Abstract
Introduction:
We sought to investigate the prognostic impact of incident left ventricular (LV) systolic dysfunction at the chronic phase of acute myocardial infarction (AMI).
Materials and methods:
Among 2,266 consecutive patients admitted for AMI, 1,330 patients with LV ejection fraction (LVEF) ≥ 40% during hospitalization who had LVEF data at 6 months after AMI were analyzed. Patients were divided into three subgroups based on LVEF at 6 months: reduced-LVEF (<40%), mid-range-LVEF (≥ 40% and < 50%) and preserved-LVEF (≥ 50%). Occurrence of a composite of hospitalization for heart failure or cardiovascular death after 6 months of AMI was the primary endpoint. The prognostic impact of LVEF at 6 months was assessed with a multivariate-adjusted Cox model.
Results:
Overall, the mean patient age was 67.5 ± 11.9 years, and LVEF during initial hospitalization was 59.4 ± 9.1%. The median (interquartile range) duration of follow-up was 3.0 (1.5–4.8) years, and the primary endpoint occurred in 35/1330 (2.6%) patients (13/69 [18.8%] in the reduced-LVEF, 9/265 [3.4%] in the mid-range-LVEF, and 13/996 [1.3%] in the preserved-LVEF category). The adjusted hazard ratio for the primary endpoint in the reduced-LVEF vs. mid-range-LVEF category and in the reduced-LVEF vs. preserved-LVEF category was 4.71 (95% confidence interval [CI], 1.83 to 12.13; p < 0.001) and 14.37 (95% CI, 5.38 to 38.36; p < 0.001), respectively.
Conclusion:
Incident LV systolic dysfunction at the chronic phase after AMI was significantly associated with long-term adverse outcomes. Even in AMI survivors without LV systolic dysfunction at the time of AMI, post-AMI reassessment and careful monitoring of LVEF are required to identify patients at risk.
Introduction
In the primary percutaneous coronary intervention (PCI) era, an increased risk of late-onset heart failure (HF) and mortality as post-acute myocardial infarction (AMI) events is still an important clinical issue. Hence, a better risk stratification system to prevent those adverse events at the remote phase of AMI is of clinical importance. At the acute phase of acute coronary syndrome (ACS) including AMI, several established risk scores, such as TIMI (1) and GRACE (2), are universally used to predict prognosis. In addition, previous reports have suggested that some clinical manifestations, such as lack of reperfusion therapy (3), frailty (4), nutritional status (5), and a combination of multiple blood variables (6, 7) obtained at the acute-phase of AMI could predict adverse events after AMI. Thus, while the prognostic value of several clinical indicators in the acute phase of AMI has been established, predictors of long-term prognosis in the chronic phase of AMI have not yet been fully established.
Left ventricular ejection fraction (LVEF) is one of the most general indicators of left ventricular (LV) systolic function and is widely available in clinical settings. Existence of LV systolic dysfunction at the acute phase of AMI is well-known as a strong predictor for adverse prognosis after AMI (8). On the other hand, LVEF often changes dynamically through chronic LV remodeling after AMI (9), and this change in LVEF during the post-AMI period also has prognostic impact (10, 11). Thus, a chronic transition to LV systolic dysfunction can occur even in patients with AMI without systolic dysfunction at the acute phase of AMI, possibly adding to the risk of adverse events at the remote phase of AMI. However, the detailed clinical features and prognostic impact of incident reduced LVEF at the remote phase of AMI remain poorly elucidated. Focusing on newly developed LV systolic dysfunction at the chronic phase of AMI may help in understanding this clinical unmet need better. Herein, we sought to clarify the clinical features of incident LV systolic dysfunction at the remote phase of AMI and its prognostic impact among AMI survivors without LV systolic dysfunction at the acute phase of AMI.
Materials and methods
Design and population
This was a single-center, retrospective, observational study performed at Miyazaki Medical Association Hospital in Japan. A total of 2,266 consecutive patients admitted for AMI, with either ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI), from February 2008 to January 2016 were eligible. Exclusion criteria were history of myocardial infarction, death within 6 months after AMI, admission due to worsening HF within 6 months after AMI, LVEF < 40% during index hospitalization, and no follow-up LVEF data at 6 months after AMI. According to LVEF at 6 months after AMI (within 1 month either side of 6 months), patients were divided into three subgroups: reduced-LVEF (< 40%), mid-range-LVEF (≥ 40% and < 50%), and preserved-LVEF (≥ 50%).
All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. All patients provided informed consent for both the procedure and the subsequent data collection and analysis for research purposes. Ethics approval was obtained from the Institutional Review Board of Miyazaki Medical Association Hospital (2019-23).
Definition and diagnosis of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction
Diagnosis of STEMI and NSTEMI, based on the 2007 universal definitions (12), was made by each cardiologist. STEMI and NSTEMI were defined as follows: for STEMI, patients had to have chest symptoms, ST-segment elevation in 2 contiguous leads or left bundle branch block, and an elevated biochemical marker of myocardial necrosis (high-sensitivity troponin T > 0.032 ng/mL or creatine phosphokinase [CPK] at least two times the upper limit of normal), whereas for NSTEMI, patients had to have chest symptoms, ST-segment depression or T-wave inversion in 2 contiguous leads, and an elevated biochemical marker of myocardial necrosis. The therapeutic strategies for AMI treatment depended on the practice of each individual cardiologist, but all treatments followed the guidelines set forth by the Japanese Circulation Society and the American College of Cardiology/American Heart Association for the diagnosis and treatment of AMI (13).
Data collection and endpoints
Data collected included clinical characteristics and demographics during initial hospitalization, such as medical history, presenting signs and symptoms, results of blood tests, electrocardiography, cardiac procedures, and clinical outcomes. Transthoracic echocardiography was also carried out during index hospitalization and at around 6 months after AMI, and LVEF was estimated by the standard biplane Simpson method. In addition, all blood biomarkers were measured within 24 h after admission as acute phase data. Clinical follow-up was carried out through clinic visits, telephone calls, and records from hospital admissions.
The primary endpoint was a composite of hospitalization for HF or cardiovascular death occurred after 6 months of AMI. The diagnosis of HF was made based on the latest local guidelines, in which HF is diagnosed by the presence of at least one sign (rales, peripheral edema, ascites, or radiographic evidence of pulmonary congestion) and one symptom (dyspnea, orthopnea or edema), regardless of ejection fraction (14). Cardiovascular death was defined as the primary cause of death determined to be atherosclerotic cardiovascular disease, arrhythmia, heart failure, or sudden cardiac death. The secondary endpoints included the individual components of the primary endpoint and all-cause death.
Statistical analysis
For continuous variables, normally distributed data are reported as the mean ± standard deviation; non-parametric data are reported as the median and interquartile range (IQR). For categorical variables, data are presented as count and percentage. Comparisons of continuous variables between groups were performed with the Wilcoxon-test or Kruskal Wallis tests, as appropriate. Comparisons of categorical variables were assessed with the chi-square or Fisher’s exact test, as appropriate. A paired sample t-test was used to compare LVEF at index hospitalization and 6 months after AMI. LVEF trajectories from index hospitalization for AMI to 6 months post-AMI were demonstrated using parallel plots. Clinical factors associated with LVEF category decline over the 6 months after AMI were assessed by logistic regression analysis adjusting for confounding factors (age, sex, STEMI, Killip class, culprit lesions (left anterior descending artery or left main trunk), use of mechanical support, maximum CPK [natural log-transformed], estimated glomerular filtration rate [eGFR], LVEF during index hospitalization and use of each medication at discharge; angiotensin-converting enzyme inhibitor [ACE-I] or angiotensin II receptor blocker [ARB] and β-blocker). The cumulative incidence of each endpoint was also calculated according to the Kaplan–Meier method, and the effects of LVEF 6 months after AMI on primary and secondary endpoints were determined with a multivariate Cox proportional hazards regression model adjusting for confounding factors (age, sex, STEMI, use of mechanical support, max CPK [natural log-transformed], eGFR, LVEF during index hospitalization and use of each medication at discharge; ACE-I or ARB and β-blocker). Time at risk was defined starting on the day of the 6-month LVEF measurement. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed with JMP® 15 (SAS Institute Inc., Cary, NC, USA).
Results
Patient clinical characteristics during index hospitalization for acute myocardial infarction
Among a total of 2,266 consecutive patients eligible for this study, a total of 936 patients were excluded; thus, a total of 1,330 patients were analyzed (Figure 1). Their background characteristics, procedural information during index hospitalization, and medications at discharge are shown in Table 1. The mean patient age was 67.5 ± 11.9 years, with 74.1% being male. Electrocardiography revealed that 68.5% were STEMI, and almost all patients (95.1%) received primary revascularization (92.3% for PCI). Most patients received standard medical therapies after AMI at discharge.
FIGURE 1
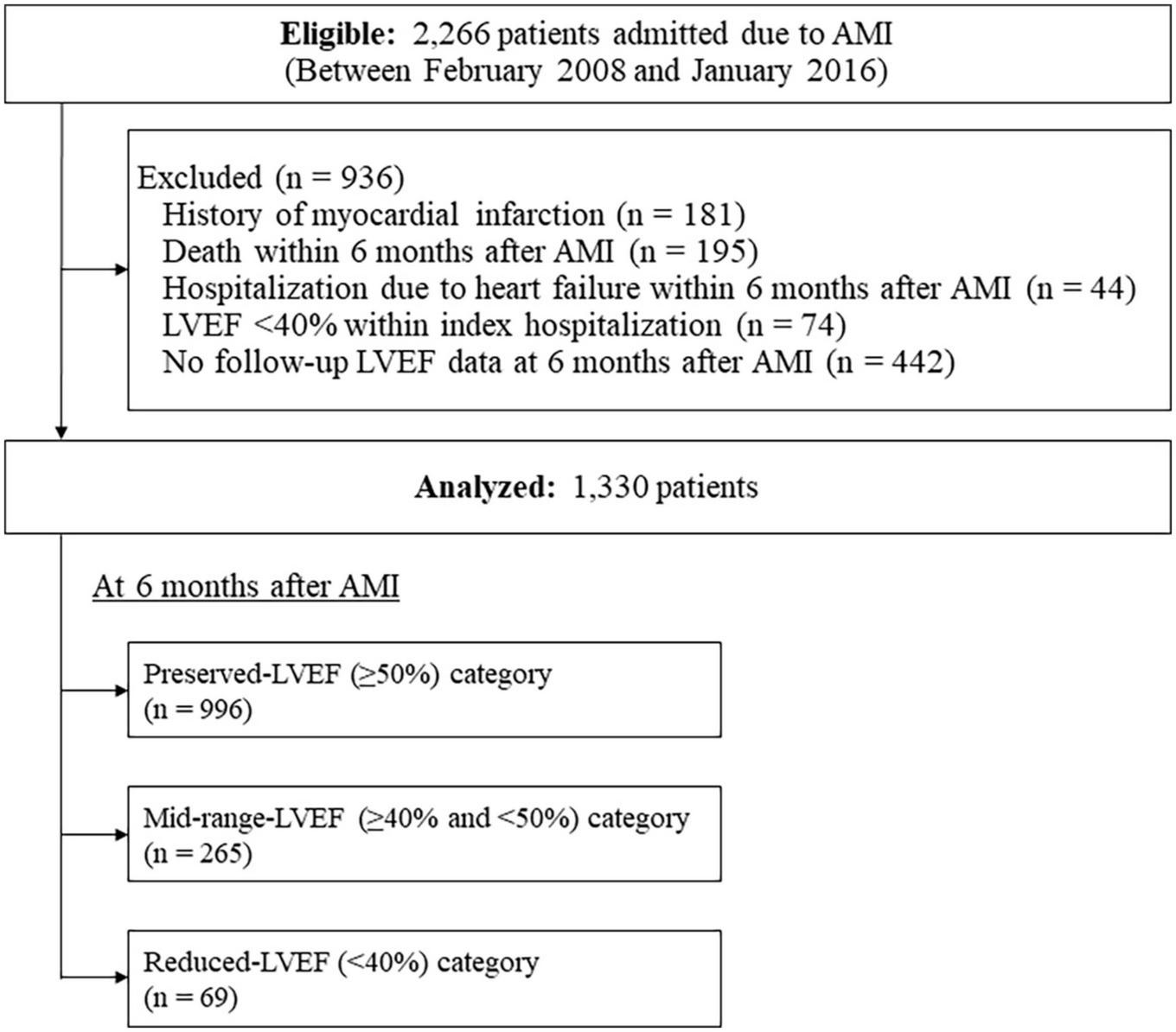
Flow diagram of the study cohort. AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction.
TABLE 1
| Variable | Overall n = 1,330 |
LVEF category 6 months after AMI |
P-value (among LVEF categories) |
||
| Preserved-LVEF (≥ 50%) n = 996 |
Mid-range-LVEF (≥ 40% and < 50%) n = 265 |
Reduced-LVEF (< 40%) n = 69 |
|||
| Age, years | 67.5 ± 11.9 | 67.5 ± 11.9 | 67.7 ± 11.7 | 68.0 ± 11.7 | 0.928 |
| Male | 986 (74.1) | 717 (72.0) | 213 (80.4) | 56 (81.2) | 0.007 |
| Body mass index, kg/m2 | 24.2 ± 3.6 | 24.2 ± 3.8 | 24.0 ± 3.4 | 24.1 ± 3.1 | 0.579 |
| eGFR, mL/min/1.73 m2 | 68.0 ± 22.0 | 68.8 ± 21.3 | 66.5 ± 23.0 | 61.6 ± 26.7 | 0.025 |
| Medical history | |||||
| Hypertension | 928 (69.8) | 713 (71.6) | 165 (62.3) | 50 (72.5) | 0.014 |
| Dyslipidemia | 695 (52.3) | 536 (53.8) | 126 (47.6) | 33 (47.8) | 0.145 |
| Diabetes mellitus | 413 (31.1) | 311 (31.2) | 84 (31.7) | 18 (26.1) | 0.642 |
| STEMI | 911 (68.5) | 649 (65.2) | 213 (80.4) | 49 (71.0) | < 0.001 |
| NSTEMI | 419 (31.5) | 347 (34.8) | 52 (19.6) | 20 (29.0) | |
| Onset-to-admission time, min | 180 (120-420) | 180 (120-420) | 180 (60-420) | 240 (120-870) | 0.108 |
| Delayed arrival (≥ 48 h after onset) | 35 (3.0) | 27 (3.0) | 7 (2.9) | 1 (1.7) | 0.804 |
| Killip class ≥ 3 | 55 (4.2) | 31 (3.1) | 16 (6.0) | 8 (11.6) | 0.003 |
| Culprit lesion | < 0.001 | ||||
| LMT | 27 (2.0) | 18 (1.8) | 8 (3.0) | 1 (1.4) | |
| LAD | 566 (42.6) | 396 (39.7) | 132 (49.8) | 38 (55.1) | |
| LCX | 165 (12.4) | 124 (12.8) | 32 (12.1) | 9 (13.0) | |
| RCA | 483 (36.3) | 383 (38.5) | 86 (32.5) | 14 (20.2) | |
| MVD and others | 89 (6.7) | 75 (8.3) | 7 (2.6) | 7 (10.1) | |
| Revascularization | 1,266 (95.1) | 949 (95.3) | 253 (95.5) | 64 (93.0) | 0.676 |
| PCI | 1,227 (92.3) | 922 (92.6) | 242 (91.3) | 63 (91.3) | 0.765 |
| CABG | 39 (2.8) | 27 (2.7) | 11 (4.2) | 1 (1.4) | 0.481 |
| Mechanical support | 108 (8.1) | 69 (6.9) | 29 (10.9) | 10 (14.5) | 0.045 |
| IABP | 108 (8.1) | 69 (6.9) | 29 (10.9) | 10 (14.5) | 0.045 |
| ECMO | 8 (0.6) | 6 (0.6) | 2 (0.8) | 0 | 0.629 |
| Peak CPK, IU/L | 1,417 (471-3,152) | 1,124 (376-,367) | 3,253 (1,159-5,319) | 2,625 (981-5,764) | < 0.001 |
| Hospital stay, days | 15 (12-19) | 14 (12–) | 17 (14-22) | 17 (14-23) | < 0.001 |
| Medication at discharge | |||||
| ACE-I or ARB | 933 (70.2) | 719 (72.2) | 175 (66.0) | 39 (56.5) | 0.007 |
| β-blocker | 619 (46.5) | 442 (44.4) | 137 (51.7) | 40 (58.0) | 0.016 |
| MRA | 108 (8.1) | 55 (5.5) | 42 (15.9) | 11 (15.9) | < 0.001 |
| Statin | 1,165 (88.0) | 886 (90.0) | 223 (84.2) | 60 (87.0) | 0.113 |
| Antiplatelet | 1,300 (97.8) | 979 (98.3) | 255 (96.2) | 66 (95.7) | 0.181 |
| LVEF during index hospitalization,% | 59.4 ± 9.1 | 61.6 ± 8.4 | 53.6 ± 7.9 | 50.2 ± 7.7 | < 0.001 |
| LVEF at 6 months after AMI,% | 49.0 ± 13.5 | 60.1 ± 6.5 | 45.5 ± 2.7 | 35.3 ± 4.0 | < 0.001 |
Patient background characteristics, procedural information, and medications at discharge.
Data for categorical variables given as number (%); data for continuous variables given as mean ± standard deviation for normal distribution or median (interquartile range) for skewed distribution. ACE-I, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CPK, creatine phosphokinase; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pumping; IU, international units; LAD, left anterior descending artery; LCX, left circumflex artery; LMT, left main trunk; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; MVD, multi-vessel disease; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-elevation myocardial infarction.
Left ventricular ejection fraction trajectories from index hospitalization for acute myocardial infarction to 6 months post-acute myocardial infarction
Overall, mean LVEF during index hospitalization and 6 months after AMI was 59.4 ± 9.1% and 49.0 ± 13.5% (p < 0.001), respectively (Table 1). The detailed trajectories of LVEF from index hospitalization to 6 months after AMI are shown in Figure 2A. A total of 69 patients (28/1,110 [2.5%] initially in the preserved-LVEF and 41/220 [18.6%] initially in the mid-range-LVEF categories) newly developed reduced-LVEF at 6 months, and a total of 170/1,100 (15.5%) patients initially in the preserved-LVEF category declined to the mid-range-LVEF category at 6 months (Figure 2B). Conversely, a total of 84/220 (38.2%) patients initially in the mid-range-LVEF category climbed from that to the preserved-LVEF category at 6 months. The LVEF categories in the other patients remained unchanged at 6 months after AMI.
FIGURE 2
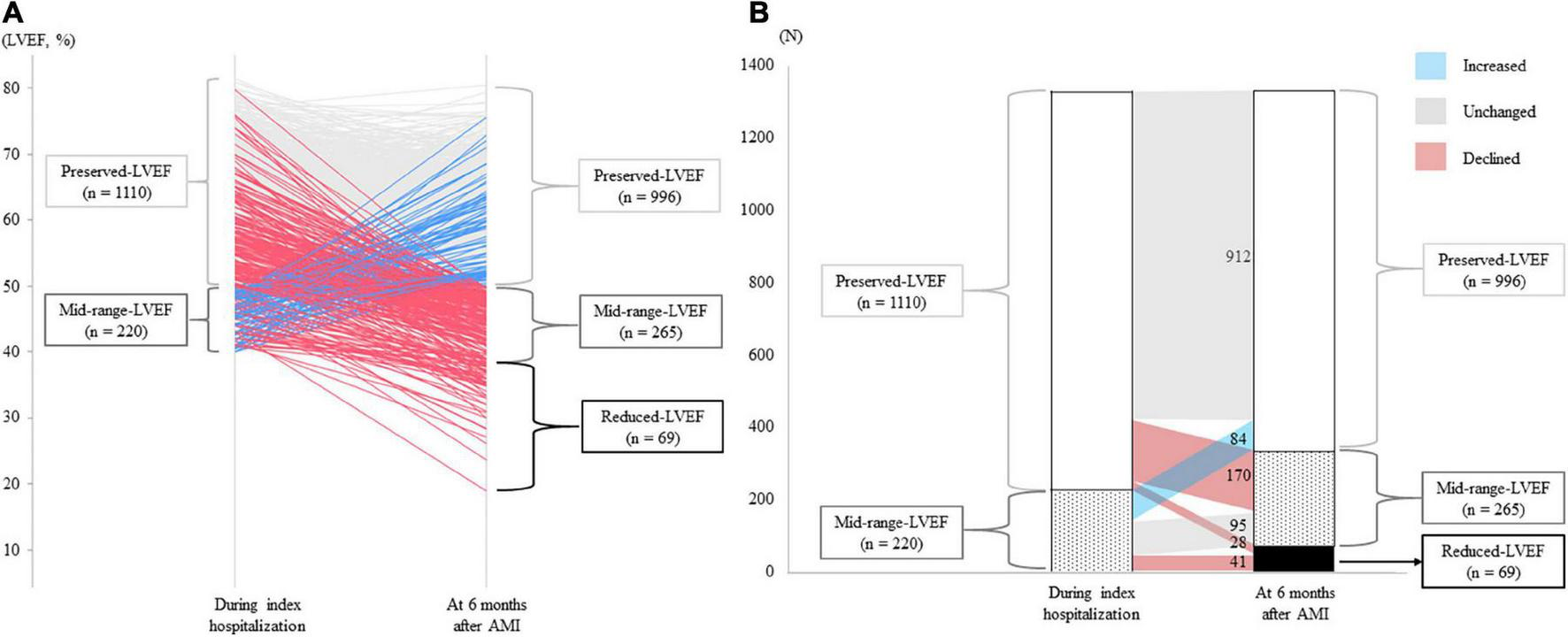
LVEF trajectories from index hospitalization for AMI to 6 months post-AMI. (A) Individual trajectories of LVEF over the 6 months after AMI. Blue, gray, red lines indicate patients with increased, unchanged, and declined LVEF, respectively. (B) Changes in LVEF categories. The numbers in the figure indicate the number of patients whose LVEF category changed or did not change 6 months after AMI. AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction.
Detailed clinical information at the time of index hospitalization in the three subgroups stratified by LVEF category at 6 months after AMI is also provided in Table 1. The subgroups with mid-range- and reduced-LVEF at 6 months after discharge, relative to the preserved-LVEF subgroup, were more likely to have a higher proportion of males, a lower eGFR, and a more severe clinical course of AMI.
The multivariate logistic regression analysis revealed that among 239 patients whose LVEF category declined at 6 months after AMI, male sex and peak CPK were independently associated with the decline, while LVEF during index hospitalization and use of ACE-I or ARB at discharge were inversely associated with a decline (Table 2). Among those factors, the use of ACE-I or ARB at discharge was solely an independent negative predictor of a two-step LVEF category decline (Table 3). Notably, ACE-I or ARB therapy was not an independent predictor of improved LVEF at 6 months, but female sex and LVEF during hospitalization were found to be associated (Supplementary Table 1).
TABLE 2
| Variable | Odds ratio | 95% CI | P-value |
| Age, per 1 year | 1.01 | 0.99-1.03 | 0.075 |
| Male | 1.76 | 1.19-2.63 | 0.005 |
| STEMI | 0.73 | 0.49-1.10 | 0.135 |
| Killip class ≥ 3 | 1.07 | 0.51-2.23 | 0.853 |
| Culprit lesion: LAD or LMT | 1.27 | 0.93-1.74 | 0.131 |
| Use of mechanical support | 0.90 | 0.51-1.60 | 0.735 |
| Peak CPK, ln U/L | 1.64 | 1.40-1.93 | < 0.001 |
| eGFR, per 1 mL/min/1.73 m2 | 0.99 | 0.99-1.00 | 0.205 |
| LVEF during index hospitalization, per 1% | 0.96 | 0.94-0.98 | < 0.001 |
| Use of ACE-I or ARB at discharge | 0.62 | 0.44-0.86 | 0.004 |
| Use of β-blocker at discharge | 1.10 | 0.80-1.51 | 0.548 |
Logistic regression analysis to identify clinical factors associated with LVEF category decline over the 6 months after AMI.
CI, confidence interval; other abbreviations, see Table 1.
TABLE 3
| Variable | One-step LVEF category decline* |
Two-step LVEF category decline** |
||||
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age, per 1 year | 1.01 | 0.99-1.03 | 0.071 | 1.00 | 0.97-1.04 | 0.897 |
| Male | 1.77 | 1.16-2.69 | 0.008 | 1.42 | 0.51-4.00 | 0.503 |
| STEMI | 0.82 | 0.53-1.26 | 0.359 | 0.47 | 0.18-1.23 | 0.125 |
| Killip class ≥ 3 | 1.09 | 0.50-2.34 | 0.833 | 0.93 | 0.18-4.81 | 0.935 |
| Culprit lesion: LAD or LMT | 1.23 | 0.88-1.70 | 0.222 | 1.39 | 0.63-3.08 | 0.421 |
| Use of mechanical support during procedures | 0.74 | 0.40-1.36 | 0.328 | 2.28 | 0.76-6.88 | 0.142 |
| Peak CPK, ln U/L | 1.63 | 1.37-1.93 | < 0.001 | 1.45 | 0.98-2.16 | 0.057 |
| eGFR, per 1 mL/min/1.73 m2 | 0.99 | 0.99-1.00 | 0.158 | 0.99 | 0.98-1.02 | 0.843 |
| LVEF during index hospitalization, per 1% | 0.96 | 0.94-0.98 | < 0.001 | 0.99 | 0.95-1.04 | 0.696 |
| Use of ACE-I or ARB at discharge | 0.70 | 0.49-0.99 | 0.044 | 0.39 | 0.17-0.86 | 0.020 |
| Use of β-blocker at discharge | 1.07 | 0.77-1.49 | 0.697 | 1.23 | 0.55-2.76 | 0.613 |
Logistic regression analysis to identify clinical factors associated with a decline in LVEF category over the 6 months after AMI.
Clinical endpoints
The median (interquartile range) duration of follow-up 6 months after AMI was 3.0 (1.5–4.8) years. Overall, the primary composite endpoint of hospitalization for HF or cardiovascular death occurred in 35/1,330 (2.6%) patients (13/996 [1.3%] in the preserved-LVEF, 9/265 [3.4%] in the mid-range-LVEF, and 13/69 [18.8%] in the reduced-LVEF categories, Log-rank p < 0.001); individual components of the primary composite endpoint occurred in 21/1,330 (1.6%) patients for hospitalization for HF and 19/1,330 (1.4%) patients for cardiovascular death (Table 4). The adjusted hazard ratio (HR) for the primary endpoint in the reduced-LVEF vs. mid-range-LVEF categories and in the reduced-LVEF vs. preserved-LVEF categories was 4.71 (95% confidence interval [CI], 1.83 to 12.13; p < 0.001) and 14.37 (95% CI, 5.38 to 38.36; p < 0.001), respectively (Figure 3A). These were almost consistent across the individual components of primary composite endpoint; hospitalization for HF and cardiovascular death (Figures 3B,C). All-cause death occurred in 50/1,330 (3.8%) patients in the overall cohort (23/996 [2.3%] in the preserved-LVEF, 15/265 [5.7%] in the mid-range-LVEF, and 12/69 [17.4%] in the reduced-LVEF categories, Log-rank p < 0.001). The adjusted HR for all-cause death in the reduced-LVEF vs. mid-range-LVEF categories and in the reduced-LVEF vs. preserved-LVEF categories was 2.16 (95% CI, 0.89 to 5.25; p = 0.087) and 6.13 (95% CI, 2.38 to 15.81; p < 0.001), respectively (Figure 3D).
TABLE 4
| Outcomes | Overall n = 1,330 |
LVEF category 6 months after AMI |
P-value (Log-rank) |
||
| Preserved-LVEF (≥ 50%) n = 996 |
Mid-range-LVEF (≥ 40% and < 50%) n = 265 |
Reduced-LVEF (< 40%) n = 69 |
|||
| Composite outcome | 35 (2.6) | 13 (1.3) | 9 (3.4) | 13 (18.8) | < 0.001 |
| Hospitalization for heart failure | 21 (1.6) | 7 (0.7) | 6 (2.3) | 8 (11.6) | < 0.001 |
| Cardiac death | 19 (1.4) | 6 (0.6) | 5 (1.9) | 8 (11.6) | < 0.001 |
| All-cause death | 50 (3.8) | 23 (2.3) | 15 (5.7) | 12 (17.4) | < 0.001 |
Clinical events.
Data are shown as number (%). Abbreviations, see Table 1.
FIGURE 3
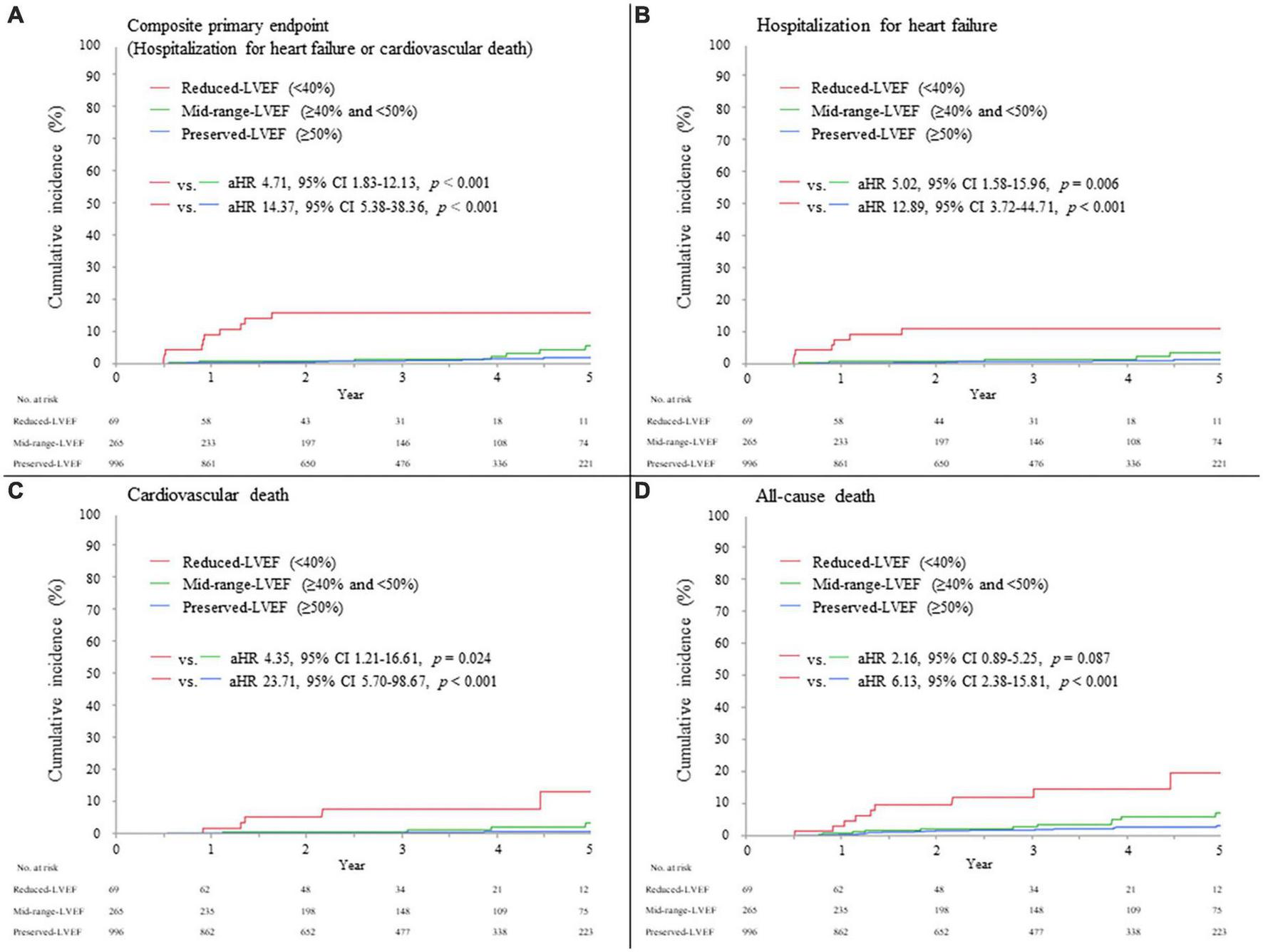
Clinical events during follow-up, according to LVEF category 6 months after AMI. (A) Composite primary endpoint (hospitalization for heart failure or cardiovascular death). (B) Hospitalization for heart failure. (C) Cardiovascular death. (D) All-cause death. The hazard ratio was adjusted by age, sex, STEMI, maximum creatine phosphokinase (natural log-transformed), LVEF during index hospitalization, eGFR, use of mechanical support, and use of each medication (ACE-I, ARB, and β-blockers) at discharge. ACE-I, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; aHR, adjusted hazard ratio; ARB, angiotensin II receptor blocker; CI, confidence interval; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction.
Discussion
This is the report to demonstrate the clinical features of chronic transit of LVEF and incident LV systolic dysfunction at the chronic phase of AMI and its long-term prognostic impact in AMI survivors. Our findings underscore the clinical importance of monitoring LVEF through the post-AMI phases, even in survivors without LV systolic dysfunction at the acute phase of AMI.
In the past two decades, widespread technical innovations in primary coronary revascularization for AMI have dramatically increased the number of AMI survivors. Accordingly, an increased risk of HF and mortality at the post-AMI phase has become an emerging clinical issue of concern, urgently requiring accurate and reliable risk stratification to predict such remote-phase adverse events (15). Traditionally, some risk prediction models, such as GRACE and TIMI, both of which consist of indicators obtained at the acute phase of AMI, have been universally used to predict the prognosis of patients with AMI (1, 2). On the other hand, such indicators obtained during the acute phase are highly variable, depending on the individual clinical situation and the course of treatment during the acute to post-AMI phases. Therefore, risk stratification based on the clinical index obtained at the chronic phase and its change from the acute to the chronic phase may contribute to the improvement of a longer-term prognostic ability for patients who have experienced AMI. However, there are few studies on long-term prognostic prediction based on the clinical data obtained at the chronic phase.
LVEF is an established indicator of LV systolic function, and LV systolic dysfunction (reduced LVEF) at the acute phase of AMI is also well-recognized as an independent predictor of adverse outcomes (16, 17). However, it is still clinically controversial whether the sole use of LVEF measured only at the acute phase is sufficient to predict the long-term prognosis (18). Moreover, substantial patients often exhibit mildly reduced- or preserved-LVEF immediately following AMI, and the majority of adverse events after AMI develop in that patient population without overt LV dysfunction at the acute-phase (19, 20). Therefore, it is clinically important to assess the mechanism by which patients without LV systolic dysfunction at the acute phase of AMI develop adverse events at the remote phase. In this context, we hypothesized that LVEF at the chronic phase would be a predictor of subsequent events in survivors with preserved LVEF at the acute phase, and then evaluated the long-term prognostic impact according to LVEF at the chronic phase and its trajectory from the acute to the chronic phases.
After the onset of AMI, immediate coronary revascularization and subsequent optimal medical therapies help to prevent adverse LV remodeling and thereby improve LV systolic function. To date, several studies of subjects with reduced LVEF at the time of AMI have demonstrated that chronic LVEF recovery was associated with better outcomes in comparison with survivors without LVEF recovery (11, 21). Chew et al. followed patients with only reduced EF during the acute phase of myocardial infarction (22). They demonstrated that the absence of LVEF recovery is associated with an increased risk of death. This suggests that patterns of chronic change in LVEF following AMI can further discriminate AMI survivors who are at increased risk of death. However, it is uncertain how the LVEF status in patients with preserved LVEF at the time of AMI transitions over time. Further, the clinical characteristics of the trajectory pattern of LVEF are unknown. Compared to a study in which AMI patients with preserved LVEF in the acute phase were excluded, the present study excluded AMI patients with reduced LVEF in the acute phase, and focused on chronic changes in AMI patients with preserved LVEF.
Our findings underscore that careful post-AMI reassessments are required to monitor the LVEF trajectory and identify potential patients who need additional medical and/or device therapies, even in patients with preserved LVEF at the time of AMI. However, despite guideline-directed recommendations (23, 24), previous studies have shown that the frequency of post-AMI LVEF reassessment was relatively low in patients with LV systolic dysfunction at the time of AMI (25, 26). A recent cohort study from Canada also demonstrated that approximately 1 in 3 patients with mildly reduced LVEF following AMI did not undergo LVEF reassessment within 6 months after AMI (27). The low frequency of post-AMI LVEF reassessment indicates a missed opportunity for appropriate care, especially for LV systolic dysfunction. Importantly, few data on the rate of post-AMI LVEF reassessment in patients with preserved LVEF at the time of AMI are currently available, and it is likely even less frequent for such patients. In addition, given our findings that incident LV systolic dysfunction 6 months after AMI was associated with poor outcomes, improvements in the quality of post-AMI management are urgently needed, including post-AMI LVEF reassessment, irrespective of LVEF status at index AMI.
The development of HF remains a major issue in AMI patients. Several clinical features, such as elevated levels of natriuretic peptides and a clinically severe AMI disease course are known to be risk factors (28). Delayed arrival causes a delay in reperfusion therapy, which often results in a larger infarct size and increased risk of HF (29). In the present study cohort, the frequency of delayed arrival after AMI onset in the subgroup with EF ≥ 40% and < 50% at 6 months after AMI was higher than that in the subgroup with reduced LVEF (< 40%) at 6 months. This might be associated with the higher peak CPK levels in the former subgroup. Several previous reports have addressed the potential risk predictors for the occurrence of early- and late-onset HF after AMI. However, the factors were diverse (30), and no specific factor was identified for either early- or late-onset HF (31, 32). In terms of echocardiographic parameters, there have been also several reports on the evaluation of chronic LVEF at a single point in time and the development of HF (33). However, LVEF dynamics and the assessment of late-onset HF according to their trajectories in the remote phase of AMI have not yet been fully studied.
In the present study, we found that the prevalence of incident LV systolic dysfunction 6 months after AMI was 5.2% among AMI survivors without LV systolic dysfunction at the time of AMI, and such patients were associated with poor long-term prognosis compared to subjects without it. This indicates the clinical need for early identification of patients at risk for LVEF decline during the chronic phase of AMI. In this context, male sex, peak CPK level, LVEF at the time of AMI, and use of ACE-I or ARB at discharge were independent predictors of LVEF decline 6 months after AMI. In particular, the use of ACE-I or ARB at discharge was solely an independent negative predictor of a two-step decline in LVEF category. In the previous PREAMI (Perindopril and Remodeling in Elderly With Acute Myocardial Infarction) study, Ferrari et al. also demonstrated that 12 months of ACE-I perindopril therapy rescued adverse LV remodeling in elderly patients with a LVEF 40% or more following AMI (34). This is likely comparable to our findings from multivariate regression analyses. Although no relationship between the prevention of adverse LV remodeling and better clinical outcomes was observed in that study, the short observation period (12 months) might have affected the outcome. Compared to that study, the strength of our study is that we reassessed the LVEF 6 months after AMI and then had a longer follow-up period (median 3 years). On the other hand, Park et al. reported that the dose of ARB had no impact on LV remodeling in patients with mid-range LVEF following AMI (35). Taken together, these findings suggest the importance of administering ACE-I or ARB, even in the absence of LV systolic dysfunction immediately after AMI.
Limitations
Some limitations must be taken into account. First, this was a retrospective, observational study carried out in a relatively small number of subjects at a single center, which limits the generalizability of our findings. It should also be noted that primary coronary revascularization and subsequent oral medication delivery were performed based on the latest local treatment guidelines. However, decision-making regarding hospitalization for HF was the choice of each physician; therefore, relevant endpoints were partly based on physicians’ subjective judgment. Second, because the study cohort included only survivors 6 months after AMI to collect remote data on LVEF, a potential selection bias should be noted. Accordingly, the occurrence of composite clinical events (hospitalization for HF and cardiovascular death) was low (2.3%) during the follow-up duration. Additionally, patients with reduced LVEF at 6 months already had worsening of some clinical indicators, such as lower EF and eGFR levels and a higher proportion of patients with Killip class ≥ 3, at index hospitalization, and this patient subgroup was therefore not entirely representative of the overall cohort. Third, our study cohort included both STEMI and NSTEMI, with two-thirds of subjects showing STEMI; this rate is higher relative to a contemporary cohort for AMI in Japan (36, 37). The prognostic impact of LVEF at the chronic phase was not investigated separately between STEMI and NSTEMI due to the limited small sample size in our cohort. Fourth, the rate of prescribing optimal drug therapy after AMI was lower than expected in our cohort. Specifically, a relatively small proportion of subjects was treated with β-blockers due mainly to tolerability, and this was similar to previous reports in Japan (36, 37). However, we cannot exclude the possibility that such incomplete implementation of optimal medical therapy after AMI might have affected the patients’ prognosis and our findings. Finally, the present analysis did not account for any clinical information that may have affected long-term prognosis in survivors of AMI, including biomarkers, at the chronic phase other than LVEF. Therefore, further studies are needed to investigate the clinical parameters related to LVEF dynamics and assess the prognostic relationships between their trajectories in the remote phase of AMI.
Conclusion
Our findings suggest that incident LV systolic dysfunction at the chronic phase after AMI was significantly associated with long-term adverse outcomes. Therefore, even in AMI survivors without LV systolic dysfunction at the time of AMI, post-AMI reassessment and careful monitoring of LVEF are required to identify patients at risk. Patient with risk factor, such as male sex and higher peak CPK should be followed more carefully.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of Miyazaki Medical Association Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GY and AT designed the research, project conception, development of overall research plan, study oversight, and wrote the manuscript. GY, KNi, NW, YS, and MN conducted the research, hands-on conduct of the experiments, and data collection. GY, AT, and AK analyzed the data or performed statistical analysis. MN and KNo revised the manuscript. GY and AT had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant Number: JP21K08130 and Takeda Science Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1009691/full#supplementary-material
References
1.
Granger CB Goldberg RJ Dabbous O Pieper KS Eagle KA Cannon CP et al Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern. Med. (2003) 163:2345–53. 10.1001/archinte.163.19.2345
2.
Abu-Assi E Ferreira-Gonzalez I Ribera A Marsal JR Cascant P Heras M et al Do GRACE (Global Registry of Acute Coronary events) risk scores still maintain their performance for predicting mortality in the era of contemporary management of acute coronary syndromes? Am Heart J. (2010) 160:826-834.e1-3. 10.1016/j.ahj.2010.06.053
3.
Boersma E Mercado N Poldermans D Gardien M Vos J Simoons ML . Acute myocardial infarction.Lancet. (2003) 361:847–58. 10.1016/S0140-6736(03)12712-2
4.
Nishihira K Yoshioka G Kuriyama N Ogata K Kimura T Matsuura H et al Impact of frailty on outcomes in elderly patients with acute myocardial infarction who undergo percutaneous coronary intervention. Eur Heart J Qual Care Clin Outcomes. (2021) 7:189–97. 10.1093/ehjqcco/qcaa018
5.
Yoshioka G Tanaka A Nishihira K Shibata Y Node K . Prognostic impact of serum albumin for developing heart failure remotely after acute myocardial infarction.Nutrients. (2020) 12:2637. 10.3390/nu12092637
6.
Goriki Y Yoshioka G Natsuaki M Shinzato K Nishihira K Kuriyama N et al Simple risk-score model for in-hospital major bleeding based on multiple blood variables in patients with acute myocardial infarction. Int J Cardiol. (2022) 346:1–7. 10.1016/j.ijcard.2021.11.046
7.
Goriki Y Tanaka A Nishihira K Kawaguchi A Natsuaki M Watanabe N et al A novel predictive model for in-hospital mortality based on a combination of multiple blood variables in patients with ST-segment-elevation myocardial infarction. J Clin Med. (2020) 9:852. 10.3390/jcm9030852
8.
Shimizu A . What are the most useful predictors of cardiac mortality in patients post myocardial infarction?Circ J. (2013) 77:319–20. 10.1253/circj.CJ-12-1542
9.
Gajarsa JJ Kloner RA . Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities.Heart Fail Rev. (2011) 16:13–21. 10.1007/s10741-010-9181-7
10.
Nienhuis MB Ottervanger JP Dambrink JH de Boer MJ Hoorntje JC Gosselink AT et al Comparative predictive value of infarct location, peak CK, and ejection fraction after primary PCI for ST elevation myocardial infarction. Coron Artery Dis. (2009) 20:9–14. 10.1097/MCA.0b013e32831bd875
11.
Chew DS Heikki H Schmidt G Kavanagh KM Dommasch M Bloch Thomsen PE et al Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol. (2018) 4:672–82. 10.1016/j.jacep.2017.12.015
12.
Thygesen K Alpert JS White HD . Universal definition of myocardial infarction.J Am Coll Cardiol. (2007) 50:2173–95. 10.1016/j.jacc.2007.09.011
13.
O’Gara PT Kushner FG Ascheim DD Casey DE Jr. Chung MK de Lemos JA et al 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. (2013) 127:e362–425.
14.
Ponikowski P Voors AA Anker SD Bueno H Cleland JGF Coats AJS et al 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. 10.1093/eurheartj/ehw128
15.
Smith LN Makam AN Darden D Mayo H Das SR Halm EA et al Acute myocardial infarction readmission risk prediction models: a systematic review of model performance. Circ Cardiovasc Qual Outcomes. (2018) 11:e003885. 10.1161/CIRCOUTCOMES.117.003885
16.
Halkin A Stone GW Dixon SR Grines CL Tcheng JE Cox DA et al Impact and determinants of left ventricular function in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction. Am J Cardiol. (2005) 96:325–31. 10.1016/j.amjcard.2005.03.069
17.
Solomon SD Skali H Anavekar NS Bourgoun M Barvik S Ghali JK et al Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation. (2005) 111:3411–9. 10.1161/CIRCULATIONAHA.104.508093
18.
Dagres N Hindricks G . Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death?Eur Heart J. (2013) 34:1964–71. 10.1093/eurheartj/eht109
19.
Gorgels AP Gijsbers C de Vreede-Swagemakers J Lousberg A Wellens HJ . Out-of-hospital cardiac arrest–the relevance of heart failure. The maastricht circulatory arrest registry.Eur Heart J. (2003) 24:1204–9. 10.1016/S0195-668X(03)00191-X
20.
Mäkikallio TH Barthel P Schneider R Bauer A Tapanainen JM Tulppo MP et al Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. (2005) 26:762–9. 10.1093/eurheartj/ehi188
21.
Parodi G Memisha G Carrabba N Signorini U Migliorini A Cerisano G et al Prevalence, predictors, time course, and long-term clinical implications of left ventricular functional recovery after mechanical reperfusion for acute myocardial infarction. Am J Cardiol. (2007) 100:1718–22. 10.1016/j.amjcard.2007.07.022
22.
Chew DS Wilton SB Kavanagh K Southern DA Tan-Mesiatowsky LE Exner DV . Left ventricular ejection fraction reassessment post-myocardial infarction: current clinical practice and determinants of adverse remodeling.Am Heart J. (2018) 198:91–6. 10.1016/j.ahj.2017.11.014
23.
O’Gara PT Kushner FG Ascheim DD Casey DE Jr. Chung MK de Lemos JA et al 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. (2013) 127:529–55.
24.
Ibanez B James S Agewall S Antunes MJ Bucciarelli-Ducci C Bueno H et al 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2018) 39:119–77.
25.
Miller AL Gosch K Daugherty SL Rathore S Peterson PN Peterson ED et al Failure to reassess ejection fraction after acute myocardial infarction in potential implantable cardioverter/defibrillator candidates: insights from the translational research investigating underlying disparities in acute myocardial infarction patients’ health status (TRIUMPH) registry. Am Heart J. (2013) 166:737–43. 10.1016/j.ahj.2013.07.019
26.
Pokorney SD Miller AL Chen AY Thomas L Fonarow GC de Lemos JA et al Reassessment of cardiac function and implantable cardioverter-defibrillator use among medicare patients with low ejection fraction after myocardial infarction. Circulation. (2017) 135:38–47. 10.1161/CIRCULATIONAHA.116.022359
27.
Wilton SB Bennett MT Parkash R Kavanagh K Jolicoeur EM Halperin F et al Variability in reassessment of left ventricular ejection fraction after myocardial infarction in the acute myocardial infarction quality assurance canada study. JAMA Netw Open. (2021) 4:e2136830. 10.1001/jamanetworkopen.2021.36830
28.
Haeck JD Verouden NJ Kuijt WJ Koch KT Van Straalen JP Fischer J et al Comparison of usefulness of N-terminal pro-brain natriuretic peptide as an independent predictor of cardiac function among admission cardiac serum biomarkers in patients with anterior wall versus nonanterior wall ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. (2010) 105:1065–9. 10.1016/j.amjcard.2009.12.003
29.
De Luca G Suryapranata H Ottervanger JP Antman EM . Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts.Circulation. (2004) 109:1223–5. 10.1161/01.CIR.0000121424.76486.20
30.
López de Sá E . [Late-onset heart failure following myocardial infarction: putting the jigsaw together].Rev Esp Cardiol. (2005) 58:1258–60. 10.1016/S1885-5857(06)60411-8
31.
Choi H Seo JY Shin J Choi BY Kim YM . A long-term incidence of heart failure and predictors following newly developed acute myocardial infarction: a 10 years retrospective cohort study with Korean national health insurance data.Int J Environ Res Public Health. (2021) 18:6207. 10.3390/ijerph18126207
32.
Sulo G Igland J Nygård O Vollset SE Ebbing M Cerqueira C et al Trends in the risk of early and late-onset heart failure as an adverse outcome of acute myocardial infarction: a cardiovascular disease in Norway project. Eur J Prev Cardiol. (2017) 24:971–80. 10.1177/2047487317698568
33.
Huang H Liu J Lei M Yang Z Bao K Li Q et al A universal new definition of heart failure with improved ejection fraction for patients with coronary artery disease. Front Physiol. (2021) 12:770650. 10.3389/fphys.2021.770650
34.
Ferrari R . Effects of angiotensin-converting enzyme inhibition with perindopril on left ventricular remodeling and clinical outcome: results of the randomized Perindopril and Remodeling in Elderly with Acute Myocardial Infarction (PREAMI) Study.Arch Intern Med. (2006) 166:659–66. 10.1001/archinte.166.6.659
35.
Park K Kim YD Kim KS Lee SH Park TH Lee SG et al The impact of a dose of the angiotensin receptor blocker valsartan on post-myocardial infarction ventricular remodelling. ESC Heart Fail. (2018) 5:354–63. 10.1002/ehf2.12249
36.
Miyachi H Takagi A Miyauchi K Yamasaki M Tanaka H Yoshikawa M et al Current characteristics and management of ST elevation and non-ST elevation myocardial infarction in the Tokyo metropolitan area: from the Tokyo CCU network registered cohort. Heart Vessels. (2016) 31:1740–51. 10.1007/s00380-015-0791-9
37.
Natsuaki M Morimoto T Watanabe H Nakagawa Y Furukawa Y Kadota K et al Ischemic and bleeding risk after percutaneous coronary intervention in patients with prior ischemic and hemorrhagic stroke. J Am Heart Assoc. (2019) 8:e013356. 10.1161/JAHA.119.013356
Summary
Keywords
acute myocardial infarction, left ventricular ejection fraction, left ventricular systolic dysfunction, prognosis, reassessment
Citation
Yoshioka G, Tanaka A, Watanabe N, Nishihira K, Natsuaki M, Kawaguchi A, Shibata Y and Node K (2022) Prognostic impact of incident left ventricular systolic dysfunction after myocardial infarction. Front. Cardiovasc. Med. 9:1009691. doi: 10.3389/fcvm.2022.1009691
Received
02 August 2022
Accepted
14 September 2022
Published
29 September 2022
Volume
9 - 2022
Edited by
Gianluca Rigatelli, Hospital Santa Maria della Misericordia of Rovigo, Italy
Reviewed by
Ratko Lasica, Clinical Center of Serbia, University of Belgrade, Serbia; Olof Gidlöf, Lund University, Sweden
Updates
Copyright
© 2022 Yoshioka, Tanaka, Watanabe, Nishihira, Natsuaki, Kawaguchi, Shibata and Node.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Goro Yoshioka, s04211090s@gmail.comAtsushi Tanaka, tanakaa2@cc.saga-u.ac.jp
This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.