Abstract
Plasma levels of the anticoagulant cofactor protein S and PROS1 mutation are reported to impart increased risk of thromboembolism in European and south east Asian populations, but the relationship is not yet documented in Han Chinese in population-based study. Therefore, we undertook a case-control study of this relationship among patients with venous thromboembolism, and probed the genetic factors contributing to low protein S deficiency. Among the 603 consecutively recruited venous thromboembolism patients, 51 (8.5%) proved to be deficient in free protein S antigen (lower than 38.6 U/dl), among whom 30 cases were identified to have a causative mutation by direct sequencing. In contrast, six cases (1.0%) of the 584 healthy controls had low free antigen levels, among whom direct sequencing confirmed disease-causing gene mutations in four controls (0.7%). After adjusting for age and gender, the odds ratio of developing venous thromboembolism in individuals with protein S deficiency based on free protein S tests was 8.1 (95% CI = 3.6–19.9, P < 0.001). Gene sequencing yielded 24 different heterozygous mutations in the 34 participants, of which 13 were newly described. 17 (50%) of the 34 mutations in our study cohort occurred in exons 12 and 13, indicating the LGR2 domain to be a hotspot mutation region for the protein. These findings are conducive to the clinical application of protein S assays for the molecular diagnosis of thrombophilia.
Introduction
Protein S is a natural vitamin K-dependent plasma glycoprotein (1, 2). Protein S acts as a cofactor of anticoagulant protease, activated protein C (APC), which inactivates the procoagulant factor Va and VIIIa to inhibit thrombin generation for regulating of coagulation (3). Protein S also has an APC-independent anticoagulant effect in inhibiting prothrombinase and the factor Xase (FXa) complex (4). Recent evidence suggests that protein S has yet another anticoagulant activity through interaction with the tissue factor pathway inhibitor (TFPI) to inhibit FXa (5, 6). In plasma, ~60% of circulating protein S is bound to C4b-bining protein (C4BP), while the remaining 40% free fractions possesses APC cofactor activity (7).
Inherited protein S deficiency (MIM 176880) is a rare autosomal dominant disorder due to various mutations in the PROS1 gene, which maps to human chromosome 3q11.2, spanning 101 kb and comprising 15 exons (8). The prevalence of protein S deficiency in healthy population reportedly broadly ranges from 0.03 to 0.13% in healthy European healthy populations, but increases to 1–13% in patients diagnosed with venous thromboembolism (9–11). New studies uncover that protein S deficiency has a relatively higher prevalence in southeast Asian general populations and patients with venous thromboembolism (12–14). Family-based studies reveal that protein S deficiency predisposes individuals to suffer venous thromboembolism, whereas the population-based studies indicate conflicting results on this association (15, 16). In addition to congenital mutations (17), oral contraceptives, pregnancy, hormone-replacement therapy, hypoxia and hepatic disorders can also decrease protein S levels (18–20).
Despite this background, there has been no study hitherto investigating the association between protein S deficiency and venous thromboembolism risk in the Chinese population. Therefore, we undertook a case-control study of the relationship between low plasma levels of free protein S, PROS1 mutation and increased risk of venous thromboembolism in a Chinese population. At the same time, we described the genetic characters of protein S deficiency in our study population.
Methods
Subjects
A total of 603 unselected patients diagnosed with symptomatic venous thromboembolism were consecutively enrolled in the study from 1 January 2014 to 12 December 2015 in Wuhan Union Hospital. The criteria for diagnosis were according to clinical manifestations, D-dimer, and imaging tests. Color Doppler Ultrasonography, Computed Tomography angiography or Magnetic Resonance venography were performed in participants with symptomatic venous thromboembolism. Five hundred eighty-four age- and sex-matched controls without an individual history of venous thromboembolism were recruited during the same period. Blood samples were drawn, immediately centrifuged, the platelet-poor plasma samples were stored at −80°C for protein S antigen assays. Separated white blood cells were extracted for genomic DNA analysis.
This study was approved by ethics committee of Union hospital affiliated to Hua Zhong University of science and technology. All methods performed in our study were exactly in accordance with the approved guidelines. Written informed consent in compliance with the Declaration of Helsinki was obtained from all participants or their legal guardians.
Sample Collection and Plasma Free Protein S Assay
Sample were collected before anticoagulant therapy or at 2 weeks after its discontinuation of anticoagulant therapy. Free protein S antigen was measured by enzyme-linked immunosorbent assay using the ZYMUTEST Free Protein S kit, following the manufacturer's instructions (Hyphen BioMed, Andresy, France).
The PROS1 Gene Analysis
The putative promoter, 5'UTR, 15 exons and their flanking regions, and 3'UTR were amplified though the Polymerase Chain Reaction (PCR) and reaction products were sequenced on an ABI PRISM 3730XL automated sequencer (Applied Biosystems). The primer-pair sequences and PCR conditions used in the amplification were as described in a previous report (21). We described novel variants according to current nomenclature conventions and the recommendations of the Human Genome Variation Society (HGVS, http://www.hgvs.org/mutnomen/). The Genebank NM_000313.3 and NP_000304.2 were used as reference sequence. Functional consequences of novel missense mutations were analyzed by in silico bioinformatics tools. MutationTaster: http://www.mutationtaster.org/; PROVEAN: http://provean.jcvi.org/index.php; PolyPhen-2: http://genetics.bwh.harvard.edu/pph2/; HomoloGene for PROS1: https://www.ncbi.nlm.nih.gov/homologene/264
Statistical Analysis
The odds ratios and 95% confidence intervals (95% CIs) evaluating the risk of venous thromboembolism associated with the plasma level of free protein S antigen or PROS1 mutations were calculate through the Chi-squared test. Multivariate logistic regression analysis was performed to adjust the odds ratios (OR) for selected confounders (age and gender). Statistical significance was accepted at P < 0.05. The statistical analyses were carried out using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of Participants
The population consisted of 603 consecutive venous thromboembolism patients and 584 controls. Female and male had similar mean free protein S level, as shown along with other results in Table 1. We established a local laboratory free protein S reference range from previous healthy individuals, whereby plasma concentrations falling below the 2.5th percentile (38.6 U/dl) were defined as protein S deficient. Among the 603 consecutively recruited venous thromboembolism patients, 51 (8.5%) proved to be deficient in free protein S antigen, among whom 30 cases were identified to have a causative mutation by direct sequencing. Six cases (1.0%) of the 584 normal controls had free antigen levels lower than 38.6 U/dl, among whom direct sequencing confirmed disease-causing gene mutations in four controls (0.7%). As shown in Figure 1, the OR of venous thromboembolism in individuals with protein S deficiency based on free protein S tests was 8.9 (95% CI = 3.8–20.9, P < 0.001), and 7.6 (95% CI = 2.6–21.7, P < 0.001) in individuals with protein S deficiency based on PROS1 gene mutations. After adjusting for age and gender, the odds ratio of developing venous thromboembolism in individuals with protein S deficiency based on free protein S tests was 8.1 (95% CI = 3.6–19.9, P < 0.001).
Table 1
| Characteristics | VTE patients | Healthy controls |
|---|---|---|
| (means of fPS level: U/dl | (means of fPS level: U/dl, | |
| N = 603) | N = 584) | |
| Age (years) | ||
| <20 | 73 ± 17, n = 8 | 97, n = 1 |
| 20~40 | 84 ± 18, n = 89 | 87 ± 13, n = 113 |
| 40~60 | 89 ± 23, n = 295 | 89 ± 13, n = 304 |
| >60 | 89 ± 20, n = 211 | 87 ± 13, n = 166 |
| Gender | ||
| Male | 87 ± 20, n = 324 | 87 ± 13, n = 292 |
| Female | 88 ± 23, n = 279 | 88 ± 13, n = 292 |
| Current smoking | ||
| Yes | 90 ± 16, n = 118 | 87 ± 13, n = 116 |
| No | 87 ± 23, n = 485 | 88 ± 13, n = 468 |
| Alcohol drinking | ||
| Yes | 87 ± 18, n = 75 | 84 ± 19, n = 64 |
| No | 88 ± 22, n = 528 | 84 ± 23, n = 520 |
| History of VTE | ||
| Yes | 84 ± 18, n = 72 | NA |
| No | 88 ± 22, n = 531 | NA |
| Malignant tumor | ||
| Yes | 91 ± 13, n = 14 | 92 ± 12, n = 3 |
| No | 88 ± 22, n = 589 | 88 ± 13, n = 581 |
| Pregnancy/puerperium | ||
| Yes | 77 ± 7, n = 5 | 80 ± 18, n = 13 |
| No | 88 ± 22, n = 598 | 88 ± 13, n = 571 |
| Recent surgery | ||
| Yes | 88 ± 17, n = 36 | 81 ± 17, n = 8 |
| No | 88 ± 22, n = 567 | 88 ± 13, n = 576 |
Free protein S levels grouped by statistic characteristics.
Recent surgery: refers to receiving surgery within 3 months.
Figure 1
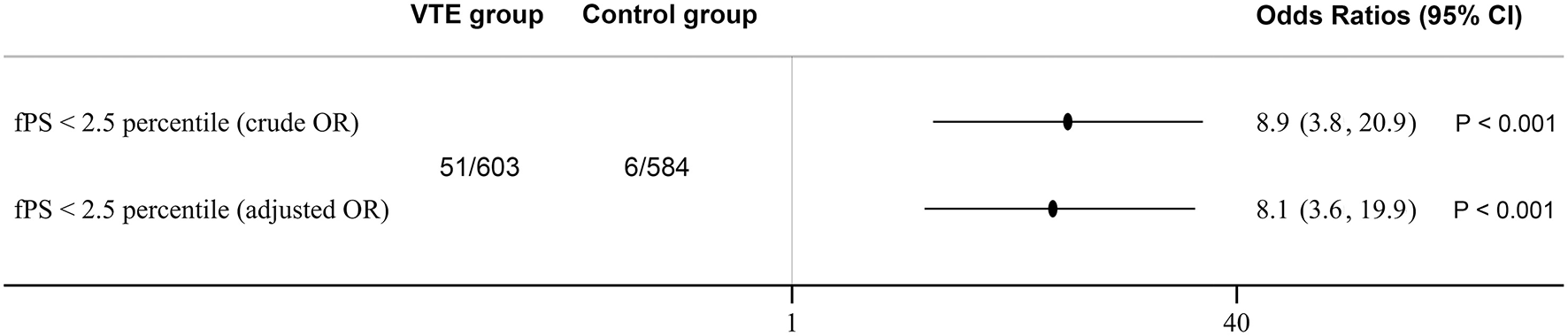
Protein S deficiency and risk of venous thromboembolism. Odds Ratios were adjusted for age and gender. 95% CI indicates 95% confidence intervals.
Genetic Analysis of PROS1
To study the genetic molecular basis of low free protein S levels, we performed DNA sequencing of the PROS1 gene in participants with free protein S level below our cut-off of 38.6 U/dL; this yielded sequence information in 51 patients with venous thromboembolism and 6 controls. In 34 of 57 sequenced participants with PS deficiency, we found a total of 24 heterozygous gene mutations, including 13 new mutations (Table 2). Four heterozygous mutations were detected in the healthy control PS-deficiency subjects. The positive rate of genetic analysis might be due to the occurrence of a causative variant in deep introns that were overlooked by the DNA sequencing, or due to gross insertion/deletion of the PROS1 gene.
Table 2
| Participant ID | Age | Sex | fPS (U/dL) | NT exchange | AA substitution | Region | Domain | Newly reported |
|---|---|---|---|---|---|---|---|---|
| VTE 1 | 57 | M | 38 | c.1454A>C | p.Tyr485Ser | E12 | LGR2 | Yes |
| VTE 2 | 68 | M | 33 | c.829C>T | p.Gln277* | E8 | EGF4 | Yes |
| VTE 3 | 42 | F | 23 | c.1424G>A | p.Cys475Tyr | E12 | LGR2 | Yes |
| VTE 4 | 50 | M | 36 | c.200A>C | p.Glu67Ala | E2 | GLA | rs766423432 |
| VTE 5 | 61 | F | 19 | c.1393G>T | p.Glu465* | E12 | LGR2 | rs199469496 |
| VTE 6 | 43 | F | 29 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
| VTE 7 | 40 | F | 30 | c.200A>C | p.Glu67Ala | E2 | GLA | rs766423432 |
| VTE 8 | 61 | M | 30 | c.1063C>T | p.Arg355Cys | E10 | LGR1 | rs387906674 |
| VTE 9 | 22 | M | 34 | c.1351C>T | p.Arg451* | E12 | LGR2 | rs5017717 |
| VTE 10 | 44 | M | 37 | c.200A>C | p.Glu67Ala | E2 | GLA | rs766423432 |
| VTE 11 | 64 | M | 33 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
| VTE 12 | 52 | M | 34 | c.1603T>G | p.Phe535Val | E13 | LGR2 | Yes |
| VTE 13 | 54 | F | 35 | c.1565T>A | p.Val522Asp | E13 | LGR2 | Yes |
| VTE 14 | 49 | M | 28 | c.203C>A | p.Ala68Asp | E2 | GLA | No |
| VTE 15 | 59 | F | 29 | c.1146_1147delAT | p.Trp383Glufs*11 | E10 | LGR1 | rs312262905 |
| VTE 16 | 70 | M | 38 | c.1351C>T | p.Arg451* | E12 | LGR2 | rs5017717 |
| VTE 17 | 39 | M | 32 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
| VTE 18 | 54 | F | 21 | c.1518G>A | p.Trp506* | E13 | LGR2 | No |
| VTE 19 | 50 | M | 29 | c.483C>A | p.Cys161* | E6 | EGF2 | Yes |
| VTE 20 | 34 | F | 30 | c.1577T>G | p.Leu526Trp | E13 | LGR2 | Yes |
| VTE 21 | 40 | M | 34 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
| VTE 22 | 35 | M | 27 | c.1704T>A | p.Cys568* | E14 | LGR2 | Yes |
| VTE 23 | 18 | F | 17 | c.365_366delGT | p.Ser122Thrfs*6 | E5 | EGF1 | Yes |
| VTE 24 | 61 | F | 17 | c.1229C>A | p.Pro410His | E11 | LGR1 | rs199469495 |
| VTE 25 | 24 | M | 20 | c.976G>T | p.Glu326* | E10 | LGR1 | Yes |
| VTE 26 | 50 | M | 33 | c.1351C>T | p.Arg451* | E12 | LGR2 | rs5017717 |
| VTE 27 | 62 | F | 37 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
| VTE 28 | 46 | M | 34 | c.1553C>T | p.Thr518Met | E13 | LGR2 | rs373336653 |
| VTE 29 | 52 | M | 29 | c.392A>G | p.Tyr131Cys | E5 | EGF1 | Yes |
| VTE 30 | 67 | F | 29 | c.1095T>G | p.Asn365Lys | E10 | LGR1 | rs199469491 |
| HC 1 | 64 | F | 31 | c.1063C>T | p.Arg355Cys | E10 | LGR1 | rs387906674 |
| HC 2 | 44 | F | 28 | c.1155+4C>T | Splicing region | E10 | LGR1 | Yes |
| HC 3 | 22 | F | 20 | c.282_282delT | p.Leu96Tyrfs*15 | E4 | TSR | Yes |
| HC 4 | 29 | M | 31 | c.1543C>T | p.Arg515Cys | E13 | LGR2 | rs199469500 |
Molecular analysis of PROS1 in participants with free protein S below the 2.5 percentile.
Mutations were designated according to the human Genome Variation Society compared with NCBI Reference Sequences NM_000313.3 and NP_000304.2. VTE, refers to venous thromboembolism patients; HC, refers to healthy control individuals; E, exon; fPS, free protein S antigen; NT, nucleotide; AA, amino acid; E, exon; I, intron; EGF, epidermal growth factor domain; TSR, thrombin-sensitive region; GLA, γ-carboxyglutamic acid domain; LGR, laminin G-type repeat domain; *nonsense mutation; M, male; F, female.
We evaluated the 6 newly discovered PROS1 gene missense mutations for pathogenicity through four functional prediction software procedures, at least three of which indicated pathogenicity for all mutations (Table 3). We did not evaluate further small deletions, non-sense mutations and previously reported detrimental variants. Four of the controls carried a variant that was identified as distinctly damaging, despite the absent phenotype of venous thromboembolism. This may be explained by that venous thromboembolism is doubtless a multifactorial disease caused by interactions between genetic and environmental factors; the controls carrying an abnormal genotype may be vulnerable to venous thromboembolism later in life, or upon exposure to some environmental factor.
Table 3
| NT exchange | AA substitution | MutationTaster | PROVEAN | PolyPhen-2 | HomoloGene |
|---|---|---|---|---|---|
| c.392A>G | p.Tyr131Cys | Disease causing (probability: 0.996) | Neutral | Probably damaging (probability: 0.993) | Highly conserved |
| c.1424G>A | p.Cys475Tyr | Disease causing (probability: 0.999) | Deleterious | Probably damaging (probability: 0.993) | Highly conserved |
| c.1454A>C | p.Tyr485Ser | Disease causing (probability: 0.999) | Deleterious | Possibly damaging (probability: 0.914) | Highly conserved |
| c.1565T>A | p.Val522Asp | Disease causing (probability: 0.999) | Deleterious | Probably damaging (probability: 0.997) | Highly conserved |
| c.1577T>G | p.Leu526Trp | Disease causing (probability: 0.999) | Deleterious | Probably damaging (probability: 0.999) | Highly conserved |
| c.1603T>G | p.Phe535Val | Disease causing (probability: 0.601) | Neutral | Possibly damaging (probability: 0.925) | Highly conserved |
In silico analysis of novel amino acid changes.
Functional consequences of novel missense mutations were analyzed by in silico bioinformatics tools. MutationTaster: http://www.mutationtaster.org/; PROVEAN: http://provean.jcvi.org/index.php; PolyPhen-2: http://genetics.bwh.harvard.edu/pph2/; HomoloGene for PROS1: https://www.ncbi.nlm.nih.gov/homologene/264; NT, nucleotide; AA, amino acid.
We were surprised to find that among the 34 participants with identified gene mutations, 11 were in exon 13 and six were in the adjacent exon 12, comprising part of the LGR2 domain of the protein. Thus, 17 (50%) of the 34 mutations in our study cohort occurred in the exons 12/13, indicating a hotspot mutation region of protein S (Figure 2).
Figure 2
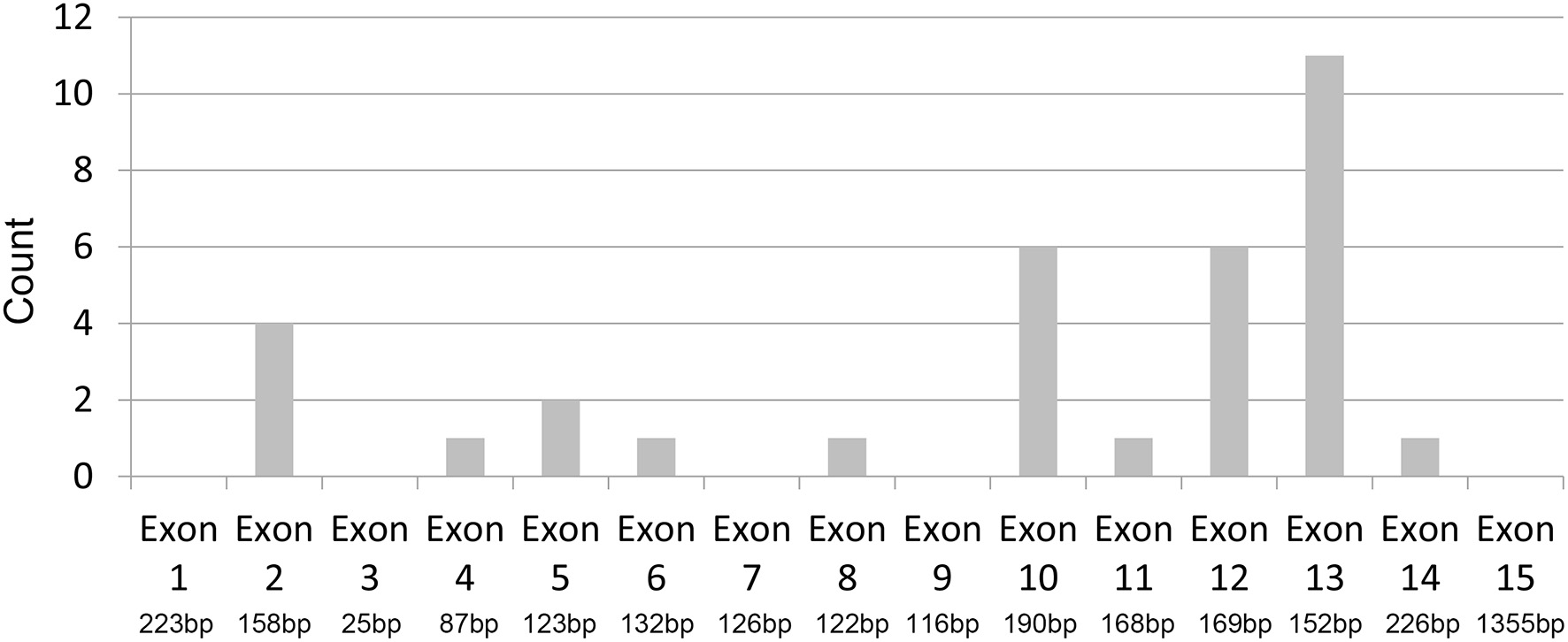
Hot-spot region of protein S mutation. Distribution of PROS1 mutations across exon 1 to 15: 50% (17/34) of the identified mutations occurred in exons 12 and 13.
Discussion
Risk factors and epidemiology of venous thromboembolism have been extensively studied in Caucasians populations during the past years. However, the corresponding state of knowledge concerning incidence and genetic factors lags behind in Asian populations. There is compelling evidence for racial and geographic differences in the hereditary risk factor and incidence of venous thromboembolism. Gain-of-function variations in procoagulant factors was the main genetic risk factor for venous thromboembolism in Caucasians, while loss of function of anticoagulation factors was the predominant cause in Asians. Specifically, deficiency of the natural anticoagulant factors protein S, protein C and antithrombin were common risk factors for venous thromboembolism in China. Among these, protein S deficiency was the more frequently compromised among the anticoagulant factors.
Protein S deficiency was established early to be risk factor associated with familial venous thromboembolism in European populations (22), as substantiated by extensive family-based studies (23). Thrombophilic subjects were 5–10-fold more likely to harbor a protein S deficiency compared to healthy relatives (24–26). Nevertheless, there have been discordant findings concerning the risk of venous thromboembolism on protein S deficiency in population-based studies. We now show that a low level of free protein S antigen imparted an increased risk of developing venous thromboembolism in a Han Chinese population, which stands in agreement with some previous research (27, 28). We wish now to emphasize some aspects of the conflicting results in the literature on protein S deficiency. First, the accurate, reproducible and reliable diagnosis of protein S deficiency is technically challenging (29). One prior study reported that only nine of 56 cases with initially low protein S antigen levels showed persistence of their decreased values, thus indicating that low findings are often a transient phenomenon (30). Furthermore, transient protein S deficiency does not seem sufficient in impart increased risk of venous thromboembolism. Second, protein S assay results differ by region, instrumentation, and clinical laboratory. Third, determining the association with risk of venous thromboembolism depends on measuring the level of protein S antigen accurately and reproducibly in reference to the normal range for the local population. Indeed, protein S antigen levels are affected not just by hereditary, but due to factors such as smoking, surgery, disease, pregnancy/puerperium, age, and sex (31). Fourth, there is some overlap of free protein S levels between healthy individuals and hereditary protein S deficiency patients with a history of venous thromboembolism (32, 33).
In the present study, diagnosis of protein S deficiency relied on the results from laboratory tests measuring protein antigen level. In our hands, the free protein S antigen assay was more accurate, reproducible, and valuable for identifying protein S deficiency status, than were the total protein S antigen assay and protein S activity test. The widely used activity assay measuring the anticoagulant function of protein S as the cofactor of APC is fraught with problems causing spuriously false low protein S values (34). Total protein S, consisting of the bound and free protein S fractions measured immunologically was not associated with risk of venous thromboembolism (35). Indeed, some individuals with hereditary and acquired protein S deficiency may manifest normal total protein S levels, while harboring decreased free protein S antigen and declining protein S activity levels (36). We performed the free protein S antigen assay to immunologically recognize only the unbound form of protein S. In previous work, the proportion of falsely low values of free protein S antigen (<1%) was distinctly lower than in activity assays (10–15%), indicating that free protein S antigen assay is a more accurate and reliable method evaluating the protein S functional state (37).
Heterozygous protein S deficiency is well-established as an autosomal dominant trait associated with an increased risk for developing venous thromboembolism (38, 39). Missense mutations, non-sense mutations and small insertion/deletions accounted the majority of defects in the present study. The latter two mutations were obviously detrimental because they contained a premature stop codon causing a truncated protein with impaired function. In this study, at least three of four in silico bioinformatic tools predicted the novel missense mutations to be harmful.
Disease-causing mutations were not identified in a large proportion of our protein S deficient subjects. This may arise due to presence of a mutation located in deep introns that are invisible to the DNA sequencing method, or due to the presence of large insertion/deletion mutations, which are a rare risk factor for developing venous thromboembolism (39). Our results were in accordance with other research showing an undetected mutation of the PROS1 gene in up to 50% of individuals with protein S deficiency (8). On the other hand, diagnosis of protein S deficiency remains a challenge due to the variable of possible genetic defects, assay performance, and various confounds such as use of oral contraceptives, surgery, infection, DIC, pregnancy/puerperium, HIV infection, and liver disease.
In summary, we investigated the effects protein S deficiency (based on plasma levels for the free antigen) and PROS1 gene mutations in a series of patients of Han ethnicity. We found a 6–8-fold elevated risk for venous thromboembolism among patients with protein S deficiency, and furthermore discovered a mutation hotspot around exons 11 and 12 of PROS1. These findings are conducive to the clinical application of protein S assays for the molecular diagnosis of thrombophilia.
Funding
This study was supported by grants from the National Natural Sciences Foundation of China (Nos. 81800132 and 81973995) and Program for HUST Academic Frontier Youth Team (No. 2018QYTD14).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number is ON730818, ON730819, ON730820, ON730821, ON730822, ON730823, ON730824, ON730825, and ON730826.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Union Hospital Affiliated to Hua Zhong University of Science and Technology. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW contributed to the study design. YW and JL collected samples, performed the experiment, and analyzed the data. LT and YH wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the participants in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Gierula M Ahnstrom J . Anticoagulant protein S-New insights on interactions and functions. J Thrombosis Haemostasis. (2020) 18:2801–11. 10.1111/jth.15025
2.
Majumder R . Regulation of venous thrombosis by platelet protein S. Blood. (2020) 135:1922–3. 10.1182/blood.2020005676
3.
Maurissen LF Thomassen MC Nicolaes GA Dahlback B Tans G Rosing J et al . Re-evaluation of the role of the protein S-C4b binding protein complex in activated protein C-catalyzed factor Va-inactivation. Blood. (2008) 111:3034–41. 10.1182/blood-2007-06-089987
4.
Wu X Dai J Xu X Li F Li L Lu Y et al . Prothrombin Arg541Trp mutation leads to defective PC (Protein C) pathway activation and constitutes a novel genetic risk factor for venous thrombosis. Arteriosclerosis Thrombosis Vasc Biol. (2020) 40:483–94. 10.1161/ATVBAHA.119.313373
5.
Reglinska-Matveyev N Andersson HM Rezende SM Dahlback B Crawley JT Lane DA et al . TFPI cofactor function of protein S: essential role of the protein S SHBG-like domain. Blood. (2014) 123:3979–87. 10.1182/blood-2014-01-551812
6.
Dahlback B Guo LJ Zoller B Tran S . New functional test for the TFPIalpha cofactor activity of Protein S working in synergy with FV-Short. J Thrombosis Haemostasis: JTH. (2019) 17:585–95. 10.1111/jth.14405
7.
Dahlback B . The tale of protein S and C4b-binding protein, a story of affection. Thrombosis Haemostasis. (2007) 98:90–6. 10.1160/TH07-04-0269
8.
Garcia de Frutos P Fuentes-Prior P Hurtado B Sala N . Molecular basis of protein S deficiency. Thrombosis Haemostasis. (2007) 98:543–56. 10.1160/TH07-03-0199
9.
Tang L Hu Y . Ethnic diversity in the genetics of venous thromboembolism. Thrombosis Haemostasis. (2015) 114:901–9. 10.1160/TH15-04-0330
10.
Lane DA Mannucci PM Bauer KA Bertina RM Bochkov NP Boulyjenkov V et al . Inherited thrombophilia: Part 1. Thrombosis Haemostasis. (1996) 76:651–62. 10.1055/s-0038-1650638
11.
Seligsohn U Lubetsky A . Genetic susceptibility to venous thrombosis. N Engl J Med. (2001) 344:1222–31. 10.1056/NEJM200104193441607
12.
Kim HJ Seo JY Lee KO Bang SH Lee ST Ki CS et al . Distinct frequencies and mutation spectrums of genetic thrombophilia in Korea in comparison with other Asian countries both in patients with thromboembolism and in the general population. Haematologica. (2014) 99:561–9. 10.3324/haematol.2013.092023
13.
Zhu T Ding Q Bai X Wang X Kaguelidou F Alberti C et al . Normal ranges and genetic variants of antithrombin, protein C and protein S in the general Chinese population. Results of the Chinese hemostasis investigation on natural anticoagulants study I group. Haematologica. (2011) 96:1033–40. 10.3324/haematol.2010.037515
14.
Li L Wu X Wu W Ding Q Cai X Wang X . Clinical manifestation and mutation spectrum of 53 unrelated pedigrees with protein S deficiency in China. Thrombosis Haemostasis. (2019) 119:449–60. 10.1055/s-0038-1677031
15.
Rezende SM Simmonds RE Lane DA . Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex. Blood. (2004) 103:1192–201. 10.1182/blood-2003-05-1551
16.
Faioni EM Valsecchi C Palla A Taioli E Razzari C Mannucci PM . Free protein S deficiency is a risk factor for venous thrombosis. Thrombosis Haemostasis. (1997) 78:1343–6. 10.1055/s-0038-1665408
17.
Tormene D Campello E Simion C Turatti G Marobin M Radu CM et al . Incidence of VTE in asymptomatic children with deficiencies of antithrombin, protein C, and protein S: a prospective cohort study. Blood Adv. (2020) 4:5442–8. 10.1182/bloodadvances.2020002781
18.
Henkens CM Bom VJ Van der Schaaf W Pelsma PM Sibinga CT de Kam PJ et al . Plasma levels of protein S. protein C, and factor X: effects of sex, hormonal state and age. Thrombosis Haemostasis. (1995) 74:1271–5. 10.1055/s-0038-1649925
19.
D'Angelo A Vigano-D'Angelo S Esmon CT Comp PC . Acquired deficiencies of protein S. Protein S activity during oral anticoagulation, in liver disease, and in disseminated intravascular coagulation. J Clin Investig. (1988) 81:1445–54. 10.1172/JCI113475
20.
Pilli VS Datta A Afreen S Catalano D Szabo G Majumder R . Hypoxia downregulates protein S expression. Blood. (2018) 132:452–55. 10.1182/blood-2018-04-841585
21.
Tang L Jian XR Hamasaki N Guo T Wang HF Lu X et al . Molecular basis of protein S deficiency in China. Am J Hematol. (2013) 88:899–905. 10.1002/ajh.23525
22.
Schwarz HP Fischer M Hopmeier P Batard MA Griffin JH . Plasma protein S deficiency in familial thrombotic disease. Blood. (1984) 64:1297–300. 10.1182/blood.V64.6.1297.1297
23.
Comp PC Nixon RR Cooper MR Esmon CT . Familial protein S deficiency is associated with recurrent thrombosis. J Clin Investig. (1984) 74:2082–8. 10.1172/JCI111632
24.
Castoldi E Maurissen LF Tormene D Spiezia L Gavasso S Radu C et al . Similar hypercoagulable state and thrombosis risk in type I and type III protein S-deficient individuals from families with mixed type I/III protein S deficiency. Haematologica. (2010) 95:1563–71. 10.3324/haematol.2010.021923
25.
Makris M Leach M Beauchamp NJ Daly ME Cooper PC Hampton KK et al . Genetic analysis, phenotypic diagnosis, and risk of venous thrombosis in families with inherited deficiencies of protein S. Blood. (2000) 95:1935–41. 10.1182/blood.V95.6.1935
26.
Ten Kate MK Platteel M Mulder R Terpstra P Nicolaes GA Reitsma PH et al . PROS1 analysis in 87 pedigrees with hereditary protein S deficiency demonstrates striking genotype-phenotype associations. Human Mutation. (2008) 29:939–47. 10.1002/humu.20687
27.
Pintao MC Ribeiro DD Bezemer ID Garcia AA de Visser MC Doggen CJ et al . Protein S levels and the risk of venous thrombosis: results from the MEGA case-control study. Blood. (2013) 122:3210–9. 10.1182/blood-2013-04-499335
28.
Koster T Rosendaal FR Briet E van der Meer FJ Colly LP Trienekens PH et al . Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study). Blood. (1995) 85:2756–61. 10.1182/blood.V85.10.2756.bloodjournal85102756
29.
Marlar RA Gausman JN Tsuda H Rollins-Raval MA Brinkman HJM . Recommendations for clinical laboratory testing for protein S deficiency: Communication from the SSC committee plasma coagulation inhibitors of the ISTH. J Thrombosis Haemostasis: JTH. (2021) 19:68–74. 10.1111/jth.15109
30.
Dykes AC Walker ID McMahon AD Islam SI Tait RC . A study of Protein S antigen levels in 3788 healthy volunteers: influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. (2001) 113:636–41. 10.1046/j.1365-2141.2001.02813.x
31.
Lijfering WM Mulder R ten Kate MK Veeger NJ Mulder AB van der Meer J . Clinical relevance of decreased free protein S levels: results from a retrospective family cohort study involving 1143 relatives. Blood. (2009) 113:1225–30. 10.1182/blood-2008-08-174128
32.
Zoller B Garcia de Frutos P Dahlback B . Evaluation of the relationship between protein S and C4b-binding protein isoforms in hereditary protein S deficiency demonstrating type I and type III deficiencies to be phenotypic variants of the same genetic disease. Blood. (1995) 85:3524–31. 10.1182/blood.V85.12.3524.bloodjournal85123524
33.
Simmonds RE Zoller B Ireland H Thompson E de Frutos PG Dahlback B et al . Genetic and phenotypic analysis of a large (122-member) protein S-deficient kindred provides an explanation for the familial coexistence of type I and type III plasma phenotypes. Blood. (1997) 89:4364–70. 10.1182/blood.V89.12.4364
34.
Mulder R Ten Kate MK Kluin-Nelemans HC Mulder AB . Low cut-off values increase diagnostic performance of protein S assays. Thrombosis Haemostasis. (2010) 104:618–25. 10.1160/TH10-02-0113
35.
ten Kate MK van der Meer J . Protein S deficiency: a clinical perspective. Haemophilia. (2008) 14:1222–8.
36.
Inherited thrombophilia: memorandum from a joint WHO/International Society on Thrombosis and Haemostasis meeting . Bull World Health Organization. (1997) 75:177–89.
37.
Marlar RA Gausman JN . Protein S abnormalities: a diagnostic nightmare. Am J Hematol. (2011) 86:418–21. 10.1002/ajh.21992
38.
Kinoshita S Iida H Inoue S Watanabe K Kurihara M Wada Y et al . Protein S and protein C gene mutations in Japanese deep vein thrombosis patients. Clin Biochem. (2005) 38:908–15. 10.1016/j.clinbiochem.2005.05.006
39.
Chen TY Su WC Tsao CJ . Incidence of thrombophilia detected in southern Taiwanese patients with venous thrombosis. Ann Hematol. (2003) 82:114–7. 10.1007/s00277-002-0603-z
Summary
Keywords
protein S deficiency, venous thromboembolism, gene mutation, odds ratio, Chinese population
Citation
Wu Y, Liu J, Zeng W, Hu B, Hu Y and Tang LV (2022) Protein S Deficiency and the Risk of Venous Thromboembolism in the Han Chinese Population. Front. Cardiovasc. Med. 8:796755. doi: 10.3389/fcvm.2021.796755
Received
17 October 2021
Accepted
13 December 2021
Published
23 June 2022
Volume
8 - 2021
Edited by
Luca Spiezia, University of Padua, Italy
Reviewed by
Koichi Kokame, National Cerebral and Cardiovascular Center, Japan; Wenman Wu, Shanghai Jiao Tong University, China
Updates
Copyright
© 2022 Wu, Liu, Zeng, Hu, Hu and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang V. Tang lancet.tang@qq.comYu Hu dr_huyu@126.com
†These authors have contributed equally to this work
This article was submitted to Thrombosis, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.