Abstract
Advances in tumor diagnosis and treatment, especially the use of targeted therapies, have remarkably improved the survival rate of patients with renal cell carcinoma (RCC), accompanied by higher hypertension (HTN) incidence among patients with RCC, reflecting the coming of a cardio-oncologic era. Therefore, for patients with RCC and HTN simultaneously, finding risk factors for the comorbidity and giving better clinical treatment have been urgent problems. In this review, we thoroughly investigated risk factors for the comorbidity of HTN and RCC based on preclinical and clinical studies. Firstly, RCC and HTN may have common risk factors, such as obesity, smoking, and other modifiable lifestyles. Secondly, RCC and HTN may lead to each other directly or indirectly by their therapies. We then discussed measures of reducing the comorbidity and treatment of HTN in patients with RCC. We also discussed the deficiency of current studies and pointed out future directions. In conclusion, this review aims to deepen the understanding of cardio-oncology and bring benefit to the population who are at high risk of getting or have already got RCC and HTN simultaneously.
Introduction
The prevalence of hypertension (HTN) and renal cell carcinoma (RCC) keeps increasing. In 2019, one-third of people between 30 and 70 years old were estimated to have HTN globally and the number has doubled from 648 to 1.2 billion in the past 3 decades (1). HTN was the most frequent comorbidity with malignant tumors, seen in 38% of patients with cancer (2). RCC accounted for about 90% of renal malignancies (3). According to GLOBOCAN in 2020, the patients with kidney cancer were more than 1.2 million and new cases were estimated to be 431,288 globally (4). RCC prevalence in the United States was increasing owing to a higher incidence which had doubled compared with the incidence in 1975 (15.6 vs. 7.1 per 100,000 persons) and longer 5-year relative survival (75.6 vs. 52.3%), reported by the SEER program (5).
Since the prevalence of HTN and RCC is increasing, patients with RCC and HTN simultaneously are estimated to increase for the following reasons: HTN is a potential risk factor for RCC (6) and RCC can cause HTN due to paraneoplastic syndrome (7), nephrectomy (8), and targeted therapies (9). Besides, prolonged survival rates and modern lifestyles may increase the comorbidity of HTN and RCC (10). The above-mentioned situation raised our questions: (1) What are the risk factors for the comorbidity of HTN and RCC in cardio-oncologic era? (2) How to decrease the comorbidity of HTN and RCC? (3) How to give better antihypertensive treatment for the patients with RCC with HTN? To answer these questions, we did a thorough search and reviewed the relationship between HTN and RCC based on clinical evidence and basic researches (Figure 1).
Figure 1
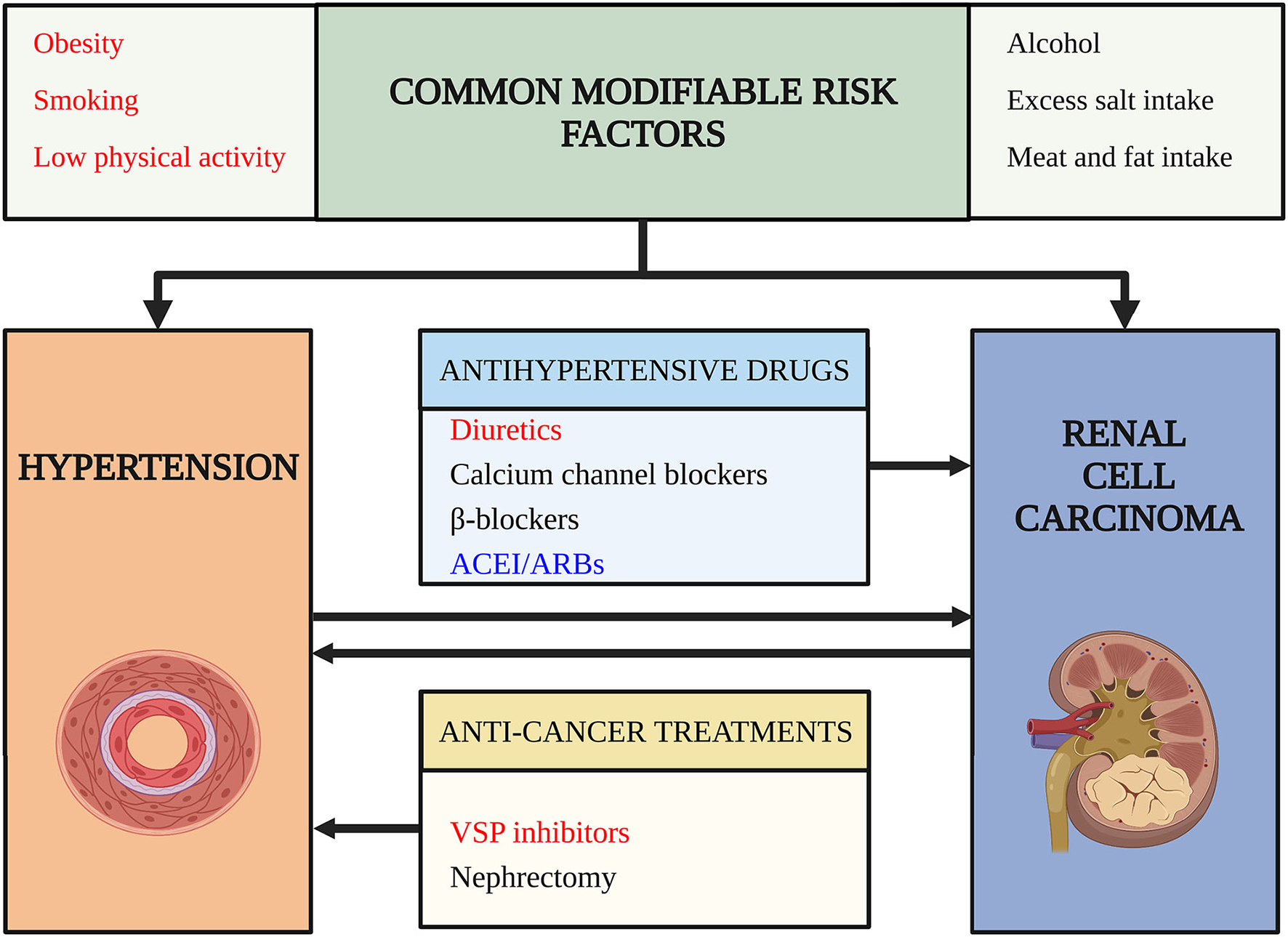
Risk factors for comorbidity of hypertension (HTN) and renal cell carcinoma (RCC). This figure outlines risk factors for the comorbidity of hypertension and renal cell carcinoma. Arrows indicate a potential causality relationship. Words in red color highlight risk factors confirmed by high-level evidence and have achieved consensus. Words in black color indicate risk factors lack strong evidence or the evidence are still conflictive. Words in blue indicate risk factors that may decrease the risk of comorbidity and have protective roles. ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockades; VSP, vascular endothelial growth factor signaling pathway.
Methods
A literature review of publications about RCC and HTN has been performed. A manuscript outline was formed before searching for relevant publications. PubMed (1946–2021) and Cochrane Library (1996–2021) were employed as the source of initial searches. Hand searching was also used to find relevant studies in PubMed and other websites (e.g., FDA and SEER). Besides, valuable publications recommended by experts were included as well. Key search words include HTN, antihypertensive agents, and kidney neoplasms. Detailed search queries and search results are available (Supplementary Material). In total, 7,279 studies were found. These studies were screened for eligibility using title and abstract. The remaining studies were then retrieved as full texts and checked with inclusion and exclusion criteria. We considered studies that were related to: (1) epidemiology about RCC or HTN; (2) risk factors causing RCC or HTN; (3) mechanisms for the formation of RCC or HTN; (4) treatment of HTN in patients with RCC. We excluded studies that were: (1) not in English; (2) duplicate; (3) clinical studies with similar results but lower evidence level or out of date; and (4) could not find full text. The review process is conducted independently by 3 authors. Discrepancies were solved by consensus.
Common Modifiable Risk Factors
Obesity, inadequate physical activity and alcohol are well-known dose-dependent risk factors for HTN (11). The relationship between smoking and HTN is complex, but it is certain that cessation of smoking can dramatically reduce the cardiovascular disease burden (12). It is noteworthy that obesity, smoking, and inadequate physical activity are also risk factors for RCC (13) while alcohol exerts a protective effect on RCC development (14). Besides, diets play important roles in HTN and RCC. For example, excess salt intake increases blood pressure (BP) (11), whereas heavy meat and fatty food are risk factors for RCC, and lack of vegetables or fruits may also increase RCC incidence (15), but the common unhealthy diets for both RCC and HTN still need further study. In summary, obesity, smoking, and inadequate physical activity are common modifiable risk factors for RCC and HTN, we will discuss the clinical evidence and potential mechanisms below.
Obesity as a Risk Factor for RCC
A meta-analysis of 24 cohort studies showed that the relative risk (RR) of kidney cancer was 35% higher (RR = 1.35, 95% CI = 1.27–1.43) in overweight [body mass index (BMI): 25–30] and 76% higher (1.76, 1.61–1.91) in patients of obesity (BMI > 30) compared with the normal weight population (16). Several indicators of obesity were used in clinical studies, such as BMI, waist and hip circumference, and body fat percentage, but the results were consistent (15). A large cohort study demonstrated that per unit increase in BMI will increase 5% risk of RCC (17). Both pre-existing obesity in adulthood and obesity near diagnosis of RCC could increase the risk of renal cancer [odds ratio (OR) = 1.6, same] (18). Of note, a cohort study in Japan demonstrated that low BMI (<21) may also increase the risk of kidney cancer [hazard ratio (HR) = 1.86; 95% CI: 1.01–3.45] compared with BMI of 23.0–24.9 (19). Interestingly, obesity was found to increase the risk of clear-cell RCC while decreasing the risk of papillary RCC (14). Such heterogeneity may be associated with demographic difference considering the fact that papillary RCC is more common in women, the older and the black (20).
Obesity-induced chronic renal hypoxia may play an oncogenic role mainly through upregulating the vascular endothelial growth factor (VEGF) pathway (21). Obesity could cause lipid peroxidation and then facilitate the formation of RCC (22). Obesity-induced renal hyperfiltration may increase the exposure to oncogenic nephrotoxins (23). Increased estrogen in adiposity patients also facilitates RCC by upregulating the insulin-like growth factor-1 (IGF-1) receptor, enhancing the oncogenic influence of IGF-1 (24). Metabolism disorders caused by obesity are also oncogenic. Overexpressed insulin and IGF-1 could promote the formation of RCC. Adiponectin, secreted by fatty tissue, is an anti-angiogenic factor by suppressing the VEGF pathway. However, the serum adiponectin is expressed lower in obesity (15). Besides, increased leptin in obesity, which is a kind of adipokine, promotes RCC by regulating VEGF, the Janus kinase/signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2 pathways (25). Obesity-induced inflammatory response increases levels of interleukin-6, which is also an oncogenic adipokine because it can protect RCC cells from immune attacks (26).
Insulin resistance and increased circulating insulin observed in obesity could induce HTN by increasing renal sodium reabsorption and activating the sympathetic nervous system (27). Elevated leptin can also promote HTN mediated by increasing sympathetic nervous system activity (28). Furthermore, a-melanocyte-stimulating hormone (a-MSH) that is a hormone secreted by melanocytes could regulate BP by suppressing adrenocorticotropic hormone. Interestingly, a-MSH could also inhibit obesity progress by targeting melanocortin 4 receptor. Therefore, it is hypothesized that sunshine may increase levels of a-MSH and then protect obese patients from inflammation-induced HTN (29).
Smoking as a Risk Factor for RCC
A meta-analysis of 24 studies reported that smokers have a higher risk for RCC (RR = 1.38, 95% CI: 1.27–1.50) and a dose-dependent relationship was seen both in men and women (30). Remarkably, passive smoking increases the risk of RCC either (31). Cessation of smoking for more than 30 years reduced 50% risk of RCC (CI: 0.3–0.8), while quitting smoking shorter than 30 years showed no significant difference in RCC incidence (32). A retrospective study revealed that smoking is positively related to increased risk of clear-cell RCC rather than papillary RCC. However, this heterogeneity may be attributable to the skewed distribution of smoking patterns (14).
Some ingredients contained in cigarettes are carcinogenic. Nicotine could induce angiogenesis of RCC, while N-nitrosamines and Benzo[α]pyrene diol epoxide correlate with renal oxidative stress which could lead to DNA damage or gene aberration, thus facilitating the formation of RCC (15). Smoking-related chronic respiratory diseases and carbon monoxide could cause hypoxia of renal tissue (10) and lipid peroxidation is another possible mechanism (22).
Grassi et al. (33) found that smoking-induced acute increase of BP attributes to higher dose of catecholamines at the neuroeffector junctions. In addition, smoking was demonstrated to cause increased arterial wave reflection and stiffness of large arteries, thus enhancing BP (34). Smokers with atherosclerotic renal artery stenosis were more common than non-smokers, and renal vascular stenosis could cause refractory HTN (35).
Low Physical Activity as a Risk Factor for RCC
Low physical activity is regarded as a risk factor for RCC (36–38). An American cohort study of 482,386 participants with a median follow-up of 8.2 years showed that the multivariate RR for those with current exercise more than 4 times per week was 0.77 (95% CI: 0.64–0.92) compared with the never exercise population. Besides, regular exercise and activity in youth is also protective (36). Similarly, a meta-analysis including 19 studies, which was conducted in 2013, showed that adequate physical activity was a protective factor for RCC (RR = 0.88, 95% CI: 0.79–0.97) (37). Physical activity like running or walking had a dose–response relationship with a decreased risk (1.9% risk decline per metabolic equivalents hour/week) of kidney cancer after adjustment for age and sex (38).
Physical activity may decrease the risk of RCC in a directly or indirectly manner. Physical activity could directly inhibit RCC formation by decreasing insulin resistance, circulating IGF-1, and lipid peroxidation (37). Some researchers thought the lack of exercise was an indirect risk factor because a low level of energy consumption could cause obesity and subsequently promotes RCC formation (13). Furthermore, more activity could prevent HTN and diabetes, which are also confounding factors (38).
The mechanisms for inactivity-induced HTN have not been clearly demonstrated. Murine studies showed that insulin resistance and imbalance of sympathetic and vagus nerves are potential reasons (39). Another animal study demonstrated that resistance training could contribute to the regulation of vessel constriction and keep luminal diameter (40). Other factors that explain inactivity-induced HTN include vascular resistance, arterial stiffness, oxidative stress, inflammation, BMI, and endothelial function (41).
HTN as a Direct Risk Factor for RCC
A meta-analysis of 18 prospective studies and 14 case–control studies showed that each 10 mmHg increase of systolic BP (SBP) led to 5% higher risk (95% CI: 1.03–1.06) of RCC and 10 mmHg increase of diastolic BP (DBP) with 7% higher risk (95% CI: 1.04–1.10) (42). However, extremely high pressure (SBP > 150 mmHg or DBP > 100 mmHg) will cause a rapid increase of RCC incidence rather than linear growth (6, 43). It is noteworthy that even high-normal BP (SBP: 130–140 mmHg, DBP: 80–90 mmHg) could increase the risk of RCC (44).
Women with HTN may be more susceptible to RCC. A recent meta-analysis showed that women with HTN have a 54% higher risk than men (RR = 63 vs. 29%), but the difference was substantially reduced (1.40, 1.12–1.74 for men and 1.54, 1.17–2.04 for women) after adjustment for age, cigarette, family history of RCC, obesity, alcohol, and physical activity (42).
Age may influence the incidence of RCC in the patients of HTN while this hypothesis is still controversial. A study suggested that HTN was not an independent risk factor for RCC in adolescence (45), while another study got the opposite conclusion that younger patients with HTN were more likely to develop RCC (44).
It is worth mentioning that HTN may have a synergistic effect with obesity on RCC formation. A prospective study showed that the risk of obesity-caused RCC will increase significantly when BP was very high (SBP > 160 mmHg or DBP > 100 mmHg) (6).
A cohort study conducted in Sweden with a mean follow-up of 16 years among 3,63,992 men using repeated measurements of BP showed that RCC incidence decreased with the reduction of BP and especially, in those with a reduction of more than 14 mmHg in DBP, the RR for RCC decreased 40% (46). Thus, HTN is a modifiable risk factor for RCC and effective control of BP is of great value.
However, some factors may influence the reliability of these researches. HTN shares several common risk factors with RCC (43), which highlights the necessity of sufficient adjustment for these confounding factors during the investigation of the causality between HTN and RCC. Besides, the way of defining and measuring HTN varies (47). Of note, if RCC were diagnosed in patients with HTN in the first several years after enrollment in a cohort, it is difficult to determine the occurrence sequence of RCC and HTN. But such bias could be avoided by excluding data of the first several years of follow-up (46). In conclusion, well-designed prospective studies are warranted to clarify their causality.
As to the mechanisms of HTN-induced RCC, HTN could result in chronic inflammation, making the kidney in a state of hypoxia and then upregulating the expression of hypoxia-inducible factors, causing overexpressed VEGF and platelet-derived growth factors which could facilitate the tumor genesis (2). Overexpressed angiotensin receptors and angiotensin-converting enzyme in the patients with HTN could upregulate the angiotensin II, and cause the overexpression of oncogenic VEGF (42, 48). In addition, HTN is related to dysfunction and remodeling of blood vessels, which could increase the number of reactive oxygen species and eventually promote the formation and progress of tumor (44). Similar to obesity, an increased level of lipid peroxidation in the patients of HTN is supposed to participate in RCC carcinogenesis (22).
Antihypertensive Drugs as a Potential Risk Factor for RCC
In general, antihypertensive drugs are not risk factors for cancers (49). However, a recent cohort study in Korea showed that the use of antihypertensive drugs in patients of HTN was related to increased risk of RCC (HR = 1.74, 95% CI: 1.64–1.84) and those with two or more classes of antihypertensive drugs have an even higher risk (HR = 1.80, 95% CI: 1.69–1.91) without adjusting for HTN (44). Another cohort study supported this result after adjusting for HTN, sex, age, BMI, and smoking (6). There seemed to be a linear relationship between the RCC incidence and the duration of antihypertensive drugs, and the risk will increase 2% per year (95% CI: 1.01–1.02) (50). However, different kinds of antihypertensive drugs play different roles in RCC development. Diuretics have been convincingly shown to be tumorigenic for kidney, while angiotensin-converting enzyme inhibitor/angiotensin receptor blockades (ACEI/ARBs) are possible anti-cancer drugs. The role of calcium channel blockers (CCB) and β-blockers is still in dispute. The tumorigenic role of these antihypertensive drugs will be elaborated below.
Diuretics
Many researchers argued that diuretics are risk factors for RCC (47, 48, 50–53). A systematic review of observational studies in 2020 found that diuretics could increase 34% RCC risk (95% CI: 1.19–1.51) (50). Another meta-analysis showed that the risk effect of diuretics was still significant after adjustment for smoking, obesity, and HTN (47). Several cohort studies and case–control studies drew similar conclusions (48, 51). Women with diuretics (OR = 1.92, 95% CI: 1.59–2.33) seemed to have a higher risk of RCC than men (OR = 1.18, 95% CI: 0.93–1.49) (47). The sexual difference may be explained by the hypothesis that estrogens could intensify the effect of thiazide in the distal tubule, and women consume more diuretics than men (52). Some possible underlying mechanisms may explain the carcinogenic role of diuretics. First, hydrochlorothiazide could be converted in the stomach to nitroso derivatives and cause genetic mutations (53). Second, diuretics may exert a little carcinogenic function on their target, the renal tubular cells (51). More detailed preclinical studies are necessary to clarify the possible tumorigenic mechanisms of diuretics.
Calcium Channel Blockers
The role of CCB on RCC carcinogenesis has not been determined yet (54). In patients without HTN, the use of CCB increased the risk of papillary RCC rather than clear-cell RCC, demonstrated by a retrospective study (55). CCB may predispose the patients with HTN to RCC by impeding DNA fragmentation and cell apoptosis (44). However, other clinical studies showed insignificant results which denied its carcinogenic role (54).
β-Blockers
The role of β-blockers for RCC incidence is less well-studied. A recent cohort study showed that β-blockers have higher HR for RCC than other antihypertensive drugs (44). However, another large cohort study showed that β-blockers may not increase the risk of total cancer incidence (56). Thus, the exact role of β-blockers as a possible cancer-promotor is far from clear.
Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockades
The role of ACEI/ARBs is still in dispute (57). A meta-analysis showed that ACEI increased the risk of RCC (RR = 1.50, 95% CI: 1.01–2.23) (58). ACEI may increase the amount of bradykinin which may facilitate RCC formation (57). Interestingly, ACEI/ARBs are also considered as possible anti-cancer drugs since overexpressed angiotensin receptors and angiotensin II is associated with upregulated VEGF (59).
Even though many clinical studies have managed to clarify the causality between antihypertensive drugs and RCC, there are still no conclusive results because of some limitations. Firstly, it is quite difficult to exclude the effect of HTN per se (10). For example, a large prospective study in 2008 showed that in those with SBP < 160 mmHg or DBP < 100 mmHg, the use of antihypertensive drugs did not show a significant difference compared with non-users while in those with poorly controlled BP, antihypertensive medication increased the risk of RCC, which highlighted the confounding role of HTN (6). Secondly, other confounding factors like age, sex, obesity, smoking, and physical activity are sometimes not adjusted because of the small sample size or poor statistical design. Thus, a well-designed large prospective clinical study is needed to clarify the relationship between antihypertensive drugs and RCC.
RCC Directly Cause HTN
The HTN directly caused by RCC is considered as a manifestation of paraneoplastic syndrome and in the population with malignant HTN, the prevalence of RCC was 1.2%, much higher than those without malignant HTN (0.01%) (7), indicating malignant HTN could be a clue for the diagnosis of RCC. The severity of paraneoplastic HTN varies and can sometimes cause refractory HTN. Most of the paraneoplastic HTN will recover after nephrectomy (60–62).
Tumor compression, renal arteriovenous fistula, and ureteral obstruction could cause renal ischemia, thus activating the rein-angiotensin-aldosterone system, leading to HTN (63, 64). Besides, ectopic hormones secretion, such as catecholamines, erythropoietin correlated with paraneoplastic HTN (7, 60). Hypercalcemia, which increased vascular resistance or indirectly increased catecholamines, could also cause HTN (61, 62). In addition, paraneoplastic nodular polyarteritis correlated with renal vascular HTN (59). It is rarely reported that brain metastasis from RCC could cause intracranial HTN by compressing dural venous sinuses (65).
Treatment of RCC Cause HTN
The treatment of RCC mainly includes surgery for localized RCC, targeted therapy, and immunotherapy for metastatic RCC (mRCC) (3). The excision of kidney jeopardizes kidney function and then increases the risk of cardiovascular disorders, such as coronary heart disease, HTN, cardiomyopathy, heart failure (HF), and dysrhythmias (66). Considering that partial nephrectomy (PN) can better preserve kidney function than radical nephrectomy (RN), PN is recommended to treat patients with early stage tumors (3). Nephrectomy-related HTN (NR-HT) has been reported by several studies, but robust high-level evidence is still needed (8, 67–69). The use of targeted therapies, especially vascular endothelial growth factor signaling pathway (VSP) inhibitors, has remarkably increase the life expectancy of patients with mRCC while the increased risk of cardiovascular events turns out to be its obvious side effect (5, 70). Apart from HTN, VSP inhibitors could also cause venous thromboembolism (VTE), HF, arterial thromboembolism (ATE), myocardial infarction (MI), long Q-T syndrome (LQTS) and Torsade de Pointes (TdP). Detailed information is listed in Table 1. Immnunotherapy is also a first-line therapy for mRCC but significant cardiovascular side effects have not been found yet (3, 70).
Table 1
| Drugs | FDA approved year | Any grade HTN (%) | Grade 3/4 HTN (%) | Other associated cardiovascular complications |
|---|---|---|---|---|
| Temsirolimus | 2007 | 7 | - | VTE, thrombophlebitis |
| Everolimus | 2009 | 1–10 | 3 | Non-infectious pneumonitis with pulmonary HTN, VTE, tachycardia, HF |
| Bevacizumab | 2004 | 4–34 | 1–11 | ATE, VTE, HF |
| Sorafenib | 2005 | 12–34 | 4–11 | MI, LQTS |
| Sunitinib | 2006 | 24–41 | 8–15 | MI, HF, cardiomyopathy, LQTS, TdP |
| Pazopanib | 2009 | 13–57 | 4 | LQTS, TdP, HF, ATE, VTE, thrombotic microangiopathy |
| Axitinib | 2012 | 40–42 | 8–16 | ATE, VTE, HF, MI |
| Lenvatinib | 2015 | 42 | 13 | cardiomyopathy, HF, ATE, LQTS |
| Cabozantinib | 2016 | 37–81 | 15–28 | MI, ATE, VTE |
| Tivozanib | 2021 | 44–45 | 12–22 | HF, MI, ATE, VTE |
Incidence of targeted therapy associated hypertension (HTN) in patients with metastatic renal cell carcinoma (mRCC).
This table shows data about incidence of tHTN collected from FDA, Phase III clinical trials and meta-analysis or other high-grade evidences. The Grade 3/4 HTN data about Temsirolimus has not been found. HTN, hypertension; VTE, venous thromboembolism; HF, heart failure; ATE, arterial thromboembolism; MI, myocardial infarction; LQTS, long Q-T syndrome; TdP, Torsade de Pointes.
Nephrectomy-Related HTN
As for PN, a cross-sectional survey showed that PN was independently associated with NR-HT (OR = 2.93, p = 0.022) (8). There are several hypotheses for NR-HT after PN. The compressed renal parenchyma due to renal hematoma, bolsters, or sclerotic tissue could cause insufficient renal perfusion and renin-angiotensin system activation, which refers to the “page kidney” hypothesis (71). In addition, vascular clamping in PN process could cause vasculitis and intimal hyperplasia, which would aggravate renal artery stenosis, resulting in the decline of glomerular capillary pressure and activated rennin-angiotensin system, leading to NR-HT (72). However, some studies drew opposite conclusions (67, 73). A retrospective study involving 264 patients with PN showed that BP had no significant change after surgery (67). A plausible explanation is that PN may treat paraneoplastic HTN, which can mask NR-HT, thus resulting in a statistically insignificant difference. Another study showed the BP decreased 1.9 mmHg (p = 0.01) in 5 years after PN and the decrease of BP is thought to be associated with more BP measurements during follow-up and increased antihypertensive medications (73). Considering the conflicting results, well-designed prospective researches are warranted for NR-HT.
As for RN, it is still uncertain for its facilitating HTN role owing to insufficient evidence. After more than 10 years of follow-up, a small cohort study showed that 40% of patients with RN developed NR-HT and the mechanisms of RN leading to NR-HT are most likely due to functional renal parenchyma deficits and secondary end-stage renal disease (68). However, another cross-sectional cohort study showed that there was no significant difference in BP among RCC patients who underwent RN (69). Besides, the circadian rhythm of BP may also be affected after bilateral RN (74).
Targeted Treatment-Related HTN
Targeted therapies for mRCC have prolonged the overall survival (OS) and progression-free survival (PFS) significantly and now have been listed as the standard treatment for mRCC (3). However, the number of patients with mRCC complicated with targeted therapy-related HTN (tHTN) as the on-target effect has dramatically increased (75).
These targeted drugs for mRCC mainly include VSP inhibitors and phosphatidylinositol-3-kinase–protein kinase B/mammalian target of rapamycin (mTOR) inhibitors. Bevacizumab is a monoclonal antibody to VEGF, often accompanied by the use of IFN-α (3). Multitargeted tyrosine kinase inhibitors (TKIs), which can bind to VEGF receptors and suppress the VEGF pathway, include sunitinib, sorafenib, pazopanib, axitinib, tivozanib, and cabozantinib (76). The mTOR inhibitor includes everolimus and temsirolimus (3). According to a report of real-world treatment patterns, the most common first-line used of targeted drugs in 2015 in the United States are sunitinib and pazopanib accounting for about 70% (77).
Strong evidence showed that targeted therapy, especially VSP inhibitors, could induce HTN. We collect data about tHTN from FDA (70), Phase III clinical trials, meta-analysis, or other high-grade evidence RCC (78–84) (Table 1). The Common Terminology Criteria for Adverse Events classified the tHTN into 5 grades. A meta-analysis of randomized controlled trials in 2015 showed that patients with TKIs have a significantly higher grade 3 or 4 HTN incidence compared with IFN-α or placebo (RR = 6.00, 95% CI: 3.36–10.69) (9). A large retrospective real-world study from 2006 to 2015 showed the total tHTN incidence rate was 69.1 per 100 patient-years and VSP inhibitors were higher than mTOR inhibitors (71.7 vs. 47.8 per patient-years) (77). The newer generation of VSP inhibitors which are more powerful to inhibit the VEGF pathway, tended to have higher HTN incidence (85). In addition, higher doses and longer duration of VSP inhibitors will increase the incidence and degree of HTN, which showed a dose-dependent relationship (2, 85, 86). Germline polymorphisms (86), high SBP at baseline (87), aging and other cardiovascular risk factors (88) may also affect the onset of tHTN. The tHTN could occur within hours or days after receiving VSP inhibitors (9) and drop quickly after drug withdrawal (89). The average onset time of tHTN was 131 days for bevacizumab (78), 116.5 days for mTOR inhibitors, and 70.0 days for VSP inhibitors (77). The newly proved lenvatinib has a median onset time of 35 days, reported by the FDA (70). The use of antihypertensive drugs may affect the onset time of severe HTN (9).
The mechanisms for tHTN are still elusive. VSP inhibitors could cause depletion of nitric oxide and prostacyclin which are vasodilators as well as increased vasoconstrictive endothelin-1 (89). In addition, increased reactive oxygen species, functional decreased microvascular density, increased vascular stiffness, and salt sensitivity are other possible reasons (90).
The tHTN could be seen as a biomarker for the on-target effect of VSP inhibitors and indicated a better prognosis (75). A multicenter retrospective study in 2020 demonstrated that patients with tHTN had higher PFS (12 months, 95% CI = 9–21 months) than those without tHTN (9 months, 95% CI: 7–12 months) (86). Similar results were shown among other TKIs and Bevacizumab (2, 75).
However, HTN that is not induced by targeted therapy could increase the risk of RCC mortality (OR = 1.75, 95% CI: 1.61–1.90) demonstrated by a review such as 6,964 patients of RCC in 2002 (91). Severe HTN in patients of RCC could cause HF, leukoencephalopathy, suspend, or cessation of targeted drugs (75, 90), which will do harm to the prognosis and well-control of BP during targeted therapy could improve prognosis (92).
Selection of Antihypertensive Drugs for tHTN
There is no conclusion about the best antihypertensive drugs for tHTN (81). The current opinion is the selection of antihypertensive drugs should be individualized, but there are indeed some preferences (93).
Angiotensin-converting enzyme inhibitors/ angiotensin receptor blockades are potential better antihypertensive drugs for VSP inhibitors users. Several retrospective studies showed that patients of mRCC treated with sunitinib or other VSP inhibitors had better OS and PFS if received ACEI/ARBs (92, 94). ACEI/ARBs may be more recommended in patients with mRCC undergoing nephrectomy, considering its renal protective function (95). ACEI/ARBs could also treat proteinuria and left ventricular systolic dysfunction induced by targeted treatment (81, 90). ACEI/ARBs may prevent sarcopenia in patients with RCC and then reduce overexposure and toxicity of TKIs which could decrease the treatment interruption rate (95). However, a case report claimed that ACEI may decrease the effect of bevacizumab in ovarian cancer (96) and another case reported that combinatorial therapy of ACEI and everolimus may increase the risk of angioedema (97). A pooled-analysis reported that baseline use of ACEI/ARB is not significantly associated with OS or PFS (81). Thus, even with much supporting evidence, the priority of ACEI/ARB in mRCC needs further studies.
Dihydropyridine CCB can control tHTN as well as other antihypertensive drugs, considering the function of inhibiting arterial wall contractility (98). In addition, CCB was thought to inhibit chemoresistance of RCC and thus enhance drug efficacy (88). Besides, animal studies showed that CCB could increase the density of micro-vessels (89). But non-dihydropyridine CCB should not be used in patients receiving VSP inhibitors because they would competitively inhibit the activity of P450 3A4, thus increasing the circulating VSP inhibitors concentrations (2).
A retrospective study showed that the patients with mRCC treated with sunitinib or pazopanib with β-blockers have better PFS and OS than other antihypertensive drugs (99). Animal studies have shown that β-blockers could inhibit the proliferation of cancer, but the anti-cancer role of β-blockers in human is still in controversy (100).
The use of diuretics should consider the probability of dehydration and electrolyte disorders, since patients treated with VSP inhibitors like sunitinib have the higher risk of diarrhea and electrolyte imbalances (95). Fluid retention due to sodium excretion depletion may explain tHTN occurred weeks later and diuretics are a potential preference in this condition (101).
Discussion
What Are the Risk Factors for Comorbidity of HTN and RCC?
The relationship between HTN and RCC is complex (Figure 1). HTN and RCC share several common modifiable risk factors, such as obesity, smoking, and low physical activity. These risk factors may induce RCC and HTN through several common mechanisms, for example, chronic inflammation, oxidative stress like lipid peroxidation, interleukin-6, insulin, IGF-1, leptin, and VEGF pathway (48). There are also some potential common risk factors, like unhealthy diet, alcohol, but need further study to confirm their roles.
Hypertension is a direct risk factor for RCC with a dose-dependent relationship. HTN may also play a synergistic role with other risk factors like obesity to facilitate RCC. Meanwhile, the risk of RCC caused by antihypertensive drugs has not been excluded and diuretics are with great suspicion to cause RCC. Notably, ACEI/ARBs are potential anti-cancer drugs considering their mechanisms of function.
Renal cell carcinoma can directly cause HTN by the formation of arteriovenous fistula, tumor compression-induced renin secretion, ectopic hormone syndromes, paraneoplastic vasculitis, and brain metastasis. Treatment of RCC can also induce HTN. Nephrectomy may affect BP. The use of targeted therapy is strongly associated with HTN. This kind of increased BP is short-term, reversible, and dose-dependent and indicates the effect of targeted therapy. As to medicine for tHTN, there is no strong evidence proving a preference for a certain kind of antihypertensive drugs.
There are some guiltless factors responsible for the increasing comorbidity. For example, population growth and aging, advances in cancer treatment and prolonged survival, widespread use of advanced imaging techniques, improved public awareness for annual medical examination (44, 48, 102).
How to Decrease the Comorbidity of HTN and RCC?
There are some factors that we can handle to decrease the comorbidity and the suggestions are discussed below (Figure 2).
Figure 2
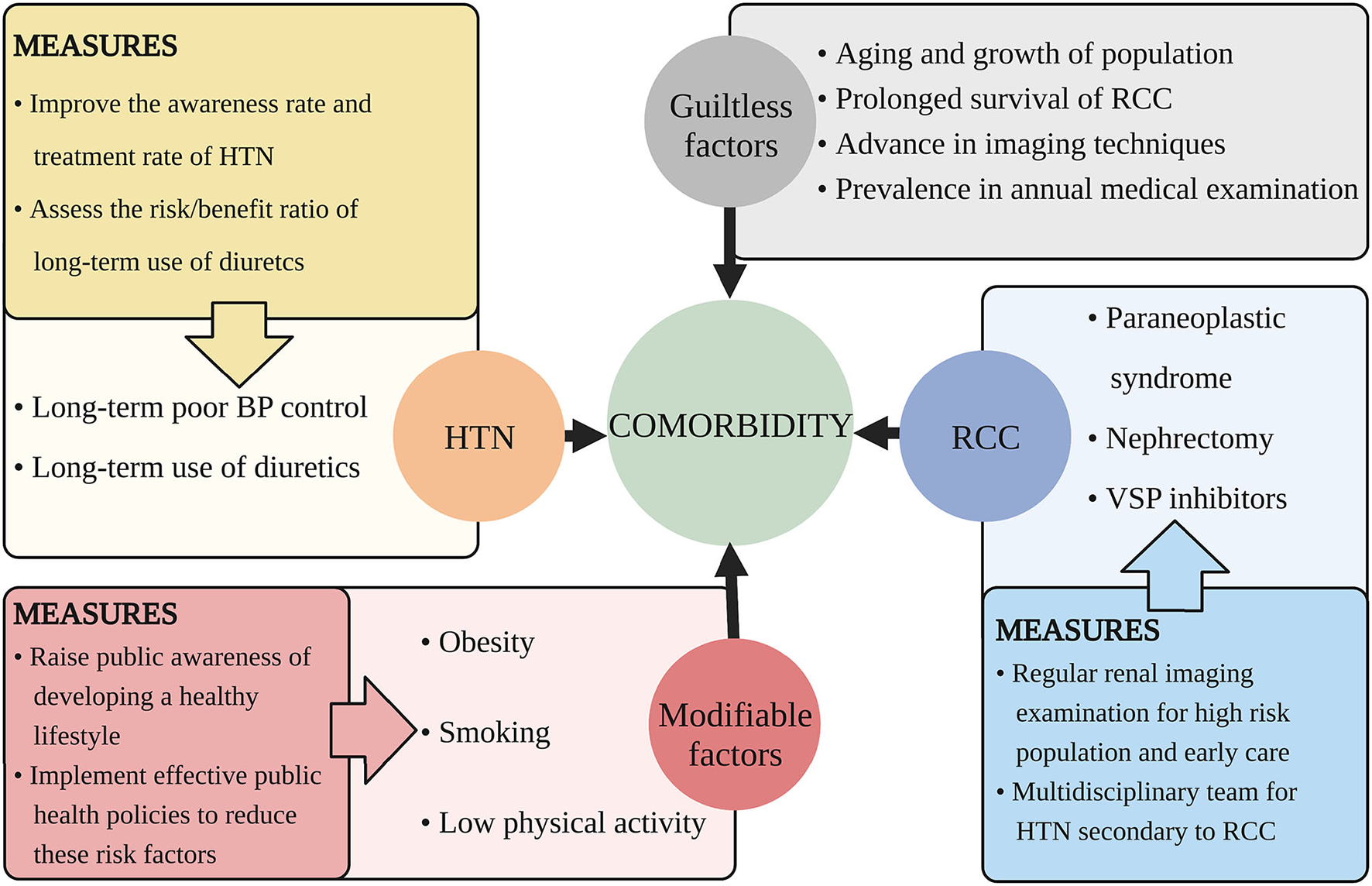
Suggested measures for decreasing the comorbidity of HTN and RCC. This figure illustrates aspects that cause the increasing comorbidity of HTN and RCC and proposes related measures. HTN, hypertension; RCC, renal cell carcinoma; BP, blood pressure; VSP, vascular endothelial growth factor signaling pathway.
Obesity, smoking, and lack of physical activity all show a dose-dependent relationship with RCC and HTN. Obesity was supposed to be associated with 78% patients of HTN, and increased 60% risk of RCC (90). The risk of RCC doubled in people smoking more than 20 cigarettes a day while cessation of smoking more than 30 years reduced the 50% risk of RCC (32). Adequate physical activity can reduce 12% risk of RCC (37). It is known that prevention is more favorable than treatment, both for patients and for society. Thus, public awareness of developing a healthy lifestyle should be raised and effective public health policies should be implemented to reduce the modifiable risk factors.
Poor control of essential HTN facilitates the rising prevalence of RCC. Globally, it is estimated that only half patients of HTN are diagnosed and one-fifth patients of HTN have well-controlled BP (1). Thus, improvement of the awareness and treatment rate of HTN is quite urgent.
Diuretics exert a potential carcinogenic effect, thus it should be prescribed after comprehensive thought of the risk/benefit ratio (103). For those with severe HF, refractory HTN, or edema, the benefit is higher than the risk. Considering the carcinogenic risk of diuretics is low and needs long-term accumulation to be significant, younger women who need decades of use of diuretics are at high risk than the elderly and if not necessary, better change to other antihypertensive drugs.
For those with obesity, smoking exposure, low activity, and unhealthy diets, the risk of RCC or HTN is high. For those with long-term HTN and diuretics history, RCC will occur in higher possibility. People with malignant HTN also have a higher incidence of RCC (7). These groups with a high risk of RCC are recommended for regular renal imaging examination. And for those already diagnosed with RCC and HTN, cardio-oncologic teams are needed to give better clinical care.
How to Give Better Antihypertensive Treatment for the Patients With RCC With HTN?
Regular and accurate BP measurement is fundamental. If the patients with RCC are treated with nephrectomy and targeted therapy, clinicians need to predict the possible changes of blood pressure and monitor BP regularly. As to tHTN, guideline recommend well-controlled BP before targeted treatment and weekly monitor during the first treatment cycle and monitor every 2–3 weeks in the remaining treatment cycle (76). For those with a history of HTN or coronary heart disease, the risk of cardiovascular event is significantly higher when receiving VSP inhibitors and should be monitored with caution (104). Hypotension may also occur as a manifestation of hypersensitivity/infusion reactions when receiving targeted therapy (70). Thus, BP should be monitored throughout the infusion process and necessary supportive care should be prepared. Notably, white-coat HTN, masked HTN may conceal the exact BP, so out-of-office measurements are also necessary (105).
Well-control of BP can improve the prognosis of RCC by preventing severe cardiovascular diseases or discontinuation of targeted drugs (104). The HTN caused by VSP inhibitors is usually mild and reversible (106). According to ACC/AHA guidelines, for people taking VSP inhibitors, the recommended BP is below 140/90 and below 130/80 if with cardiovascular risk factors (105). However, there is limited evidence to support this antihypertensive target. The patients with RCC with VSP inhibitors developed stage I HTN or DBP increased >20 mmHg should use antihypertensive drugs (107). The pros and cons of each kind of antihypertensive drugs have been discussed above. If the HTN could not be controlled well with a single agent, consider combined therapy methods. If the HTN is uncontrolled with end organ damage, the cessation of VSP inhibitors is recommended (108). Paraneoplastic HTN is usually reversible after renal tumor removal and there is a lack of evidence for antihypertensive therapies for NR-HT. Besides, better pain control and psychotherapy are necessary in the control of BP in the patients with RCC (2).
However, in view of the lack of high-level evidence for the management of HTN in the patients with RCC and different comorbidity conditions of patients, the strategy of blood pressure control is often best guided by a team of oncologist, cardiologist, and clinical pharmacist (108). It is necessary to improve the understanding of “cardio-oncology” among health professionals. The term “cardio-oncology” highlights the complex relationship between cardiovascular diseases and cancer, and encourages the corporation of cardiovascular specialists and oncologists to give better clinical care for cancer survivors.
Conclusions
In modern society, owing to the change of lifestyle and use of VSPs, the number of patients with HTN and RCC simultaneously is increasing, which turns out to be a heavy disease burden. This review thoroughly investigated the relationship between RCC and HTN from basic, epidemiological, and clinical aspects, aiming to deepen the understanding of the comorbidity and benefit of these patients. However, many problems remain to be resolved. Apart from obesity, smoking, and low physical activity, there are still other possible common modifiable risk factors without robust evidence. Besides, the exact roles of antihypertensive drugs on tumor formation are uncertain and high quality evidence regarding the management of HTN secondary to RCC is far from enough to generate guidance for clinicians. Thus, we appealed to the corporation of basic scientists, public health officers, oncologists, cardiologists, and other health experts to solve these cardio-oncologic problems.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
ZB and JY: conception and thoroughly searching related papers. ZB, YX, MH, DL, HW, HL, and JY: drafting of the manuscript or revising it critically for important intellectual content. YX and ZB: drawing illustrations. JY: final approval of the manuscript submitted. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Jiawei Huang, Wei Zhu, and Fanzhou Zeng, for their kind advice on medical writing support. The authors thank BioRender.com because the pictures were drawn by their software.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.810262/full#supplementary-material
- ACEI
angiotensin-converting enzyme inhibitor
- a-MSH
a-melanocyte-stimulating hormone
- ARB
angiotensin receptor blockade
- BMI
body mass index
- BP
blood pressure
- CCB
calcium channel blocker
- DBP
diastolic blood pressure
- HR
hazard ratio
- HTN
hypertension
- IGF-1
insulin-like growth factor-1
- mRCC
metastatic RCC
- mTOR
mammalian target of rapamycin
- OR
odds ratio
- OS
overall survival
- PFS
progression-free survival
- PN
partial nephrectomy
- RCC
renal cell carcinoma
- RR
relative risk
- RN
radical nephrectomy
- SBP
systolic blood pressure
- tHTN
targeted therapy-related HTN
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
- VSP
vascular endothelial growth factor signaling pathway.
Abbreviations
References
1.
NCD Risk Factor Collaboration . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. 10.1016/s0140-6736(21)01330-1
2.
Kidoguchi S Sugano N Tokudome G Yokoo T Yano Y Hatake K et al . New concept of onco-hypertension and future perspectives. Hypertension. (2021) 77:16–27. 10.1161/hypertensionaha.120.16044
3.
Ljungberg B Albiges L Abu-Ghanem Y Bensalah K Dabestani S Fernández-Pello S et al . European Association of Urology Guidelines on renal cell carcinoma: the 2019 update. Eur Urol. (2019) 75:799–810. 10.1016/j.eururo.2019.02.011
4.
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660
5.
SEER Program . Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Available online at: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed October 13, 2021).
6.
Weikert S Boeing H Pischon T Weikert C Olsen A Tjonneland A et al . Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. (2008) 167:438–46. 10.1093/aje/kwm321
7.
Handler J . Renal cell carcinoma and hypertension. J Clin Hypertens. (2005) 7:249–51. 10.1111/j.1524-6175.2005.04108.x
8.
Fukushima H Inoue M Kijima T Yoshida S Yokoyama M Ishioka J et al . Incidence and risk factors of hypertension following partial nephrectomy in patients with renal tumors: a cross-sectional study of postoperative home blood pressure and antihypertensive medications. Clin Genitourin Cancer. (2020) 18:e619–28. 10.1016/j.clgc.2020.02.004
9.
Tan Q Wang W Long Y Chen G . Therapeutic effects and associated adverse events of multikinase inhibitors in metastatic renal cell carcinoma: a meta-analysis. Exp Ther Med. (2015) 9:2275–80. 10.3892/etm.2015.2427
10.
Chow WH Dong LM Devesa SS . Epidemiology and risk factors for kidney cancer. Nat Rev Urol. (2010) 7:245–57. 10.1038/nrurol.2010.46
11.
Carey RM Muntner P Bosworth HB Whelton PK . Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. (2018) 72:1278–93. 10.1016/j.jacc.2018.07.008
12.
Njølstad I Arnesen E Lund-Larsen PG . Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation. (1996) 93:450–6. 10.1161/01.CIR.93.3.450
13.
Washio M Mori M Mikami K Miki T Watanabe Y Nakao M et al . Cigarette smoking and other risk factors for kidney cancer death in a Japanese population: Japan Collaborative Cohort Study for evaluation of cancer risk (JACC study). Asian Pac J Cancer Prev. (2014) 14:6523–8. 10.7314/apjcp.2013.14.11.6523
14.
van de Pol JA George L van den Brandt PA Baldewijns M Schouten LJ . Etiologic heterogeneity of clear-cell and papillary renal cell carcinoma in the Netherlands Cohort Study. Int J Cancer. (2021) 148:67–76. 10.1002/ijc.33193
15.
Kabaria R Klaassen Z Terris MK . Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis. (2016) 9:45–52. 10.2147/ijnrd.S75916
16.
Liu X Sun Q Hou H Zhu K Wang Q Liu H et al . The association between BMI and kidney cancer risk: an updated dose-response meta-analysis in accordance with PRISMA guideline. Medicine. (2018) 97:e12860. 10.1097/MD.0000000000012860
17.
Bjørge T Tretli S Engeland A . Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. (2004) 160:1168–76. 10.1093/aje
18.
Beebe-Dimmer JL Colt JS Ruterbusch JJ Keele GR Purdue MP Wacholder S et al . Body mass index and renal cell cancer: the influence of race and sex. Epidemiology. (2012) 23:821–8. 10.1097/EDE.0b013e31826b7fe9
19.
Sawada N Inoue M Sasazuki S Iwasaki M Yamaji T Shimazu T et al . Body mass index and subsequent risk of kidney cancer: a prospective cohort study in Japan. Ann Epidemiol. (2010) 20:466–72. 10.1016/j.annepidem.2010.03.008
20.
Purdue MP Moore LE Merino MJ Boffetta P Colt JS Schwartz KL et al . An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer. (2013) 132:2640–7. 10.1002/ijc.27934
21.
Rini BI Small EJ . Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. (2005) 23:1028–43. 10.1200/JCO.2005.01.186
22.
Gago-Dominguez M Castelao JE . Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med. (2006) 40:721–33. 10.1016/j.freeradbiomed.2005.09.026
23.
Griffin KA Kramer H Bidani AK . Adverse renal consequences of obesity. Am J Physiol Renal Physiol. (2008) 294:F685–96. 10.1152/ajprenal.00324.2007
24.
Sun L Chao F Luo B Ye D Zhao J Zhang Q et al . Impact of estrogen on the relationship between obesity and renal cell carcinoma risk in women. EBioMedicine. (2018) 34:108–12. 10.1016/j.ebiom.2018.07.010
25.
Klinghoffer Z Yang B Kapoor A Pinthus JH . Obesity and renal cell carcinoma: epidemiology, underlying mechanisms and management considerations. Expert Rev Anticancer Ther. (2009) 9:975–87. 10.1586/era.09.51
26.
Yao X Huang J Zhong H Shen N Faggioni R Fung M et al . Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. (2014) 141:125–39. 10.1016/j.pharmthera.2013.09.004
27.
Salvetti A Brogi G Di Legge V Bernini GP . The inter-relationship between insulin resistance and hypertension. Drugs. (1993) 46(Suppl 2):149–59. 10.2165/00003495-199300462-00024
28.
Shek EW Brands MW Hall JE . Chronic leptin infusion increases arterial pressure. Hypertension. (1998) 31:409–14. 10.1161/01.hyp.31.1.409
29.
Kotsis V Nilsson P Grassi G Mancia G Redon J Luft F et al . New developments in the pathogenesis of obesity-induced hypertension. J Hypertens. (2015) 33:1499–508. 10.1097/hjh.0000000000000645
30.
Hunt JD van der Hel OL McMillan GP Boffetta P Brennan P . Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. (2005) 114:101–8. 10.1002/ijc.20618
31.
Hu J Ugnat A-M . Active and passive smoking and risk of renal cell carcinoma in Canada. Eur J Cancer. (2005) 41:770–8. 10.1016/j.ejca.2005.01.003
32.
Parker AS Cerhan JR Janney CA Lynch CF Cantor KP . Smoking cessation and renal cell carcinoma. Ann Epidemiol. (2003) 13:245–51. 10.1016/s1047-2797(02)00271-5
33.
Grassi G Seravalle G Calhoun DA Bolla GB Giannattasio C Marabini M et al . Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation. (1994) 90:248–53. 10.1161/01.cir.90.1.248
34.
Virdis A Giannarelli C Neves MF Taddei S Ghiadoni L . Cigarette smoking and hypertension. Curr Pharm Des. (2010) 16:2518–25. 10.2174/138161210792062920
35.
Mackay A Brown JJ Cumming AM Isles C Lever AF Robertson JI . Smoking and renal artery stenosis. Br Med J. (1979) 2:770. 10.1136/bmj.2.6193.770
36.
Moore SC Chow WH Schatzkin A Adams KF Park Y Ballard-Barbash R et al . Physical activity during adulthood and adolescence in relation to renal cell cancer. Am J Epidemiol. (2008) 168:149–57. 10.1093/aje/kwn102
37.
Behrens G Leitzmann MF . The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. (2013) 108:798–811. 10.1038/bjc.2013.37
38.
Williams PT . Reduced risk of incident kidney cancer from walking and running. Med Sci Sports Exerc. (2014) 46:312–7. 10.1249/MSS.0b013e3182a4e89c
39.
Moraes-Silva IC Mostarda C Moreira ED Silva KA dos Santos F Angelis K de et al . Preventive role of exercise training in autonomic, hemodynamic, and metabolic parameters in rats under high risk of metabolic syndrome development. J Appl Physiol. (2013) 114:786–91. 10.1152/japplphysiol.00586.2012
40.
Araujo AJ de Santos AC Souza KD Aires MB Santana-Filho VJ Fioretto ET et al . Resistance training controls arterial blood pressure in rats with L-NAME- induced hypertension. Arq Bras Cardiol. (2013) 100:339–46. 10.5935/abc.20130051
41.
Diaz KM Shimbo D . Physical activity and the prevention of hypertension. Curr Hypertens Rep. (2013) 15:659–68. 10.1007/s11906-013-0386-8
42.
Seretis A Cividini S Markozannes G Tseretopoulou X Lopez DS Ntzani EE et al . Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. (2019) 9:8565. 10.1038/s41598-019-45014-4
43.
Hidayat K Du X Zou SY Shi BM . Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. (2017) 35:1333–44. 10.1097/hjh.0000000000001286
44.
Kim CS Han KD Choi HS Bae EH Ma SK Kim SW . Association of hypertension and blood pressure with kidney cancer risk: a nationwide population-based cohort study. Hypertension. (2020) 75:1439–46. 10.1161/hypertensionaha.120.14820
45.
Leiba A Kark JD Afek A Derazne E Keinan-Boker L Shamiss A et al . Hypertension in adolescence is not an independent risk factor for renal cancer: a cohort study of 918,965 males. J Am Soc Hypertens. (2013) 7:283–8. 10.1016/j.jash.2013.04.003
46.
Chow WH Gridley G Fraumeni JF Jr. Järvholm B . Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. (2000) 343:1305–11. 10.1056/nejm200011023431804
47.
Corrao G Scotti L Bagnardi V Sega R . Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf. (2007) 2:125–33. 10.2174/157488607780598296
48.
Koene RJ Prizment AE Blaes A Konety SH . Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. 10.1161/circulationaha.115.020406
49.
Copland E Canoy D Nazarzadeh M Bidel Z Ramakrishnan R Woodward M et al . Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol. (2021) 22:558–70. 10.1016/s1470-2045(21)00033-4
50.
Xie Y Xu P Wang M Zheng Y Tian T Yang S et al . Antihypertensive medications are associated with the risk of kidney and bladder cancer: a systematic review and meta-analysis. Aging. (2020) 12:1545–62. 10.18632/aging.102699
51.
Schouten LJ van Dijk BA Oosterwijk E Hulsbergen-van de Kaa CA Kiemeney LA Goldbohm RA et al . Hypertension, antihypertensives and mutations in the Von Hippel-Lindau gene in renal cell carcinoma: results from the Netherlands Cohort Study. J Hypertens. (2005) 23:1997–2004. 10.1097/01.hjh.0000186023.74245.48
52.
Messerli FH . Diuretic therapy and renal cell carcinoma—another controversy?Eur Heart J. (1999) 20:1441–2. 10.1053/euhj.1999.1534
53.
Messerli FH . Risk factors for renal cell carcinoma: hypertension or diuretics?Kidney Int. (2005) 67:774–5. 10.1111/j.1523-1755.2005.67190.x
54.
Grossman E Messerli FH Goldbourt U . Antihypertensive therapy and the risk of malignancies. Eur Heart J. (2001) 22:1343–52. 10.1053/euhj.2001.2729
55.
Colt JS Hofmann JN Schwartz K Chow WH Graubard BI Davis F et al . Antihypertensive medication use and risk of renal cell carcinoma. Cancer Causes Control. (2017) 28:289–97. 10.1007/s10552-017-0857-3
56.
Hole DJ Hawthorne VM Isles CG McGhee SM Robertson JW Gillis CR et al . Incidence of and mortality from cancer in hypertensive patients. BMJ. (1993) 306:609. 10.1136/bmj.306.6878.609
57.
Sobczuk P Szczylik C Porta C Czarnecka AM . Renin angiotensin system deregulation as renal cancer risk factor. Oncol Lett. (2017) 14:5059–68. 10.3892/ol.2017.6826
58.
Yoon C Yang H-S Jeon I Chang Y Park SM . Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. CMAJ. (2011) 183:E1073–84. 10.1503/cmaj.101497
59.
Miyajima A Kikuchi E Kosaka T Oya M . Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in urogenital cancer. Rev Recent Clin Trials. (2009) 4:75–8. 10.2174/157488709788185996
60.
Rosenbach LM Xefteris ED . Erythrocytosis associated with carcinoma of the kidney. JAMA. (1961) 176:136–7. 10.1001/jama.1961.63040150001012
61.
Eiam-Ong S Eiam-Ong S Punsin P Sitprija V Chaiyabutr N . Acute hypercalcemia-induced hypertension: the roles of calcium channel and alpha-1 adrenergic receptor. J Med Assoc Thai. (2004) 87:410–8.
62.
Ahmed U Chatterjee T Kandula M . Polyarteritis Nodosa: an unusual case of paraneoplastic process in renal cell carcinoma. J Community Hosp Intern Med Perspect. (2020) 10:73–5. 10.1080/20009666.2019.1703374
63.
Dahl T Eide I Fryjordet A . Hypernephroma and hypertension. Two case reports. Acta Med Scand. (1981) 209:121–4.
64.
Ogunmola OJ Onyema C Babatunde Bakare TI Olabinri EO Bamigboye-Taiwo OT Adaje AO et al . A 10-year old girl with resistant hypertension without significant indication of an underlying renal cell carcinoma, misdiagnosed as malaria. Am J Case Rep. (2019) 20:1434–9. 10.12659/AJCR.916588
65.
Marvin E Synkowski J Benko M . Tumor cerebri: Metastatic renal cell carcinoma with dural venous sinus compression leading to intracranial hypertension; a case report. Surg Neurol Int. (2017) 8:175. 10.4103/sni.sni_69_17
66.
Capitanio U Terrone C Antonelli A Minervini A Volpe A Furlan M et al . Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a-T1b renal mass and normal preoperative renal function. Eur Urol. (2015) 67:683–9. 10.1016/j.eururo.2014.09.027
67.
Lawrentschuk N Trottier G Mayo K Rendon RA . Effects of partial nephrectomy on postoperative blood pressure. Korean J Urol. (2012) 53:154–8. 10.4111/kju.2012.53.3.154
68.
Nestler S Levien P Neisius A Thomas C Kamal MM Hampel C et al . Incidence of cardiovascular events after nephrectomy - a single centre, matched pair analysis between donor and tumour nephrectomy in a long term follow-up. Urol Int. (2016) 97:142–7. 10.1159/000446248
69.
Inoue M Fujii Y Yokoyama M Saito K Numao N Kihara K . Progression of hypertension after partial nephrectomy in patients with renal tumors: a preliminary report. Int J Urol. (2015) 22:797–8. 10.1111/iju.12794
70.
U.S. Food and Drug administration . Drugs@FDA: FDA-Approved Drugs. Available online at: https://www.accessdata.fda.gov/scripts/cder/daf (accessed October 15, 2021).
71.
Smyth A Collins CS Thorsteinsdottir B Madsen BE Oliveira GH Kane G et al . Page kidney: etiology, renal function outcomes and risk for future hypertension. J Clin Hypertens. (2012) 14:216–21. 10.1111/j.1751-7176.2012.00601.x
72.
Ploth DW Fitzgibbon W . Pathophysiology of altered renal function in renal vascular hypertension. Am J Kidney Dis. (1994) 24:652–9. 10.1016/S0272-6386(12)80227-7
73.
Gupta N Ganesan V Gao TM Zabell J Campbell SC Haber GP . The effect of partial nephrectomy on blood pressure in patients with solitary kidney. World J Urol. (2021) 39:1577–82. 10.1007/s00345-020-03354-1
74.
Uzu T Takeji M Kanasaki M Isshiki K Araki S Sugiomoto T et al . Change in circadian rhythm of blood pressure by bilateral radical nephrectomy and haemodialysis: a case report. J Hum Hypertens. (2006) 20:549–50. 10.1038/sj.jhh.1002027
75.
Liu Y Zhou L Chen Y Liao B Ye D Wang K et al . Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol. (2019) 19:49. 10.1186/s12894-019-0481-5
76.
Jesus-Gonzalez N de Robinson E Moslehi J Humphreys BD . Management of antiangiogenic therapy-induced hypertension. Hypertension. (2012) 60:607–15. 10.1161/hypertensionaha.112.196774
77.
Pal S Gong J Mhatre SK Lin SW Surinach A Ogale S et al . Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer. (2019) 19:548. 10.1186/s12885-019-5716-z
78.
Shepard DR Garcia JA . Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert Rev Anticancer Ther. (2009) 9:795–805. 10.1586/era.09.29
79.
Di Lorenzo G Porta C Bellmunt J Sternberg C Kirkali Z Staehler M et al . Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. (2011) 59:526–40. 10.1016/j.eururo.2011.01.002
80.
Kirkali Z . Adverse events from targeted therapies in advanced renal cell carcinoma: the impact on long-term use. BJU Int. (2011) 107:1722–32. 10.1111/j.1464-410X.2010.09985.x
81.
Derosa L Izzedine H Albiges L Escudier B . Hypertension and angiotensin system inhibitors in patients with metastatic renal cell carcinoma. Oncol Rev. (2016) 10:298. 10.4081/oncol.2016.298
82.
Schmidinger M Danesi R . Management of adverse events associated with cabozantinib therapy in renal cell carcinoma. Oncologist. (2018) 23:306–15. 10.1634/theoncologist.2017-0335
83.
Bæk Møller N Budolfsen C Grimm D Krüger M Infanger M Wehland M et al . Drug-induced hypertension caused by multikinase inhibitors (sorafenib, sunitinib, lenvatinib and axitinib) in renal cell carcinoma treatment. Int J Mol Sci. (2019) 20:4712. 10.3390/ijms20194712
84.
Campia U Moslehi JJ Amiri-Kordestani L Barac A Beckman JA Chism DD et al . Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. (2019) 139:e579–602. 10.1161/cir.0000000000000641
85.
Versmissen J Mirabito Colafella KM Koolen SL Danser AH . Vascular cardio-oncology: vascular endothelial growth factor inhibitors and hypertension. Cardiovasc Res. (2019) 115:904–14. 10.1093/cvr/cvz022
86.
Dienstmann R Braña I Rodon J Tabernero J . Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. (2011) 16:1729–40. 10.1634/theoncologist.2011-0163
87.
Izumi K Itai S Takahashi Y Maolake A Namiki M . Predictive factor and antihypertensive usage of tyrosine kinase inhibitor-induced hypertension in kidney cancer patients. Oncol Lett. (2014) 8:305–8. 10.3892/ol.2014.2060
88.
Milan A Puglisi E Ferrari L Bruno G Losano I Veglio F . Arterial hypertension and cancer. Int J Cancer. (2014) 134:2269–77. 10.1002/ijc.28334
89.
Ptinopoulou AG Sprangers B . Tyrosine kinase inhibitor-induced hypertension-marker of anti-tumour treatment efficacy or cardiovascular risk factor?Clin Kidney J. (2021) 14:14–7. 10.1093/ckj/sfaa174
90.
van Dorst DC Dobbin SJ Neves KB Herrmann J Herrmann SM Versmissen J et al . Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. (2021) 128:1040–61. 10.1161/circresaha.121.318051
91.
Grossman E Messerli FH Boyko V Goldbourt U . Is there an association between hypertension and cancer mortality?Am J Med. (2002) 112:479–86. 10.1016/s0002-9343(02)01049-5
92.
Penttilä P Rautiola J Poussa T Peltola K Bono P . Angiotensin inhibitors as treatment of sunitinib/pazopanib-induced hypertension in metastatic renal cell carcinoma. Clin Genitourin Cancer. (2017) 15:384–90.e3. 10.1016/j.clgc.2016.12.016
93.
Cohen JB Geara AS Hogan JJ Townsend RR . Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncol. (2019) 1:238–51. 10.1016/j.jaccao.2019.11.009
94.
Yang J Yang X Gao L Zhang J Yi C Huang Y . The role of the renin-angiotensin system inhibitors in malignancy: a review. Am J Cancer Res. (2021) 11:884–97.
95.
Porta C Szczylik C . Tolerability of first-line therapy for metastatic renal cell carcinoma. Cancer Treat Rev. (2009) 35:297–307. 10.1016/j.ctrv.2008.12.003
96.
Emile G Pujade-Lauraine E Alexandre J . Should we use the angiotensin-converting enzyme inhibitors for the treatment of anti-VEGF-induced hypertension?Ann Oncol. (2014) 25:1669–70. 10.1093/annonc/mdu197
97.
Rothermundt C Gillessen S . Angioedema in a patient with renal cell cancer treated with everolimus in combination with an angiotensin-converting enzyme inhibitor. J Clin Oncol. (2013) 31:e57–8. 10.1200/jco.2012.44.5106
98.
Ivanyi P Beutel G Drewes N Pirr J Kielstein JT Morgan M et al . Therapy of treatment-related hypertension in metastatic renal-cell cancer patients receiving sunitinib. Clin Genitourin Cancer. (2017) 15:280–90.e3. 10.1016/j.clgc.2016.10.004
99.
Fiala O Ostašov P Rozsypalová A Hora M Šorejs O Šustr J et al . Impact of concomitant cardiovascular medication on survival of metastatic renal cell carcinoma patients treated with sunitinib or pazopanib in the first line. Target Oncol. (2021) 16:643–52. 10.1007/s11523-021-00829-y
100.
Masur K Niggemann B Zanker KS Entschladen F . Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. (2001) 61:2866–9.
101.
Saito K Fujii H Kono K Hirabayashi K Yamatani S Watanabe K et al . Changes in blood pressure during treatment with the tyrosine kinase inhibitor lenvatinib. Clin Kidney J. (2021) 14:325–31. 10.1093/ckj/sfaa137
102.
Miller KD Nogueira L Mariotto AB Rowland JH Yabroff KR Alfano CM et al . Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. (2019) 69:363–85. 10.3322/caac.21565
103.
Grossman E Messerli FH . Diuretics and renal cell carcinoma—what is the risk/benefit ration?Kidney Int. (1999) 56:1603–4. 10.1046/j.1523-1755.1999.00713-3.x
104.
Di Lorenzo G Autorino R Bruni G Cartenì G Ricevuto E Tudini M et al . Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. (2009) 20:1535–42. 10.1093/annonc/mdp025
105.
Marvel FA Whelton SP Blumenthal RS . A cardio-oncology cardiovascular prevention framework. JACC CardioOncol. (2019) 1:252–5. 10.1016/j.jaccao.2019.11.012
106.
Schmidinger M Zielinski CC Vogl UM Bojic A Bojic M Schukro C et al . Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. (2008) 26:5204–12. 10.1200/jco.2007.15.6331
107.
Maitland ML Bakris GL Black HR Chen HX Durand JB Elliott WJ et al . Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. (2010) 102:596–604. 10.1093/jnci/djq091
108.
Essa H Dobson R Wright D Lip GY . Hypertension management in cardio-oncology. J Hum Hypertens. (2020) 34:673–81. 10.1038/s41371-020-0391-8
Summary
Keywords
hypertension, kidney cancer, comorbidity, targeted therapy, antihypertensive drug, cardio-oncology
Citation
Ba Z, Xiao Y, He M, Liu D, Wang H, Liang H and Yuan J (2022) Risk Factors for the Comorbidity of Hypertension and Renal Cell Carcinoma in the Cardio-Oncologic Era and Treatment for Tumor-Induced Hypertension. Front. Cardiovasc. Med. 9:810262. doi: 10.3389/fcvm.2022.810262
Received
06 November 2021
Accepted
17 January 2022
Published
17 February 2022
Volume
9 - 2022
Edited by
Dong Han, People's Liberation Army General Hospital, China
Reviewed by
Keyue Ding, Queen's University, Canada; Aditi Jain, Institute for Stem Cell Science and Regenerative Medicine (inStem), India
Updates
Copyright
© 2022 Ba, Xiao, He, Liu, Wang, Liang and Yuan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiansong Yuan jsyuantg@163.com
This article was submitted to Cardio-Oncology, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.