Abstract
Objective: Atrial fibrillation (AF) and venous thromboembolism (VTE) share several risk factors related to arterial thromboembolism. No study has reported the differential contribution to arterial thromboembolic events and mortality between these two conditions in the same population. We therefore assessed the differential arterial thromboembolic events between AF and VTE.
Methods: We included AF and VTE national cohorts derived from Taiwan National Health Insurance Research Database between 2001 and 2013. The eligible population was 314,861 patients in the AF cohort and 41,102 patients in the VTE cohort. The primary outcome was arterial thromboembolic events, including ischemic stroke, extracranial arterial thromboembolism (ECATE) and myocardial infarction (MI). Secondary outcomes were all-cause mortality and cardiovascular death.
Results: After a 1:1 propensity matching, 32,688 patients in either group were analyzed. The risk of arterial thromboembolic events was lower in the VTE cohort than that in the AF cohort (subdistribution hazard ratio [SHR], 0.60; 95% confidence interval [CI], 0.57–0.62). The risk of ischemic stroke (SHR, 0.44; 95% CI, 0.42–0.46) and MI (SHR, 0.80; 95% CI, 0.72–0.89) were lower in the VTE cohort, while the risk of ECATE (SHR, 1.23; 95% CI, 1.14–1.33; particularly lower extremities) was higher in the VTE cohort. All-cause mortality rate was higher in the VTE cohort (HR, 1.18; 95% CI, 1.15–1.21) while the risk of cardiovascular death was lower in the VTE cohort (HR, 0.96; 95% CI, 0.93–0.995).
Conclusions: Patients with AF had higher risks of arterial thromboembolic events compared to patients with VTE, despite having risk factors in common. The VTE cohort had higher risks of all-cause mortality and ECATE, particularly lower extremity events, compared to AF patients. The differential manifestations of thromboembolism sequelae and mortality between AF and VTE patients merit further investigation.
Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke, systemic thromboembolic events, and mortality (1). Long-term anticoagulation therapy, particularly with direct oral anticoagulants (DOACs), significantly reduces the risk of stroke and mortality (2). In terms of venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), the duration of anticoagulation therapy to prevent recurrences takes into consideration the risk of recurrent VTE and the risk of bleeding (3).
AF and VTE have many pathophysiological and clinical risk factors in common. In terms of pathophysiology, the pathogenesis of arterial thromboembolism in AF has been associated with a prothrombotic state by fulfilling Virchow's triad for thrombogenesis, i.e., with abnormal blood flow (stasis) in the atria, vessel wall abnormalities and abnormal blood constituents (coagulation factors) as well as inflammation (4). Likewise, the pathogenesis of arterial thromboembolism in VTE has been associated with a prothrombotic state, i.e., with abnormal blood flow (stasis) in the vessels, vessel wall abnormalities and abnormal blood constituents (coagulation factors) as well as inflammation (5, 6). Several studies showed that VTE increases risk of atherothrombotic cardiovascular events, including myocardial infarction (MI) (7). In terms of contributing factors, AF and VTE also share similar comorbidities (3, 6), such as age, hypertension, smoking, diabetes, and obesity (8–10), peripheral artery disease (11) and malignancy (12). Moreover, one community registry study reported that AF and VTE independently contributed to each other (13). However, the duration of prescribing anticoagulation is quite different between AF and VTE in current practice. Long-term anticoagulation should be prescribed for AF patients (2, 14) whereas more limited-duration of anticoagulation is sometimes prescribed for VTE patients unless there are high risk features for recurrence (3, 15).
We hypothesized that AF and VTE, despite sharing many pathophysiological and clinical risk factors, have different duration of prescribing anticoagulation and should have differential contribution to arterial thromboembolic events and mortality in the same population. Accordingly, we tested this hypothesis in a nationwide cohort study of VTE and AF patients from the Taiwan National Health Insurance Database.
Methods
The data of this national retrospective cohort study was retrieved from the Taiwan National Health Insurance Research Database (NHIRD) released by the Taiwan National Health Research Institutes. The National Health Insurance system is a mandatory universal health insurance program that offers comprehensive medical care coverage to nearly all Taiwan residents since the inception of the program in March 1995. In the NHIRD, the patients' original identification numbers are encrypted and the encrypting procedure is consistent, so that linking claims belonging to the same enrollee is feasible and can be followed longitudinally. The available health care information included complete outpatient visits, hospitalization, and diseases, which were registered using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (16). In addition, medication prescriptions are also recorded. Patients with newly diagnosis of AF and VTE were included in this study. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201900915B1).
Identification of Patients With VTE and AF
This study included national AF and VTE cohorts. Patients with AF were identified with ≥2 times outpatient visits or in a discharge diagnosis using the ICD-9 CM diagnostic code of 427.31 between 2001 and 2013. Patients with VTE were identified using the discharge diagnosis (ICD-9-CM: 453 for DVT and 415.1 for PE) with use of anticoagulation during admission between 2001 and December 31, 2013. In the AF cohort, we excluded patients who were under age of 20 years old and were diagnosed as VTE historically (the diagnosis could be tracked up to year 1997) or in follow-up period. In the VTE cohort, we excluded patients who were under age of 20 years old and were diagnosed as AF historically (the diagnosis could be tracked up to year 1997) or in the follow-up period. In order to compare the differences in the clinical outcomes after developing AF and VTE, we excluded those who died at the index admission in both cohorts. Finally, 314,861 AF patients without VTE and 41,102 VTE patients without AF were included in this study (Figure 1).
Figure 1
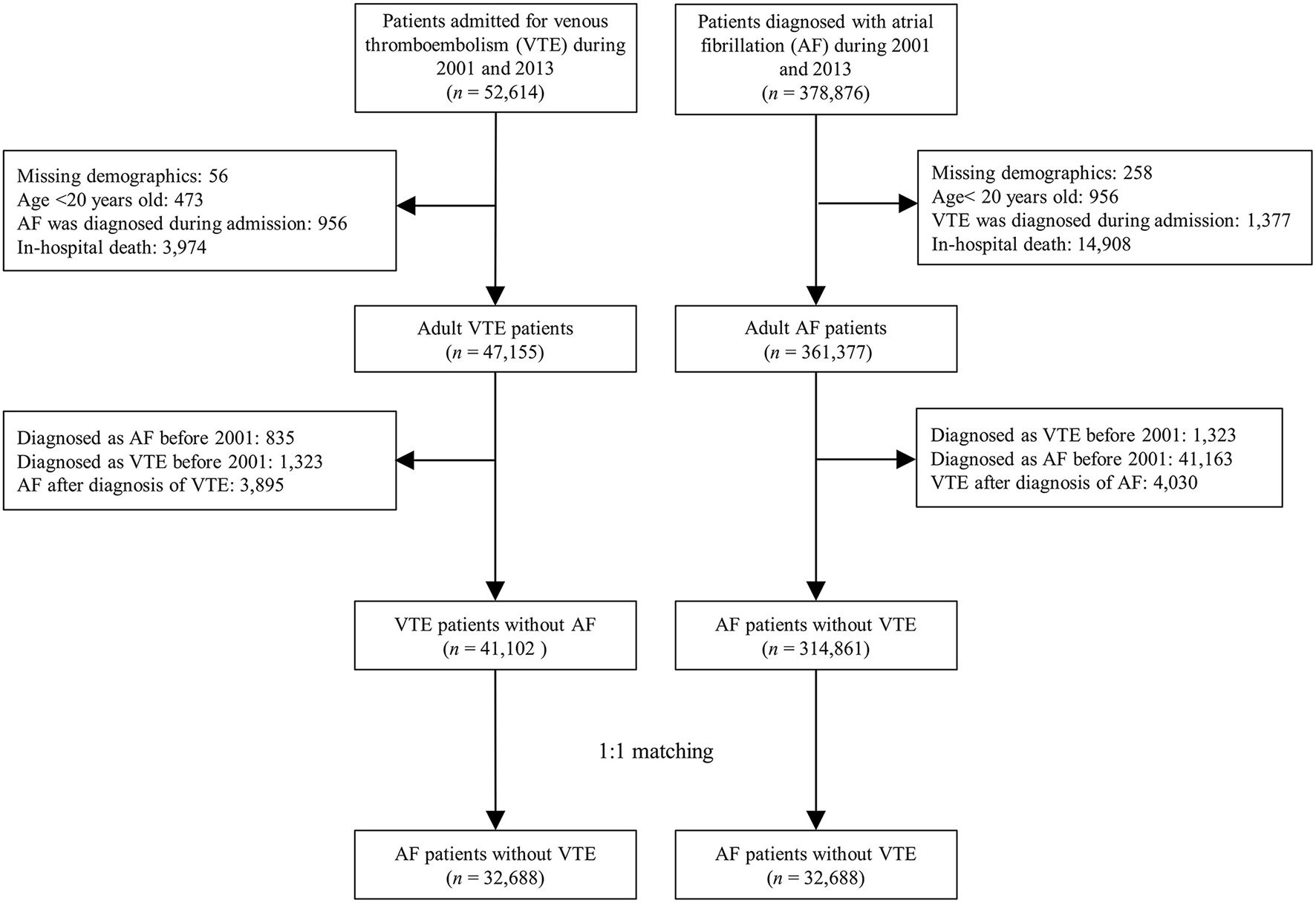
Flowchart for selection of the study patients.
Covariates
Covariates were age, sex, eighteen comorbidities, Charlson Comorbidity Index score, four historical events, and fourteen kinds of medications (Table 1). Comorbidities were recognized with at least two clinic visits or any inpatient record in the previous year before the index date. Historical events were detected using any inpatient diagnosis before the index date which could be tracked up to year 1997. The use of medication was extracted within 3 months after index date. All the information about medications were extracted from the claims data of outpatient visits or the refill for chronic illness in the pharmacy by using the Anatomical Therapeutic Chemical codes or the Taiwan NHI reimbursement code.
Table 1
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
|
VTE
(n = 41,102) |
AF
(n = 314,861) |
STD |
VTE
(n = 32,688) |
AF
(n = 32,688) |
STD | |
| Age (years) | 64.3 ± 16.4 | 71.2 ± 13.5 | −0.46 | 66.7 ± 15.4 | 67.4 ± 14.6 | −0.04 |
| Age group | ||||||
| <65 years | 18,837 (45.8) | 89,486 (28.4) | 0.37 | 13,087 (40.0) | 12,464 (38.1) | 0.04 |
| 65-74 years | 9,526 (23.2) | 82,459 (26.2) | −0.07 | 8,082 (24.7) | 8,422 (25.8) | −0.02 |
| ≥ 75 years | 12,739 (31.0) | 142,916 (45.4) | −0.30 | 11,519 (35.2) | 11,802 (36.1) | −0.02 |
| Male sex | 19,152 (46.6) | 174,954 (55.6) | −0.18 | 16,123 (49.3) | 16,752 (51.2) | −0.04 |
| Types of VTE | ||||||
| Pulmonary embolism (PE) | 9,143 (22.2) | - | 7,702 (23.6) | - | ||
| Deep vein thrombosis (DVT) | 29,512 (71.8) | - | 23,081 (70.6) | - | ||
| DVT + PE | 2,447 (6.0) | - | 1,905 (5.8) | - | ||
| Comorbid conditions | ||||||
| Hypertension | 21,262 (51.7) | 191,993 (61.0) | −0.19 | 18,355 (56.2) | 18,942 (57.9) | −0.04 |
| Diabetes mellitus | 10,800 (26.3) | 75,303 (23.9) | 0.05 | 8,877 (27.2) | 8,880 (27.2) | <0.01 |
| Ischemic heart disease | 8,845 (21.5) | 110,828 (35.2) | −0.31 | 8,023 (24.5) | 8,695 (26.6) | −0.05 |
| Dyslipidemia | 7,382 (18.0) | 52,525 (16.7) | 0.03 | 6,133 (18.8) | 6,423 (19.6) | −0.02 |
| Gout | 4,104 (10.0) | 31,540 (10.0) | <0.01 | 3,463 (10.6) | 3,526 (10.8) | −0.01 |
| COPD | 5,630 (13.7) | 58,210 (18.5) | −0.13 | 4,843 (14.8) | 4,793 (14.7) | <0.01 |
| Peripheral artery disease | 2,802 (6.8) | 11,400 (3.6) | 0.14 | 2,052 (6.3) | 2,041 (6.2) | <0.01 |
| Chronic kidney disease | 7,190 (17.5) | 45,300 (14.4) | 0.08 | 5,803 (17.8) | 5,758 (17.6) | <0.01 |
| Dialysis | 1,209 (2.9) | 8,190 (2.6) | 0.02 | 1,034 (3.2) | 1,065 (3.3) | −0.01 |
| Cancer | 8,847 (21.5) | 19,901 (6.3) | 0.45 | 5,030 (15.4) | 4,727 (14.5) | 0.03 |
| Auto-immune disease | 811 (2.0) | 1,891 (0.6) | 0.12 | 424 (1.3) | 393 (1.2) | 0.01 |
| Hepatitis C virus infection | 835 (2.0) | 4,841 (1.5) | 0.04 | 626 (1.9) | 593 (1.8) | 0.01 |
| Paralysis | 3,245 (7.9) | 22,491 (7.1) | 0.03 | 2,705 (8.3) | 2,810 (8.6) | −0.01 |
| Osteoporosis | 3,578 (8.7) | 19,662 (6.2) | 0.09 | 2,702 (8.3) | 2,503 (7.7) | 0.02 |
| Charlson comorbidity index score | 3.0 ± 3.0 | 2.0 ± 2.0 | 0.40 | 2.7 ± 2.7 | 2.7 ± 2.7 | <0.01 |
| History of disease | ||||||
| Prior any stroke | 5,600 (13.6) | 46,969 (14.9) | −0.04 | 4,933 (15.1) | 5,230 (16.0) | −0.03 |
| Prior ischemic stroke or systemic thromboembolism | 5,506 (13.4) | 45,757 (14.5) | −0.03 | 4,832 (14.8) | 5,228 (16.0) | −0.03 |
| Old MI | 1,249 (3.0) | 13,564 (4.3) | −0.07 | 1,140 (3.5) | 1,240 (3.8) | −0.02 |
| Heart failure admission | 3,654 (8.9) | 40,121 (12.7) | −0.12 | 3,331 (10.2) | 3,608 (11.0) | −0.03 |
| Antithrombotic therapy within 3 months after index date | ||||||
| None | 9,640 (23.5) | 119,550 (38.0) | −0.32 | 9,475 (29.0) | 9,012 (27.6) | 0.03 |
| Antiplatelet | 3,192 (7.8) | 148,174 (47.1) | −0.98 | 3,189 (9.8) | 3,364 (10.3) | −0.02 |
| Anticoagulant | 28,270 (68.8) | 47,137 (15.0) | 1.30 | 20,024 (61.3) | 20,312 (62.1) | −0.02 |
| Medication | ||||||
| ACEi or ARB | 10,771 (26.2) | 137,957 (43.8) | −0.38 | 9,956 (30.5) | 11,094 (33.9) | −0.07 |
| Beta blocker | 8,044 (19.6) | 115,262 (36.6) | −0.39 | 7,496 (22.9) | 8,337 (25.5) | −0.06 |
| DCCB | 9,594 (23.3) | 85,295 (27.1) | −0.09 | 8,193 (25.1) | 8,447 (25.8) | −0.02 |
| Diuretic | 9,849 (24.0) | 80,723 (25.6) | −0.04 | 7,870 (24.1) | 8,237 (25.2) | −0.03 |
| Metformin | 4,078 (9.9) | 35,237 (11.2) | −0.04 | 3,428 (10.5) | 3,540 (10.8) | −0.01 |
| TZD | 656 (1.6) | 4,942 (1.6) | <0.01 | 520 (1.6) | 506 (1.5) | <0.01 |
| DPP4i | 774 (1.9) | 5,783 (1.8) | <0.01 | 681 (2.1) | 742 (2.3) | −0.01 |
| Insulin | 1,900 (4.6) | 10,125 (3.2) | 0.07 | 1,476 (4.5) | 1,458 (4.5) | <0.01 |
| Estrogen | 946 (2.3) | 4,105 (1.3) | 0.08 | 577 (1.8) | 504 (1.5) | 0.02 |
| Antidepressants | 3,728 (9.1) | 20,564 (6.5) | 0.09 | 2,754 (8.4) | 2,747 (8.4) | <0.01 |
| Statin | 4,289 (10.4) | 39,303 (12.5) | −0.06 | 3,846 (11.8) | 4,264 (13.0) | −0.04 |
| Digoxin | 1,068 (2.6) | 73,147 (23.2) | −0.65 | 1,067 (3.3) | 1,257 (3.8) | −0.03 |
| Follow up year | 3.8 ± 3.5 | 4.2 ± 3.4 | −0.12 | 3.7 ± 3.4 | 3.8 ± 3.4 | −0.03 |
Baseline characteristics of the patients diagnosed with AF or VTE.
VTE, venous thromboembolism; AF, atrial fibrillation; STD, standardized difference; COPD, chronic obstructive pulmonary disease; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DCCB, dihydropyrinde calcium channel blocker; TZD, thiazolidinedione; DDP4i, dipeptidyl peptidase 4 inhibitor.
Data were presented as frequency (percentage) or mean ± standard deviation.
Outcomes
The primary outcome was arterial thromboembolic events, including ischemic stroke, myocardial infarction (MI) and extracranial arterial thromboembolism (ECATE). Extracranial arterial thromboembolism included arterial thromboembolic occlusion of an extremity or extracranial vital organ, including kidney, intestine, and spleen. The occurrence of ischemic stroke and MI was defined as the principal discharge diagnosis of hospitalization. The occurrence of ECATE was defined as the principal or secondary diagnoses of hospitalization. Secondary outcomes were all-cause mortality and cardiovascular death. All-cause mortality was defined as withdrawal from the NHI program (17). The definition of cardiovascular (CV) death was the criteria of the Standardized Definitions for Cardiovascular and Stroke Endpoint Events in Clinical Trials by the FDA in the United States. Each patient was followed from the discharge date of index admission to the date of event occurrence, date of death, or December 31, 2013.
Ascertainment of VTE, AF, Ischemic Stroke, MI, and ECATE
The validation of AF has been assessed and presented in our previous reports, with a high positive predictive value (PPV) of 89% (18). The accuracy of VTE was reliable in the Taiwan insurance claim system and some published studies also used the same diagnosis method (11, 19). Ischemic stroke and MI were also validated with high PPVs (20, 21). In terms of ECATE, a validation study was conducted at our medical center, randomly sampling 100 hospitalizations for systemic thromboembolism who were selected using the same criteria as mentioned in this study. After experienced physicians (YSL and VCCW) reviewed the medical records and all imaging results, including vascular duplex, computed tomography angiography and intervention reports, the PPV of systemic thromboembolism was 88% (data not shown).
Statistics
There would be substantial difference in the baseline characteristics between study groups (i.e., VTE and AF cohorts). Therefore, we performed 1:1 ratio propensity score matching to make the covariates balanced between groups. The propensity score was the predicted probability to be in the one group (i.e., VTE) given the values of covariates using the multivariable logistic regression without considering interaction effects. The variables selected to calculate propensity score were listed in Table 1 where the follow-up year was replaced with the index date (Table 1). The matching was processed using a greedy nearest neighbor algorithm with a caliper of 0.2 times of the standard deviation of the logit of propensity score, with random matching order and without replacement. The quality of matching was checked using the absolute value of standardized difference (STD) between the groups, where a value <0.1 was considered negligible difference. We additionally performed three propensity score matchings to compare the PE-only cohort with the DVT-only cohort, the DVT-only cohort with the AF cohort and the PE-only cohort with the AF cohort, respectively.
As to the time to fatal outcomes (i.e., all-cause mortality and cardiovascular death), the risks between the groups were compared by the Cox proportional hazard model. The incidences of time to non-fatal outcomes (e.g., ischemic stroke or MI) between groups were compared by the Fine and Gray subdistribution hazard model which considered all-cause mortality a competing risk. The within-pair clustering of outcomes after propensity score matching was accounted for by using a robust standard error (22). Finally, we performed a subgroup analysis stratified by the use of oral anticoagulant within 3 months after the index date. A two-sided P-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
This study enrolled 314,861 AF patients (mean age of 71.2 ± 13.5 years) and 41,102 VTE patients (mean age of 64.3 ± 16.4 years). These two cohorts were different in age distribution, whereby the VTE cohort was predominantly age < 65 years while the AF cohort was predominantly age ≥ 75 years (Table 1). The AF cohort had significantly greater prevalence of hypertension, ischemic heart disease, chronic obstructive pulmonary disease (COPD), hyperthyroidism and heart failure, while VTE cohort had higher prevalence of peripheral artery disease, cancer and auto-immune disease. In terms of medications, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, beta blockers and digoxin were more frequently prescribed in the AF population. In this study, anticoagulant use was continuous in the AF cohorts while the duration of anticoagulant was at least for 3~6 months in the VTE cohort and 38.6% of VTE patients had the duration of anticoagulant use for more than 6 months. In addition, the available follow-up period in AF cohort were longer than that in VTE cohort (AF cohort vs. VTE cohort: 4.2 ± 3.4 vs. 3.8 ± 3.5 years, standardized difference: −0.12).
After matching, 32,688 patients in either cohort were well-balanced in baseline characteristics, including follow-up period (Table 1). In patients with DVT-only vs. AF, the AF cohorts had higher incidence of ischemic heart disease, heart failure and COPD while DVT-only cohort had higher incidence of cancer, auto-immune disease and peripheral artery disease (Supplementary Table 1). In patients with PE-only vs. AF, the PE-only cohort had a higher incidence of cancer (Supplementary Table 2). In terms of the PE-only vs. DVT-only cohorts, the PE-only cohort had higher incidence of ischemic heart disease, heart failure and COPD while DVT-only cohort had higher prevalence of cancer and peripheral artery disease (Supplementary Table 3).
Outcomes Between VTE and AF Cohorts
The outcomes between VTE and AF cohort obtained after propensity matching are shown in Table 2; Figure 2. The risk of the arterial thromboembolic events was lower in the VTE cohort [subdistribution hazard ratio (SHR), 0.60; 95% confidence interval (CI), 0.57–0.62] (Figure 2A), as were the risks of ischemic stroke (SHR, 0.44; 95% CI, 0.42–0.46) (Figure 2B) and MI (SHR, 0.80; 95% CI, 0.72–0.89). The risks of ECATE (SHR, 1.23; 95% CI, 1.14–1.33) (Figure 2C) and all-cause mortality rate (HR, 1.18; 95% CI, 1.15–1.21) were higher in VTE cohort (Figure 2E), although the latter had lower CV death (HR, 0.96; 95% CI, 0.93–0.995) (Figure 2D).
Table 2
| Before propensity matching | After propensity matching | |||||
|---|---|---|---|---|---|---|
| Outcome |
VTE
(n = 41,102) |
AF
(n = 314,861) |
VTE
(n = 32,688) |
AF
(n = 32,688) |
HR or SHR of VTE
(95% CI) |
P |
| Arterial thromboembolic events | 4,864 (11.8) | 61,684 (19.6) | 4,143 (12.7) | 6,383 (19.5) | 0.60 (0.57–0.62) | <0.001 |
| Ischemic stroke | 2,881 (7.0) | 47,867 (15.2) | 2,481 (7.6) | 5,141 (15.7) | 0.44 (0.42–0.46) | <0.001 |
| Extracranial arterial thromboembolism | 1,725 (4.2) | 10,026 (3.2) | 1,410 (4.3) | 1,122 (3.4) | 1.23 (1.14–1.33) | <0.001 |
| Lower extremity thromboembolism | 1,435 (3.5) | 8,361 (2.7) | 1,184 (3.6) | 917 (2.8) | 1.26 (1.16–1.37) | <0.001 |
| Non-lower extremity thromboembolism | 365 (0.9) | 2,329 (0.7) | 288 (0.88) | 284 (0.87) | 0.99 (0.84–1.16) | 0.867 |
| Myocardial infarction | 690 (1.7) | 9,718 (3.1) | 619 (1.9) | 741 (2.3) | 0.80 (0.72–0.89) | <0.001 |
| Secondary outcomes | ||||||
| All-cause mortality | 18,098 (44.0) | 135,551 (43.1) | 14,188 (43.4) | 12,345 (37.8) | 1.18 (1.15–1.21) | <0.001 |
| Cardiovascular death | 7,321 (17.8) | 73,344(23.3) | 5,958 (18.2) | 6,376 (19.5) | 0.96 (0.93–0.995) | 0.025 |
| Non-cardiovascular death | 10,777 (26.2) | 62,207 (19.8) | 8,230 (25.2) | 5,969 (18.3) | 1.42 (1.37–1.47) | <0.001 |
Follow-up outcomes in patients with VTE versus those with AF.
VTE, venous thromboembolism; AF, atrial fibrillation; HR, hazard ratio; SHR, subdistribution hazard ratio; CI, confidence interval.
Data were presented as frequency (percentage).
Figure 2
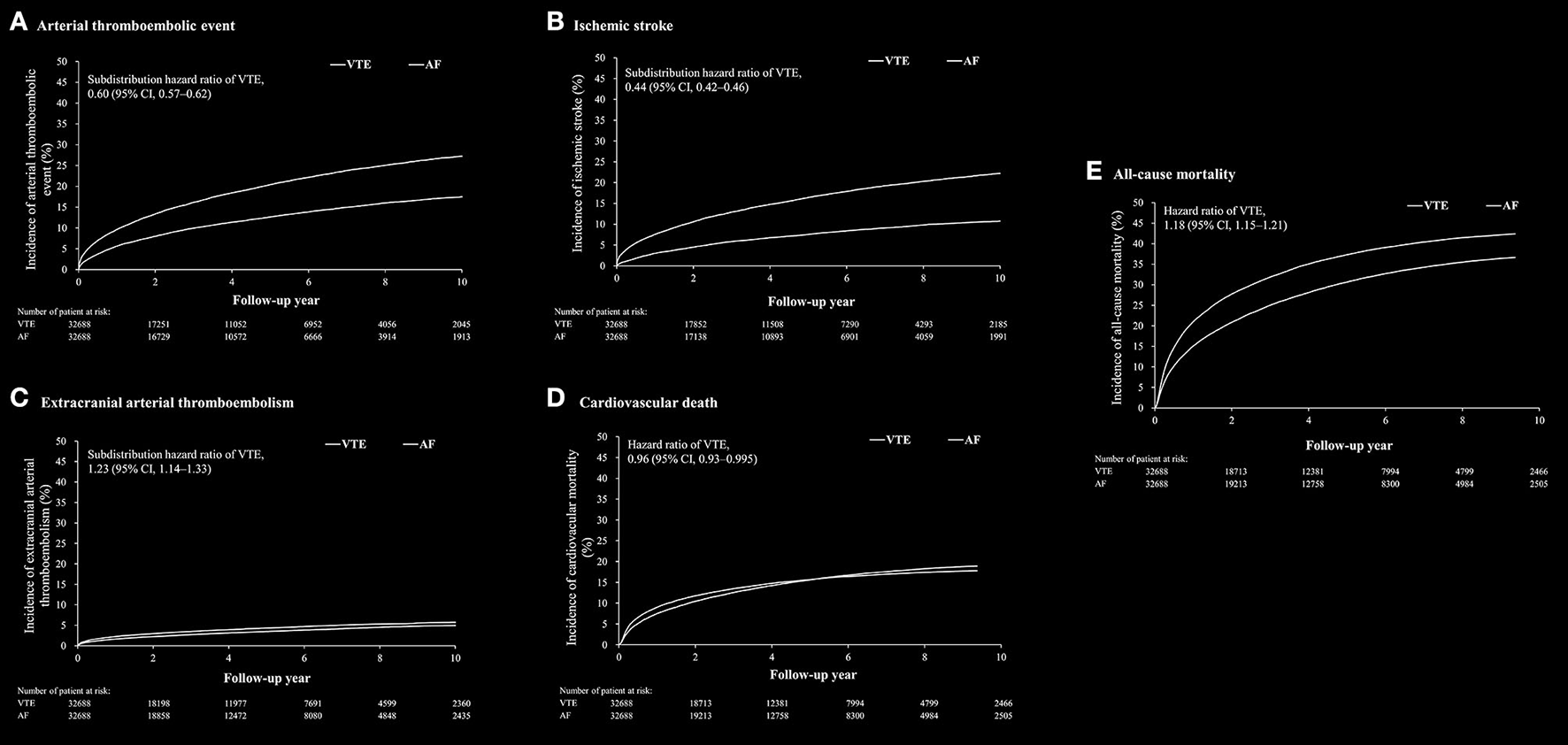
The cumulative incidence rate of arterial thromboembolic event (A), ischemic stroke (B), extracranial arterial thromboembolic events (C), cardiovascular death (D) and all-cause mortality (E) after propensity score matching between patients with venous thromboembolism (VTE) and patients with atrial fibrillation.
In subgroup analysis of the cause of death after propensity matching, the percentage of CV death was significant higher in AF cohort than in VTE cohort (VTE cohort vs. AF cohort: 56.4 vs. 66.6%, P < 0.001) (Supplementary Table 4). The percentages of death related to cancer and infection other than pneumonia among the causes of non-CV death were significantly higher in the VTE cohort than in the AF cohort.
Furthermore, the outcomes between the subgroups stratified according to the use of anticoagulation therapy within 3 months after index date were analyzed. The impact of anticoagulant use on the association between AF/VTE and the risk of arterial thromboembolic events was very significant (non-anticoagulant user: SHR, 0.89; 95% CI, 0.83–0.96; anticoagulant user: SHR, 0.49; 95% CI, 0.47–0.52; P interaction < 0.001). In terms of individual arterial thromboembolic events and mortality, anticoagulant use also had a significant impact on the association between AF/VTE and these events, except for MI (Table 3).
Table 3
| Outcome/ Subgroup |
VTE
(n = 32,688) |
AF
(n = 32,688) |
HR or SHR of VTE (95% CI) | P for interaction |
|---|---|---|---|---|
| Arterial thromboembolic events | <0.001 | |||
| Non-anticoagulant user | 1,422 (11.2) | 1,583 (12.8) | 0.89 (0.83–0.96) | |
| Anticoagulant user | 2,721 (13.6) | 4,800 (23.6) | 0.49 (0.47–0.52) | |
| Ischemic stroke | <0.001 | |||
| Non-anticoagulant user | 822 (6.5) | 1,141 (9.2) | 0.82 (0.75–0.89) | |
| Anticoagulant user | 1,659 (8.3) | 4,000 (19.7) | 0.37 (0.35–0.39) | |
| Extracranial arterial thromboembolism | <0.001 | |||
| Non-anticoagulant user | 497 (3.9) | 328 (2.7) | 1.53 (1.33–1.75) | |
| Anticoagulant user | 913 (4.6) | 794 (3.9) | 1.10 (1.001–1.21) | |
| Lower extremity thromboembolism | <0.001 | |||
| Non-anticoagulant user | 422 (3.3) | 272 (2.2) | 1.56 (1.34–1.82) | |
| Anticoagulant user | 762 (3.8) | 645 (3.2) | 1.13 (1.02–1.26) | |
| Non-lower extremity thromboembolism | 0.016 | |||
| Non-anticoagulant user | 96 (0.8) | 71 (0.6) | 1.35 (0.99–1.84) | |
| Anticoagulant user | 192 (1.0) | 213 (1.0) | 0.86 (0.71–1.05) | |
| Myocardial infarction | 0.349 | |||
| Non-anticoagulant user | 231 (1.8) | 269 (2.2) | 0.86 (0.72–1.02) | |
| Anticoagulant user | 388 (1.9) | 472 (2.3) | 0.77 (0.68–0.88) | |
| All-cause mortality | <0.001 | |||
| Non-anticoagulant user | 7,519 (59.4) | 6,324 (51.1) | 1.37 (1.32–1.42) | |
| Anticoagulant user | 6,669 (33.3) | 6,021 (29.6) | 1.07 (1.04–1.11) | |
| Cardiovascular death | <0.001 | |||
| Non-anticoagulant user | 2,676 (21.1) | 2,619 (21.2) | 1.19 (1.12–1.25) | |
| Anticoagulant user | 2,769 (13.8) | 3,301 (16.3) | 0.81 (0.77–0.85) | |
| Non-cardiovascular death | 0.020 | |||
| Non-anticoagulant user | 4,843 (38.2) | 3,705 (29.9) | 1.50 (1.44–1.57) | |
| Anticoagulant user | 3,900 (19.5) | 2,720 (13.4) | 1.39 (1.33–1.46) |
Subgroup analysis of long-term outcomes of the VTE versus AF patients stratified by use of anticoagulation therapy within 3 months after index date in the propensity score matched cohort.
VTE, venous thromboembolism; AF, atrial fibrillation; HR, hazard ratio; SHR, subdistribution hazard ratio; CI, confidence interval.
Outcomes Between DVT-Only and AF Cohorts
After propensity matching, there was no substantial difference in the baseline characteristics between the DVT-only and AF cohorts (Supplementary Table 1). Clinical outcomes between DVT-only and AF cohorts are shown in Supplementary Table 5.
The incidence of arterial thromboembolic event was lower in the DVT-only cohort than that in the AF cohort (SHR, 0.62; 95% CI, 0.59–0.65) (Supplementary Figure 1A). The risks of ischemic stroke (SHR, 0.45; 95% CI, 0.43–0.48) (Supplementary Figure 1B) and MI (SHR, 0.76; 95% CI, 0.67–0.86) were lower, while the risk of ECATE was higher, in the DVT-only cohort (SHR, 1.31; 95% CI, 1.20–1.43) (Supplementary Figure 1C). All-cause mortality rates were higher in the DVT-only cohort (HR, 1.14; 95% CI, 1.11–1.17), while the rate of CV death was lower (HR, 0.89; 95% CI, 0.85–0.92) (Supplementary Figures 1D,E).
Outcomes Between PE-Only and AF Cohorts
After propensity matching, there was no substantial difference in the baseline characteristics between the PE alone and AF cohorts (Supplementary Table 2). Clinical outcomes between PE-only and AF cohorts were shown in Supplementary Table 6. The incidence of the arterial thromboembolic event was lower in the PE-only cohort than that in AF cohort (SHR, 0.52; 95% CI, 0.48–0.56) (Supplementary Figure 2A). The risk of ischemic stroke was lower in the PE-only cohort (SHR, 0.41; 95% CI, 0.37–0.45) (Supplementary Figure 2B) with no differences in ECATE (Supplementary Figure 2C) and MI event rates (Supplementary Table 6). The PE-only cohort had higher all-cause mortality (HR, 1.26; 95% CI, 1.20–1.32) and CV death (HR, 1.14; 95% CI, 1.07–1.22) than the AF cohort (Supplementary Figures 2D,E).
Outcomes Between PE-Only and DVT-Only Cohorts
After propensity matching, there was no substantial difference in the baseline characteristics between the PE-only and DVT-only cohorts (Supplementary Table 3). Clinical outcomes between PE-only and DVT-only cohorts were shown in Supplementary Table 7. The arterial thromboembolic event was lower in the PE-only cohort than in the DVT-only cohort (SHR, 0.80; 95% CI, 0.73–0.88) (Figure 3A). The risks of ischemic stroke (SHR, 0.82; 95% CI, 0.74–0.92) and ECATE, including lower extremity events, (SHR, 0.69; 95% CI, 0.58–0.82) were lower in the PE-only cohort than in the DVT-only cohort (Figures 3B,C). The risks of all-cause mortality (HR, 1.08; 95% CI, 1.03–1.13) and CV death (HR, 1.28; 95% CI, 1.19–1.37) were higher in the PE-only cohort compared to the DVT-only cohort (Figures 3D,E).
Figure 3
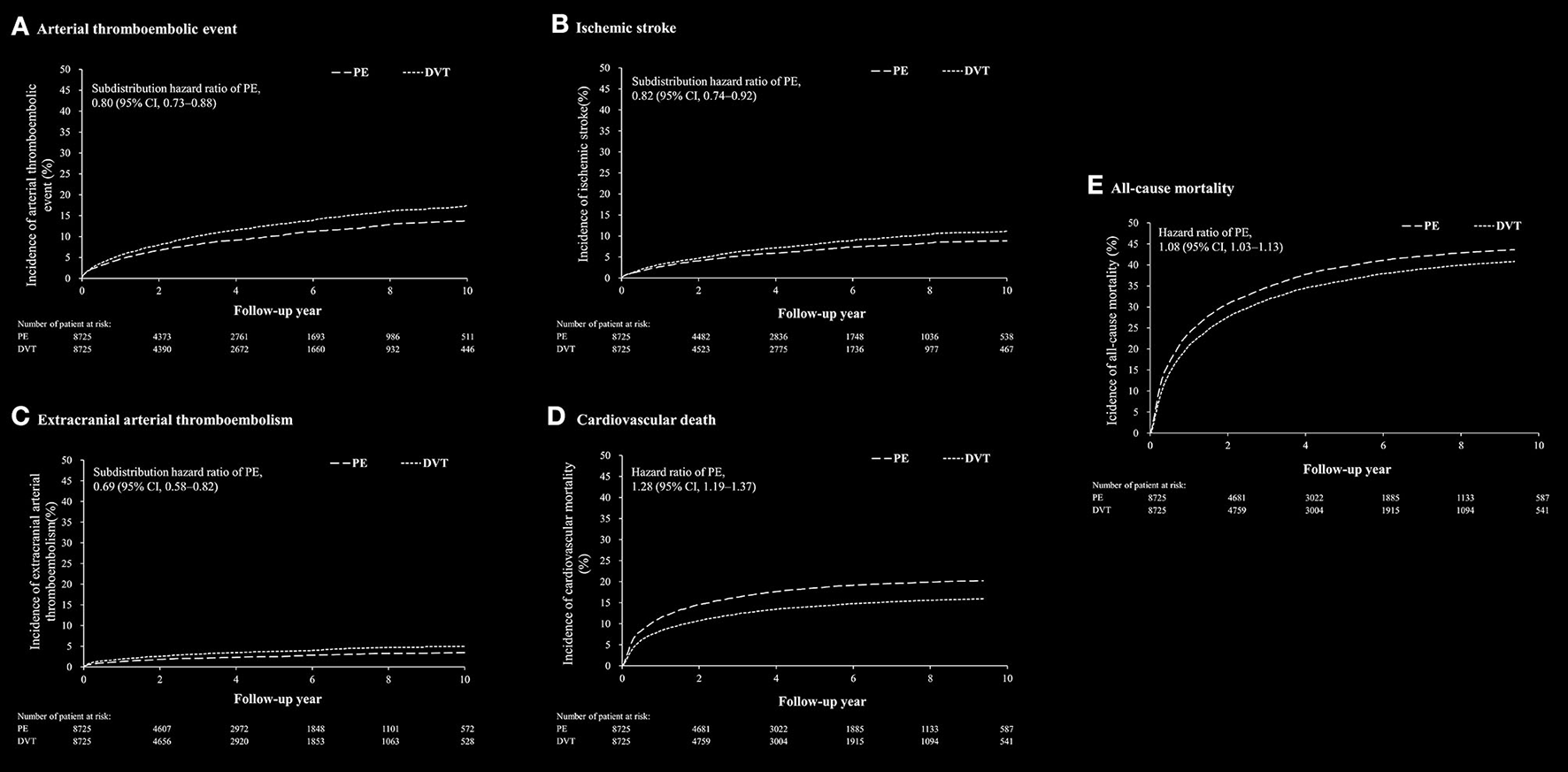
The cumulative incidence rate of arterial thromboembolic event (A), ischemic stroke (B), extracranial arterial thromboembolic events (C), cardiovascular death (D) and all-cause mortality (E) after propensity score matching between patients with pulmonary embolism (PE) alone and patients with deep vein thrombosis (DVT) alone.
Discussion
This retrospective 10-year nationwide cohort study enrolled two national cohorts shows that the arterial thromboembolic events, ischemic stroke and MI, were higher in matched patients with AF cohort than those with VTE cohort. Second, the VTE cohort had higher incidence of ECATE than AF cohort, particularly lower extremity thromboembolism. Third, the AF cohort had higher incidence of CV death, but lower incidence of all-cause mortality compared to the VTE cohort (Figure 4; Supplementary Table 8). In subgroup analyses comparing the DVT-only, PE-only and AF cohorts, the AF patients had highest incidence of ischemic stroke among the three cohorts and had similar incidence of MI compared to patients with PE-only. Patients with DVT-only had highest incidence of ECATE among the three cohorts, particularly lower extremity thromboembolic event. In terms of mortality, patients with PE-only had highest incidence of CV death and all-cause mortality (Supplementary Table 8).
Figure 4
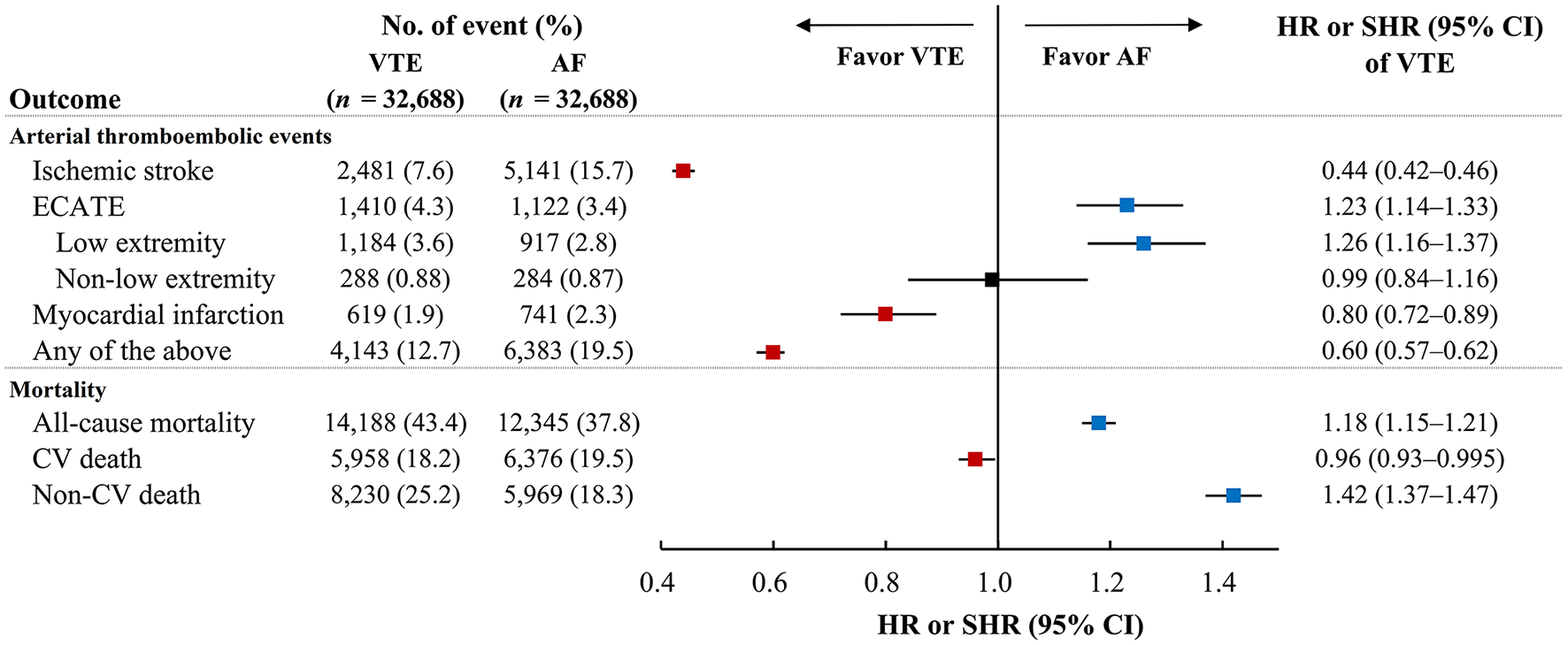
The incidence of arterial thromboembolic events and mortality between VTE and AF cohorts. The incidence of ischemic stroke and myocardial infarction were lower in VTE cohort than AF cohort but ECATE, particularly in low extremity, was lower in AF cohort than VTE cohort. In terms of mortality, CV death was lower in VTE cohorts than AF cohort while all-cause mortality and non-CV death were lower in AF cohort than VTE cohort. AF, atrial fibrillation; CV, cardiovascular; ECATE, extracranial arterial thromboembolic event; VTE, venous thromboembolism.
A national 20-year observational study demonstrated that patients with VTE had a 1.26-1.31-fold increased risk of subsequent arterial thromboembolic events, including MI and stroke (23). Schulman et al. also showed that VTE was associated with a 1.28-fold increased risk of MI or stroke over a 10-year follow-up period (24). Epidemiological studies and meta-analysis have also recognized that AF is independently associated with a five-fold increased risk of stroke (1), 1.47-fold increased risk of MI (25), and a two-fold increased risk of mortality (26).
Although VTE and AF contribute to similar arterial thromboembolic events, we are unaware of any study that has compared the different presentations of arterial thromboembolic events between VTE and AF patients, from the same population cohort. Based on our study, AF contributed to more arterial thromboembolic events while VTE contributed to greater all-cause mortality. In terms of the causes of mortality, more AF patients (66.6%) died from cardiovascular death than VTE patients (56.4%) while more VTE patients (13.7%) died from cancer than AF patients (8.6%) (Supplementary Table 4). Our results were generally consistent with other studies in terms of causes of death in VTE and AF patients (27).
As mentioned above there are many parallels between the epidemiology of risk factors and associated pathogenesis of thrombosis in AF and VTE (4, 6, 8, 10, 28). Nonetheless, our study showed some differential distributions of baseline characteristics between VTE and AF cohorts. Of note, the prevalence of peripheral artery disease and cancer was higher in VTE cohort than AF cohort. In contrast, the prevalence of ischemic heart disease and heart failure was higher in AF cohort than VTE cohort. Importantly, even after propensity matching, AF cohort had higher risks of arterial thromboembolic event, ischemic stroke and MI compared to the VTE cohort. VTE cohort had higher all-cause mortality while AF cohort had higher CV mortality. Therefore, VTE and AF patients have different risks in presentations related to arterial thromboembolic events.
Long-term anticoagulation therapy to prevent arterial thromboembolism is a well-established strategy in AF population (2) and a net clinical benefit more than 5 years with DOACs is still evident (14). On the other hand, long-term anticoagulation was not recommended in VTE population due to uncertain net clinical benefit in the era of vitamin K antagonists (VKA, e.g., warfarin) (15). However, some studies have shown that extended treatment with DOAC for 6-15 months resulted in less recurrent VTE events than no treatment, and had less bleeding events compared to VKA (29–32). Of note, extended low-dose aspirin in VTE patients for up to 4 years results in a significant reduction in the rate of major vascular events, with improved net clinical benefit in the ASPIRE study (33). Moreover, our study showed that anticoagulant use had a significant impact on the association between AF/VTE and individual arterial thromboembolic events and all-cause and cardiovascular mortality (Table 3). Lifelong anticoagulation is indicated in AF patients with high CHA2DS2-VASc score (≥2). The differential manifestations of thromboembolism sequelae and mortality between AF and VTE cohorts merit further investigation of an extended period or lifelong anticoagulation in VTE patients and validation in other ethnic population.
Our study has several limitations. First, we could not clearly identify the prevalence of provoked and unprovoked VTE in our VTE cohort. Several observational studies have reported that unprovoked VTE does not contribute to the same risk as provoked VTE in terms of clinical outcomes, including arterial thromboembolic events. However, a 20-year national observational cohort study reported no significant differences in arterial CV events between provoked and unprovoked VTE (23). In addition, the distinction between provoked/unprovoked PE is no longer supported by the 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism (34). Furthermore, clinical presentations/manifestations and laboratory data were not available in NHIRD and such information might affect the outcomes of VTE, especially those with PE (34). In order to reduce the bias, we excluded those died during hospitalization and within 3 months after discharge. Second, differentiating subtypes of AF (paroxysmal, sustained) cannot be performed because this information was not available in our national database. Although the incidence of ischemic stroke is considered to be generally lower in paroxysmal AF patients than in patients with sustained AF (35), it should not affect the outcomes between AF and VTE in such a large volume study. Third, the duration of anticoagulation therapy was different between AF vs. VTE cohorts (2, 34). We also did not compare the outcomes between AF and VTE cohorts in individual different scenarios with different durations of anticoagulation therapy. Furthermore, although there were different frequencies of anticoagulants and antiplatelets between AF and VTE cohorts before propensity matching (Table 1), our conclusion was based on the results derived from the study population after propensity matching (Tables 1, 2). Therefore, the unbalanced prescription of anticoagulants and antiplatelets between AF and VTE cohorts before propensity matching should not influence our main results derived from the study population after propensity matching. Fourth, VTE cohort had a higher incidence of lower extremity thromboembolic events than AF cohort. We could not completely exclude the possibility of more image studies performed in the VTE cohort to reveal a higher incidence rate of lower extremity thromboembolic events. Finally, propensity score matching was used to reduce the potential confounding variables in this study. However, there were potential unknown variables in the study population for matching and comparison.
Conclusion
Patients with AF had higher risks of arterial thromboembolic events (ischemic stroke and MI) compared to patients with VTE, despite having risk factors in common. The VTE cohort had higher risks of all-cause mortality and ECATE, particularly lower extremity events, compared to AF patients. The differential manifestations of thromboembolism sequelae and mortality between AF and VTE patients merit further investigation of an extended period or lifelong anticoagulation in VTE patients.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The data underlying this study is from the Taiwan's National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html). Requests to access these datasets should be directed to (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Ethics statement
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital: IRB number: 201900915B1. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-SL, M-SL, GL, and M-CC: study concept and design. VW, Y-LC, and J-JC: acquisition of data. Y-SL, M-SL, and P-HC: analysis and interpretation of data. Y-SL, GL, and M-CC: manuscript draft. GL and M-CC: critical revision of the manuscript for important intellectual content. All authors reviewed the manuscript and completed final approval.
Acknowledgments
We would like to thank Alfred Hsing-Fen Lin and Ben Yu-Lin Chou for the statistical assistance during the completion of this manuscript.
Conflict of interest
GL is a Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo. Speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.775564/full#supplementary-material
References
1.
Wolf PA Abbott RD Kannel BW . Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. (1991) 22:983-8. 10.1161/01.STR.22.8.983
2.
January CT Wann LS Calkins H Chen LY Cigarroa JE Cleveland JC Jr. et al . 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2019) 74:104-32. 10.1016/j.jacc.2019.01.011
3.
Witt DM Nieuwlaat R Clark NP Ansell J Holbrook A Skov J et al . American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. (2018) 2:3257-91. 10.1182/bloodadvances.2018024893
4.
Khan AA Lip GYH . The prothrombotic state in atrial fibrillation: pathophysiological and management implications. Cardiovasc Res. (2019) 115:31-45. 10.1093/cvr/cvy272
5.
Esmon CT . Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. (2009) 23:225-9. 10.1016/j.blre.2009.07.002
6.
Riva N Donadini MP Ageno W . Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. (2015) 113:1176-83. 10.1160/TH14-06-0563
7.
Franchini M Mannucci MP . Venous and arterial thrombosis: different sides of the same coin?Eur J Intern Med. (2008) 19:476-81. 10.1016/j.ejim.2007.10.019
8.
Ageno W Becattini C Brighton T Selby R Kamphuisen WP . Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. (2008) 117:93-102. 10.1161/CIRCULATIONAHA.107.709204
9.
Gregson J Kaptoge S Bolton T Pennells L Willeit P Burgess S et al . Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. (2019) 4:163-73. 10.1001/jamacardio.2018.4537
10.
Anumonwo JM Kalifa J . Risk factors and genetics of atrial fibrillation. Heart Fail Clin. (2016) 12:157-66. 10.1016/j.hfc.2015.08.013
11.
Chang SL Huang YL Lee MC Hu S Hsiao YC Chang SW et al . Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. (2018) 319:807-17. 10.1001/jama.2018.0246
12.
Farmakis D Parissis J Filippatos G . Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. (2014) 63:945-53. 10.1016/j.jacc.2013.11.026
13.
Lutsey PL Norby FL Alonso A Cushman M Chen LY Michos ED et al . Atrial fibrillation and venous thromboembolism: evidence of bidirectionality in the Atherosclerosis Risk in Communities Study. J Thromb Haemost. (2018) 16:670-9. 10.1111/jth.13974
14.
Connolly SJ Wallentin L Ezekowitz MD Eikelboom J Oldgren J Reilly PA et al . The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) Study. Circulation. (2013) 128:237-43. 10.1161/CIRCULATIONAHA.112.001139
15.
Robertson L Yeoh SE Ramli A . Secondary prevention of recurrent venous thromboembolism after initial oral anticoagulation therapy in patients with unprovoked venous thromboembolism. Cochrane Database Syst Rev. (2017) 12:Cd011088. 10.1002/14651858.CD011088.pub2
16.
Lin LY Warren-Gash C Smeeth L Chen CP . Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. (2018) 40:e2018062. 10.4178/epih.e2018062
17.
Wu CY Chen YJ Ho HJ Hsu YC Kuo KN Wu MS et al . Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. (2012) 308:1906-14. 10.1001/2012.jama.11975
18.
Chang CH Lee YC Tsai CT Chang SN Chung YH Lin MS et al . Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. (2014) 232:224-30. 10.1016/j.atherosclerosis.2013.11.036
19.
Wang CC Lin CL Wang GJ Chang CT Sung FC Kao HC . Atrial fibrillation associated with increased risk of venous thromboembolism. A population-based cohort study. Thromb Haemost. (2015) 113:185-92. 10.1160/TH14-05-0405
20.
Hsieh CY Chen CH Li CY Lai LM . Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. (2015) 114:254-9. 10.1016/j.jfma.2013.09.009
21.
Cheng CL Lee CH Chen PS Li YH Lin SJ Yang HY . Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. (2014) 24:500-7. 10.2188/jea.JE20140076
22.
Austin PC Fine PJ . Propensity-score matching with competing risks in survival analysis. Stat Med. (2019) 38:751-77. 10.1002/sim.8008
23.
Sorensen HT Horvath-Puho E Pedersen L Baron JA Prandoni P . Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. (2007) 370:1773-9. 10.1016/S0140-6736(07)61745-0
24.
Schulman S Lindmarker P Holmstrom M Larfars G Carlsson A Nicol P et al . Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. (2006) 4:734-42. 10.1111/j.1538-7836.2006.01795.x
25.
Guo XY Li N Du X Bai R Yu RH Long DY Tang RB et al . Atrial fibrillation is associated with an increased risk of myocardial infarction: insights from a meta-analysis. Atherosclerosis. (2016) 254:1-7. 10.1016/j.atherosclerosis.2016.09.011
26.
Benjamin EJ Wolf PA D'Agostino RB Silbershatz H Kannel WB Levy D . Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. (1998) 98:946-52. 10.1161/01.CIR.98.10.946
27.
Novo G Lee E Choi E-K Han K-D Lee H Choe W-S et al . Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS ONE. (2018) 13:e0209687. 10.1371/journal.pone.0209687
28.
Heit JA . Epidemiology of venous thromboembolism. Nat Rev Cardiol. (2015) 12:464-74. 10.1038/nrcardio.2015.83
29.
Schulman S Kearon C Kakkar AK Schellong S Eriksson H Baanstra D et al . Goldhaber: Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. (2013) 368:709-18. 10.1056/NEJMoa1113697
30.
Agnelli G Buller HR Cohen A Curto M Gallus AS Johnson M et al . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. (2013) 368:699-708. 10.1056/NEJMoa1207541
31.
Weitz JI Lensing AWA Prins MH Bauersachs R Beyer-Westendorf J Bounameaux H et al . Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. (2017) 376:1211-22. 10.1056/NEJMoa1700518
32.
Raskob G Ageno W Cohen AT Brekelmans MP Grosso MA Segers A et al . Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol. (2016) 3:e228-36. 10.1016/S2352-3026(16)00023-5
33.
Brighton TA Eikelboom JW Mann K Mister R Gallus A Ockelford P et al . Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. (2012) 367:1979-87. 10.1056/NEJMoa1210384
34.
Konstantinides SV Meyer G Becattini C Bueno H Geersing GJ Harjola VP et al . 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. (2019) 54:1901647. 10.1183/13993003.01647-2019
35.
Hohnloser SH Pajitnev D Pogue J Healey JS Pfeffer MA Yusuf S et al . Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. (2007) 50:2156-61. 10.1016/j.jacc.2007.07.076
Summary
Keywords
atrial fibrillation, arterial thromboembolic event (ATE), venous thromboembolism (VTE), mortality, stroke, myocardial infarction (MI)
Citation
Lin Y-S, Lin M-S, Wu VC-C, Chen Y-L, Chang J-J, Chu P-H, Lip GYH and Chen M-C (2021) Differential Presentations of Arterial Thromboembolic Events Between Venous Thromboembolism and Atrial Fibrillation Patients. Front. Cardiovasc. Med. 8:775564. doi: 10.3389/fcvm.2021.775564
Received
14 September 2021
Accepted
15 November 2021
Published
06 December 2021
Volume
8 - 2021
Edited by
Roberto Pola, Università Cattolica del Sacro Cuore, Italy
Reviewed by
Marieke J. H. A. Kruip, Erasmus Medical Center, Netherlands; Håkan Wallen, Karolinska Institutet (KI), Sweden
Updates
Copyright
© 2021 Lin, Lin, Wu, Chen, Chang, Chu, Lip and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mien-Cheng Chen chenmien@ms76.hinet.net
†These authors share senior authorship
This article was submitted to Thrombosis, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.