Abstract
Venous thromboembolism is a major concern during pregnancy as well as in the postpartum period. In acute proximal deep venous thrombosis, endovascular recanalization with locally administered thrombolytic agents has evolved as therapeutic alternative to anticoagulation alone. However, data on the bleeding risk of thrombolysis in the postpartum period is limited. We addressed the key clinical question of safety outcomes of catheter-directed thrombolysis (CDT) in the peri- and postpartum period. Therefore, we performed a non-exhaustive literature review and illustrated the delicate management of a patient with postpartum acute iliofemoral thrombosis treated with CDT and endovascular revascularization with thrombectomy, balloon angioplasty and stenting.
Introduction
Venous thromboembolism is a major concern during pregnancy and the postpartum period and represents the main cause of mortality during the postpartum in developed countries (1). The risk of venous thromboembolism is fivefold increased during pregnancy and up to 60-fold in the postpartum period (2, 3). The annual incidence amounts to 200 cases per 100,000 women (4).
The thrombophilic condition during pregnancy and postpartum period is mainly the result of a hormone-related hypercoagulability with elevated concentrations of factors VII, VIII, and X, fibrinogen and von Willebrand factor. In addition, intrinsic thrombolytic capacity is decreased due to higher levels of plasminogen activator inhibitor (5). These hemostatic changes occur in preparation of delivery and are supposed to be protective against peripartum hemorrhages. However, these prothrombotic changes persist for several weeks after delivery. Besides these systemic risk factors for VTE, local factors impairing venous inflow play an important role, in particular venous compression by the growing fetus (6). These local factors explain in part the higher rate of proximal deep venous thrombosis (DVT) during late stage pregnancy.
Proximal DVT is associated with a high morbidity. Postthrombotic syndrome (PTS) represents the most important long-term complication of DVT that is associated with a significant reduction in the quality of life. As many as 50% of patients with iliofemoral DVT develop a severe PTS (7).
To prevent PTS after acute proximal DVT, catheter directed thrombolysis (CDT), i.e., endovascular recanalization with locally administered thrombolytic agents, which is followed by mechanical thrombectomy and stent placement in most of the cases, has been suggested as a therapeutic alternative to anticoagulation alone (8, 9). However, bleeding complications are a major adverse effect of any thrombolysis and must be weighed against the benefits of quick clot removal (10). There is paucity of data on thrombolysis after DVT in the high risk constellation of postpartum patients. Therefore, we addressed the key clinical question of safety outcomes of CDT in the peri- and postpartum period and performed a non-exhaustive literature review.
Literature Review
We performed a review of the literature on CDT in the postpartum period, applying the following search terms in the PubMed database: [(peripartum OR postpartum OR pregnancy) AND thrombolysis], without any further search limits. After the primary query, the obtained abstracts were screened manually whether deep vein thrombosis was the underlying clinical condition and which complications and which other therapies, e.g., thrombectomy, stenting, were applied.
Results
We found 26 reports on single patients or small patient series, totaling 31 patients who had been treated with CDT in the postpartum period. No larger patient series and no randomized controlled trials were available. The mean age of the patients treated was 26.9 years. The time after delivery when thrombolysis was performed varied between 2 and 42 days. Only in one case, thrombolysis was already started during pregnancy (9th gestational week) but the pregnancy was terminated after the treatment. The most frequently used thrombolytic agent was alteplase (15 cases), followed by urokinase (12 cases), and streptokinase (5 cases). Duration of the thrombolytic therapy was agent-dependent and varied from 20 to 30 h for alteplase, 16 to 72 h for urokinase, and 106 to 140 h for streptokinase, respectively. For alteplase the dose was 0.01 mg/kg/h (4 cases), 0.02 mg/kg/h (4 cases), and 1 mg/h (6 cases). For streptokinase and urokinase, mostly 100,000 U/h were used (only in two cases the dosage was slightly higher). A simultaneous unfractionated heparin treatment was routinely administered during thrombolysis except for 4 cases where this information on adjunctive anticoagulation was missing. Of 31 patients analyzed, 22 were treated successfully (<30% residual luminal area narrowing), three patients were treated partially successful. In 6 cases the outcome was not described. Eighteen patients underwent balloon angioplasty with stent placement in 10 cases. Three patients were exclusively treated with thrombolytic therapy. In 10 patients adjunctive therapies besides thrombolysis were not described. Criteria for/against angioplasty and/or stenting were mostly not described. When described, the most reason for angioplasty was stenosis and for stenting was the presence of a May Thurner Syndrom. In one case, an early re-thrombosis occurred (after angioplasty plus stenting). Minor bleedings occurred in 5 cases only and no major or life-threatening bleeding complications were registered. Other complications as anemia, thrombocytopenia, and hemolysis were described casually but no life threatening situations occurred. In more than half of our reviewed patients, no information on PTS was documented. Nevertheless, in the remaining 15 patients, 14 did not develop PTS subsequently, and only one patient showed signs of mild PTS in the further course. Results of our literature review are summarized in Table 1 (11–19). Additionally we illustrated the delicate management of a patient with postpartum acute iliofemoral thrombosis treated with CDT in Figure 1.
TABLE 1
| Thrombolytic agent |
||||||||||||||
| Patient | References | Age | Mode of delivery |
Time of thrombolysis (days after delivery) | Agent | Bolus | Dosis | Duration (h) | Anticoagulation | Additional intervention | IVC filter insertion prior CDT | Outcome | Complications | PTS |
| 1 | (11) | Mean 30 (28–33) | Cesarean | <42 | Alteplase | 5 mg | 0.01 mg/kg/h | 20–24 | UFH | NS | No | Successful | None | NS |
| 2 | (11) | Mean 30 (28–33) | Vaginal | <42 | Alteplase | 5 mg | 0.01 mg/kg/h | 20–24 | UFH | NS | No | Successful | None | NS |
| 3 | (11) | Mean 30 (28–33) | Vaginal | <42 | Alteplase | 5 mg | 0.01 mg/kg/h | 20–24 | UFH | NS | No | Successful | None | NS |
| 4 | (11) | Mean 30 (28–33) | Vaginal | <42 | Alteplase | 5 mg | 0.01 mg/kg/h | 20–24 | UFH | NS | No | Successful | None | NS |
| 5 | (19) | 24 | Vaginal | 8 | Streptokinase | NS | 100,000 U/h | 120 | UFH | PTA | No | Partial successful | None | Mild PTS at 6 Mo |
| 6 | (19) | 22 | Vaginal | 7 | Streptokinase | NS | 100,000 U/h | 140 | UFH | PTA | Yes | Successful | Minor bleeding | No PTS at 6 Mo |
| 7 | (19) | 20 | Vaginal | 7 | Streptokinase | NS | 100,000 U/h | 110 | UFH | PTA | No | Successful | Minor bleeding | No PTS at 6 Mo |
| 8 | (19) | 23 | Vaginal | 10 | Streptokinase/Urokinase | NS | 100,000 U/h | 120 | UFH | PTA | No | Successful | Minor bleeding | No PTS at 6 Mo |
| 9 | (19) | 29 | Abortion (Second trimester) | 10 | Streptokinase | NS | 100,000 U/h | 120 | UFH | PTA | Yes | Successful | Minor bleeding | No PTS at 6 Mo |
| 10 | (16) | 26 | vaginal | 14 | Alteplase | 5 mg | 0.02 mg/kg/h | 20 | NS | None | No | Successful | None | NS |
| 11–16 | (14) | Mean 28 (21–42) | NS | <42 | Alteplase | NS | 1 mg/h | mean 30 | UFH | NS | 1 of 6 yes | NS | None | NS |
| 17 | (15) | 26 | Vaginal | 14 | Alteplase | 5 mg | 0.02 mg/kg/h | 22 | NS | None | No | Successful | None | NS |
| 18 | (15) | 34 | No information | 35 | Alteplase | 5 mg | 0.02 mg/kg/h | 23 | NS | None | No | Successful | None | NS |
| 19 | (15) | 30 | Vaginal | 47 | Alteplase | 5 mg | 0.02 mg/kg/h | 24 | NS | PTA and Stent | No | Successful | None | NS |
| 20 | (13) | 24 | Cesarean | 20 | Alteplase | NS | NS | 18 | UFH | PTA and Stent | Yes | Successful | None | NS |
| 21 | (12) | 35 | Vaginal | 3 | Urokinase | 300,000 U | 100,000 U/h | 31 | UFH | PTA and Stent | Yes | Successful | No major, calf hematoma | No PTS at 16 Mo |
| 22 | (12) | 22 | Vaginal | 28 | Urokinase | 300,000 U | 100,000 U/h | 49 | UFH | PTA and Stent | Yes | 70% patency | No major, early rethrombosis | No PTS at 12 Mo |
| 23 | (12) | 30 | Vaginal | 14 | Urokinase | 300,000 U | 100,000 U/h | 16 | UFH | PTA and Stent | Yes | Successful | None | No PTS at 39 Mo |
| 24 | (12) | 26 | No information | 21 | Urokinase | 300,000 U | 100,000 U/h | 26 | UFH | PTA | Yes | Successful | No major, hemolysis | No PTS at 20 Mo |
| 25 | (12) | 27 | Cesarean | 42 | Urokinase | 300,000 U | 100,000 U/h | 72 | UFH | PTA and Stent | Yes | Successful | None | No PTS at 56 Mo |
| 26 | (12) | 21 | Vaginal | 11 | Urokinase | 300,000 U | 100,000 U/h | 24 | UFH | PTA and Stent | Yes | Successful | No major, embolism trapped by filter | No PTS at 18 Mo |
| 27 | (12) | 27 | Cesarean | 12 | Urokinase | 300,000 U | 100,000 U/h | 48 | UFH | PTA and Stent | Yes | Successful | None | No PTS at 26 Mo |
| 28 | (12) | 23 | Vaginal | 14 | Urokinase | 300,000 U | 100,000 U/h | 27 | UFH | PTA and Stent | Yes | Successful | No major, early rethrombosis | No PTS at 12 Mo |
| 29 | (12) | 29 | Cesarean | 14 | Urokinase | 300,000 U | 100,000 U/h | 46 | UFH | PTA and Stent | Yes | 70% patency | None | No PTS at 14 Mo |
| 30 | (18) | 22 | Vaginal | 15 | Streptokinase | NS | 150,000 U/h | 106 | UFH | Thrombus Aspiration/PTA | Yes | Successful | No attributable to thrombolysis | NS |
| 31 | (17) | 30 | Vaginal (twins) | 2 | Urokinase | NS | 137,500 U/h | 48 | UFH | PTA | No | Successful | No major, minor bleeding | NS |
Summary of literature review.
GW, Gestational week; NS, not specified; IVC, inferior vena cava; REF, reference; PTS, postthrombotic syndrom.
FIGURE 1
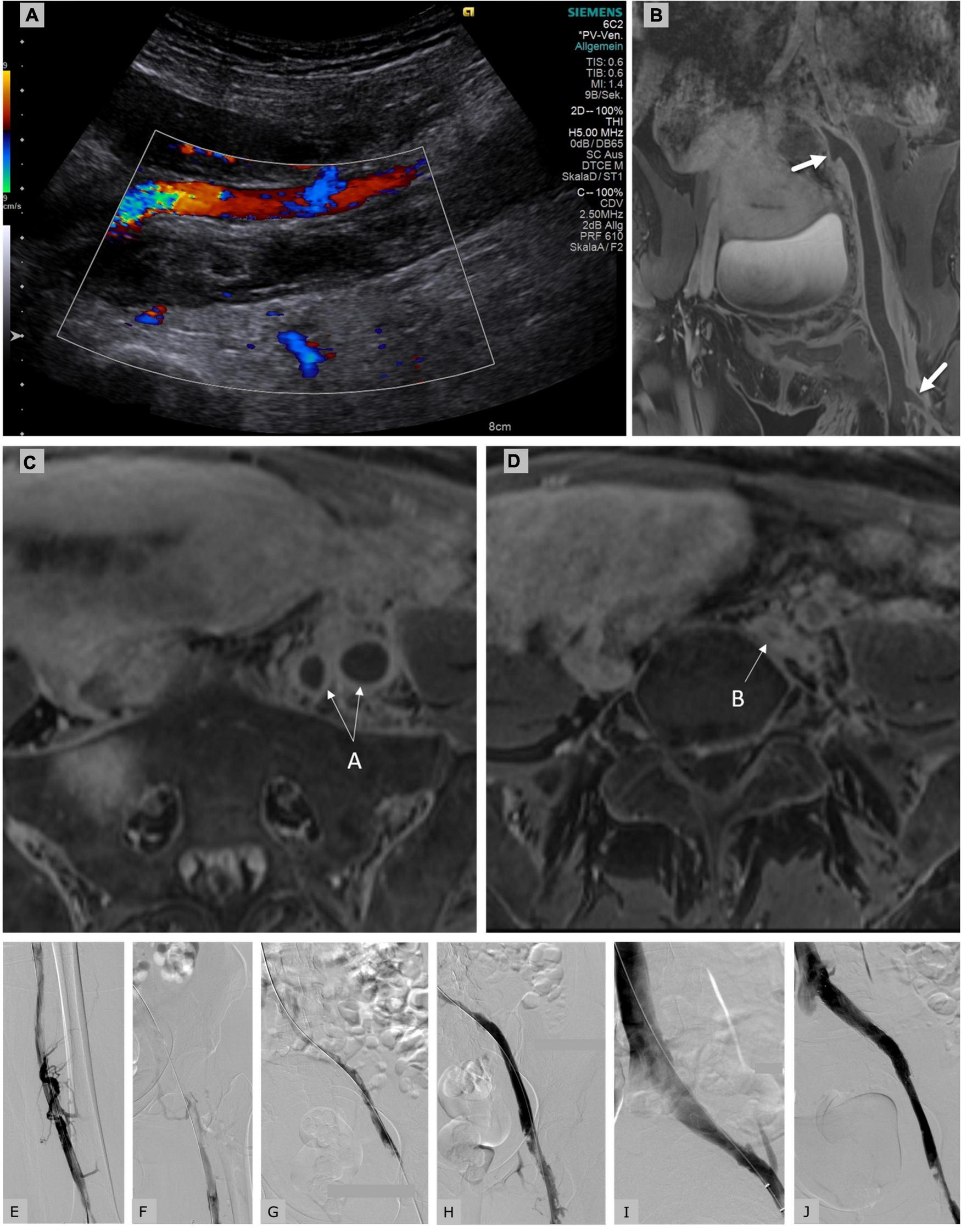
Duplex sonography (A) and MR-phlebography (B) of a 31-year-old female patient 10 days postpartum showing an iliofemoral thrombosis with involvement of the internal iliac vein (proximal arrow) and the deep femoral vein (distal arrow) down to the left popliteal vein. Clinically the left leg was tender and cool with slight sensible, but no motoric impairment; imminent phlegmasia was suspected. The patient was placed on a therapeutic dose of intravenously administered unfractionated heparin (Liquemin®, Drossapharm, Basel, Switzerland) which was adjusted according to repeated aPTT measurements. In the absence of clinical signs of bleeding and with a hemoglobin level within the normal range, catheter-directed thrombolysis (CDT) was performed via transcutaneous access of left occluded popliteal vein. After a bolus of 10 mg, a continuous infusion of alteplase (Actilyse®, Boehringer Ingelheim, Basel, Switzerland) was initiated at 2 mg/h for 5 h, and then reduced to 1 mg/h for 10 h. A total dose of 30 mg alteplase was applied. Insertion of an inferior vena cava filter was rejected after interdisciplinary discussion. Breast-feeding was paused for 24 h, but breast milk was collected before CDT to be fed to the infant later. aPTT levels were always documented to be within the therapeutic range (64–85 s) that is 1.5–2.5 times more than the baseline aPTT of 30 s. After discontinuation of thrombolysis the patient was placed on anticoagulation with subcutaneously administered enoxaparin 0.9 mg/kg bid (Clexane®, Sanofi, Vernier, Switzerland). (C) MR-phlebography of the same patient showing the transversal view of the enlarged thrombosed external and internal iliac veins (arrow A). (D) Proximal common iliac vein compression in the context of May-Thurner syndrome (arrow B). Initial phlebography of the partially occluded femoral veins (E) and of the occluded iliac veins (F) showing fresh thrombus. Control venography of our patient after catheter-directed thrombolysis (G), after Angiojet® thrombectomy [Angiojet Zelante® (8F), Boston Scientific, Larlborough, United States] (H), after balloon angioplasty (I), after stenting (Sinus obliquus 16/100, Optimed®, Ettlingen, Germany) of the left common iliac vein which was compressed in the context of underlying May-Thurner syndrome with residual thrombosis in the common femoral vein (J). The patient was discharged under anticoagulant treatment with enoxaparin 1.7 mg/kg qd for 3 months. No bleeding complications occurred neither during thrombolysis, pharmacomechanical thrombectomy nor during anticoagulation. No venous thrombembolic events occurred. Stent patency was confirmed sonographically after 3 months. After 4 months of treatment, the patient showed no signs or symptoms of PTS (Villalta-Score 0 point) and identical leg circumferences.
Discussion
The aim of CDT therapy in proximal DVT is to restore venous patency in imminent phlegmasia as soon as possible, to preserve arterial perfusion, to attenuate symptoms, to prevent local thrombosis progression and pulmonary embolism and to preserve valvular function in order to reduce the risk of PTS. In case of May-Thurner stenosis as an additional thrombotic risk factor besides the transient risk of hormonal changes during pregnancy and the postpartum period, anticoagulation may be discontinued after 3 months of anticoagulation if May-Thurner lesion was successfully treated by stent placement and an unrestricted venous inflow has been documented before discontinuation of anticoagulation.
There is paucity of data on safety regarding systemic or local thrombolysis in pregnancy or in the postpartum period. Several guidelines consider thrombolysis during pregnancy as a relative but not as an absolute contraindication, recommendations regarding the postpartum period are not specified (20–22). Among the most relevant adverse effects during any thrombolysis are hemorrhagic complications. While the complication rate of systemic thrombolytic therapy does not seem to be higher in pregnant compared to non-pregnant women (major and minor bleeding complications have a rate of 8% each), (23) the major bleeding risk was much greater in the postpartum (58%) than in the antepartum period (18%) (24). The difference is due to the recent delivery or cesarean section and most postpartum bleedings presented as vaginal or intraabdominal hemorrhages with the highest bleeding risk for cesarean section (24, 25). Measures to control postpartum bleedings comprise manual uterus compression, intrauterine tamponade, uterotonic drugs, blood transfusions, and endovascular interventions, e.g., uterine artery embolization, laparotomy, hysterectomy, and recombinant factor VIIa (25).
When performing low-doses of locally administered thrombolytics with CDT in the postpartum period, there is a major concern with an increased bleeding risk from the uterus. It is known that CDT plus anticoagulation confers a significant increase in the occurrence of major bleeding events compared to anticoagulation alone (26). Our literature research revealed 31 published cases, none of whom suffered severe bleeding complications. In order to minimize bleeding risk, it should be respected that the incidence of bleeding complications falls with increasing time interval between delivery and start of thrombolysis (25). Regarding hemostatic management, it is important to consider that global coagulation assays, e.g., aPTT-measurements may be confounded by thrombolytic therapy and changes of coagulation factors in pregnancy and postpartum (5, 27–31). In that case, use of a chromogenic anti-factor Xa assay instead, which provides more stable and predictable therapy adjustment should be considered (32–35). Fibrinogen measurement during alteplase thrombolysis is recommended because bleeding events have been significantly associated with the percent reduction in fibrinogen and have a likelihood ration of 1.4 when fibrinogen level is < 150 mg/dl (36).
However, if bleeding risk is deemed to be too high by the treating physician, single-session mechanical thrombectomy may represent a viable strategy that avoids thrombolytic therapy and its incurring bleeding risk completely and warrants quick restoration of venous inflow (37).
Vena cava filter (VCF) placement should be subject to interdisciplinary discussion because the incidence of symptomatic pulmonary embolism (PE) during CDT is low (38, 39) and the benefit of VCF placement before CDT remains unclear (39–41) and it is currently not standard practice (42). Furthermore, VCF use had no associated benefit regarding in-hospital mortality and contrariwise showed an increased incidence of procedure-related hematoma and a prolongation of hospital stay (42). A current meta-analysis on efficacy and safety of VCF placement for prevention of PE showed a reduction in the number of subsequent PE by 50% and an increase of the risk subsequent DVT by 70%. PE-related and all-cause mortality had no significant benefit (38).
Breast-feeding is another important consideration in this clinical scenario. It is known that alteplase does not cross the placenta because of its molecular size of 59 kDa (43), but it remains uncertain whether alteplase passes into breast milk in humans. However, absorption is unlikely because alteplase is destroyed in the infant’s gastrointestinal tract (43). The short half-life of 3.5–5.0 min explain why we pragmatically decided to pause breast-feeding for 24 h.
As has been shown in a recent meta-analysis, percutaneous endovenous intervention in conjunction with anticoagulation is associated with a significant reduction in PTS, a lower rate of venous obstruction and a lower rate of recurrent DVT (26). Conflicting results of the recently completed Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial on CDT in deep vein thrombosis that showed no statistically significant difference in PTS between patients with anticoagulation plus CDT vs. patients with anticoagulation alone (44) must be interpreted with caution. ATTRACT was limited by a high rate of ascending femoropopliteal thromboses, a high rate of implanted not dedicated venous stents, the lack of clear stenting criteria and a lower overall stenting rate which may have hampered the results (45). In patients with an extended iliofemoral thrombosis, imminent phlegmasia and May Thurner syndrome the availability of specifically configured reinforced bifurcational venous stents, may provide good clinical results with prevention of future PTS. However, it must be acknowledged that the reviewed case reports are subject to selection bias, which limits their generalizability.
In conclusion, CDT together with catheter-directed thrombectomy and additional stenting of residual stenotic lesions is effective and safe in postpartum patients with iliofemoral DVT. So far, encouraging case reports from the literature have not documented major bleeding complications during CDT > 2 days postpartum. To definitely clarify this issue larger case series and eventually randomized controlled trials appear necessary.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
Open access funding was provided by the University of Bern.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Sullivan EA Ford JB Chambers G Slaytor EK . Maternal mortality in Australia, 1973-1996.Aust N Zealand J Obstetr Gynaecol. (2004) 44:452–7. 10.1111/j.1479-828X.2004.00313.x
2.
Pomp ER Lenselink AM Rosendaal FR Doggen CJ . Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study.J Thromb Haemost. (2008) 6:632–7. 10.1111/j.1538-7836.2008.02921.x
3.
Ray JG Chan WS . Deep vein thrombosis during pregnancy and the puerperium: a meta-analysis of the period of risk and the leg of presentation.Obstetr Gynecol Survey. (1999) 54:265–71. 10.1097/00006254-199904000-00023
4.
Heit JA Kobbervig CE James AH Petterson TM Bailey KR Melton LJ III. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. (2005) 143:697–706. 10.7326/0003-4819-143-10-200511150-00006
5.
Hellgren M . Hemostasis during normal pregnancy and puerperium.Semin Thromb Hemost. (2003) 29:125–30. 10.1055/s-2003-38897
6.
Nazzal M El-Fedaly M Kazan V Qu W Renno AW Al-Natour M et al Incidence and clinical significance of iliac vein compression. Vascular. (2015) 23:337–43. 10.1177/1708538114551194
7.
Cogo A Lensing AW Prandoni P Hirsh J . Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound.Arch Intern Med. (1993) 153:2777–80.
8.
Enden T Haig Y Klow NE Slagsvold CE Sandvik L Ghanima W et al Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. (2012) 379:31–8. 10.1016/S0140-6736(11)61753-4
9.
Engelberger RP Fahrni J Willenberg T Baumann F Spirk D Diehm N et al Fixed low-dose ultrasound-assisted catheter-directed thrombolysis followed by routine stenting of residual stenosis for acute ilio-femoral deep-vein thrombosis. Thromb Haemost. (2014) 111:1153–60. 10.1160/TH13-11-0932
10.
Wells PS Forgie MA Rodger MA . Treatment of venous thromboembolism.JAMA. (2014) 311:717–28.
11.
Acharya G Singh K Hansen JB Kumar S Maltau JM . Catheter-directed thrombolysis for the management of postpartum deep venous thrombosis.Acta Obstetr Gynecol Scand. (2005) 84:155–8.
12.
Bloom AI Farkas A Kalish Y Elchalal U Spectre G . Pharmacomechanical catheter-directed thrombolysis for pregnancy-related iliofemoral deep vein thrombosis.J Vasc Interv Radiol. (2015) 26:992–1000. 10.1016/j.jvir.2015.03.001
13.
Demir MC Kucur D Cakir E Aksu NM Onur MR Sabuncu T et al May-Thurner syndrome: a curious syndrome in the ED. Am J Emerg Med. (2016) 34:1920.e1–3. 10.1016/j.ajem.2016.02.045
14.
Demirturk OS Oguzkurt L Coskun I Gulcan O . Endovascular treatment and the long-term results of postpartum deep vein thrombosis in 18 patients.Diagn Interv Radiol. (2012) 18:587–93. 10.4261/1305-3825.DIR.5808-12.1
15.
Dumantepe M Arif TI Ilhan Y Ozdemir A Azmi O . Endovascular treatment of postpartum deep venous thrombosis: report of three cases.Vascular. (2013) 21:380–5. 10.1177/1708538112472155
16.
Dumantepe M Tarhan IA Yurdakul I Ozler A . Ultrasound-accelerated catheter-directed thrombolysis for the management of postpartum deep venous thrombosis.J Obstetr Gynaecol Res. (2013) 39:1065–9. 10.1111/jog.12002
17.
Patterson DE Raviola CA D’Orazio EA Buch C Calligaro KD Dougherty MJ et al Thrombolytic and endovascular treatment of peripartum iliac vein thrombosis: a case report. J Vasc Surg. (1996) 24:1030–3. 10.1016/s0741-5214(96)70049-6
18.
Srinivas BC Patra S Agrawal N Manjunath CN . Successful catheter directed thrombolysis in postpartum deep venous thrombosis complicated by nicoumalone-induced skin necrosis and failure in retrieval of inferior vena caval filter.BMJ Case Rep. (2013) 2013:bcr2013010489. 10.1136/bcr-2013-010489
19.
Srinivas BC Patra S Nagesh CM Reddy B Manjunath CN . Catheter-directed thrombolysis is a safe and alternative therapeutic approach in the management of postpartum lower limb deep venous thrombosis.Int J Angiol. (2015) 24:292–5. 10.1055/s-0034-1374807
20.
Kearon C Akl EA Comerota AJ Prandoni P Bounameaux H Goldhaber SZ et al Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2012) 141:e419S–96S. 10.1378/chest.11-2301
21.
Royal College of Obstetricians and Gynaecologists. The Acute Management of Thrombosis and Embolism During Pregnancy and the Puerperium. Green-top Guideline No. 37b. London: RCOG (2015).
22.
Antman EM Anbe DT Armstrong PW Bates ER Green LA Hand M et al ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary. A report of the American college of cardiology/American heart association task force on practice guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. (2004) 44:671–719.
23.
Sousa Gomes M Guimaraes M Montenegro N . Thrombolysis in pregnancy: a literature review.J Matern Fetal Neonatal Med. (2019) 32:2418–28.
24.
Martillotti G Boehlen F Robert-Ebadi H Jastrow N Righini M Blondon M . Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review.J Thromb Haemost. (2017) 15:1942–50. 10.1111/jth.13802
25.
Akazawa M Nishida M . Thrombolysis with intravenous recombinant tissue plasminogen activator during early postpartum period: a review of the literature.Acta Obstetr Gynecol Scand. (2017) 96:529–35. 10.1111/aogs.13116
26.
Wang CN Deng HR . Percutaneous endovenous intervention plus anticoagulation versus anticoagulation alone for treating patients with proximal deep vein thrombosis: a meta-analysis and systematic review.Ann Vasc Surg. (2018) 49:39–48. 10.1016/j.avsg.2017.09.027
27.
Granger CB Becker R Tracy RP Califf RM Topol EJ Pieper KS et al Thrombin generation, inhibition and clinical outcomes in patients with acute myocardial infarction treated with thrombolytic therapy and heparin: results from the GUSTO-I trial. GUSTO-I hemostasis substudy group. Global utilization of streptokinase and TPA for occluded coronary arteries. J Am Coll Cardiol. (1998) 31:497–505. 10.1016/s0735-1097(97)00539-1
28.
Bick RLSJ . Thrombolytic therapy and its uses.Lab Med. (1995) 26:330–7.
29.
Bell WR . Laboratory monitoring of thrombolytic therapy.Clin Lab Med. (1995) 15:165–78.
30.
Perry DJGC . Acquired bleeding disorders. In: PorwitAMcCulloughJErberWNeditors. Blood and Bone Marrow Pathology (Second Edition). (Vol. 2). London: Churchill Livingstone (2011). p. 565–82.
31.
Shafer KE Santoro SA Sobel BE Jaffe AS . Monitoring activity of fibrinolytic agents. A therapeutic challenge.Am J Med. (1984) 76:879–86.
32.
Guervil DJ Rosenberg AF Winterstein AG Harris NS Johns TE Zumberg MS . Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion.Ann Pharmacother. (2011) 45:861–8. 10.1345/aph.1Q161
33.
Hirsh J Warkentin TE Shaughnessy SG Anand SS Halperin JL Raschke R et al Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. (2001) 119:64s–94s.
34.
Clark NP Delate T Cleary SJ Witt DM . Analysis of unfractionated heparin dose requirements to target therapeutic anti-Xa intensity during pregnancy.Thromb Res. (2010) 125:402–5. 10.1016/j.thromres.2009.07.014
35.
Arachchillage DRJ Kamani F Deplano S Banya W Laffan M . Should we abandon the APTT for monitoring unfractionated heparin?Thromb Res. (2017) 157:157–61. 10.1016/j.thromres.2017.07.006
36.
Hirsch DR Goldhaber SZ . Bleeding time and other laboratory tests to monitor the safety and efficacy of thrombolytic therapy.Chest. (1990) 97:124s–31s. 10.1378/chest.97.4_supplement.124s
37.
Loffroy R Falvo N Guillen K Galland C Baudot X Demaistre E et al Single-session percutaneous mechanical thrombectomy using the aspirex®S device plus stenting for acute iliofemoral deep vein thrombosis: safety, efficacy, and mid-term outcomes. Diagnostics. (2020) 10:544–54. 10.3390/diagnostics10080544
38.
Bikdeli B Chatterjee S Desai NR Kirtane AJ Desai MM Bracken MB et al Inferior vena cava filters to prevent pulmonary embolism: systematic review and meta-analysis. J Am Coll Cardiol. (2017) 70:1587–97. 10.1016/j.jacc.2017.07.775
39.
Mewissen MW Seabrook GR Meissner MH Cynamon J Labropoulos N Haughton SH . Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry.Radiology. (1999) 211:39–49. 10.1148/radiology.211.1.r99ap4739
40.
Protack CD Bakken AM Patel N Saad WE Waldman DL Davies MG . Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement.J Vasc Surg. (2007) 45:992–7. 10.1016/j.jvs.2007.01.012
41.
Kolbel T Lindh M Holst J Uher P Eriksson KF Sonesson B et al Extensive acute deep vein thrombosis of the iliocaval segment: midterm results of thrombolysis and stent placement. J Vasc Interv Radiol. (2007) 18:243–50. 10.1016/j.jvir.2006.12.002
42.
Akhtar OS Lakhter V Zack CJ Hussain H Aggarwal V Oliveros E et al Contemporary trends and comparative outcomes with adjunctive inferior vena cava filter placement in patients undergoing catheter-directed thrombolysis for deep vein thrombosis in the United States: insights from the national inpatient sample. JACC Cardiovasc Interv. (2018) 11:1390–7. 10.1016/j.jcin.2018.04.048
43.
National Library of Medicine. Alteplase. Drugs and Lactation Database (LactMed). Bethesda, MD: National Library of Medicine (2006).
44.
Vedantham S Goldhaber SZ Julian JA Kahn SR Jaff MR Cohen DJ et al Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. (2017) 377:2240–52.
45.
O’Sullivan GJ de Graaf R Black SA . Just how attractive is the ATTRACT trial?Cardiovasc Intervent Radiol. (2018) 41:1313–7.
Summary
Keywords
venous thromboembolism, iliofemoral deep vein thrombosis, catheter-directed thrombolysis, pregnancy, postpartum
Citation
Girona M, Säly C, Makaloski V, Baumgartner I and Schindewolf M (2022) Catheter-Directed Thrombolysis for Postpartum Deep Venous Thrombosis. Front. Cardiovasc. Med. 9:814057. doi: 10.3389/fcvm.2022.814057
Received
12 November 2021
Accepted
16 March 2022
Published
26 April 2022
Volume
9 - 2022
Edited by
Salah D. Qanadli, University of Lausanne, Switzerland
Reviewed by
Oliver Königsbrügge, Medical University of Vienna, Austria; Romaric Loffroy, Centre Hospitalier Regional Universitaire de Dijon, France
Updates
Copyright
© 2022 Girona, Säly, Makaloski, Baumgartner and Schindewolf.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Schindewolf, marc.schindewolf@insel.ch
This article was submitted to Atherosclerosis and Vascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.