Abstract
Cardiac arrhythmias (CAs) are generally caused by disruption of the cardiac conduction system; interleukin-2 (IL-2) is a key player in the pathological process of CAs. This study aimed to investigate the molecular mechanism underlying the regulation of IL-2 and the sodium channel current of sodium voltage-gated channel beta subunit 3 (SCN3B) by miR-190a-5p in the progression of CAs. ELISA results suggested the concentration of peripheral blood serum IL-2 in patients with atrial fibrillation (AF) to be increased compared to that in normal controls; fluorescence in situ hybridization indicated that the expression of IL-2 in the cardiac tissues of patients with AF to be upregulated and that miR-190a-5p to be downregulated. Luciferase reporter assay, quantitative real-time-PCR, and whole-cell patch-clamp experiments confirmed the downregulation of IL-2 by miR-190a-5p and influence of the latter on the sodium current of SCN3B. Overall, miR-190a-5p suppressed the increase in SCN3B sodium current caused by endogenous IL-2, whereas miR-190a-5p inhibitor significantly reversed this effect. IL-2 was demonstrated to be directly regulated by miR-190a-5p. We, therefore, concluded that the miR-190a-5p/IL-2/SCN3B pathway could be involved in the pathogenesis of CAs and miR-190a-5p might acts as a potential protective factor in pathogenesis of CAs.
Introduction
Interleukin-2 (IL-2), a proinflammatory factor, is predominantly secreted by activated T lymphocytes (1). The vital role of IL-2 is to stimulate the proliferation of T cells and generate effector and memory T cells (2). Serum IL-2 levels are associated with multiple cardiovascular diseases such as coronary artery disease and cardiac arrhythmias (CAs) (3).
Atrial fibrillation (AF) and ventricular tachycardia/ventricular fibrillation (VT/VF) are the two main types of CAs that are highly associated with the level of serum IL-2 (4–6). IL-2 has also been associated with the recurrence of AF in patients undergoing catheter ablation (5). In addition, high-dose IL-2 therapy has been significantly associated with cardiac toxicities in patients with cancer, often resulting in lethal arrhythmias (6–8). However, the underlying mechanism of IL-2 in the pathogenesis of CAs still remains unclear.
Dysfunction of ion channels plays an important role in the etiology of CAs (9). Family-based genetic studies have revealed that both loss-of-function and gain-of-function mutations of genes encoding ion channels can lead to CAs including AF (10) as well as VT/VF (11, 12). IL-2 has been confirmed to regulate the expression of the voltage-gated sodium channel (Nav1.5) complex including sodium voltage-gated channel alpha subunit 3 (SCN3A), sodium voltage-gated channel beta subunit 3 (SCN3B), and sodium voltage-gated channel alpha subunit 4 (SCN4A). In particular, the expression of SCN3B has been reported to be upregulated by IL-2 via the p53 pathway. Overexpression of SCN3B has been found to play a gain-of-function-like effect, increase the fast inward cardiac sodium current-INa, and possibly cause CAs (13). However, the reasons behind the increased serum IL-2 levels and the potential regulatory factor(s) related to the expression of IL-2 in patients with CAs are still unknown.
The expression of miR-190a-5p is altered in response to hypoxia and circulating miR-190a-5p is a possible biomarker of chronic heart failure (14). IL-2 has been observed to be possibly regulated by miR-190-5p, as per TargetScan 7.2 (15). These reports prompted us to evaluate the effect of miR-190a-5p on IL-2 expression in the progress of CAs. This study aimed to: (1) evaluate the expression of peripheral blood serum IL-2 in patients with AF, (2) verify the correlation between IL-2 and miR-190-5p expression in myocardial tissues of patients with AF, (3) clarify the role of miR-190a-5p in regulating the abnormal electrical activity of sodium current density in SCN3B, and (4) reveal the possibility of miR-190a-5p being a protective factor against the progress of CAs through negative regulation of IL-2.
Materials and Methods
Ethics Statement
This study involving human participants was reviewed and approved by the appropriate Local Institutional Review Board on human subject research at Huazhong University of Science and Technology and the First Affiliated Hospital of Xiamen University and also conformed to the guidelines set forth by the Declaration of Helsinki. The patients/participants (legal guardian/next of kin) provided a written informed consent to participate in this study. This study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Sample Collection
Peripheral blood serum samples of 61 clinical subjects, including patients with AF (n = 34) (Table 1) and normal controls (n = 27) with normal ECG, were collected from Tongji Hospital Affiliated to Huazhong University of Science and Technology. Human cardiac tissues from patients with AF (n = 4) and those without AF (non-AF) (n = 4) were collected from the First Affiliated Hospital of Xiamen University. All the patients with AF were diagnosed by visual inspection of the ECG.
Table 1
| ID No. | Review or not | Hospitalized No. | Gender | Related clinical symptoms |
|---|---|---|---|---|
| 160101 | No | 1254639 | Male | Permanent AF, New York Heart Function Classification (NYHA) class III, chronic obstructive emphysema with acute exacerbation, coronary atherosclerotic heart disease, arrhythmia. |
| 160112 | No | 1254948 | Male | AF, wide QRS complex tachycardia (ventricular tachycardia), acute coronary syndrome, hypertension grade 3, type 2 diabetes, cerebral infarction, liver insufficiency, renal insufficiency. |
| 160118 | No | 1254970 | Male | AF, coronary atherosclerotic heart disease, NYHA class III. |
| 160122 | No | 1254513 | Female | AF, rheumatic heart disease, rheumatic mitral stenosis with insufficiency, aortic regurgitation, NYHA class III, type 2 diabetes, coronary atherosclerosis, hypertension grade 3 (extremely high risk), anemia. |
| 160125 | No | 1254710 | Female | AF, coronary heart disease, acute coronary syndrome, NYHA class II, hypertension grade 3(extremely high risk), herpes. |
| 160128 | No | 1255356 | Female | AF, coronary heart disease, angina pectoris, arrhythmia, post-percutaneous coronary intervention (PCI), NYHA class III, hypertension level 3. |
| 160130 | No | 1255576 | Female | AF, coronary heart disease, hypertension level 2. |
| 160155 | No | 1255273 | Male | AF, coronary heart disease, arrhythmia, NYHA class II, hypertension, digestive system disease. |
| 160160 | No | 1256003 | Male | AF, heart valve disease, moderate mitral regurgitation, coronary heart disease, arrhythmia, NYHA class III, connective tissue disease, lung infection. |
| 160170 | No | 1255921 | Male | AF, arrhythmia, paroxysmal supraventricular tachycardia. |
| 160200 | No | 1255236 | Female | Persistent AF, hypertension grade 3, arrhythmia, post implantation of permanent cardiac pacemaker. |
| 160208 | Yes (160168) | 1256341 | Male | AF, hypertension grade 3, hypertensive heart disease, coronary atherosclerotic heart disease, prior myocardial infarction, NYHA class II, arrhythmia, chronic bronchitis. |
| 160226 | No | 1257212 | Female | AF, coronary heart disease, valvular heart disease, moderate tricuspid regurgitation, NYHA class III, hypertension grade 3, type 2 diabetes, renal artery stenosis. |
| 160232 | No | 1256854 | Male | AF, arrhythmia, coronary heart disease, schistosomiasis liver disease, splenectomy, hepatitis B. |
| 160234 | No | 1257535 | Male | AF, arrhythmia, hypertension grade 3, type 2 diabetes, hyperuricemia, hyperlipidemia. |
| 160262 | No | 1258107 | Male | AF, paroxysmal supraventricular tachycardia, NYHA class II. |
| 160269 | No | 1257444 | Male | AF, arrhythmia. |
| 160271 | No | 1258363 | Male | AF with long intervals, coronary atherosclerotic heart disease, NYHA class III, emphysema, lung infection, hypertension grade 3 (very high risk). |
| 160281 | No | 1258826 | Male | Paroxysmal AF, hypertension. |
| 160285 | No | 1258637 | Male | AF, arrhythmia, ventricular premature beats, bronchial asthma. |
| 160292 | No | 1258209 | Male | AF, cerebral infarction, coronary heart disease, pacemaker implantation, type 2 diabetes. |
| 160297 | No | 1257856 | Female | AF, coronary heart disease, hypertension grade 3 (very high-risk group), type 2 diabetes, peripheral neuropathy. |
| 160309 | No | 1258911 | Female | AF, arrhythmia, paroxysmal supraventricular tachycardia, frequent premature ventricular, hypertension grade 3. |
| 160449 | Yes (160271) | 1264487 | Male | AF, coronary atherosclerotic heart disease, NYHA class III, chronic obstructive pulmonary disease, hypertension grade 3 (extremely high risk). |
| 160015P1 | No | 1253229 | Male | AF, coronary heart disease, arrhythmia, lung infection. |
| 160024P1 | No | 1252735 | Female | AF, coronary heart disease, post- PCI, arrhythmia, NYHA class III, radiofrequency ablation of atrial flutter. |
| 160027P1 | No | 1253389 | Female | AF, hypertension grade 3, hypertensive heart disease, NYHA class III, coronary atherosclerotic heart disease, chronic renal insufficiency, sequelae of cerebral infarction. |
| 160035P1 | No | 1253856 | Male | Paroxysmal AF, arrhythmia, hypertension grade 3. |
| 160043P1 | No | 1253789 | Male | AF, arrhythmia, paroxysmal atrial flutter, post cardiac radiofrequency ablation, hypertension grade 3 (extremely high-risk). |
| 160049P1 | No | 1253907 | Male | AF, coronary atherosclerotic heart disease, unstable angina pectoris, NYHA class II, hypertension grade 3, sick sinus syndrome, after permanent cardiac single-chamber pacemaker implantation, type 2 diabetes. |
| 160050P1 | No | 1253643 | Female | AF, coronary heart disease, arrhythmia, NYHA class II. |
| 160056P1 | No | 1254269 | Male | AF, hypertension grade 3, coronary heart disease, prior myocardial infarction (2013), ventricular aneurysm, post-PCI, significant sinus bradycardia, permanent cardiac pacemaker implantation. |
| 160081P1 | No | 1254498 | Female | AF, coronary atherosclerotic heart disease, prior myocardial infarction, post-PCI, NYHA class IV, hypertension grade 3, senile dementia. |
| 160088P1 | No | 1254245 | Female | Paroxysmal AF, arrhythmia, post radiofrequency ablation, hypertension, coronary atherosclerosis. |
| 160092P1 | No | 1254441 | Female | Paroxysmal AF, hypertension grade 3, type 2 diabetes, chronic renal insufficiency, hypothyroidism, ankylosing spondylitis, lung infection. |
Clinical information of patients with AF.
Bioinformatics-Based Prediction
TargetScan 7.2 (http://www.targetscan.org/vert72/) was used to predict the potential microRNAs [miRNA(s)] directly regulating the downstream target gene IL-2 and the specific binding site(s) of miRNA(s) (or seed sequences) in the 3'-untranslated region (UTR) of IL-2 (Table 2, marked in blue).
Table 2
| Positions in 3'-UTR and miRNAs | Predicted consequential pairing of target region (top) and miRNA (bottom) | Site type | Context++ score | Context++ score percentile | Weighted context++ score | Conserved branch length | PCT |
|---|---|---|---|---|---|---|---|
| 74–81 miR-181a-5p |
5'...UUUUAUAUUUAUUA... 3' UGAGUGGCUGUCGA |
8mer | −0.46 | 99 | −0.46 | 1.034 | <0.1 |
| 215–221 miR-181a-5p |
5'...UAUUUAUUAUUAUU... 3' UGAGUGGCUGUCGA |
7mer-m8 | −0.25 | 95 | −0.25 | 0.284 | <0.1 |
| 74–81 miR-181b-5p |
5'...UUUUAUAUUUAUUGUA... 3' UGGGUGGCUGUCGUUA |
8mer | −0.44 | 98 | −0.44 | 1.034 | <0.1 |
| 215–221 miR-181b-5p |
5'...UAUUUAUUAUUAUGUU.. . 3' UGGGUGGCUGUCGUUA |
7mer-m8 | −0.23 | 94 | −0.23 | 0.284 | <0.1 |
| 74–81 miR-181c-5p |
5'...UUUUAUAUUUAUUA... 3' UGAGUGGCUGUA |
8mer | −0.46 | 99 | −0.46 | 1.034 | <0.1 |
| 215–221 miR-181c-5p |
5'...UAUUUAUUAUUAUU... 3' UGAGUGGCUGUCA |
7mer-m8 | −0.25 | 95 | −0.25 | 0.284 | <0.1 |
| 74–81 miR-181d-5p |
5'...UUUUAUAUUUAUUGUA... 3' UGGGUGGCUGUUGUUA |
8mer | −0.44 | 98 | −0.44 | 1.034 | <0.1 |
| 215–221 miR-181d-5p |
5'...UAUUUAUUAUUAUGUU.. . 3' UGGGUGGCUGUUGUUA |
7mer-m8 | −0.23 | 94 | −0.23 | 0.284 | <0.1 |
| 24–31 miR-190a-5p |
5'...UGCUUCCCACUUAAAA... 3' UGGAUUAUAUAGUUU |
8mer | −0.68 | 99 | −0.68 | 0.100 | <0.1 |
| 24–31 miR-190b |
5'...UGCUUCCCACUUAAAA... 3' UUGGGUUAUAGUUU |
8mer | −0.69 | 99 | −0.69 | 0.100 | <0.1 |
Binding sites (blue) of miR-181 and miR-191 in the 3'-untranslated region (UTR) of interleukin-2 (IL-2).
Cell Lines and miRNAs
AC16 cell line is a type of human myocardial cells that is commonly used to study the cardiovascular diseases in vitro. In this study, human Raji cell line was used to verify the results acquired from AC16 cells. AC16 and Raji cell lines were purchased from American Type Culture Collection (ATCC, Rockville, Maryland, USA). HEK293 and HEK293 cells stably overexpressing SCN5A (HEK293/Nav1.5) were available in our own laboratory (16). AC16, HEK293, and HEK293/Nav1.5 cells were cultured in Dulbecco's Modified Eagle's Medium (Gibco Life Technologies, Grand Island, Nebraska, USA) and Raji cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium at 37°C with 5% carbon dioxide (CO2).
All the miRNA mimics, inhibitors, and negative controls were purchased from RiboBio Corporation Limited (Guangzhou, China).
Double Luciferase Reporter Assay
The PCR product (282 bp) of the 3'-UTR of IL-2, containing the predicted binding sites of miR-181 (miR-181a-5p, miR-181b-5p, miR-181c-5p, and miR-181d-5p) and miR-190 (miR-190a-5p and miR-190b-5p) families, was subcloned into the empty vector of pMIR-REPORT (Applied Biosystems, Foster City, California, USA), named IL-2-pMIR-REPORT-WT, to screen for the effective miRNA(s) targeting IL-2. Subsequently, the binding site was mutated (IL-2-pMIR-REPORT-MU) by site-directed mutagenesis to further confirm the binding site of miR-190a-5p.
To screen for miRNA(s) that targeted IL-2, HEK293 cells, cultured at 37°C with 5% CO2 for 24 h, were co-transfected with: (1) pMIR-REPORT + miR-NC, (2) IL-2-pMIR-REPORT-WT + miR-NC, (3) pMIR-REPORT + miRNA libraries (miR-181 or miR-190), and (4) IL-2-pMIR-REPORT-WT + miRNA libraries (miR-181 or miR-190).
To further confirm the relationship between miR-190a-5p and IL-2, HEK293 cells, at 37°C with 5% CO2 for 24 h, were co-transfected with: (1) pMIR-REPORT + inhibitor-NC, (2) IL-2-pMIR-REPORT-WT + inhibitor-NC, (3) pMIR-REPORT + miR-190a-5p inhibitor, (4) IL-2-pMIR-REPORT-WT + miR-190a-5p inhibitor, (5) IL-2-pMIR-REPORT-MU + miR-NC, and (6) IL-2-pMIR-REPORT-MU + miR-190a-5p. After 48 h, luciferase signal intensity was measured using the Dual-Luciferase Reporter Assay System (PR-E1910, Promega, Madison, Wisconsin, USA) on GloMax 20/20 (Promega, Madison, Wisconsin, USA). Relative luciferase intensity was normalized to Renilla luciferase activity.
Quantitative Real-Time-PCR (qRT-PCR)
AC16 cells and Raji cells, cultured at 37°C with 5% CO2 for 24 h, were transfected with miR-190a-5p mimic/inhibitor or miR-NC/inhibitor-NC (RiboBio Corporation Limited, Guangzhou, China) for 48 h. The cells were then lysed using RNAiso Plus (Takara Biomedical Technology, Dalian, China). Total RNA was extracted and reverse transcribed into complementary DNA (cDNA) using the M-MLV Reverse Transcription Kit (Vazyme, Nanjing, China) in accordance with the instructions of the manufacturer. qRT-PCR was conducted with AceQ qPCR SYBR Green Master Mix (Q141-02/03, Vazyme, Nanjing, China) on the ABI StepOnePlus™ Real-Time PCR System. The primer sequence used was as follows: IL-2: 5′-AGGCCACAGAACTGAAAC-3′ (Forward), 5′-TTACGTTGATATTGCTGATTA-3′ (Reverse); SCN3B: 5′-GCCTTCAATAGATTGTTTCCCCT-3′ (Forward), 5′-CTCGGGCCTGTAGAACCAT-3′ (Reverse); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 5′-GGAGCGAGATCCCTCCAAAAT-3′ (Forward), 5′-GGCT GTTGTCATACTTCTCATGG-3′ (Reverse). For reverse transcription and qRT-PCR of miR-190a-5p, the Bulge-Loop™ qRT-PCR primer sets of miR-190a-5p and U6 were used (RiboBio Corporation Limited, Guangzhou, China).
Enzyme-Linked Immunosorbent Assay
The concentration of IL-2 in peripheral blood serum of clinical samples was detected by ELISA using the Chemiluminescent Immunoassay Kit for IL-2 (SCA073Hu, Houston, Texas, USA) according to the instructions of the manufacturer.
AC16 and Raji cells were plated in 6-well plates at 37°C with 5% CO2 for 24 h and treated with miR-190a-5p mimic/miR-NC or miR-190a-5p inhibitor/inhibitor-NC for 48 h. Thereafter, the culture supernatant was collected and concentration of IL-2 was determined by ELISA.
Whole-Cell Patch-Clamp Experiments
Nav1.5 is a sodium channel subunit that generates sodium current in the heart. Sodium current is commonly analyzed by patch-clamping of HEK293 cells (17). Change in sodium current density in SCN3B was detected by whole-cell patch-clamp technique using HEK293/Nav1.5 cells. After being cultured for 24 h, 2 μg pEGFP-N1/pEGFP-N1-SCN3B (13), miR-190a-5p mimic/miR-NC, or miR-190a-5p inhibitor/inhibitor-NC was co-transfected at 70–80% confluence. After 48 h, green fluorescent protein (GFP)-positive cells were selected for electrophysiological studies according to the standardized experimental procedures (17).
Fluorescence in situ Hybridization
Human cardiac tissues from patients with AF and from non-AF controls (Table 3) were fixed with 4% paraformaldehyde for fluorescence in situ hybridization (FISH). Briefly, paraffin-embedded sections of cardiac tissues were subjected to high-pressure antigen retrieval in citrate buffer (pH 6.0). Sections were blocked in 5% bovine serum albumin (BSA), incubated with IL-2 primary antibodies (NBP2-16948, NOVUS, Colorado, USA), and then incubated with Cy3-conjugated goat antirabbit immunoglobulin G (IgG) (H + L) (BA1032; Boster Biological Technology Corporation Ltd., Wuhan, China). Next, the sections were incubated with miR-190a-5p Dig-labeled antisense probe (5′-ACCUAAUAUAUCAAACAUAUCA-3′) and then treated with Alexa Fluor 488-labeled goat antirabbit IgG (H + L) (A0423, Beyotime, Shanghai, China). Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:2,000). The PANNORAMIC MIDI II (3DHISTECH, Budapest, Hungary, UK) was used to detect images of the slides.
Table 3
| Patient ID | Hospitalized no. | Sex | Age (years) | AF | Other cardiovascular diseases | Tissue source |
|---|---|---|---|---|---|---|
| Patient 1 | 552954 | Female | 53 | Yes | Heart valve disease, heart enlargement | Tendons from valvula bicuspidalis |
| Patient 2 | 554552 | Female | 65 | Yes | Heart valve disease, cardiac insufficiency | Right auricle |
| Patient 3 | 442617 | Female | 59 | Yes | Heart valve disease, rheumatic heart disease | Right auricle |
| Patient 4 | 568001 | Male | 39 | Yes | Heart valve disease, rheumatic heart disease, heart enlargement, cardiac insufficiency | Left auricle |
| Control 1 | 553053 | Male | 0.4 | No | Congenital heart disease, ventricular septal defect | Right auricle |
| Control 2 | 556812 | Male | 4 | No | ventricular septal defect | Right auricle |
| Control 3 | 558147 | Male | 0.5 | No | Congenital heart disease, ventricular septal defect | Right auricle |
| Control 4 | 561361 | Male | 4 | No | Congenital heart disease, ventricular septal defect | Right auricle |
Clinical information of subjects for fluorescence in situ hybridization (FISH).
Data Analysis
Data are presented as the mean ± SEM. The GraphPad Prism version 6 (San Diego, California, USA) was used for statistical analysis. The one-way ANOVA and the Student's two-tailed t-tests were used for multiple-group comparisons and between-group comparisons, respectively. Statistically significant difference was considered at p < 0.05 (*p < 0.05, **p < 0.01).
Results
Serum IL-2 Concentration Increased in Patients With AF
Many inflammatory markers, including IL-2, are associated with the presence or outcome of AF (18). To confirm the relationship between IL-2 and AF, an ELISA was performed to detect the change in IL-2 concentration in the peripheral blood serum of patients with AF. Results demonstrated that the level of IL-2 in the peripheral blood serum of patients with AF increased by 42.38% compared to that in the normal control group (0.9980 ± 0.03558 vs. 1.4210 ± 0.1163 pg ml−1, **p < 0.01, Figure 1A), suggesting that the increase in IL-2 levels in the peripheral blood serum of patients with AF may be related to the inflammatory changes caused by AF.
Figure 1
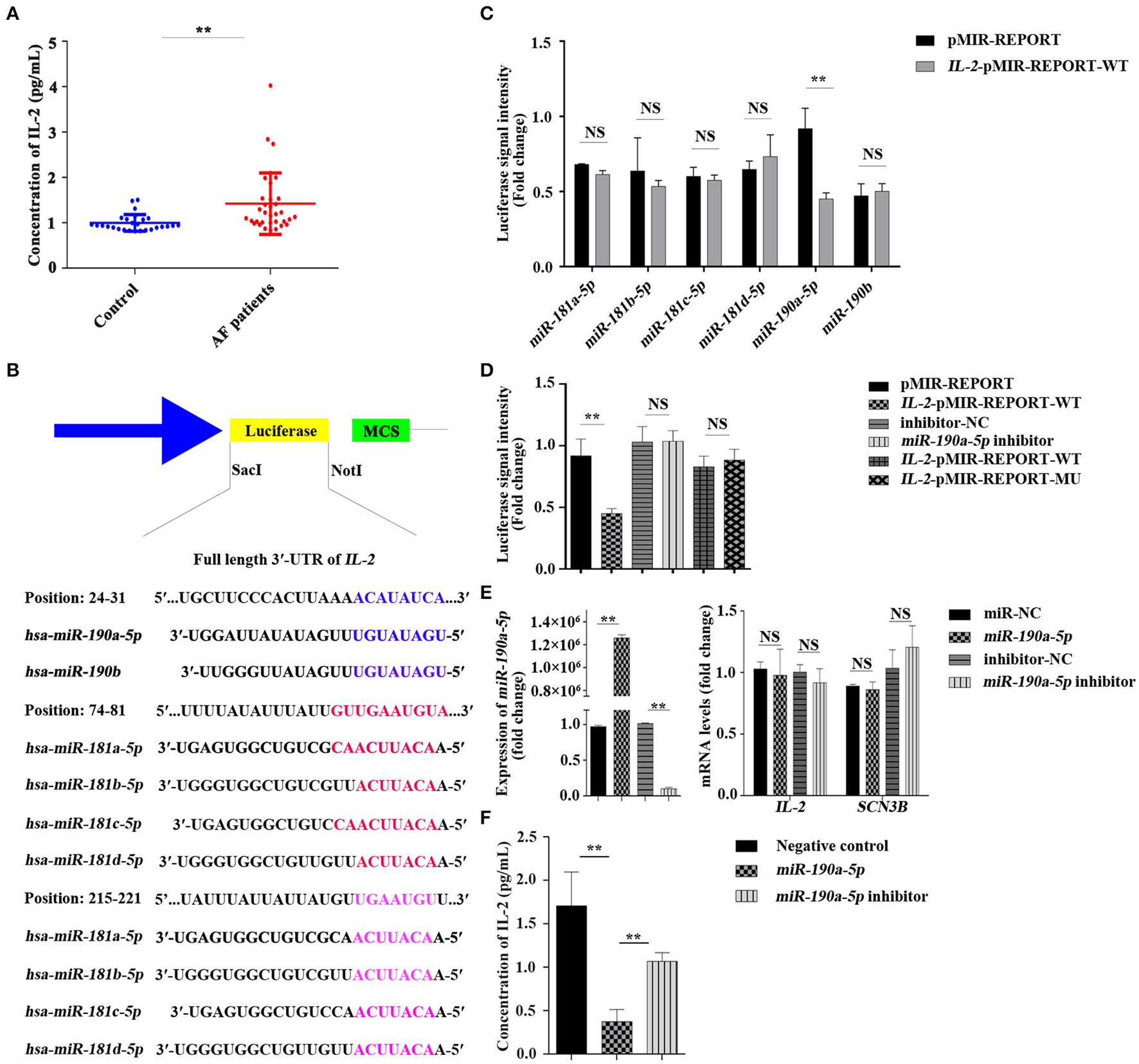
Concentration of interleukin-2 (IL-2) in the peripheral blood serum of patients with atrial fibrillation (AF) was increased and IL-2 was negatively regulated by miR-190a-5p. (A) Level of IL-2 was increased in the peripheral blood serum of patients with AF than that of the normal control group (**p < 0.01 vs. control group). (B) The binding sites of miR-181 and miR-190 families in the 3'-untranslated region (UTR) of IL-2. (C,D) Relative luciferase signal intensity in HEK293 cells (NS, no significant difference, **p < 0.01 vs. pMIR-REPORT). (E) Relative expression of miR-190a-5p, IL-2, and sodium voltage-gated channel beta subunit 3 (SCN3B) in AC16 cells transfected with miR-190a-5p mimic/inhibitor or miR-NC/inhibitor-negative control (NC) (**p < 0.01 vs. miR-NC, NS vs. inhibitor-NC). (F) Concentration of IL-2 in culture medium of AC16 cells treated with miR-190a-5p mimic/inhibitor or Negative control (NC) (**p < 0.01 vs. NC, **p < 0.01 vs. miR-190a-5p). Data are represented as means ± SEM.
Interleukin-2 Was Negatively Regulated by miR-190a-5p
Online bioinformatics-based prediction indicated that miR-180 or miR-190 families may target the downstream IL-2 (Figure 1B) either alone or together. Results of the double luciferase reporter assay confirmed that luciferase signal intensity from the vector carrying the IL-2 wild-type reporter gene (IL-2-pMIR-REPORT-WT) was notably decreased by 50.92% after transfection with miR-190a-5p mimic, though not with other miRNAs, compared to that of pMIR-REPORT (**p < 0.01, Figure 1C), whereas the miR-190a-5p inhibitor passivated this effect (p > 0.5, Figure 1D). The binding site (5′-ACAUAUCA-3′) was mutated (5′-GTGGCGTC-3′) to further verify the targeting of miR-190a-5p to IL-2. Luciferase signal intensity from the vector carrying the IL-2 mutant reporter gene (IL-2-pMIR-REPORT-MU) was not significantly different from that carrying IL-2-pMIR-REPORT-WT (p > 0.5, Figure 1D).
AC16 and Raji cells, seeded in 6-well plates at 37°C with 5% CO2 for 24 h, were transfected with miR-190a-5p mimic/inhibitor or miR-NC/inhibitor-NC for 48 h. The adherent cells were collected to detect the expression of miR-190a-5p, IL-2, and SCN3B by qRT-PCR and the culture medium was retained for detection of IL-2 concentration by ELISA. Results of qRT-PCR revealed that, compared to miR-NC, miR-190a-5p was overexpressed by 1298127.275-fold in AC16 cells (**p < 0.01, Figure 1E) and 422.22-fold in Raji cells (**p < 0.01, Supplementary Figure 1A). The expression of miR-190a-5p was remarkably downregulated by 90.13% when treated with miR-190a-5p inhibitor in AC16 cells than when treated with inhibitor-NC (**p < 0.01, Figure 1E); it did not change significantly after transfection with either inhibitor-NC or miR-190a-5p inhibitor in Raji cells (Supplementary Figure 1A); the differential effects of miR-190a-5p inhibitor may be due to the differences in cell types. The expression of IL-2 did not change when cells (both AC16 and Raji cells) were treated with miR-190a-5p mimics or inhibitors (Figure 1E and Supplementary Figure 1B) and the expression of SCN3B, after transfection with miR-190a-5p mimic or inhibitor, was similar to that of IL-2 in AC16 cells (Figure 1E). In AC16 cells, the concentration of IL-2 in the medium supernatant was decreased by 78.11% when treated with miR-190a-5p (1.707 ± 0.3904 vs. 0.3737 ± 0.1397 pg ml−1) (**p < 0.01, Figure 1F), whereas treatment with miR-190a-5p inhibitor increased the concentration of IL-2 by 65.01% (0.3737 ± 0.1397 vs. 1.068 ± 0.1001 pg ml−1, **p < 0.01, Figure 1F). In Raji cells, the concentration of IL-2 in the medium supernatant decreased by 11.73% when treated with miR-190a-5p (163.7 ± 4.070 vs. 144.5 ± 1.703 pg ml−1, **p < 0.01, Supplementary Figure 1C); however, treatment with miR-190a-5p inhibitor did not affect the secretion of IL-2 (Supplementary Figure 1C). These data collectively indicated that miR-190a-5p may affect the expression of IL-2 by directly targeting the 3′-UTR binding site of IL-2.
miR-190a-5p Reversed the Increased Sodium Current (INa) Caused by Increased Endogenous IL-2
Changes in sodium current were analyzed by whole-cell patch clamping in HEK293/Nav1.5 cells. In order to investigate whether miR-190a-5p affected the sodium current density in SCN3B at the endogenous level, pEGFP-N1/pEGFP-N1-SCN3B, miR-190a-5p mimic/miR-NC, or miR-190a-5p inhibitor/inhibitor-NC was co-transfected in HEK293/Nav1.5 cells. After treatment with miR-190a-5p, the sodium current density (expressed as the normalized peak current relative to the battery capacitance, pA/pF) decreased by 59.98% (**p < 0.01) over the entire test potential range; it increased 2.04-fold in the cells transfected with miR-190a-5p inhibitor (**p < 0.01, Figures 2A,C,E). miR-NC or inhibitor-NC failed to affect the sodium currents (Figures 2B,D,F). The results suggested that miR-190a-5p may downregulate the endogenous expression of IL-2, which induces abnormal sodium channel current of SCN3B, eventually accelerating the pathogenesis of CAs.
Figure 2
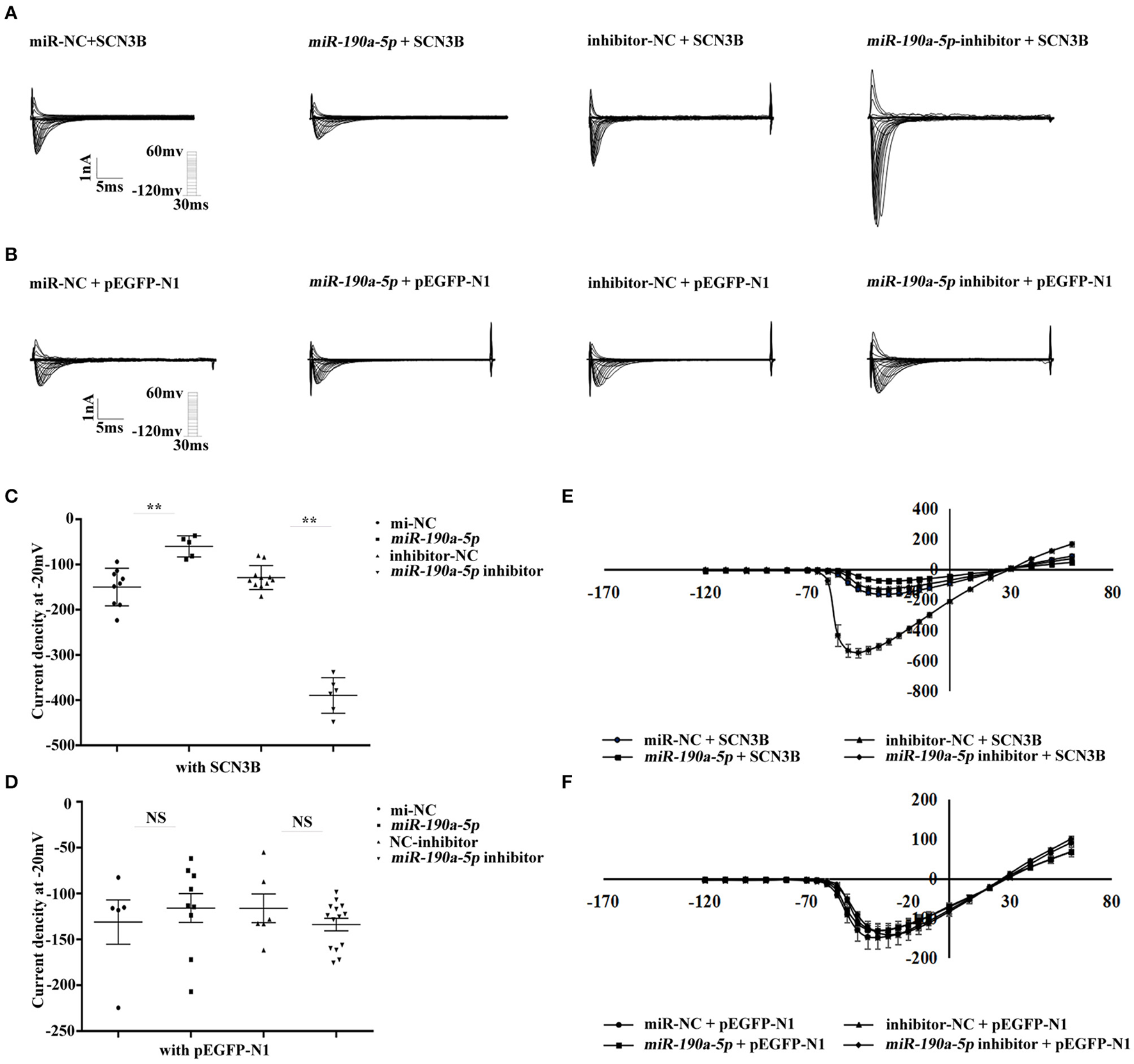
miR-190a-5p reduced the sodium current density of SCN3B. (A,C,E) Representative traces of sodium currents, histogram of sodium current densities at −20 mV, and IV relation for peak sodium current Nav1.5 from HEK293/Nav1.5 cells, respectively, transfected with: (1) miR-NC + SCN3B, (2) miR-190a-5p + SCN3B, (3) inhibitor-NC + SCN3B, and (4) miR-190a-5p inhibitor + SCN3B (**p < 0.01 vs. miR-NC, **p < 0.01 vs. inhibitor-NC). Data are represented as means ± SEM. (B,D,F) Representative traces of sodium currents, histogram of sodium current densities at −20 mV, and IV relation for peak sodium current Nav1.5 from HEK293/Nav1.5 cells, respectively, transfected with: (1) miR-NC + pEGFP-N1, (2) miR-190a-5p + pEGFP-N1, (3) inhibitor-NC + pEGFP-N1, and (4) miR-190a-5p inhibitor + pEGFP-N1 (NS, no significant difference vs. miR-NC, NS vs. inhibitor-NC). Data are represented as means ± SEM.
miR-190a-5p Decreased While IL-2 Increased in Human AF Cardiac Tissues
Interleukin-2 has been verified to be a direct target of miR-190a-5p at the cellular level. However, co-localization of miR-190a-5p and IL-2 needed to be confirmed in human AF cardiac tissues. Results of co-immunofluorescence of miR-190a-5p and IL-2 revealed that miR-190a-5p (green) was downregulated, while IL-2 (red) was upregulated in the AF group compared to that in the non-AF group (Figure 3), suggesting the negative regulatory relationship between miR-190a-5p and IL-2.
Figure 3
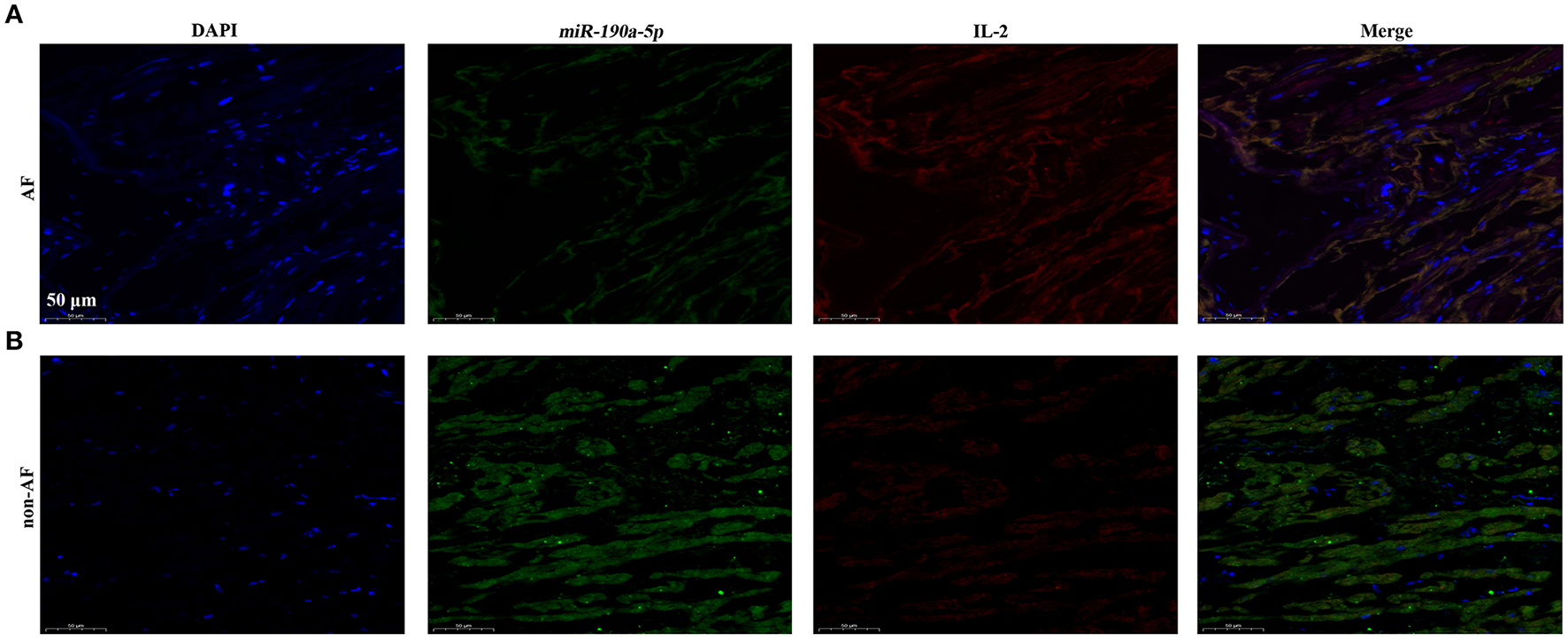
In cardiac tissues of patients with AF, miR-190a-5p was downregulated while IL-2 was upregulated. (A) Immunofluorescence co-staining of miR-190a-5p and IL-2 in human cardiac tissues of patients with AF. (B) Immunofluorescence co-staining of miR-190a-5p and IL-2 in human cardiac tissues from non-AF control. Scale bar = 50 μm, n = 4 per group.
Discussion
In this study, the level of peripheral blood serum IL-2 was found to be increased by 42.38% in patients with AF compared to that in normal controls (**p < 0.01, Figure 1A); miR-190a-5p was downregulated, while IL-2 was upregulated in human AF cardiac tissues compared to that in non-AF controls (Figure 3). Furthermore, after treatment with miR-190a-5p, the sodium current density decreased by 59.98% over the entire test potential range; it increased 2.04-fold in the cells transfected with miR-190a-5p inhibitor (Figures 2A,C,E). Based on such evidence, we confirmed for the first time that miR-190a-5p may play a role in the causation of CAs by negatively regulating IL-2 and reducing the density of sodium current peaks produced by SCN3B.
miR-190 (gene ID* 406965), including two major mature forms of miR-190-5p and miR-190-3p, is located near the long arm of chromosome 15 (15q22.2) (19). The biological function of miR-190-3p has rarely been investigated. Jin et al. had confirmed that miR-190-3p participates in the formation of glioma via the prostate androgen-regulated transcript 1 (PART1)/miR-190a-3p/phosphatase and tensin homolog deleted on chromosome ten (PTEN)/phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathway (20). Experimental evidence had indicated miR-190-5p to be associated with various human diseases such as diabetic neuropathic pain (21), breast cancer (22), Parkinson's disease (23), pulmonary arterial hypertension (24), and diabetes mellitus (25). However, whether miR-190a-5p is involved in the occurrence of CAs is not yet clear and the specific molecular mechanism needs to be further explored.
Levels of IL-2 have been associated with reduced incidence of postoperative AF and supraventricular tachycardia (6, 26). In this in-vivo study, the level of IL-2 was remarkably increased in the peripheral blood of patients with AF; moreover, miR-190a-5p was downregulated, while IL-2 was upregulated in human AF cardiac tissues. In in-vitro experiments, miR-190a-5p did not affect the transcription level of IL-2 in AC16 and Raji cells, whereas it significantly reduced both the secretion of IL-2 in the medium supernatant of both kinds of cells and the INa of SCN3B in HEK293/Nav1.5 cells, which, in turn, was aggravated by inhibition of miR-190a-5p. The results, therefore, suggested that miR-190a-5p might play an important role in the progression of CAs by negatively regulating IL-2.
Furthermore, miR-190a-5p was shown to decrease the sodium current intensity of SCN3B, while its inhibition significantly increased the same. Olesen et al. had demonstrated that R6K, L10P, and M161T in SCN3B (NM_018400) were associated with early lone AF (27). Valdivia et al. had reported a V54G mutation in SCN3B of a patient diagnosed with idiopathic ventricular fibrillation (IVF) and indicated this mutation to cause “loss-of-function” of SCN3B by decreasing INa by 70 and 90% in HEK293 and primate fibroblastoid COS cells, respectively (28). In Japan, V110I in SCN3B is a relatively common cause of SCN5A-negative Brugada syndrome, which eventually results in a decrease in sodium current due to the lack of cell surface expression of Nav1.5 (29). This evidence supported the fact that SCN3B is closely related to CAs. Further, IL-2 increases the current intensity of SCN3B via the p53 pathway (13). Therefore, miR-190a-5p may partially rescue the abnormal cardiac electrical activity of patients with CAs, to some extent, by reducing the expression of IL-2. Furthermore, we speculated that there may be an important role of other miRNA(s) or genes in the pathological process of CAs via regulation of the current intensity of SCN3B or the stability of ion channels; this mechanism needs to be further explored and studied in future.
This study has some limitations. miR-190a-5p has been suggested to possibly play a role in protecting against CAs; however, the relationship between miR-190a-5p and IL-2 did not display one-to-one stoichiometry. Furthermore, one specific miRNA may simultaneously target multiple genes. The existing results did not exclude whether miR-190a-5p targets one or more other genes concurrently while exerting a protective role in the pathological process of CAs. The role of miR-190a-5p and other target genes, besides IL-2, in the pathological progress of CAs and the specific molecular mechanism, would need further exploration. Although both miR-190a-5p and IL-2 are closely associated with CAs, whether the expression of IL-2 is affected by miR-190a-5p in the long run and the exact role of miR-190a-5p and IL-2 in patients with CAs would require further evidence in future prospective clinical studies. Considering that CAs are divided into many types, whether miR-190a-5p and IL-2 play identical roles in many other CAs remains to be addressed. Since the sequence of miR-190a-5p binding to IL-2 is not well-conserved across humans, mice, and rats, it cannot be verified by animal model experiments. Some other miRNAs, which are higher more conserved across mammalian species, might also play an important role in affecting the expression of IL-2 in AF; therefore, future studies would need to be performed to discover the potential candidates. In this study, only eight tissue samples (auricle or valvula bicuspidalis) were collected, due to the major challenge of collecting human cardiac tissue samples. In addition, sex-related differences exist in a wide variety of CAs (30–32). Therefore, the relatively low volume of data in human AF study was the major limitation of this study. In future, more cardiac tissue samples from patients with AF and from non-AF controls would need to be collected to further confirm the accuracy of FISH results. Expansion of the clinical sample size would be necessary in future studies.
In conclusion, this study confirmed that miR-190a-5p may partially block the abnormal electrical activity of SCN3B in the progression of CAs and the possible pathway could be via miR-190a-5p/IL-2/SCN3B. Although the specific mechanism needs to be confirmed in further experiments, miR-190a-5p may still be regarded as a potential clinical target for CAs.
Funding
This study was funded by grants from the National Natural Science Foundation of China (Grant Nos. 81700300, 82000321, 81700302, 81770652, and 81800296) and the Natural Science Foundation of Hubei Province (2017CFB322).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This study involving human participants was reviewed and approved by the appropriate Local Institutional Review Board on human subject research at Huazhong University of Science and Technology and the First Affiliated Hospital of Xiamen University and also conformed to the guidelines set forth by the Declaration of Helsinki. The patients/participants (legal guardian/next of kin) provided a written informed consent to participate in this study. This study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Author contributions
QL, ZZ, SC, and YZ performed the experimental work. ZZ, MW, MZ, CY, XW, YC, and ZH provided the clinical samples and assisted in analysis. YZ, QL, ZZ, DJ, DD, YH, ZH, and XT analyzed the data and provided advice. YZ, ZC, and XT designed the experiments. QL, YZ, and XT wrote the manuscript, obtained funding for this project, directed, and supervised the study. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We are grateful to all the subjects who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer LG declared a shared affiliation with one of the authors, QL to the handling editor at time of review.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.795675/full#supplementary-material
References
1.
Nelson B.H . IL-2, regulatory T cells, and tolerance. J Immunol. (2004) 172:3983–8. 10.4049/jimmunol.172.7.3983
2.
Abbas AK Trotta E Simeonov D Marson A Bluestone JA . Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. (2018) 3:eaat1482. 10.1126/sciimmunol.aat1482
3.
Mizia-Stec K Gasior Z Zahorska-Markiewicz B Janowska J Szulc A Jastrzebska-Maj E et al . Serum tumour necrosis factor-alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron Artery Dis. (2003) 14:431–8. 10.1097/00019501-200309000-00003
4.
Ognibene FP Rosenberg SA Lotze M Skibber J Parker MM Shelhamer JH et al . Interleukin-2 administration causes reversible hemodynamic changes and left ventricular dysfunction similar to those seen in septic shock. Chest. (1988) 94:750–4. 10.1378/chest.94.4.750
5.
Cabrera-Bueno F Medina-Palomo C Ruiz-Salas A Flores A Rodriguez-Losada N Barrera A et al . Serum levels of interleukin-2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine. (2015) 73:74–8. 10.1016/j.cyto.2015.01.026
6.
Guglin M Aljayeh M Saiyad S Ali R Curtis AB . Introducing a new entity: chemotherapy-induced arrhythmia. Europace. (2009) 11:1579–86. 10.1093/europace/eup300
7.
Wu S Sarcon A Do K Shinbane J Doshi R Van Herle H . A case of myocarditis and near-lethal arrhythmia associated with interleukin-2 therapy. J Investig Med High Impact Case Rep. (2018) 6:2324709617749622. 10.1177/2324709617749622
8.
Ravaud A Delva R Gomez F Chevreau C Douillard JY Peny J et al . Subcutaneous interleukin-2 and interferon alpha in the treatment of patients with metastatic renal cell carcinoma-Less efficacy compared with intravenous interleukin-2 and interferon alpha. Results of a multicenter Phase II trial from the Groupe Francais d'Immunotherapie. Cancer. (2002) 95:2324–30. 10.1002/cncr.10968
9.
Wilde A Amin AS . Clinical spectrum of SCN5A mutations: long QT syndrome, brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. (2018) 4:569–79. 10.1016/j.jacep.2018.03.006
10.
Thireau J Pasquie JL Martel E Le Guennec JY Richard S . New drugs vs. old concepts: a fresh look at antiarrhythmics. Pharmacol Ther. (2011) 132:125–45. 10.1016/j.pharmthera.2011.03.003
11.
Hu D Viskin S Oliva A Carrier T Cordeiro JM Barajas-Martinez H et al . Novel mutation in the SCN5A gene associated with arrhythmic storm development during acute myocardial infarction. Heart Rhythm. (2007) 4:1072–80. 10.1016/j.hrthm.2007.03.040
12.
Karagueuzian HS Pezhouman A Angelini M Olcese R . Enhanced late Na and Ca currents as effective antiarrhythmic drug targets. Front Pharmacol. (2017) 8:36. 10.3389/fphar.2017.00036
13.
Zhao Y Sun Q Zeng Z Li Q Zhou S Zhou M et al . Regulation of SCN3B/scn3b by interleukin 2 (IL-2): IL-2 modulates SCN3B/scn3b transcript expression and increases sodium current in myocardial cells. BMC Cardiovasc Disord. (2016) 16:1. 10.1186/s12872-015-0179-x
14.
Sun B Meng M Wei J Wang S . Long noncoding RNA PVT1 contributes to vascular endothelial cell proliferation via inhibition of miR-190a-5p in diagnostic biomarker evaluation of chronic heart failure. Exp Ther Med. (2020) 19:3348–54. 10.3892/etm.2020.8599
15.
Agarwal V Bell GW Nam JW Bartel DP . Predicting effective microRNA target sites in mammalian mRNAs. Elife. (2015) 4:e05005. 10.7554/eLife.05005
16.
Huang Y Wang Z Liu Y Xiong H Zhao Y Wu L et al . alphaB-Crystallin Interacts with Nav1.5 and Regulates Ubiquitination and Internalization of Cell Surface Nav1.5. J Biol Chem. (2016) 291:11030–41. 10.1074/jbc.M115.695080
17.
Zhao Y Huang Y Li W Wang Z Zhan S Zhou M et al . Huang, Post-transcriptional regulation of cardiac sodium channel gene SCN5A expression and function by miR-192-5p. Biochim Biophys Acta. (2015) 1852:2024–34. 10.1016/j.bbadis.2015.07.016
18.
Guo Y Lip GY Apostolakis S . Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60:2263–70. 10.1016/j.jacc.2012.04.063
19.
Yu Y Cao XC . miR-190-5p in human diseases. Cancer Cell Int. (2019) 19:257. 10.1186/s12935-019-0984-x
20.
Jin Z Piao L Sun G Lv C Jing Y Jin R . Long non-coding RNA PART1 exerts tumor suppressive functions in glioma via sponging miR-190a-3p and inactivation of PTEN/AKT pathway. Onco Targets Ther. (2020) 13:1073–86. 10.2147/OTT.S232848
21.
Gong Q Lu Z Huang Q Ruan L Chen J Liang Y et al . Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem Biophys Res Commun. (2015) 456:615–20. 10.1016/j.bbrc.2014.12.004
22.
Yu Y Luo W Yang ZJ Chi JR Li YR Ding Y et al . miR-190 suppresses breast cancer metastasis by regulation of TGF-beta-induced epithelial-mesenchymal transition. Mol Cancer. (2018) 17:70. 10.1186/s12943-018-0818-9
23.
Sun Q Wang S Chen J Cai H Huang W Zhang Y et al . MicroRNA-190 alleviates neuronal damage and inhibits neuroinflammation via Nlrp3 in MPTP-induced Parkinson's disease mouse model. J Cell Physiol. (2019) 234:23379–87. 10.1002/jcp.28907
24.
Blissenbach B Nakas CT Kronke M Geiser T Merz TM Pichler HJ . Hypoxia-induced changes in plasma micro-RNAs correlate with pulmonary artery pressure at high altitude. Am J Physiol Lung Cell Mol Physiol. (2018). 314:L157–64. 10.1152/ajplung.00146.2017
25.
Mirra P Nigro C Prevenzano I Procopio T Leone A Raciti GA et al . The role of miR-190a in methylglyoxal-induced insulin resistance in endothelial cells. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:440–9. 10.1016/j.bbadis.2016.11.018
26.
Hak L Mysliwska J Wieckiewicz J Szyndler K Siebert J Rogowski J . Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J Interferon Cytokine Res. (2009) 29:327–32. 10.1089/jir.2008.0082.2906
27.
Olesen MS Jespersen T Nielsen JB Liang B Moller DV Hedley P et al . Mutations in sodium channel beta-subunit SCN3B are associated with early-onset lone atrial fibrillation. Cardiovasc Res. (2011) 89:786–93. 10.1093/cvr/cvq348
28.
Valdivia CR Medeiros-Domingo A Ye B Shen WK Algiers TJ Ackerman MJ et al . Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. (2010) 86:392–400. 10.1093/cvr/cvp417
29.
Ishikawa T Takahashi N Ohno S Sakurada H Nakamura K On On YK et al . Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ J. (2013) 77:959–67. 10.1253/circj.CJ-12-0995
30.
Goldenberg I Moss AJ . Long QT syndrome. J Am Coll Cardiol. (2008) 51:2291–300. 10.1016/j.jacc.2008.02.068
31.
van Rijsingen IA Nannenberg EA Arbustini E Elliott PM Mogensen J Hermans-van Ast JF et al . Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail. (2013) 15:376–84. 10.1093/eurjhf/hfs191
32.
Jang JH Shin SH Beak YS Ko KY Kwon SW Park SD et al . Impact of gender on heart failure presentation in non-obstructive hypertrophic cardiomyopathy. Heart Vessels. (2020) 35:214–22. 10.1007/s00380-019-01492-0
Summary
Keywords
cardiac arrhythmias, inflammation, interleukin-2, microRNA, sodium channel current, SCN3B
Citation
Li Q, Zhang Z, Chen S, Huang Z, Wang M, Zhou M, Yu C, Wang X, Chen Y, Jiang D, Du D, Huang Y, Tu X, Chen Z and Zhao Y (2022) miR-190a-5p Partially Represses the Abnormal Electrical Activity of SCN3B in Cardiac Arrhythmias by Downregulation of IL-2. Front. Cardiovasc. Med. 8:795675. doi: 10.3389/fcvm.2021.795675
Received
26 October 2021
Accepted
07 December 2021
Published
10 January 2022
Volume
8 - 2021
Edited by
Andrew Landstrom, Duke University, United States
Reviewed by
Estefania Lozano Velasco, University of East Anglia, United Kingdom; Lu Gao, The First Affiliated Hospital of Zhengzhou University, China; Qinghua Cui, Peking University, China
Updates
Copyright
© 2022 Li, Zhang, Chen, Huang, Wang, Zhou, Yu, Wang, Chen, Jiang, Du, Huang, Tu, Chen and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Zhao yyzhao@tjh.tjmu.edu.cnXin Tu xtu@hust.edu.cnZhishui Chen zschen@tjh.tjmu.edu.cn
†These authors have contributed equally to this work
This article was submitted to Cardiovascular Genetics and Systems Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.