies of KD are largely lacking, the increasing number of publications from Africa are encouraging and portend enhanced detection of KD in near future (7, 8).
Latin America
ARF
The highest population-based annual incidence of ARF (~360/100,000 children aged 10–20 years) in Latin America has been documented in Belo Horizonte, Brazil during the year 1992 (69, 72). However, the study was carried out in a limited population only and the duration of the study was from March to December 1992 (72). Other studies from the region have noted at least a 5-fold lower incidence of ARF. In Cuban province of Pinar del Rio, a marked reduction in incidence of ARF (in the age group 5-25 years) was noted during 1986 to 1996 wherein the incidence rate decreased from 18.6/100,000 to 2.5/100,000, respectively (73). In the French Caribbean islands of Martinique and Guadeloupe, the incidence of ARF (in the age group of <20 years) in 1982–83 was 19.6/100,000 and 17.4/100,000, respectively. By 1992, the incidence was reduced by about 4- to 5-fold in both the regions as a result of a decade-long educational program (74). A study from Chile has noted a gradual decline in the incidence of ARF from 3/100,000 inhabitants in 1979 to 0 in 1998 (75). During 1994–99, a hospital-based study from Mexico noted incidence of first episode of ARF to be 660/100,000 cases admitted to the hospital (700/100,000 children and adolescents aged 5–20 years) (76). The incidence of ARF during this period was noted to be lower than the early 1970s when it was 1060/100,000 subjects (76, 77).
KD
The exact incidence of KD in many Latin American countries remains unknown. For precise estimation of disease burden, a research network was officially formed in 2013 comprising of 20 countries (78). Using the nationwide hospital-based data, the incidence of KD in Chilean children <5 years of age was estimated to be 5.7/100,000 in 2001–2004, 8.4/100,000 in 2005–2007, and 10.4/100,000 in 2009–2011 (79, 80). The highest incidence of KD (19.8/100,000) was noted in Eastern Metropolitan Health District region that, notably, had the highest socioeconomic status in the country (80).
The trends in the incidence of KD and ARF in regions classified as “high-risk” for ARF are summarized in Supplementary Tables 4, 5.
Discussion
KD and ARF are the two most common causes of acquired heart disease in the pediatric population (1–3). ARF is an important complication of β-hemolytic group-A streptococcal infection that may lead to life-long cardiac morbidity primarily due to sequelae of valvular involvement (2). In contrast, KD is a medium vessel vasculitis of unknown etiology that has the predilection to involve coronary arteries and myocardium (3, 81). As we have reviewed, the incidence of ARF is on the decline in majority of the “high-risk” regions. This could be due to general improvement in health and sanitation facilities and effective public health programs. However, robust population-based data on the incidence of ARF are lacking from many high-risk regions with the most notable exception of Northern Australia – the region with the highest incidence of ARF amongst the Indigenous population. Even within Northern Australia, the incidence of ARF is seemingly high in regions where there is stringent data collection (58). Based on this corollary, KD may be under-recognized in the Indigenous population (64, 66). The robust ARF surveillance network may be leveraged to know the incidence of KD specifically amongst the Indigenous population. It would be intriguing to know the epidemiology of KD in populations with the highest incidence of ARF.
The most notable cardiac sequel of KD is the formation of coronary artery aneurysms. These aneurysms may get thrombosed in the acute stage and even lead to myocardial infarction (3). Myocardial involvement (myocarditis), increasingly thought to be universal in KD, may be severe enough to lead to severe cardiogenic shock (81). Long-term cardiac complications of KD include the risk the premature coronary artery disease, early myocardial infarction, and possibly cardiomyopathy (3, 81). It is imperative to mention that the diagnosis of KD may easily be missed especially in developing countries. Lack of awareness, poor healthcare infrastructure, and increased burden of infectious diseases (many of tropical infections may mimic KD) are some of the major contributing factors. The available diagnostic criteria for KD are based on constellation of clinical features and there is lack of a specific diagnostic test. Besides, epidemiological data collection in many developing countries remains suboptimal. These issues can further compound the problem of underdiagnosis of KD in developing countries. Nevertheless, increase in the incidence of KD has still been noted in the developing countries which has been attributed to industrialization and urbanization, besides the increase in the general awareness regarding the disease (82, 83). In the developed world, has already overtaken ARF as the leading cause of acquired heart disease in children. The developing countries also seem to be on a similar trend.
Recognition of KD and ARF in the pediatric population would help to reduce the burden of acquired heart disease in a significant proportion of the economically productive age group. This would have immense implications for the developing countries many of which face significant economic constraints (82). Surveillance networks simultaneously assessing the incidence of KD and ARF would be an effective option to tackle the major chunk of acquired heart disease in children in these regions.
Finally, multisystem inflammatory syndrome in children (MIS-C) post-SARS-CoV-2 usually presents with KD-like features. Myocardial dysfunction is seen in approximately half, whereas, coronary artery involvement is seen in about one-tenth (84). The SARS-CoV-2 pandemic has resulted in a spike in the incidence of KD in many countries around the globe with may further add to the burden of acquired heart disease in children in the near future. On a global stage, especially in the developing countries, the young hearts faced a dual challenge of ARF and KD even before the SARS-CoV-2 pandemic. MIS-C post-SARS-CoV-2 has compounded the challenge of mitigating the problem of acquired heart disease in children. A dedicated collaborative effort is required on a global level to manage the predicament of acquired heart disease in children.
Author Contributions
AB: writing of initial draft of manuscript, editing and revision of manuscript at all stages of its production, review of literature, drawing of figures, and final approval. SM: writing of initial draft of the manuscript, contributed to editing of manuscript, review of literature, and final approval. PB, AS, and RK: contributed to editing of manuscript, data collection, and final approval. PV: contributed to editing of manuscript, critical revision of the manuscript at all stages of production, review of literature, and final approval. SS: contributed to editing of manuscript, revision of the manuscript, and its final approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.694393/full#supplementary-material
Supplementary Table 1. American Heart Association (2015) Revised Jones criteria for the diagnosis of acute rheumatic fever (ARF).
Supplementary Table 2. American Heart Association (2017) criteria for diagnosis of Kawasaki disease (KD).
Supplementary Table 3. Japanese criteria (2020) for diagnosis of Kawasaki disease (KD).
Supplementary Table 4. Summary of trends in incidence of acute rheumatic fever in “high-risk” regions.
Supplementary Table 5. Summary of trends in incidence of Kawasaki disease in children below 5 years of age (unless specified otherwise) in regions classified as “high-risk” for acute rheumatic fever.
References
1. Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. (2016) 2:15084. doi: 10.1038/nrdp.2015.84
2. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. (2017) 377:713–22. doi: 10.1056/NEJMoa1603693
3. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. American heart association rheumatic fever, endocarditis, and kawasaki disease committee of the council on cardiovascular disease in the young; council on cardiovascular and stroke nursing; council on cardiovascular surgery and anesthesia; and council on epidemiology and prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
4. Curry C, Zuhlke L, Mocumbi A, Kennedy N. Acquired heart disease in low-income and middle-income countries. Arch Dis Child. (2018) 103:73–7. doi: 10.1136/archdischild-2016-312521
5. Singh S, Jindal AK, Pilania RK. Diagnosis of kawasaki disease. Int J Rheum Dis. (2018) 21:36–44. doi: 10.1111/1756-185X.13224
6. Al-Ammouri I, Al-Wahsh S, Khuri-Bulos N. Kawasaki disease in Jordan: demographics, presentation, and outcome. Cardiol Young. (2012) 22:390–5. doi: 10.1017/S1047951111001818
7. Ben Chehida A, Ben Messaoud S, Ben Abdelaziz R, Boudabous H, Oujra M, Ben Turkia H, et al. High frequency of cardiovascular complications in Tunisian Kawasaki disease patients: need for a further awareness. J Trop Pediatr. (2019) 65:217–23. doi: 10.1093/tropej/fmy036
8. Boudiaf H, Achir M. The clinical profile of Kawasaki disease in Algerian children: a single institution experience. J Trop Pediatr. (2016) 62:139–43. doi: 10.1093/tropej/fmv090
9. Vijayvergiya R, Bhattad S, Varma S, Singhal M, Gordon J, Singh S. Presentation of missed childhood Kawasaki disease in adults: experience from a tertiary care center in north India. Int J Rheum Dis. (2017) 20:1023–7. doi: 10.1111/1756-185X.13073
10. Wilder MS, Palinkas LA, Kao AS, Bastian JF, Turner CL, Burns JC. Delayed diagnosis by physicians contributes to the development of coronary artery aneurysms in children with Kawasaki syndrome. Pediatr Infect Dis J. (2007) 26:256–60. doi: 10.1097/01.inf.0000256783.57041.66
11. Hormell RS. Notes on the history of rheumatism and gout. N Engl J Med. (1940) 223:754–60. doi: 10.1056/NEJM194011072231903
12. Hartung EF. History of the use of colchicum and related medicaments in gout: with suggestions for further research. Ann Rheum Dis. (1954) 13:190. doi: 10.1136/ard.13.3.190
13. Pechey J. The Whole Works of That Excellent Practical Physician, Dr. (1711). Available online at: https://www.loc.gov/item/40021032/ (accessed January 15, 2021).
14. Harvey G. The Vanities of Philosophy and Physick. 2nd ed (1700). Available online at: https://books.google.com/ (accessed January 15, 2021).
15. Snyder JR. Rheumatism of childhood. JAMA. (1907) XLVIII:490–3. doi: 10.1001/jama.1907.25220320028002g
16. Dunn CH. The clinical aspects of rheumatic fever in childhood, and their significance in the question of specific etiology. JAMA. (1907) XLVIII:493–501. doi: 10.1001/jama.1907.25220320031002h
17. Jones TD. The diagnosis of rheumatic fever. JAMA. (1944) 126:481–4. doi: 10.1001/jama.1944.02850430015005
18. Bland EF, Jones TD. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. Circulation. (1951) 4:836–43. doi: 10.1161/01.CIR.4.6.836
19. Dajani AS, Ayoub E, Bierman FZ, Bisno AL, Denny FW, Durack DT, et al. Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA. (1992) 268:2069–73. doi: 10.1001/jama.1992.03490150121036
20. Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, et al. American heart association committee on rheumatic fever, endocarditis, and kawasaki disease of the council on cardiovascular disease in the young. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. (2015) 131:1806–18. doi: 10.1161/CIR.0000000000000205
21. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. (1967) 16:178–222.
22. Melish ME. Kawasaki syndrome: a new infectious disease? J Infect Dis. (1981) 143:317–24. doi: 10.1093/infdis/143.3.317
23. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. (1974) 54:271–6.
24. Kato H, Koike S, Yamamoto M, Ito Y, Yano E. Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J Pediatr. (1975) 86:892–8. doi: 10.1016/S0022-3476(75)80220-4
25. Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. (1984) 2:1055–8. doi: 10.1016/S0140-6736(84)91504-6
26. Kobayashi T, Ayusawa M, Suzuki H, Abe J, Ito S, Kato T, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int. (2020) 62:1135–8. doi: 10.1111/ped.14326
27. Koshi G, Benjamin V, Cherian G. Rheumatic fever and rheumatic heart disease in rural South Indian children. Bull World Health Organ. (1981) 59:599–603.
28. Grover A, Dhawan A, Iyengar SD, Anand IS, Wahi PL, Ganguly NK. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bull World Health Organ. (1993) 71:59–66.
29. Negi PC, Kanwar A, Chauhan R, Asotra S, Thakur JS, Bhardwaj AK. Epidemiological trends of RF/RHD in school children of Shimla in north India. Indian J Med Res. (2013) 137:1121–7. Available online at: https://www.ijmr.org.in/text.asp?2013/137/6/1121/114429
30. Thakur JS, Negi PC, Ahluwalia SK, Vaidya NK. Epidemiological survey of rheumatic heart disease among school children in the Shimla hills of northern India: prevalence and risk factors. J Epidemiol Community Health. (1996) 50:62–7. doi: 10.1136/jech.50.1.62
31. Negi PC, Sondhi S, Asotra S, Mahajan K, Mehta A. Current status of rheumatic heart disease in India. Indian Heart J. (2019) 71:85–90. doi: 10.1016/j.ihj.2018.12.007
32. Singh S, Kawasaki T. Kawasaki disease in India, lessons learnt over the last 20 years. Indian Pediatr. (2016) 53:119–24. doi: 10.1007/s13312-016-0804-5
33. Singh S, Aulakh R, Bhalla AK, Suri D, Manojkumar R, Narula N, et al. Is Kawasaki disease incidence rising in Chandigarh, North India? Arch Dis Child. (2011) 96:137–40. doi: 10.1136/adc.2010.194001
34. Singh S, Bhattad S. Kawasaki disease incidence at Chandigarh, North India, during 2009-2014. Rheumatol Int. (2016) 36:1391–7. doi: 10.1007/s00296-016-3543-y
35. Singh S, Aulakh R, Kawasaki T. Kawasaki disease and the emerging coronary artery disease epidemic in India: is there a correlation? Indian J Pediatr. (2014) 81:328–32. doi: 10.1007/s12098-013-1229-y
36. Sharma D, Iqbal F, Narayan Dev C, Bora S, Hoque RA, Kom LB. Clinical profile, treatment and outcome of Kawasaki disease: a single-center experience from a tertiary care referral center of Assam, north-east India. Int J Rheum Dis. (2021) 24:391–96. doi: 10.1111/1756-185X.14059
37. Nandi A, Pal P, Basu S. A comparison of serum IL6 and CRP levels with respect to coronary changes and treatment response in Kawasaki disease patients: a prospective study. Rheumatol Int. (2019) 39:1797–801. doi: 10.1007/s00296-019-04375-9
38. Chen X, Zhang M, Huang D, Huang M, Xiong Y, Xie M, et al. An epidemiologic investigation of acute rheumatic fever and rheumatic heart disease among students aged 5-18 in west area of Sichuan Province. Sichuan Da Xue Xue Bao Yi Xue Ban. (2003) 34:533–5. Available online at: https://pubmed.ncbi.nlm.nih.gov/12910712/
39. Chen L, Xie X, Gu J, Xu L, Yang X, Yu B. Changes of manifestations of 122 patients with rheumatic fever in South China during last decade. Rheumatol Int. (2009) 30:239–43. doi: 10.1007/s00296-009-0944-1
40. Zhendong H, Xuxu R, Runchao C. An updated epidemiologic survey of acute rheumatic fever among school-age children in China. Zhonghua xin xue Guan Bing za zhi. (1998) 26. Available online at: https://europepmc.org/article/cba/586014
41. Woo KS, Kong SM, Wai KH. The changing prevalence and pattern of acute rheumatic fever and rheumatic heart disease in Hong Kong–(1968-1978). Aust N Z J Med. (1983) 13:151–6. doi: 10.1111/j.1445-5994.1983.tb02671.x
42. Ma XJ, Yu CY, Huang M, Chen SB, Huang MR, Huang GY, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2003 through 2007. Chin Med J. (2010) 123:2629–34. doi: 10.3760/cma.j.issn.0366-6999.2010.19.002
43. Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, et al. Shanghai Kawasaki disease research group. Epidemiologic features of Kawasaki Disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. (2016) 35:7–12. doi: 10.1097/INF.0000000000000914
44. Xie LP, Yan WL, Huang M, Huang MR, Chen S, Huang GY, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J Epidemiol. (2020) 30:429–35. doi: 10.2188/jea.JE20190065
45. Du ZD, Zhang T, Liang L, Meng X, Li T, Kawasaki T, et al. Epidemiologic picture of Kawasaki disease in Beijing from 1995 through 1999. Pediatr Infect Dis J. (2002) 21:103–7. doi: 10.1097/00006454-200202000-00004
46. Du ZD, Zhao D, Du J, Zhang YL, Lin Y, Liu C, et al. Beijing Kawasaki Research Group. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. (2007) 26:449–51. doi: 10.1097/01.inf.0000261196.79223.18
47. Zhang X, Zhang Z, Liu S, Sun J. Epidemiologic survey of Kawasaki disease in Jilin from 1999 through 2008. Pediatr Cardiol. (2012) 33:272–9. doi: 10.1007/s00246-011-0121-7
48. Zhang X, Liang Y, Feng W, Su X, Zhu H. Epidemiologic survey of Kawasaki disease in inner mongolia, China, between 2001 and 2013. Exp Ther Med. (2016) 12:1220–4. doi: 10.3892/etm.2016.3393
49. Jiao F, Yang L, Li Y, Qiao J, Guo X, Zhang T, et al. Epidemiologic and clinical characteristics of Kawasaki disease in Shaanxi Province, China, 1993-1997. J Trop Pediatr. (2001) 47:54–6. doi: 10.1093/tropej/47.1.54
50. Li XH, Li XJ, Li H, Xu M, Zhou M. Epidemiological survey of Kawasaki disease in Sichuan province of China. J Trop Pediatr. (2008) 54:133–6. doi: 10.1093/tropej/fmm085
51. Patten BR. Rheumatic fever in the West Kimberley. Med J Aust. (1981) 1:11–5. doi: 10.5694/j.1326-5377.1981.tb135997.x
52. Richmond P, Harris L. Rheumatic fever in the Kimberley region of Western Australia. J Trop Pediatr. (1998) 44:148–52. doi: 10.1093/tropej/44.3.148
53. Murdoch J, Davis S, Forrester J, Masuda L, Reeve C. Acute rheumatic fever and rheumatic heart disease in the Kimberley: using hospitalisation data to find cases and describe trends. Aust N Z J Public Health. (2015) 39:38–43. doi: 10.1111/1753-6405.12240
54. Davies SB, Hofer A, Reeve C. Mortality attributable to rheumatic heart disease in the Kimberley: a data linkage approach. Intern Med J. (2014) 44:1074–80. doi: 10.1111/imj.12540
55. Hanna JN, Heazlewood RJ. The epidemiology of acute rheumatic fever in indigenous people in north Queensland. Aust N Z J Public Health. (2005) 29:313–7. doi: 10.1111/j.1467-842X.2005.tb00199.x
56. Hanna JN, Clark MF. Acute rheumatic fever in indigenous people in North Queensland: some good news at last? Med J Aust. (2010) 192:581–4. doi: 10.5694/j.1326-5377.2010.tb03641.x
57. Brennan RE, Patel MS. Acute rheumatic fever and rheumatic heart disease in a rural central Australian aboriginal community. Med J Aust. (1990) 153:335:338–9. doi: 10.5694/j.1326-5377.1990.tb136942.x
58. Carapetis JR, Currie BJ, Mathews JD. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population? Epidemiol Infect. (2000) 124:239–44. doi: 10.1017/S0950268800003514
59. Carapetis JR, Currie BJ. Mortality due to acute rheumatic fever and rheumatic heart disease in the Northern Territory: a preventable cause of death in aboriginal people. Aust N Z J Public Health. (1999) 23:159–63. doi: 10.1111/j.1467-842X.1999.tb01227.x
60. Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. (2013) 128:492–501. doi: 10.1161/CIRCULATIONAHA.113.001477
61. Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Stacey I, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc. (2020) 9:e016851. doi: 10.1161/JAHA.120.016851
62. Smith PK, Goldwater PN. Kawasaki disease in Adelaide: a review. J Paediatr Child Health. (1993) 29:126–31. doi: 10.1111/j.1440-1754.1993.tb00464.x
63. Royle JA, Williams K, Elliott E, Sholler G, Nolan T, Allen R, et al. Kawasaki disease in Australia, 1993-95. Arch Dis Child. (1998) 78:33–9. doi: 10.1136/adc.78.1.33
64. Saundankar J, Yim D, Itotoh B, Payne R, Maslin K, Jape G, et al. The epidemiology and clinical features of Kawasaki disease in Australia. Pediatrics. (2014) 133:e1009–14. doi: 10.1542/peds.2013-2936
65. Ferreira D, Ng R, Lai E, Singh-Grewal D, Kehr J, Collins N, et al. Kawasaki disease in the Australian population: an Australian tertiary hospital experience. Heart Lung Circ. (2021) 30:996–1001. doi: 10.1016/j.hlc.2020.12.016
66. O'Brien K. Australian hospitalisations for Kawasaki disease, 1993-1994 to 2017-2018. J Paediatr Child Health. (2020) 56:1126–33. doi: 10.1111/jpc.14847
67. Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. (2008) 94:1534–40. doi: 10.1136/hrt.2007.141309
68. Kechrid A, Kharrat H, Bousnina S, Kriz P, Kaplan EL. Acute rheumatic fever in Tunisia. Serotypes of group A streptococci associated with rheumatic fever. Adv Exp Med Biol. (1997) 418:121–3. doi: 10.1007/978-1-4899-1825-3_29
69. World Health Organization? The Current Evidence For the Burden of Group A Streptococcal Diseases. World Health Organization. (2005). Available online at: https://apps.who.int/iris/handle/10665/69063. (accessed Match 21, 2021).
70. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. (2005) 5:685–94. doi: 10.1016/S1473-3099(05)70267-X
71. Gorrab AA, Fournier A, Bouaziz AA, Spigelblatt L, Scuccimarri R, Mrabet A, et al. incidence rate and epidemiological and clinical aspects of Kawasaki disease in children of Maghrebi origin in the province of Quebec, Canada, compared to the country of origin. Glob Pediatr Health. (2016) 3:2333794X16630670. doi: 10.1177/2333794X16630670
72. Alves Meira ZM, de Castilho SR, Lins Barros MV, Maria Vitarelli A, Diniz Capanema F, Moreira NS, et al. Prevalence of rheumatic fever in children from a public high school in Belo Horizonte. Arq Bras Cardiol. (1995) 65:331–4.
73. Nordet P, Lopez R, Dueñas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986-1996-2002). Cardiovasc J Afr. (2008) 19:135–40. Available online at: https://pubmed.ncbi.nlm.nih.gov/18568172/
74. Bach JF, Chalons S, Forier E, Elana G, Jouanelle J, Kayemba S, et al. 10-year educational programme aimed at rheumatic fever in two French Caribbean islands. Lancet. (1996) 347:644–8. doi: 10.1016/S0140-6736(96)91202-7
75. Luque C, Cisternas FA, Araya M. Changes in the patterns of disease after the epidemiological transition in health in Chile, 1950-2003. Rev Med Chil. (2006) 134:703–12. doi: 10.4067/S0034-98872006000600005
76. Soto López ME, Cordera González de Cosío F, Estrada L, Guel L, Abud Mendoza C, Reyes PA. Rheumatic fever in the 5-year period of 1994-1999 at 2 hospitals in San Luis Potosi and Mexico D.F. Arch Cardiol Mex. (2001) 71:127–35. Available online at: https://pubmed.ncbi.nlm.nih.gov/18568172/
77. Kuri J, Fernández de la Vega P, Mata LA, Attie F, Zamora C. Study of the 1st outbreak of rheumatic fever. Long-term observation and clinical evolution of 40 patients. Arch Inst Cardiol Mex. (1977) 47:19–36.
78. González-Mata A, Ulloa-Gutiérrez R, Brea J, Soza G, Tremoulet AH. Origin and importance of the Latin American Kawasaki disease network (REKAMLATINA). Rev Chilena Infectol. (2014) 31:330–2. doi: 10.4067/S0716-10182014000300012
79. Borzutzky A, Hoyos-Bachiloglu R, Cerda J, Talesnik E. Rising hospitalization rates of Kawasaki Disease in Chile between 2001 and 2007. Rheumatol Int. (2012) 32:2491–5. doi: 10.1007/s00296-011-2050-4
80. Hoyos-Bachiloglu R, García Á, Morales PS, Cerda J, Talesnik E, Borzutzky A. Geographic distribution of Kawasaki disease throughout Chile. Rev Chilena Infectol. (2016) 33:12–8. doi: 10.4067/S0716-10182016000100002
81. Dionne A, Dahdah N. Myocarditis and Kawasaki disease. Int J Rheum Dis. (2018) 21:45–9. doi: 10.1111/1756-185X.13219
82. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. (2015) 100:1084–8. doi: 10.1136/archdischild-2014-307536
83. Elakabawi K, Lin J, Jiao F, Guo N, Yuan Z. Kawasaki disease: global burden and genetic background. Cardiol Res. (2020) 11:9–14. doi: 10.14740/cr993
Keywords: epidemiology, fever, heart, incidence, Kawasaki disease, rheumatic, trend
Citation: Banday AZ, Mondal S, Barman P, Sil A, Kumrah R, Vignesh P and Singh S (2021) What Lies Ahead for Young Hearts in the 21st Century – Is It Double Trouble of Acute Rheumatic Fever and Kawasaki Disease in Developing Countries? Front. Cardiovasc. Med. 8:694393. doi: 10.3389/fcvm.2021.694393
Received: 13 April 2021; Accepted: 01 June 2021;
Published: 24 June 2021.
Edited by:
Roney Orismar Sampaio, University of São Paulo, BrazilReviewed by:
Vitor Emer Egypto Rosa, Universidade de São Paulo, BrazilJohn Bernard Lawrenson, Stellenbosch University, South Africa
Copyright © 2021 Banday, Mondal, Barman, Sil, Kumrah, Vignesh and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pandiarajan Vignesh, dmlnaW1tY0BnbWFpbC5jb20=
†These authors share first authorship
 Aaqib Zaffar Banday
Aaqib Zaffar Banday Sanjib Mondal
Sanjib Mondal Prabal Barman
Prabal Barman Archan Sil
Archan Sil Rajni Kumrah
Rajni Kumrah Pandiarajan Vignesh
Pandiarajan Vignesh Surjit Singh
Surjit Singh